Abstract
Background
Endometrial cancer (EC) is the most common gynecological malignancy with high incidence of metastasis, while the mechanism of metastasis in EC is not clear.
Methods
Immunohistochemistry and real-time PCR assays were used to assess expression of SOX17 in paraffin-embedded tissues from EC patients and in EC cells. The migration of EC cells was assessed by wound-healing and Transwell assays as well as in an in vitro study of nude mice. In addition, the expression of specific proteins was analyzed by Western blot.
Results
We observed that SOX17 expression levels were relatively high in stage I EC specimens, and were significantly correlated with the epithelial cadherin (E-cadherin) and β-catenin expression. Additionally, stage II EC patients whose specimens had relatively high SOX17 expression levels had better outcomes. Wound-healing and Transwell assays and in vivo murine experiments revealed that SOX17 inhibited EC cell migration. Meanwhile, SOX17 increased expression of E-cadherin and decreased expression of β-catenin and proteins in the Wnt signaling pathway. Moreover, LiCl (β-catenin activator) enhanced the regulatory effects of SOX17 on the expression of E-cadherin, promigratory cadherin, vimentin, and proteins in the Wnt signaling pathway, while XAV93920 (β-catenin inhibitor) exerted the opposite effect. The SOX17 N-terminus was proved to be necessary for these effects. Mechanistic investigations suggested SOX17 inhibits EC cell migration by inactivating the Wnt/β-catenin–epithelial mesenchymal transition (EMT) axis in EC cells.
Conclusion
We uncovered a common SOX17–β-catenin–EMT mechanism underlying EC cell migration.
Keywords: SOX17, endometrial cancer (EC), migration, high-mobility group (HMG)
Introduction
Endometrial cancer (EC) is the most common gynecological malignancy, with an estimated 54,870 new cases and 10,170 deaths in the US in 2015.1 Type I EC, which is also known as endometrioid EC, accounts for 70–80% of all EC cases. Additionally, in up to 30% cases of endometrioid EC, the malignancy has already invaded the myometrium upon diagnosis, subsequently leading to local or extra-pelvic metastasis, which is the most fatal consequence of endometrial carcinogenesis.2 The poor survival rate associated with advanced EC can be attributed to the fact that the mechanisms underlying its invasiveness and metastasis have not been sufficiently characterized. Thus, identifying novel molecular targets as markers for metastasis and for diagnostic of high-risk patients is extremely meaningful in clinic.
The epithelial–mesenchymal transition (EMT) is critical for endowing incipient cancer cells with invasive and metastatic properties.3 In tumor cells, an essential EMT step involves a decrease in epithelial cadherin (E-cadherin) abundance.4 A lack of E-cadherin expression is associated with a change from purely membranous expression to membrane–cytoplasmic expression, and is apparently critical for the progression of EC.2,5
β-Catenin was firstly isolated as the intracellular domain that binds E-cadherin to the cytoskeleton.6 Decreased E-cadherin expression activates β-catenin and promotes tumor invasiveness and metastasis.7 Moreover, β-catenin mediates Wnt/β-catenin signaling, which is a key element related to the induction of EMT. It has been reported that nuclear β-catenin accumulates in EC8 and the improper activation of the Wnt/β-catenin signaling pathway is essential for EC, especially type I EC.9 Sex-determining region Y-box 17 (SOX17) has been identified as an important antagonist and inhibitor of the canonical Wnt signaling pathway and suppresser of β-catenin activity.10,11 In EC, SOX17 downregulates the expression of Mastermind like 3 (MAML3), and subsequently inhibits the activation of β-catenin and the Wnt signaling pathway.12
Previous studies revealed that SOX17 is a member of a transcription factor superfamily and contributes to diverse developmental processes and disease contexts.13,14 SOX17 protein comprises 414 amino acids, and includes a high mobility group (HMG) box domain, which influences embryogenesis.15 Other studies demonstrated that SOX17 plays an important role during the development of various types of cancers,16 including colorectal cancer, breast cancer, gastric cancer, and hepatocellular carcinoma.17–20 Although 8% of EC specimens harbor a mutated SOX17,21 the regulatory effects of SOX17 on EMT in EC remain unclear.
In this study, we investigated whether SOX17 facilitates EMT and affects EC metastasis. We observed that downregulated SOX17 increases β-catenin expression, thereby decreasing E-cadherin expression and ultimately enabling EC cell migration.
Materials And Methods
Cells, Patients, And Samples
This study was approved by our hospital’s Protection of Human Subjects Committee and was performed according to the relevant guidelines. Specimens were acquired with written informed consent from patients at the Shanghai First Maternity and Infant Hospital Affiliated with Shanghai Tongji University. The study was conducted in accordance with the Declaration of Helsinki.
We analyzed the paraffin-embedded tissues of 90 patients who underwent surgery between January 2009 and January 2012, of whom 42 patients had stage I EC and 48 patients had stage II EC. The tumors were assigned stages (I or II) and histological grades (G2 or G3) based on the 2009 criteria of the International Federation of Gynecology and Obstetrics surgical staging system.22 None of the patients had undergone hormone therapy or radiotherapy before surgery. All of the patients provided written informed consent during follow-up. Follow-up data came from outpatient medical records and telephone inquiries, and updated until January 31, 2015.
Additionally, the human EC cell lines Ishikawa, HEC-1B, AN3CA, RL95-2, and KLE were obtained and maintained as recommended by the China Center for Type Culture Collection.
Immunohistochemistry Analysis
An immunohistochemistry (IHC) analysis was performed according to the manufacturer’s instructions with the following antibodies specific for target proteins: SOX17 (ab84990; Abcam, Cambridge, UK), E-cadherin (ab15148; Abcam), and β-catenin (ab16051; Abcam). Scoring was performed independently by two pathologists who were blinded to the clinical and pathological data. Protein staining was evaluated as previously described.23
RNA Extraction And Analysis
Total RNA was extracted and reverse transcribed as previously described.24 GAPDH was used as the reference gene. The following primer pairs were used for PCR amplifications: human SOX17, forward: 5′-ATCCTCAGACTCCTGGG TTT-3′, reverse: 5′-ACTGTTCAAGTGGCAGACAAA-3′; human E-cadherin, forward: 5′-CTTTCAAACACAGCCACGG-3′, reverse: 5′-GGTTGATCCTTATCGG TCACA G-3′; human promigratory cadherin (N-cadherin) gene, forward: 5′-ATCCTCAGACTCCTGGGTTT-3′, reverse: 5′-ACTGTTCAAGTGGCAGACAA A-3′; human CTNNB1, forward: 5′-CAATGGCTTGGAATGAGACTG-3′, reverse: 5′-ACCAGAGTGAAAAGAACGATAGC-3′; human C-myc, forward: 5′-CAAGACT CCAGCGCCTTCTC-3′, reverse: 5′-CTTCCTCATCTTCTTGTTCCT CC-3′; and GAPDH, forward: 5′-GGCT CCCTTGGGTATATGGT-3′, reverse: 5′-TTGATTTTGG AGGGATCTCG-3′.
Western Blot Analysis
A Western blot analysis was performed as previously described.25 with the following primary antibodies specific for target proteins: SOX17 (ab84990; Abcam), E-cadherin (ab15148; Abcam), N-cadherin (ab76057; Abcam), β-catenin (ab16051; Abcam), C-myc (ab32072; Abcam), and GAPDH (A01020; Sigma-Aldrich, Carlsbad, CA).
Cell Culture And Establishment Of SOX17-Expressing Tumor Cell Clones
All cell lines were obtained from the China Center for Type Culture Collection. Tumor cell clones overexpressing or underexpressing SOX17 were established as previously described.26
SOX17 RNA-Interference And Overexpression
We designed an oligonucleotide to target the SOX17 mRNA based on a sequence in the GenBank database (accession number NM022454). The RNA-interference target sequence was as follows: 5′-GGTATATTACTGCAACTAT-3′. A short hairpin RNA (shRNA) sequence was cloned into the pCMV6-Entry vector (Cat. PS100001; Origene, Rockville, MD). Positive clones were identified by PCR and sequenced. A SOX17 cDNA plasmid was purchased from Origene (Cat. RC220888).
Cell Migration Assays
To conduct a Transwell migration assay, 2 × 104 cells were added to individual upper chambers of a Transwell plate (Corning, Corning, NY), and the lower chambers were stained after an 24-h incubation.
Wound-Healing Assay
Cells were seeded in a 6-well plate and grown to 90–95% confluency. Plate surfaces were scratched with a pipette tip and photographed at 0 and 24 h.
Nude Mice Study
All animal experiments were performed in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals, with the approval of the Scientific Investigation Board of Tongji University School of Medicine. We purchased 4-6-week-old BALB/c nude mice from the Shanghai Laboratory Animal Center. HEC-1B cells stably expressing SOX17 or cells in which SOX17 was knocked down were suspended in PBS at a density of 3 × 107 cells/mL, after which a 200-μl aliquot of each cell solution was injected into the abdomen of nude mice (n = 5). The mice were euthanized 28 days later and their tumors were excised.
Statistical Analysis
All experiments were repeated in triplicate. Data are expressed as the mean ± standard deviation. Significant differences between two groups were determined based on Student’s t-test. The association between SOX17 expression and clinicopathological parameters was examined by the Chi-square test. Survival analysis adopted Kaplan-Meier test. A P value < 0.05 was used as the threshold for determining significance.
Results
SOX17 Expression Decreased In Stage II EC
An IHC analysis of the paraffin-embedded tissues revealed that the SOX17 expression levels were significantly lower in stage II EC than in stage I EC (Figure 1A). The expression of β-catenin was negatively correlated with the SOX17 and E-cadherin levels. The expression of E-cadherin was higher in stage I EC tissues than in stage II EC tissues. The correlations between clinicopathological features and SOX17 expression are summarized in Table 1. Low SOX17 levels were observed in the stage II EC samples. We selected the cases of stage II EC, and analyzed the relationship between SOX17 expression and patient survival. As expected, increasing SOX17 expression was associated with a higher survival rate (Figure 1B).
Figure 1.
Decrease in SOX17 levels in stage II endometrial cancer (EC). (A) Immunohistochemistry analysis of SOX17 expressions in EC tissues. The SOX17 protein abundance was higher in stage I EC tissues. Original magnification: ×400; bar = 50 μm. (B) Influence of SOX17 production on the overall survival of stage II EC patients. Increasing SOX17 levels extended the survival time. *P < 0.05.
Table 1.
Clinical Features Of Patients
| Feature | All | SOX17 Expression | P | |
|---|---|---|---|---|
| Low | High | |||
| Age (year) | 0.894 | |||
| <50 | 37 | 10 | 27 | |
| ≥50 | 53 | 15 | 38 | |
| Stage | 0.024 | |||
| I | 42 | 19 | 23 | |
| II | 48 | 33 | 15 | |
| Grade | 0.814 | |||
| II | 45 | 13 | 32 | |
| III | 45 | 12 | 33 | |
SOX17 Expression In EC Cells
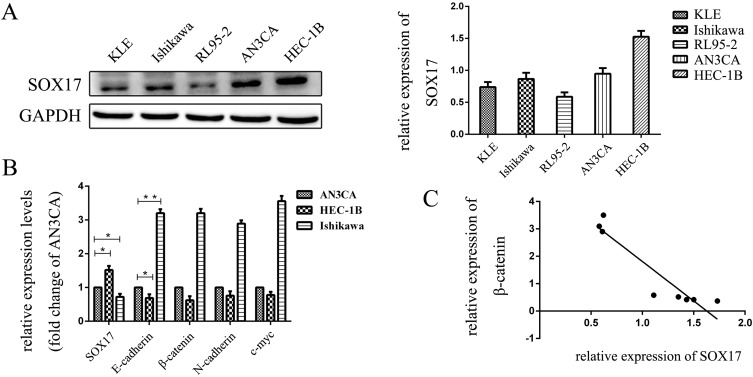
We assayed the expression level of SOX17 in EC cell lines and determined that SOX17 expression was highest in HEC-1B cells, while it was relatively low in RL95-2 and KLE cells. Therefore, we chose HEC-1B and Ishikawa as the study cell lines (Figure 2A). To confirm the relationship between SOX17 and β-catenin in EC cells, we performed real-time PCR to analyze the expression levels of the genes encoding these two proteins in EC cells. A relative decrease in SOX17 expression was observed in HEC-1B cells (defined as a decrease in SOX17 expression in AN3CA cells). In addition, Figure 2B indicates that there is a fold-change increase for SOX17 expression relative to the corresponding expression level in AN3CA, as well as a relative decrease in the β-catenin gene expression level, while a opposite pattern was observed in Ishikawa cells (Figure 2B and C).
Figure 2.
The expression of SOX17 in endometrial cancer (EC) cells. (A) Western blot analysis of SOX17 expression in EC cells. (B) PCR analysis of SOX17, β-catenin, N-cadherin, vimentin, and C-myc mRNA expression in EC cells relative to the GAPDH mRNA level. (C) PCR results indicated that low SOX17 expression levels were correlated with increased E-cadherin and β-catenin gene expression in EC cell lines. SOX17 and β-catenin gene expression levels were inversely correlated in human EC cells (R = −0.936; P = 0.001). *P < 0.05, **P < 0.01.
Since SOX17 is correlated with higher expression in the stage 1 endometrial cancer, we also assayed the effect of the estrogen and progesterone on SOX17 expression. No obvious effect was observed in HEC-1B and Ishikawa cells when estrogen 10nM or progesterone 10nM were used to treat (Supple Figure 1).
SOX17 Inhibited The Migration And Invasiveness Of EC Cell Lines
Next, we performed wound-healing and Transwell migration assays to analyze the influence of SOX17 on the migration of EC cells, and cell line HEC-1B and Ishikawa were selected as model cell lines. Specifically, the wound-healing assay revealed that SOX17-overexpressing cells migrated slower than the controls, while the SOX17-knockdown cells migrated faster (Figure 3A). The Transwell migration assay confirmed this observation. After 24 h, we stained the lower Transwell chamber (Figure 3B). Relative to the number of invaded control cells, fewer SOX17-overexpressing cells and more SOX17-knockdown cells were invaded. The increases and decreases in the number of invasive HEC-1B cells were 35 ± 4.6 and 120 ± 4.7, respectively, and the increases and decreases in the number of invasive Ishikawa cells were 48 ± 3.9 and 115 ± 5.1, respectively.
Figure 3.
SOX17 inhibits endometrial cancer (EC) cell mobility. (A) A wound-healing assay revealed the effect of SOX17 on cell migration. During a 24-h period, SOX17-overexpressing cells spread slower than the controls, whereas cells in which SOX17 was silenced by shRNA exhibited significantly greater cell mobility. (B) A Transwell migration assay indicated that SOX17 significantly inhibited the migration of EC cells. (C) SOX17 influenced the migration of tumor cells in vivo. After stably transfecting HEC-1B cells with either SOX17 or SOX17 shRNA, mice were inoculated with HEC-1B-SOX17, HEC-1B-SOX17shRNA, or HEC-1B-vector (control). Tumors of the HEC-1B-SOX17-treated group were smaller than those of the HEC-1B-SOX17shRNA-treated group. (D) Tumor volumes of the HEC-1B-SOX17-treated group were smaller than those of the HEC-1B-SOX17shRNA-treated group. (E) Tumor weights of the HEC-1B-SOX17-treated group were lower than those of the HEC-1B-SOX17shRNA-treated group. (F) Real-time PCR analysis confirmed that E-cadherin mRNA levels were higher, while β-catenin mRNA expression was lower, in the HEC-1B-SOX17-treated group. (G) Tumor E-cadherin expression was higher in the HEC-1B-SOX17-treated group than in the HEC-1B-SOX17shRNA-treated or control groups according to an immunohistochemistry analysis. *P < 0.05,**P < 0.01.
SOX17 Inhibited The Migration Of EC Cell Lines In Vivo
To further investigate the effects of SOX17 on the migration of EC cells, we performed an in vivo experiment involving the peritoneal implantation of transduced cells into BALB/c nude mice (Figure 3C). Tumor sizes and volumes in the SOX17 group were significantly smaller than those of the control group (Figure 3D and E). To further explore the mechanism underlying this phenomenon, we analyzed the expression of the genes encoding SOX17, E-cadherin, and β-catenin using real-time PCR (Figure 3F). Additionally, an IHC analysis of the tumor samples confirmed that tumor growth was inhibited in the SOX17 group (Figure 3G). The tumors from the SOX17 group contained significantly higher E-cadherin levels and lower β-catenin levels compared with the control group.
The N-Terminus Of SOX17 Is Necessary For The Inhibitory Activity Of The Protein
The SOX17 protein consists of 414 amino acids, including the HMG box domain, which contributes to embryogenesis. The N-terminus of SOX17, which comprises 132 amino acids, can specifically bind to the DNA element AACAAT.27 It has been reported that SOX17 can interact with β-catenin and T-cell factor (TCF)/lymphoid enhancer factor (LEF) proteins via the HMG box. To examine the combined structure of SOX17 and β-catenin, we developed plasmids carrying the SOX17 sequence that encode the N-terminus (amino acids 1–132) or C-terminus (amino acids 201–414) of SOX17 as well as hemagglutinin (HA) tags, and subsequently confirmed HA expression in a Western blot (Figure 4A and B).
Figure 4.
The SOX17 N-terminus is necessary for the inhibitory activity of the protein. (A) Structures of the SOX17 N-terminus and C-terminus. Plasmids carrying sequences encoding the SOX17 N-terminus or C-terminus and the HA tag were developed. (B) Western blotting involving HA antibodies verified the effects of the transfection with SOX17-N or SOX17-C. (C) Wound-healing assay revealed that the SOX17 N-terminus inhibited endometrial cancer (EC) cell migration, whereas the SOX17 C-terminus did not. (D) Transwell migration assay indicated that SOX17-C significantly inhibited EC cell migration. *P < 0.05, **P < 0.01.
We performed wound-healing and Transwell migration assays to analyze the influence of the SOX17 N-terminus on EC cell migration. Cells overexpressing the SOX17 sequence encoding the N-terminus migrated slower than the control cells. Additionally, compared with the control cells, SOX17-knockdown cells were less invasive and fewer of them migrated. In addition, the SOX17 C-terminus did not influence EC cell migration (Figure 4C and D).
SOX17 Increased E-Cadherin Expression And Suppressed EMT In A Process Requiring The SOX17 N-Terminus
We evaluated the effects of SOX17 on EMT and observed that E-cadherin expression increased in SOX17-overexpressing cells but decreased in EC cells after the SOX17 RNA-interference treatment. The expression of vimentin and β-catenin was retained in SOX17-overexpressing cells, but induced in SOX17-silenced cells (Figure 5A). To investigate the role of SOX17 in EMT, we generated HEC-1B and Ishikawa cell lines overexpressing SOX17. Interestingly, upregulated SOX17 expression led to increased E-cadherin gene expression levels, but decreased expression levels of the related genes (N-cadherin and vimentin), relative to the corresponding expression levels in cells bearing the empty vector (Figure 5B–D).
Figure 5.
SOX17 suppresses E-cadherin expression and epithelial mesenchymal transition. (A) Immunofluorescence analysis revealed that E-cadherin, vimentin, and β-catenin co-localized in Ishikawa and HEC-1B cell nuclei. Increased SOX17 expression decreased vimentin and β-catenin expression. (B) At 48 h after the transfection with SOX17, E-cadherin mRNA levels increased, while those of β-catenin, N-cadherin, vimentin, and C-myc decreased. (C) At 48 h after the transfection with SOX17 shRNA, E-cadherin mRNA levels increased, while the β-catenin, N-cadherin, vimentin, and C-myc mRNA levels decreased. (D) Western blotting at 72 h after the transfection with either SOX17 or SOX17 shRNA indicated the protein levels reflected the changes in mRNA levels. *P < 0.05, **P < 0.01.
Compared with the corresponding expression level in the control cells, the knockdown of SOX17 considerably decreased the expression of E-cadherin, which encodes a typical epithelial marker. In contrast, the knockdown of SOX17 was associated with an increase in the expression of the related genes N-cadherin and vimentin (Figure 5B–D).
An assay of the expression of E-cadherin, N-cadherin, vimentin, and C-myc revealed that the SOX17 N-terminus increased the abundance of E-cadherin at the mRNA and protein levels, but decreased the abundances of β-catenin, N-cadherin, vimentin, and C-myc at the mRNA level (Figure 6A, B and C). This effect was eliminated following the application of a β-catenin inhibitorc XAV93920, while the application of a β-catenin activator LiCl enhanced the effect (Figure 6A and B). The changes in the protein levels were consistent with the changes in the mRNA levels, as determined in a Western blot (Figure 6C).
Figure 6.
N-terminus of SOX17 suppresses E-cadherin expression and epithelial mesenchymal transition. (A) Real-time PCR analysis at 48 h after the transfection with the SOX17-N plasmid indicated E-cadherin mRNA levels increased, while the β-catenin, N-cadherin, vimentin, and C-myc mRNA levels decreased. (B) At 48 h after the transfection with the SOX17-C plasmid, mRNA levels were not significantly affected. (C) Proteins were detected at 72 h after the transfection with either the SOX17-N plasmid or the SOX17-C plasmid. *P < 0.05.
SOX17 Overexpression Decreased C-myc Levels, But Increased E-Cadherin Expression Via The Wnt/β-Catenin Pathway
The C-myc gene is targeted by the Wnt signaling pathway, and β-catenin enhances C-myc transcriptional activity. Because E-cadherin production is apparently suppressed by β-catenin,7 we focused on the mechanism by which SOX17 regulates the β-catenin–E-cadherin axis. Thus, we analyzed the SOX17-induced expression of genes encoding E-cadherin, N-cadherin, vimentin, and C-myc. Our data indicated that SOX17 upregulated the expression of the E-cadherin gene, while exerting an opposite effect on the genes encoding β-catenin, N-cadherin, vimentin, and C-myc (Figure 7A). This effect was eliminated in response to a β-catenin inhibitor, while a β-catenin activator enhanced the effect (Figure 7B). Western blot assay revealed that the changes in protein levels were consistent with the changes in mRNA levels (Figure 7B). We performed a Transwell assay to further verify the key effect of β-catenin on SOX17 that influences EC cell migration. The addition of a β-catenin inhibitor after the SOX17 transfection decreased cell migration, while the addition of a β-catenin activator had the opposite effect (Figure 7C). Meanwhile, as expected, SOX17 RNA-interference inversely changed expression when the β-catenin inhibitor or activator was applied (Figure 8A–C).
Figure 7.
SOX17 inactivates Wnt-based epithelial mesenchymal transition signaling in endometrial cancer (EC) cells. (A) Cells were transfected with SOX17, and then 24 h later, they were treated with XAV93920 (β-catenin inhibitor) or LiCl (β-catenin activator) for 24 h, after which mRNA levels were assayed. The increase in E-cadherin expression in cells transfected with SOX17 was inhibited by XAV93920. (B) Proteins were analyzed by Western blot. (C) Migration of cells after the SOX17 transfection and treatment with XAV93920 or LiCl. *P < 0.05, **P < 0.01.
Figure 8.
N-terminus of SOX17 inactivates Wnt-based epithelial mesenchymal transition signaling in endometrial cancer (EC) cells. (A) mRNA levels were detected after the SOX17 shRNA transfection and XAV93920 or LiCl treatment. (B) Changes in protein levels are shown. (C) Migration of EC cells after the transfection and subsequent treatment. *P < 0.05, **P < 0.01.
Discussion
In this study, we observed that SOX17 expression was relatively low in late-stage EC tissue, and were negatively correlated with E-cadherin expression. Our hypothesis that SOX17 is involved in the mechanism mediating cancer migration was verified by wound-healing and Transwell assays as well as an in vivo animal experiment. To assess the molecular mechanisms underlying EC cell invasiveness, we focused on β-catenin. We observed the strengthened or diminished effects by β-catenin activator and inhibitor accordingly. We also proved, for the first time, that the SOX17 N-terminus is required for EC cell migration. Additionally, we demonstrated the effect of SOX17 on EC metastasis for the first time.
Cadherin switching is necessary for the increased motility associated with EMT.28 Additionally, E-cadherin is converted to mesenchymal N-cadherin, which promotes tumor invasiveness and metastasis.29 In the present study, SOX17 suppressed EC cell migration and invasion in vivo and in vitro, increased E-cadherin mRNA and protein expression, and decreased N-cadherin expression. These results support the crutial role for SOX17 in EMT. To the best of our knowledge, this is the first study to reveal the effect of SOX17 on EMT.
Approximately 8% of EC specimens harbor a mutated SOX17,21 and SOX17 mutation was significantly associated with microsatellite instability (MSI) which present in about 30% of endometrioid endometrial carcinomas (EECs).30 In the samlpes used in this study from 90 EC patients, 25.6% of the stage I samples and 16.7% of the stage II samples expressed SOX17. Higher SOX17 muations associated with MSI were found in stage II samples.31 Functional assessment analysis showed that hotspot missense mutations have the transcriptional activities, but neither of the frameshift mutant proteins.31
Nuclear β-catenin reportedly accumulates in EC8 and we determined that SOX17 levels are negatively correlated with β-catenin levels. Additionally, SOX17 negatively regulates the β-catenin/TCF transcriptional activity in the Wnt/β-catenin signal transduction pathway.11,18 A recent study reported that SOX17 binds to the β-catenin gene promoter and inhibits tumor formation by suppressing β-catenin expression in cervical cancer.32 The essential SOX17 residues for this process are Lys512, Asp513, and Glu514.33 SOX17-HMG box domain mutations weaken the interaction with β-catenin, and hinder the singal transduction.30 In endometrial cancer, SOX17 binded to the MAML3 promoter, and MAML3 interacted with β-catenin, then SOX17 decreased Wnt pathway protein expression and suppressed EC cell proliferation.12
Our results also verified that SOX17 co-localizes with β-catenin and suppresses its activity. The Wnt/β-catenin pathway is a key factor that induces EMT. The activated Wnt pathway also induces β-catenin dissociation from the adherens junction complex and the translocation into the nucleus.34 In the present study, XAV93920 was used to inhibit the Wnt/β-catenin pathway, while LiCl was used as the activator.35 The XAV93920 treatment inhibited the SOX17 effect on EMT, while the LiCl treatment had the opposite effect. These results suggest that the interaction with β-catenin is critical for the SOX17-induced inhibition of EMT.
Several studies have shown that the C-myc gene is the downstream target of β-catenin and is involved in the induction of the EMT phenotype in tumors.36 In our study, SOX17 decreased mRNA and protein expression of C-myc according to levels of β-catenin. Moreover, the β-catenin inhibitor or activator suppressed or enhanced the effect of SOX17 on C-myc expression, implying that SOX17 influences EMT through the Wnt signaling pathway.
An earlier investigation confirmed that SOX17 interacts with TCF/LEF family members via its HMG box domain.37 The HMG box, comprising 79 amino acids, binds to the minor groove of specific DNA sequences38,39 and exerts its transcriptional effects through its interactions with co-activators or co-repressors.40 We added the HA tag to the SOX17 N-terminus containing an HMG domain. We observed that the SOX17 C-terminus lacking an HMG domain had no effect on EMT, while the N-terminus with an HMG domain was necessary for the SOX17-mediated effect on EMT.
Funding
This study was supported by the National Natural Science Foundation of China (81871126) and the Foundation Project of Shanghai Municipal Commission of Health and Family Planning (201640248).
Disclosure
The authors declare no conflicts of interest in this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332 [DOI] [PubMed] [Google Scholar]
- 2.Makker A, Goel MM. Tumor progression, metastasis, and modulators of epithelial-mesenchymal transition in endometrioid endometrial carcinoma: an update. Endocr Relat Cancer. 2016;23(2):R85–R111. doi: 10.1530/ERC-15-0218 [DOI] [PubMed] [Google Scholar]
- 3.Huang L, Wu RL, Xu AM. Epithelial-mesenchymal transition in gastric cancer. Am J Transl Res. 2015;7(11):2141–2158. [PMC free article] [PubMed] [Google Scholar]
- 4.Harris TJ, Tepass U. Adherens junctions: from molecules to morphogenesis. Nat Rev Mol Cell Biol. 2010;11(7):502–514. doi: 10.1038/nrm2927 [DOI] [PubMed] [Google Scholar]
- 5.Ahmed AR, Muhammad EM. E-Cadherin and CD10 expression in atypical hyperplastic and malignant endometrial lesions. J Egypt Natl Canc Inst. 2014;26(4):211–217. doi: 10.1016/j.jnci.2014.08.002 [DOI] [PubMed] [Google Scholar]
- 6.Aberle H, Butz S, Stappert J, Weissig H, Kemler R, Hoschuetzky H. Assembly of the cadherin-catenin complex in vitro with recombinant proteins. J Cell Sci. 1994;107(PT12):3655–3663. [DOI] [PubMed] [Google Scholar]
- 7.Yang J, Weinberg RA. Epithelial-mesenchymal transtition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14(6):818–829. doi: 10.1016/j.devcel.2008.05.009 [DOI] [PubMed] [Google Scholar]
- 8.Peiro G, Peiro FM, Ortiz-Martinez F, et al. Association of mammalian target of rapamycin with aggressive type II endometrial carcinomas and poor outcome: a potential target treatment. Hum Pathol. 2013;44(2):218–225. doi: 10.1016/j.humpath.2012.05.008 [DOI] [PubMed] [Google Scholar]
- 9.Markowska A, Pawalowska M, Lubin J, Markowska J. Signalling pathways in endometrial cancer. Contemp Oncol (Pozn). 2014;18(3):143–148. doi: 10.5114/wo.2014.43154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamachi Y, Kondoh H. Sox proteins: regulators of cell fate specification and differentiation. Development. 2013;140(20):4129–4144. doi: 10.1242/dev.091793 [DOI] [PubMed] [Google Scholar]
- 11.Jia Y, Yang Y, Liu S, Herman JG, Lu F, Guo M. SOX17 antagonizes WNT/β-catenin signaling pathway in hepatocellular carcinoma. Epigenetics. 2010;5(8):743–749. doi: 10.4161/epi.5.8.13104 [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Bao W, Wang K, et al. SOX17 is a tumor supperssor in endometrial cancer. Oncotarget. 2016;7(46):76036–76046. doi: 10.18632/oncotarget.12582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakajima-Takagi Y, Osawa M, Oshima M, et al. Role of SOX17 in hematopoietic development from human embryonic stem cells. Blood. 2013;121(3):447–458. doi: 10.1182/blood-2012-05-431403 [DOI] [PubMed] [Google Scholar]
- 14.Choi E, Kraus MR, Lemaire LA, et al. Dual lineage-specific expression of Sox17 during mouse embryogenesis. Stem Cells. 2012;30(10):2297–2308. doi: 10.1002/stem.1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katoh M. Molecular cloning and characterization of human SOX17. Int J Mol Med. 2002;9(2):153–157. [PubMed] [Google Scholar]
- 16.Dong C, Wilhelm D, Koopman P. Sox genes and cancer. Cytogenet Genome Res. 2004;105(2–4):442–447. doi: 10.1159/000078217 [DOI] [PubMed] [Google Scholar]
- 17.Zhang W, Glockner SC, Guo M, et al. Epigenetic inactivation of the canonical Wnt antagonist SRY-box containing gene 17 in colorectal cancer. Cancer Res. 2008;68(8):2764–2772. doi: 10.1158/0008-5472.CAN-07-6349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu DY, Wang ZM, Chen L, et al. Sox17, the canonical Wnt antagonist, is epigenetically inactivated by promoter methylation in human breast cancer. Breast Cancer Res Treat. 2010;119(3):601–612. doi: 10.1007/s10549-009-0339-8 [DOI] [PubMed] [Google Scholar]
- 19.Kuo IY, Wu CC, Chang JM, et al. Low SOX17 expression is a prognostic factor and drives transcriptional dysregulation and esophageal cancer progression. Int J Cancer. 2014;135(3):563–573. doi: 10.1002/ijc.28695 [DOI] [PubMed] [Google Scholar]
- 20.Yang T, Li XN, Li L, et al. Sox17 inhibits hepatocellular carcinoma progression by downregulation of KIF14 expression. Tumour Biol. 2014;35(11):11199–11207. doi: 10.1007/s13277-014-2398-7 [DOI] [PubMed] [Google Scholar]
- 21.Cancer Genome Atlas Research Network. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang X, Hu G, Xu C, et al. HZ08 reverse the aneuploidy-induced cisplatin-resistance in gastric cancer by modulating the p53 pathway. Eur J Pharmacol. 2013;720(1–3):84–97. doi: 10.1016/j.ejphar.2013.10.045 [DOI] [PubMed] [Google Scholar]
- 23.Zhou WJ, Geng ZH, Chi S, et al. Slit-Robo signaling induces malignant transformation through Hakai-mediated E-cadherin degradation during colorectal epithelial cell carcinogenesis. Cell Res. 2011;21(4):609–626. doi: 10.1038/cr.2011.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adams JM. Ways of dying: multiple pathways to apoptosis. Genes Dev. 2003;17(20):2481–2495. doi: 10.1101/gad.1126903 [DOI] [PubMed] [Google Scholar]
- 25.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305(5684):626–629. doi: 10.1126/science.1099320 [DOI] [PubMed] [Google Scholar]
- 26.Jurgensmeier JM, Xie Z, Deveraux Q, Ellerby L, Bredesen D, Reed JC. Bax directly induces release of cytochrome c from isolated mitochondria. Proc Natl Acad Sci USA. 1998;95(9):4997–5002. doi: 10.1073/pnas.95.9.4997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mertin S, McDowall SG, Harley VR. The DNA-binding specificity of SOX9 and other SOX proteins. Nucleic Acids Res. 1999;27(5):1359–1364. doi: 10.1093/nar/27.5.1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith A, Teknos NT, Pan Q. Epithelial to mesenchymal transition in head and neck squamous cell carcinoma. Oral Oncol. 2013;49(4):287–292. doi: 10.1016/j.oraloncology.2012.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shiozaki H, Oka H, Inoue M, Tamura S, Monden M. E-cadherin mediated adhesion system in cancer cells. Cancer. 1996;77(8 Suppl):1605–1613. doi: [DOI] [PubMed] [Google Scholar]
- 30.Walker CJ, O’Hern MJ, Serna VA, et al. Novel SOX17 frameshift mutations in endometrial cancer are functionally distinct from recurrentmissense mutations. Oncotarget. 2017;8(40):68758–68768. doi: 10.18632/oncotarget.20213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McMeekin DS, Tritchler DL, Cohn DE, et al. Clinicopathologic significance of mismatch repair defects in endometrial cancer: an NRGOncology/Gynecologic Oncology Group Study. J Clin Oncol. 2016;34(25):3062–3068. doi: 10.1200/JCO.2016.67.8722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li L, Yang WT, Zheng PS, Liu XF. SOX17 restrains proliferation and tumor formation by down-regulation activity of the Wnt/β-catenin signaling pathway via trans-suppressing β-catenin in cervical cancer. Cell Death Dis. 2018;9(7):741. doi: 10.1038/s41419-018-0782-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Banerjee A, Ray S. Structural insight, mutation and interactions in human Beta-catenin and SOX17 protein: a molecular-level outlook for organogenesis. Gene. 2017;610:118–126. doi: 10.1016/j.gene.2017.01.026 [DOI] [PubMed] [Google Scholar]
- 34.Cheung CT, Bendris N, Paul C, et al. Cyclin A2 modulates EMT via β-catenin and phospholipase C pathways. Carcinogenesis. 2015;36(8):914–924. doi: 10.1093/carcin/bgv069 [DOI] [PubMed] [Google Scholar]
- 35.Yang T, Zhang H, Qiu H, et al. EFEMP1 is repressed by estrogen and inhibits the epithelial-mesenchymal transition via Wnt/β-catenin signaling in endometrial carcinoma. Oncotarget. 2016;7(18):25712–25725. doi: 10.18632/oncotarget.8263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu W, Kovacevic Z, Peng Z, et al. The molecular effect of metastasis suppressors on Src signaling and tumorigenesis: new therapeutic targets. Oncotarget. 2015;6(34):35522–35541. doi: 10.18632/oncotarget.5849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zorn AM, Barish GD, Williams BO, Lavender P, Klymkowsky MW, Varmus HE. Regulation of Wnt signaling by Sox proteins: XSox17 alpha/beta and XSox3 physically interact with beta-catenin. Mol Cell. 1999;4(4):487–498. doi: 10.1016/s1097-2765(00)80200-2 [DOI] [PubMed] [Google Scholar]
- 38.Palasingam P, Jauch R, Ng CK, Kolatkar PR. The structure of Sox 17 bound to DNA reveals a conserved bending topology but selective protein interaction platforms. J Mol Biol. 2009;388(3):619–630. doi: 10.1016/j.jmb.2009.03.055 [DOI] [PubMed] [Google Scholar]
- 39.Francois M, Koopman P, Beltrame M. SoxF genes: key players in the development of the cardio-vascular system. Int J Biochem Cell Biol. 2010;42(3):445–448. doi: 10.1016/j.biocel.2009.08.017 [DOI] [PubMed] [Google Scholar]
- 40.Fores M, Simon-Carrasco L, Ajuria L, et al. A new mode of DNA binding distinguishes Capicua from other HMG-box factors and explains its mutation patterns in cancer. PLoS Genet. 2017;13(3):e1006622. doi: 10.1371/journal.pgen.1006622 [DOI] [PMC free article] [PubMed] [Google Scholar]