Abstract
The best-known appetite-regulating factors identified in rodents are leptin, an appetite inhibitor, and ghrelin, an appetite stimulator. Rare cases of loss-of-functions mutations affecting leptin and its receptor, as well as polymorphisms concerning ghrelin and its receptor, have been documented in human obesity, apparently validating the relevance of leptin and ghrelin for human physiology. Paradoxically, however, the overwhelming majority of obese individuals manifest high leptin and low ghrelin plasma levels, suggesting that both factors are not directly disease-relevant. We recently discovered that acyl-CoA-binding protein (ACBP), also known as diazepam-binding inhibitor (DBI), acts as an efficient lipogenic and appetite stimulator in mice. Indeed, in response to starvation, ACBP/DBI is released from tissues in an autophagy-dependent fashion and increases in the plasma. Intravenous injection of ACBP/DBI stimulates feeding behavior through a reduction of circulating glucose levels, and consequent activation of orexigenic neurons in the hypothalamus. In contrast, neutralization of ACBP/DBI abolishes the hyperphagia observed after starvation of mice. Of note, ACBP/DBI is increased in the plasma of obese persons and mice, pointing to a convergence (rather than divergence) between its role in appetite stimulation and human obesity. Based on our results, we postulate a novel ‘hunger reflex' in which starvation induces a surge in extracellular ACBP/DBI, which in turn stimulates feeding behavior. Thus, ACBP/DBI might be the elusive ‘hunger factor' that explains increased food uptake in obesity.
Keywords: appetite control, diazepam binding protein, metabolism, obesity, unconventional protein secretion
Overweight has beaten undernutrition as the most frequent pathological state throughout the world, affecting close to 25% of the adult population. This has severe implications for global health, given that obesity is the major risk factor for most if not all non-communicable diseases, including the entire spectrum of cardiovascular, neoplastic, metabolic and neurodegenerative diseases. Among the G20 countries, the US is the uncontested leader (adult obesity rate ~36%) followed by countries with a rate of 30-35% (Saudi Arabia, Turkey), a large group of countries with a rate of 20-30% (Argentina, Australia, Brazil, Canada, France, Germany, Mexico, Russia, South Africa, United Kingdom), one European Country that is just undercutting 20% (Italy) and a group of Asian Countries with obesity rates well under 10% (China, India, Indonesia, Japan, South Korea). These numbers (http://worldpopulationreview.com) eloquently underscore the cause of the obesity pandemic, which is the Western lifestyle characterized by excessive consumption of calories (and in particular carbohydrates and ultra-processed food) coupled to sedentarism, as well as the failure of public health education [1–4].
In spite of the extremely high prevalence of obesity, multiple studies have been designed to define genetically determined ‘risk factors' that would explain why only one third of the population reaches a body mass index (BMI) >30 [5–7]. Such studies were spurred by the discovery of leptin, the satiety hormone. Loss-of-function mutation of leptin in mice causes hyperphagy and obesity (in Ob/Ob mice), as does that of its receptor (in Db/Db mice) [8, 9]. Later exceptionally rare cases of human obesity with mutations in the genes coding for leptin or its receptor were described [10, 11]. However, the vast majority of obese patients exhibit an increase in circulating leptin levels (perhaps as a failing homeostatic mechanism in which leptin levels are upregulated yet fail to tame appetite), meaning that leptin deficiency is not a major pathogenic factor in obesity [12–14]. Other studies have led to the discovery of a major appetite-stimulatory factor, ghrelin, in rodents [15]. However, obese patients exhibit a decrease in circulating ghrelin levels (again, likely as a failing homeostatic mechanism), indicating that excessive ghrelin cannot be the cause of human obesity [16, 17]. Moreover, in patients with anorexia nervosa, ghrelin levels are paradoxically high [18, 19], while leptin levels are paradoxically low [20, 21]. These results indicate that major appetite control circuitries discovered in mice cannot be pharmacologically manipulated to prevent or treat human eating disorders.
In eukaryotes, autophagy is the phylogenetically most ancient response to dwindling nutrient resources, allowing cells and organisms to sequester and to digest non-essential macromolecules contained in the cytoplasm [22, 23]. Continuous or periodic stimulation of autophagy by caloric restriction or intermittent fasting, respectively, improves the fitness of model organisms ranging from yeast to primates [24–28]. Indeed, autophagy is the mechanisms through which constant or periodic limitations in food access increases the healthspan and lifespan of model organisms [29–31]. Caloric excess suppresses autophagy, thereby abolishing an important cytoplasmic recycling mechanism, favoring the storage of excessive lipid in a variety of cell types, reducing cellular and organismal fitness, and likely precipitating the manifestation of age-related diseases, which are the ‘co-morbidities' of obesity [32, 33]. Indeed, obesity is linked to a state of autophagic suppression [34] and autophagy induction by pharmacologic manipulations has anti-obesity effects [35], suggesting that autophagy inhibition is causally involved in the pathogenic cascade that leads to supraphysiological adiposity [33, 36].
Intrigued by these insights, we have been attempting to develop ‘caloric restriction mimetics' (CRMs), i.e. pharmacological agents that mimic the biochemical effects of caloric restriction [37–39]. Nutrient deprivation causes autophagy induction through the depletion of the cytosolic pool of acetyl coenzyme A (AcCoA), resulting in deacetylation of cytoplasmic proteins (including a number of proteins involved in the regulation and execution of autophagy), thereby stimulating autophagic flux [40, 41]. CRMs mimic the effect of caloric restriction because they inhibit enzymes that generate AcCoA (such as ATP citrate lyase) or that use AcCoA for protein acetylation (such as the EP300 acetyltransferase) or, alternatively, stimulate deacetylases (such as sirtuin 1), resulting in autophagy induction [38, 39]. The collection of CRMs includes several compounds reputed for their capacity to extend healthspan and/or lifespan such as aspirin [42], chalcones [43], resveratrol [44] and spermidine [30, 45]. This latter agent is a natural polyamine present in food items. Epidemiological studies suggest that ingestion of high levels of spermidine reduces overall mortality as well as disease-specific mortality from cancer and cardiovascular disorders [46–48], supporting prior evidence in yeast, nematodes, fruit flies and mice that spermidine delays age-associated disease and death [30, 45].
Given that autophagy seems to antagonize obesity-associated disease pathogenesis [35, 49], we searched for novel ways to stimulate this process. Back in 2010, several groups reported that fungal species can release one particular protein, acyl-coenzyme A binding protein (ACBP, also known as diazepam-binding inhibitor, DBI) in an autophagy-dependent fashion [50–52]. Based on the fact that cell stress is usually communicated to other cells, a phenomenon that can be referred to ‘inside-outside communication' [53], we wondered whether extracellular ACBP/DBI protein might be a target for modulating autophagy or even impact on pathogenic processes. In mice and humans, ACBP/DBI is ubiquitously expressed (though particularly high in adipocytes, https://www.proteinatlas.org/). As indicated by its dual name, ACBP/DBI has two functions, as an intracellular buffer and transporter for acyl coenzyme A, and as a modulator of benzodiazepine receptors (and in particular the gamma-aminobutyric acid (GABA) A receptor) [54–56]. Intriguingly, we found that any type of human or murine cell released ACBP/DBI upon starvation (which is the most physiological stimulus of autophagy) in vitro and in vivo through a process that can be inhibited by deletion of essential autophagy genes or by pharmacological autophagy inhibitors [57]. Thus, the autophagy-associated release of intracellular ACBP/DBI into the extracellular space appears to be a general, phylogenetically conserved phenomenon that applies to both fungal and mammalian systems.
We then set out to determine the effects of ACBP/DBI on autophagy and general metabolism. Interestingly, in human and mouse cell cultures the depletion of intracellular and extracellular ACBP/DBI do not have the same effects on autophagy. Depletion of intracellular ACBP/DBI by small interfering RNAs (siRNAs) inhibits autophagy, while neutralization of extracellular ACBP/DBI with suitable antibodies stimulates autophagy [57]. These results may be interpreted to mean that the autophagy-associated secretion of ACBP/DBI is involved in a negative feedback loop limiting autophagy. In mice, fasting was associated with an increase in the plasma concentration of ACBP/DBI, and intravenous injection of recombinant ACBP/DBI protein inhibited starvation-induced autophagy, while ACBP/DBI neutralization (by means of an intraperitoneally injected antibody) enhanced autophagy [57].
Intravenous injection of recombinant ACBP/DBI protein had multiple effects on metabolism (Fig. 1) including a rapid (30 min) increase in the expression of the glucose transporter GLUT1 on hepatocytes. This was accompanied by a reduction in plasma glucose levels that could be prevented by GLUT1 inhibitors. Experiments involving isotope-labelled glucose revealed the presence of labeled-glucose in the adipose tissue a few hours after ACBP/DBI injection. ACBP/DBI concomitantly inhibited fatty acid oxidation. Most importantly, mice injected with ACBP/DBI manifested a close-to-immediate (30 min) hyperphagic response that was accompanied by the activation of orexigenic neurons in the hypothalamus. When glucose levels were maintained in an artificial fashion (by injection of glucose into the peritoneal cavity) both hyperphagy and the activation of orexigenic neurons were prevented, suggesting that the effects of ACBP/DBI on central appetite control were secondary to its metabolic effects on peripheral tissues. Of note, in this time frame ACBP/DBI injection did not affect insulin or ghrelin levels. Of note, sustained overexpression of a transgene coding for ACBP/DBI in hepatocytes was sufficient to cause a significant increase in weight gain coupled to an augmentation of perigonadal and visceral adiposity [57].
Figure 1. FIGURE 1: Effects of recombinant ACBP/DBI in mice.
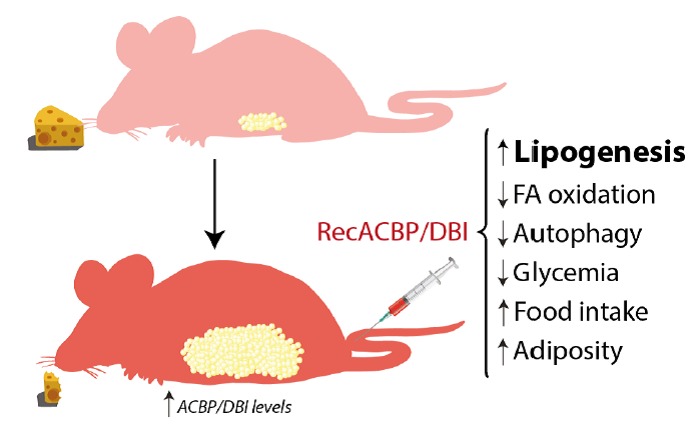
Metabolic effects detailed after intravenous injection of recombinant ACBP/DBI (recACBP/DBI) protein, including an increase of lipogenesis, food intake and consequent adiposity and decrease of autophagy, glycemia and fatty acid (FA) oxidation.
Altogether, the aforementioned data suggest that ACBP/DBI is an orexigenic and obesogenic factor. In accord with this interpretation, neutralization of ACBP/DBI had anorexigenic and lipolytic effects (Fig. 2). Thus, the hyperphagic response of mice that had been starved for 24 hours (which causes ~10% weight loss) was largely abolished by intraperitoneal injection of neutralizing ACBP/DBI antibodies, which, in parallel, prevented the reduction of glycemia that normally accompanies a 24-hour fasting period, caused a decrease in circulating insulin levels, and caused the activation of anorexigenic neurons in the hypothalamus. In mice, ACBP/DBI neutralization led to increased lipolysis from white adipose tissue, an increase in gluconeogenesis from glycerol (which may explain the maintenance of glucose levels), as well as an important raise in fatty acid oxidation. ACBP/DBI neutralization could be achieved for longer periods by a specific immunization protocol designed to break autotolerance and to elicit autoantibodies against ACBP/DBI. The surge in neutralizing ACBP/DBI autoantibodies led to a reduction in weight gain induced by high-fat diet (in normal mice) or by feeding a normal diet to leptin-deficient (Ob/Ob) mice. These effects were accompanied by a reduction in the abundance of white adipose tissue, a reduction in the median diameter of adipocytes, browning of fat, amelioration of the glucose tolerance test, as well as a reduction of hepatosteatosis. Of note, an inducible whole-body knockout of ACBP/DBI recapitulated many of these features, suggesting that the predominant effect of both (intracellular + extracellular) pools of ACBP/DBI is indeed orexigenic and obesogenic [57]. In a plausible scenario, ACBP/DBI would be involved in a ‘hunger reflex' in which starvation leads to a transient, autophagy-dependent release of ACBP/DBI from tissues, and extracellular ACBP/DBI then causes metabolic changes that ultimately stimulate feeding behavior, favor lipo-anabolic reactions and inhibit catabolic pathways including autophagy (Fig. 3).
Figure 2. FIGURE 2: ACBP/DBI neutralization and its effects in mice.
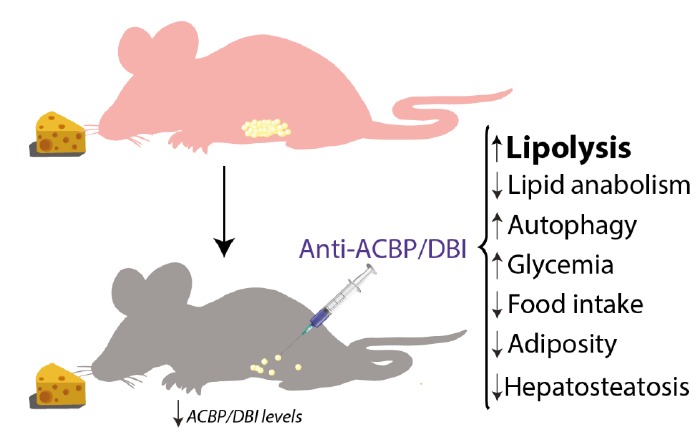
Metabolic effects observed after intraperitoneal injection of neutralizing anti-ACBP/DBI antibody, including an increase of lipolysis, glycemia and autophagy and decrease of lipogenesis, food intake, hepatosteatosis and body weight.
Figure 3. FIGURE 3: The ‘hunger reflex'.
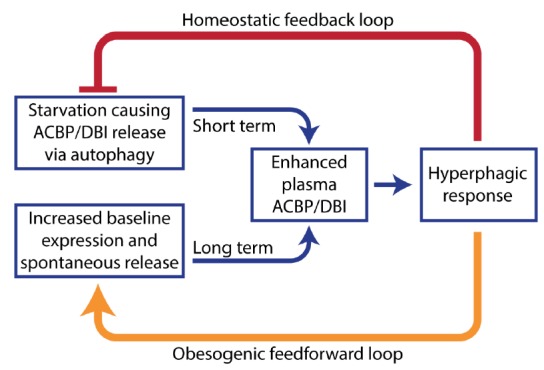
Hypothetical explanation of the pathogenesis of obesity. Extracellular ACBP/DBI participates in a short-term homeostatic feedback loop to link starvation to food intake. However, obesity causes an increase in ACBP/DBI expression and its spontaneous release that leads to chronic hyperphagy, thus locking the disease in a pathogenic feedforward loop.
We also examined the levels of ACBP/DBI expression in patients with anorexia and obesity. Of note, we found a high (Spearman r>0.8) positive correlation between the body mass index and plasma ACBP/DBI levels across several patient cohorts. Thus, anorexic patients manifested subnormal ACBP/DBI plasma concentrations, while obese individuals were characterized by supranormal ACBP/DBI. After successful bariatric surgery ACBP/DBI levels decline when patients lose weight, but increase again when they relapse. Dietary interventions that cause transient weight loss also temporarily reduce ACBP/DBI mRNA expression in the periumbilical fat tissue [57]. In mice, we observed a similar trend. Murine obesity was associated with higher ACBP/DBI plasma concentrations, as well as with increased ACBP/DBI mRNA and protein expression in the liver and white adipose tissue. In obese humans, we found a positive association between, on one hand, plasma ACBP/DBI and, on the other hand, fasting insulin levels as well as aspartate transaminases (AST). Thus, ACBP/DBI correlates with laboratory parameters indicative of insulin-resistant (type 2) diabetes and liver damage [57]. However, such clinical observations do not allow to establish any cause-effect relationships beyond these correlations.
Altogether, these results support the notion that ACBP/DBI has not only an obesogenic function in mice but that it is indeed increased in obesity in humans. Thus, at difference with leptin and ghrelin, ACBP/DBI exhibits a concordant (rather than discordant) behavior in mice and in humans with eating disorders (Table 1). At this stage, we postulate that ACBP/DBI may well be the elusive ‘hunger factor' that is elevated in obesity. Obviously, clinical studies must be designed to neutralize ACBP/DBI or its receptor and to validate this assumption.
Table 1.
Comparison among major appetite control systems in mice and human obesity.
| Mouse | Human | Observation | |
|---|---|---|---|
| Leptin | Genetic deficiency of leptin or its receptor causes hyperphagy and obesity. | High in obesity Low in anorexia nervosa |
Paradoxical association of human hyperphagy with high levels of an anorexigenic factor. |
| Ghrelin | Administration of ghrelin causes hy-perphagy and obesity. | Low in obesity High in anorexia nervosa |
Paradoxical association of human hyperphagy with low levels of an orexigenic factor. |
| ACBP/DBI | ACBP/DBI administration causes hy-perphagy and obesity, while neutrali-zation of ACBP/DBI is anorexigenic. | High in obesity Low in anorexia nervosa |
Concordant association of human hyperphagy with high levels of an orexigenic factor. |
Acknowledgments
GK is supported by the Ligue contre le Cancer (équipe labellisée); Agence National de la Recherche (ANR) – Projets blancs; ANR under the frame of E-Rare-2, the ERA-Net for Research on Rare Diseases; Association pour la recherche sur le cancer (ARC); Association “Le Cancer du Sein, Parlons-en!”, Cancéropôle Ile-de-France; Chancelerie des universités de Paris (Legs Poix), Fondation pour la Recherche Médicale (FRM); a donation by Elior; European Research Area Network on Cardiovascular Diseases (ERA-CVD, MINOTAUR); Gustave Roussy Odyssea, the European Union Horizon 2020 Project Oncobiome; Fondation Carrefour; High-end Foreign Expert Program in China (GDW20171100085), Institut National du Cancer (INCa); Inserm (HTE); Inserm Transfert, Institut Universitaire de France; LeDucq Foundation; the LabEx Immuno-Oncology (ANR-18-IDEX-0001); the RHU Torino Lumière; the Seerave Foundation; the SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); and the SIRIC Cancer Research and Personalized Medicine (CARPEM). F.M. is grateful to the Austrian Science Fund FWF (SFB LIPOTOX F3007 & F3012, W1226, P29203, P29262, P27893, P 31727 and the Austrian Federal Ministry of Education, Science and Research and the University of Graz for grants “Unkonventionelle Forschung-InterFast” and “flysleep” (BMWFW-80.109/0001-WF/V/3b/2015) as well as the field of excellence program BioHealth. We acknowledge support from NAWI Graz and the BioTechMed-Graz flagship project “EPIAge”.
Abbreviations:
- ACBP
– Acyl-CoA-binding protein,
- AcCoA
– acetyl coenzyme A,
- CRM
– caloric restriction mimetic,
- DBI
– diazepam-binding protein.
REFERENCES
- 1.Bovet P, Chiolero A, Gedeon J. Health Effects of Overweight and Obesity in 195 Countries. N Engl J Med. 2017;377(15):1495–1496. doi: 10.1056/NEJMc1710026. [DOI] [PubMed] [Google Scholar]
- 2.Geserick M, Vogel M, Gausche R, Lipek T, Spielau U, Keller E, Pfaffle R, Kiess W, Korner A. Acceleration of BMI in Early Childhood and Risk of Sustained Obesity. N Engl J Med. 2018;379(14):1303–1312. doi: 10.1056/NEJMoa1803527. [DOI] [PubMed] [Google Scholar]
- 3.Kroemer G, Lopez-Otin C, Madeo F, de Cabo R. Carbotoxicity-Noxious Effects of Carbohydrates. Cell. 2018;175(3):605–614. doi: 10.1016/j.cell.2018.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baur LA, Garnett SP. Early childhood - a critical period for obesity prevention. Nat Rev Endocrinol. 2018;15(1):5–6. doi: 10.1038/s41574-018-0131-0. [DOI] [PubMed] [Google Scholar]
- 5.Robinson MR, Hemani G, Medina-Gomez C, Mezzavilla M, Esko T, Shakhbazov K, Powell JE, Vinkhuyzen A, Berndt SI, Gustafsson S, Justice AE, Kahali B, Locke AE, Pers TH, Vedantam S, Wood AR, van Rheenen W, Andreassen OA, Gasparini P, Metspalu A, Berg LH, Veldink JH, Rivadeneira F, Werge TM, Abecasis GR, Boomsma DI, Chasman DI, de Geus EJ, Frayling TM, Hirschhorn JN, et al. Population genetic differentiation of height and body mass index across Europe. Nat Genet. 2015;47(11):1357–1362. doi: 10.1038/ng.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zillikens MC, Demissie S, Hsu YH, Yerges-Armstrong LM, Chou WC, Stolk L, Livshits G, Broer L, Johnson T, Koller DL, Kutalik Z, Luan J, Malkin I, Ried JS, Smith AV, Thorleifsson G, Vandenput L, Hua Zhao J, Zhang W, Aghdassi A, Akesson K, Amin N, Baier LJ, Barroso I, Bennett DA, Bertram L, Biffar R, Bochud M, Boehnke M, Borecki IB, et al. Large meta-analysis of genome-wide association studies identifies five loci for lean body mass. Nat Commun. 2017;8(1):80. doi: 10.1038/s41467-017-00031-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turcot V, Lu Y, Highland HM, Schurmann C, Justice AE, Fine RS, Bradfield JP, Esko T, Giri A, Graff M, Guo X, Hendricks AE, Karaderi T, Lempradl A, Locke AE, Mahajan A, Marouli E, Sivapalaratnam S, Young KL, Alfred T, Feitosa MF, Masca NGD, Manning AK, Medina-Gomez C, Mudgal P, Ng MCY, Reiner AP, Vedantam S, Willems SM, Winkler TW, et al. Protein-altering variants associated with body mass index implicate pathways that control energy intake and expenditure in obesity. Nat Genet. 2018;50(1):26–41. doi: 10.1038/s41588-017-0011-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269(5223):543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 9.Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, Friedman JM. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379(6566):632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- 10.Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ, Sewter CP, Digby JE, Mohammed SN, Hurst JA, Cheetham CH, Earley AR, Barnett AH, Prins JB, O'Rahilly S. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387(6636):903–908. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 11.Clement K, Vaisse C, Lahlou N, Cabrol S, Pelloux V, Cassuto D, Gourmelen M, Dina C, Chambaz J, Lacorte JM, Basdevant A, Bougneres P, Lebouc Y, Froguel P, Guy-Grand B. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature. 1998;392(6674):398–401. doi: 10.1038/32911. [DOI] [PubMed] [Google Scholar]
- 12.Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, Hughes IA, McCamish MA, O'Rahilly S. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med. 1999;341(12):879–884. doi: 10.1056/NEJM199909163411204. [DOI] [PubMed] [Google Scholar]
- 13.Einerhand MP, Bakx TA, Valerio D. IL-6 production by retrovirus packaging cells and cultured bone marrow cells. Hum Gene Ther. 1991;2(4):301–306. doi: 10.1089/hum.1991.2.4-301. [DOI] [PubMed] [Google Scholar]
- 14.Pan WW, Myers MG., Jr. Leptin and the maintenance of elevated body weight. Nat Rev Neurosci. 2018;19(2):95–105. doi: 10.1038/nrn.2017.168. [DOI] [PubMed] [Google Scholar]
- 15.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407(6806):908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 16.Tschop M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001;50(4):707–709. doi: 10.2337/diabetes.50.4.707. [DOI] [PubMed] [Google Scholar]
- 17.Makris MC, Alexandrou A, Papatsoutsos EG, Malietzis G, Tsilimigras DI, Guerron AD, Moris D. Ghrelin and Obesity: Identifying Gaps and Dispelling Myths. A Reappraisal. In Vivo. 2017;31(6):1047–1050. doi: 10.21873/invivo.11168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, Suda M, Koh T, Natsui K, Toyooka S, Shirakami G, Usui T, Shimatsu A, Doi K, Hosoda H, Kojima M, Kangawa K, Nakao K. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab. 2001;86(10):4753–4758. doi: 10.1210/jcem.86.10.7885. [DOI] [PubMed] [Google Scholar]
- 19.Otto B, Cuntz U, Fruehauf E, Wawarta R, Folwaczny C, Riepl RL, Heiman ML, Lehnert P, Fichter M, Tschop M. Weight gain decreases elevated plasma ghrelin concentrations of patients with anorexia nervosa. Eur J Endocrinol. 2001;145(5):669–673. doi: 10.1530/eje-1450669. [DOI] [PubMed] [Google Scholar]
- 20.Hebebrand J, Blum WF, Barth N, Coners H, Englaro P, Juul A, Ziegler A, Warnke A, Rascher W, Remschmidt H. Leptin levels in patients with anorexia nervosa are reduced in the acute stage and elevated upon short-term weight restoration. Mol Psychiatry. 1997;2(4):330–334. doi: 10.1038/sj.mp.4000282. [DOI] [PubMed] [Google Scholar]
- 21.Ferron F, Considine RV, Peino R, Lado IG, Dieguez C, Casanueva FF. Serum leptin concentrations in patients with anorexia nervosa, bulimia nervosa and non-specific eating disorders correlate with the body mass index but are independent of the respective disease. Clin Endocrinol. 1997;46(3):289–293. doi: 10.1046/j.1365-2265.1997.1260938.x. [DOI] [PubMed] [Google Scholar]
- 22.Galluzzi L, Pietrocola F, Levine B, Kroemer G. Metabolic control of autophagy. Cell. 2014;159(6):1263–1276. doi: 10.1016/j.cell.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levine B, Kroemer G. Biological Functions of Autophagy Genes: A Disease Perspective. Cell. 2019;176(1-2):11–42. doi: 10.1016/j.cell.2018.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Cabo R, Carmona-Gutierrez D, Bernier M, Hall MN, Madeo F. The search for antiaging interventions: from elixirs to fasting regimens. Cell. 2014;157(7):1515–1526. doi: 10.1016/j.cell.2014.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitchell SJ, Madrigal-Matute J, Scheibye-Knudsen M, Fang E, Aon M, Gonzalez-Reyes JA, Cortassa S, Kaushik S, Gonzalez-Freire M, Patel B, Wahl D, Ali A, Calvo-Rubio M, Buron MI, Guiterrez V, Ward TM, Palacios HH, Cai H, Frederick DW, Hine C, Broeskamp F, Habering L, Dawson J, Beasley TM, Wan J, Ikeno Y, Hubbard G, Becker KG, Zhang Y, Bohr VA, et al. Effects of Sex, Strain, and Energy Intake on Hallmarks of Aging in Mice. Cell Metab. 2016;23(6):1093–1112. doi: 10.1016/j.cmet.2016.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev. 2017;39:46–58. doi: 10.1016/j.arr.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mattson MP, Moehl K, Ghena N, Schmaedick M, Cheng A. Intermittent metabolic switching, neuroplasticity and brain health. Nat Rev Neurosci. 2018;19(2):63–80. doi: 10.1038/nrn.2017.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stekovic S, Hofer SJ, Tripolt N, Aon MA, Royer P, Pein L, Stadler JT, Pendl T, Prietl B, Url J, Schroeder S, Tadic J, Eisenberg T, Magnes C, Stumpe M, Zuegner E, Bordag N, Riedl R, Schmidt A, Kolesnik E, Verheyen N, Springer A, Madl T, Sinner F, de Cabo R, Kroemer G, Obermayer-Pietsch B, Dengjel J, Sourij H, Pieber TR, Madeo F. Alternate day fasting improves physiological and molecular markers of aging in healthy, non-obese humans. Cell Metabolism. 2019;30(3):462–476. doi: 10.1016/j.cmet.2019.07.016. [DOI] [PubMed] [Google Scholar]
- 29.Tavernarakis N, Pasparaki A, Tasdemir E, Maiuri MC, Kroemer G. The effects of p53 on whole organism longevity are mediated by autophagy. Autophagy. 2008;4(7):870–873. doi: 10.4161/auto.6730. [DOI] [PubMed] [Google Scholar]
- 30.Eisenberg T, Knauer H, Schauer A, Buttner S, Ruckenstuhl C, Carmona-Gutierrez D, Ring J, Schroeder S, Magnes C, Antonacci L, Fussi H, Deszcz L, Hartl R, Schraml E, Criollo A, Megalou E, Weiskopf D, Laun P, Heeren G, Breitenbach M, Grubeck-Loebenstein B, Herker E, Fahrenkrog B, Frohlich KU, Sinner F, Tavernarakis N, Minois N, Kroemer G, Madeo F. Induction of autophagy by spermidine promotes longevity. Nat Cell Biol. 2009;11(11):1305–1314. doi: 10.1038/ncb1975. [DOI] [PubMed] [Google Scholar]
- 31.Morselli E, Maiuri MC, Markaki M, Megalou E, Pasparaki A, Palikaras K, Criollo A, Galluzzi L, Malik SA, Vitale I, Michaud M, Madeo F, Tavernarakis N, Kroemer G. Caloric restriction and resveratrol promote longevity through the Sirtuin-1-dependent induction of autophagy. Cell Death Dis. 2010;1:e10. doi: 10.1038/cddis.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopez-Otin C, Galluzzi L, Freije JMP, Madeo F, Kroemer G. Metabolic Control of Longevity. Cell. 2016;166(4):802–821. doi: 10.1016/j.cell.2016.07.031. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Sowers JR, Ren J. Targeting autophagy in obesity: from pathophysiology to management. Nat Rev Endocrinol. 2018;14(6):356–376. doi: 10.1038/s41574-018-0009-1. [DOI] [PubMed] [Google Scholar]
- 34.Nunez CE, Rodrigues VS, Gomes FS, Moura RF, Victorio SC, Bombassaro B, Chaim EA, Pareja JC, Geloneze B, Velloso LA, Araujo EP. Defective regulation of adipose tissue autophagy in obesity. Int J Obes. 2013;37(11):1473–1480. doi: 10.1038/ijo.2013.27. [DOI] [PubMed] [Google Scholar]
- 35.Fernandez AF, Barcena C, Martinez-Garcia GG, Tamargo-Gomez I, Suarez MF, Pietrocola F, Castoldi F, Esteban L, Sierra-Filardi E, Boya P, Lopez-Otin C, Kroemer G, Marino G. Autophagy couteracts weight gain, lipotoxicity and pancreatic beta-cell death upon hypercaloric pro-diabetic regimens. Cell Death Dis. 2017;8(8):e2970. doi: 10.1038/cddis.2017.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sciarretta S, Volpe M, Sadoshima J. Is reactivation of autophagy a possible therapeutic solution for obesity and metabolic syndrome? Autophagy. 2012;8(8):1252–1254. doi: 10.4161/auto.20670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Madeo F, Tavernarakis N, Kroemer G. Can autophagy promote longevity? Nat Cell Biol. 2010;12(9):842–846. doi: 10.1038/ncb0910-842. [DOI] [PubMed] [Google Scholar]
- 38.Madeo F, Pietrocola F, Eisenberg T, Kroemer G. Caloric restriction mimetics: towards a molecular definition. Nat Rev Drug Discov. 2014;13(10):727–740. doi: 10.1038/nrd4391. [DOI] [PubMed] [Google Scholar]
- 39.Madeo F, Carmona-Gutierrez D, Hofer SJ, Kroemer G. Caloric Restriction Mimetics against Age-Associated Disease: Targets, Mechanisms, and Therapeutic Potential. Cell Metab. 2019;29(3):592–610. doi: 10.1016/j.cmet.2019.01.018. [DOI] [PubMed] [Google Scholar]
- 40.Marino G, Pietrocola F, Eisenberg T, Kong Y, Malik SA, Andryushkova A, Schroeder S, Pendl T, Harger A, Niso-Santano M, Zamzami N, Scoazec M, Durand S, Enot DP, Fernandez AF, Martins I, Kepp O, Senovilla L, Bauvy C, Morselli E, Vacchelli E, Bennetzen M, Magnes C, Sinner F, Pieber T, Lopez-Otin C, Maiuri MC, Codogno P, Andersen JS, Hill JA, et al. Regulation of autophagy by cytosolic acetyl-coenzyme A. Mol Cell. 2014;53(5):710–725. doi: 10.1016/j.molcel.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 41.Eisenberg T, Schroeder S, Andryushkova A, Pendl T, Kuttner V, Bhukel A, Marino G, Pietrocola F, Harger A, Zimmermann A, Moustafa T, Sprenger A, Jany E, Buttner S, Carmona-Gutierrez D, Ruckenstuhl C, Ring J, Reichelt W, Schimmel K, Leeb T, Moser C, Schatz S, Kamolz LP, Magnes C, Sinner F, Sedej S, Frohlich KU, Juhasz G, Pieber TR, Dengjel J, et al. Nucleocytosolic depletion of the energy metabolite acetyl-coenzyme a stimulates autophagy and prolongs lifespan. Cell Metab. 2014;19(3):431–444. doi: 10.1016/j.cmet.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pietrocola F, Castoldi F, Markaki M, Lachkar S, Chen G, Enot DP, Durand S, Bossut N, Tong M, Malik SA, Loos F, Dupont N, Marino G, Abdelkader N, Madeo F, Maiuri MC, Kroemer R, Codogno P, Sadoshima J, Tavernarakis N, Kroemer G. Aspirin Recapitulates Features of Caloric Restriction. Cell Rep. 2018;22(9):2395–2407. doi: 10.1016/j.celrep.2018.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carmona-Gutierrez D, Zimmermann A, Kainz K, Pietrocola F, Chen G, Maglioni S, Schiavi A, Nah J, Mertel S, Beuschel CB, Castoldi F, Sica V, Trausinger G, Raml R, Sommer C, Schroeder S, Hofer SJ, Bauer MA, Pendl T, Tadic J, Dammbrueck C, Hu Z, Ruckenstuhl C, Eisenberg T, Durand S, Bossut N, Aprahamian F, Abdellatif M, Sedej S, Enot DP, et al. The flavonoid 4,4′-dimethoxychalcone promotes autophagy-dependent longevity across species. Nat Commun. 2019;10(1):651. doi: 10.1038/s41467-019-08555-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morselli E, Marino G, Bennetzen MV, Eisenberg T, Megalou E, Schroeder S, Cabrera S, Benit P, Rustin P, Criollo A, Kepp O, Galluzzi L, Shen S, Malik SA, Maiuri MC, Horio Y, Lopez-Otin C, Andersen JS, Tavernarakis N, Madeo F, Kroemer G. Spermidine and resveratrol induce autophagy by distinct pathways converging on the acetylproteome. J Cell Biol. 2011;192(4):615–629. doi: 10.1083/jcb.201008167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Madeo F, Eisenberg T, Pietrocola F, Kroemer G. Spermidine in health and disease. Science. 2018;359(6374) doi: 10.1126/science.aan2788. [DOI] [PubMed] [Google Scholar]
- 46.Eisenberg T, Abdellatif M, Schroeder S, Primessnig U, Stekovic S, Pendl T, Harger A, Schipke J, Zimmermann A, Schmidt A, Tong M, Ruckenstuhl C, Dammbrueck C, Gross AS, Herbst V, Magnes C, Trausinger G, Narath S, Meinitzer A, Hu Z, Kirsch A, Eller K, Carmona-Gutierrez D, Buttner S, Pietrocola F, Knittelfelder O, Schrepfer E, Rockenfeller P, Simonini C, Rahn A, et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat Med. 2016;22(12):1428–1438. doi: 10.1038/nm.4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kiechl S, Pechlaner R, Willeit P, Notdurfter M, Paulweber B, Willeit K, Werner P, Ruckenstuhl C, Iglseder B, Weger S, Mairhofer B, Gartner M, Kedenko L, Chmelikova M, Stekovic S, Stuppner H, Oberhollenzer F, Kroemer G, Mayr M, Eisenberg T, Tilg H, Madeo F, Willeit J. Higher spermidine intake is linked to lower mortality: a prospective population-based study. Am J Clin Nutr. 2018;108(2):371–380. doi: 10.1093/ajcn/nqy102. [DOI] [PubMed] [Google Scholar]
- 48.Pietrocola F, Castoldi F, Kepp O, Carmona-Gutierrez D, Madeo F, Kroemer G. Spermidine reduces cancer-related mortality in humans. Autophagy. 2019;15(2):362–365. doi: 10.1080/15548627.2018.1539592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lim H, Lee MS. Amelioration of obesity-induced diabetes by a novel autophagy enhancer. Cell Stress. 2018;2(7):181–183. doi: 10.15698/cst2018.07.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Manjithaya R, Anjard C, Loomis WF, Subramani S. Unconventional secretion of Pichia pastoris Acb1 is dependent on GRASP protein, peroxisomal functions, and autophagosome formation. J Cell Biol. 2010;188(4):537–546. doi: 10.1083/jcb.200911149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duran JM, Anjard C, Stefan C, Loomis WF, Malhotra V. Unconventional secretion of Acb1 is mediated by autophagosomes. J Cell Biol. 2010;188(4):527–536. doi: 10.1083/jcb.200911154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abrahamsen H, Stenmark H. Protein secretion: unconventional exit by exophagy. Curr Biol. 2010;20(9):R415–418. doi: 10.1016/j.cub.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 53.Galluzzi L, Yamazaki T, Kroemer G. Linking cellular stress responses to systemic homeostasis. Nat Rev Mol Cell Biol. 2018;19(11):731–745. doi: 10.1038/s41580-018-0068-0. [DOI] [PubMed] [Google Scholar]
- 54.Knudsen J, Neergaard TB, Gaigg B, Jensen MV, Hansen JK. Role of acyl-CoA binding protein in acyl-CoA metabolism and acyl-CoA-mediated cell signaling. J Nutr. 2000;130(2S Suppl):294S–298S. doi: 10.1093/jn/130.2.294S. [DOI] [PubMed] [Google Scholar]
- 55.Neess D, Bek S, Engelsby H, Gallego SF, Faergeman NJ. Long-chain acyl-CoA esters in metabolism and signaling: Role of acyl-CoA binding proteins. Prog Lipid Res. 2015;59:1–25. doi: 10.1016/j.plipres.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 56.Farzampour Z, Reimer RJ, Huguenard J. Endozepines. Adv Pharmacol. 2015;72:147–164. doi: 10.1016/bs.apha.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bravo-San Pedro JM, Sica V, Martins I, Pol J, Loos F, Maiuri MC, Durand S, Bossut N, Aprahamian F, Anagnostopoulos G, Niso-Santano M, Aranda F, Ramirez-Pardo I, Lallement J, Denom J, Boedec E, Gorwood P, Ramoz N, Clement K, Pelloux V, Rohia A, Pattou F, Raverdy V, Caiazzo R, Denis RGP, Boya P, Galluzzi L, Madeo F, Migrenne-Li S, Cruciani-Guglielmacci C, et al. Acyl-CoA-Binding Protein Is a Lipogenic Factor that Triggers Food Intake and Obesity. Cell Metab. 2019. [DOI] [PubMed]


