Abstract
Infectious bursal disease (IBD), caused by infectious bursal disease virus (IBDV), is characterized by severe immunosuppression in young chicks of 3 to 6 week age group. Although vaccines are available to prevent IBD, outbreaks of disease are still noticed in the field among vaccinated flocks. Further, the birds surviving IBD become susceptible to secondary infections caused by various viral and bacterial agents. This study assessed the immunoprophylactic potential of Cytosine-guanosinedeoxynucleotide (CpG) oligodeoxynucleotides (ODN) and Tinospora cordifolia stem aqueous extract in the specific pathogen free (SPF) chicks, experimentally infected with very virulent IBDV (vvIBDV). Both of these agents (CpG ODN and herbal extract) showed significant increase in the IFN-γ, IL-2, IL-4, and IL-1 levels in the peripheral blood mononuclear cells (PBMCs) (p < 0.05) of chickens in the treatment groups following IBD infection. Further we found significant reduction in mortality rate in vvIBDV infected chicks treated with either, or in combination, compared with the birds of control group. Additionally, the adjuvant or immune enhancing potential of these two immunomodulatory agents with the commercially available IBDV vaccine was determined in chicks. The augmentation of vaccine response in terms of an enhanced antibody titer after vaccination, along with either or a combination of the two agents was noticed. The findings provide a way forward to counter the menace of IBDV in the poultry sector through use of these herbal or synthetic immunomodulatory supplements.
Keywords: IBDV, Tinospora cordifolia, TLR agonist, CpG ODN, immunomodulator, adjuvant
1. Introduction
Infectious bursal disease (IBD), caused by IBD virus (IBDV), is an acute, highly contagious, and immunosuppressive disease particularly affecting chicks of 3 to 6 weeks age worldwide. The disease results in severe economic loss to poultry farmers. IBDV is a non-enveloped, double stranded RNA virus belonging to the genus Avibirnavirus of the family Birnaviridae [1]. This virus primarily inflicts cytolysis of dividing cells in primary lymphoid organ, bursa of Fabricius (BF) in chicks, leading to severe immunosuppression [2], and fatal complications, such as high mortality, poor weight gain, and condemnation of the carcasses because of hemorrhages in the skeletal muscles of IBD affected chickens [3]. Transmission of IBDV is via the fecal-oral route. Out of two serotypes of IBDV, serotype 1 is pathogenic, while serotype 2 is non-pathogenic. Serotype 1 is further classified into classical, intermediate, and very virulent strains [4]. IBDV infection prevails in vaccinated broiler stock in Indian states throughout the year with the highest incidence in monsoon season (July to October) [5]. Although live attenuated vaccines are available to prevent IBD, field outbreaks are not uncommon in vaccinated flocks, which might be due to the emergence of antigenic variants of IBDV in the field settings [6]. Besides the direct consequences, IBD outbreak also lead to the vaccination failures of other diseases in the recovered birds, due to immunosuppression [7], thus extending the negative impact of IBD over poultry sector.
Augmentation of innate immune responses through immunomodulatory supplements may help to overcome such challenges in the management of IBDV induced infection and associated secondary complications. Amid several agents known to augment the innate immune response, Toll like receptors’ (TLR) agonists and some herbs can further improvise an appropriate adaptive immune response, countering the invading pathogen efficiently [8,9]. TLRs, the key sensors of innate immunity, are evolutionary conserved pattern recognition receptors (PRRs) found in both mammals and avian species [10]. They recognize conserved structural motifs on various pathogens, termed pathogen associated molecular patterns (PAMPs). Recognition of PAMPs by TLRs on immune cells leads to activation of the signaling pathways, and provokes cellular activation and release of cytokines [11]. This further activates the adaptive immune system, due to the maturation of antigen presenting cells. Owing to said properties, the TLR ligands can be employed either as prophylactic agents or as vaccine adjuvants against various pathogens [12,13,14]. The chicken’s TLR repertoire consists of 10 TLRs; viz., TLR1LA, TLR1LB, TLR2A, TLR2B, TLR3, TLR4, TLR5, TLR7, TLR15, and TLR21 [15]. Avian TLR21 is a cytoplasmic receptor which recognizes microbial DNA (containing unique CpG dinucleotide motifs) as a danger signal, similarly to typical TLR9 in mammalian species. Recognition of CpG DNA by TLR21 induces activation of NF-ĸB pathway, leading to up regulation of pro-inflammatory molecules, including nitric oxide (NO), and recruitment of innate immune cells [16]. Such activation of TLR21 has shown an antiviral effect against viruses, such as against the avian influenza virus (AIV) in birds [17]. CpG ODN has also shown promising results to enhance the efficacy of the IBDV DNA vaccine and attenuated vaccine, compared to the use of the live vaccine used alone [18,19].
Herbs have been used widely in India under the ayurvedic system of medicine since time immemorial. Several studies have proven the immunomodulatory potential of various herbs; to name few, Astragalus sp, Withania somnifera, Tinospora cordifolia, Azadirachta indica, and Phyllanthus emblica. [20,21,22]. Amongst them, T. cordifolia (guduchi, common name) has been well established for potent immunomodulatory properties [23,24]. Guduchi aqueous extract has been shown to activate macrophages, which form the first line of defense against pathogens invading the living system [25]. Guduchi has shown an antiviral effect against chicken infectious anemia virus (CIAV) infection in poultry, along with an immunomodulatory effect to overcome immunosuppression caused by this virus [21].
In this study, we established the interaction of CpG ODN (TLR21 agonist) and guduchi aqueous extract in providing protection against IBDV, and their mechanism of interaction. Furthermore, adjuvant action of CpG ODN and guduchi aqueous extract with commercially available IBDV vaccine was also evaluated. To the best of our knowledge, this is the first study demonstrating the interaction of any TLR agonist and herbal extract for providing protection against a viral infection in chicken.
2. Materials and Methods
2.1. Experimental Animals
Specific pathogen free (SPF) eggs (n = 170) were procured from Venky’s Pvt. Ltd., (Pune, India) and hatched at the Central Avian Research Institute (CARI), Izatnagar. The chicks were maintained at the experimental animal shed facility of the Avian Disease Section, ICAR-IVRI, Izatnagar, maintaining required pathogen free settings. The experiment had prior approval from the institute animal ethics committee (number F. 26-1/2015-16/JD(R)).
2.2. Virus
A very virulent strain of IBDV (vvIBDV) (GenBank accession: KM205793.1 (VP2 gene)), maintained in the viral laboratory of the Avian Disease Section, was used for the experimental infection after passaging in three-week-old SPF White Leghorn (WL) chicken. At 72 h post-inoculation, the bursae were harvested, chopped, and homogenized in peptone broth containing penicillin and streptomycin (1000 mg/mL each). The filtered supernatant obtained from the bursal homogenate was used as a source of the vvIBDV for further work. The virus suspension was titrated in 10 day-old SPF embryonated chicken eggs by inoculation into the chorioallantoic membrane route [26].
2.3. TLR21 Agonist
Chicken TLR21 agonist (CpG ODN) was procured from Alpha Diagnostic Intl Inc, San Antonio, TX, USA. (Cat. #ODN2007-1), and was used in the study after making appropriate dilutions.
2.4. Preparation of T. Cordifolia Aqueous Extract
T. cordifolia stem powder was procured commercially (Herbal Hills, Mumbai, India) and was used for preparing an aqueous extract for further use in the experiment. Aqueous extract was prepared by boiling stem powder of this plant in water at a 1:5 ratio, at 100 °C for 30 min [27], followed by filtration of the mixture. Filtrate was stored in refrigerator until further use.
2.5. Vaccines
Commercially available IBDV (Intermediate and Intermediate Plus) vaccines (Venky’s India Pvt. Ltd., Pune, India) and Newcastle disease virus (NDV) vaccine, lentogenic ‘F’ strain (Biomed Pvt. Ltd., Ghaziabad, India) were used in the present study.
2.6. ELISA Kit
The ELISA kit for IBDV was procured from IDEXX Laboratories, Westbrook, MA, USA.
2.7. Primers
The published oligonucleotide primers selected for chicken specific genes; viz., β-actin, IL-2, IFN-γ, IL-4, IL-1β, and vvIBD-VP2 (Table 1), were used in the study. All primers were synthesized from Eurofins Genomics India Pvt. Ltd. (Bangalore, India).
Table 1.
Details of the oligonucleotide primer sequence used for chicken specific genes in qReal-time PCR.
| Name of the Gene | Primer sequence (5′ → 3′) | Reference |
|---|---|---|
| IL-2 | TTCTGGGACCACTGTATGCTCTT TACCGACAAAGTGAGAATCAATCAG |
AF000631.1-Gene bank accession no. |
| IL-4 | GGAGAGCATCCGGATAGTGA TGACGCATGTTGAGGAAGAG |
[28] |
| IFN-γ | AAGTCAAAGCCGCACATCAAAC CTGGATTCTCAAGTCGTTCATCG |
X99774.1-Gene bank accession no. |
| β-actin | TATGTGCAAGGCCGGTTTC TGTCTTTCTGGCCCATACCAA |
[29] |
| IL-1β | GGATTCTGAGCACACCACAGT TCTGGTTGATGTCGAAGATGTC |
[28] |
| VVIBD-VP2 gene | CCTGGCTCAATTGTGGGTGCTCA GGCGTTTATGGTTCCGTTTAGTGC |
Custom designed |
2.8. Experiment 1
A total of 100 SPF WL birds, three-weeks old, were randomly divided into five groups; viz. A, B, C, D, and E (n = 20/group). Group A was kept as a negative control and was given water in a pulsating manner (over a period of eight hours), 10 mL/bird/day orally, to mimic the T. cordifolia extract treatment (control for group C). Group B (positive virus control) was given phosphate buffered saline (PBS, pH 7.2) via intramuscular route at 4 weeks of age. Birds in group C were given T. cordifolia aqueous extract from day one until 4 weeks of age in a pulsating manner (over a period of eight hours), 10 mL/bird/day orally. Group D was given CpG ODN, 50 µg/bird via intramuscular injection at 4 weeks of age. Group E was given both aqueous extract, prepared as above, from one day old, until 4 weeks of age, in a pulsating manner, 10 mL/bird/day; and CpG ODN, 50 µg/bird via intramuscular route at 4 weeks of age. Birds in group B, C, D, and E were challenged with 105 ELD50 of vvIBDV at 24 h post-injection of CpG ODN through the ocular route and observed for the clinical signs and mortality until 10 days post-challenge.
2.8.1. Collection of Samples
Blood samples (n = 6/group) were collected in heparinized vials (20 IU/mL) at the 4th (before challenge), 5th (7 days post challenge (DPC)), and 6th (14 DPC) week of age. On day four post-challenge, three birds from each group (A–E) were sacrificed for the collection of bursa. A piece of bursal tissue was stored in 10% neutral buffered formalin for histopathology (HP), and the remaining half of the bursal tissue was stored in an RNA later (Qiagen India Pvt. Ltd., New Delhi, India) solution at −80 °C for cytokine expression studies.
2.8.2. Isolation of Chicken Peripheral Blood Mononuclear Cells (PBMCs)
PBMCs were isolated from the blood, as described previously [30]. Briefly, blood was layered over an equal volume of Ficoll Hypaque (Sigma Chemical Co., St. Louis, MO, USA) with a density of 1.077 g/mL and kept for centrifugation at 500× g for 45 min for phase separation. The interface layer containing the PBMCs was aspirated carefully and washed in sterile PBS twice, and finally cell pellet was suspended in 1 mL sterile PBS. Cell viability was checked by trypan blue dye exclusion method, and around 1 × 106 cells from each sample were stored in a 1 mL RiboZol (Genetix Biotech, New Delhi, India) at −80 °C until RNA extraction.
2.8.3. RNA Extraction and cDNA Synthesis
Total RNA was extracted from the PBMCs and bursal tissue, using RiboZol reagent as per manufacturer’s guidelines. Briefly, bursal tissue was homogenized in RiboZol (1:10) and 250 µL PBMCs mixed with 750 µL RiboZol, followed by addition of chloroform (200 µL) and centrifuged at 12,000 rpm for 15 min at 4 °C for phase separation. Aqueous phases were precipitated with 500 µL isopropanol followed by centrifugation (12,000 rpm for 15 min at 4 °C), supernatants were removed, and the RNA pellets were washed carefully with 75% ethanol and centrifuged (7500 rpm for 5 min at 4 °C). After removing ethanol, RNA pellets were dissolved in RNase free water, warmed at 55–60 °C and then stored at −20 °C, until further use. RNA purity and quantity were determined by Nano-drop spectrophotometer analyzer (Thermo Fischer Scientific, Waltham, MA, USA) at A260/280.
Reverse transcription (RT) for the first strand synthesis was carried out by using Revert-Aid Reverse Transcriptase (Thermo Scientific, Waltham, MA, USA) in a standard 20 µL reaction mixture. To a sterile nuclease free 0.2 mL PCR tube, the reaction mixture was prepared using: RNA template (1 μg), Random Hexamer Primer 100 µM (0.2 μg/μL, 1.0 μL), dNTP mix (10 mM each) (2.0 μL) and nuclease free water (NFW) (to make up to 20 µL volume). The mixture was incubated at 70 °C for 5 min, followed by immediate snap chilling in ice. Then reagents were added as follows, 5X RT buffer (4.0 μL), RiboLock RNase inhibitor (40 U/μL, 0.5 μl) and RevertAid Reverse Transcriptase (200 U/μL, 1.0 μL). RNA was subsequently reverse-transcribed at 25 °C for 10 min, and 42 °C for 60 min, followed by 10 min at 70 °C to denature the enzyme. All these steps were carried out in a thermal cycler (QB 96 Server Gradient Thermal Cycler, Quanta Biotech Ltd., Surrey, UK). The cDNA samples were cooled at 4 °C and stored at −20 °C.
2.8.4. Quantitative Real-Time PCR
SYBR green-based quantitative real-time PCR was performed using Stratagene MX300 5P for studying the expression of different immune response genes. β-actin was used as the internal control gene. Each sample was run in duplicate and the total reaction volume was 20 µL, containing SYBR green master mix (Thermo Fischer Scientific, Lithuania, Waltham, MA, USA), 10 µL, template cDNA, 1 µL, gene specific forward and reverse primers, 0.5 µL each, and NFW, 8 µL. Real-time PCR conditions were one cycle at 95 °C for 10 min, 40 cycles of 95 °C for 15 s, 60 °C for 15 s, 72 °C for 15 s and one cycle 95 °C for 1 min; and a 65–95 °C ramp for melt curve analysis to check the amplicon specificity.
2.8.5. Evaluation of the Humoral Immune Response Using ELISA
The humoral immune response against vvIBDV was assessed using an ELISA kit (IDEXX Laboratories, Westbrook, ME, USA) following the manufacturer’s protocol on collected sera samples. Briefly, antigen coated plates were taken, and sample positions were recorded, followed by the addition of 100 µL of undiluted negative control (NC), 100 µL of undiluted positive control (PC), and 100 µL of respective diluted samples (1:500) into appropriate wells. Plates were incubated for 30 min at 18–26 °C, followed by removal of the solution in each well and thrice washing with distilled water (350 µL/well). 100 µL of conjugate was dispensed into each well, incubated for 30 min at 18–26 °C, and followed by washing, as previously explained. 100 µL of TMB substrate was dispensed into each well, incubated for 15 min at 18–26 °C and finally 100 µL of stop solution was dispensed. Absorbance was recorded at 650 nm using the ELISA reader.
The antibody titer was calculated as per the equations provided in the ELISA kit;
Controls
Samples
where, S/P = sample to positive ratio
Antibody titer is the antilog of that value.
2.8.6. Protection Study against the vvIBDV Challenge
The protection study included observation of clinical signs and mortality up to ten days post-challenge, viral load estimation, and gross and histopathological examination of bursae.
Gross and Histopathological Evaluation
Necropsy was done on dead birds to record the gross lesion and post-mortem diagnosis. Bursae from dead as well as sacrificed birds were processed for routine paraffin embedding technique. Tissue sections of 4–5 mm thickness were stained with hematoxylin and eosin (H&E) for microscopic evaluation. Bursal lesions (BLS) were scored on a scale of 0 to 5 (0: no lesion, 1: slight change, 2: scattered or partial bursal damage, 3: 50% or less follicle damage, 4: 51–75% follicle damage, and 5: 76–100% bursal damage) by a trained avian pathologist, following the blind folded method, as described elsewhere [31].
Viral Load Estimation
RNA samples from bursal tissues collected at 4 DPC of vvIBDV infection from all the groups was used for cDNA synthesis, followed by quantitative real-time PCR for IBDV specific gene (VP2). Real-time PCR conditions included one cycle at 95 °C for 8 min, 40 cycles of 94 °C for 1 min, 57 °C for 50 s, 72 °C for 50 s, and one cycle of 95 °C for 1 min; and 65–95 °C for melt curve analysis to check the amplicon specificity. For evaluating the concentrations of virus in bursal samples, a standard curve was prepared for the VP2 gene amplified in the pDrive Cloning Vector.
2.8.7. Assessment of Protection against NDV
On day 10 post-vvIBDV challenge, the surviving birds were vaccinated with Newcastle disease virus (NDV) vaccine, lentogenic ‘F’ strain (Biomed Pvt. Ltd., Ghaziabad, India) through the ocular route with the recommended dose. Sera were collected at weekly intervals (n = 5/group) for three weeks post-vaccination, and stored at −20 °C until further use.
2.8.8. Evaluation of the Humoral Immune Response against the NDV Vaccine
The humoral immune response against the NDV vaccine was measured by hemagglutination inhibition (HI) test, according to the OIE-World organization for animal health recommended protocol [32], using antigen prepared from the lentogenic strain (F) of NDV and 1% chicken RBCs.
2.9. Experiment 2
The evaluation of the immune-enhancing effect of T. cordifolia and a TLR21 agonist (CpG ODN) on IBDV vaccine responses in chicks.
Fifty SPF WL birds of two-week age were randomly divided into five groups; viz. A, B, C, D, and E (n = 10/group). Group A was kept as a negative control. Group B was kept as a positive control. Birds in group C were given T. cordifolia aqueous extract from day one until 4 weeks of age in a pulsating manner (over a period of eight hours), 10 mL/bird/day orally. Group D was given CpG ODN, 10 µg/bird via the intramuscular route at 2 weeks, repeated at 4 weeks of age. Group E was given both aqueous extract, prepared as above, from day one until 4 weeks of age in a pulsating manner (over a period of eight hours), 10 mL/bird/day; and CpG ODN, 10 µg/bird via the intramuscular route at 2 weeks, and again at 4 weeks of age. Chicks in group B to E were vaccinated with IBDV intermediate (live attenuated, each dose of vaccine containing ≥ 102 EID50 of IBD Intermediate standard strain) vaccine at 2 weeks and a booster dose at 4 weeks of age, with IBDV intermediate plus vaccine (each dose of vaccine containing Intermediate Plus strain ≥ 102 EID50), while group A acted as negative control.
Blood samples without anticoagulant (n = 6/group) were collected from birds in all the groups pre-vaccination; and at 7, 14, 21, and 28 days post-vaccination (DPV) in vaccinated birds, for assessing the IBDV specific antibody responses. IBDV specific antibody responses were measured using a commercially available ELISA kit (IDEXX), as described in the earlier procedure.
2.10. Data Analysis
Relative expressions in target genes were analyzed based on the 2−ΔΔCT method, previously described by Pfaffl, 2001 [33], normalizing against the β-actin gene and expressing the outcome as fold change over the control gene. All the data are expressed as mean ± SEM. All the results were analyzed using the statistical software GraphPad Prism version 8.1.2. Two-way analysis of variance (ANOVA) was used to determine the statistically significant differences in mean values between the groups (except immune response genes in PBMCs ex vivo, which were analyzed using the ‘t’ test). The control group was considered a calibrator for relative quantification of mRNA expression. Multiple comparisons between groups were done through Tukey’s post hoc test. Values of p < 0.05 were considered significant.
3. Results
3.1. Immune Response Genes in PBMCs Ex Vivo
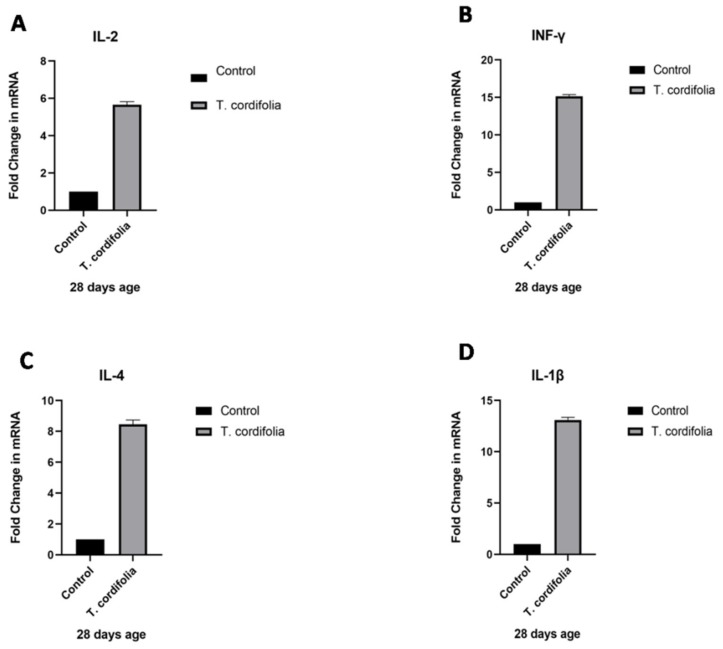
The guduchi treated (after oral administration of T. cordifolia aqueous extract to birds at 10 mL/day/bird from one day old to 4 weeks of age) group showed significant increases (p < 0.05) in IL-2 (5.657 ± 0.1663), IFN-γ (15.15 ± 0.2288), IL-4 (8.458 ± 0.277), and IL-1β (13.09 ± 0.2681) expressions in chicken PBMCs compared to the control group (Table 2, Figure 1A–D). Though a significant increase in the immune response gene expression is observed, there were still no clinical symptoms of illness observed in treated chicks, thereby suggesting that T. cordifolia extract usage is apparently safer in chicks without any autoimmunity or immunopathological changes
Table 2.
Quantitative real time PCR analysis of immune response genes (expressed as fold change in the immune response gene transcripts) in chicken PBMCs after T. cordifolia treatment at 10 mL/bird/day from day old to 4 week of age.
| S. number | Group | 1L-2 | IFN-γ | IL-4 | IL-1β |
|---|---|---|---|---|---|
| 1. | Control | 1 a ± 0.00 | 1 a ± 0.00 | 1 a ± 0.00 | 1 a ± 0.00 |
| 2. | T. cordifolia | 5.657 b ± 0.1663 | 15.15 b ± 0.2288 | 8.458 b ± 0.277 | 13.09 b ± 0.2681 |
a,b Means bearing different superscripts column-wise differ significantly (p < 0.05).
Figure 1.
(A–D) Quantitative real-time PCR analysis of Immune response gene transcripts (expressed as fold change in the immune response gene transcripts) in PBMCs ex vivo in specific pathogen free (SPF) WL chickens post T. cordifolia treatment for 4 weeks.
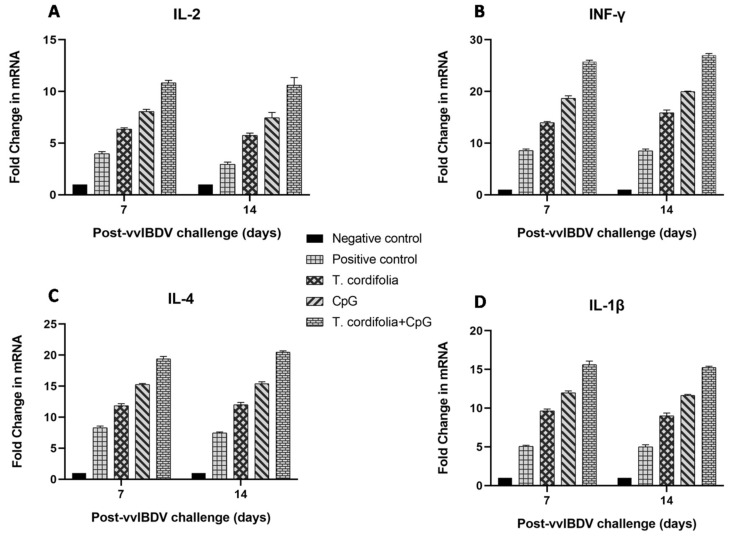
The group E showed an increase (p < 0.05) in IL-2 expression in PBMCs at 7 DPC (10.840 ± 0.232) and 14 DPC (10.620 ± 0.723) which was the only increase, and was followed by group D, which showed significant differences from groups A, B, and C at 7 DPC (8.068 ± 0.207) and 14 DPC (7.462 ± 0.507) (Table 3, Figure 2A).
Table 3.
Quantitative real-time PCR analysis of IL-2 expression in PBMCs of chickens challenged with very virulent, infections bursal disease virus (vvIBDV) at different days post challenge (DPC).
| S. number | Group | 7 DPC | 14 DPC |
|---|---|---|---|
| 1. | Negative control (Group A) | 1 a ± 0.00 | 1 a ± 0.00 |
| 2. | Positive control (Group B) | 3.977 b ± 0.200 | 2.957 b ± 0.210 |
| 3. | T. cordifolia + virus challenge (Group C) | 6.350 c ± 0.132 | 5.740 c ± 0.231 |
| 4. | CpG ODN + virus challenge (Group D) | 8.068 d ± 0.207 | 7.462 d ± 0.507 |
| 5. | T. cordifolia + CpG ODN + challenge (Group E) | 10.840 e ± 0.232 | 10.620 e ± 0.723 |
a–e Means bearing different superscripts column-wise differ significantly (p < 0.05). The results are expressed as fold changes to the immune response gene transcripts relative to the control group.
Figure 2.
(A–D). Quantitative real-time PCR analysis of immune response gene transcripts in PBMCs ex vivo in SPF WL chickens 7 and 14 days post-vvIBDV challenge. The result is expressed as fold change in the immune response gene transcripts relative to the control group.
The group E showed an increase (p < 0.05) in IFN-γ expression in PBMCs at 7 DPC (25.702 ± 0.328) and 14 DPC (26.982 ± 0.345) which was the only increase, and was followed by group D, which showed significant differences from groups A, B, or C at 7 DPC (18.672 ± 0.490) and 14 DPC (19.973 ± 0.094) (Table 4, Figure 2B).
Table 4.
Quantitative real time PCR analysis of IFN-γ expression in PBMCs of chickens challenged with vvIBDV at different DPC.
| S. number | Group | 7 DPC | 14 DPC |
|---|---|---|---|
| 1. | Negative control (Group A) | 1 a ± 0.00 | 1 a ± 0.00 |
| 2. | Positive control (Group B) | 8.570 b ± 0.303 | 8.480 b ± 0.376 |
| 3. | T. cordifolia + virus challenge (Group C) | 13.953 c ± 0.224 | 15.892 c ± 0.509 |
| 4. | CpG ODN + virus challenge (Group D) | 18.672 d ± 0.490 | 19.973 d ± 0.094 |
| 5. | T. cordifolia + CpG ODN + challenge (Group E) | 25.702 e ± 0.328 | 26.982 e ± 0.345 |
a–e Means bearing different superscripts column-wise differ significantly (p < 0.05). The results are expressed as fold changes to the immune response gene transcripts relative to the control group.
The group E showed an increase (p < 0.05) in IL-4 expression in PBMCs at 7 DPC (19.400 ± 0.376) and 14 DPC (20.468 ± 0.207). Group D, followed, but showed significant differences from groups A, B, and C at 7 DPC (15.265 ± 0.162) and 14 DPC (15.397 ± 0.293) (Table 5, Figure 2C).
Table 5.
Quantitative real time PCR analysis of IL-4 expression in PBMCs of chickens challenged with vvIBDV at different DPC.
| S. number | Group | 7 DPC | 14 DPC |
|---|---|---|---|
| 1. | Negative control (Group A) | 1 a ± 0.00 | 1 a ± 0.00 |
| 2. | Positive control (Group B) | 8.288 b ± 0.294 | 7.447 b ± 0.172 |
| 3. | T. cordifolia + virus challenge (Group C) | 11.870 c ± 0.315 | 12.017 c ± 0.364 |
| 4. | CpG ODN + virus challenge (Group D) | 15.265 d ± 0.162 | 15.397 d ± 0.293 |
| 5. | T. cordifolia + CpG ODN + challenge (Group E) | 19.400 e ± 0.376 | 20.468 e ± 0.207 |
a–e Means bearing different superscripts column-wise differ significantly (p < 0.05). The results are expressed as fold changes to the immune response gene transcripts relative to the control group.
The group E showed an increase (p < 0.05) in IL-1β expression in PBMCs at 7 DPC (15.628 ± 0.444) and 14 DPC (15.247 ± 0.169) which was the only increase, followed by group D which showed significant differences from groups A, B, and C at 7 DPC (11.987 ± 0.243) and 14 DPC (11.660 ± 0.123) (Table 6, Figure 2D).
Table 6.
Quantitative real time PCR analysis of IL-1β expression in PBMCs of chickens challenged with vvIBDV at different DPC.
| S. number | Group | 7 DPC | 14 DPC |
|---|---|---|---|
| 1. | Negative control (Group A) | 1 a ± 0.00 | 1 a ± 0.00 |
| 2. | Positive control (Group B) | 5.077 b ± 0.128 | 4.992 b ± 0.276 |
| 3. | T. cordifolia + virus challenge (Group C) | 9.650 c ± 0.240 | 8.987 c ± 0.390 |
| 4. | CpG ODN + virus challenge (Group D) | 11.987 d ± 0.243 | 11.660 d ± 0.123 |
| 5. | T. cordifolia + CpG ODN + challenge (Group E) | 15.628 e ± 0.444 | 15.247 e ± 0.169 |
a–e Means bearing different superscripts column-wise differ significantly (p < 0.05). The results are expressed as fold changes to the immune response gene transcripts relative to the control group.
3.2. Immune Response Genes in the bursa of Fabricius Ex Vivo
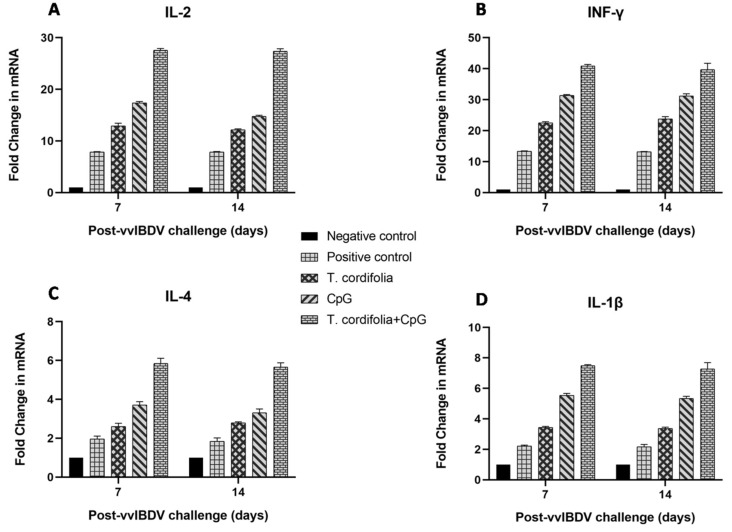
The group E showed an increase (p < 0.05) in IL-2 expression in PBMCs at 7 DPC (27.550 ± 0.363) and 14 DPC (27.370 ± 0.496), which was followed by group D, which showed significant difference from group A, B and C at 7 DPC (17.397 ± 0.232) and 14 DPC (14.797 ± 0.189) (Table 7, Figure 3A).
Table 7.
Quantitative real time PCR analysis of IL-2 expression in the bursa of Fabricius of chickens challenged with vvIBDV at different DPC.
| S. number | Group | 7 DPC | 14 DPC |
|---|---|---|---|
| 1. | Negative control (Group A) | 1 a ± 0.00 | 1 a ± 0.00 |
| 2. | Positive control (Group B) | 7.838 b ± 0.104 | 7.840 b ± 0.127 |
| 3. | T. cordifolia + virus challenge (Group C) | 12.912 c ± 0.524 | 12.148 c ± 0.188 |
| 4. | CpG ODN + virus challenge (Group D) | 17.397 d ± 0.232 | 14.797 d ± 0.189 |
| 5. | T. cordifolia + CpG ODN + challenge (Group E) | 27.550 e ± 0.363 | 27.370 e ± 0.496 |
a–e Means bearing different superscripts column-wise differ significantly (p < 0.05). The results are expressed as fold changes to the immune response gene transcripts relative to the control group.
Figure 3.
(A–D). Quantitative real-time PCR analysis of immune response gene transcripts in the bursa of Fabricius ex vivo, in SPF WL chickens 7 and 14 days post-vvIBDV challenge. The results are expressed as fold changes to the immune response gene transcripts relative to the control group.
Group E showed an increase (p < 0.05) in IFN-γ expression in PBMCs at 7 DPC (40.892 ± 0.446) and 14 DPC (39.693 ± 2.031) which was the only increase, followed by group D, which showed significant difference from group A, B and C at 7 DPC (31.348 ± 0.298) and 14 DPC (31.232 ± 0.659) (Table 8, Figure 3B).
Table 8.
Quantitative real time PCR analysis of IFN-γ expression in the bursa of Fabricius of chickens challenged with vvIBDV at different DPC.
| S. number | Group | 7 DPC | 14 DPC |
|---|---|---|---|
| 1. | Negative control (Group A) | 1 a ± 0.00 | 1 a ± 0.00 |
| 2. | Positive control (Group B) | 13.322 b ± 0.154 | 13.137 b ± 0.153 |
| 3. | T. cordifolia + virus challenge (Group C) | 22.490 c ± 0.407 | 23.758 c ± 0.766 |
| 4. | CpG ODN + virus challenge (Group D) | 31.348 d ± 0.298 | 31.232 d ± 0.659 |
| 5. | T. cordifolia + CpG ODN + challenge (Group E) | 40.892 e ± 0.446 | 39.693 e ± 2.031 |
a–e Means bearing different superscripts column-wise differ significantly (p < 0.05). The results are expressed as fold changes to the immune response gene transcripts relative to the control group.
Group E showed an increase (p < 0.05) in IL-4 expression in PBMCs at 7 DPC (5.842 ± 0.272) and 14 DPC (5.663 ± 0.211) which was the only increase, followed by group D, which showed significant differences from groups A, B, and C at 7 DPC (3.712 ± 0.172) and 14 DPC (3.310 ± 0.195) (Table 9, Figure 3C).
Table 9.
Quantitative real time PCR analysis of IL-4 expression in the bursa of Fabricius of chickens challenged with vvIBDV at different DPC.
| S. number | Group | 7 DPC | 14 DPC |
|---|---|---|---|
| 1. | Negative control (Group A) | 1 a ± 0.00 | 1 a ± 0.00 |
| 2. | Positive control (Group B) | 1.962 b ± 0.145 | 1.838 b ± 0.178 |
| 3. | T. cordifolia + virus challenge (Group C) | 2.600 c ± 0.169 | 2.788 c ± 0.056 |
| 4. | CpG ODN + virus challenge (Group D) | 3.712 d ± 0.172 | 3.310 d ± 0.195 |
| 5. | T. cordifolia + CpG ODN + challenge (Group E) | 5.842 e ± 0.272 | 5.663 e ± 0.211 |
a–e Means bearing different superscripts column-wise differ significantly (p < 0.05). The result is expressed as fold change in the immune response gene transcripts relative to control group.
Group E showed an increase (p < 0.05) in IL-1β expression in PBMCs at 7 DPC (7.498 ± 0.054) and 14 DPC (7.275 ± 0.413) which was the only increase, followed by group D, which showed significant differences from groups A, B, and C at 7 DPC (5.543 ± 0.128) and 14 DPC (5.345 ± 0.133) (Table 10, Figure 3D).
Table 10.
Quantitative real time PCR analysis of IL-1β expression in the bursa of Fabricius of chickens challenged with vvIBDV at different DPC.
| S. number | Group | 7 DPC | 14 DPC |
|---|---|---|---|
| 1. | Negative control (Group A) | 1 a ± 0.00 | 1 a ± 0.00 |
| 2. | Positive control (Group B) | 2.222 b ± 0.055 | 2.165 b ± 0.157 |
| 3. | T. cordifolia + virus challenge (Group C) | 3.427 c ± 0.075 | 3.360 c ± 0.092 |
| 4. | CpG ODN + virus challenge (Group D) | 5.543 d ± 0.128 | 5.345 d ± 0.133 |
| 5. | T. cordifolia + CpG ODN + challenge (Group E) | 7.498 e ± 0.054 | 7.275 e ± 0.413 |
a–e Means bearing different superscripts column-wise differ significantly (p < 0.05). The results are expressed as fold changes to the immune response gene transcripts relative to the control group.
3.3. The Humoral Immune Response (vvIBDV Post Challenge, Based on an Antibody Titer Estimated Using an ELISA Kit)
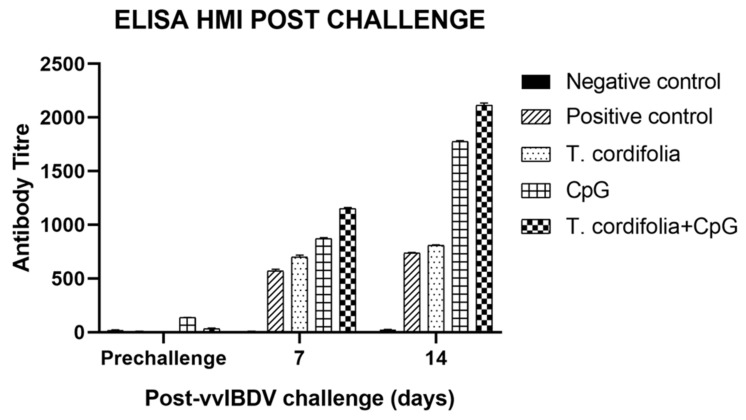
Group E showed a significant difference (p < 0.05) from other groups at 7 DPC (1152.477 ± 8.040) and 14 DPC (2115.643 ± 18.462), with the maximum antibody titer being estimated in this group. Following E, was group D, which differed significantly (p < 0.01) at 7DPC (871.597 ± 7.625) and 14 DPC (1776.421 ± 7.847) from group C and group B. Additionally, in both group D and group E antibody titer significantly increased (p < 0.01) from 7 DPC to 14 DPC. Group C also showed a significant increase (p < 0.01) in antibody titer 7 DPC (699.968 ± 17.517) and 14 DPC (809.135 ± 3.712) from the positive virus control (group B) (Table 11, Figure 4).
Table 11.
Serum antibody titer (mean ± SE of antilog of Log10 titer) in chickens challenged with vvIBDV at different DPC by ELISA kit method.
| S. number | Group | Pre-Challenge | 7 DPC | 14 DPC |
|---|---|---|---|---|
| 1. | Negative control (Group A) | 21.345 a ± 0.767 | 8.070 a ± 1.702 | 25.658 a ± 1.397 |
| 2. | Positive control (Group B) | 8.773 b ± 0.659 | 569.689 b ± 15.910 | 737.271 b ± 6.345 |
| 3. | T. cordifolia + virus challenge (Group C) | 7.048 c ± 0.400 | 699.968 c ± 17.517 | 809.135 c ± 3.712 |
| 4. | CpG ODN + virus challenge (Group D) | 135.942 d ± 2.754 | 871.597 d ± 7.625 | 1776.421 d ± 7.847 |
| 5. | T. cordifolia + CpG ODN + challenge (Group E) | 35.355 e ± 2.604 | 1152.477 e ± 8.040 | 2115.643 e ± 18.462 |
a–e Means bearing different superscripts column-wise differ significantly (p < 0.05).
Figure 4.
Serum antibody titer in chickens challenged with vvIBDV at different DPC by ELISA kit method (antibody titer is expressed as mean ± SE of antilog of Log10 titer).
3.4. Protection against vvIBDV Challenge
-
(a)
Clinical signs and mortality: No clinical signs and mortality were observed in any of the birds for the first 24 h post challenge with vvIBDV. After 48 h, affected birds showed clinical signs, such as drowsiness, depression, ruffled feathers, inability to move, and severe prostration. Group E showed the highest protection (100%), followed by group D (80%), and group C (50%), whereas 100% mortality was observed in group B (Table 12).
-
(b)
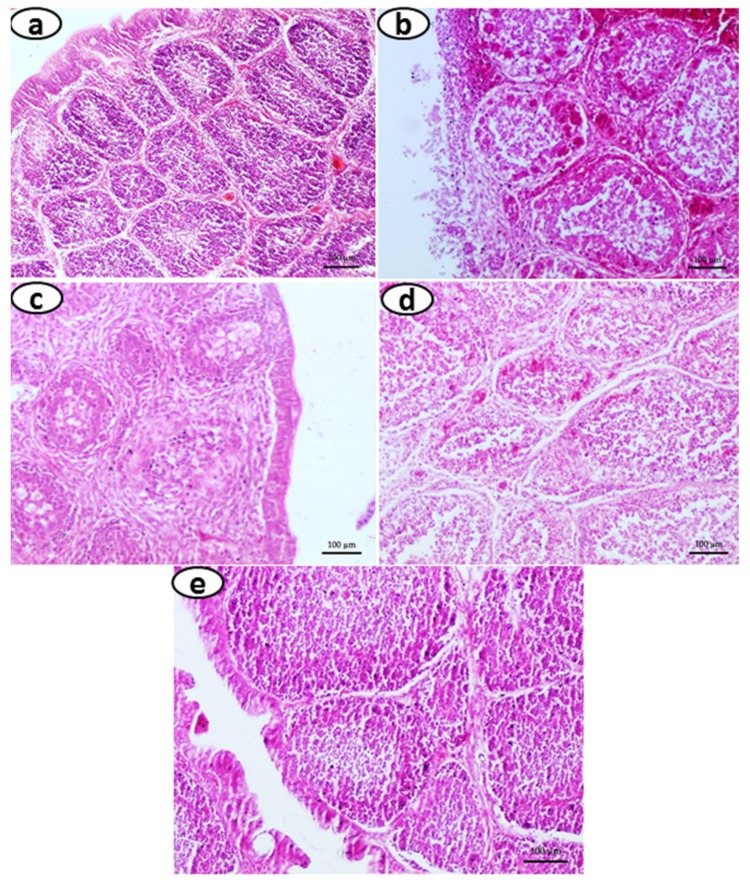
Gross lesions and Histopathology: In group A (negative control), the bursa of Fabricius revealed apparently normal histo-architecture, characterized by intact lining epithelia, lymphoid follicles with sufficient numbers of lymphocytes in the cortex, and medulla with thin connective tissue stroma separating each follicle (Figure 5a). No pathological lesions could be detected in the bursa of Fabricius of the control group. In group B (positive virus control), the lesions in the bursa of Fabricius included moderate to severe hemorrhages, edema, and partial to complete destruction of bursal follicles, with or without perifollicular fibrosis. Severe lymphocytolysis with marked perifollicular fibroplasia was also observed in bursa of Fabricius (Figure 5b). In group C, there were hemorrhages, and there was mild to moderate lymphocytolysis, marked reticuloendothelial cell hyperplasia, and congestion in stromal blood vessels (Figure 5c). In group D, the bursa of Fabricius exhibited very little histopathological alterations, characterized by mild peri-follicular edema and reticuloendothelial cell hyperplasia (Figure 5d). In group E, the histo-architecture of the bursa of Fabricius was intact. No pathologically significant alterations in the stromal connective tissue were observed (Figure 5e). But in 20% of the birds, a mild degree of edema in the bursal follicles and only mild depletion of lymphocytes in the medulla were observed.
-
(c)
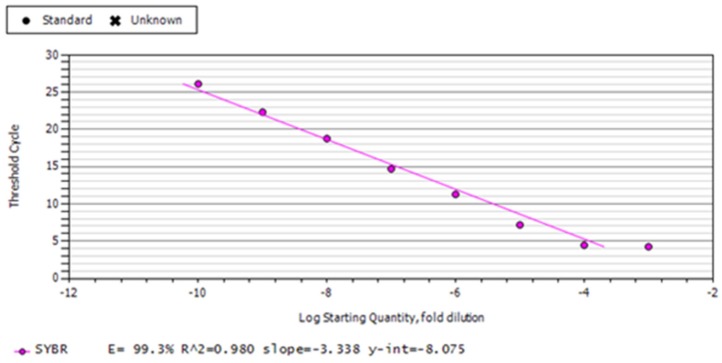
Viral load: Real time quantification of viral load from bursa of Fabricius in birds from groups A and C to E was done by using VP2 as the target gene, cloned in a pDrive Cloning Vector (not done in birds from group B as none of those survived the virus challenge). The viral copy number of samples was calculated by interpolating the samples’ Ct values with Ct value of serially diluted VP2 cloned plasmid. The group E showed the lowest (p < 0.05) of copy number of all the groups at 4 DPC (2.647 ± 0.277), followed by the group D (3.977 ± 0.222), and then by group C (5.910 ± 0.262). The highest copy number was found in group A (8.840 ± 0.180) (Table 13, Figure 6).
-
(d)
Humoral immune response analysis after NDV vaccination: The birds treated with both T. cordifolia and CpG ODN (group E) prior to the vvIBDV challenge showed a highly significant increase in HI titer, more so than those of groups C and D at two and three weeks post-NDV vaccination (p < 0.05) (Table 14). The antibody titer in group A and E was almost similar at two and three weeks post-NDV vaccination. However, no significant difference was noticed at one week post-NDV vaccination among all the groups.
Table 12.
Protection observed in different vvIBDV challenge groups.
| S. Number | Group | Total Number of Birds Challenged | Total Number of Birds Live | Total Number of Birds Dead | Percentage of Live Birds |
|---|---|---|---|---|---|
| 1. | Negative control (Group A) | --- | 20 | --- | 100% |
| 2. | Positive control (Group B) | 20 | --- | 20 | 0% |
| 3. | T. cordifolia + virus challenge (Group C) | 20 | 10 | 10 | 50% |
| 4. | CpG ODN + virus challenge (Group D) | 20 | 16 | 4 | 80% |
| 5. | T. cordifolia + CpG ODN + challenge (Group E) | 20 | 20 | --- | 100% |
Figure 5.
Section of bursa of Fabricius showing (a) Normal histo-architecture characterized by bursal follicles with intact cortex and medulla, along with lining epithelium; (b) Bursal follicles with severe lymphoid depletion in the cortex and medulla, severe congestion, and hemorrhages are evident. Loss of plical lining epithelium is seen; (c) moderate to severe lymphoid depletion in the bursal follicles, along with normal lining of plical epithelium and peri-follicular fibrosis is evident; (d) moderate lymphoid depletion in the bursal follicles along with mild peri-follicular fibrosis; (e) apparently normal histo-architecture, characterized by bursal follicles with intact cortexes and mild lymphoid depletion in the medulla, along with intact lining epithelium (H&E 20X).
Table 13.
Viral load (mean log10 copy number ± SE) in the bursa of Fabricius (gene copy number by micrograms of total RNA) of chickens in different groups challenged with vvIBDV at 4 DPC.
| S. Number | Group | 4 DPC |
|---|---|---|
| 1. | Negative control (Group A) | 8.840 a ± 0.180 |
| 2. | T. cordifolia + virus challenge (Group C) | 5.910 b ± 0.262 |
| 3. | CpG ODN + virus challenge (Group D) | 3.977 c ± 0.222 |
| 4. | T. cordifolia + CpG ODN + challenge (Group E) | 2.647 d ± 0.277 |
a–d Means bearing different superscripts column-wise differ significantly (p < 0.05).
Figure 6.
Standard curve with VP2 gene (189 bp) cloned in a pDrive cloning vector for viral load estimation.
Table 14.
Hemagglutination inhibition (HI) antibody titer (mean log2 HI titer ± SE) against the New Castle disease vaccine in T. cordifolia and/or CpG treated and vvIBDV challenged WL chicken.
| S. Number | Group | 1 Wk Post NDV Vaccination | 2 Wk Post NDV Vaccination | 3 Wk Post NDV Vaccination |
|---|---|---|---|---|
| 1. | Negative control (Group A) | 1.314 a ± 0.014 | 3.684 a ± 0.029 | 6.788 a ± 0.061 |
| 2. | T. cordifolia + virus challenge (Group C) | 1.116 b ± 0.072 | 2.668 b ± 0.143 | 5.518 b ± 0.157 |
| 3. | CpG ODN + virus challenge (Group D) | 1.204 c ± 0.061 | 2.820 c ± 0.051 | 5.908 c ± 0.064 |
| 4. | T. cordifolia + CpG ODN + challenge (Group E) | 1.298 d ± 0.018 | 3.640 d ± 0.034 | 6.750 d ± 0.077 |
a–d Means bearing different superscripts column-wise differ significantly (p < 0.001).
3.5. The Assessment of the Humoral Immune Response after Vaccination by ELISA Kit
The results of ELISA for serum antibody titer estimation after IBDV vaccination are shown in Table 15 and Figure 7.
Table 15.
Serum antibody titers (mean ± SE of antilog of Log10 titer) in chickens vaccinated with IBDV vaccine at different DPV by ELISA kit method.
| S. Number | Group | Pre-Vaccination | 7 DPV | 14 DPV | 21 DPV | 28 DPV |
|---|---|---|---|---|---|---|
| 1. | Negative control | 127.367 a±1.735 | 16.476 a±0.895 | 18.914 a±0.087 | 23.683 a±0.591 | 57.440 a±2.618 |
| 2. | Positive control | 5.545 b ± 0.109 | 404.624 b ± 1.797 | 466.811 b ± 6.808 | 576.626 b ± 8.290 | 728.446 b ± 3.561 |
| 3. | T. cordifolia + vaccine | 6.340 c ± 0.195 | 525.834 c ± 3.913 | 602.523 c ± 5.427 | 716.618 c ± 11.656 | 944.575 c ± 12.222 |
| 4. | CpG ODN + vaccine | 19.897 d ± 0.190 | 587.897 d ± 11.084 | 717.898 d ± 11.729 | 1217.303 d ± 18.584 | 1857.785 d ± 4.094 |
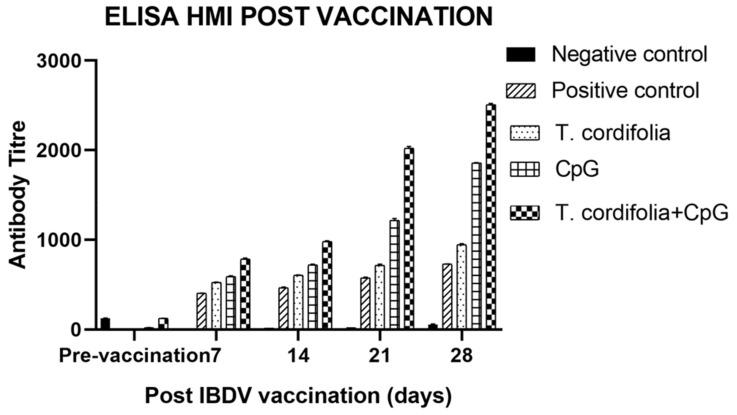
| 5. | T. cordifolia + CpG ODN + vaccine | 124.547 e ± 1.614 | 784.827 e ± 12.559 | 979.217 e ± 9.894 | 2020.827 e ± 20.893 | 2509.359 e ± 12.774 |
a–e Means bearing different superscripts column-wise differ significantly (p < 0.001).
Figure 7.
Serum antibody titer in chickens vaccinated with IBDV at different DPV by ELISA kit method. Antibody titer is expressed as mean ± SE of antilog of Log10 titer.
The group E showed a significant difference (p < 0.05) from other groups at 7 DPV (784.827 ± 12.559), 14 DPV (979.217 ± 9.894), 21 DPV (2020.827 ± 20.893), and 28 DPV (2509.359 ± 12.774), with the maximum antibody titer being estimated in this group; followed by the group D which differed significantly (p < 0.01) at 7DPV (587.897 ± 11.084), 14 DPV (717.898 ± 11.729), 21 DPV (1217.303 ± 18.584), and 28 DPV (1857.785 ± 4.094) from group C and group B. Furthermore, in both group D and group E, antibody titer significantly increased (p < 0.01) from 7 DPV to 28 DPV. Group C also showed a significant increase (p < 0.01) in antibody titer at 7 DPV (525.834 ± 3.913), 14 DPV (602.523 ± 5.427), 21 DPV (716.618 ± 11.656), and 28 DPV (944.575 ± 12.222), from group B.
4. Discussion
Infectious bursal disease virus induced immunosuppressive disease is one of the serious hurdles faced by commercial poultry producers throughout the world. This virus causes severe acute infection in young chickens, affecting mainly the bursa of Fabricius, leading to death of lymphoid cells [34]. IBDV is ubiquitous, and most often chickens acquire infection via the oral route. Viral replication occurs in bursa of Fabricius, and IgM bearing B cells are the target of this virus [35]. Immunosuppression induced by this virus affects chickens in serious ways; viz., increased incidence of secondary infections, poor feed conversion, reduction in protective immune response generated by other vaccines, etc. [19]. Though vaccination is imperative in preventing the incidences of infectious diseases, at certain times this procedure fails in the field. Similarly, vaccines available to protect IBDV challenge are not fully protective, despite the high antibody titer generated by them [36]. Live vaccines available induce bursal atrophy, along with possessing unstable antigenic and pathogenic characteristics [34]. This leads to increase in susceptibility to other secondary bacterial infections [37]. Thus, raising the need to find immunomodulatory agents which may act as standalone armor to combat such pathogens, or when used with vaccines, to increase their effectiveness in the host.
For time immemorial, herbs have been used in India traditionally for their immunomodulatory and prophylactic potential against various pathogens. T. cordifolia is one such herb with immense immunomodulatory potential in animals and poultry [21,24]. Previous studies on T. cordifolia supplementation in broilers indicated its use as an alternative to an antibiotic growth promoter and immunostimulator, showing positive impacts on feed conversion ratio and overall growth improvement without any negative effects or toxicity [38,39]. Similarly, in the present study, T. cordifolia extract feeding for chicks up to 4 weeks of age does not show any observable ill effects. Thus, its use was found safe for chickens, without causing any harm to them with autoimmunity or immunopathology due to the stimulation of immune system.
In this study, the aqueous extract of T. cordifolia stem powder was given from one day old until 4 weeks of age. Expressions of IL-2, IFN-γ, IL-4, and IL-1β were estimated in chicken PBMCs at 4 weeks of age. The results indicated significant up-regulation of these cytokine genes in PBMCs isolated from supplemented birds, compared with the control. These results are in accordance with the study where polysaccharide (G1-4A arabinogalactan polysaccharide) derived from T. cordifolia was found to stimulate murine macrophages leading to up-regulation of IL-2, IFN-γ, IL-4, and IL-1β in vitro [40]. Therefore, these results are indicative of the immunomodulatory potential of this herb in chickens, through alteration of the expressions of immune response cytokines. Recent studies have also shown the immunomodulatory potential of T. cordifolia stem hydro-alcoholic extract in chickens against a 2.4-dinitrofluorobenzene (DNFB) skin sensitization test, as seen in the form of increases in skin thickness [41]. T. cordifolia extract supplementation in chicks has also shown protection against Escherichia coli infection by increasing the E. coli specific antibody titer and lymphocyte proliferation response [42]. It has been found that G1-4A, an arabinogalactan polysaccharide from the stem of T. cordifolia, responsible for its immunomodulatory potential, acts by activating the B cells polyclonally, via an increase in CD69 expression in lymphocytes. TLR4 on B lymphocytes and macrophages acts as a receptor for G1-4A polysaccharide, activating these immune cells via TLR4/MyD88 dependent manner [40,43]. Similarly, G1-4A leads to enhanced antigen presentation from dendritic cells, and further activation of cytotoxic T cells [44]. Apart from that, there is one more component identified from T. cordifolia stem extract, termed immunomodulatory protein (single chain acidified protein, 25 kDa) which is reported to possess lymphoproliferative and macrophage stimulating properties [23].
TLRs are the best characterized component of the innate immune system, and have been reported in wide range of species, from arthropods to humans. Microbes stimulate TLRs with their PAMPs which in turn activates both the innate and acquired immune responses [45]. The signals generated out of stimulating these TLRs induce the appropriate types of immune responses, providing protection against relevant pathogens. Based on the immunostimulatory properties of TLR agonists, attempts have been made to employ them as an adjuvant with various vaccine antigens. Chicken TLR agonists have been used successfully as vaccine adjuvants [12,46] and prophylactic agents [13,47]. Previous studies already established the role of CpG ODN (TLR21 agonist) as an immunostimulant in chickens leading to increased protection against various pathogens [48,49]. CpG ODN provides antiviral immunity by enhanced recruitment of macrophages, up-regulation of INF-γ and cluster differentiation of CD8/4+ T lymphocytes [49]. Apart from enhancing cytokine expression, CpG ODN administration has shown an anti-microbial effect by the enrichment of immunological niches (macrophages, CD4, and CD8 T cells population) in various lymphoid organs, such as the spleen and thymus [50].
In this experiment, T. cordifolia aqueous extract (group C) and CpG ODN (group D) were evaluated for their prophylactic efficacy against the vvIBDV challenge in chickens at 4 weeks of age. Moreover, their combined effect to protect against the vvIBDV challenge (group E) was also evaluated. Protection was evaluated based on the cellular immune response, humoral response, histopathological, and the protection study. Cellular immune response was evaluated through the quantitative real-time PCR based expression of cytokines (IFN-γ, IL-2) in chicken PBMCs and bursa of Fabricius. Expression levels of IL-4 and IL-1β was also assessed in chicken PBMCs and bursa of Fabricius using qRT-PCR. Humoral immune response was assessed using an ELISA kit for estimating the antibody titer.
The results of the current study indicated highest expression levels of IL-2, IFN-γ, IL-4, and IL-1β in the PBMCs and bursa of Fabricius in group E, followed by group D, and then group C. This indicates that the three groups treated with immunomodulatory agent (either alone or in combination) showed more expression of cytokines under study than the positive virus control (group B) at both 7 DPC and 14 DPC. Significant increases in the expression levels of IL-2 and IFN-γ cytokines in group E point to the additive influence of CpG ODN and T. cordifolia extract in stimulating PBMCs and immune cells in the bursa of Fabricius towards a Th1 response, thereby stimulating a cell mediated immune response in a chicken, when challenged with vvIBDV. IL-2 is expressed exclusively by T-lymphocytes, and it promotes the growth of T-lymphocytes, thereby enhancing cell mediated immunity [51]. IFN-γ is a type 2 interferon and a hallmark cytokine for TH1 cells [52], producing pleiotropic effects on immune cells; viz., antiviral activity, stimulation of macrophages and natural killer cells, and increased expression of major histocompatibility antigens [53]. Thus, induction of IFN-γ by T. cordifolia aqueous extract and TLR 21 agonist suggests that they enhance the ability of the immune system to combat various intracellular pathogens in chickens, which is biased towards Th1 response. This biased Th1 immune response is essential to combat intracellular pathogens such as viruses [54]. Since stimulation of intra-bursal T lymphocytes plays an important role in clearing viral infection and promoting recovery from infection [55], the ability of CpG ODN and T. cordifolia aqueous extract in inducing TH1 responses, as observed in the present study, suggests the mechanism of their protective effects against vvIBDV in the treated chickens.
The immune system is regulated between TH1 and TH2 responses and the polarization of the responses is largely based on antigen-specific TH cells. TH1 cells drive cell-mediated, inflammatory responses, while TH2 cells drive responses against helminthic worms, extracellular bacteria, and some viral infections. TH1 cells typically produce IFN-γ, while TH2 cells typically produce IL-4 [56]. IL-4 is a pleiotropic lymphokine produced by Th2 cells which plays an important role in the immune system [57]. MHC II is an important molecule for the antigen presentation of B cells. IL-4 enhances expression of MHC II and IL-4 receptors on B lymphocytes and these receptors have an important role in B cell function. Chicken bone marrow cells cultured in the presence of recombinant chicken IL-4 get transformed into dendritic cells and express increased MHC II [58]. MHC II is required to present exogenous antigens processed by endocytic pathway and promote TH2 mediated immunity [59]. IL4 expression in this study shows a stimulatory effect of immune modulatory supplements over IL4 cytokine (as evidenced from its significant increase in groups E, D, and C), thus indicating activated B lymphocyte responses and humoral immunity against vvIBDV challenge. Thus, the findings of this study suggest that TLR 21 agonist and T. cordifolia aqueous extract can induce a mixed and more balanced TH1 and TH2 response which is vital to protect a host against distinct types of microbes, such as viruses and worms.
Besides the above described adaptive responses, the influences of CpG ODN and T. cordifolia aqueous extract in stimulating macrophages could be evaluated from the expression of the pro-inflammatory cytokine, chicken IL-1β, which is a mammalian homolog produced from avian macrophages [60]. IL-1β acts as a chemotactic factor for macrophages at the site of injury where they differentiate into tissue macrophages. These tissue macrophages carry out various functions, such as the release of nitric oxide (NO) which is needed for viral destruction, phagocytosis of antigens, and antigen processing and presentation [60].
Humoral immune response stimulation in vvIBDV challenged birds was evaluated through estimating the antibody titer using an ELISA kit. The antibody titer was found to be highest in group E, followed by group D, and then group C. The immunomodulatory agent treated groups showed significantly higher IBDV specific antibody titers than the positive virus control group at both 7 DPC and 14 DPC. This correlates with the level of IL-4 levels estimated by qRT-PCR. Anti-IBDV antibody plays an important role in protecting the chicken against IBDV infection [61].
Bursal damage, as seen histopathologically, was found to be least in group E, followed by groups D and C, respectively. Positive control (group B) showed extensive bursal damage. The least bursal damage in group E might be due to the highest virus clearance in their bursa. This clearance of virus is due to the stimulation of the cell mediated immune response as indicated by high IFN-γ and IL-2 expression levels in both their bursa and PBMCs [62].
Moreover, this study provides evidence that the challenging of SPF chickens with vvIBDV in group E induced an obvious protective immune response to avoid an otherwise lethal outcome. The protection of 100% from vvIBD virus was achieved in the group E which received CpG ODN along with T. cordifolia aqueous extract. In groups C and D, 50% and 80% protection was observed, respectively. In the positive virus control (group B), 100% mortality was observed during the observation period. These birds showed characteristics signs of IBDV infection, such as depression, drowsiness, inability to move, ruffled feathers, and severe prostration. On necropsy examination of birds in group B, characteristics of IBDV lesions were found, such as hemorrhages in the thigh muscles, splenomegaly, and swollen and hemorrhagic bursae. The bursal folds were extensively hemorrhagic and contained variable amounts of blood clotting.
Further, immunosuppression, which is the most serious impact of IBDV infection, was found to be diluted in immunomodulatory agent treated groups, with the highest dilution in group E, followed by group D, and then group C. This finding is significant, since vaccination with live vaccines leaves birds protected to some extent, but birds have lowered immunity, and further vaccination against other diseases fails, leaving them vulnerable to various pathogens.
Antibody response was highest after IBDV vaccine in group E, followed by group D, and then group C. The immunomodulatory agent treated groups showed significantly higher IBDV specific antibody titers than positive vaccine control group at 7 DPV, 14 DPV, 21 DPV, and 28 DPV. Thus, both the immunomodulatory agents possess adjuvant activity with IBDV vaccine. Such adjuvants with balanced stimulation of the immune response and with less adverse effects are highly desirable to achieve efficient protection against infectious diseases.
5. Conclusions
To conclude, we suggest that T. cordifolia and CpG ODN be used as prophylactic agents, and as adjuvants in poultry for viral diseases. They both have immunomodulatory potential via the TLR mediated pathway. There is evidence for using a combination of TLR agonist as an adjuvant in vaccines for a better immune response [63]. However, TLR agonists are costly compared with herbal preparations. If one of the synthetic TLR agonists acting via one TLR can be substituted with the herb acting through the same TLR pathway, then the cost of the vaccine can be further reduced. To further understand the mechanism of synergy between this herb and CpG ODN, studies must be designed to evaluate various intermediate adaptor molecules and their interaction in immune cells. Other herbal preparations and TLR agonists can also be evaluated for their synergistic effects of stimulating the immune response against various infectious diseases. Evaluation of herbs and their extracts to modulate the innate immune response will be helpful, as they are cheap and easily available to farmers for use in the form of immunomodulatory agents, to protect the poultry flock against a variety of infectious diseases.
Acknowledgments
The authors are thankful to the director, Indian Veterinary Research Institute, and the Indian Council of Medical Research (ICMR) for providing the funds and facilities for the research.
Author Contributions
All the authors contributed substantially to the conceptualization, methodology, analysis, interpretation, and the preparation of manuscript; conceptualization, S.S. and K.D.; methodology, S.S. and K.D.; software, H.A.S. and S.K.L.; validation, P.M. and A.K.M.; formal analysis, Y.P.S.M.; investigation, S.S. and S.K.L.; resources, R.K.S. and K.P.S.; data curation, S.S., H.A.S., and K.D.; writing—original draft preparation, S.S.; writing—reviewing and editing, S.S., S.K.L., P.M., Y.P.S.M., and K.D.; visualization, K.P.S.; supervision, R.S.
Funding
The study was financially supported by the Indian Veterinary Research Institute and the Indian Council of Medical Research (ICMR).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Smith J., Sadeyen J.R., Butter C., Kaiser P., Burt D.W. Analysis of the early immune response to infection by infectious bursal disease virus in chickens differing in their resistance to the disease. J. Virol. 2015;89:2469–2482. doi: 10.1128/JVI.02828-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ciccone N.A., Smith L.P., Mwangi W., Boyd A., Broadbent A.J., Smith A.L., Nair V. Early pathogenesis during infectious bursal disease in susceptible chickens is associated with changes in B cell genomic methylation and loss of genome integrity. Dev. Comp. Immunol. 2017;73:169–174. doi: 10.1016/j.dci.2017.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zachar T., Popowich S., Goodhope B., Knezacek T., Ojkic D., Willson P., Ahmed K.A., Gomis S. A 5-year study of the incidence and economic impact of variant infectious bursal disease viruses on broiler production in Saskatchewan, Canada. Can. J. Vet. Res. 2016;80:255–261. [PMC free article] [PubMed] [Google Scholar]
- 4.Jayasundara J.M.K.G.K., Walkden-Brown S.W., Katz M.E., Islam A.F.M.F., Renz K.G., McNally J., Hunt P.W. Pathogenicity, tissue distribution, shedding and environmental detection of two strains of IBDV following infection of chickens at 0 and 14 days of age. Avian Pathol. 2017;46:242–255. doi: 10.1080/03079457.2016.1248898. [DOI] [PubMed] [Google Scholar]
- 5.Macwan T.S., Dave C.J., Joshi B.P., Ghodasara D.J. Epidemiological Study on Infectious Bursal Disease in Broilers. Indian J. Vet. Sci. Biotechnol. 2019;14:68–72. doi: 10.21887/ijvsbt.14.3.17. [DOI] [Google Scholar]
- 6.Dulwich K.L., Giotis E.S., Gray A., Nair V., Skinner M.A., Broadbent A.J. Differential gene expression in chicken primary B cells infected ex vivo with attenuated and very virulent strains of infectious bursal disease virus (IBDV) J. Gen. Virol. 2017;98:2918–2930. doi: 10.1099/jgv.0.000979. [DOI] [PubMed] [Google Scholar]
- 7.Spackman E., Stephens C.B., Pantin-Jackwood M.J. The effect of infectious bursal disease virus–induced immunosuppression on vaccination against highly pathogenic avian influenza virus. Avian Dis. 2017;62:36–44. doi: 10.1637/11769-110717-Reg.1. [DOI] [PubMed] [Google Scholar]
- 8.Tartey S., Takeuchi O. Pathogen recognition and toll-like receptor targeted therapeutics in innate immune cells. Int. Rev. Immunol. 2017;36:57–73. doi: 10.1080/08830185.2016.1261318. [DOI] [PubMed] [Google Scholar]
- 9.Musthafa M.S., Asgari S.M., Kurian A., Elumalai P., Ali A.R.J., Paray B.A., Al-Sadoon M.K. Protective efficacy of Mucuna pruriens (L.) seed meal enriched diet on growth performance, innate immunity, and disease resistance in Oreochromis mossambicus against Aeromonas hydrophila. Fish Shellfish Immunol. 2018;75:374–380. doi: 10.1016/j.fsi.2018.02.031. [DOI] [PubMed] [Google Scholar]
- 10.Hajishengallis G., Lambris J.D. More than complementing Tolls: Complement–Toll-like receptor synergy and crosstalk in innate immunity and inflammation. Immunol. Rev. 2016;274:233–244. doi: 10.1111/imr.12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Nardo D. Toll-like receptors: Activation, signalling and transcriptional modulation. Cytokine. 2015;74:181–189. doi: 10.1016/j.cyto.2015.02.025. [DOI] [PubMed] [Google Scholar]
- 12.Sachan S., Ramakrishnan S., Annamalai A., Sharma B.K., Malik H., Saravanan B.C., Jain L., Saxena M., Kumar A., Krishnaswamy N. Adjuvant potential of resiquimod with inactivated Newcastle disease vaccine and its mechanism of action in chicken. Vaccine. 2015;33:4526–4532. doi: 10.1016/j.vaccine.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 13.Annamalai A., Ramakrishnan S., Sachan S., Kumar B.A., Sharma B.K., Kumar V., Palanivelu M., Varghese B.P., Kumar A., Saravanan B.C., et al. Prophylactic potential of resiquimod against very virulent infectious bursal disease virus (vvIBDV) challenge in the chicken. Vet. Microbiol. 2016;187:21–30. doi: 10.1016/j.vetmic.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Bavananthasivam J., Kulkarni R.R., Read L., Sharif S. Reduction of Marek’s Disease Virus Infection by Toll-Like Receptor Ligands in Chicken Embryo Fibroblast Cells. Viral Immunol. 2018;31:389–396. doi: 10.1089/vim.2017.0195. [DOI] [PubMed] [Google Scholar]
- 15.Taha-abdelaziz K., Alkie T.N., Hodgins D.C., Shojadoost B., Sharif S. Characterization of host responses induced by Toll-like receptor ligands in chicken cecal tonsil cells. Vet. Immunol. Immunopathol. 2016;174:19–25. doi: 10.1016/j.vetimm.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Keestra A.M., de Zoete M.R., Bouwman L.I., van Putten J.P. Chicken TLR21 is an innate CpG DNA receptor distinct from mammalian TLR9. J. Immunol. 2010;185:460–467. doi: 10.4049/jimmunol.0901921. [DOI] [PubMed] [Google Scholar]
- 17.Abdul-Cader M.S., Ahmed-Hassan H., Amarasinghe A., Nagy E., Sharif S., Abdul-Careem M.F. Toll-like receptor (TLR) 21 signalling-mediated antiviral response against avian influenza virus infection correlates with macrophage recruitment and nitric oxide production. J. Gen. Virol. 2017;98:1209–1223. doi: 10.1099/jgv.0.000787. [DOI] [PubMed] [Google Scholar]
- 18.Wang X., Jiang P., Deen S., Wu J., Liu X., Xu J. Efficacy of DNA vaccines against infectious bursal disease virus in chickens enhanced by coadministration with CpG oligodeoxynucleotide. Avian Dis. 2003;47:1305–1312. doi: 10.1637/6045. [DOI] [PubMed] [Google Scholar]
- 19.Li L., Pielsticker C., Han Z., Kubasova T., Rychlik I., Kaspers B., Rautenschlein S. Infectious bursal disease virus inoculation infection modifies Campylobacter jejuni–host interaction in broilers. Gut Pathogs. 2018;10:13. doi: 10.1186/s13099-018-0241-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paul R., Khanna A. A study of immunomodulatory effects of aqueous extract of phyllanthus emblica (amla) leaf as a dietary herbal constituent in clarias batrachus by analysing the haematological parameters. Int. Res. J. Pharm. 2016;7:33–36. doi: 10.7897/2230-8407.0716. [DOI] [Google Scholar]
- 21.Latheef S.K., Dhama K., Samad H.A., Wani M.Y., Kumar M.A., Palanivelu M., Malik Y.S., Singh S.D., Singh R. Immunomodulatory and prophylactic efficacy of herbal extracts against experimentally induced chicken infectious anaemia in chicks: Assessing the viral load and cell mediated immunity. Virus Dis. 2017;28:115–120. doi: 10.1007/s13337-016-0355-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou X., Liu Z., Long T., Zhou L., Bao Y. Immunomodulatory effects of herbal formula of astragalus polysaccharide (APS) and polysaccharopeptide (PSP) in mice with lung cancer. Int. J. Biol. Macromol. 2018;106:596–601. doi: 10.1016/j.ijbiomac.2017.08.054. [DOI] [PubMed] [Google Scholar]
- 23.Aranha I., Clement F., Venkatesh Y.P. Immunostimulatory properties of the major protein from the stem of the Ayurvedic medicinal herb, guduchi (Tinospora cordifolia) J. Ethnopharmacol. 2012;139:366–372. doi: 10.1016/j.jep.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 24.Sharma A., Chadha N.K., Das S.K., Sen A., Roy S.D., Chanu T.I., Sawant P.B., Prakash C. Tinospora cordifolia extract induced effects on cellular immune reactions of labeo rohita (hamilton) challenged against aeromonas hydrophila. Int. J. Pure Appl. Biosci. 2017;5:765–775. doi: 10.18782/2320-7051.5120. [DOI] [Google Scholar]
- 25.More P., Pai K. Immunomodulatory effects of Tinospora cordifolia (Guduchi) on macrophage activation. Biol. Med. 2011;3:134–140. [Google Scholar]
- 26.Reed L.J., Muench H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938;27:493–497. doi: 10.1093/oxfordjournals.aje.a118408. [DOI] [Google Scholar]
- 27.Ranjith M.S., Ranjit singh A.J.A., Shankar S.G., Vijayalaksmi G.S., Deepa K., Sidhu H.S. Enhanced Phagocytosis and Antibody Production by Tinospora cordifolia-A new dimension in Immunomodulation. African J. Biotechnol. 2008;7:081–085. [Google Scholar]
- 28.Nang N.T., Lee J.S., Song B.M., Kang Y.M., Kim H.S., Seo S.H. Induction of inflammatory cytokines and toll-like receptors in chickens infected with avian H9N2 influenza virus. Vet. Res. 2011;42:64. doi: 10.1186/1297-9716-42-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fan Y.F., Hou Z.C., Yi G.Q., Xu G.Y., Yang N. The sodium channel gene family is specifically expressed in hen uterus and associated with eggshell quality traits. BMC Genet. 2013;14:90. doi: 10.1186/1471-2156-14-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He H., Crippen T.L., Farnell M.B., Kogut M.H. Identification of CpG oligodeoxynucleotide motifs that stimulate nitric oxide and cytokine production in avian macrophage and peripheral blood mononuclear cells. Dev. Comp. Immunol. 2003;27:621–627. doi: 10.1016/S0145-305X(03)00013-2. [DOI] [PubMed] [Google Scholar]
- 31.Gao H., Li K., Gao L., Qi X., Gao Y., Qin L., Wang Y., Wang X. DNA prime–protein boost vaccination enhances protective immunity against infectious bursal disease virus in chickens. Vet. Microbiol. 2013;164:9–17. doi: 10.1016/j.vetmic.2013.01.027. [DOI] [PubMed] [Google Scholar]
- 32.[OIE] World Organisation for Animal Health . Biological Standards Commission. 7th ed. World Organization for Animal Health; Paris, France: 2012. Manual of diagnostic tests and vaccines for terrestrial animals: Mammals, birds and bees. [Google Scholar]
- 33.Pfaffl M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Killian M.P., Boviez J.D., Gambarotta M., Lombardo D.M. Induction of apoptosis in the bursa of Fabricius by vaccination against Gumboro disease. Avian Pathol. 2017;46:526–534. doi: 10.1080/03079457.2017.1322684. [DOI] [PubMed] [Google Scholar]
- 35.Sharma J.M., Kim I.J., Rautenschlein S., Yeh H.Y. Infectious bursal disease virus of chickens: Pathogenesis and immunosuppression. Dev. Comp. Immunol. 2000;24:223–235. doi: 10.1016/S0145-305X(99)00074-9. [DOI] [PubMed] [Google Scholar]
- 36.Mahmood M.S., Siddique M., Hussain I., Khan A., Mansoor M.K. Protection capability of recombinant plasmid DNA vaccine containing VP2 gene of very virulent infectious bursal disease virus in chickens adjuvanted with CpG oligodeoxynucleotide. Vaccine. 2006;24:4838–4846. doi: 10.1016/j.vaccine.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 37.Arafat N., Eladl A.H., Mahgoub H., El-Shafei R.A. Effect of infectious bursal disease (IBD) vaccine on Salmonella Enteritidis infected chickens. Vaccine. 2017;35:3682–3689. doi: 10.1016/j.vaccine.2017.04.076. [DOI] [PubMed] [Google Scholar]
- 38.Singh S. Effect of dietary inclusion of Giloy (Tinospora cordifolia) stem powder on growth performance and metabolizability in broilers. J. Entomol. Zool. Stud. 2018;6:36–40. [Google Scholar]
- 39.Rajkumar R.S., Yadav A.S., Kirupasankar M., Saxena V.K., Sangeeta S. Effect of Tinospora cordifolia supplementation on immunity of broiler chicks. Indian Vet. J. 2009;86:1244–1245. [Google Scholar]
- 40.Gupta P.K., Rajan M.G.R., Kulkarni S. Activation of murine macrophages by G1-4A, a polysaccharide from Tinospora cordifolia, in TLR4/MyD88 dependent manner. Int. Immunopharmacol. 2017;50:168–177. doi: 10.1016/j.intimp.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 41.Shraddha N., Koley K.M., Choudhary M., Durga C., Veerendra K. Comparative study of immunomodulatory effect of Tinospora cordifolia stem and Azadirachta indica leaf extract in broiler chicks. Vet. Pract. 2017;18:286–288. [Google Scholar]
- 42.Jakhar K.K. Ph.D. Thesis. LUVAS; Hisar, Haryana, India: 2016. Clinico-Pathological Studies on Escherichia coli Infection in Broiler Chickens Fed on Probiotic and Tinospora cordifolia Extract. [Google Scholar]
- 43.Raghu R., Sharma D., Ramakrishnan R., Khanam S., Chintalwar G.J., Sainis K.B. Molecular events in the activation of B cells and macrophages by a non-microbial TLR4 agonist, G1-4A from Tinospora cordifolia. Immunol. Lett. 2009;123:60–71. doi: 10.1016/j.imlet.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 44.Pandey V.K., Shankar B.S., Sainis K.B. G1-4 A, an arabinogalactan polysaccharide from Tinospora cordifolia increases dendritic cell immunogenicity in a murine lymphoma model. Int. Immunopharmacol. 2012;14:641–649. doi: 10.1016/j.intimp.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 45.Gerdol M., Venier P., Edomi P., Pallavicini A. Diversity and evolution of TIR-domain-containing proteins in bivalves and Metazoa: New insights from comparative genomics. Dev. Comp. Immunol. 2017;70:145–164. doi: 10.1016/j.dci.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 46.Bhardwaj R., Verma R., Deka D., Dubey P.P., Arora J.S., Sethi R.S., Tolenkhomba T.C., Mukhopadhyay C.S. Validation of immunomodulatory effects of lipopolysaccharide through expression profiling of Th1 and Th2 biased genes in Newcastle disease virus vaccinated indigenous chicken. Vet. World. 2018;11:437. doi: 10.14202/vetworld.2018.437-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bavananthasivam J., Read L., Astill J., Yitbarek A., Alkie T.N., Abdul-Careem M.F., Wootton S.K., Behboudi S., Sharif S. The effects of in ovo administration of encapsulated Toll-like receptor 21 ligand as an adjuvant with Marek’s disease vaccine. Sci. Rep. 2018;8:16370. doi: 10.1038/s41598-018-34760-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goonewardene K.B., Popowich S., Gunawardana T., Gupta A., Kurukulasuriya S., Karunarathna R., Chow-Lockerbie B., Ahmed K.A., Tikoo S.K., Foldvari M., et al. Intrapulmonary delivery of CpG-ODN microdroplets provides protection against Escherichia coli septicemia in neonatal broiler chickens. Avian Dis. 2017;61:503–511. doi: 10.1637/11684-060617-Reg.1. [DOI] [PubMed] [Google Scholar]
- 49.De Silva Senapathi U., Abdul-Cader M., Amarasinghe A., van Marle G., Czub M., Gomis S., Abdul-Careem M. The in ovo delivery of CpG oligonucleotides protects against infectious bronchitis with the recruitment of immune cells into the respiratory tract of chickens. Viruses. 2018;10:635. doi: 10.3390/v10110635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gunawardana T., Ahmed K.A., Goonewardene K., Popowich S., Kurukulasuriya S., Karunarathna R., Gupta A., Lockerbie B., Foldvari M., Tikoo S.K., et al. Synthetic CpG-ODN rapidly enriches immune compartments in neonatal chicks to induce protective immunity against bacterial infections. Sci. Rep. 2019;9:341. doi: 10.1038/s41598-018-36588-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lillehoj H.S., Min W., Choi K.D., Babu U.S., Burnside J., Miyamoto T., Rosenthal B.M., Lillehoj E.P. Molecular, cellular, and functional characterization of chicken cytokines homologous to mammalian IL-15 and IL-2. Vet. Immunol. Immunopathol. 2001;82:229–244. doi: 10.1016/S0165-2427(01)00360-9. [DOI] [PubMed] [Google Scholar]
- 52.Sharma A., Saqib M., Sheikh J.A., Ehtesham N.Z., Bhaskar S., Chaudhuri T.K., Hasnain S.E. Mycobacterium indicus pranii protein MIP_05962 induces Th1 cell mediated immune response in mice. Int. J. Med. Microbiol. 2018;308:1000–1008. doi: 10.1016/j.ijmm.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 53.Song B., Li X., Ma J., Yu L., Feng Z., Liu Z., Cui Y. Prokaryotic expression and anti-IBDV activity of chicken interleukin-18 and interferon-γ. Cytogenet. Genome Res. 2017;153:36–45. doi: 10.1159/000481522. [DOI] [PubMed] [Google Scholar]
- 54.Shojadoost B., Kulkarni R.R., Brisbin J.T., Quinteiro-Filho W., Alkie T.N., Sharif S. Interactions between lactobacilli and chicken macrophages induce antiviral responses against avian influenza virus. Res. Vet. Sci. 2017;125:441–450. doi: 10.1016/j.rvsc.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 55.Teo K.Y.W., Keong Y.S., Wei T.S., Omar A.R., Alitheen N.B. Relationship between virus replication and apoptosis events in IgM+ cells from chicken spleen and bursa of Fabricius infected with malaysia strain of very virulent infectious bursal disease virus. Acta Sci. Vet. 2016;44:1–9. doi: 10.22456/1679-9216.81295. [DOI] [Google Scholar]
- 56.Raphael I., Nalawade S., Eagar T.N., Forsthuber T.G. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine. 2015;74:5–17. doi: 10.1016/j.cyto.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang T., Secombes C.J. The evolution of IL-4 and IL-13 and their receptor subunits. Cytokine. 2015;75:8–13. doi: 10.1016/j.cyto.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 58.Wu Z., Rothwell L., Young J.R., Kaufman J., Butter C., Kaiser P. Generation and characterization of chicken bone marrow-derived dendritic cells. Immunology. 2010;129:133–145. doi: 10.1111/j.1365-2567.2009.03129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schuijs M.J., Hammad H., Lambrecht B.N. Professional and ‘Amateur’Antigen-Presenting Cells In Type 2 Immunity. Trends Immunol. 2018;40:22–34. doi: 10.1016/j.it.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Amarasinghe A., Abdul-Cader M.S., Almatrouk Z., van der Meer F., Cork S.C., Gomis S., Abdul-Careem M.F. Induction of innate host responses characterized by production of interleukin (IL)-1β and recruitment of macrophages to the respiratory tract of chickens following infection with infectious bronchitis virus (IBV) Vet. Microbiol. 2018;215:1–10. doi: 10.1016/j.vetmic.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 61.Yeh H.Y., Rautenschlein S., Sharma J.M. Protective immunity against infectious bursal disease virus in chickens in the absence of virus-specific antibodies. Vet. Immunol. Immunopathol. 2002;89:149–158. doi: 10.1016/S0165-2427(02)00206-4. [DOI] [PubMed] [Google Scholar]
- 62.Rautenschlein S., Yeh H.Y., Njenga M.K., Sharma J.M. Role of intrabursal T cells in infectious bursal disease virus (IBDV) infection: T cells promote viral clearance but delay follicular recovery. Arch. Virol. 2002;147:285–304. doi: 10.1007/s705-002-8320-2. [DOI] [PubMed] [Google Scholar]
- 63.Gupta S.K., Singh L.V., Chellappa M.M., Dey S. Toll-like receptor ligands and their combinations as adjuvants-current research and its relevance in chickens. World’s Poult. Sci. J. 2015;71:95–110. doi: 10.1017/S0043933915000094. [DOI] [Google Scholar]