Significance
The existence of female orgasm is intriguing for 2 reasons: On the one hand, female orgasm is not necessary for female reproductive success, and on the other hand, this neuro-endocrine reflex is too complex to be an evolutionary accident. This led to many proposed evolutionary explanations, most of which have little empirical support. We previously proposed that female orgasm uses a mechanism that originated for inducing ovulation during copulation: A mechanism that still exists in many animals but lost its role in others. Here we provide experimental evidence, strengthening the likelihood that female orgasm evolved from copulation-induced ovulation. This finding helps interpreting otherwise difficult to explain aspects of female sexuality, such as the low rate of female orgasm during intercourse.
Keywords: fluoxetine, induced ovulation, process homology, anorgasmia, female sexuality
Abstract
The ovulatory homolog model of female orgasm posits that the neuro-endocrine mechanisms underlying female orgasm evolved from and are homologous to the mechanisms mediating copulation-induced ovulation in some mammals. This model predicts that pharmacological agents that affect human orgasm, such as fluoxetine, should also affect ovulation in animals with copulation-induced ovulation, such as rabbits. We tested this prediction by treating rabbits with daily doses of fluoxetine for 2 wk and found that fluoxetine treatment reduces the number of ovulations postcopulation by 30%. In a second experiment we tested whether this result was mediated by an effect on the brain or via peripheral serotonin functions. We treated animals with fluoxetine and induced ovulation with a single injection of human chorionic gonadotropin. In this experiment ovulation rate was nominally reduced by only 8%, which is statistically not significant. We conclude that the effect of fluoxetine on copulation-induced ovulation rate supports the ovulatory homolog model of female orgasm, suggesting that female orgasm has very deep evolutionary roots among the early eutherian mammals.
Ever since the beginnings of comparative biology with Aristotle (384 to 322 BC), the biological significance of female orgasm has been controversial (1, 2). Orgasm is a complex neuro-endocrine process (3, 4) and such complexity of a biological trait usually points to an important functional role, since complex traits do not originate by chance without strong selection in their favor (5). Yet, women can conceive and deliver healthy offspring without the need for orgasm at any stage of the reproductive process. The second reason that makes it difficult to identify a biological role for female orgasm is the highly variable incidence rate of female orgasm during reproductively relevant penetrative intercourse (6). A trait with an important biological function is expected to be more stable, since natural selection should favor the reliable execution or implementation of that trait. A large number of ideas have been proposed trying to explain the evolutionary origin and the function of female orgasm. That literature has been reviewed repeatedly, with largely negative results (2, 7). Here we are presenting the results of an experimental test of one such hypothesis, the ovulatory homolog model of female orgasm (OHM) (8–10).
The OHM proposes that the neuro-endocrine mechanisms underlying female orgasm originated in mammals where ovulation is induced by copulatory stimulation. Copulation-induced ovulation (CIO) is best characterized in rabbits, and is also found in cats, ferrets, and camel, to name a few (11). In contrast, humans, great apes, as well as rodents and others have endogenous ovulation, and copulation is not necessary for ovulation but only for fertilization and other nonovulatory effects. An example is the need for copulation for the maintenance of functional corpus luteum in mice and rat (12–15). To better understand the evolution of ovulation, we previously performed a phylogenetic analysis of ovulation types by tracing them on the phylogenetic tree of therian mammals. The distribution of the trait across species for which information is available suggests that CIO was present in early branching lineages as well as many later ones, implying that it is ancestral in eutherian mammals and that endogenous ovulation is derived (8). This result is further supported by the fact that in several endogenously ovulating species ovulation can also be triggered under specific circumstances, implying that induced ovulation is an older, still latent mechanism (8). These results support the idea that female orgasm consists of a copulation-induced reflex that originally had a role in triggering ovulation. If this inference were correct, then both female orgasm in women and CIO might still retain core commonalities that would be affected by the same pharmacological agents. This idea offers an opportunity to experimentally test the OHM hypothesis.
A well-known class of pharmacological agents negatively affecting orgasm in humans are the selective serotonin reuptake inhibitors (SSRIs), such as fluoxetine (16–19). If SSRIs inhibit orgasm in humans and if female orgasm is homologous to CIO, then SSRIs might be expected to have a high probability of reducing ovulation rate in animals with CIO. In this paper we test this prediction by treating female rabbits with daily doses of fluoxetine for 14 d and observing its effect on ovulation after copulation. We found that the number of ovulations after copulation is reduced in females treated with fluoxetine compared to control animals. In contrast, no significant effect was found in treated females when ovulation was induced by human chorionic gonadotropin (hCG) injections, circumventing the central nervous system (CNS). This suggests that fluoxetine affects ovulation in rabbits via the central nervous segment of CIO rather than the peripheral female organs (e.g., ovaries). We thus conclude that our data supports the hypothesis that female orgasm and CIO are homologous processes.
Results
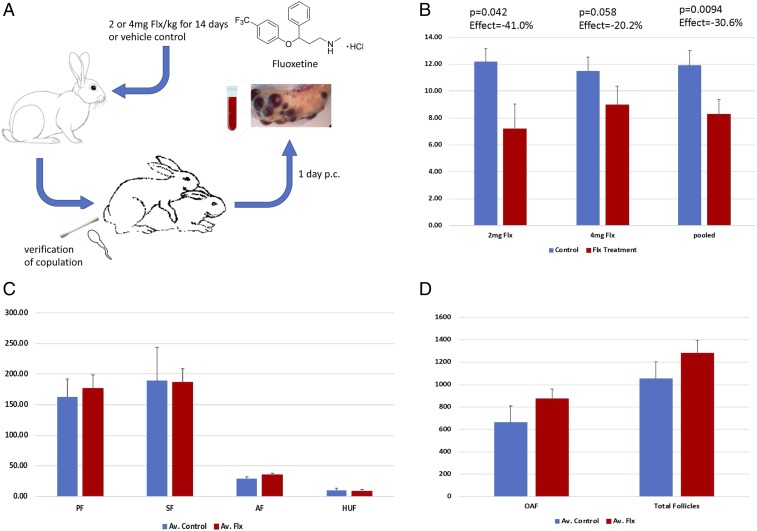
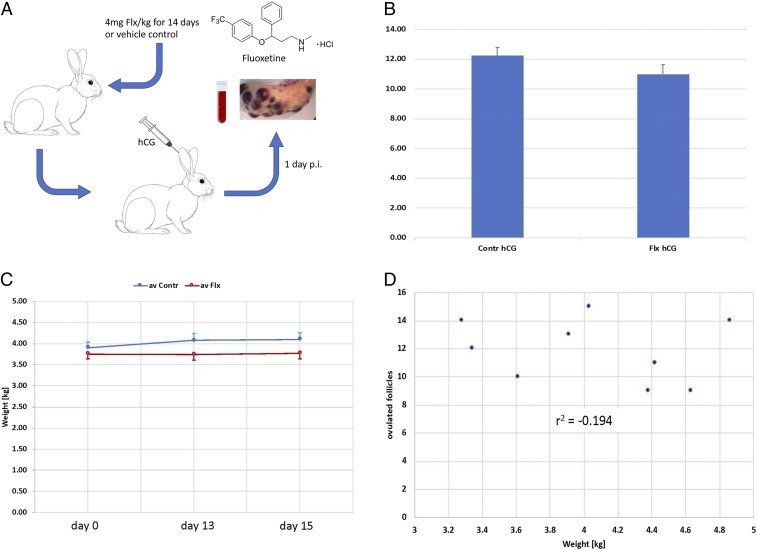
In order to test whether fluoxetine affects CIO in rabbits, we performed 2 kinds of experiments. In Exp. 1 (Fig. 1A), we treated female rabbits with daily oral fluoxetine doses given in Critical Care formulation for 14 d at 2 mg or 4 mg of fluoxetine per kilogram of body weight (Flx/kg). On the last day of fluoxetine treatment, the female was mated to a male rabbit. In all experiments the same male rabbit (also known as “Frank”) was used. A vaginal smear showing sperm confirmed successful copulation. One day after copulation the female was euthanized, and the number of ovulations assessed by counting the corpora hemorrhagica (but not hemorrhagic unovulated follicles, HUFs). A second experiment was performed to test whether the effect of fluoxetine on ovulation was caused by the local effects of fluoxetine on the ovary. Exp. 2 was done in the same way as Exp. 1 with 4 mg Flx/kg and day, except that ovulation was induced by injection of hCG instead of copulation (Fig. 2A).
Fig. 1.
Exp. 1: The effect of fluoxetine on CIO. (A) Design of Exp. 1: Adult female rabbits were fed Critical Care formulation with or without fluoxetine for 2 wk. After 2 wk the females were mated, and successful copulation was verified by a vaginal swab. One day after copulation blood was collected, the animal was euthanized, and the ovaries were retrieved for further analysis. (B) Results from 2 sets of experiments, one with 2 mg Flx/kg and 4 mg Flx/kg and vehicle control animals. In both sets of experiments the number of ovulations is less than the control animals (see text for details). (C) Effect of fluoxetine treatment on other follicle types in the ovary. Estimates for all follicle types are indistinguishable between control and treatment groups with the possible exception of AFs (see text for details). (D) OAF and total follicle number in control and treated animals. No statistically significant differences were discovered.
Fig. 2.
Exp. 2: The effect of fluoxetine on hormone-induced ovulation and other potentially confounding factors. (A) Design of Exp. 2: Adult female rabbits were fed Critical Care formulation with 4 mg Flx/kg or vehicle control for 2 wk. On day 14 the females were injected with hCG and 1 d after hCG injection (p.i.) blood was taken and the animal euthanized, and the ovaries retrieved for further analysis. (B) Number of ovulations in control and hCG-injected animals. There is a nominal but statistically insignificant decrease of about 8% in number of ovulations after fluoxetine treatment compared to all control animals. (C) Body weight in fluoxetine and vehicle control animals. The experimental group did not change body weight between the beginning of the treatment and the day of the ovulation test, day 13 or day 15 after ovulation. The control animals gained a small amount of weight, on average. (D) Relationship between body weight of control animals and the number of ovulations. No correlation was found that would suggest that body weight is a confounding factor in assessing experimental effects on ovulation rate.
Exp. 1: Effect of Fluoxetine on CIO.
Exp. 1 was performed in 2 sets, one with 2 mg Flx/kg (Ncont. = 5, Ntreatment = 5) and one with 4 mg Flx/kg (Ncont. = 4, Ntreatment = 7) (Fig. 1B). The average total numbers of ovulations from both ovaries in the control groups were about 12 (avC1 = 12.2 ± 0.95 SEM, avC2 = 11.5 ± 1.03 SEM) and are statistically indistinguishable between the 2 experimental sets (P = 0.68, t test; P = 0.624, Mann–Whitney U [MWU] test). In both treatment groups the number of ovulations was smaller than that in the control groups, with averages of 7.2 ± 1.84 SEM and 9.0 ± 1.47 SEM for 2 mg and 4 mg Flx/kg, respectively. The difference between the 2 treatment groups is not significant (P = 0.50 t test; P = 0.33 MWU test). Given that the control and treatment groups of both dosages are statistically indistinguishable, we pooled both the control and the treatment groups and obtained a pooled average for the control treatments of 11.89 ± 0.71 SEM and 8.25 ± 1.14 SEM for the fluoxetine-treated animals. The measured effect of fluoxetine on ovulation was −30.6% with a t test P = 9.39 × 10−3 and MWU test P = 2.5 × 10−2.
In order to determine the effect of fluoxetine treatment on the composition of the follicle populations in the ovary, 1 ovary each from 6 control animals and 9 fluoxetine-treated animals were paraffin-embedded and serially sectioned, and the number of various follicle types sampled (Materials and Methods): primary follicles (PF), secondary follicles (SF), antral follicles (AF), HUFs, and old atretic follicles (OAF) (Fig. 1 C and D). The numbers of follicle types were comparable between control and fluoxetine-treated animals, and not statistically significant. A possible exception is the AFs, which show a nominal increase of 20% in fluoxetine-treated animals. A closer inspection of the data revealed 2 outliers, defined as larger or smaller than the interval of 2 SDs from the mean. Removing these 2 cases, one in the control group and one in the treatment group, leads to a highly significant difference (P value of 3.59 × 10−3 by t test and P = 3.41 × 10−3 according to the nonparametric MWU test). It is thus possible that fluoxetine treatment leads to higher number of AFs after CIO. This could be due to the lower number of ovulated follicles under fluoxetine treatment, leaving behind a larger number of preovulatory follicles.
Exp. 2: hCG-Induced Ovulation.
While we observed a reduction of the number of ovulations in fluoxetine-treated animals after copulation, the OHM model predicts that the fluoxetine effect should be mediated through the CNS rather than through the direct effects of fluoxetine on the ovary. To test whether the fluoxetine effect depends on the copulation-induced neuro-endocrine reflex, we performed an additional experiment where ovulation was induced by injection of hCG (50 IU per animal) (Fig. 2A).
The number of ovulations induced by hCG in females with and without fluoxetine treatment is similar (Fig. 2B), with controls showing on average 12.25 ± 0.54 SEM (n = 4) ovulations and the treated animals 11.00 ± 0.63 SEM (n = 5), which is a difference of 10.2%. The t test yields a P = 0.228, and the MWU test P = 0.270. The average number of ovulations in control animals is remarkably similar to that in copulation-induced control animals of 11.89, and the 2 are statistically indistinguishable (P = 0.721, t test). To investigate whether the lack of significance of the control and the fluoxetine-treated animals with hCG is due to the small number of control animals, we pooled all control observations (n = 13) and performed another t test. Even with the larger control sample, the P value dropped only to 0.145. The critical question, however, is whether the fluoxetine treatment leads to lower number of ovulations induced by copulation than by hCG.
When the fluoxetine-treated hCG-induced animals are compared to the fluoxetine-treated copulation-induced animals, the number of ovulations in the latter (CIO) was 25% lower than that of hCG-induced animals, which was associated with a P = 0.0326 (t test). When the 2 observations in the CIO group with very low ovulations (0 and 1) were eliminated, the P value dropped to 0.0126, even though the average increased from 8.25 to 9.80. This is due to the fact that the 2 low outlier values inflated the estimated SD of the CIO sample and thus inflated the P value. We thus conclude that at least the majority of the fluoxetine effect observed with CIO cannot be explained by the peripheral, direct effects of fluoxetine on the ovary, and are thus likely due to effects of fluoxetine on the CNS.
Experimental Robustness and Confounding Factors.
Since female fertility also depends on body weight and fluoxetine at high doses is known to cause weight loss in rabbits (20), we wanted to investigate whether the reduced number of ovulations in our CIO experiments could be explained by an indirect effect of fluoxetine on body weight.
The fluoxetine-treated animals do not show any significant weight change on average (Fig. 2C), probably due to the compensatory effect of the Critical Care formulation that we used to administer fluoxetine. In contrast, the control animals, which received the same amount of Critical Care formulation but no fluoxetine, gained weight slightly, from 3.9 kg to 4.1 kg (∼5% on average), with the control animals being on average about 8% heavier than the fluoxetine-treated animals.
The body weight of the control animals on day 13, the day before copulation, was quite variable, ranging from 3.3 kg to 4.8 kg (almost +50% of the weight of lightest animal), with some animals gaining weight while others remained constant. This sample allowed us to test whether body weight, within this range, affects CIO. We found no systematic relationship between body weight and number of ovulations caused by copulation (Fig. 2D), and a correlation of −0.194. We conclude that body weight differences caused by our treatment regime are unlikely able to explain the differences in CIO numbers between control and fluoxetine-treated animals.
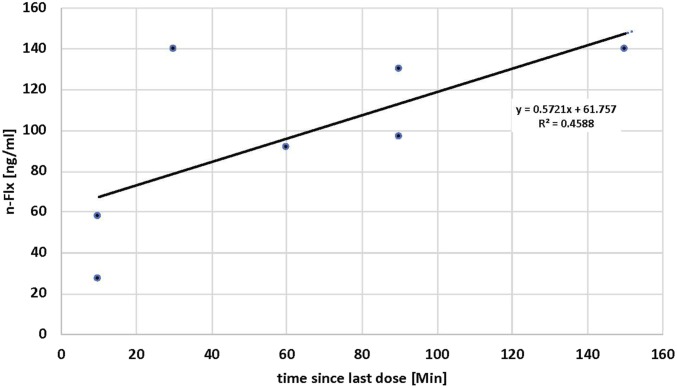
In order to assess the effectiveness of our fluoxetine administration, we measured fluoxetine and norfluoxetine (i.e., the biologically active metabolite) levels in ear vein blood on day 13, after various amounts of time since the last administration of fluoxetine. To our surprise, fluoxetine was undetectable even at >2 h since the last feeding. Nevertheless, norfluoxetine levels increased over this time course (Fig. 3), showing that fluoxetine is reaching the blood stream but is quickly metabolized to norfluoxetine, probably because blood from the gut primarily passes directly to the liver through the hepatic portal vein.
Fig. 3.
Serum concentration of norfluoxetine at various time points after the last administration of the fluoxetine/Critical Care mixture. No fluoxetine was found over this time span, and norfluoxetine shows an increase from about 40 ng/mL to about 120 ng/mL.
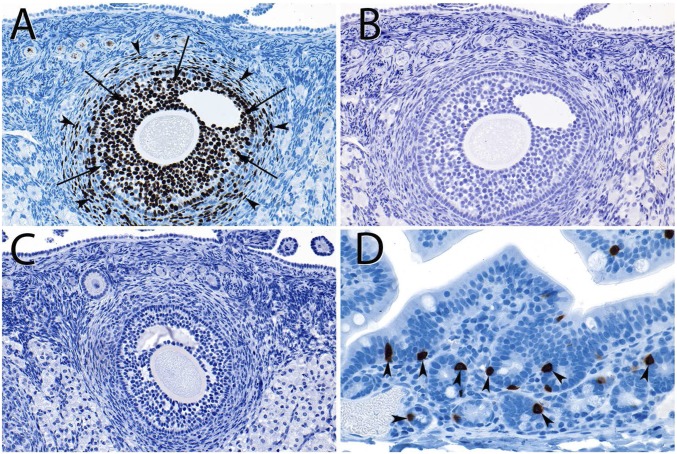
Fluoxetine acts by inhibiting the serotonin uptake via the serotonin transporter SERT in the cell membrane, and thus affecting serotonin levels at the synapse, but also other tissues (Discussion). In another approach, to understand whether fluoxetine can affect ovulation at the peripheral level of the ovary, we therefore performed serotonin (5-HT) accumulation experiments in which we incubated fresh harvested rabbit ovaries in cell culture media with 10, 30, and 100 μM 5-HT and immunostained for 5-HT accumulation. There is no staining for 5-HT, neither in untreated tissues (Fig. 4B) nor in tissue incubated in 100 μM 5-HT for 1 h (Fig. 4 C and D shows chrome-affine cells in the rabbit gut as positive control). We suggest that there is no evidence for cells in the rabbit ovary that accumulate serotonin and would thus be directly affected by fluoxetine.
Fig. 4.
Histology and immunohistochemistry of rabbit ovary. (A) Proliferative activity (Ki67) in granulosa cells (arrows) and the thecal stroma (arrowheads). (B) Staining for serotonin in the same follicle reveals no immunoreactivity. (C) Serotonin antibody staining after incubation of the ovary in 100 μM 5-HT for 1 h at 37 °C. No serotonin immunoreactivity is detected. (D) Rabbit colon stained in the same way as the ovaries in A and B, serving as a positive control, revealed strongly stained enterochromaffin cells (arrowheads). (Magnification: A–C, 20×; D, 40×.)
Finally, we assessed the effectiveness of fluoxetine treatment by measuring 5-HT in blood serum using ELISA. We found that in fluoxetine-treated animals the average level of 5-HT was higher by 75%, but the measurements were quite variable between animals so that the P value was only 0.072 based on MWU test (SI Appendix, Fig. S1).
Discussion
The current study presents experimental support for the homology between human female orgasm and the induction of ovulation in reflex ovulators. Fluoxetine, known for causing anorgasmia in humans (16–18), reduced the rate of CIO in rabbit by 30%. This effect is notable, as fluoxetine is metabolized very quickly by rabbits. Despite 14-d treatment, fluoxetine did not accumulate in rabbit serum. This observation is consistent with previous reports, showing that only the metabolite norfluoxetine remains in rabbit plasma 24 h after last administration, even when administering intravenously (21). Importantly, Yee et al. (21) have shown that the detectable norfluoxetine in rabbit after 24 h is the less-active chiral isoform R-norfluoxetine (R-norfluoxetine is ∼20-fold less effective than S-norfluoxetine). The rate of breakdown of fluoxetine is likely higher in oral administration, shortening the time of passage via the hepatic portal vein to the liver, the main site of fluoxetine metabolism. In contrast to the high metabolic rate in rabbits, the half-life of fluoxetine in humans is 1 to 4 d (22). Nevertheless, increasing the dosage of fluoxetine in rabbits beyond what we used in this study is not feasible, as toxic effects may confound the effect on ovulation. Doses greater than 2.5 mg/kg have been shown to induce weight loss and anorexia in rabbits, and those beyond 15 mg/kg lead to abortions and maternal mortality (20). To counteract the possibility of weight loss in the present study, fluoxetine was fed with the Critical Care formulation, resulting in slight weight increase in the control group, and maintenance of steady weights in the treatment groups (Fig. 2C), without affecting the difference in ovulation rates, as shown (Fig. 2D). Relative to dosages used in human therapy (20 to 80 mg/d, which corresponds to 0.3 to 1.2 mg/kg for a 70-kg adult), the dosages used in this study (2 and 4 mg/kg) are 2- to 4-fold higher. Despite lower dosages human, steady plasma levels are maintained at >100 ng/mL for fluoxetine (>120 ng/mL norfluoxetine) and are reached 2 to 4 wk after the beginning of treatment (22–24), while no fluoxetine accumulation can be detected in rabbits (present study). The observed effect of fluoxetine on ovulation rates in rabbits is thus particularly remarkable as it is detectable despite the high metabolic clearance rate.
It is important to briefly address the question whether the observed effect can be considered to support homology, in particular in the face of the observation that fluoxetine reduces ovulation rate rather than preventing it completely. In the absence of detailed knowledge of the mechanisms, we can only speculate about the proximate reasons for this result. One notable fact is that also in women, SSRI do not always and fully prevent orgasm (19). Another factor that likely contributes to the modest effect size of fluoxetine on ovulation in rabbits is the higher rate of fluoxetine metabolism in rabbits (21) (Results). Finally, homology does not imply identity of the compared processes (25) but only derivation from the same process in the common ancestor of humans and rabbits. For example, we know that forelimbs of birds and frogs are homologous, but we do not require that they look identical. With that in mind, it is not particularly problematic that the processes in the extant species are affected to different degree in humans and rabbits. On the basis of these arguments and the previously published evidence (8), we propose that this experiment affects a shared mechanism of both female orgasm and induced ovulation, and thus supports the homology between the 2.
Fluoxetine has been previously associated with effects on peripheral tissues (i.e., outside the central nervous tissue), which will be discussed below. To test whether the negative effect of fluoxetine on ovulation rate specifically is caused by effects on the peripheral organs rather than effects on the CNS, as predicted from the OHM hypothesis, we conducted an additional experiment (Exp. 2) using hCG to induce ovulation instead of inducing it by copulation. This hormone is a member of the same structurally similar group of hormones as LH and is widely used for inducing ovulation in rabbits (26–31). As hCG acts downstream of the CNS, this experimental setting circumvents the CNS-mediated need for copulatory stimulation. As confirmed by the comparison of the control groups of both experiments, induction of ovulation with hCG has replicated the copulatory induction well in terms of the ovulatory rate. This suggests that hCG is equally effective in causing ovulation as copulation and thus allows for a fair comparison of the fluoxetine effects between these 2 experiments. The nominal effect of fluoxetine on ovulation (approximately −8% when compared to all control animals) is smaller than that with copulation (approximately −30%) and not significant, suggesting that the CNS mediated the largest fraction of the fluoxetine effect found with CIO. Even though the lack of statistical significance in the hGC-induced rabbits could be in part due to the lower number of animals used in this compared to the previous experiment, the measured effect size of fluoxetine treatment is much lower and is thus insufficient to explain the effect of fluoxetine on CIO. The overall results are thus consistent with the ovulatory homolog model of female orgasm predicting a brain-mediated reduction of ovulation in fluoxetine-treated rabbits.
Weak or absent peripheral effect is also confirmed in our study by the histological assessment of ovaries. In most aspects, no significant changes were detected because of the treatment, except for a possible increase in the number of AFs in the treatment group. This group comprises a broad range of maturation stages of follicles, which precedes ovulation. In rabbits, the follicles undergo a surge of maturation following the hormonal stimulus of copulation (32, 33), and hence the results are consistent with fluoxetine affecting either the final stage of follicle development, or ovulation itself. Furthermore, we investigated whether there are cells in the rabbit ovary that could be affected by fluoxetine: That is, cells that can accumulate serotonin from the extracellular space. We incubated fresh ovary slices with up to 100 μM serotonin for 1 h and then immunostained for serotonin. Neither fresh ovaries nor serotonin-incubated ovarian tissues show evidence for cells that accumulate serotonin and would thus be affected by fluoxetine.
SSRI drugs such as fluoxetine are commonly thought to function by inhibiting the serotonin transporter SERT, a membrane protein involved in the transport of neurotransmitter serotonin from the synaptic cleft, thereby changing the serotonin levels and persistence in the synaptic cleft (34). It has been shown that administration of the SSRI drug Sertraline for 28 d in rabbits increases the serotonin levels in brain (35). Serotonin does not cross the blood–brain barrier; however, it is abundantly present also in the peripheral tissues (e.g., gut), is accumulated by blood platelets, and uses the same transporter to cross membranes in the periphery as it does in the CNS. It has been shown that, following the fluoxetine administration, serotonin levels increase in blood plasma and serum (35, 36) while they decrease in the platelets (36). The opposite result reached by Alvarez et al. (37) may be due to difficulties entirely separating platelets and plasma (38). As platelets take up serotonin from the serum and do not synthesize it themselves, their decrease in serotonin is likely due to the inhibition of the same serotonin transporter (SERT) in the platelet plasma membrane as is involved in synaptic reuptake.
The effect of elevated serotonin on reproduction has been widely studied, both in relation to and also independently of SSRIs. Serotonin has been shown to participate in the modulation of gonadotropin-releasing hormone (GnRH) secretion at the hypothalamic level (39) as well as the function of anterior pituitary in some species (40). Serotonin, its transporter, and receptors have been found in mouse ovaries, and serotonin has been shown to affect steroidogenesis in cow and rat females (41, 42). Ovarian serotonin is dynamically regulated and was observed in rat to peak at estrous (43). Soliman and Huston (44) observed the inhibitory effect of serotonin on ovulation in domestic fowl and suggested that it occurs by modulation of LH. In rabbit, Currie et al. (45) reported that when serotonin is given to female rabbits at 4 mg/kg in the marginal ear vein, it prevents ovulation completely, and at doses of 1 to 3 mg/kg it reduces the number of ova shed by inhibiting the release of LH by the anterior pituitary. Mishra et al. (46) have shown that intracerebroventricular administration of serotonin prevents coitally induced ovulation in rabbits, but it does not inhibit the ovulation induced by cupric acetate. The latter, however, can be inhibited by intraperitoneal injection of serotonin, suggesting both central and peripheral effects of serotonin.
Given the effect of fluoxetine on serotonin levels and the involvement of neuronal and peripheral serotonin in reproduction, it is perhaps not surprising that effects of fluoxetine on reproduction have been observed. For example, Rasmussen et al. (47) used fluoxetine for pharmacological manipulation of rat brain serotonin and observed that fluoxetine causes decrease in the magnitude, but not frequency, of LH pulses. Increased prolactin levels are another well-known effect of fluoxetine, suggesting dysregulation in the hypothalamus (48, 49). Imposing fluoxetine treatment on prepubertal rats, Romero-Reyes et al. (50) noted an increase in numbers of atretic follicles as well as oocyte fragmentation in female rats at first estrous. Furthermore, Moore et al. (51) found that perinatal fluoxetine exposure lengthens estrous cycles of the offspring and changes their follicular development. Effects on reproduction have also been noticed in fish: Exposure of sexually mature female zebrafish to 32 µg fluoxetine per liter water for 7 d resulted in 4.5-fold decrease in the average number of spawned eggs, decreased ovarian levels of estradiol, and decreased LH-receptor expression (52).
While the potential mechanisms that mediate the CNS effects of fluoxetine, namely serotonin levels and LH, are documented in the literature, they were not assessed in detail in this study. The LH peak is acute and short-lived and hence measuring LH levels is sensitive to individual variation in timing, requiring a continuous blood draw. Serotonin levels in the blood serum show a trend of increased values after fluoxetine treatment but also a large amount of variation. Due to the large amounts of serotonin in platelets, the serum samples easily become contaminated with serotonin released from platelets, rendering the serotonin measurements difficult to replicate. Nevertheless, despite these caveats, given the abovementioned effects of serotonin and fluoxetine on female reproduction documented in the literature, the results of our experiment are broadly consistent with the published literature. We thus suggest that our results are consistent with previous work and are experimentally robust.
We argue that our results with fluoxetine in rabbits support the hypothesis that copulatory ovulation in rabbits is homologous to female orgasm in humans. This amounts to a homology statement among 2 functional processes, those of copulatory ovulation and orgasm. While homology of anatomical structures, cell types, and genes is well understood and criteria for its validity broadly accepted, criteria for validating homology hypotheses among functional processes are less well established (see refs. 25 and 53). One possible candidate criterion for functional homology has been suggested by Alan Love (53): “Any account of activity-function homology must incorporate a notion of sameness based on underlying causal processes.” In that sense, the case of CIO and orgasm, the homology hypothesis implies an underlying homology of the neuro-endocrine structures supporting these 2 processes in humans and rabbits. While the exact structural basis of these 2 processes is not known (4) at a level of detail that would make a direct comparison feasible, testing this homology hypothesis with experimental manipulations is the most direct approach currently available. A concern with this approach, however, is that an experimental intervention that leads to the loss or attenuation of a functionality could be due to unspecific toxic side effects of the treatment. We attempted to control for this possibility by monitoring changes in body weight of the animals, where we did not find significant differences. Moreover, given the relatively specific effects of fluoxetine in humans (20) and other mammals, it is unlikely that our observations are due to an unspecific toxic side effect of this molecule. Hence, we suggest that similarity of effects of experimental manipulations in different species can be used to test hypotheses about the homology of functional processes. One can see the logic of this approach as similar to finding a common developmental/genetic basis for homologous anatomical structures, an approach deeply rooted in the tradition of comparative developmental biology.
Materials and Methods
Animals.
New Zealand white rabbits at 8 to18 mo of age, weighing 3.76 ± 0.69 kg, were obtained from Covance (2 females) and Charles River (20 females, 1 male). They were singly housed in floor pens and fed rabbit pellet and hay ad libitum.
Fluoxetine Treatment.
The study consists of three 14-d experiments (Exps. 1a and 1b and Exp. 2), each involving separate fluoxetine-treated and control groups of rabbits. The fluoxetine treatment in first experiment consisted of daily doses of 2 mg/kg fluoxetine, and the remaining 2 fluoxetine-treatment groups received 4 mg/kg of fluoxetine a day. All treatments took place in the morning. Exp. 1a involved 10 rabbits (5 treatment/5 controls), Exp. 1b involved 11 rabbits (7 treatment/4 controls), and Exp. 2 involved 9 rabbits (5 treatment/4 controls). Treatment consisted of fluoxetine oral solution (USP NDC 54838-523-40), which was mixed with Oxbow Critical Care Fine Grind Pet Supplement and administered by a needleless syringe. For the control rabbits, Critical Care was mixed with water to suitable thickness. Syringes were coded at preparation before assignment to the animals to ensure that experimenters were blinded as to the attribution of the individual rabbits to treatment group for the length of the experiment.
Induction of Ovulation.
Copulation (Exps. 1a and 1b).
On day 14 of treatment, the females were brought to the male’s cage for mating at midmorning. The experiment was designed such that only 2 to 3 females were at 14 d of treatment in the same day, to allow for maximum of 3 matings per day, for which the same male was used throughout the study. Successful copulations occurred within the first minute of encounter, requiring a single mount. In 2 cases in which the females were unwilling to mate on the designated day, another day of treatment was administered, and mating was attempted on the next day. All mattings were confirmed by the presence of sperm in vaginal swabs.
hCG injections (Exp. 2).
On day 14 of treatment, a 24-gauge ear catheter was placed into the marginal ear vein without sedation. Next, 50 IU (0.25 mL) of hCG were injected and flushed subsequently with 1 mL of saline.
Harvest.
Blood collection.
For blood collection, 2 mL of blood was drawn with a 25-gauge needle from the central ear artery before the experiment, after the treatment on day 14, 1 h after mating, and again 22 to 26 h after mating. Blood was collected in serum collector tubes (BD Microtainer REF 365967), left to clot for 30 to 120 min, centrifuged for 30 min at 3,000 × g, and serum removed for storage at −80 °C. An additional 3 mL of blood was collected for fluoxetine levels determination on day 13 in EDTA tubes, clotted for 1 to 2 h, and centrifuged for 10 min at 1,300 × g. Serum was stored at −80 °C until shipment to Arup Laboratories for fluoxetine and a metabolite quantitative serum test.
Ovaries.
The rabbits were sedated with ketamine and euthanized with pentobarbital cardiac injection ∼24 (range 22 to 26) h postcopulation (ovulation occurs ∼10 h postcopulation). Both ovaries were extracted and preserved in 4% PFA (below), and the number of ovulated follicles on their surfaces were counted immediately under dissecting microscope. The stigmas of ovulated follicles on the ovary are clearly visible as light red spots, corpora hemorrhagica.
Histology.
Ovaries and uterus were collected at necropsy and left to fix overnight in 4% PFA, after which they were transferred via gradually increasing concentrations to 70% ethanol on the next day. One ovary from each rabbit was processed, embedded in paraffin, and cut in total at 7 µm, then mounted 4 to 5 cuts per TruBond 380-coated slide. To establish a comparable proxy for the number of follicles, every fifth slide was stained with Mayer H&E stain, and a single slice per stained slide was drawn under the microscope (i.e., every 20th cut or every 130 µm). The follicles were then evaluated from the drawings. Thereby the follicles of the following groups were counted: Primary, secondary, ovulated, antral, OAFs, and hemorrhagic anovulatory follicles (following general criteria in ref. 54). The large follicles that could be traced beyond the subsequent counted slides were carefully traced; smaller follicles were counted as independent on every slide.
To our knowledge, there are no reported systematic differences in follicle development between left and right ovary, and similarly, we found none in terms of number of ovulations. However, focusing on one side, while significantly reducing the effort necessary, also did increase the variation and reduce the statistical power to detect differences between control and treatment groups with respect to these other follicle types. Fresh ovulations are easily counted on the surface of the ovary and for those the reported numbers are the sum from both ovaries.
Immunohistochemistry.
Immunohistochemistry was performed as previously described (55), with the following modifications. Antigen retrieval was performed with a Cusinart model STM-1000 steamer. Following antigen retrieval, the slides were incubated for 30 min with 0.03% hydrogen peroxide in DDH2O. The slides were then incubated overnight at 4 °C with either 1 µg/mL antiserotonin primary antibody, clone 5-HT-H209, catalog number M0758 (Dako); 0.1 µg/mL anti-Ki67 antibody, catalog number M7240 (Dako); or normal mouse ascites negative control sera, catalog number M8273 (Sigma-Aldrich). The following day, slides were washed with PBS-tween, and PBS-tween–BSA for 5 min each. Anti-mouse HRP-polymer, catalog number MHRP520 (Biocare Medical) was applied to the slides for 60 min. After washes with PBS-tween and PBS-tween–BSA, the antigen–antibody binding was visualized with DAB (Biocare BDB2004). Slides were then counterstained with hematoxylin, dehydrated, and coverslipped.
Serotonin Essay.
A competitive serotonin enzyme-linked immunosorbent assay kit (ab133053) was used to detect serum serotonin levels, following the manufacturer`s manual. The serum samples were diluted 1:50 for the assessment. The results were read at 405 nm immediately after stopping the enzyme reaction, using an automatic plate reader with Gen5 software.
All animal procedures conducted were part of the approved animal use protocol #IACUC2016-0053 at Cincinnati Children’s Hospital Medical Center (56).
Supplementary Material
Acknowledgments
Partial funding came from a crowd funding effort on “Experiment.com” to M.P. and G.P.W., https://experiment.com/, as well as the science development fund of Yale University.
Footnotes
The authors declare no conflict of interest.
See QnAs on page 20250.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1910295116/-/DCSupplemental.
References
- 1.Leroi A. M., The Lagoon. How Aristotle Invented Science (Viking, New York, NY, 2014). [Google Scholar]
- 2.Lloyd E. A., The Case of Female Orgasm: Bias in the Science of Evolution (Harvard University Press, Cambridge, 2005). [Google Scholar]
- 3.Komisaruk B. R., Beyer C., Whipple B., Orgasm. Psychologist 21, 100–103 (2008). [Google Scholar]
- 4.Komisaruk B., Beyer-Flores C., Whipple B., The Science of Orgasm (The Johns Hopkins University Press, Baltimore, 2006). [Google Scholar]
- 5.Futuyma D. J., Evolutionary Biology (Sinauer Associates, Sunderland, Mass, ed. 3, 1998). [Google Scholar]
- 6.Wallen K., Lloyd E. A., Clitoral variability compared with penile variability supports nonadaptation of female orgasm. Evol. Dev. 10, 1–2 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Levin R. J., The human female orgasm: A critical evaluation of its proposed reproductive functions. Sex. Relationship Ther. 26, 301–314 (2011). [Google Scholar]
- 8.Pavličev M., Wagner G., The evolutionary origin of female orgasm. J. Exp. Zoolog. B Mol. Dev. Evol. 326, 326–337 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Wagner G. P., Pavličev M., Origin, function, and effects of female orgasm: All three are different. J. Exp. Zoolog. B Mol. Dev. Evol. 328, 299–303 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Wagner G. P., Pavličev M., What the evolution of female orgasm teaches us. J. Exp. Zoolog. B Mol. Dev. Evol. 326, 325 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Bakker J., Baum M. J., Neuroendocrine regulation of GnRH release in induced ovulators. Front. Neuroendocrinol. 21, 220–262 (2000). [DOI] [PubMed] [Google Scholar]
- 12.Yang J. J., Larsen C. M., Grattan D. R., Erskine M. S., Mating-induced neuroendocrine responses during pseudopregnancy in the female mouse. J. Neuroendocrinol. 21, 30–39 (2009). [DOI] [PubMed] [Google Scholar]
- 13.Erskine M. S., Lehmann M. L., Cameron N. M., Polston E. K., Co-regulation of female sexual behavior and pregnancy induction: An exploratory synthesis. Behav. Brain Res. 153, 295–315 (2004). [DOI] [PubMed] [Google Scholar]
- 14.Smith M. S., Freeman M. E., Neill J. D., The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: Prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology 96, 219–226 (1975). [DOI] [PubMed] [Google Scholar]
- 15.Freeman M. E., Kanyicska B., Lerant A., Nagy G., Prolactin: Structure, function, and regulation of secretion. Physiol. Rev. 80, 1523–1631 (2000). [DOI] [PubMed] [Google Scholar]
- 16.Montejo-González A. L., et al. , SSRI-induced sexual dysfunction: Fluoxetine, paroxetine, sertraline, and fluvoxamine in a prospective, multicenter, and descriptive clinical study of 344 patients. J. Sex Marital Ther. 23, 176–194 (1997). [DOI] [PubMed] [Google Scholar]
- 17.Hensley P. L., Nurnberg H. G., SSRI sexual dysfunction: A female perspective. J. Sex Marital Ther. 28 (suppl. 1), 143–153 (2002). [DOI] [PubMed] [Google Scholar]
- 18.Carek D. J., SSRI and sexual functioning. J. Am. Acad. Child Adolesc. Psychiatry 35, 1106–1107 (1996). [DOI] [PubMed] [Google Scholar]
- 19.Balon R., SSRI-associated sexual dysfunction. Am. J. Psychiatry 163, 1504–1509, quiz 1664 (2006). [DOI] [PubMed] [Google Scholar]
- 20.National Toxology Program , “NTP-CERHR monograph on the potential human reproductive and developmental effects of fluoxetine” (Publication 05-4471, NIH, 2004; https://ntp.niehs.nih.gov/ntp/ohat/fluoxetine/fluoxetine_monograph.pdf). [PubMed] [Google Scholar]
- 21.Yee L., Wong S. H., Skrinska V. A., Chiral high-performance liquid chromatographic analysis of fluoxetine and norfluoxetine in rabbit plasma, urine, and vitreous humor using an acetylated beta-cyclodextrin column. J. Anal. Toxicol. 24, 651–655 (2000). [DOI] [PubMed] [Google Scholar]
- 22.Altamura A. C., Moro A. R., Percudani M., Clinical pharmacokinetics of fluoxetine. Clin. Pharmacokinet. 26, 201–214 (1994). [DOI] [PubMed] [Google Scholar]
- 23.Bergstrom R. F., Lemberger L., Farid N. A., Wolen R. L., Clinical pharmacology and pharmacokinetics of fluoxetine: A review. Br. J. Psychiatry 153 (suppl. 3), 47–50 (1988). [PubMed] [Google Scholar]
- 24.Amsterdam J. D., et al. , Fluoxetine and norfluoxetine plasma concentrations in major depression: A multicenter study. Am. J. Psychiatry 154, 963–969 (1997). [DOI] [PubMed] [Google Scholar]
- 25.Gilbert S. F., Bolker J. A., “Homologies of process and modular elements of embryonic construction” in The Character Concept In Evolutionary Biology, Wagner G. P., Ed. (Academic Press, San Diego, CA, 2001), pp. 435–454. [DOI] [PubMed] [Google Scholar]
- 26.Bomsel-Helmreich O., Vu N Huyen L., Durand-Gasselin I., Effects of varying doses of HCG on the evolution of preovulatory rabbit follicles and oocytes. Hum. Reprod. 4, 636–642 (1989). [DOI] [PubMed] [Google Scholar]
- 27.Kranzfelder D., Korr H., Mestwerdt W., Maurer-Schultze B., Follicle growth in the ovary of the rabbit after ovulation-inducing application of human chorionic gonadotropin. Cell Tissue Res. 238, 611–620 (1984). [DOI] [PubMed] [Google Scholar]
- 28.Asch R. H., Fernandez E. O., Smith C. G., Pauerstein C. J., Precoital single doses of delta9-tetrahydrocannabinol block ovulation in the rabbit. Fertil. Steril. 31, 331–334 (1979). [PubMed] [Google Scholar]
- 29.Kaufmann R. A., Savoy-Moore R. T., Subramanian M. G., Moghissi K. S., Cocaine inhibits mating-induced, but not human chorionic gonadotropin-stimulated, ovulation in the rabbit. Biol. Reprod. 46, 641–647 (1992). [DOI] [PubMed] [Google Scholar]
- 30.Dal Bosco A., Rebollar P. G., Boiti C., Zerani M., Castellini C., Ovulation induction in rabbit does: Current knowledge and perspectives. Anim. Reprod. Sci. 129, 106–117 (2011). [DOI] [PubMed] [Google Scholar]
- 31.Hulot F., Mariana J. C., Cattiau G., HCG-induced ovulation in two rabbit breeds: Effects of dose, season and sexual behaviour. Livest. Prod. Sci. 20, 257–267 (1988). [Google Scholar]
- 32.Cherney D. D., Didio L. J., Motta P., The development of rabbit ovarian follicles following copulation. Fertil. Steril. 26, 257–270 (1975). [DOI] [PubMed] [Google Scholar]
- 33.Pincus G., Enzmann E. V., The growth, maturation and atresia of ovarian eggs in the rabbit. J. Morphol. 61, 351–383 (1937). [Google Scholar]
- 34.Frazer A., Pharmacology of antidepressants. J. Clin. Psychopharmacol. 17 (suppl. 1), 2S–18S (1997). [DOI] [PubMed] [Google Scholar]
- 35.Ebuehi O. A. T., Ikanone C. E., Balogun A. A., Akinwande A. I., Famuyiwa O. O., Effects of administration of sertraline, clozapine, amitriptyline and imipramine on brain serotonine, liver enzymes and blood chemistry of rabbit. Int. J. Biol. Chem. Sci. 3, 85–94 (2009). [Google Scholar]
- 36.Blardi P., et al. , Serotonin and fluoxetine levels in plasma and platelets after fluoxetine treatment in depressive patients. J. Clin. Psychopharmacol. 22, 131–136 (2002). [DOI] [PubMed] [Google Scholar]
- 37.Alvarez J. C., et al. , Plasma serotonin level after 1 day of fluoxetine treatment: A biological predictor for antidepressant response? Psychopharmacology (Berl.) 143, 97–101 (1999). [DOI] [PubMed] [Google Scholar]
- 38.Brand T., Anderson G. M., The measurement of platelet-poor plasma serotonin: A systematic review of prior reports and recommendations for improved analysis. Clin. Chem. 57, 1376–1386 (2011). [DOI] [PubMed] [Google Scholar]
- 39.Wada K., et al. , Serotonin (5-HT) receptor subtypes mediate specific modes of 5-HT-induced signaling and regulation of neurosecretion in gonadotropin-releasing hormone neurons. Mol. Endocrinol. 20, 125–135 (2006). [DOI] [PubMed] [Google Scholar]
- 40.Johns M. A., Azmitia E. C., Krieger D. T., Specific in vitro uptake of serotonin by cells in the anterior pituitary of the rat. Endocrinology 110, 754–760 (1982). [DOI] [PubMed] [Google Scholar]
- 41.Battista P. J., Condon W. A., Serotonin-induced stimulation of progesterone production by cow luteal cells in vitro. J. Reprod. Fertil. 76, 231–238 (1986). [DOI] [PubMed] [Google Scholar]
- 42.Moran M. J., et al. , Effects of systemic administration or intrabursal injection of serotonin on puberty, first ovulation and follicular development in rats. Reprod. Fertil. Dev. 25, 1105–1114 (2013). [DOI] [PubMed] [Google Scholar]
- 43.Clausell D. E., Soliman K. F., Ovarian serotonin content in relation to ovulation. Experientia 34, 410–411 (1978). [DOI] [PubMed] [Google Scholar]
- 44.Soliman K. F., Huston T. M., Effect of serotonin on ovulation in the fowl. Poult. Sci. 55, 1051–1056 (1976). [DOI] [PubMed] [Google Scholar]
- 45.Currie G. N., Black D. L., Armstrong D. T., Greep R. O., Blockade of ovulation in the rabbit with catecholamines and sympathomimetics. Proc. Soc. Exp. Biol. Med. 130, 598–602 (1969). [DOI] [PubMed] [Google Scholar]
- 46.Mishra N., Tangri K. K., Bhargava K. P., Gupta M. L., Evidence for the inhibitory effect of 5-hydroxytryptamne at central and peripheral sites on ovulation in rabbits. Clin. Exp. Pharmacol. Physiol. 17, 595–599 (1990). [DOI] [PubMed] [Google Scholar]
- 47.Rasmussen D. D., Jacobs W., Kissinger P. T., Malven P. V., Plasma luteinizing hormone in ovariectomized rats following pharmacologic manipulation of endogenous brain serotonin. Brain Res. 229, 230–235 (1981). [DOI] [PubMed] [Google Scholar]
- 48.Clemens J. A., Sawyer B. D., Cerimele B., Further evidence that serotonin is a neurotransmitter involved in the control of prolactin secretion. Endocrinology 100, 692–698 (1977). [DOI] [PubMed] [Google Scholar]
- 49.Krulich L., The effect of a serotonin uptake inhibitor (Lilly 110140) on the sercretion of prolactin in the rat. Life Sci. 17, 1141–1144 (1975). [DOI] [PubMed] [Google Scholar]
- 50.Romero-Reyes J., Cárdenas M., Damián-Matsumura P., Domínguez R., Ayala M. E., Inhibition of serotonin reuptake in the prepubertal rat ovary by fluoxetine and effects on ovarian functions. Reprod. Toxicol. 59, 80–88 (2016). [DOI] [PubMed] [Google Scholar]
- 51.Moore C. J., et al. , Perinatal administration of a selective serotonin reuptake inhibitor induces impairments in reproductive function and follicular dynamics in female rat offspring. Reprod. Sci. 22, 1297–1311 (2015). [DOI] [PubMed] [Google Scholar]
- 52.Lister A., Regan C., Van Zwol J., Van Der Kraak G., Inhibition of egg production in zebrafish by fluoxetine and municipal effluents: A mechanistic evaluation. Aquat. Toxicol. 95, 320–329 (2009). [DOI] [PubMed] [Google Scholar]
- 53.Love A. C., Functional homology and homology of function: Biological concepts and philosophical consequences. Biol. Philos. 22, 691–708 (2007). [Google Scholar]
- 54.Uslu B., et al. , Quantifying growing versus non-growing ovarian follicles in the mouse. J. Ovarian Res. 10, 3 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kliman H. J., et al. , Pathway of maternal serotonin to the human embryo and fetus. Endocrinology 159, 1609–1629 (2018). [DOI] [PubMed] [Google Scholar]
- 56.National Research Council , Guide for the Care and Use of Laboratory Animals (National Academies Press, Washington, DC, ed 8, 2011). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.