Significance
Lipid metabolism is crucial to many (patho-)physiological processes, including inflammation. In particular, cholesterol biosynthesis has emerged as an exciting novel therapeutic target in numerous recent studies. Much is known about the early steps in cholesterol biosynthesis; however, the later steps have hitherto largely been neglected. Here, we investigated the druggability of distal cholesterol biosynthesis using a selective and potent chemical probe (SH42). This inhibition leads to the accumulation of the bioactive metabolite desmosterol, boosting the biosynthesis of polyunsaturated fatty acids (PUFA) and the downstream production of antiinflammatory mediators. Our report integrates distal cholesterol biosynthesis and its intermediate desmosterol with endogenous PUFA biosynthesis and resolution of inflammation, rendering this pathway attractive for further developments in the context of proresolving therapies.
Keywords: inflammation resolution, desmosterol, cholesterol, lipid mediator, PUFA
Abstract
Targeting metabolism through bioactive key metabolites is an upcoming future therapeutic strategy. We questioned how modifying intracellular lipid metabolism could be a possible means for alleviating inflammation. Using a recently developed chemical probe (SH42), we inhibited distal cholesterol biosynthesis through selective inhibition of Δ24-dehydrocholesterol reductase (DHCR24). Inhibition of DHCR24 led to an antiinflammatory/proresolving phenotype in a murine peritonitis model. Subsequently, we investigated several omics layers in order to link our phenotypic observations with key metabolic alterations. Lipidomic analysis revealed a significant increase in endogenous polyunsaturated fatty acid (PUFA) biosynthesis. These data integrated with gene expression analysis, revealing increased expression of the desaturase Fads6 and the key proresolving enzyme Alox-12/15. Protein array analysis, as well as immune cell phenotype and functional analysis, substantiated these results confirming the antiinflammatory/proresolving phenotype. Ultimately, lipid mediator (LM) analysis revealed the increased production of bioactive lipids, channeling the observed metabolic alterations into a key class of metabolites known for their capacity to change the inflammatory phenotype.
In recent years, our knowledge of human lipid metabolism has significantly increased, particularly its role in controlling and defining biological phenotypes. The most important example is possibly the role of immune cell metabolism in cancer (1). Consequently, this has triggered scientists to develop novel technologies for studying the interactions between metabolome and phenotype (2). Moreover, metabolic reprogramming has become accepted as a possible therapeutic strategy (3, 4). In particular, lipid metabolism has long been recognized for its important role in inflammatory processes (5, 6). However, to date, most therapeutic strategies target specific enzymes or receptors rather than attempting to comprehensively understand and modify lipid metabolism as a key process for the production and homoeostasis of a plethora of lipid-derived mediators. In this context, we aimed at modifying inflammation through global lipid metabolism changes. We hypothesize that global changes in lipid metabolism can be controlled in such a way that an intrinsically antiinflammatory/proresolving phenotype is observed. We argue that this would require a shift of lipid metabolism toward the increased production of antiinflammatory and proresolving mediators. As n3-polyunsaturated fatty acids (PUFAs) have particularly been described as important precursors of several antiinflammatory and proresolving mediators (7), we therefore questioned how their endogenous production and metabolism could be boosted, especially during an inflammatory event.
Mainly through the recent work of Glass and coworkers (8, 9), we identified Δ24-dehydrocholesterol reductase (DHCR24; also called Seladin-1) as a promising target for controlling lipid metabolism, possibly allowing its reprogramming. DHCR24 is the terminal enzyme in cholesterol biosynthesis, catalyzing the transformation of desmosterol to cholesterol (Fig. 1A). Of the 10 enzymes involved in distal cholesterol biosynthesis, starting with squalene, DHCR24 has recently taken center stage in several diseases. This enzyme has been linked to Alzheimer’s disease (AD), oncogenic and oxidative stress (10), hepatitis C virus (HCV) infections (11), differentiation of T helper-17 cells (12), development of foam cells (13), and prostate cancer (14). While the role of DHCR24 in AD is controversially discussed, it has been postulated as a possible drug target for HCV infections (11) as well as arteriosclerosis (8). Interestingly, this suggests a pronounced involvement of DHCR24 in inflammatory processes, as can be further underlined by the actions of its substrate, desmosterol. Desmosterol has been shown to interact with the liver X receptors (LXRs), the adenosine 5′-triphosphate–binding cassette transporter A1 (ABCA1), the sterol response element-binding protein (SREBP), and RORγ (8, 12, 15, 16). In addition, it has been proven that activation of LXRs has a marked influence on immunological functions and PUFA biosynthesis, particularly within macrophages (MΦ) (15, 17). Importantly, PUFAs are substrates for the synthesis of immunologically relevant lipid mediators (LMs) involved in onset and offset of inflammation (7).
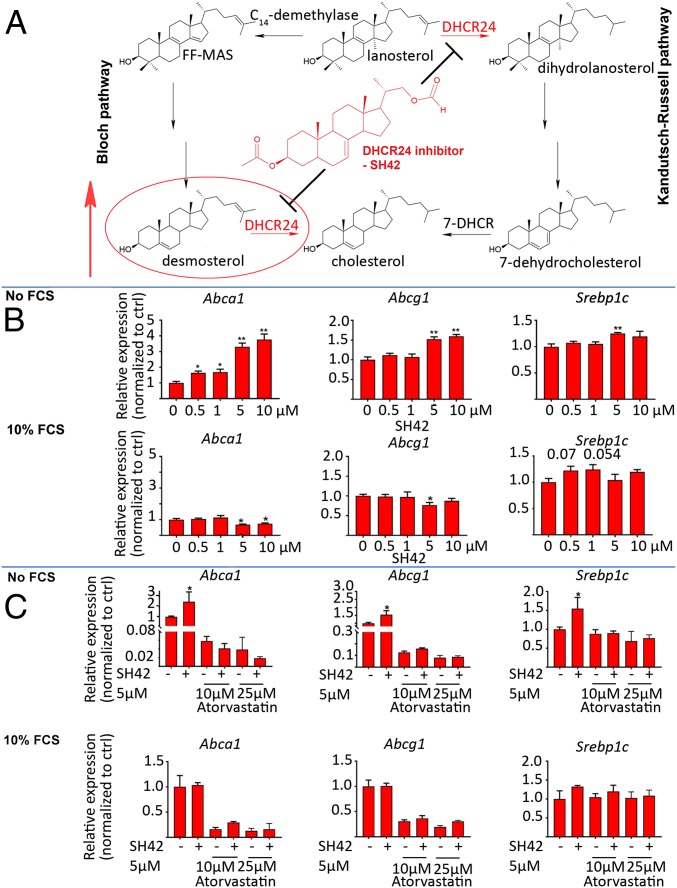
Fig. 1.
Distal cholesterol biosynthesis, structure of chemical probe SH42, and selectivity assessment. (A) Structure and action of SH42; the red circle emphasizes desmosterol, accumulating due to blockage of DHCR24. 7-DHCR; 7-dehydrocholesterol reductase; FF-MAS, follicular fluid meiosis-activating sterol. (B) LXR target gene activation (qPCR) with (inactive cholesterol biosynthesis) and without (active cholesterol biosynthesis) FCS. ctrl, control. (C) LXR target gene regulation under cotreatment with atorvastatin. For all experiments, results are expressed as mean ± SEM (n = 5). *P < 0.05, **P < 0.01; nonparametric Student’s t test (Mann–Whitney) comparing each group separately with the control group (no treatment).
When focusing on LXRs as master regulators of the aforementioned effects, there is no doubt about the possible value of LXR agonists in the context of several diseases (18). Nevertheless, no LXR agonist has yet reached the clinic. The main drawback of unspecific LXR activators can be attributed to SREBP1c activation, resulting in severely increased hepatic lipogenesis (19). This has led researchers to develop specific LXR-β agonists, as well as tissue- and cell-specific drug formulations (18, 20). In contrast, desmosterol has been described as an LXR agonist and selective interaction partner of SREBP in that it can likely activate a subset of SREBP1 target genes, despite inhibiting SREBP1 binding to response elements. In turn, DCHR24 integrates cholesterol biosynthesis, LXR activation, and inflammation. This raises the question of whether the endogenous substrate of DHCR24, desmosterol, might be an attractive candidate, fulfilling important requirements for successful LXR activation: (1) Desmosterol has been shown to bind to LXRs in the low micromolar range (16), (2) it possesses a selective SREBP interaction potential, and (3) it inhibits inflammatory gene expression (8). Recently these processes have been demonstrated in MΦ using desmosterol and desmosterol mimetics (9).
Along these lines, we reasoned that inhibition of DHCR24, leading to controlled desmosterol accumulation, is ideally suited as a master regulator of lipid metabolism, allowing initiation of an antiinflammatory and proresolving phenotype. We therefore investigated the metabolic and immunological consequences, as well as the antiinflammatory/proresolving potential, of controlled desmosterol accumulation through selective inhibition of DHCR24 using a recently published, highly in vivo active chemical probe (SH42) (half-maximal inhibitory concentration = 5 nM) (21). Initially, we evaluated the cross-reactivity of SH42 with LXRs and a set of additional transcription factors; next, we investigated the probes’ effects on the course of zymosan A (zyA)-induced peritoneal inflammation. Subsequently, lipidomic analysis was carried out in order to understand the observed phenotypic changes on a molecular level. We quantitatively assessed more than 900 lipid species in blood, liver, and peritoneal lavage samples. We monitored serum and peritoneal lavage chemokine and gene expression profiles and conducted protein array analysis. Using tandem mass spectrometry (MS) analysis, we investigated PUFA levels and the production of specialized proresolving mediators (SPMs) and tissue-regenerative mediators. Ultimately, we investigated the therapeutic use of SH42 in animal experiments and investigated functional and phenotypic immune cell responses.
Results
The Chemical Probe SH42 Does Not Interact Directly with LXRs.
To exclude direct LXR activation caused by the employed chemical probe SH42 (Fig. 1), we investigated the regulation of well-known LXR target genes under treatment with this compound. Since cholesterol biosynthesis in cell culture is only active when extracellular cholesterol is low (22), we treated RAW264.7 MΦ with high concentrations of SH42 in the presence and absence of fetal calf serum (FCS) as an exogenous source of cholesterol. As can be seen in Fig. 1, the LXR target genes (23) Abca1 and Abcg1 (Srebp1c only at 5 μM) are only activated when no extracellular cholesterol is available. In turn, SH42 blocks cholesterol biosynthesis (21) and causes LXR activation via desmosterol accumulation, but not in a direct fashion; otherwise, target gene regulation would also take place when extracellular cholesterol is available. In order to further strengthen this line of argumentation, we repeated the incubation of RAW264.7 MΦ with and without FCS under cotreatment with the cholesterol-lowering drug atorvastatin, which interferes with proximal cholesterol biosynthesis. By doing so, the accumulation of steroidal precursors, such as desmosterol, was prevented. As can be seen in Fig. 1, atorvastatin did block SH42-regulated target gene activation when no extracellular cholesterol (FCS) was present. Again, from these results, we conclude that SH42 causes LXR target gene regulation only in conjunction with activated cholesterol biosynthesis, hence not directly interacting with LXRs but via a desmosterol-related loop. As an additional line of evidence, we repeated the aforementioned experiments in combination with the LXR inverse agonist/antagonist GSK2033 (24). As can be seen in SI Appendix, Fig. S1, treatment with GSK2033 inhibits the expression of LXR target genes (e.g., Abca1, Abcg1) mediated by SH42, without affecting Srebp1c gene expression. In addition, we monitored possible cross-reactivities with other nuclear receptors, namely, farnesoid X receptor (FXR), pregnane X receptor (PXR), and retinoid X receptor (RXR). We monitored the target gene expression for Shp (25) (FXR), Cyp7a1 (26) (FXR and PXR), Cyp3a11 (27) (PXR), and Cyp27a1 (28, 29) (PXR and RXR). As can be seen in SI Appendix, Figs. S1 and S2, SH42 did not induce target gene expression of any of these transcription factors. As previously reported, SH42 does pose selective actions on DHCR24 within distal cholesterol biosynthesis (21). Taken together, these results provide evidence that the actions of SH42 are mediated by a desmosterol-related loop without a direct interaction with the transcription factors tested here.
Inhibition of DCHR24 Leads to Increased Inflammation Resolution and Selectively Decreases Proinflammatory Cell Influx.
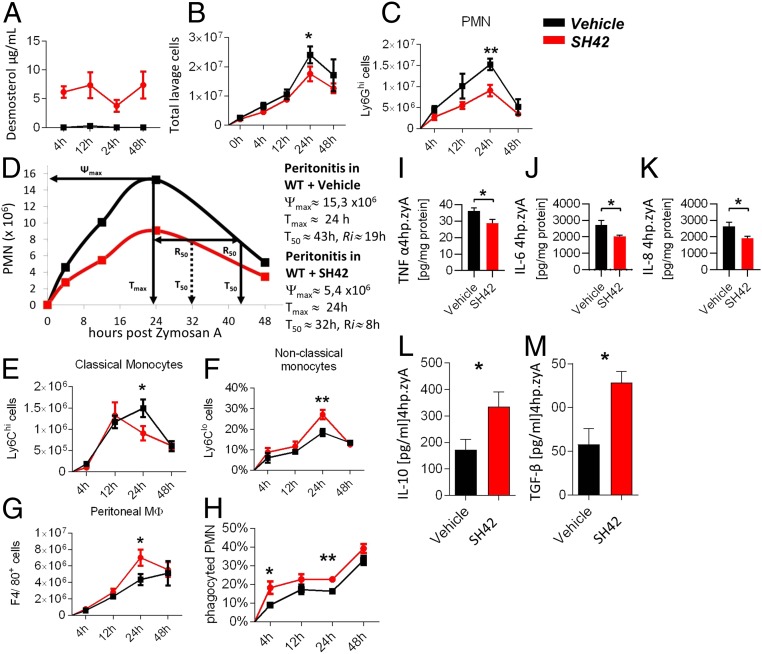
Next, we aimed at verifying that inhibition of DHCR24 indeed has a profound effect on acute inflammation. One of the most frequently used animal models for such investigations is the murine zyA-induced peritonitis model (30). Mice were treated once daily (intraperitoneally [i.p.]) with 0.5 mg of SH42 for 3 d, followed by i.p. injection of zyA. This was an empirically found dose of SH42 at which we did not observe any acute or long-term toxicity (up to 8 wk). Desmosterol levels in whole blood, liver, and peritoneal exudate were monitored using gas chromatography (GC)-MS analysis (22). Blood desmosterol levels proved stable at ∼5 μg/mL (13 μM) (Fig. 2A), while desmosterol was basically absent in the control group. Cellular influx into the peritoneal cavity was monitored using fluorescence-activated cell sorting (FACS) analysis. As can be seen in Fig. 2 B–H, treatment with SH42 did cause a decrease in leukocyte influx (Fig. 2B), with a specific decrease in polymorphonuclear cells (PMNs) (Ly6Ghi) in the early phase of acute inflammation (Fig. 2C). To quantify the kinetics of leukocytes, we determined the resolution index (Ri) (31) displaying a 58% reduction in Ri from 19 h to 8 h in mice treated with SH42. This indicated a strong acceleration of inflammation resolution (Fig. 2D). When focusing on the resolution phase, in which monocytes and MΦ dominate, we found a marked reduction in classical Ly6Chi monocytes (Fig. 2E) and a significant increase in the nonclassical Ly6Clo monocytes (Fig. 2F) and MΦ (Fig. 2G), which were accompanied by a significant enhancement in MΦ clearance of apoptotic PMNs (Fig. 2H). Next, we analyzed pro- and antiinflammatory cytokine levels. As can be seen in Fig. 2 I–K, proinflammatory cytokines were down-regulated, while antiinflammatory/proresolving cytokines (Fig. 2 L and M) were up-regulated. This observation correlated well with the fact that exudate levels of interleukin-10 (IL-10) and transforming growth factor-β (TGF-β), known to be crucial in the resolution of acute inflammation and tissue regeneration, were strongly enhanced (Fig. 2 L and M). Together, these data indicate that SH42 displays an antiinflammatory, proresolving, and proregenerative impact during acute inflammation. While it has been shown before that activation of LXRs in the zyA-induced murine peritonitis model alleviates inflammation (32), it is important to note that the chemical probe SH42 does not possess direct LXR activation capacity as detailed above. In turn, the effects described here on cellular influx and population observed in the murine peritonitis model can likely be attributed to selective inhibition of DHCR24. This is followed by increased levels of desmosterol, which modulates inflammatory outcome by its known actions on LXRs (13, 16).
Fig. 2.
Peritoneal cell analysis. Red lines and circles represent SH42 treatment, and black lines and squares represent vehicle injection. C57BL/6 mice were exposed to zyA-induced peritonitis after 3 d of treatment with SH42, and peritoneal lavages were collected at 4, 12, 24, and 48 h. (A) Blood desmosterol levels after the indicated time points (x axis). (B) Total leukocytes were enumerated by light microscopy. (C) Cell numbers of PMN Ly6Ghi cells. (D) Ri values. Ψmax, maximum PMN numbers; Tmax, the time point of maximum PMN numbers; T50, the time point where PMN are reduced to 50%. (E) Cell numbers of classical Ly6Chi monocytes. (F) Cell numbers of nonclassical Ly6Clow monocytes. (G) Cell numbers of F4/80+ peritoneal MΦ. (H) Cell numbers of monocyte-derived MΦ efferocytosis as analyzed by FACS through intracellular staining of PMNs. (I) TNF-α analysis. (J) IL-6 analysis. (K) IL-8 analysis. (L and M) IL-10 and TGF-β levels all measured in the peritoneal fluids (4 h after zyA). The results represent at least 2 independent experiments and are expressed as mean ± SEM (n = 9 to 11 per group). All experiments were analyzed by means of an unpaired Student’s t test, 1 per time point. *P < 0.05; **P < 0.01. Further details are provided in the main text.
Lipidomic Analysis of Serum, Liver, and Residential Peritoneal Cells.
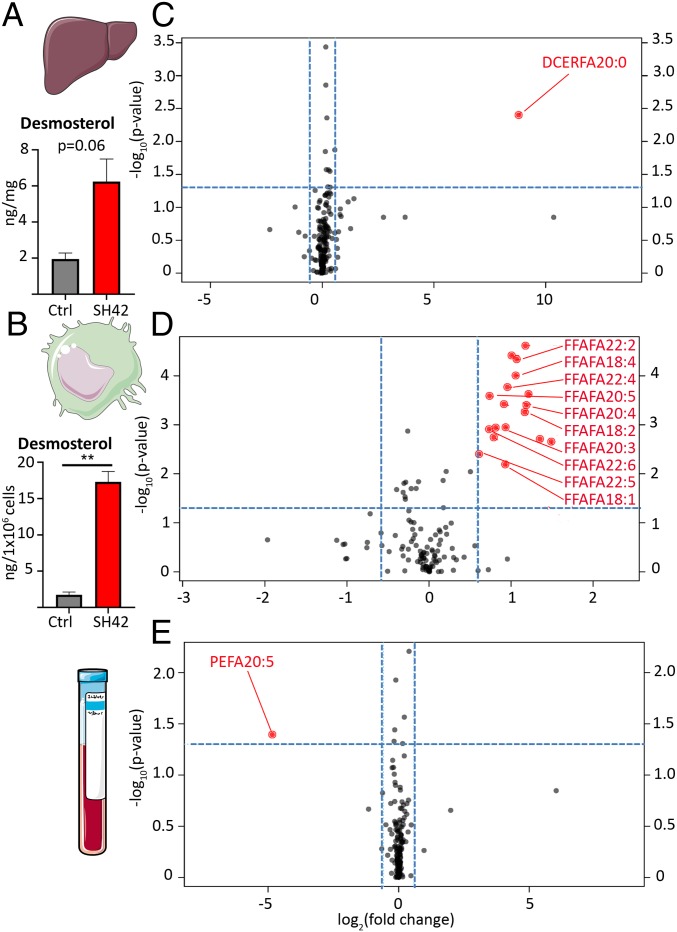
Next, we aimed to obtain a better understanding of the underlying molecular factors possibly explaining our findings. In first instance, we targeted lipid biosynthesis modulation under treatment with SH42. We furthermore aimed to exclude classical side reactions observed for LXR agonists, such as hypertriglyceridemia and increased lipogenesis (19). Initially, we monitored liver desmosterol levels, averaging 6.2 ng/mg in the treatment group and 1.9 ng/mg in the control group (Fig. 3A). Next, we investigated desmosterol levels in the resident peritoneal cell fraction, averaging 17.3 versus 1.8 ng/1 × 106 cells in the control group (Fig. 3B). Using our established GC-MS assay in the scan mode (22), we also screened for other steroidal precursors of cholesterol (33) as well as oxysterols, neither of which could be detected under our experimental conditions. Additionally, we found increased blood and serum desmosterol levels under treatment with SH42, as described above and elsewhere (21). To gain further insights into molecular changes linked to SH42 treatment, we carried out comprehensive quantitative lipidomic analysis of serum and liver samples after a 3-d treatment with SH42 (without zyA) (34). The serum lipid composition showed a significant reduction in the cholesteryl ester (CE) fraction, accompanied by a strong trend toward higher free fatty acid (FFA) levels (SI Appendix, Fig. S3). This finding is in line with SH42 blocking cholesterol biosynthesis, leading to lower CE levels. Importantly, no significant increase in the serum triacylglyceride (TAG) fraction was obtained, which is a known side effect of nonselective LXR agonists (19). When evaluating serum lipid concentrations, we found a significant increase in the FFA concentration with a median of 441 nmol/g in the SH42 group versus 363 nmol/g in the control group (SI Appendix, Fig. S4). Additionally, we investigated the liver lipidome under treatment with SH42. As can be seen in SI Appendix, Fig. S3, a reduced diacylglyceride (DAG) and TAG fraction was observed. This observation was also true for the absolute concentrations of DAG and TAG lipids found per gram of liver tissue, being 956 and 3,718 nmol/g (DAG and TAG) for SH42-treated mice and 1,351 and 6,205 nmol/g for control mice, respectively (complete lipidomic data are provided in Datasets S1–S4). To gain more mechanistic insights and shed light on the effects of SH42 on the course of zyA-induced peritonitis, we carried out fatty acid (FA) compositional analysis of serum, liver, and residential peritoneal cells. As LXRs have been described to control FA elongation and desaturation (15), we were particularly interested in the FA composition of these lipidomes as we expected to find specific metabolic adaptations under treatment with SH42. As can be seen in Fig. 3 C–E, we did indeed observe significant changes in the FA composition, mainly in residential peritoneal cells. Our main finding was an increased content of PUFA in the FFA lipid fraction, including important precursor FAs for the downstream biosynthesis of SPMs such as, for example, eicosapentaenoic acid (FA20:5) and docosahexaenoic acid (FA22:6). Further details are shown in Fig. 3.
Fig. 3.
Analysis of the fatty acid composition represented in the different lipidomes and desmosterol after a 3-d treatment with SH42. (A) Liver desmosterol levels (n = 3 to 5). Ctrl, control. (B) Desmosterol amounts found in the residential peritoneal cell fraction (n = 4 to 5). (C–E) Volcano plot analysis of the FA composition of the different lipidomes; each black dot represents a lipid species linked to a specific FA. Lipids are marked in red (red dot). When a fold change >1.5 and P value <0.05 were observed, vehicle groups were compared with SH42 treatment. The FFA-free FA lipid class is denoted as FA, followed by the carbon number and double-bond count (e.g., FFAFA204 is free arachidonic acid with 20 carbons and 4 double bonds). Liver lipidomic (C, Upper), residential peritoneal cell lipidomic (D, Middle) and serum lipidomic (E) analyses are shown. The blue dotted lines show the fold change and P value thresholds. The control group was considered as the reference in all comparisons. Results are expressed as mean ± SEM. In A and B, **P < 0.01; nonparametric Student’s t test (Mann–Whitney).
Gene Expression Analysis in Liver and Peritoneal Cells.
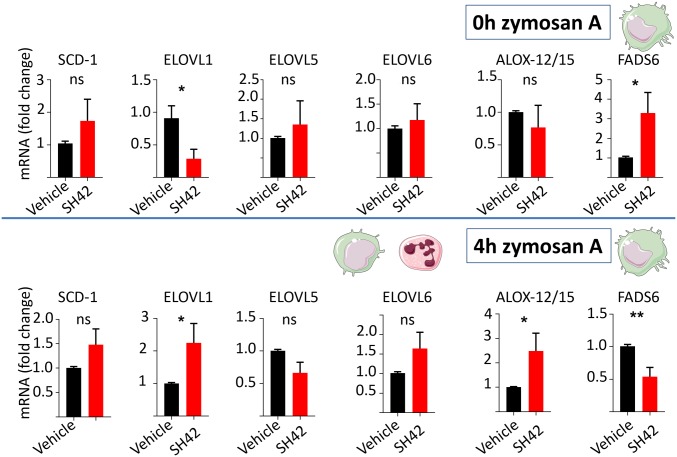
To substantiate our findings, we next performed gene regulation analysis (qPCR) in liver and peritoneal exudate cells. It has recently been reported that desmosterol and desmosterol mimetics have a cell type-specific effect on LXR target gene regulation. Muse et al. (9) showed that desmosterol selectively activates the LXR target gene ABCA1 (Abca1) in MΦ but not in hepatocytes. Along these lines, we tested the effects of our chemical probe SH42 in vivo in peritoneal lavage cells and liver homogenates. As indicated in SI Appendix, Fig. S5, the chemical inhibition of DHCR24 decreased expression of Abca1 alongside Fasn without affecting expression of Scd in liver homogenates. Gene expression analysis in peritoneal cells focused on Scd-1, FA desaturase 6 (Fads6), Elovl1, Elovl5, Elovl6, and Alox-12/15. We chose Fads6 because it is one of the rate-limiting steps in endogenous PUFA biosynthesis (35). Further, we monitored Elovl5 as it is particularly involved in PUFA elongation and Elovl6, which is an important gene (enzyme) for the elongation of medium- to long-chain FA (36). Alox-12/15 was monitored as an important gene related to PUFA metabolism and, in particular, the production of SPMs. As can be seen in Fig. 4, when comparing 3-d SH42-treated mice with vehicle control, we observed a trend for increased expression of Scd-1 (P = 0.19) as well a significant increase for Fads6 expression at time point 0. This finding, together with our lipidomic data (Fig. 3), pinpointed to an involvement of Fads6 in increased endogenous PUFA production predominantly in residential peritoneal cells. Our findings are vastly in line with a previous report on the activation of PUFA biosynthesis mediated by the nonselective LXR agonist T0901317 (15). Next, we aimed at comparing the changes found in residential peritoneal cells with infiltrating cells during zyA-induced peritonitis. We carried out qPCR analysis of the same genes 4 h after injecting zyA into the peritoneum. As depicted in Fig. 4, Fads6 was now down-regulated in the treatment group, while Elovl-1 and, in particular, Alox-12/15 became up-regulated at the 4-h time point. Taken together, our data, in combination with an earlier report on the activation of PUFA biosynthesis by T0901317 (15), point to increased PUFA biosynthesis mediated by SH42 treatment. Mainly, the finding that Alox-12/15 became up-regulated in the treatment group 4 h after zyA administration made us interested in a possible link between our initial observations in the zyA-induced self-limiting murine peritonitis model (Fig. 2) and increased endogenous PUFA production, as well as the possibly altered downstream production of important PUFA metabolites such as eicosanoids and docosanoids.
Fig. 4.
mRNA expression under treatment with SH42. mRNA expression was determined in peritoneal lavage cells from 3-d treated mice versus vehicle at time point 0 h of zyA-induced peritonitis and at time point 4 h of zyA-induced peritonitis. Results are expressed as mean ± SEM (n = 10 to 15). *P < 0.05, **P < 0.01; nonparametric Student’s t test (Mann–Whitney).
Inhibition of DHCR24 Results in Increased Biosynthesis of Specialized Proresolving Mediators.
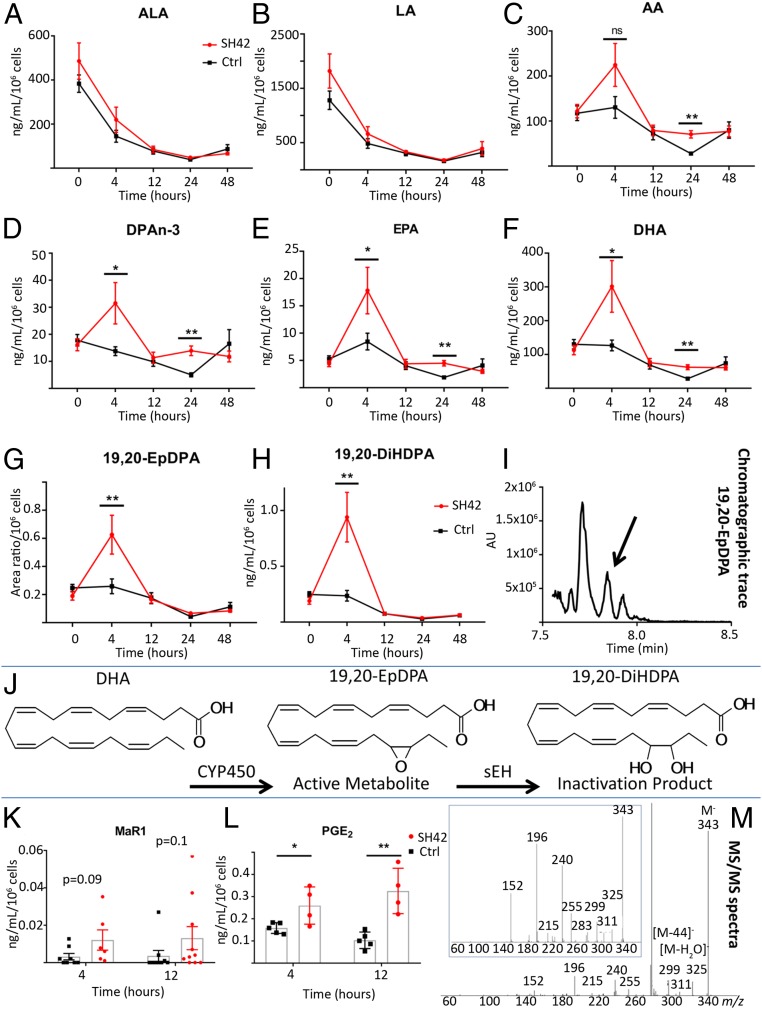
Based on findings that PUFAs are well-known precursors for the biosynthesis of SPMs and other antiinflammatory lipids (7) and on our own lipidomic and qPCR data obtained from residential peritoneal cells, we questioned whether inhibition of DHCR24 and the effects described here are linked by an increased production of proresolving and antiinflammatory lipids. To investigate this hypothesis, we used a standardized liquid chromatography (LC)-tandem MS (MS/MS) approach (37) and profiled peritoneal LM concentrations. As can be seen in Fig. 5, we observed higher PUFA levels particularly at the early 4-h time point, while other FAs such as linoleic acid and α-linolenic acid remained basically unchanged between the treatment and control groups. In view of several recent reports about the immunological function of cytochrome P450-derived epoxy metabolites, we monitored not only SPMs (resolvins) but also 19,20-epoxy docosapentaenoic acid (19,20-EpDPA) and its inactivation product 19,20-dihydroxydocosapentaenoic acid (19,20-DiHDPA) (38, 39). As the mediators identified here are known to actively control inflammation resolution and cellular influx (39), we postulated that their increased biosynthesis under treatment with SH42 might pose a link between LXR activation, increased production of antiinflammatory and proresolving LMs, and alleviated inflammatory processes. In this context, we also monitored prostaglandin E2 (PGE2) and leukotriene B4 (LTB4) as established mediators of inflammation (resolution) (40). As can be seen in Fig. 5L and SI Appendix, Fig. S6, PGE2 was significantly up-regulated in the treatment group, while LTB4 showed a strong trend (P = 0.06) toward higher concentrations. For the identification of maresin 1 (MaR1), please refer to the MS/MS analysis and comparison with genuine synthetic standard as provided in SI Appendix, Fig. S7. The relative retention times, relative to the internal standard d4-LTB4, for MaR1 observed in our samples were 0.997 and 0.998 for an authentic standard. In order to substantiate our findings, we carried out a validation experiment at the 4- and 12-h time points for docosahexaenoic acid and its bioactive mediators MaR1 and 19,20-EpDPA. These validation experiments confirmed our findings, as can be seen in SI Appendix, Fig. S8.
Fig. 5.
Changes of free FA and the downstream metabolites 19,20-EpDPA and 19,20-DiHDPA in peritoneal lavages in the zyA-induced peritonitis model, with and without SH42 treatment. For all experiments, n = 6 to 10; data were obtained from 2 independent experiments with an individual n = 3 to 5 (single experiment for PGE2 with n = 4 to 5). *P < 0.05, **P < 0.01; nonparametric Student’s t test. (A–H) Longitudinal behavior of various FAs during zyA-induced peritonitis with and without SH42 treatment (nonparametric Student’s t test [Mann–Whitney]). Ctrl, control. (I) Chromatographic trace showing the transition m/z 343→281 was used to detect 19,20-EpDPA. AU, arbitrary units. (J) Enzymatic formation of 19,20-EpDPA and 19,20-DiHDPA catalyzed by CYP450 and soluble epoxide hydrolase. (K and L) Levels of MaR1 and PGE2 observed at the 4-h and 12-h time points. Results are expressed as mean ± SEM. *P < 0.05, **P < 0.01; nonparametric Students t test (Mann–Whitney). (M) MS/MS spectra identifying 19,20-EpDPA compared with an authentic standard (1 ng/mL, blue boxed inlay).
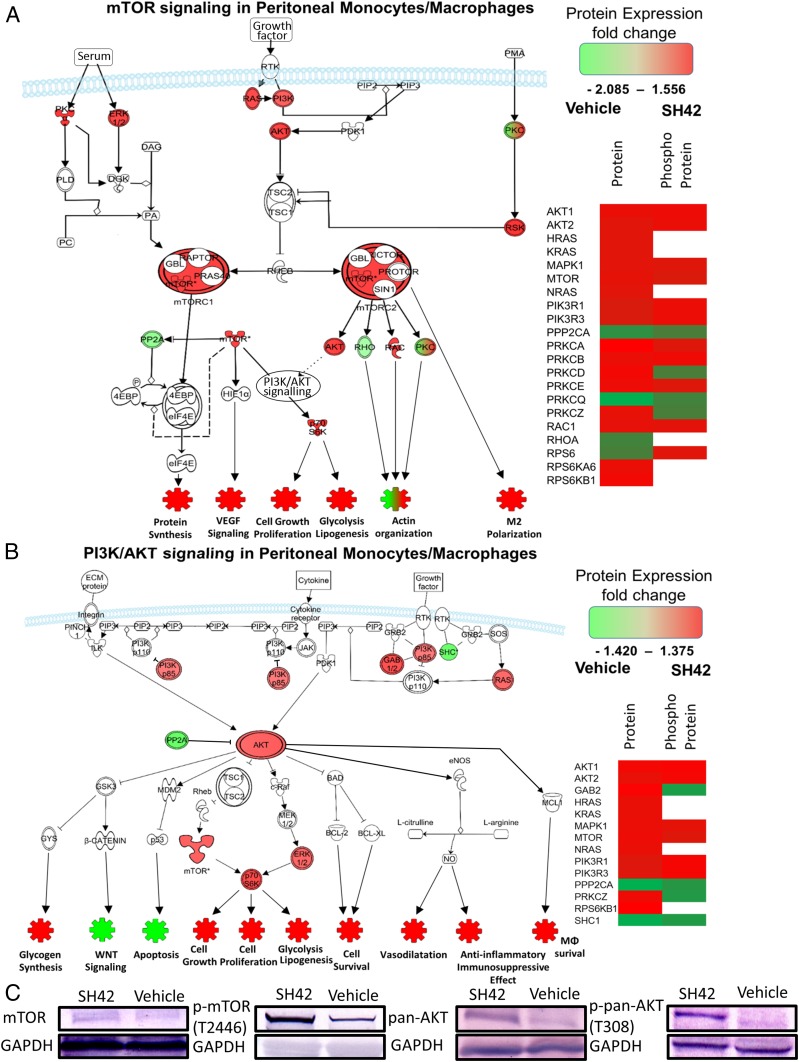
Protein Array Analysis of the Phosphatidylinositol 3-Kinase/Protein Kinase B and Mammalian Target of Rapamycin Pathways.
In order to expand on these findings and shed more light on the effects of accumulating desmosterol (not cholesterol) under SH42 treatment on the proteomic level, we next carried out protein array analysis of residential peritoneal monocytes/MΦ comparing SH42 treatment and vehicle. We focused on the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) and mammalian target of rapamycin (mTOR) pathways, as they are crucial in restricting proinflammatory responses, thereby promoting antiinflammatory/proresolving responses and activating monocyte/MΦ differentiation and polarization toward a proresolving phenotype (41). To understand if and how SH42 affects these pathways, we carried out protein array analysis of peritoneal monocytes after a 3-d treatment with SH42 (Fig. 6). In the treatment group, marked changes in the expression and phosphorylation of key proteins in both the AKT/PI3K and mTOR pathways were observed. As can be seen in Fig. 6 A and B, most signaling pathways downstream of AKT did point to antiinflammatory effects and to changes especially in MΦ survival, cell proliferation, cell growth, and gluconeogenesis. In order to substantiate our protein array data, we also carried out Western blot analysis of mTOR and AKT as well as their phosphorylated variants (Fig. 6C). Besides these aspects, SH42 regulated the activation of the mTOR pathway and particularly RICTOR signaling, which plays an important role in regulating MΦ metabolism to promote M2 polarization (42). Notably, the latter observation is in line with our results observed by lipidomic analysis. This finding was reflected in increased levels of Alox-12/15 and, finally, enhanced generation of SPMs, such as MaR1, suggesting that SH42 induces polarization toward the M2 prohealing and proresolving phenotype. The results obtained for the entire protein array are presented in Dataset S5.
Fig. 6.
Protein array analysis of the mTOR (A) and PI3K/AKT (B) pathways. (Left) Main proteins involved in the mTOR and PI3K/AKT pathways are shown alongside their known physiological functions. Increased protein levels for the SH42 group are shown in red, and decreased proteins are shown in green. (Right) Corresponding protein fold-changes, including phosphoprotein. (C) Western blot analysis.
Therapeutic Treatment with SH42.
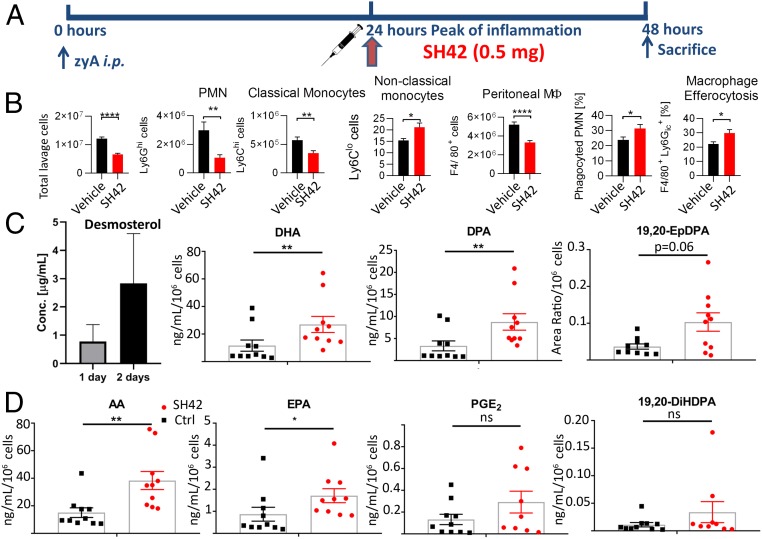
Next, we questioned whether SH42 would also exert acute treatment effects in addition to prophylactic treatment effects. For this, we chose the 24-h time point resembling the maximum cellular influx after zyA injection (Fig. 2). Using in vivo mouse experiments, we initially monitored blood desmosterol levels after a single (1 d) or repeated (2 d) injections of SH42 in order to investigate whether desmosterol accumulates after a single dose only. This experiment was crucial to correctly interpret the results of the treatment study. If no desmosterol would have accumulated after a single SH42 injection, no causal link could be made between desmosterol and the observed biological phenotype. If so, treatment effects would likely have to be attributed to SH42 itself. As can be seen in Fig. 7, we did indeed observe increased blood desmosterol levels after a single dose of SH42, which further increased after the second dose (without treatment, desmosterol is undetectable in blood samples as outlined in Fig. 2A). Subsequently, we monitored changes in LM biosynthesis and cellular influx at the 48-h time point. As can be seen in Fig. 7 C and D, significantly increased concentrations of several PUFAs and LMs were observed. Additionally, cell numbers and populations were significantly altered in the treatment group (Fig. 7B), vastly resembling the results of the prophylactic treatment. Taken together, this points to the fact that SH42 can be used in an antiinflammatory therapeutic setting.
Fig. 7.
Therapeutic application of SH42. (A) Experimental setup of SH42 administration: 24 h after zyA-induced inflammation, 0.5 mg of SH42 was administered i.v. via the tail vein. Twenty-four hours later at the 48-h time point, mice were killed and peritoneal cells were analyzed by FACS analysis. (B, Left to Right) FACS results: total number of peritoneal lavage cells, PMNs, classical monocytes, nonclassical monocytes, peritoneal MΦ, percentage of phagocyted PMNs, and MΦ efferocytosis. (C and D) Levels of blood desmosterol after 1-d and 2-d treatments, followed by PUFA and LM levels in the peritoneal exudates at the 48-h time point. Results are expressed as mean ± SEM (n = 4 to 5 for desmosterol analyses and n = 9 to 10 for FACS and LM analysis, combined from 2 individual experiments). *P < 0.05, **P < 0.01, ****P < 0.001; nonparametric Student’s t test (Mann–Whitney) for LM and desmosterol analysis and unpaired Student’s t test for cellular analysis. ns, not significant.
Proresolving Effects and the Role of LXRs and 12/15-Lipoxygenase.
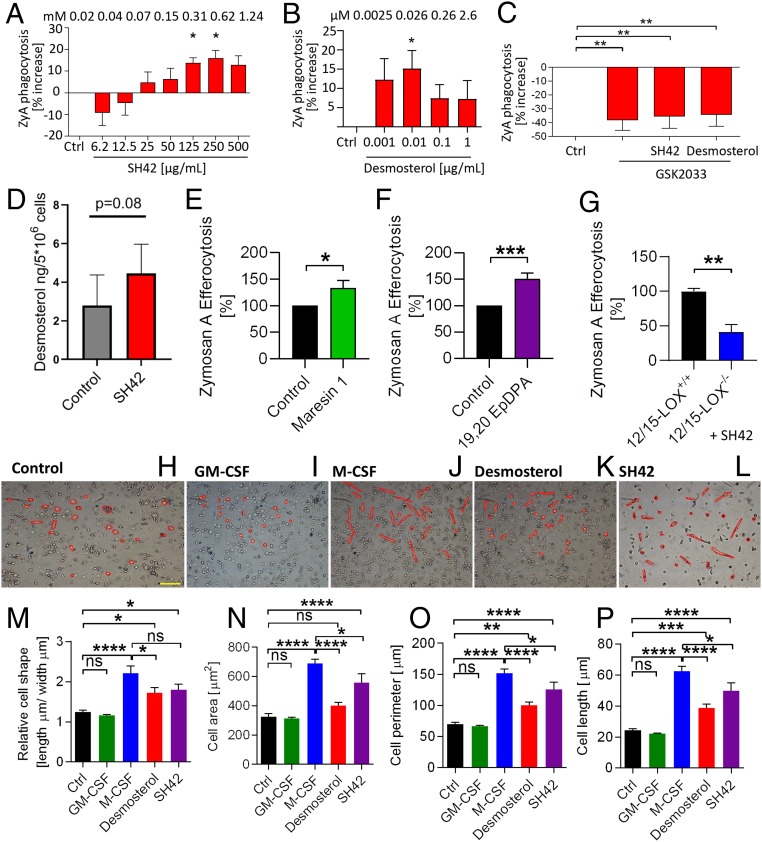
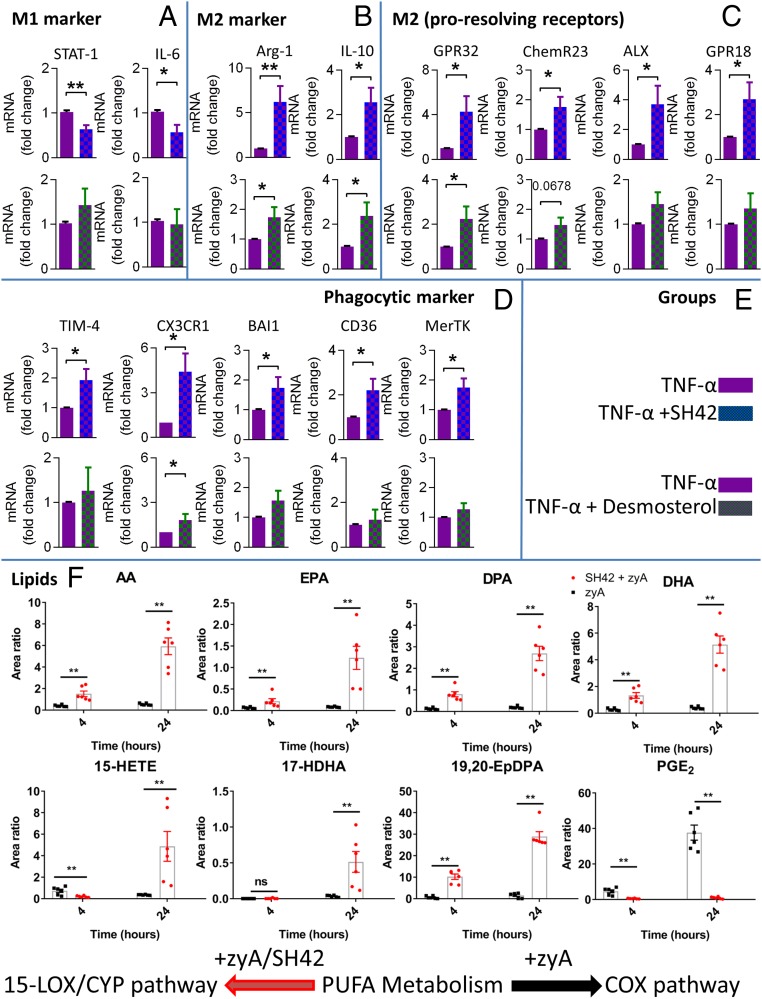
Ultimately, we aimed at sketching a more functional link between increased desmosterol levels, the increased production of antiinflammatory lipids, and inflammation resolution, as well giving some insight into effects observed in human-derived MΦ. To provide these relations, we focused our efforts on MΦ phenotype, polarization, phagocytosis, and the roles of LXRs and 12/15-lipoxygenase (12/15-LOX) (43), as well as activation of the cytochrome P450 (CYP), 15-LOX, and cyclooxygenase (COX) pathways in human MΦ. Phagocytosis (efferocytosis) is a crucial process during inflammation resolution. We initially investigated the effects of SH42 and desmosterol on the phagocytic capacity of human monocyte-derived MΦ. As can be seen in Fig. 8, SH42 was capable of inducing increased phagocytosis starting from ∼125 μg/mL, while desmosterol showed a positive effect already at doses of no more than 0.01 μg/mL This points to the fact that desmosterol is the actual mediator of the observed effects (Fig. 8 A and B). When coincubating with the LXR antagonist GSK2033, phagocytosis was blocked, and this effect could not be rescued by either SH42 or desmosterol (Fig. 8C). In turn, this points to a possible role of LXRs during this process. To provide additional proof that desmosterol is the actual mediator of the positive effects of SH42, we quantified desmosterol after a 2-h incubation of human monocyte-derived MΦ with high concentrations of SH42. As can be seen in Fig. 8D, we could indeed observe a strong trend toward increased desmosterol concentrations. The other suspected mediators for the described effects of SH42 are MaR1 and 19,20-EpDPA; hence, we subsequently investigated the effects of these lipids on MΦ phagocytosis (Fig. 8 E and F). This showed that both lipids positively influenced phagocytosis at low concentrations (10 ng/mL). While 19,20-EpDPA is a CYP product, MaR1 is biosynthesized by the actions of 12/15-LOX. To this end, we investigated the effects of SH42 on MΦ isolated from either wild-type or 12/15-knockout (KO) mice, as we had no KO model related to the production of 19,20-EpDPA available. As can be seen in Fig. 8G, treatment of 12/15-LOX KO MΦ did result in diminished phagocytosis. As a next step in integrating our results with inflammation resolution, we focused on monocyte differentiation and MΦ phenotypes as both processes are crucial for inflammation resolution. As cell shape and MΦ phenotype are correlated (44), we carried out cell shape analysis assessing monocyte differentiation toward the M1 or M2 phenotype (45). As can be seen in Fig. 8 H–P, a 7-d treatment of human-derived monocytes (peripheral blood mononuclear cells [PBMCs]) did give a phenotype closely resembling the macrophage colony-stimulating factor (M-CSF) M2 phenotype. As expected, the effects of desmosterol were significantly lower than observed for SH42, as desmosterol will be further converted into cholesterol if no DCHR24 inhibitor is present (Fig. 8 K–P). In order to gain more proof that desmosterol and SH42 influence MΦ polarization, we assessed messenger RNA (mRNA) expression markers for both the M1 and M2 phenotypes. As can be seen in Fig. 9 A and B, the M2 markers Arg-1 and IL-10 were especially increased after treatment with both SH42 and desmosterol. Next, as several specific surface receptors are known to play an important role in mediating inflammation resolution (46), we quantified the corresponding mRNA expression and found a significant up-regulation of several important receptors (mRNA) (Fig. 9C). In order to further substantiate our functional phagocytosis data, we also monitored the expression of well-described phagocytic markers (47). As we had observed a number of MΦ-related functional changes associated with SH42 and desmosterol treatment, we wondered if we could metabolically integrate these findings with the 15-LOX, CYP, and COX pathways. Indeed, when MΦ were treated with zyA with and without SH42, dramatic metabolic changes could be observed. While zyA-treated MΦ relied mainly on COX-driven PUFA metabolism producing high amounts of PGE2, cotreatment with SH42 not only increased the intracellular amount of free PUFA but also caused a marked shift toward the CYP and 15-LOX pathways. The latter shows that treatment with SH42 stimulates a metabolic shift toward an antiinflammatory phenotype as shown by increased levels of the 15-LOX pathway markers 15-HETE (15-hydroxyeicosatetraenoic acid) and 17-HDHA (17-hydroxydocosahexaenoic acid), as well as the CYP-derived LM 19,20-EpDPA (Fig. 9F). For validation, we repeated the experiment with the LOX inhibitor baicalein and could block the actions of SH42 (SI Appendix, Fig. S10).
Fig. 8.
Functional studies of SH42, 19,20-EpDPA, and MaR1. (A) Phagocytosis under treatment with SH42. Ctrl, control. (B) Phagocytosis with desmosterol treatment. (C) Phagocytosis under cotreatment with GSK2033. Results in A–C represent at least 2 independent experiments and are expressed as mean ± SEM (n = 9 to 11 per group); 1-way ANOVA with Dunnett’s multiple comparison test. (D) Desmosterol analysis in SH42-treated MΦ (2 h); nonparametric Student’s t test. (E and F) Phagocytosis with MaR1 (E) and phagocytosis with 19,20-EpDPA (F) treatment. (G) Phagocytosis comparing wild-type and 12/15-LOX KO MΦ under treatment (both) with SH42. (E–G) Unpaired 2-tailed Student’s t test. Cell shape analysis of human PBMCs stimulated for 7 d with vehicle (H), GM-CSF (I), M-CSF (J), desmosterol (K), or SH42 (L) is shown. (Scale bar: 100 μm.) (M–P) Statistical analysis of cell shape, cell area, cell perimeter, and cell length (n = 3 to 11 independent experiments expressed as mean ± SEM). ns, not significant. (A–F and M–P) *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; 1-way ANOVA with Bonferroni correction. Detailed information about the experimental procedures used for cell shape analysis in H–L can be found in SI Appendix, S9.
Fig. 9.
Analysis of MΦ phenotype and PUFA, 15-LOX, CYP, and COX pathway markers. M1 MΦ were stimulated with TNF-α and, in combination with SH42 or desmosterol gene expression, were analyzed for M1 markers (A), M2 markers (B), proresolving receptors (C), and phagocytic markers (D). (E) Groups are described; all mRNA levels were quantified by RT-PCR (n = 9 to 11). The results are representative of 3 to 11 independent experiments and are expressed as the mean ± SEM. *P < 0.05, **P < 0.01; unpaired 2-tailed Student’s t test. (F) LC-MS/MS analysis of PUFA, 15-LOX, CYP, and COX pathway markers (n = 6 for all experiments, with 3 biological replicates and 2 technical repeats; mean ± SEM). **P < 0.01, nonparametric Student’s t test (Mann–Whitney). ns, not significant.
Discussion
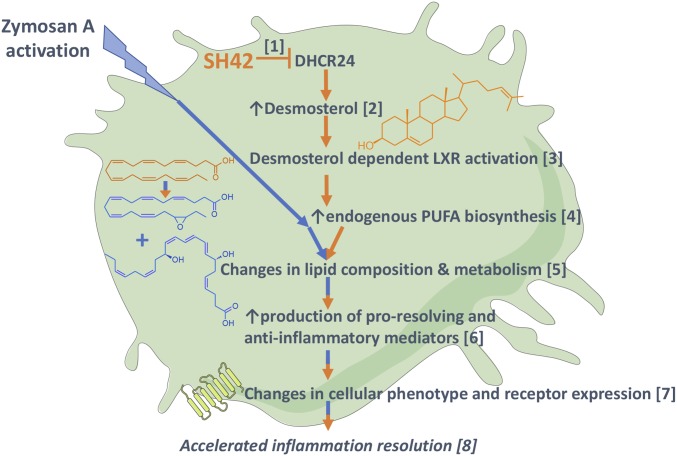
A summary of the observed effects of SH42 and the mechanism of its proresolving effect described here can be found in Fig. 10.
Fig. 10.
Overview and summary of the observed actions of SH42 in a schematic cell. The effects of SH42 are shown in orange, zyA activation is shown in blue, and combined effects are shown in orange/blue. SH42 selectively blocks DHCR24 [1]. Inhibition of DHCR24 leads to increased levels of desmosterol [2]. Desmosterol activates LXRs [3]. LXR activation leads to increased endogenous PUFA biosynthesis [4]. LXR activation leads to changes in lipid composition and metabolism [5]. The altered lipidome results in increased production of antiinflammatory and proresolving LMs after zyA stimulation [6]. LMs are known effectors of immune cell phenotype and function; we also observed significant changes in their corresponding receptor expression [7]. Taken together, these actions result in accelerated inflammation resolution and modulation [8].
The fact that DHCR24 has been proposed as a potential drug target for several diseases made us interested in the metabolic and immunological role of DHCR24 during inflammation. Using a recently developed highly selective and potent DHCR24 inhibitor (SH42) as a chemical probe (21), we aimed at investigating the consequences of controlled DHCR24 inhibition. In order to separate direct and indirect effects on DHCR24/LXRs, it was mandatory to establish the probes’ selectivity, allowing for streamlined data interpretation without observing possibly mixed activities. Moreover, we wanted to exclude known side effects from nonselective LXR activation (19), aiming to exploit the selective functions of desmosterol. We particularly aimed at boosting endogenous PUFA biosynthesis and identified desmosterol as a suitable endogenous metabolite mediating such effects. In order to test this hypothesis, we initially investigated the probe’s influence on the course of zyA-induced peritonitis (30). SH42 did have a marked influence on relevant numbers and types of inflammation-related immune cells. Particularly antiinflammatory/tissue-regenerative cell types were found up-regulated upon treatment with SH42. In line with these findings, increased levels of IL-10 and TGF-β and increased efferocytotic capacity were found. In order to shed some light on the underlying molecular mechanisms, we carried out quantitative lipidomic analysis.
Monitoring almost 1,000 individual lipid species, we observed increased amounts of longer chain FA as well as increased PUFA content. This change in lipid metabolism, which was mainly observed in residential peritoneal cells, proved that our hypothesis of boosting PUFA biosynthesis through the controlled accumulation of desmosterol is possible. As SPMs are well-known and characterized downstream products of PUFA (7), we rationalized that altered lipid metabolism, leading to increased endogenous PUFA production, might ultimately lead to changed LM profiles during onset and offset of inflammation. Indeed, we found a significant increase in the gene expression of the desaturase Fads6 in residential peritoneal cells (Fig. 4) from mice treated with SH42. After inducing inflammation, a significant difference between vehicle and SH42 treatment in the expression of the key proresolving enzyme Alox-12/15 was observed. This result clearly pointed to a molecular modulation of the course of murine peritoneal inflammation. The increased expression of Alox-12/15 found in the peritoneal cavity might reflect an altered reaction to zyA stimulation, mediated through Toll-like receptor (TLR) stimulation. Ito et al. (48) showed that LXRs control a plethora of genes involved in lipid metabolism; this function correlates with their ability to modulate membrane lipid organization and thereby influence TLR signaling (18, 23). A direct link between LXRs and Alox-12/15 has been established, particularly in human M2 MΦ (49). However, to our knowledge, no report has yet pointed out an LXR-dependent increase in the expression of peritoneal Alox-12/15. Nevertheless, it seems conceivable that changes in lipid metabolism, caused by desmosterol-driven LXR activation, might ultimately translate into a differential response to zyA stimulation. This might explain altered gene expression profiles at the early 4-h time point.
Having observed these marked changes in lipid metabolism and gene expression of related enzymes, we next investigated PUFA metabolism. Using a standardized LC-MS/MS platform, we analyzed peritoneal LM profiles under treatment with SH42 during murine peritonitis. As can be seen in Fig. 5, we observed increased free PUFA levels in mice under treatment with SH42 at the 4-h time point, and this finding correlated well with our lipidomic results. It is likely that the increased levels of PUFA stem from the altered lipid metabolism/composition of the residential MΦ, as described by lipidomic and gene expression analysis. Additionally, increased levels of the well-established eicosanoids LTB4 and PGE2, as well as the proresolving docosanoid MaR1 and the epoxy metabolite 19,20-EpDPA and its inactive metabolite 19,20-DiHDPA, were observed. LTB4 levels were enhanced at the 4-h time point in the treatment group, with a rapid decrease toward the 12-h time point. The levels of PGE2 remained elevated at 12 h in the treatment group. Although this finding might initially seem counterintuitive, it is important to realize that PGE2 has been described as an important mediator of LM class switching in the early phase of inflammatory processes (40). Moreover, several recent reports have underlined possible antiinflammatory effects exerted by PGE2 (50). In turn, the increased levels of PGE2 found at 4 and 12 h are likely contributing to the observed effects of SH42. Additionally, the increased production of MaR1 was in line with the altered gene expression of Alox-12/15, a key enzyme necessary for the biosynthesis of antiinflammatory/proresolving mediators. With respect to the involved receptors, to our knowledge, no definite receptor has yet been identified for 19,20-EpDPA (51). However, MaR1 has been shown to be a partial agonist of recombinant human BLT1 receptor, likely antagonizing the actions of the proinflammatory mediator LTB4 (52). The dissociation constant (Kd) values for several proresolving LMs are described to be in the pico- to nanomolar range (53), a concentration range that can possibly be reached in our model. Along this line, we argued that Alox-12/15–derived LMs, as well as the increased concentrations of epoxy metabolites (i.e., 19,20-EpDPA), are the final downstream products possibly explaining the actions of SH42 (38).
In order to gain more insight on the proteomic level, we carried out protein array analysis of the AKT/PI3K and mTOR pathways (Fig. 6) in residential peritoneal cells. Also, on the proteomic level, we found a significant skewing toward a more antiinflammatory phenotype. Interestingly, these results point to an intertwined change of lipidome and proteome, jointly changing the metabolism and actions of peritoneal monocytes, thereby mediating the actions of SH42. It seems plausible that these observations are mediated by changes in lipid metabolism and possibly lipid rafts, altering cellular signaling. Integration of LXR and AKT signaling mediated by changes in lipid rafts has been described in cancer cells (54). However, to our knowledge, the control of AKT/PI3K and mTOR in a DHCR24/LXR-dependent fashion has not yet been shown.
Next, we tested if SH42 would also prove beneficial in a therapeutic setting and carried out additional mouse experiments in order to gain more mechanistic and functional insights into the relation between desmosterol, LXRs, and the observed antiinflammatory effects. As can be seen in Fig. 7, SH42 did also give rise to antiinflammatory effects when administered at the peak of inflammation (24-h time point). It was important to see that after a single dose of SH42, we could already observe the accumulation of desmosterol, as, otherwise, possible antiinflammatory effects would have had to be attributed to SH42 itself. Nevertheless, it is important to stress that we cannot entirely exclude other biological effects related to SH42, beyond inhibiting DHCR24. Finally, we investigated the mechanistic relationship between SH42, desmosterol, MaR1, 19,20-EpDPA, LXRs, and ALOX-12/15. For this, we chose monocyte differentiation and polarization, as well as MΦ phagocytosis, as functional readouts, 2 important hallmarks of inflammation resolution. As can be seen in Fig. 8, MaR1, 19,20-EpDPA, SH42, and desmosterol were capable of inducing the proresolving M2 MΦ type, as well as enhancing phagocytosis. Much higher doses of SH42 were needed when compared with desmosterol, MaR1, and 19,20-EpDPA. This speaks for the fact that desmosterol, MaR1, and 19,20-EpDPA are the actual mediators of the effects observed under SH42 treatment. We substantiated our findings by gene expression analysis, showing a shift toward the prohealing MΦ phenotype (Fig. 9). Additionally, our functional findings were accompanied by an increased expression of several important proresolving receptors (GPR32, ChemR23, ALX, and GPR18), strengthening our line of argumentation. These receptors have just recently been shown to be involved in inflammation resolution in a human skin blister model (55). Ultimately, we monitored important CYP, 15-LOX, and COX pathway markers under treatment of human MΦ with zyA, with and without SH42. We found a dramatic shift toward the 15-LOX and CYP pathways under cotreatment with SH42 (Fig. 9). MΦ only stimulated with zyA almost solely relied on COX metabolism, producing large amounts of PGE2. The aforementioned findings might lead to novel treatment opportunities, as polymorphisms in the 15-LOX pathway have been linked to the development of asthma, arthritis, and atherosclerosis in humans. Products of this pathway (MaR1 and LXA4) have been described in a human skin blister model (55, 56).
In summary (Fig. 10), we describe here the antiinflammatory effects of SH42 as a highly potent and selective inhibitor of DHCR24. SH42 leads to accumulation of desmosterol, subsequently activating LXRs and affecting PUFA metabolism mainly in residential monocytes. These changes are downstream-translated into the increased production of proresolving mediators, likely mediating the observed effects. In turn, targeting lipid metabolism at a presumably distinct point (e.g., cholesterol biosynthesis) can have profound effects on the general lipid composition, with fundamental consequences for cellular phenotypes and inflammatory outcome. Endogenous n3-PUFA production has long been considered negligible when compared with dietary sources. It has been established that humans almost entirely depend on the dietary uptake of n3-PUFA, and the possibility of boosting endogenous n3-PUFA biosynthesis to target crucial physiological processes such as inflammation resolution is vastly unexplored. However, our data presented here strongly indicate that interfering with lipid metabolism at an appropriate junction can trigger endogenous PUFA biosynthesis and the downstream production of proresolving lipids, which, in turn, translates into important functional and phenotypic changes. We are confident that our study can form the fundament for a better understanding of the interplay between cholesterol biosynthesis, LXRs, and the production of proresolving lipids, possibly posing a novel means for targeting inflammatory diseases.
Materials and Methods
The chemical probe SH42 was prepared as described elsewhere (21). All animal experiments were approved by the Local Ethical Commission of the Leiden University Medical Center or the Regierungspräsidium Tübingen. All chemicals were from Sigma–Aldrich and were of pro analysi or LC-MS grade, if not stated otherwise.
Human MΦ Differentiation, Polarization, and Lipid Analysis.
Human PBMCs were isolated from human leukapheresis collars from the Blood Bank of Eberhard-Karls University of Tübingen. For differentiation experiments, cells were cultured in RPMI medium 1640 alone, with 10 ng/mL human recombinant granulocyte/macrophage (GM)-CSF (Macs Milteny), 100 ng/mL M-CSF (Macs Milteny), 0.01 μg/mL desmosterol, or 250 μg/mL SH42 at 37 °C for 7 d. For polarization experiments, M1 MΦ (monocytes cultured with GM-CSF for 7 d) were stimulated with tumor necrosis factor-α (TNF-α; 100 ng/mL; Promokine) with and without SH42 (250 μg/mL) or desmosterol (0.01 μg/mL) for 24 h before transcriptional analysis. For LM analysis, 5 × 106 M1 MΦ were stimulated with SH42 (250 μg/mL) and/or in combination with zyA (50 μg/mL) for the indicated time points (4 and 24 h), quenched with 1.5 mL of ice-cold methanol, and kept at −80 °C until analysis, as described below. In an additional set of experiments, baicalein (1 μg/mL) was added together with SH42 and zyA, followed by incubation for 24 h.
LXR Selectivity Assessment.
RAW 264.7 cells were maintained in high-glucose Dulbecco’s modified Eagle’s medium (DMEM) with 10% FCS and 1% penicillin/streptomycin under a 5% CO2-humidified atmosphere at 37 °C. Before treatment, cells were seeded into 24-well plates and incubated for at least 12 h. Different concentrations of SH42 (dissolved in ethanol; 0, 0.5, 1, 5, and 10 μM) were tested, and treated cells were incubated for 8 h with 10% FCS or 0.5% bovine serum albumin (BSA) in DMEM. In the FCS-starved groups, medium contained 0.5% BSA and 1% penicillin/streptomycin DMEM for 8 h before SH42 treatment. In the atorvastatin-treated groups, cells were pretreated with atorvastatin (dissolved in DMSO; 10/25 μM) for 8 h before SH42 treatment. Inhibitor experiments with GSK 2033 were carried out as follows. Cells were treated as described above. In the GSK 2033-treated groups, cells were pretreated with GSK 2033 (5 μM) for 2 h before SH42 treatment. Cells were ultimately incubated with SH42 (5 μM) with or without pretreatment for 8 h with either 10% FCS or 0.5% BSA in DMEM (no FCS). Control groups were adjusted to 0.1% ethanol and/or DMSO if necessary. RNA was isolated using Tripure RNA Isolation Reagent (Roche) according to the manufacturer’s protocol. Total RNA (1 μg) was reverse-transcribed using M-MLV (Moloney murine leukemia virus) Reverse Transcriptase (Promega) for RT-qPCR analysis according to the manufacturer’s instructions to produce complementary DNA. mRNA expressions were normalized to β-actin and cyclophilin A mRNA expressions and expressed as fold change compared with the vehicle-treated group using the ΔΔCT method. Primer sequences can be found in SI Appendix, S11.
GC-MS Analysis of Desmosterol Levels.
Desmosterol levels were determined as described elsewhere, with some modifications (21). A detailed description of the method is provided in SI Appendix, S12.
zyA-Induced Peritonitis and FACS Analysis.
All animal protocols were performed in accordance with the regulations of the Regierungspräsidium Tübingen and the local ethics committee. For all experiments, SH42 was prepared in a 50% sterile Cremophor solution (Merck) to a final concentration of 50 μg/μL. Six- to 8-wk-old female C57BL/6 mice (Charles River Laboratories) were injected intravenously (i.v.) via the tail vein. Mice were injected daily i.v. with SH42 (0.5 mg) or vehicle control in a total volume of 150 μL for a period of 3 d. The mice were then injected i.p. with 1 mL of zyA (1 mg/mL; Z4250; Sigma–Aldrich). Peritoneal lavages (obtained in 4 mL of phosphate-buffered saline [PBS] without calcium and magnesium [PBS−/−; Sigma]) and tissues were obtained at 4, 12, 24, and 48 h. Collected exudates were counted using a hemocytometer. A 2-mL peritoneal lavage aliquot was then spun down (300 × g, 5 min, 4 °C), and the pellet was resuspended in 300 μL of flow cytometry staining buffer (FCSB) (1× PBS−/−, 1% BSA, 2 mM NaN3). A 100-μL sample volume was then transferred to a 96-well plate (round bottom). Antibodies were diluted with FCSB before use. For differential cell counting, cells were blocked with mouse anti-CD16/CD32 (101320; BioLegend; 1:50 [vol/vol]) antibodies for 10 min at room temperature and then stained with anti-mouse e450-F4/80 (48-4801-82; eBioscience; 1:100 [vol/vol]), Allophycocyanin-Ly6G (APC-Ly6G) (127614; BioLegend; 1:250 [vol/vol]) and FITC-Ly6C (128006; BioLegend; 1:250 [vol/vol]) antibodies for 30 min at 4 °C. For determination of MΦ phagocytosis of apoptotic PMNs, cells were permeabilized using a fixation and permeabilization kit (554714; Becton Dickinson) according to the manufacturer’s instructions, following staining with anti-mouse PerCP-Cy5.5–conjugated anti-Ly6G (127616; BioLegend; 15:1,000 [vol/vol]) for 30 min at 4 °C. Data acquisition was performed on a FACSCanto II flow cytometer (Becton Dickinson ) using FACSDiva software (Becton Dickinson), and data were analyzed with FlowJo (TreeStar). The gating strategy can be found in SI Appendix, S13 (45). For therapeutic treatment with SH42, peritonitis was initiated as described above using 1 mL of a zyA solution. A single dose of SH42 was then administered i.v. 24 h later, and mice were killed at the 48-h time point.
Determination of IL-10, IL-6, IL-8, TNF-α, and TGF-β.
Analysis was carried out according to the manufacturer’s instructions using a standard enzyme-linked immunosorbent assay (R&D Systems).
Transcriptional Analysis of Peritoneal Cells and MΦ.
Human PBMCs were differentiated for 7 d in RPMI 1640 supplemented with GM-CSF (10 ng/mL) and then treated with TNF-α (100 ng/mL), SH42 (250 μg/mL), and desmosterol (0.01 μg/mL). Primer sequences can be found in SI Appendix, S11
Peritoneal lavages from SH42- and vehicle-treated mice were used following 0 and 4 h of zyA-induced peritonitis prior to transcriptional analysis. Murine 18S expression as a housekeeping gene was evaluated. Primer sequences can be found in SI Appendix, S11.
Protein Array Analysis.
Peritoneal monocytes/MΦ from 3-d SH42- and vehicle-treated mice were used following 4 h of zyA-induced peritonitis. Protein and phosphorylation (TGF-β Phospho Antibody Array, PTG176; FullMoonBioscience) profiling of peritoneal monocytes (pooled lavages from 5 mice per condition) was performed according to the manufacturer’s instructions. Image acquisition was performed by the manufacturer. A detailed description is provided in SI Appendix, S14.
Human and Murine MΦ Phagocytosis.
Human MΦ were prepared from PBMCs using the Histopaque (Sigma–Aldrich) method and differentiated with GM-CSF; subsequently, 0.1 × 106 cells per well were incubated with test substances as stated. Murine MΦ were obtained by peritoneal lavage and plated on 48-well plates for 1 h in PBS with calcium and magnesium to allow adherence. Next, MΦ were stimulated with SH42 (250 μg/mL), MaR1 (10 ng/mL), or 19,20 EpDPA (10 ng/mL) as indicated. GSK 2033 was used at a concentration of 200 nM. Fluorescent zyA particles (Z2841; Thermo Fisher Scientific) were added at a 1:30 ratio (MΦ/zyA) and incubated at 37 °C for 60 min to allow phagocytosis. Fluorescence was determined using a fluorescent plate reader (Tecan).
Western Blot Analysis.
Peritoneal monocytes/MΦ from 3-d SH42- and vehicle-treated mice were used following 4 h of zyA-induced peritonitis. Cell lysates were obtained using radioimmunoprecipitation buffer (89900; Thermo Fisher Scientific), determination of protein quantity was performed using the standard protocol of the Pierce BCA Protein Assay Kit (23225; Thermo Fisher Scientific), and protein levels were normalized and loaded in NuPAGE Bis-Tris gels (NP0335BOX; Thermo Fisher Scientific) and blotted on polyvinylidene difluoride membranes. The following antibodies were used: anti-mTOR (2972; Cell Signaling Technology), anti–phospho-mTOR (T2446) (ab63552; Abcam), anti–pan-AKT (ab8805; Abcam), and anti–phospho-pan AKT (T308) (ab38449; Abcam); for a loading control, an anti-GAPDH antibody (G9545; Sigma–Aldrich) was used. A goat anti-rabbit antibody (375699; ABIN) conjugated with alkaline phosphatase was used as a secondary antibody.
Quantitative Lipidomic Analysis.
Quantitative lipidomic analysis was carried out as described elsewhere (34), with some modification. A detailed description of the method is provided in SI Appendix, S15.
Targeted LM Analysis.
Targeted LM analysis was carried out as described elsewhere (45). Further details are available in SI Appendix, S16 and Table S1, and raw data are available in Dataset S6.
Supplementary Material
Acknowledgments
We thank Shane Soogea, Evelyne Steenvoorden, and Marieke Heijink for their help with lipid analysis. We thank Marije Kuipers for help with the artwork of Fig. 10. This work was supported by 2 grants from the Deutsche Forschungsgemeinschaft (DFG; German Research Foundation): DFG-MI 1506/5-1 and Projektnummer 374031971–TRR 240 (to V.M.); and IZKF-Fortüne Grant 2377-0-0 (to A.K.). This research was also supported by the Enabling Technologies Hotels Programme-ZonMW Grant 4350004007 (to Y.W. and M.G.). Y.W. is supported by VENI Grant 91617027 from the Netherlands Organization for Scientific Research-ZonMW. M.G. is supported by and coordinator of the Horizon2020 Innovative Training Network (ITN) project, ArthritisHeal 812890.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1911992116/-/DCSupplemental.
References
- 1.Pavlova N. N., Thompson C. B., The emerging hallmarks of cancer metabolism. Cell Metab. 23, 27–47 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rinschen M. M., Ivanisevic J., Giera M., Siuzdak G., Identification of bioactive metabolites using activity metabolomics. Nat. Rev. Mol. Cell Biol. 20, 353–367 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luengo A., Gui D. Y., Vander Heiden M. G., Targeting metabolism for cancer therapy. Cell Chem. Biol. 24, 1161–1180 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hiebert P., Werner S., Targeting metabolism to treat psoriasis. Nat. Med. 24, 537–539 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Serhan C. N., Discovery of specialized pro-resolving mediators marks the dawn of resolution physiology and pharmacology. Mol. Aspects Med. 58, 1–11 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Diepen J. A., Berbée J. F. P., Havekes L. M., Rensen P. C. N., Interactions between inflammation and lipid metabolism: Relevance for efficacy of anti-inflammatory drugs in the treatment of atherosclerosis. Atherosclerosis 228, 306–315 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Serhan C. N., Petasis N. A., Resolvins and protectins in inflammation resolution. Chem. Rev. 111, 5922–5943 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spann N. J., Glass C. K., Sterols and oxysterols in immune cell function. Nat. Immunol. 14, 893–900 (2013). [DOI] [PubMed] [Google Scholar]
- 9.Muse E. D., et al. , Cell-specific discrimination of desmosterol and desmosterol mimetics confers selective regulation of LXR and SREBP in macrophages. Proc. Natl. Acad. Sci. U.S.A. 115, E4680–E4689 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu C., Miloslavskaya I., Demontis S., Maestro R., Galaktionov K., Regulation of cellular response to oncogenic and oxidative stress by Seladin-1. Nature 432, 640–645 (2004). [DOI] [PubMed] [Google Scholar]
- 11.Takano T., et al. , Augmentation of DHCR24 expression by hepatitis C virus infection facilitates viral replication in hepatocytes. J. Hepatol. 55, 512–521 (2011). [DOI] [PubMed] [Google Scholar]
- 12.Cederbaum A. I., Molecular mechanisms of the microsomal mixed function oxidases and biological and pathological implications. Redox Biol. 4, 60–73 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spann N. J., et al. , Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell 151, 138–152 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo H., et al. , Modulation of long noncoding RNAs by risk SNPs underlying genetic predispositions to prostate cancer. Nat. Genet. 48, 1142–1150 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Varin A., et al. , Liver X receptor activation promotes polyunsaturated fatty acid synthesis in macrophages: Relevance in the context of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 35, 1357–1365 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Yang C., et al. , Sterol intermediates from cholesterol biosynthetic pathway as liver X receptor ligands. J. Biol. Chem. 281, 27816–27826 (2006). [DOI] [PubMed] [Google Scholar]
- 17.Oishi Y., et al. , SREBP1 contributes to resolution of pro-inflammatory TLR4 signaling by reprogramming fatty acid metabolism. Cell Metab. 25, 412–427 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong C., Tontonoz P., Liver X receptors in lipid metabolism: Opportunities for drug discovery. Nat. Rev. Drug Discov. 13, 433–444 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Schultz J. R., et al. , Role of LXRs in control of lipogenesis. Genes Dev. 14, 2831–2838 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gadde S., et al. , Development of therapeutic polymeric nanoparticles for the resolution of inflammation. Adv. Healthc. Mater. 3, 1448–1456 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Müller C., et al. , New chemotype of selective and potent inhibitors of human delta 24-dehydrocholesterol reductase. Eur. J. Med. Chem. 140, 305–320 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Giera M., Plössl F., Bracher F., Fast and easy in vitro screening assay for cholesterol biosynthesis inhibitors in the post-squalene pathway. Steroids 72, 633–642 (2007). [DOI] [PubMed] [Google Scholar]
- 23.Calkin A. C., Tontonoz P., Transcriptional integration of metabolism by the nuclear sterol-activated receptors LXR and FXR. Nat. Rev. Mol. Cell Biol. 13, 213–224 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zuercher W. J., et al. , Discovery of tertiary sulfonamides as potent liver X receptor antagonists. J. Med. Chem. 53, 3412–3416 (2010). [DOI] [PubMed] [Google Scholar]
- 25.Jiao Y., Lu Y., Li X. Y., Farnesoid X receptor: A master regulator of hepatic triglyceride and glucose homeostasis. Acta Pharmacol. Sin. 36, 44–50 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiang J. Y. L., Regulation of bile acid synthesis: Pathways, nuclear receptors, and mechanisms. J. Hepatol. 40, 539–551 (2004). [DOI] [PubMed] [Google Scholar]
- 27.Zhou J., et al. , A novel pregnane X receptor-mediated and sterol regulatory element-binding protein-independent lipogenic pathway. J. Biol. Chem. 281, 15013–15020 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li T., Chen W., Chiang J. Y. L., PXR induces CYP27A1 and regulates cholesterol metabolism in the intestine. J. Lipid Res. 48, 373–384 (2007). [DOI] [PubMed] [Google Scholar]
- 29.Quinn C. M., Jessup W., Wong J., Kritharides L., Brown A. J., Expression and regulation of sterol 27-hydroxylase (CYP27A1) in human macrophages: A role for RXR and PPARgamma ligands. Biochem. J. 385, 823–830 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cash J. L., White G. E., Greaves D. R., “Zymosan‐induced peritonitis as a simple experimental system for the study of inflammation” in Methods in Enzymology, Handel T. M., Hamel D. J., Eds. (Academic Press, 2009), vol. 461, chap. 17 pp. 379–396. [DOI] [PubMed] [Google Scholar]
- 31.Serhan C. N., Levy B. D., Resolvins in inflammation: Emergence of the pro-resolving superfamily of mediators. J. Clin. Invest. 128, 2657–2669 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi J.-Y., et al. , Mer signaling increases the abundance of the transcription factor LXR to promote the resolution of acute sterile inflammation. Sci. Signal. 8, ra21 (2015). [DOI] [PubMed] [Google Scholar]
- 33.Mailänder-Sánchez D., et al. , Antifungal defense of probiotic Lactobacillus rhamnosus GG is mediated by blocking adhesion and nutrient depletion. PLoS One 12, e0184438 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ubhi B. K., “Direct infusion-tandem mass spectrometry (DI-MS/MS) analysis of complex lipids in human plasma and serum using the lipidyzer platform” in Clinical Metabolomics: Methods and Protocols, Giera M., Ed. (Springer, New York, NY, 2018), pp. 227–236. [DOI] [PubMed] [Google Scholar]
- 35.He C., et al. , Inhibiting delta-6 desaturase activity suppresses tumor growth in mice. PLoS One 7, e47567 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsuzaka T., Shimano H., Elovl6: A new player in fatty acid metabolism and insulin sensitivity. J. Mol. Med. (Berl.) 87, 379–384 (2009). [DOI] [PubMed] [Google Scholar]
- 37.Schlegel M., et al. , The neuroimmune guidance cue netrin-1 controls resolution programs and promotes liver regeneration. Hepatology 63, 1689–1705 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Shimanaka Y., et al. , Omega-3 fatty acid epoxides are autocrine mediators that control the magnitude of IgE-mediated mast cell activation. Nat. Med. 23, 1287–1297 (2017). [DOI] [PubMed] [Google Scholar]
- 39.Gilroy D. W., et al. , CYP450-derived oxylipins mediate inflammatory resolution. Proc. Natl. Acad. Sci. U.S.A. 113, E3240–E3249 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levy B. D., Clish C. B., Schmidt B., Gronert K., Serhan C. N., Lipid mediator class switching during acute inflammation: Signals in resolution. Nat. Immunol. 2, 612–619 (2001). [DOI] [PubMed] [Google Scholar]
- 41.Manning B. D., Toker A., AKT/PKB signaling: Navigating the network. Cell 169, 381–405 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Covarrubias A. J., et al. , Akt-mTORC1 signaling regulates Acly to integrate metabolic input to control of macrophage activation. eLife 5, e11612 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Greenlee-Wacker M. C., Clearance of apoptotic neutrophils and resolution of inflammation. Immunol. Rev. 273, 357–370 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McWhorter F. Y., Wang T., Nguyen P., Chung T., Liu W. F., Modulation of macrophage phenotype by cell shape. Proc. Natl. Acad. Sci. U.S.A. 110, 17253–17258 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Körner A., et al. , Sympathetic nervous system controls resolution of inflammation via regulation of repulsive guidance molecule A. Nat. Commun. 10, 633 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buckley C. D., Gilroy D. W., Serhan C. N., Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity 40, 315–327 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gordon S., Phagocytosis: An immunobiologic process. Immunity 44, 463–475 (2016). [DOI] [PubMed] [Google Scholar]
- 48.Ito A., et al. , LXRs link metabolism to inflammation through Abca1-dependent regulation of membrane composition and TLR signaling. eLife 4, e08009 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang J. T., et al. , Interleukin-4-dependent production of PPAR-γ ligands in macrophages by 12/15-lipoxygenase. Nature 400, 378–382 (1999). [DOI] [PubMed] [Google Scholar]
- 50.FitzGerald G. A., BIOMEDICINE. Bringing PGE2 in from the cold. Science 348, 1208–1209 (2015). [DOI] [PubMed] [Google Scholar]
- 51.Bishop-Bailey D., Thomson S., Askari A., Faulkner A., Wheeler-Jones C., Lipid-metabolizing CYPs in the regulation and dysregulation of metabolism. Annu. Rev. Nutr. 34, 261–279 (2014). [DOI] [PubMed] [Google Scholar]
- 52.Colas R. A., et al. , Identification and actions of the maresin 1 metabolome in infectious inflammation. J. Immunol. 197, 4444–4452 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spite M., Clària J., Serhan C. N., Resolvins, specialized proresolving lipid mediators, and their potential roles in metabolic diseases. Cell Metab. 19, 21–36 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pommier A. J. C., et al. , Liver X Receptor activation downregulates AKT survival signaling in lipid rafts and induces apoptosis of prostate cancer cells. Oncogene 29, 2712–2723 (2010). [DOI] [PubMed] [Google Scholar]
- 55.Motwani M. P., et al. , Pro-resolving mediators promote resolution in a human skin model of UV-killed Escherichia coli-driven acute inflammation. JCI Insight 3, 94463 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wittwer J., Marti-Jaun J., Hersberger M., Functional polymorphism in ALOX15 results in increased allele-specific transcription in macrophages through binding of the transcription factor SPI1. Hum. Mutat. 27, 78–87 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.