Abstract
Due to the necessity of iron for a variety of cellular functions, the developing mammalian organism is vulnerable to iron deficiency, hence causing structural abnormalities and physiological malfunctioning in organs, which are particularly dependent on adequate iron stores, such as the brain. In early embryonic life, iron is already needed for proper development of the brain with the proliferation, migration, and differentiation of neuro-progenitor cells. This is underpinned by the widespread expression of transferrin receptors in the developing brain, which, in later life, is restricted to cells of the blood–brain and blood–cerebrospinal fluid barriers and neuronal cells, hence ensuring a sustained iron supply to the brain, even in the fully developed brain. In embryonic human life, iron deficiency is thought to result in a lower brain weight, with the impaired formation of myelin. Studies of fully developed infants that have experienced iron deficiency during development reveal the chronic and irreversible impairment of cognitive, memory, and motor skills, indicating widespread effects on the human brain. This review highlights the major findings of recent decades on the effects of gestational and lactational iron deficiency on the developing human brain. The findings are correlated to findings of experimental animals ranging from rodents to domestic pigs and non-human primates. The results point towards significant effects of iron deficiency on the developing brain. Evidence would be stronger with more studies addressing the human brain in real-time and the development of blood biomarkers of cerebral disturbance in iron deficiency. Cerebral iron deficiency is expected to be curable with iron substitution therapy, as the brain, privileged by the cerebral vascular transferrin receptor expression, is expected to facilitate iron extraction from the circulation and enable transport further into the brain.
Keywords: developmental, iron deficiency anemia, neonatal, transferrin receptor, treatment
1. Introduction
Iron deficiency is the most common type of malnutrition in humans [1,2,3,4]. Iron deficiency occurs due to an inadequate intake, excess loss, or increased need, and gradually leads to insufficient functions of many organs, including the bone marrow, and as a consequence, iron deficiency leads to iron deficiency anemia [5,6,7]. According to the WHO, anemia affects 1.8 billion people worldwide, equivalent to approximately 25% of the world’s population. Among this group, approximately 0.5 billion are women of a reproductive age, and in developing countries, the incidence of anemia is even higher and varies during pregnancy from 35% to 56% for Africa, 37% to 75% in Asia, and 37% to 52% in Latin America.
Women of a reproductive age are particularly at risk for developing iron deficiency due to menstrual bleeding and pregnancy. The prevalence of iron deficiency anemia in pregnant women is high, even in industrialized countries with well-established iron supplementation policies, e.g., in Denmark, where iron deficiency with anemia affects roughly 12% of pregnant women. The state of pregnancy greatly increases the demand of iron used in foetal organogenesis and growth. The pregnant body prioritizes the fetal iron supply over the maternal utilization up until a threshold of the maternal iron stores being adequate, with a maternal plasma ferritin concentration of approximately 14 µg/L [8]. When the maternal iron stores exceed this threshold, the placenta actively transports iron into the fetal circulation to ensure adequate iron supply to the fetus [9].
Iron deficiency can adversely affect brain development in fetuses and infants. Whereas most knowledge on the fetal needs for iron during pregnancy has been obtained within hematological, nephrology, and gastrointestinal disciplines, the impact on the fetal brain of maternal iron deficiency during pregnancy remains quite understudied. A higher turnover of iron in the developing brain [10], in addition to the widespread expression of iron-containing proteins, nonetheless dictate the importance of iron for the developing brain [11,12,13,14]. This is particularly underscored by the profound expression of transferrin receptor 1 by dividing neuroprogenitor cells [15,16] and signifies that cells of the developing brain with respect to their need for iron share a range of common conditions with precursor cells participating in bone marrow erythropoiesis and the formation of duodenal enterocytes of the fully developed organism. This review aims to summarize the current evidence on the significance of iron for the developing brain, how iron deficiency may impair functioning of the central nervous system (CNS) in the human brain and brains of experimental animals, and which therapeutic advances available can prevent damage to the developing CNS.
1.1. Transport of Iron into the Brain
The brain acquires iron during life in a privileged manner in that its capillary endothelial cells are the only endothelial cells of the entire body that express transferrin receptors [17]. Iron circulates in blood exclusively bound to transferrin, unless pathological conditions like hemochromatosis occurs, which will result in the presence of low-molecular-weight forms of non-transferrin bound iron. The brain capillary endothelial cells form the blood–brain barrier (BBB) that prevents paracellular, non-specific entry of the iron-containing transferrin into the brain [17]. Rather, the brain capillary endothelium regulates iron transport into the brain via the expression of transferrin receptors [9,13,17]. Iron-transferrin attaches to the transferrin receptor, which results in the formation of endocytic vesicles. Recent studies have also shown that the transferrin receptor of the BBB can bind and take up circulating ferritin [18,19,20,21]. These vesicles are slightly acidic, and the lower pH reduces the binding affinity of iron to transferrin, which loosens their binding. The iron, present on its ferric form, is reduced to ferrous iron, which can be transported out of the endosome and into the cytosol by divalent metal transporter 1 (DMT1) [22]. The release of iron from transferrin within the endosome causes the iron-free apo-transferrin to detach from the transferrin receptor, which allows unbound apo-transferrin to recycle to the luminal surface [17].
Unbound ferrous iron is a potent pro-oxidative molecule that needs immediate oxidation [23,24]. Consequently, ferrous iron is either oxidized within the cytosol by ferrous oxidases, e.g., ceruloplasmin, or gets transported into the brain’s extracellular space via the efflux transporter ferroportin, while undergoing oxidation during passage of the cellular membrane [23]. The iron transported across the brain endothelium accordingly occurs in a non-transferrin bound iron form and thereby is a candidate for binding to transferrin present within the brain extracellular space [17].
Iron in the cytosol participates in essential metabolic processes, e.g., participation in mitochondrial respiration via incorporation mitochondrial enzymes. Many cell types of the body also store residual iron as ferritin-iron, as ferritin also has pro-oxidant activity and is capable of oxidizing ferrous iron to store around 4500 iron atoms [25,26]. Of note, however, is that brain capillary endothelial cells hardly express ferritin, except for during development [27], suggesting that virtually all iron present within the brain capillary endothelial cells is immediately directed toward transport across the BBB to ensure its function further inside the brain.
Iron is also transferred to the brain via transfer across choroid plexus epithelial cells that form the blood–cerebrospinal fluid (CSF) barrier [17]. Like the endothelial cells forming the BBB, the epithelial cells of the choroid plexus also express transferrin receptors [24], but the quantitative relevance of the choroid plexus for iron transport into the brain is of less significance due to their much lower surface than that of brain endothelial cells of the BBB. The choroid plexus nonetheless very likely makes an important contribution to cerebral iron homeostasis, as transferrin of the blood plasma is filtered through the blood–CSF barrier and enters the brain ventricles, while transferrin in parallel is also synthesized and secreted from the choroid plexus to enter the brain ventricles [24]. In sum, this suggests that transferrin of the brain ventricle, and likely also elsewhere in the brain’s extracellular space, is derived from the choroid plexus. In the extracellular compartment of the brain, transferrin is needed to capture non-transferrin-bound iron transported across the BBB or released from neurons and glia. The need for transferrin in the brain’s extracellular space is further underscored by the presence of transferrin receptors on neurons [28]. Surprisingly, transferrin receptors and DMT1 are hardly detected on major glial cells like astrocytes, oligodendrocytes, and microglia [23,28], which suggests that iron enters glial cells as non-transferrin-bound iron, possibly via specific transporters like ZIP14 [29].
1.2. Transport of Iron into the Developing and Iron-Deficient Brain
The uptake of iron-containing transferrin at the BBB and blood–CSF barriers and the further transportation of iron into the brain are dramatically upregulated in the developing brain [30,31,32]. The higher iron uptake strongly correlates to a higher expression of transferrin receptors by brain endothelial cells in the developing brain, as evidenced from studies on the rodent brain [30,31]. The upregulated iron transport is attributable to a generally higher need for iron as the progenitor cells of the brain proliferate and differentiate into their final phenotypes [15,16,32,33]. Interestingly, as the cerebral turnover of iron is extremely low and ceases with increasing age, virtually all the iron transported across the brain barriers during development is believed to remain within the brain [10,11,12].
Correspondingly, when the events take place during development, the brain also adapts to conditions with deprivation in iron accessibility by the upregulation of transferrin receptors [28]. When cerebral iron deficiency occurs, the brain profoundly increases the internalization of transferrin receptors in the capillary endothelial cells. The brain also increases the expression of transferrin receptors in neurons in iron deficiency, whereas glial cells, even in stages with robust iron deficiency, fail to express transferrin receptors [28].
Combining iron deficiency with stages of development produces the maximal demand for the brain to mobilize transferrin receptors, but in this context, it is of note that there seems to be an upper limit for the extent to which the brain can adapt. Supporting this notion, the brain failed to increase the expression of transferrin receptors when iron deficiency was subjected to experimental animals during development [31,34]. Therefore, the failure to further increase transferrin receptor expression suggests that the developing brain is particularly vulnerable to severe iron deficiency.
1.3. The Significance of Iron for Precursor Cells of the Developing Brain
The availability of iron for the brains’ cells must be adequate to undertake several iron-dependent processes, not only to ensure important functions such as cellular division and differentiation, but also the development of the entire brain [9,11,12], e.g., (i) the complex cellular architecture consisting of neuronal axons ensheathed with myelin synthetized by oligodendrocytes, (ii) the complicated brain–barrier interface supported by astrocytes and pericytes to regulate transport in and out of the brain, and (iii) the establishment of an innate immune system in the brain via the formation of microglia.
The significance of iron for maintaining cellular functions has been covered in former reviews [11,12,35,36,37]. Iron denotes an essential part as the co-factor of several proteins that can be organized into four groups: Non-enzymatic iron-containing proteins; enzymes that use iron-sulfur as a co-factor; enzymes with an iron-containing heme group; iron-containing enzymes without heme or an iron-containing sulfur group. Together, these four groups of proteins undertake essential iron-dependent cellular events, i.e., electron transfer in the mitochondria, regulation of the expression levels of several genes, regulation of cellular division and differentiation, the binding and transport of oxygen, the synthesis of neurotransmitters (in particular serotonin, norepinephrine, and dopamine), the packaging of neurotransmitters in the axon terminal, the reuptake and degradation of neurotransmitters, and the co-factor function for peroxide- and nitrous oxide-generating enzymes for the functioning of immune cells and the intracellular killing of pathogens [11,12,35,36,37]. More specifically, for the developing brain, these above-mentioned cellular iron-dependent processes make their contribution to adequately ensure series of important events ranging from early formation of the neuronal tube to later differentiation of neuronal precursor cells into neurons and glial cells. Iron is very important for the formation of the neuronal tube, of which the formation is abrogated during a conditional lack of transferrin receptors [15,16]. Severe iron deficiency early in life is also expected to impair the forming brain due to the loss of function of the iron-containing enzyme ribonucleotide reductase that is essential for cellular division [38]. This leads to the major concern that remains unexploited, which predicts that developmental iron deficiency during early gestation can cause a permanent reduction in the number of neuronal and glial cells in spite of iron being supplied later in life, e.g., by admitting iron to the neonate [39]. Concerning glial cells, their formation in the developing brain depends on iron-containing enzymes to ensure cellular division and differentiation. Regarding oligodendrocytes, the lack of iron availability during development is thought to significantly affect their capability to form myelin [11,12,40], and current hypotheses concern whether the formation of myelin is permanently affected, even if the iron supply is restored later in life, hence hinting towards a certain time-window during development where iron availability must be adequate to promote myelination [41].
1.4. Translational Models of the Brain Development
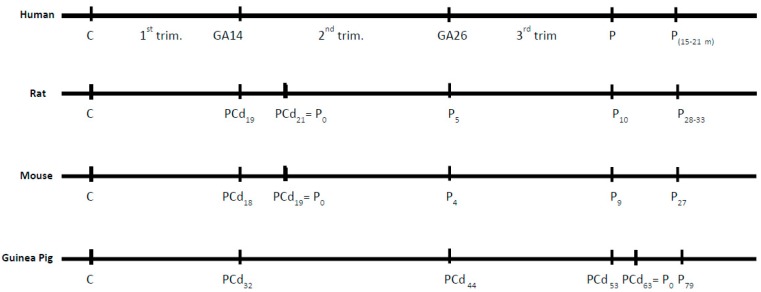
The effects of iron deficiency on the brain will likely manifest, with the earlier the impacts taking place representing the worst condition. The gestational ages vary dramatically between mammalian species, which is very important to notify when comparing experimental data on the effects of iron deficiency.
The normal brain development of different mammalian species can be compared (http://www.translatingtime.org/translate) [42]. Events like neurogenesis and myelination in the brains of rats and mice time wise are almost identical (Figure 1), but differences dramatically occur when comparing rodents and humans. Figure 1 also shows that myelination of the human brain compared to myelination in rats and mice is clearly different regarding both timing and the time period of gestation, i.e., myelination of the human brain takes places around post-conception (PC) day 250, which translates to PC day 30 in the rat. The latter is very important to emphasize, because it shows that opposed to the human brain, myelination takes places after birth in the rodent (around P7), which must be taken into account when designing an experimental study on brain development with the purpose of detecting correlations between species.
Figure 1.
Comparison of the developmental ages of the human, rat, mouse, and guinea pig with respect to myelination of the entire brain. C = day of conception. Psuffixnr = postnatal day. PCd = post-conception day. P = partus. GA = human gestation week corresponding to PCd. trim. = Trimester.
1.5. Evidence of Deleterious Effects of Iron Deficiency on the Developing Brain
The effects of iron deficiency on the gastro-intestinal tract and hematological system are well-described, with the effects being reversible. In contrast, the developing CNS differs from many organs of the body as the impacts are much more prone to be irreversible, even when iron supplies are restored, because the neurons are post-mitotic from the time of birth. The following paragraphs will outline the studies that have been conducted to delineate the effects of iron deficiency on the developing brain, with an emphasis on whether effects were reversible or irreversible. To facilitate the translational relevance, the different species are reported separately based on changes in neuronal and glial functioning, and probable changes in behavior. The selected studies were identified based on a search strategy using PubMed to identify primary research on experimental animal studies and human studies using the following MESH words: iron deficiency, development, brain, or neuro, which revealed approximately 60 relevant studies on experimental animals and observations of the developing human brain during the most recent two decades.
1.6. Experimental Animals
The experimental animal data point towards significant effects on the brain during periods of iron deficiency during both the gestational period and after birth (Table 1). The data on small rodents like rats and mice clearly show that the most dramatic effects on the brain development occur when iron deficiency is introduced in pregnancy, whereas data from larger animals like the domestic pig and non-human primates show an influence when iron deficiency is introduced to the offspring (Table 1).
Table 1.
Studies in experimental animals showing cerebral effects of iron deficiency (ID) subjected to pregnant females or their offspring. References are listed chronologically after species rather than after specific topics, as the many studies addressed more than a single objective. Most data were obtained from studies on rats. Abbreviations: ABR, auditory brainstem responses; DPOAE, distortion product of otoacoustic emissions; IHC, immunohistochemistry; PUFA, long-chain polyunsaturated fatty acids.
| Species | Study Design | Method | Offspring Age | Conclusion | Reference |
|---|---|---|---|---|---|
| Rat | ID before conception + Gestational ID | Electrophysiological recordings |
P15–P30, P65 | Late term effects on synapses in hippocampus in spite of cerebral iron repletion |
[44] |
| ID before conception | Behavior | P10–Adult | Some persistent effects in spite of iron repletion | [45] | |
| ID before conception + Gestational ID |
Brain iron Neurotransmitters |
P35 | Behavioral impairments related to persistent loss in dopamine in spite of brain iron reversal |
[46] | |
| Gestational ID + Lactational ID |
mRNA | P6–P21 | ID from E15 leads to alteration in tyrosine hydroxylase and reversibility in behavior |
[47] | |
| Gestational ID + Lactational ID |
mRNA, proteins morphology |
P7–P15; P30 | Lower BDNF, impaired neuronal differentiation | [48] | |
| ID before conception + Gestational ID |
Behavior | Adult | Detrimental effects of behavioral tasks, sex dependency | [49] | |
| Gestational ID | Myelination | P25 | Impaired myelination with correlation to impairment | [50] | |
| Gestational ID | mRNA, proteins | P32–P69 | Effect of behavior, no effects on motor skills in hippocampus | [51] | |
| Gestational ID + Lactational ID |
Behavior | P65 | Permanent changes in behavioral tasks | [52] | |
| Gestational ID + Lactational ID |
mRNA morphology |
P7–P65 | Permanent changes in mRNA of neuronal markers and dendritic branching in spite of postnatal reversal to normal diet | [53] | |
| Gestational ID | mRNA, T3, T4 | P12 | Marked reduction in T3, T4 | [54] | |
| Rat | ID before conception + Gestational ID |
ABR, DPOAE | P0–P45 | First trimester displays profound changes in auditory brain stem response | [55] |
| Gestational ID + Lactational ID |
MRI, NMR | P7–56 | Restoration of brain iron, permanent size reduction in hippocampus and neurochemical hall-markers in spite of postnatal reversal to normal diet | [56] | |
| Gestational ID | mRNA | P7–P56 | Impaired formation of neuronal network and impaired neuronal plasticity in spite of postnatal reversal to normal diet | [57] | |
| Gestational ID | Morphology | P21–P40 | 25% reduction in dendritic length 20% reduction in axonal diameter |
[58] | |
| ID before conception + Gestational ID |
ABR | P40 | Increased ABR latencies in ID depending on stage of ID | [59] | |
| Gestational ID + Lactational ID |
mRNA | P10–P15 | Elevated angiogenic/vasculogenic signaling with increased blood vessel complexity | [60] | |
| ID before conception + gestational ID |
mRNA, T3, T4 | E13–P10 | Marked reduction in T3, T4 Lowering of thyroid hormone responsive genes |
[61] | |
| Embryonic brain | mRNA | Not available (Cultures at E16) |
DFO-induced ID lowers expression of series of markers of dendritic and synaptic development, and mitochondrial function | [62] | |
| Gestational ID | Tactile stimuli | P1–P32 | Tactile stimuli reverse defect myelination and alteration in oligodendrocytes and microglia, but not astrocytes | [63] | |
| Rat | Gestational ID | Pro/anti-oxidant | P0–P70 | Age- and iron-dependent levels of oxidative stress profiling | [64] |
| Gestational ID | mRNA, IHC | P21, P35 | Defect myelination, alteration in glial cells | [65] | |
| Mouse | Gestational ID Brain iron |
Hematology | E17–E18 | Effect of brain weight, lower brain iron | [66] |
| ID in offspring | Brain iron | Adult | Correction of cerebral ID with parenteral iron | [67] | |
| Guinea Pig | Gestational ID + Lactational ID |
ABR | P9–P24 | Effect of ABR in ID Part restoration with PUFA treatment |
[68] |
| Gestational ID + Lactational ID |
ABR | P24 | Effect of ABR in ID | [69,70] | |
| Domestic Pig | Gestational ID + Lactational ID |
Cognitive tasks | 0–4 weeks after birth | No cognitive deficits | [71] |
| ID in offspring | MRI | 0–6 weeks after birth | Cerebral ID, alteration in brain tissue composition persists in spite of iron repletion |
[72] | |
| Lactational ID | RNA analysis | 4 weeks after birth | Change in hippocampal DNA methylation and gene regulation | [73] | |
| Gestational ID | MRI, IHC | PD 2–30 | ID after PD 14 detriments white matter | [74] | |
| Monkey | ID in offspring | 1H NMR | Infancy | Change in metabolomic profile in CSF | [75] |
| ID in offspring | Proteomic | Infancy | Persistent change in proteomic profile in CSF | [76] | |
| ID in offspring | 1H NMR | Infancy | Metabolomic profile in CSF predicts effects of ID on brain iron metabolism | [77] | |
| ID in offspring | Cognitive tasks | Infancy | Only initial cognitive + behavioral deficits | [78] | |
| Gestational ID + Lactational ID |
Cognitive tasks | Infancy | Cognitive and emotional effects present, but vary with protocol | [79] |
Rodents, especially the laboratory rat, denote the most popular experimental animal for studies of iron deficiency. The gestational period in the rat principally covers the first two trimesters in humans, with the transition between 1st and 2nd trimesters occurring only two days before delivery, whereas the third trimester in the human is reflected in the first weeks after birth in the rat (Figure 1).
The latter points towards a significant difference in the possibilities to compare human and rodent studies, as feeding of this early postnatal rodent no longer occurs via the transfer of nutrients across the placenta, but instead relies on a functioning gastrointestinal system of the neonate. The absorption of iron mainly occurs in the proximal duodenum and is regulated by the iron availability of the duodenal enterocytes. These are mainly under regulation of the general iron status in the neonate via signaling via circulatory levels of hepcidin, which is a hormone synthetized and released from the liver in response to inflammatory stimuli and high circulatory levels of iron [43]. However, inflammation in the neonate may lead to increased levels of hepcidin, as this will negatively affect iron uptake from the gut [6], and hence the rodent as a model of development equal to the third semester in the human fetus represents a model of potential risk. On the other hand, for the study of the effects of iron deficiency during development, the early postnatal rodent represents an accessible model with many possibilities for intervention.
The studies pertained on the developing rodent brain all point towards a deleterious effect of dietary iron deficiency subjected to the mother during pregnancy (Table 1). The effects range from observations based on a direct comparison with normal fed mothers to reports on permanent effects on the brain of the offspring in spite of iron being admitted even early after birth. The effects on the developing rat brain concern structural, biochemical, and behavioral impairments (Table 1). Structurally, influences include structural defects in general brain development [48], and more specifically, the development of dendritic length and arborization, and effects on the formation of synapses [44,53,57,58]. A particular focus in many studies has been the effects of changes in the expression level of genes related to the functioning of synaptic transmission [55,59], vascularization [55], and hormones improving metabolism [61]. Studies have also reported on defects in the synthesis of monoaminergic neurotransmitters [46,47,57], and growth factors [48]. Additionally, studies have reported on behavioral disturbances [44,49,51,52]. A single study has reported on the impaired development of glial cells [65] and the impaired formation of myelin has also been reported [44,63,65], suggesting that earlier studies demonstrating that changes in the profiles of fatty acids in phospholipids are present in iron deficiency relate back to the functioning of the developing oligodendrocytes [11,12,13,40].
The significance of dietary iron deficiency on the developing mouse has gained less attention than that of the rat. Iron deficiency negatively affects the brain weight, iron content, and formation of oligodendrocytes and their myelination [66,80]. As previously mentioned, genetic depletion of the transferrin receptor in the mouse results in severe fetal effects and impaired neurotransmitter formation [15,16]. In the guinea pig, a series of studies have been made on neural transmission in the brain stem, and reportedly deleterious effects of iron deficiency were partly restored by dietary supplementation with polyunsaturated fatty acids, indicating a beneficial effect on otherwise impaired myelination [72,73,74].
Most studies of larger animals have been conducted in postnatal animals, which, for practical reasons, make this approach durable [8]. Studies of gestational iron deficiency performed in the domestic pig report on impaired myelination, but without cognitive effects [72]. Impaired myelination was also reported in piglets, who were only subjected to postnatal iron deficiency [75]. Interestingly, epigenetic regulation is also affected when iron deficiency is present in the piglet brain [74]. Another intriguing study using MRI reports on permanent changes in the brain of the domestic pig in spite of the reversal of brain iron with dietary treatment [73].
In terms of the non-human primate brain, a single study has reported on cognitive effects following gestational iron deficiency, but the effects were not consistent and were largely dependent on the induced dietary regimen [79]. Studies on iron deficiency induced in the offspring have demonstrated that this led to significant changes in nuclear magnetic resonance (NMR)-detectable metabolites and proteomic profiles in CSF, clearly hinting towards impaired cerebral metabolism [76,77]. Another study has concluded that iron deficiency subjected to the offspring led to behavioral deficits that were compensable, suggesting that the effects of iron deficiency were less deteriorating [78].
1.7. The Developing Human Brain
In humans, the brain forms very early, and maternal iron deficiency is likely to impair the developing brain during the entire period of pregnancy. Compiling the studies reporting the negative impact of iron deficiency on the formation of the developing brain in experimental animals, the following factors stand out as being particularly important: The timing and the severity of the iron deficiency regimens. These factors are also very important to keep in mind when considering the impact of iron deficiency or iron deficiency with anaemia in the human brain, as they are likely to be the most determinant concerning whether damage is at risk of being irreversible [81,82]. The effects of iron deficiency on brain development were suggested to include the genesis of dendrites and synapses, hence clearly addressing the effects of iron deficiency on differentiation during formation of the human brain, and specifically suggesting an impact on particular brain regions such as the cerebral cortex (i.e., frontal cortex, prefrontal-striatal network, auditive cortex), hippocampus, and striatum [3,82]. Prior studies were clearly limited in access to measurements on brain functioning in real-time and merely relied on correlations between the iron status measured in blood and putative changes in behavior. Infants with low cord-blood s-ferritin and haemoglobin were prone to negative emotions, and they were less alert and difficult to sooth, and in a 5-year follow-up, the children had poorer behaviour and development outcomes, trouble with auditory language skills, and fine motor skills [82,83,84]. A single trial showed that maternal anaemia in pregnancy could be linked to 14% of cases of mental retardation at a 7-year follow-up. It should, however, also be kept in mind that iron deficiency in humans is not likely to be as extreme as can be instituted in experimental animals, and this should indeed be kept in mind when translating data from animal models to hypotheses in human physiology. A valid indicator of the severity in humans is seen when iron deficiency is complicated with anemia. In this situation, the iron transport to the fetus will be prioritized over the maternal iron need unless a certain threshold (ferritin ≈ 14 µg/L) is met [8,83]. Furthermore, in severe cases of iron deficiency with anemia, fetal erythropoiesis is more highly prioritized than neurodevelopment [33,58].
The data obtained from several studies in humans all congregate towards the conclusion that there are significant effects on brain development, both pre-and postnatally (Table 2). Compared to the more extreme situations that invariably relate to the experimental animals, the impairment in iron statuses is not so dramatic in the human brain, and hence also the reported results [83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106]. The studies of humans have mainly involved structural analyses on brain growth in real-time [83,84,85,86,87,88,89], neurophysiological measurements of basic functions related to myelination and cranial nerve development [90,91,92,93,94,95,96], and neuropsychological tests of cognition, memory, and personal traits [97,98,99,100,101,102,103,104,105,106].
Table 2.
Studies of humans showing cerebral effects of iron deficiency (ID) or iron deficiency with anemia (IDA) subjected to pregnant females or their offspring. References are listed after specific topics. Abbreviations: ABR, auditory brainstem responses; LBW, low birth weight; No. F/O, numbers of patients (females/offspring); PND, postnatal day; VEP, visually evoked potentials.
| Study Objective | Evidence of ID | Infant Age | No. F/O | Conclusion | Reference |
|---|---|---|---|---|---|
| Fetal brain development | |||||
| Normal development | Maternal IDA | PND 3–5 days | /70 | Maternal IDA adversely affects l hippocampal morphogenesis and fetal production of BDNF | [83] |
| Normal development | Maternal IDA | 18 months | 331/ | Maternal ID at 34 weeks associated with lower motor scores | [84] |
| Normal development | Normal iron status | 7–11 years | /39 | MRI iron content in basal ganglia influences spatial intelligence | [85] |
| Brain connectivity | Infant IDA | Mean 21.5 years | /31 | Different patterns of functional connectivity between former IDA and control young adults | [86] |
| Risk of schizophrenia | Maternal IDA | Prospective study | /6872 | Maternal ID as risk factor for schizophrenia in offspring | [87] |
| Cerebral functions | IDA in adults | Adult | /2957 | IDA associated with increases in psychiatric disorders | [88] |
| Autism | Infant IDA | 2–7 years | /102 | No evidence between IDA and autism | [89] |
| Basic cerebral functions | |||||
| ABR | LBW | PND 42–6 months | /285 | Iron supplements did not improve ABR, but ABR was discarded as measure of impairment in ID | [90] |
| ABR | Maternal IDA | PND 2, 3 months | ABR closely related to severity of maternal and neonatal iron status | [91] | |
| ABR | Infant IDA | 6–24 months | Prolonged latencies in ABR traces in IDA | [92] | |
| ABR | Infant IDA | <48 h | /90 | Latent iron deficiency associated with abnormal ABR | [93] |
| VEP | Infant IDA | 6–24 months | /50 | Negative correlation between severity of IDA and VEP latencies | [94] |
| Eye-blinking rates | Infant IDA | 9–10 months | 61 | Increased eye-blink rats consistent with low dopamine function in IDA | [95] |
| EEG recordings | Infant IDA | 0, 9 months | /80 | ID associated with EEG asymmetry | [96] |
| Memory | |||||
| Memory | Infant IDA | 8–10 years | /201 | Iron supplementation substantially restores cognitive capabilities | [97] |
| Execution, memory | Infant IDA | 19 years | /114 | Chronic impairment of functions related to frontostriatal-connections (executive functions), and hippocampus (recognition memory) | [98] |
| Recognition memory | Infant IDA | 6–18 months | /209 | Sustained effects on memory in 10-year follow-up in spite of oral supplement in early life | [99] |
| Higher cerebral functions | |||||
| Social-emotional behavior | Infant IDA | 9–10 years | /77 | Social-emotional behavior associated with ID | [100] |
| Behavior | Normal | 6–8 years | /264 | Fe supplementation in pregnancy without consistent effect on behavior | [101] |
| Cognition | Infant IDA | 1–3 years | /3 | Improvement in cognition once iron stores were restored | [102] |
| Cognition | Infant IDA | Mean age 12.0 | Reduced cognitive performance | [103] | |
| Cognition | Infant IDA | 12 months | 828/828 | No effect of IDA on cognition or motor development | [104] |
| ADHD symptomology, IQ | Infant IDA | 2.5–5 years | /123 | Effects of early deprivation and ID on ADHD symptoms and IQ years after adoption | [105] |
| ADHD symptomology | Infant IDA | Mean age 11.0 | IDA associated with ADHD | [106] |
Concerning the studies on the development of the brain that took morphological approaches, reports indicate that brain volumes, neurogenesis, and iron content are reduced [83,84,85]. This also led to permanent defects in neuronal connectivity years after developmental iron deficiency was recovered [91], which was further associated with a higher risk for psychiatric disorders [87,88], but not for causing autism [89].
Influences of impaired sensory function and neuronal development were also attributed to developmental iron deficiency using auditory brainstem responses (ABR) [90,91,92,93]. However, at least one study brought the reliability of this measure into question [90] and hinted that changes in ABR can have other explanation. Other studies taking a neurophysiological approach showed impairment in the visual input and general brain activity [94,95,96], suggesting that, e.g., dopaminergic neurotransmission is affected [95].
Examining higher functional tasks, the impact on structures and their function in the forebrain have been reported in many studies of the hippocampus (various memory tasks) [97,98,99] and cerebral cortex (social behavior, cognition, association with ADHD) [100,102,103,105,106]. Conversely, behavior was reportedly not affected in two other studies [101,102], which suggest that effects on the developing brain could be subtle unless dramatic maternal iron deficiency occurs.
2. Conclusions
The human brain develops throughout the gestational period, ranging from the formation and proliferation of neuroprogenitor cells, to their later migration, and later differentiation into fully developed neurons and glial cells. Severe iron deficiency can negatively impact cell division, neurotic outgrowth and formation of the neuronal network, and myelination in glial cells. Experimental studies in animals, especially the laboratory rat, clearly support that these cellular events can be impacted by developmental iron deficiency. In the human brain, where events in the third semester are reflected in the initial postnatal weeks, reports also point towards the negative impact of iron deficiency during development. The quality of the identified studies reported here, including the number of involved subjects, appears valid, but some limitations subtract the possibilities for overall conclusions. The translational value of the result of the experimental animal is high, but more data obtained in higher animals with a longer gestation than the rodent brain would be appreciated. Concerning the human data, a certain shortage in the number of available studies prevails and more studies monitoring the cerebral function postnatally are needed. With respect to the validity of the results, it must also be emphasized that publication bias may exist towards the demonstration of effects of iron deficiency on brain development. This would leave out negative results that may remain unpublished, and this may play an important role as scientific results on the developing human brain are rather scarce. Investigations on the brain in the gestational period are obviously very complicated, so research on biomarkers from the umbilical cord or chorion villus biopsy would be highly appreciated.
In terms of the prevention of iron deficiency, strategies have not yet been developed to specifically address the developing brain. Supplementation with oral or parenteral iron is possible in pregnancy and postnatally [2], and strategies to halt iron deficiency anemia will likely also improve cerebral iron deficiency as the brain is able to extract iron from the blood due to the expression of transferrin receptors on brain capillaries [39]. Parenteral iron supplementation is being assayed in pregnant women and women with post-partum hemorrhage to generally improve their iron status [2,4,107,108], and this will likely also improve the cerebral iron status.
Abbreviations
| BBB | blood–brain barrier |
| CNS | central nervous system |
| CSF | cerebrospinal fluid |
Author Contributions
Conceptualization, V.M. and T.M. writing—original draft preparation, V.M. and T.M.; Writing—review and editing C.H., A.B.P. and L.L.T.
Funding
V.M. is a PhD student funded by the Innovation Fund Denmark (grant No. 5189-00027) and Pharmacosmos.
Conflicts of Interest
Authors declare no conflicts of interest with respect to this review manuscript. L.L.T. and V.M. are employed by Pharmacosmos. C.H. served on advisory boards for Pharmacosmos.
References
- 1.Zimmermann M.B., Hurrell R.F. Nutritional iron deficiency. Lancet. 2007;370:511–520. doi: 10.1016/S0140-6736(07)61235-5. [DOI] [PubMed] [Google Scholar]
- 2.Breymann C., Bian X.M., Blanco-Capito L.R., Chong C., Mahmud G., Rehman R. Expert recommendations for the diagnosis and treatment of iron-deficiency anemia during pregnancy and the postpartum period in the Asia-Pacific region. J. Perinat. Med. 2011;39:113–121. doi: 10.1515/jpm.2010.132. [DOI] [PubMed] [Google Scholar]
- 3.Hernandez-Martinez C., Canals J., Aranda N., Ribot B., Escribano J., Arija V. Effects of iron deficiency on neonatal behavior at different stages of pregnancy. Early Hum. Dev. 2011;87:165–169. doi: 10.1016/j.earlhumdev.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Khalafallah A., Dennis A., Bates J., Bates G., Robertson I.K., Smith L., Ball M.J., Seaton D., Brain T., Rasko J.E. A prospective randomized, controlled trial of intravenous versus oral iron for moderate iron deficiency anaemia of pregnancy. J. Int. Med. 2010;268:286–295. doi: 10.1111/j.1365-2796.2010.02251.x. [DOI] [PubMed] [Google Scholar]
- 5.Milman N. Serum ferritin in Danes: Studies of iron status from infancy to old age, during blood donation and pregnancy. Int. J. Hematol. 1996;63:103–135. doi: 10.1016/0925-5710(95)00426-2. [DOI] [PubMed] [Google Scholar]
- 6.Camaschella C. Iron deficiency. Blood. 2019;133:30–39. doi: 10.1182/blood-2018-05-815944. [DOI] [PubMed] [Google Scholar]
- 7.Bashiri A., Burstein E., Sheiner E., Mazor M. Anemia during pregnancy and treatment with intravenous iron: Review of the literature. Eur. J. Obs. Gyn. Reprod. Biol. 2003;110:2–7. doi: 10.1016/S0301-2115(03)00113-1. [DOI] [PubMed] [Google Scholar]
- 8.Shao J., Lou J., Rao R., Georgieff M.K., Kaciroti N., Felt B.T., Zhao Z.Y., Lozoff B. Maternal serum ferritin concentration is positively associated with newborn iron stores in women with low ferritin status in late pregnancy. J. Nutr. 2012;142:2004–2049. doi: 10.3945/jn.112.162362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duck K.A., Connor J.R. Iron uptake and transport across physiological barriers. Biometals. 2016;29:573–591. doi: 10.1007/s10534-016-9952-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dallman P.R., Spirito R.A. Brain iron in the rat: Extremely slow turnover in normal rats may explain long-lasting effects of early iron deficiency. J. Nutr. 1977;107:1075–1081. doi: 10.1093/jn/107.6.1075. [DOI] [PubMed] [Google Scholar]
- 11.Beard J.L. Iron biology in immune function, muscle metabolism and neuronal functioning. J. Nutr. 2001;131:568S–579S. doi: 10.1093/jn/131.2.568S. [DOI] [PubMed] [Google Scholar]
- 12.Beard J. Iron deficiency alters brain development and functioning. J. Nutr. 2003;133(Suppl. 1):1468s–1472s. doi: 10.1093/jn/133.5.1468S. [DOI] [PubMed] [Google Scholar]
- 13.Moos T., Morgan E.H. A morphological study of the developmentally regulated transport of iron into the brain. Dev. Neurosci. 2002;24:99–105. doi: 10.1159/000065702. [DOI] [PubMed] [Google Scholar]
- 14.Siddappa A.M., Rao R., Long J.D., Widness J.A., Georgieff M.K. The assessment of newborn iron stores at birth: A review of the literature and standards for ferritin concentrations. Neonatology. 2007;92:73–82. doi: 10.1159/000100805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoyle C., Henderson D.J., Matthews D.J., Copp A.J. Transferrin and its receptor in the development of genetically determined neural tube defects in the mouse embryo. Dev. Dyn. 1996;207:35–46. doi: 10.1002/(SICI)1097-0177(199609)207:1<35::AID-AJA4>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 16.Matak P., Matak A., Moustafa S., Aryal D.K., Benner E.J., Wetsel W., Andrews N. Disrupted iron homeostasis causes dopaminergic neurodegeneration in mice. Proc. Natl. Acad. Sci. USA. 2016;113:3428–3435. doi: 10.1073/pnas.1519473113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jefferies W.A., Brandon M.R., Hunt S.V., Williams A.F., Gatter K.C., Mason D.Y. Transferrin receptor on endothelium of brain capillaries. Nature. 1984;312:162–163. doi: 10.1038/312162a0. [DOI] [PubMed] [Google Scholar]
- 18.Meyron-Holtz E.G., Cohen L.A., Fahoum L., Haimovich Y., Lifshitz L., Magid-Gold I., Stuemler T., Truman-Rosentsvit M. Ferritin polarization and iron transport across monolayer epithelial barriers in mammals. Front. Pharmacol. 2014;5:194. doi: 10.3389/fphar.2014.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan K., Jia X., Zhou M., Wang K., Conde J., He J., Tian J., Yan X. Ferritin nanocarrier traverses the blood brain barrier and kills glioma. ACS Nano. 2018;12:4105–4115. doi: 10.1021/acsnano.7b06969. [DOI] [PubMed] [Google Scholar]
- 20.Fiandra L., Mazzucchelli S., Truffi M., Bellini M., Sorrentino L., Corsi F. In vitro permeation of FITC-loaded ferritins across a rat blood-brain barrier: A model to study the delivery of nanoformulated molecules. J. Vis. Exp. 2016;114 doi: 10.3791/54279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiou B., Neal E.H., Bowman A.B., Lippmann E.S., Simpson I.A., Connor J.R. Endothelial cells are critical regulators of iron transport in a model of the human blood-brain barrier. J. Cereb. Blood Flow Metab. 2018 doi: 10.1177/0271678X18783372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skjørringe T., Burkhart A., Johnsen K.B., Moos T. Divalent metal transporter 1 (DMT1) in the brain: Implications for a role in iron transport at the blood-brain barrier, and neuronal and glial pathology. Front. Mol. Neurosci. 2015;8:19. doi: 10.3389/fnmol.2015.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burkhart A., Skjørringe T., Johnsen K.B., Siupka P., Thomsen L.B., Nielsen M.S., Thomsen L.L., Moos T. Expression of iron-related proteins at the neurovascular unit supports reduction and reoxidation of iron for transport through the blood-brain barrier. Mol. Neurobiol. 2016;53:7237–7253. doi: 10.1007/s12035-015-9582-7. [DOI] [PubMed] [Google Scholar]
- 24.Skjørringe T., Møller L.B., Moos T. Impairment of interrelated iron- and copper homeostatic mechanisms in brain contributes to the pathogenesis of neurodegenerative disorders. Front. Pharmacol. 2012;3:169. doi: 10.3389/fphar.2012.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Theil E.T. Ferritin: Structure, gene regulation, and cellular function in animals, plants, and microorganisms. Ann. Rev. Biochem. 1987;56:289–315. doi: 10.1146/annurev.bi.56.070187.001445. [DOI] [PubMed] [Google Scholar]
- 26.d’Estaintot B.L., Santambrogio P., Granier T., Gallois B., Chevalier J.M., Precigoux G., Levi S., Arosio P. Crystal structure and biochemical properties of the human mitochondrial ferritin and its mutant Ser144Ala. J. Mol. Biol. 2004;340:277–293. doi: 10.1016/j.jmb.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 27.Møllgård K., Dziegielewska K.M., Saunders N.R., Zakut H., Soreq H. Synthesis and localization of plasma proteins in the developing human brain. Integrity of the fetal blood-brain barrier to endogenous proteins of hepatic origin. Dev. Biol. 1988;128:207–221. doi: 10.1016/0012-1606(88)90283-7. [DOI] [PubMed] [Google Scholar]
- 28.Moos T., Oates P.S., Morgan E.H. Expression of the neuronal transferrin receptor is age dependent and susceptible to iron deficiency. J. Comp. Neurol. 1998;398:420–430. doi: 10.1002/(SICI)1096-9861(19980831)398:3<420::AID-CNE8>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 29.Knutson M.D. Non-transferrin-bound iron transporters. Free Radic. Biol. Med. 2019;133:101–111. doi: 10.1016/j.freeradbiomed.2018.10.413. [DOI] [PubMed] [Google Scholar]
- 30.Taylor E.M., Morgan E.H. Developmental changes in transferrin and iron uptake by the brain in the rat. Dev. Brain Res. 1990;55:35–42. doi: 10.1016/0165-3806(90)90103-6. [DOI] [PubMed] [Google Scholar]
- 31.Moos T., Morgan E.H. Restricted transport of anti-transferrin receptor antibody [OX26] through the blood-brain barrier in the rat. J. Neurochem. 2001;79:119–129. doi: 10.1046/j.1471-4159.2001.00541.x. [DOI] [PubMed] [Google Scholar]
- 32.Chen Q., Connor J.R., Beard J.L. Brain iron, transferrin and ferritin concentrations are altered in developing iron-deficient rats. J. Nutr. 1995;125:1529–1535. doi: 10.1093/jn/125.6.1529. [DOI] [PubMed] [Google Scholar]
- 33.Laskey J., Webb I., Schulman H.M., Ponka P. Evidence that transferrin supports cell proliferation by supplying iron for DNA synthesis. Exp. Cell Res. 1988;176:87–95. doi: 10.1016/0014-4827(88)90123-1. [DOI] [PubMed] [Google Scholar]
- 34.Taylor E.M., Crowe A., Morgan E.H. Transferrin and iron uptake by the brain: Effects of altered iron status. J. Neurochem. 1991;57:1584–1592. doi: 10.1111/j.1471-4159.1991.tb06355.x. [DOI] [PubMed] [Google Scholar]
- 35.Richard C., John M., Wrigglesworth H.B. Iron-dependent enzymes in mammalian systems. In: Ponka P., Schulman H.M., Woodworth R.C., Richter G.W., editors. Transport and Storage; Chapter: Iron-Dependent Enzymes in Mammalian Systems. CRC Press; Stockholm, Sweden: 1990. pp. 17–39. [Google Scholar]
- 36.Lozoff B., Georgieff M.K. Iron deficiency and brain development. Semin. Pediatr. Neurol. 2006;13:158–165. doi: 10.1016/j.spen.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 37.Georgieff M.K. Nutrition and the developing brain: Nutrient priorities and measurement. Am. J. Clin. Nutr. 2007;85:614–620. doi: 10.1093/ajcn/85.2.614S. [DOI] [PubMed] [Google Scholar]
- 38.Wright J.A., Chan A.K., Choy B.K., Hurta R.A., McClarty G.A., Tagger A.Y. Regulation and drug resistance mechanisms of mammalian ribonucleotide reductase, and the significance to DNA synthesis. Biochem. Cell Biol. 1990;68:1364–1371. doi: 10.1139/o90-199. [DOI] [PubMed] [Google Scholar]
- 39.Moos T., Skjorringe T., Thomsen L.L. Iron deficiency and iron treatment in the fetal developing brain—A pilot study introducing an experimental rat model. Reprod. Health. 2018;15(Suppl. 1):93. doi: 10.1186/s12978-018-0537-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oloyede O.B., Folayan A.T., Odutuga A.A. Effects of low-iron status and deficiency of essential fatty acids on some biochemical constituents of rat brain. Biochem. Int. 1992;27:913–922. [PubMed] [Google Scholar]
- 41.Möller H.E., Bossoni L., Connor J.R., Crichton R.R., Does M.D., Ward R.J., Zecca L., Zucca F.A., Ronen I. Iron, myelin, and the brain: Neuroimaging meets neurobiology. Trends Neurosci. 2019;42:384–401. doi: 10.1016/j.tins.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 42.Workman A.D., Charvet C.J., Clancy B., Darlington R.B., Finlay B.L. Modeling transformations of neurodevelopmental sequences across mammalian species. J. Neurosci. 2013;33:7368–7383. doi: 10.1523/JNEUROSCI.5746-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ganz T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood. 2003;102:783–788. doi: 10.1182/blood-2003-03-0672. [DOI] [PubMed] [Google Scholar]
- 44.Jorgenson L.A., Sun M., O’Connor M., Georgieff M.K. Fetal iron deficiency disrupts the maturation of synaptic function and efficacy in area CA1 of the developing rat hippocampus. Hippocampus. 2005;15:1094–1102. doi: 10.1002/hipo.20128. [DOI] [PubMed] [Google Scholar]
- 45.Eseh R., Zimmerberg B. Age-dependent effects of gestational and lactational iron deficiency on anxiety behavior in rats. Behav. Brain Res. 2005;164:214–221. doi: 10.1016/j.bbr.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 46.Felt B.T., Beard J.L., Schallert T., Shao J., Aldridge J.W., Connor J.R., Georgieff M.K., Lozoff B. Persistent neurochemical and behavioral abnormalities in adulthood despite early iron supplementation for perinatal iron deficiency anemia in rats. Behav. Brain Res. 2006;171:261–270. doi: 10.1016/j.bbr.2006.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Unger E.L., Paul T., Murray-Kolb L.E., Felt B., Jones B.C., Beard J.L. Early iron deficiency alters sensorimotor development and brain monoamines in rats. J. Nutr. 2007;137:118–124. doi: 10.1093/jn/137.1.118. [DOI] [PubMed] [Google Scholar]
- 48.Tran P.V., Carlson E.S., Fretham S.J., Georgieff M.K. Early-life iron deficiency anemia alters neurotrophic factor expression and hippocampal neuron differentiation in male rats. J. Nutr. 2008;138:2495–2501. doi: 10.3945/jn.108.091553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bourque S.L., Iqbal U., Reynolds J.N., Adams M.A., Nakatsu K. Perinatal iron deficiency affects locomotor behavior and water maze performance in adult male and female rats. J. Nutr. 2008;138:931–937. doi: 10.1093/jn/138.5.931. [DOI] [PubMed] [Google Scholar]
- 50.Wu L.L., Zhang L., Shao J., Qin Y.F., Yang R.W., Zhao Z.Y. Effect of perinatal iron deficiency on myelination and associated behaviors in rat pups. Behav. Brain Res. 2008;188:263–270. doi: 10.1016/j.bbr.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 51.McEchron M.D., Cheng A.Y., Liu H., Connor J.R., Gilmartin M.R. Perinatal nutritional iron deficiency permanently impairs hippocampus-dependent trace fear conditioning in rats. Nutr. Neurosci. 2005;8:195–206. doi: 10.1080/10284150500162952. [DOI] [PubMed] [Google Scholar]
- 52.Schmidt A.T., Ladwig E.K., Wobken J.D., Grove W.M., Georgieff M.K. Delayed alternation performance in rats following recovery from early iron deficiency. Physiol. Behav. 2010;101:503–508. doi: 10.1016/j.physbeh.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brunette K.E., Tranm P.V., Wobken J.D., Carlson E.S., Georgieff M.K. Gestational and neonatal iron deficiency alters apical dendrite structure of CA1 pyramidal neurons in adult rat hippocampus. Dev. Neurosci. 2010;32:238–248. doi: 10.1159/000314341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bastian T.W., Prohaska J.R., Georgieff M.K., Anderson G.W. Perinatal iron and copper deficiencies alter neonatal rat circulating and brain thyroid hormone concentrations. Endocrinology. 2010;151:4055–4065. doi: 10.1210/en.2010-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mihaila C., Schramm J., Strathmann F.G., Lee D.L., Gelein R.M., Luebke A.E., Mayer-Pröschel M. Identifying a window of vulnerability during fetal development in a maternal iron restriction model. PLoS ONE. 2011;6:e17483. doi: 10.1371/journal.pone.0017483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rao R., Tkac I., Schmidt A.T., Georgieff M.K. Fetal and neonatal iron deficiency causes volume loss and alters the neurochemical profile of the adult rat hippocampus. Nutr. Neurosci. 2011;14:59–65. doi: 10.1179/1476830511Y.0000000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Callahan L.S., Thibert K.A., Wobken J.D., Georgieff M.K. Early-life iron deficiency anemia alters the development and long-term expression of parvalbumin and perineuronal nets in the rat hippocampus. Dev. Neurosci. 2013;35:427–436. doi: 10.1159/000354178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Greminger A.R., Lee D.L., Shrager P., Mayer-Proschel M. Gestational iron deficiency differentially alters the structure and function of white and gray matter brain regions of developing rats. J. Nutr. 2014;144:1058–1066. doi: 10.3945/jn.113.187732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Greminger A.R., Mayer-Proschel M. Identifying the threshold of iron deficiency in the central nervous system of the rat by the auditory brainstem response. ASN Neuro. 2015;7 doi: 10.1177/1759091415569911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bastian T.W., Santarriaga S., Nguyen T.A., Prohaska J.R., Georgieff M.K., Anderson G.W. Fetal and neonatal iron deficiency but not copper deficiency increases vascular complexity in the developing rat brain. Nutr. Neurosci. 2015;18:365–375. doi: 10.1179/1476830515Y.0000000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hu X., Wang R., Shan Z., Dong Y., Zheng H., Jesse F.F., Rao E., Takahashi E., Li W., Teng W., et al. Perinatal iron deficiency-induced hypothyroxinemia impairs early brain development regardless of normal iron levels in the neonatal brain. Thyroid. 2016;26:891–900. doi: 10.1089/thy.2015.0293. [DOI] [PubMed] [Google Scholar]
- 62.Bastian T.W., von Hohenberg W.C., Mickelson D.J., Lanier L.M., Georgieff M.K. Iron deficiency impairs developing hippocampal neuron gene expression, energy metabolism, and dendrite complexity. Dev. Neurosci. 2016;38:264–276. doi: 10.1159/000448514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Horiquini-Barbosa E., Gibb R., Kolb B., Bray D., Lachat J.J. Tactile stimulation partially prevents neurodevelopmental changes in visual tract caused by early iron deficiency. Brain Res. 2017;1657:130–139. doi: 10.1016/j.brainres.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 64.Vieyra-Reyes P., Millan-Aldaco D., Palomero-Rivero M., Jimenez-Garces C., Hernandez-Gonzalez M., Caballero-Villarraso J. An iron-deficient diet during development induces oxidative stress in relation to age and gender in Wistar rats. J. Physiol. Biochem. 2017;73:99–110. doi: 10.1007/s13105-016-0529-x. [DOI] [PubMed] [Google Scholar]
- 65.Rosato-Siri M.V., Marziali L., Guitart M.E., Badaracco M.E., Puntel M., Pitossi F., Correale J., Pasquini J.M. Iron availability compromises not only oligodendrocytes but also astrocytes and microglial cells. Mol. Neurobiol. 2018;55:1068–1081. doi: 10.1007/s12035-016-0369-2. [DOI] [PubMed] [Google Scholar]
- 66.Hubbard A.C., Bandyopadhyay S., Wojczyk B.S., Spitalnik S.L., Hod E.A., Prestia K.A. Effect of dietary iron on fetal growth in pregnant mice. Comp. Med. 2013;63:127–135. [PMC free article] [PubMed] [Google Scholar]
- 67.Unger E.L., Earley C.J., Thomsen L.L., Jones B.C., Allen R.P. Effects of IV iron isomaltoside-1000 treatment on regional brain iron status in an iron-deficient animal. Neuroscience. 2013;246:179–185. doi: 10.1016/j.neuroscience.2013.04.049. [DOI] [PubMed] [Google Scholar]
- 68.Jougleux J.L., Rioux F.M., Church M.W., Fiset S., Jacques H., Surette M.E. Dietary LC-PUFA in iron-deficient anaemic pregnant and lactating guinea pigs induce minor defects in the offsprings’ auditory brainstem responses. Nutr. Neurosci. 2016;19:447–460. doi: 10.1179/1476830514Y.0000000140. [DOI] [PubMed] [Google Scholar]
- 69.Jougleux J.L., Rioux F.M., Church M.W., Fiset S., Surette M.E. Mild maternal iron deficiency anemia during pregnancy and lactation in guinea pigs causes abnormal auditory function in the offspring. J. Nutr. 2011;141:1390–1395. doi: 10.3945/jn.110.135715. [DOI] [PubMed] [Google Scholar]
- 70.Jougleux J.L., Rioux F.M., Church M.W., Fiset S., Surette M.E. Mild iron deficiency anaemia during pregnancy and lactation in guinea pigs alters amplitudes and auditory nerve velocity, but not brainstem transmission times in the offspring’s auditory brainstem response. Nutr. Neurosci. 2014;17:37–47. doi: 10.1179/1476830513Y.0000000067. [DOI] [PubMed] [Google Scholar]
- 71.Antonides A., van Laarhoven S., van der Staay F.J., Nordquist R.E. Non-anemic iron deficiency from birth to weaning does not impair growth or memory in piglets. Front. Behav. Neurosci. 2016;10:112. doi: 10.3389/fnbeh.2016.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mudd A.T., Fil J.E., Knight L.C., Dilger R.N. Dietary iron repletion following early-life dietary iron deficiency does not correct regional volumetric or diffusion tensor changes in the developing pig brain. Front. Neurol. 2017;8:735. doi: 10.3389/fneur.2017.00735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schachtschneider K.M., Liu Y., Rund L.A., Madsen O., Johnson R.W., Groenen M.A., Schook L.B. Impact of neonatal iron deficiency on hippocampal DNA methylation and gene transcription in a porcine biomedical model of cognitive development. BMC Genom. 2016;17:856. doi: 10.1186/s12864-016-3216-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leyshon B.J., Radlowski E.C., Mudd A.T., Steelman A.J., Johnson R.W. Postnatal iron deficiency alters brain development in piglets. J. Nutr. 2016;146:1420–1427. doi: 10.3945/jn.115.223636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rao R., Ennis K., Oz G., Lubach G.R., Georgieff M.K., Coe C.L. Metabolomic analysis of cerebrospinal fluid indicates iron deficiency compromises cerebral energy metabolism in the infant monkey. Neurochem. Res. 2013;38:573–580. doi: 10.1007/s11064-012-0950-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rao R., Ennis K., Lubach G.R., Lock E.F., Georgieff M.K., Coe C.L. Metabolomic analysis of CSF indicates brain metabolic impairment precedes hematological indices of anemia in the iron-deficient infant monkey. Nutr. Neurosci. 2018;21:40–48. doi: 10.1080/1028415X.2016.1217119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Patton S.M., Coe C.L., Lubach G.R., Connor J.R. Quantitative proteomic analyses of cerebrospinal fluid using iTRAQ in a primate model of iron deficiency anemia. Dev. Neurosci. 2012;34:354–365. doi: 10.1159/000341919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lubach G.R., Coe C.L. Selective impairment of cognitive performance in the young monkey following recovery from iron deficiency. J. Dev. Behav. Pediatr. JDBP. 2008;29:11–17. doi: 10.1097/DBP.0b013e31815f24a9. [DOI] [PubMed] [Google Scholar]
- 79.Golub M.S., Hogrefe C.E., Germann S.L., Capitanio J.P., Lozoff B. Behavioral consequences of developmental iron deficiency in infant rhesus monkeys. Neurotox. Teratol. 2006;28:3–17. doi: 10.1016/j.ntt.2005.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guitart M.E., Vence M., Correale J., Pasquini J.M., Rosato-Siri M.V. Ontogenetic oligodendrocyte maturation through gestational iron deprivation: The road not taken. Glia. 2019;67:1760–1774. doi: 10.1002/glia.23647. [DOI] [PubMed] [Google Scholar]
- 81.Szudzik M., Starzyński R.R., Jończy A., Mazgaj R., Lenartowicz M., Lipiński P. Iron supplementation in suckling piglets: An ostensibly easy therapy of neonatal iron deficiency anemia. Pharmaceuticals. 2018;11:128. doi: 10.3390/ph11040128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lozoff B. Iron deficiency and child development. Food Nutr. Bull. 2007;28(Suppl. 4):S560–S671. doi: 10.1177/15648265070284S409. [DOI] [PubMed] [Google Scholar]
- 83.Basu S., Kumar D., Anupurba S., Verma A., Kumar A. Effect of maternal iron deficiency anemia on fetal neural development. J. Perinatol. 2018;38:233–239. doi: 10.1038/s41372-017-0023-5. [DOI] [PubMed] [Google Scholar]
- 84.Berglund S.K., Torres-Espinola F.J., Garcia-Valdes L., Segura M., Martinez-Zaldivar C., Padilla C., Rueda R., Pérez García M., McArdle H.J., Campoy C. The impacts of maternal iron deficiency and being overweight during pregnancy on neurodevelopment of the offspring. Br. J. Nutr. 2017;118:533–540. doi: 10.1017/S0007114517002410. [DOI] [PubMed] [Google Scholar]
- 85.Carpenter K.L.H., Li W., Wei H., Wu B., Xiao X., Liu C., Worley G., Egger H.L. Magnetic susceptibility of brain iron is associated with childhood spatial IQ. Neuro Image. 2016;132:167–174. doi: 10.1016/j.neuroimage.2016.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Algarin C., Karunakaran K.D., Reyes S., Morales C., Lozoff B., Peirano P., Biswal B. Differences on brain connectivity in adulthood are present in subjects with iron deficiency anemia in infancy. Front. Aging Neurosci. 2017;9:54. doi: 10.3389/fnagi.2017.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Insel B.J., Schaefer C.A., McKeague I.W., Susser E.S., Brown A.S. Maternal iron deficiency and the risk of schizophrenia in offspring. Arch. Gen. Psychiatry. 2008;65:1136–1144. doi: 10.1001/archpsyc.65.10.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen M.H., Su T.P., Chen Y.S., Hsu J.W., Huang K.L., Chang W.H., Chen T.J., Bai Y.M. Association between psychiatric disorders and iron deficiency anemia among children and adolescents: A nationwide population-based study. BMC Psychiatry. 2013;13:161. doi: 10.1186/1471-244X-13-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lane R., Kessler R., Buckley A.W., Rodriguez A., Farmer C., Thurm A., Swedo S., Felt B. Evaluation of periodic limb movements in sleep and iron status in children with autism. Pediatr. Neurol. 2015;53:343–349. doi: 10.1016/j.pediatrneurol.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Berglund S.K., Westrup B., Haraldsson E., Engstrom B., Domellof M. Effects of iron supplementation on auditory brainstem response in marginally LBW infants. Pediatr. Res. 2011;70:601–606. doi: 10.1203/PDR.0b013e3182320cd0. [DOI] [PubMed] [Google Scholar]
- 91.ElAlfy M.S., El-Farrash R.A., Taha H.M., Ismail E.A., Mokhtar N.A. Auditory brainstem response in full-term neonates born to mothers with iron deficiency anemia: Relation to disease severity. J. Matern.-Fetal Neonatal Med. 2018;4:1–8. doi: 10.1080/14767058.2018.1533940. [DOI] [PubMed] [Google Scholar]
- 92.Sundagumaran H., Seethapathy J. Auditory brainstem response in infants with iron deficiency anemia. Int. J. Pediatr. Otorhinolaryngol. 2019;117:78–81. doi: 10.1016/j.ijporl.2018.11.017. [DOI] [PubMed] [Google Scholar]
- 93.Choudhury V., Amin S.B., Agarwal A., Srivastava L.M., Soni A., Saluja S. Latent iron deficiency at birth influences auditory neural maturation in late preterm and term infants. Am. J. Clin. Nutr. 2015;102:1030–1034. doi: 10.3945/ajcn.115.113084. [DOI] [PubMed] [Google Scholar]
- 94.Monga M., Walia V., Gandhi A., Chandra J., Sharma S. Effect of iron deficiency anemia on visual evoked potential of growing children. Brain Dev. 2010;32:213–216. doi: 10.1016/j.braindev.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 95.Lozoff B., Armony-Sivan R., Kaciroti N., Jing Y., Golub M., Jacobson S.W. Eye-blinking rates are slower in infants with iron-deficiency anemia than in nonanemic iron-deficient or iron-sufficient infants. J. Nutr. 2010;140:1057–1061. doi: 10.3945/jn.110.120964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Amony-Sivan R., Zhu B., Clark K.M., Richards B., Ji C., Kaciroti N., Shao J., Lozoff B. Iron deficiency (ID) at both birth and 9 months predicts right frontal EEG asymmetry in infancy. Dev. Psychobiol. 2016;58:462–470. doi: 10.1002/dev.21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Otero G.A., Pliego-Rivero F.B., Porcayo-Mercado R., Mendieta-Alcantara G. Working memory impairment and recovery in iron deficient children. Clin. Neurophysiol. 2008;119:1739–1746. doi: 10.1016/j.clinph.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 98.Lukowski A.F., Koss M., Burden M.J., Jonides J., Nelson C.A., Kaciroti N., Jimenez E., Lozoff B. Iron deficiency in infancy and neurocognitive functioning at 19 years: Evidence of long-term deficits in executive function and recognition memory. Nutr. Neurosci. 2010;13:54–70. doi: 10.1179/147683010X12611460763689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Congdon E.L., Westerlund A., Algarin C.R., Peirano P.D., Gregas M., Lozoff B., Nelson C.A. Iron deficiency in infancy is associated with altered neural correlates of recognition memory at 10 years. J. Pediatr. 2012;160:1027–1033. doi: 10.1016/j.jpeds.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lozoff B., Clark K.M., Jing Y., Armony-Sivan R., Angelilli M.L., Jacobson S.W. Dose-response relationships between iron deficiency with or without anemia and infant social-emotional behavior. J. Pediatr. 2008;152:696–702. doi: 10.1016/j.jpeds.2007.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Parsons A.G., Zhou S.J., Spurrier N.J., Makrides M. Effect of iron supplementation during pregnancy on the behaviour of children at early school age: Long-term follow-up of a randomised controlled trial. Br. J. Nutr. 2008;99:1133–1139. doi: 10.1017/S0007114507853359. [DOI] [PubMed] [Google Scholar]
- 102.Qubty W., Renaud D.L. Cognitive impairment associated with low ferritin responsive to iron supplementation. Pediatr. Neurol. 2014;51:831–833. doi: 10.1016/j.pediatrneurol.2014.08.035. [DOI] [PubMed] [Google Scholar]
- 103.Ji X., Cui N., Liu J. Neurocognitive function is associated with serum iron status in early adolescents. Biol. Res. Nurs. 2017;19:269–277. doi: 10.1177/1099800417690828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mireku M.O., Davidson L.L., Boivin M.J., Zoumenou R., Massougbodji A., Cot M., Bodeau-Livinec F. Prenatal iron deficiency, neonatal ferritin, and infant cognitive function. Pediatrics. 2016;138 doi: 10.1542/peds.2016-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Doom J.R., Georgieff M.K., Gunnar M.R. Institutional care and iron deficiency increase ADHD symptomology and lower IQ 2.5–5 years post-adoption. Dev. Sci. 2015;18:484–494. doi: 10.1111/desc.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Islam K., Seth S., Saha S., Roy A., Das R., Datta A.K. A study on association of iron deficiency with attention deficit hyperactivity disorder in a tertiary care center. Ind. J. Psychiatry. 2018;60:131–134. doi: 10.4103/psychiatry.IndianJPsychiatry_197_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Holm C., Thomsen L.L., Norgaard A., Langhoff-Roos J. Single-dose intravenous iron infusion or oral iron for treatment of fatigue after postpartum haemorrhage: A randomized controlled trial. Vox Sang. 2017;112:219–228. doi: 10.1111/vox.12477. [DOI] [PubMed] [Google Scholar]
- 108.Markova V., Norgaard A., Jørgensen K.J., Langhoff-Roos J. Treatment for women with postpartum iron deficiency anaemia. Cochrane Database Syst. Rev. 2015;13:CD010861. doi: 10.1002/14651858.CD010861.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]