Significance
Mast cells are key effector cells of the innate immune system and have been shown to orchestrate host–defense responses against a variety of pathogens. In this report, we demonstrate the importance of mast cells for the control of bacterial wound infections, by which mast cell-derived IL-6 turns keratinocytes into better bacterial killers.
Keywords: mast cells, skin wound infection, bacterial infection, innate immunity, pseudomonas aeruginosa
Abstract
Skin wound infections are a significant health problem, and antibiotic resistance is on the rise. Mast cells (MCs) have been shown to contribute to host–defense responses in certain bacterial infections, but their role in skin wound superinfection is unknown. We subjected 2 MC-deficient mouse strains to Pseudomonas aeruginosa skin wound infection and found significantly delayed wound closure in infected skin wounds. This delay was associated with impaired bacterial clearance in the absence of MCs. Engraftment of MCs restored both bacterial clearance and wound closure. Bacterial killing was dependent on IL-6 released from MCs, and engraftment with IL-6–deficient MCs failed to control wound infection. Treatment with recombinant IL-6 enhanced bacterial killing and resulted in the control of wound infection and normal wound healing in vivo. Taken together, our results demonstrate a defense mechanism for boosting host innate immune responses, namely effects of MC-derived IL-6 on antimicrobial functions of keratinocytes.
Bacterial skin wound infection is a serious health risk in which a wound fails to heal, leading to increased patient distress, prolonged hospital stays, and increased mortality and health care costs (1, 2). Pseudomonas aeruginosa, a Gram-negative bacterium, is the second most common cause of nosocomial skin wound infections and is associated with high morbidity and mortality (3). P. aeruginosa presents a rising therapeutic challenge due to its ability to form biofilms and increasing antibiotic resistance (4). Therefore, effective treatment, combined with rapid wound closure, is essential to restrain such infections.
Mast cells (MCs) are key effector cells of the innate immune system (5, 6). MCs have been shown to be involved in the defense of bacterial infections by releasing soluble factors that recruit or activate immune cells such as neutrophils (7), dendritic cells, and T cells. They also can contribute to antibacterial defense by acting as phagocytes, releasing antimicrobial peptides, and forming extracellular traps (8). By contrast, the role of MCs in skin wound healing is controversial. Whereas some studies indicate that MCs are essential in certain models of wound healing (9–12), others found that skin wound closure may not be affected by the absence of MCs (13–15).
However, there have been no reports investigating the potential roles of MCs during skin wound infection. Therefore, we addressed this clinically relevant topic and identified, and characterized in detail, a mechanism by which MCs can control bacterial skin wound infections and promote wound healing.
Results
MC-Deficient Mice but Not WT Mice Exhibit Impaired Skin Wound Healing in Response to Skin Wound Infection with P. aeruginosa.
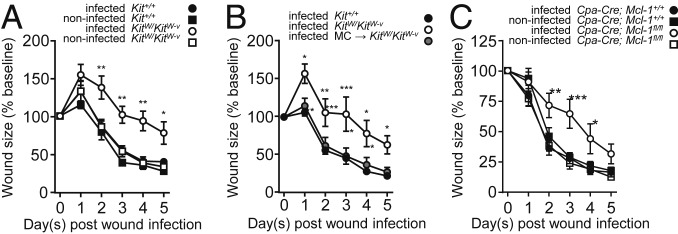
To investigate the role of MCs in a physiological model of skin wound infection, skin wounds of MC-deficient and WT mice were inoculated with P. aeruginosa mimicking a naturally occurring infection route. Wound closure was evaluated by lesion size over time. As early as 1 d after skin wound infection, MC-deficient KitW/KitW-v mice exhibited increased wound size compared to infected WT mice, indicative of impaired wound closure (Fig. 1A). This difference in wound size between KitW/KitW-v and WT mice was most pronounced between day 2 and day 4 after wound infection with an average of 111 ± 9% of the initial wound size in MC-deficient mice as compared with 61 ± 5% in WT mice (Fig. 1A). By contrast, wound closure in noninfected mice did not differ in size between the 2 genotypes, indicating that MCs are dispensable for normal closure of noninfected wounds in this model.
Fig. 1.
MCs are required for normal healing of P. aeruginosa-infected wounds. MC-deficient mice show delayed wound closure after local P. aeruginosa skin wound infection. (A–C) Wound closure kinetics of infected or noninfected PBS-treated skin wounds of Kit+/+ (n = 9 PBS, n = 7 infected) mice and KitW/KitW-v mice (n = 5 PBS, n = 5 infected) (A); Kit+/+ mice (n = 9) , KitW/KitW-v mice (n = 7), and KitW/KitW-v mice engrafted intradermally with bone marrow-derived cultured MCs (MCs → KitW/KitW-v) (n = 8) (B), and MC-deficient Cpa3-Cre; Mcl-1fl/fl mice (n = 6 PBS, n = 9 infected) compared to Cpa3-Cre; Mcl-1+/+ mice (n = 7 PBS, n = 8 infected) (C). Data are expressed as mean ± SEM and pooled from 3 independent experiments each, analyzed using 2-way repeated measure ANOVA followed by Bonferroni post hoc test. *P < 0.05; **P < 0.01; ***P < 0.001; n.s., not significant.
To determine whether the impaired wound healing was only associated with the absence of MCs in KitW/KitW-v mice, some KitW/KitW-v mice were reconstituted with bone marrow-derived cultured MCs (BMCMCs). Local adoptive transfer of BMCMCs to the skin of KitWKitW-v mice 4 wk before wounding restored MC numbers (SI Appendix, Fig. S6A) and normalized wound closure of infected skin wounds (Fig. 1B). The wound closure kinetics of KitW/KitW-v + BMCMCs mice closely resembled those of WT mice (Fig. 1B). This demonstrates that MCs are required for normal healing of skin wounds infected with P. aeruginosa.
We further confirmed our finding that the MC deficiency, but not MC-independent defects of the KitW/KitW-v mouse model, was responsible for the delayed wound closure by testing a non-c-Kit–dependent model of MC deficiency, the Cpa3-Cre; Mcl-1fl/fl mouse (Fig. 1C and SI Appendix, Fig. S6B). Upon skin wound infection with P. aeruginosa, MC-deficient Cpa3-Cre; Mcl-1fl/fl mice exhibited larger wounds compared with WT mice, peaking at day 3 with a difference of 40 ± 13% between the genotypes. By contrast, no significant differences in wound closure kinetics was noted in the sham-infected mice.
MC-Deficient Mice Show Higher Bacterial Counts Compared to WT Mice in Response to Topical Wound Infection with P. aeruginosa.
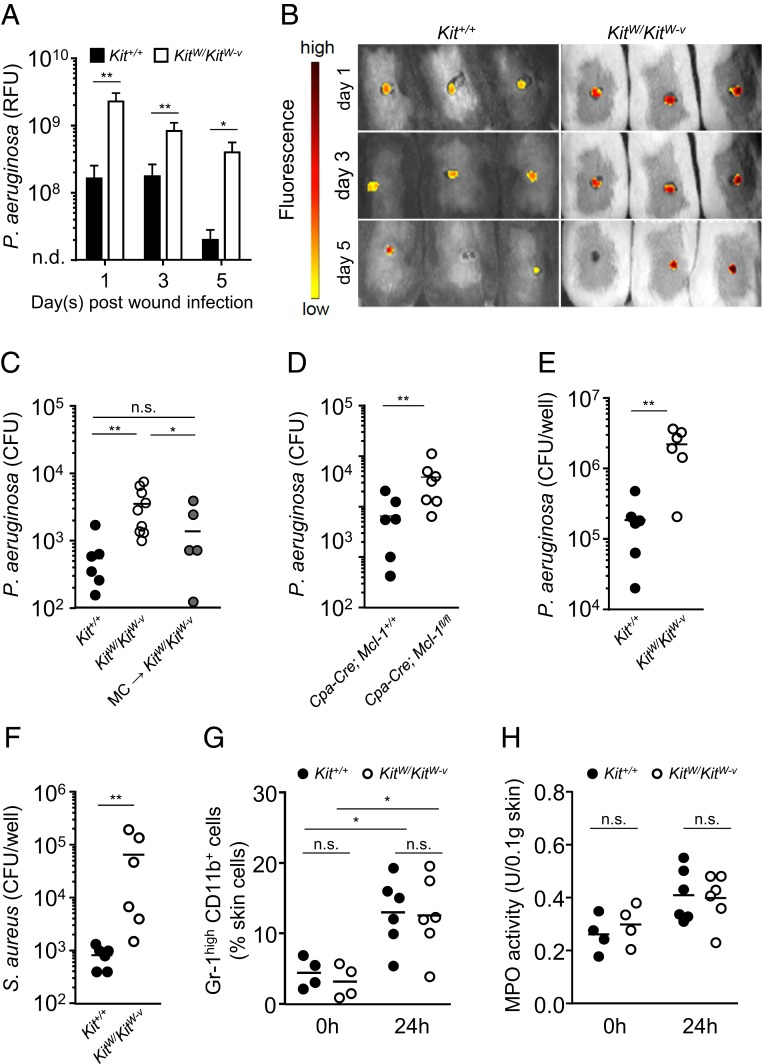
To identify the mechanisms of impaired healing of P. aeruginosa-infected skin wounds, we analyzed the skin bacterial load during the course of infection. Using in vivo live imaging of fluorescently labeled bacteria, the bacterial load within skin wounds was monitored over 5 d following wound infection. KitWKitW-v mice exhibited a 14-fold higher bacterial load in skin wounds than the corresponding WT mice on day 1 after skin wound infection (Fig. 2 A and B). Although bacterial numbers decreased in both genotypes over time, KitW/KitW-v mice showed a 20-fold higher bacterial load at day 5. To ascertain whether the increased bacterial load consisted of viable bacteria, we conducted plate counts at day 1 after skin wound infection. Lysates of infected skin wounds of KitW/KitW-v mice contained 7 times more P. aeruginosa compared with the WT mice. To assess whether this antibacterial effect required MCs, intradermal reconstitution of KitW/KitW-v with MCs was carried out, which resulted in a reduced bacterial load to approximately 2-fold WT levels (Fig. 2C). In accord with these findings, MC-deficient Cpa3-Cre; Mcl-1fl/fl mice exhibited a 5-fold increase in bacterial load compared with WT mice (Fig. 2D).
Fig. 2.
MCs control P. aeruginosa numbers in P. aeruginosa-infected skin wounds. MC-deficient mice exhibit reduced bacterial clearance after skin wound infection in vivo and ex vivo. (A) Bacterial load measured as relative fluorescence units (RFU) during skin wound infection of Kit+/+ mice (n = 9) and KitW/KitW-v mice (n = 9) assessed by in vivo live imaging using fluorescent bacteria-binding substrate. Data are shown as means ± SEM with pooled from 3 independent experiments analyzed by Mann–Whitney U test. (B) Representative images of infected wounds assessed by luminescence. KitW/KitW-v mice exhibit a higher bacterial load (red) than Kit+/+ mice (yellow). (C and D) Colony forming units (CFUs) of skin homogenates normalized to 0.1 g of skin after 24 h of skin wound infection derived from Kit+/+ mice (n = 7), KitW/KitW-v mice (n = 9), and KitW/KitW-v mice engrafted intradermally with bone marrow-derived MCs (MCs → KitW/KitW-v) (n = 6) (C), Cpa3-Cre; Mcl-1fl/fl mice (n = 7) and Cpa3-Cre; Mcl-1+/+ mice (n = 7) (D). Data pooled from 3 independent experiments analyzed by Mann–Whitney U test. (E and F) CFUs of supernatant derived from Kit+/+ (n = 6) and KitW/KitW-v (n = 6) skin biopsies after 3 h ex vivo infection with P. aeruginosa (E) and S. aureus (F). Data pooled from 3 independent experiments analyzed by Mann–Whitney U test. (G) Neutrophil numbers (Gr-1high, CD11b+) in infected skin wounds of Kit+/+ mice (n = 6) and KitW/KitW-v mice (n = 6) by FACS analysis. Data are expressed as mean per mouse and pooled from 3 independent experiments analyzed by Mann–Whitney U test. (H) Myeloperoxidase activity of skin homogenates normalized to 0.1 g of skin from Kit+/+ mice (n = 6) and KitW/KitW-v mice (n = 6). Data are expressed as mean per mouse and pooled from 2 independent experiments analyzed by Mann–Whitney U test. *P < 0.05; **P < 0.01; ***P < 0.001; n.s., not significant; n.d., not detectable.
Next, we analyzed whether this antibacterial effect of MCs is dependent on immune cell recruitment. For this purpose, 4-mm skin biopsies derived from KitW/KitW-v vs. WT mice were infected ex vivo with P. aeruginosa. Infection of skin explants derived from KitWKitW-v mice showed 12 times reduced bacterial clearance compared to WT skin explants (Fig. 2E), suggesting that MC-mediated bacterial clearance in this setting is not dependent on immune cell recruitment. Impaired bacterial elimination in the absence of MCs was also detected for Gram-positive Staphylococcus aureus (Fig. 2F). Supporting the evidence that reduced neutrophil recruitment in KitW/KitW-v mice was not the underlying cause of the impaired bacterial clearance, we analyzed neutrophil numbers and myeloperoxidase (MPO) activity within skin wounds upon infection. In both MC-deficient and WT mice, neutrophil numbers as well as MPO activity (a marker of neutrophil activity) were increased but not different between the genotypes (Fig. 2 G and H). These findings point toward an important role of MCs in bacterial clearance that appears to be independent of immune cell recruitment and the type of bacteria.
Antimicrobial Response to P. aeruginosa Infection Depends on MC–Keratinocyte Interaction.
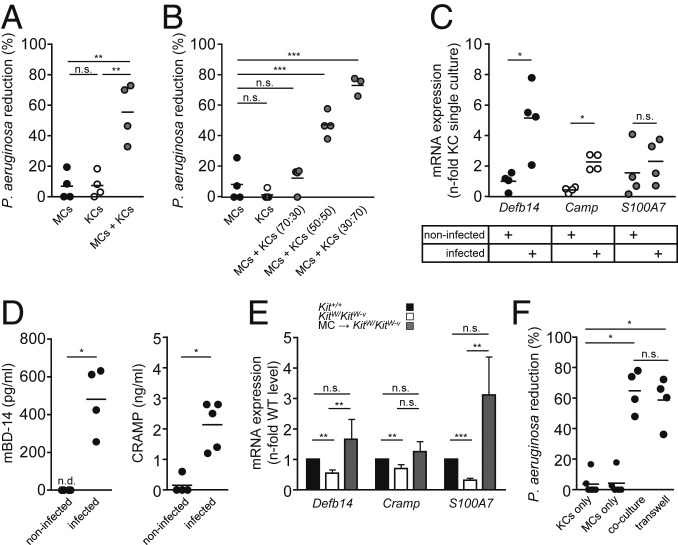
As our results pointed to a MC-mediated antibacterial effect, we investigated the direct antibacterial effects of MCs in vitro. However, MCs infected with P. aeruginosa were not able to reduce numbers of bacteria (Fig. 3A). Therefore, we hypothesized that MCs might stimulate epidermal cells to elicit the antimicrobial activity. We cocultured mouse MCs together with mouse keratinocytes (KCs) and analyzed the effects on P. aeruginosa. Upon their infection with P. aeruginosa, an increase of 56 ± 10% in the antibacterial effect within cocultures of MCs and KCs was observed compared with single cultures of MCs or KCs (Fig. 3A). This antibacterial effect was amplified by increasing the KC/MC ratio of the coculture (Fig. 3B), supporting the conclusion that the antimicrobial effects were KC-derived.
Fig. 3.
P. aeruginosa reduction involves MC–KC interaction. Reduction of P. aeruginosa numbers in vitro requires MC–KC interaction. (A and B) P. aeruginosa CFU from cell culture supernatant after 3 h in vitro infection of either KCs or MCs alone compared with MC-KC cocultures (A) or MC-KC cocultures with different cell ratios (B). Each data point represents an individual experiment, data are expressed as means, pooled from 4 independent experiments and analyzed using one-way ANOVA followed by Tukey’s post hoc test. (C) mRNA of AMPs expressed in KCs during coculture with MCs with or without infection with P. aeruginosa. Values are normalized to those of the internal control gene beta-actin and represented as fold change relative to that of noninfected KC controls, which was converted to 1. Each data point represents an individual experiment, data are expressed as means, pooled from 4 independent experiments and analyzed by Student’s t test. (D) Protein levels of secreted AMPs measured in supernatants of infected or noninfected MC-KC cocultures. Each data point represents an individual experiment, data are expressed as means, pooled from 4 independent experiments and analyzed by Student’s t test (mBD-14) and Mann–Whitney U test for CRAMP. (E) AMP expression analysis of skin wound samples 24 h after skin wound infection in Kit+/+ mice, KitW/KitW-v (n = 7) mice, and KitW/KitW-v mice, which had been intradermally engrafted with MCs (MCs → KitW/KitW-v) (n = 5). Values are normalized to those of the internal control gene beta-actin and represented as fold change relative to Kit+/+ mice, which was converted to 1. (F) P. aeruginosa CFU from culture supernatant of MC-KC cocultures and transwell cultures. Each data point represents an individual experiment, data are expressed as means, pooled from 4 independent experiments and analyzed by Mann–Whitney U test. Data are shown as means ± SEM and analyzed with Mann–Whitney U test. *P < 0.05; **P < 0.01; ***P < 0.001; n.s., not significant; n.d., not detectable.
Since KCs are a major source of antimicrobial peptides (AMPs), we analyzed the production and release of AMPs by KCs. Upon infection of the coculture, KCs increased expression of the mBD-14 gene (Defb14) 4-fold as well as CRAMP (Camp) 2-fold. The expression level of Psoriasin (S100A7), however, remained unchanged (Fig. 3C). To confirm that these AMPs are in fact secreted, we measured protein levels of mBD-14 and CRAMP. Analysis of the supernatants of previously infected MC-KCs cocultures by enzyme-linked immunosorbent assay (ELISA) allowed for the detection of mBD-14 and CRAMP protein. While mBD-14 showed a moderate increase in protein levels (of 385 ± 118 pg/mL) a significant release of CRAMP (2,140 ± 348 pg/mL) was observed (Fig. 3D). Moreover, we analyzed AMP expression after skin wound infection in the MC-deficient mouse model. Upon P. aeruginosa skin wound infection, MC-deficient KitW/KitW-v mice showed consistently lower levels of mBD-14, CRAMP, and Psoriasin compared to WT or MC reconstituted mice at day 1 after skin wound infection (Fig. 3E).
We next determined whether the collaborative antibacterial activity of MCs and KCs required direct cell contact or was mediated by a soluble factor. We used transwell inserts to separate MCs and KCs and found that the transwell inserts did not hinder the bacterial reduction ability of the coculture. We concluded that direct cell-cell contact between the 2 cell types was not essential for the observed antibacterial effect (Fig. 3F). These findings suggest that MCs protect mice from P. aeruginosa skin wound infection by secreting mediators that can induce antibacterial effects of KCs.
MC-Derived IL-6 Is Required for a KC Antimicrobial Response.
The antibacterial effect of KCs upon infection with P. aeruginosa only became apparent when they were cocultured with MCs. To identify the MC mediators involved in the induction of antibacterial KC effects, we analyzed the supernatants of infected MC-KC cocultures and found high levels of interleukin (IL)-6 (SI Appendix, Fig. S1A). To identify the source of IL-6 secretion, we performed expression analysis of IL-6 mRNA transcripts from MCs and KCs derived from infected cocultures. IL-6 mRNA expression levels were 36-fold higher in MCs as compared with KCs, suggesting that MCs, but not KCs, predominantly produce and release IL-6 when cocultures are infected with P. aeruginosa (SI Appendix, Fig. S1B). Notably, IL-6 production by MCs was dependent on coculture with KCs, indicating that KC-derived signals are required to induce IL-6 production by MCs (SI Appendix, Fig. S1B). Further characterization of such KC-derived signals suggests that the release of IL-1 family members by KCs infected with PA is able to stimulate IL-6 production in MCs by a MyD88-dependent pathway (SI Appendix, Fig. S2 A–F), thereby augmenting bacterial clearance (SI Appendix, Fig. S2G). Although IL-33 stimulation of MCs results in high levels of IL-6 (SI Appendix, Fig. S2E), bacterial clearance and the release of IL-6 by MCs seems to be independent from their expression of the IL-33/ST2 receptor (SI Appendix, Fig. S2 H and J).
Next, we investigated the importance of MC-derived IL-6 for the collaborative antimicrobial effect of MC-KC cocultures using MCs derived from bone marrow of IL-6–deficient mice. Cocultures of ll6−/− MCs and KCs, in contrast to cocultures with WT MCs, were unable to reduce numbers of P. aeruginosa in MC-KC cocultures (SI Appendix, Fig. S1C). Moreover, we analyzed the AMP mRNA expression levels of KCs in MC-KC cocultures containing Il6−/− MCs and found no up-regulation of AMP expression in KCs upon infection with P. aeruginosa compared with cocultures containing WT MCs (SI Appendix, Fig. S1D).
To provide proof of concept that IL-6 is required for an optimal defense response from KCs upon infection, we stimulated KCs with recombinant IL-6 (recIL-6). Pretreatment of KCs with recIL-6 induced a significant bacterial reduction in vitro compared with vehicle treatment in a concentration-dependent manner with 50 ng of recIL-6 leading to an almost complete clearance of P. aeruginosa (87 ± 6%) (SI Appendix, Fig. S1E). This was in line with the finding that KCs showed a strong induction of AMPs mRNA levels upon recIL-6 stimulation. Moreover, mRNA levels of Defb14 as well as CRAMP mRNA were induced by 5-fold and 6-fold compared with vehicle-treated KCs (SI Appendix, Fig. S1F), respectively.
MC-Derived IL-6 Protects from Skin Wound Infections with P. aeruginosa.
As we had demonstrated that the antimicrobial capacity of KCs was strongly dependent on MC-derived IL-6 stimulation in vitro, we hypothesized that the antimicrobial effects observed in skin wound infections are also mediated by MC-derived IL-6 in vivo. We analyzed skin wounds in MC-deficient KitW/KitW-v and WT mice for their content of IL-6 upon P. aeruginosa infection and found that MC-deficient KitW/KitW-v mice exhibit significantly lower IL-6 levels compared with WT mice (SI Appendix, Fig. S3A). Adoptive transfer of WT MCs restored IL-6 levels in P. aeruginosa-infected skin wounds of KitW/KitW-v mice to WT levels. In contrast, reconstitution with Il6−/− MCs resulted in 4-fold lower levels of IL-6 within the P. aeruginosa-infected skin wounds, comparable to the IL-6 levels detected in MC-deficient KitW/KitW-v mice (SI Appendix, Fig. S3A). This indicated that the increase of IL-6 levels in P. aeruginosa-infected skin wounds depends on IL-6 released by MCs.
We next determined the bacterial load in P. aeruginosa-infected wounds of MC-deficient mice and MC-deficient mice that had received IL-6–deficient or WT MCs. Compared to MC-deficient KitW/KitW-v mice, WT mice and MC-deficient KitW/KitW-v mice reconstituted with WT MCs exhibited a similar reduction in bacterial numbers (SI Appendix, Fig. S3B). In contrast, adoptive transfer of MCs lacking IL-6 resulted in a 14-fold higher bacterial burden within the skin wounds compared with WT mice (SI Appendix, Fig. S3B). The bacterial load of MC-deficient KitW/KitW-v reconstituted with Il6−/− MCs was comparable to that in MC-deficient KitW/KitW-v mice (SI Appendix, Fig. S3B).
Furthermore, we investigated the impact of MC-derived IL-6 on skin wound closure upon P. aeruginosa infection. In contrast to the adoptive transfer of WT MCs, MC-deficient KitW/KitW-v mice reconstituted with Il6−/− MCs exhibited larger wounds compared to WT mice. Reconstitution with Il6−/− MCs did not lead to an improvement in wound healing compared with nonreconstituted MC-deficient mice at any time point during wound healing (SI Appendix, Fig. S3C). To further support the importance of MC-derived IL-6 during P. aeruginosa skin wound infection, we generated a MC-specific Il-6 knockout mouse model (Cpa-Cre; Il-6fl/fl). Subjecting these mice to skin wound infection resulted in significantly impaired wound healing, which confirmed, in mice with normal c-Kit, the relevance of MC-derived IL-6 for normal healing of infected skin wounds (SI Appendix, Fig. S3D).
Recombinant IL-6 Treatment Stimulates the Antibacterial Response in Mice and Humans.
We next tested whether the topical application of recombinant IL-6 (recIL-6) can compensate for MC deficiency in the promotion of antibacterial responses to wound infection with P. aeruginosa. We showed that pretreatment with IL-6 improved wound closure of infected skin wounds in MC-deficient KitW/KitW-v mice and normalized wound healing to WT levels (SI Appendix, Fig. S4A). By contrast, vehicle pretreatment of MC-deficient mice did not improve wound closure of infected skin wounds. However, IL-6 pretreatment of WT mice did not further improve the closure of infected skin wounds. Moreover, pretreatment of MC-deficient mice with recIL-6 also significantly reduced the bacterial load of skin wounds, as compared with vehicle-treated littermates (SI Appendix, Fig. S4B). RecIL-6 pretreatment normalized the bacterial load of skin wounds in KitW/KitW-v mice to 1.4-fold that of WT mice. Interestingly, recIL-6 treatment also reduced the bacterial load of skin wounds of MC-competent WT mice 4-fold compared with vehicle-treated WT mice.
Since pretreatment with recIL-6 significantly improved wound healing of MC-deficient mice, and also reduced bacteria within skin wounds of both MC-deficient and WT mice, we aimed to translate these findings into the human system. Therefore, we performed in vitro infection experiments using human KCs. We observed a 13-fold increase in the reduction of P. aeruginosa after recIL-6 pretreatment compared to vehicle-treated controls (SI Appendix, Fig. S4C). Furthermore, we could show that IL-6 pretreatment also improves host–defense responses in human skin explants as compared with vehicle-treated controls (SI Appendix, Fig. S4D). Taken together, these data demonstrate that recIL-6 pretreatment can induce antibacterial effects in mice and human KCs, as well as in human skin explants.
Discussion
In this study, we demonstrate a previously unreported role of MCs in the control of skin wound infection and identify MC-derived IL-6 as a crucial driver of an antibacterial innate immune response in infected skin wounds (SI Appendix, Fig. S5). IL-6 has been previously implicated in wound healing, and increased IL-6 levels have been reported in different models of mouse and human skin injury (16–19). The role of MCs in defense against pathogens also has been well established in several infection models (8). In response to various pathogens, MCs have been shown to exhibit an important function in facilitating the recruitment of other immune cells, i.e., neutrophils, macrophages, and T cells, to the site of infection (20–24). Interestingly, in the model of bacterial skin wound infection reported herein, the control of bacterial burden appeared to be independent from the influx of neutrophils (Fig. 2 G and H) but was mediated by MC-derived IL-6–driven production and release of antimicrobial peptides by KCs (Fig. 3 C–E). In addition, this effect was not limited to a specific pathogen, since both Gram-negative P. aeruginosa and Gram-positive S. aureus provoked a similar response (Fig. 2 E and F).
In a recent study, MC-derived IL-6 was also found to augment host defense to herpes simplex virus (HSV) infection. HSV was not able to directly induce the production of IL-6 by MCs but did so by an IL-33–dependent mechanism, most likely initiated by infected KCs. Blocking the IL-33 receptor T1/ST2 on BMCMCs significantly reduced IL-6 production by BMCMCs (25). Notably, we also observed an early increase of IL-33 in MC-KC cocultures infected with P. aeruginosa. However, IL-6 release was not reduced in T1/ST2−/− MCs stimulated with the coculture supernatant. This may be explained by the fact that in addition to IL-33, other members of the IL-1 family, i.e., IL-1α/β and IL-36, were simultaneously released, suggesting a biologically redundant response compensating for the lack of IL-33 signaling via the T1/ST2 receptor (SI Appendix, Fig. S2).
The relevance of MCs in wound healing is still regarded as controversial. Our group has previously shown that the healing of skin wounds is delayed in the absence of MCs (10). As demonstrated by the results of the current study, this effect was likely due to the control of the bacterial burden on skin wounds, since we found that the healing of noninfected wounds was not delayed in either Kit-dependent or Kit-independent MC-deficient mice. Our finding that MCs are not significantly involved in the closure of noninfected wounds is in accord with several other studies investigating wound healing in Kit-independent MC-deficient mouse models (13–15).
IL-6 previously has been described to augment the production of antimicrobial peptides in a skin graft model (26). We, therefore, hypothesized that MC-derived IL-6 may be crucial for the induction of antimicrobial peptides in KCs. In fact, we were able to show that MC-derived IL-6 induces antimicrobial peptide production and enhances the antibacterial capacity of KCs in vitro and in vivo.
IL-6 has also been reported to be expressed in human wounds (27). Interestingly, patients treated with the humanized neutralizing anti-IL6 receptor antibody Tocilizumab exhibit a noticeable increase in the frequency of skin and s.c. infections (28). In our study, the ability of IL-6 to improve bacterial clearance was also not limited to the mouse system as treatment of human KCs and human skin explants with recombinant human IL-6 significantly reduced bacterial numbers in the supernatant (SI Appendix, Fig. S4 C and D).
In conclusion, our findings show that MC-derived IL-6 is crucial for the control of skin wound infections and for the healing of infected wounds. These results may allow for the development of novel and effective approaches for the prevention and treatment of bacterial skin, and other, infections, which are urgently needed in this time of increasing antibiotic resistance.
Materials and Methods
Animals.
Mice were obtained from breeding colonies of the animal facilities of Charité–Universitätsmedizin Berlin and all animal experimentation were approved by the ethics committee of the state of Berlin, Landesamt für Gesundheit und Soziales (LAGeSo). Where indicated, engraftment of back skin was performed by intradermal injection of 106 BMCMCs (29, 30). Full details can be found in SI Appendix, SI Materials and Methods.
Model of Skin Wound Infection with P. aeruginosa.
Skin wounds were created on the lower back using a 6-mm biopsy punch as described previously (10). Full details can be found in SI Appendix, SI Materials and Methods.
In Vitro Bacterial Infection Assays.
Pam212 mouse KCs, human HaCaT cells, and human skin explants were infected with P. aeruginosa. For IL-6 treatment: 50 ng/mL (or as indicated) recIL-6 was added 1 h prior to infection. Full details can be found in SI Appendix, SI Materials and Methods.
Quantitative Real-Time PCR, ELISA, and MPO Assay.
Full details can be found in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Sina Heydrich and Evelin Hagen for their excellent technical support and the Danish Molecular Biomedical Imaging Centre of the faculties of Natural Sciences and Health Sciences at the University of Southern Denmark, Odense for the support using in vivo imaging. This work was supported by Deutsche Forschungsgemeinschaft Grants SI1407/2 (to F.S.) and SPP1394, the Else Kröner-Fresenius Stiftung, and NIH Grants NIAMS R01 AR067145 and NIAID R01 AI132494 (to S.J.G.). This work also benefitted from the strategic funding of the European Cooperation in Science and Technology Action BM1007. Portions of this work have appeared in the dissertations of C.Z. and D.T.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1908816116/-/DCSupplemental.
References
- 1.Mustoe T., Understanding chronic wounds: A unifying hypothesis on their pathogenesis and implications for therapy. Am. J. Surg. 187, 65S–70S (2004). [DOI] [PubMed] [Google Scholar]
- 2.Plowman R., The socioeconomic burden of hospital acquired infection. Euro Surveill. 5, 49–50 (2000). [DOI] [PubMed] [Google Scholar]
- 3.Lister P. D., Wolter D. J., Hanson N. D., Antibacterial-resistant Pseudomonas aeruginosa: Clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin. Microbiol. Rev. 22, 582–610 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serra R., et al. , Chronic wound infections: The role of Pseudomonas aeruginosa and Staphylococcus aureus. Expert Rev. Anti Infect. Ther. 13, 605–613 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Metz M., Maurer M., Mast cells–Key effector cells in immune responses. Trends Immunol. 28, 234–241 (2007). [DOI] [PubMed] [Google Scholar]
- 6.Metz M., Siebenhaar F., Maurer M., Mast cell functions in the innate skin immune system. Immunobiology 213, 251–260 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Siebenhaar F., et al. , Control of Pseudomonas aeruginosa skin infections in mice is mast cell-dependent. Am. J. Pathol. 170, 1910–1916 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abraham S. N., St John A. L., Mast cell-orchestrated immunity to pathogens. Nat. Rev. Immunol. 10, 440–452 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shiota N., et al. , Pathophysiological role of skin mast cells in wound healing after scald injury: Study with mast cell-deficient W/W(V) mice. Int. Arch. Allergy Immunol. 151, 80–88 (2010). [DOI] [PubMed] [Google Scholar]
- 10.Weller K., Foitzik K., Paus R., Syska W., Maurer M., Mast cells are required for normal healing of skin wounds in mice. FASEB J. 20, 2366–2368 (2006). [DOI] [PubMed] [Google Scholar]
- 11.Younan G. J., et al. , Mast cells are required in the proliferation and remodeling phases of microdeformational wound therapy. Plast. Reconstr. Surg. 128, 649e–658e (2011). [DOI] [PubMed] [Google Scholar]
- 12.Succar J., et al. , The role of mouse mast cell proteases in the proliferative phase of wound healing in microdeformational wound therapy. Plast. Reconstr. Surg. 134, 459–467 (2014). [DOI] [PubMed] [Google Scholar]
- 13.Antsiferova M., et al. , Mast cells are dispensable for normal and activin-promoted wound healing and skin carcinogenesis. J. Immunol. 191, 6147–6155 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Nauta A. C., et al. , Evidence that mast cells are not required for healing of splinted cutaneous excisional wounds in mice. PLoS One 8, e59167 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willenborg S., et al. , Genetic ablation of mast cells redefines the role of mast cells in skin wound healing and bleomycin-induced fibrosis. J. Invest. Dermatol. 134, 2005–2015 (2014). [DOI] [PubMed] [Google Scholar]
- 16.McFarland-Mancini M. M., et al. , Differences in wound healing in mice with deficiency of IL-6 versus IL-6 receptor. J. immunol. 184, 7219–7228 (2010). [DOI] [PubMed] [Google Scholar]
- 17.Arranz-Valsero I., Soriano-Romaní L., García-Posadas L., López-García A., Diebold Y., IL-6 as a corneal wound healing mediator in an in vitro scratch assay. Exp. Eye Res. 125, 183–192 (2014). [DOI] [PubMed] [Google Scholar]
- 18.Gallo R. L., et al. , Endothelial cell surface alkaline phosphatase activity is induced by IL-6 released during wound repair. J. Invest. Dermatol. 109, 597–603 (1997). [DOI] [PubMed] [Google Scholar]
- 19.Lin Z. Q., Kondo T., Ishida Y., Takayasu T., Mukaida N., Essential involvement of IL-6 in the skin wound-healing process as evidenced by delayed wound healing in IL-6-deficient mice. J. Leukoc. Biol. 73, 713–721 (2003). [DOI] [PubMed] [Google Scholar]
- 20.Chan C. Y., St John A. L., Abraham S. N., Plasticity in mast cell responses during bacterial infections. Curr. Opin. Microbiol. 15, 78–84 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi H. W., Abraham S. N., Mast cell mediator responses and their suppression by pathogenic and commensal microorganisms. Mol. Immunol. 63, 74–79 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnzon C. F., Rönnberg E., Pejler G., The role of mast cells in bacterial infection. Am. J. Pathol. 186, 4–14 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Shelburne C. P., Abraham S. N., The mast cell in innate and adaptive immunity. Adv. Exp. Med. Biol. 716, 162–185 (2011). [DOI] [PubMed] [Google Scholar]
- 24.Trivedi N. H., et al. , Mast cells: Multitalented facilitators of protection against bacterial pathogens. Expert Rev. Clin. Immunol. 9, 129–138 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aoki R., et al. , Mast cells play a key role in host defense against herpes simplex virus infection through TNF-α and IL-6 production. J. Invest. Dermatol. 133, 2170–2179 (2013). [DOI] [PubMed] [Google Scholar]
- 26.Erdag G., Morgan J. R., Interleukin-1alpha and interleukin-6 enhance the antibacterial properties of cultured composite keratinocyte grafts. Ann. Surg. 235, 113–124 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grellner W., Time-dependent immunohistochemical detection of proinflammatory cytokines (IL-1beta, IL-6, TNF-alpha) in human skin wounds. Forensic Sci. Int. 130, 90–96 (2002). [DOI] [PubMed] [Google Scholar]
- 28.Jones G., Ding C., Tocilizumab: A review of its safety and efficacy in rheumatoid arthritis. Clin. Med. Insights Arthritis Musculoskelet. Disord. 3, 81–89 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lilla J. N., et al. , Reduced mast cell and basophil numbers and function in Cpa3-Cre; Mcl-1fl/fl mice. Blood 118, 6930–6938 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quintana A., et al. , Astrocyte-specific deficiency of interleukin-6 and its receptor reveal specific roles in survival, body weight and behavior. Brain Behav. Immun. 27, 162–173 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.