Significance
Currently, there are no therapies available to mitigate intestinal damage after radiation injury. Efforts to study and design new therapies are hampered by a lack of models that can be readily adopted to study therapeutic targets. Here we describe a preclinical platform to evaluate therapeutic countermeasures against intestinal radiation injury in vivo in a mouse model that permits inducible and reversible gene suppression following radiation exposure. We demonstrate that transient intestinal Apc suppression stimulates intestinal regeneration and mitigates lethality after radiation intestinal injury, thus validating pulsed Wnt pathway agonism as a therapeutic strategy. This platform can be readily adopted to study theoretically any gene of interest associated with the biology and treatment of intestinal radiation injury.
Keywords: radiation mitigator, radiation-induced gastrointestinal syndrome, radiation enteritis, intestinal regeneration, Wnt signaling
Abstract
Radiation-induced gastrointestinal syndrome (RIGS) is a limiting factor for therapeutic abdominopelvic radiation and is predicted to be a major source of morbidity in the event of a nuclear accident or radiological terrorism. In this study, we developed an in vivo mouse-modeling platform that enables spatial and temporal manipulation of potential RIGS targets in mice following whole-abdomen irradiation without the confounding effects of concomitant hematopoietic syndrome that occur following whole-body irradiation. We then tested the utility of this platform to explore the effects of transient Wnt pathway activation on intestinal regeneration and animal recovery following induction of RIGS. Our results demonstrate that intestinal epithelial suppression of adenomatous polyposis coli (Apc) mitigates RIGS lethality in vivo after lethal ionizing radiation injury-induced intestinal epithelial damage. These results highlight the potential of short-term Wnt agonism as a therapeutic target and establish a platform to evaluate other strategies to stimulate intestinal regeneration after ionizing radiation damage.
Whole-body radiation exposure can result in a myriad of deleterious effects, largely affecting the hematopoietic and gastrointestinal systems (1, 2). Ultimately, this can result in severe organ dysfunction and/or death (1, 2). Radiation-induced gastrointestinal syndrome (RIGS) presents a major limitation for delivering tumoricidal radiation therapy to the abdomen and pelvis and is predicted to be a significant source of morbidity and mortality in the event of nuclear accidents or radiological terrorism (3, 4). While hematopoietic syndrome can be rescued by supportive care (i.e., hydration and/or antibiotics) or hematopoietic stem cell transplantation, there are no effective therapies to mitigate RIGS (5).
Intestinal epithelial regeneration after radiation injury is dependent on intestinal stem cell (ISC) repopulation and regeneration of the differentiated cells that populate the functional intestinal villus (6, 7). Lgr5 is a Wnt target gene that marks a population of self-renewing and multipotent ISCs (8). While Lgr5+ cells are dispensable for intestinal homeostasis, depletion of Lgr5+ ISCs dramatically impairs intestinal regeneration following radiation damage (9). Indeed, studies suggest that Wnt pathway agonists, such as the Wnt modulator R-spondin (Rspo), can enhance intestinal cell proliferation in clonogenic survival assays and reduce gastrointestinal injury in mice (9–17). The mechanism of protection remains elusive, as systemic Wnt potentiation can result in a wide range of effects in various organ systems (18–21).
A major limitation in the advancement of new therapies against RIGS is the lack of facile model systems in which to study RIGS pathology and to validate new therapeutic targets. Given the complexity of the intestinal tissue and the underlying pathology of RIGS, it seems unlikely that cell culture systems alone can predict the initial response and potential for regeneration associated with intestinal radiation damage. However, whole-body radiation doses necessary to elicit RIGS in mice invariably result in concomitant hematopoietic syndrome, which precludes analysis of RIGS unless animals are concomitantly given bone marrow transplantation (1, 2). In addition, while genetic perturbation studies represent an extremely powerful approach in validating therapeutic targets, conditional gene deletions are tedious to produce in the germline setting, and it is difficult to engineer models in which the putative target can be acutely deleted after a pathology-inducing stimulus (22–24). Furthermore, it is not feasible to assess the consequences of transient target inhibition in these models, as strategies to restore endogenous gene function after gene excision are neither effective nor routine.
Herein, we sought to develop a model of focal irradiation to permit escalating abdominopelvic irradiation while minimizing bone marrow damage and concomitant hematopoietic syndrome. Additionally, we incorporated an inducible short-hairpin RNA (shRNA) platform that enables inducible and reversible gene suppression at any time pre- or postradiation exposure. This platform was used to evaluate the impact of local Wnt pathway activation on intestinal epithelial regeneration after ionizing radiation (IR). The combination of abdominal restricted radiation exposure and focused genetic tools demonstrates that suppression of adenomatous polyposis coli (Apc) in the gut augments intestinal regeneration and mitigates lethality after ablative radiation injury, thus validating a new approach for studying the biology and treatment of RIGS.
Results
Whole-Abdomen Irradiation Elicits Radiation-Induced Gastrointestinal Syndrome, but Not Hematopoietic Syndrome.
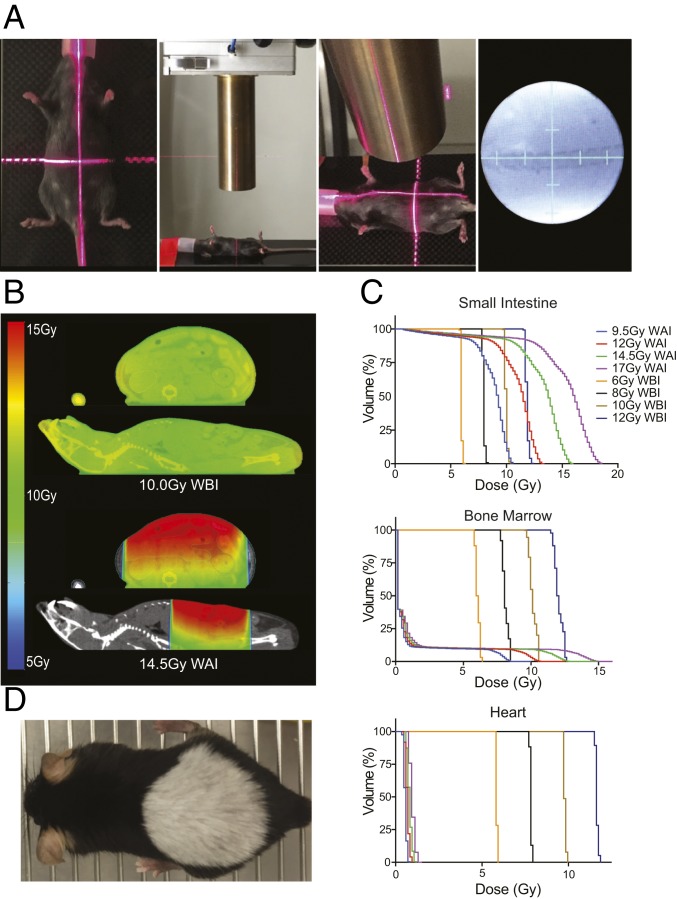
Given limitations of in vivo models to study intestinal regeneration after lethal radiation injury, we set out to develop a strategy to elicit radiation-induced gastrointestinal syndrome (RIGS) in the absence of hematopoietic syndrome. In previous work, this has been accomplished through sub–total-body gamma irradiation using lead to shield the front limbs and head, thereby sparing bone marrow that would otherwise be targeted by whole-body irradiation (WBI) or through the utilization of abdominal focused radiation (22, 25). While cesium irradiators have been the standard source of radiation exposure, the Department of Energy (DOE)/National Nuclear Security Administration (NNSA) has implemented a Cesium Irradiator Replacement Project (CIRM) with the hope to eliminate all Cs-137 irradiators nationwide (26). As such, we sought to utilize a small-animal linear accelerator to develop a highly reproducible whole-abdomen irradiation (WAI) system (Fig. 1A). The utilization of intravenous, intraperitoneal, and/or oral contrast, as appropriate, allowed the delineation of normal organs by microcomputerized topography (CT), which thus permitted real-time animal dosimetry. As expected, and in contrast to WBI, WAI allowed escalating IR dose to the small intestine while minimizing radiation therapy (RT) dose to the normal healthy bone marrow (Fig. 1 B and C and SI Appendix, Fig. S1). This relative organ sparing was confirmed for thoracic organs (i.e., heart and lungs) and abdominopelvic organs (i.e., liver, stomach, large intestine, and bladder) (Fig. 1C and SI Appendix, Fig. S2).
Fig. 1.
Whole-abdomen irradiation permits escalating RT dose to the small intestine while minimizing RT dose to normal healthy bone marrow. (A) Anesthetized mice were aligned with laser coordinate positioning, a 25-mm diameter collimator was utilized to localize the radiation to the abdomen, treatment depth was verified, and fluoroscopic images were obtained to confirm mouse positioning relative to bony anatomy. (B) Whole-mouse microcomputerized topography scans were obtained to allow animal dosimetry and normal organ identification. Representative images of 5 replicates. (C) Small intestine, bone marrow, and heart dose–volume histograms were calculated. (D) RT-induced canities at 1 y after shApc induction and 14.5 Gy WAI.
Given potential differences in the relative biological effectiveness (RBE) of Cs-137 gamma rays as compared to orthovoltage X-rays (i.e., greater dose deposition in bone marrow and lower dose deposition in visceral organs), we next tested the biological response to WAI as compared to WBI in our small-animal linear accelerator system (27). Serial monitoring of complete blood counts (CBC) after WBI and WAI noted significant differences in hematocrit, platelets, neutrophils, and lymphocytes (Fig. 2A and SI Appendix, Fig. S3A). Postmortem analysis of bone marrow from the femur revealed significant hypocellularity in mice treated with WBI as compared to an active bone marrow in mice treated with WAI (Fig. 2B). This was reflected in the substantial sparing of hematopoietic stem and progenitor cells in the WAI setting (SI Appendix, Fig. S3B). Thus, the utilization of focused radiotherapy with an orthovoltage linear accelerator permits escalating abdominopelvic irradiation while maintaining significant bone marrow function.
Fig. 2.
Whole-abdominal irradiation elicits radiation-induced gastrointestinal syndrome (RIGS) but does not result in hematopoietic syndrome. (A) Notable differences in serial hematocrit and lymphocyte counts after whole-body and whole-abdominal irradiation. Five mice per cohort. (B) Appreciable differences in femur bone marrow cellularity between mice treated with whole-abdominal and whole-body irradiation at 7 d. Representative image of 5 biological replicates. (C) Kaplan–Meier survival after escalating dose of whole-abdomen irradiation (n ≥ 6 per group representing at ≥2 [range; 2 to 6] independent experiments depending on the dose). (D) Absolute change in weight at 8 d after escalating WAI dose (n ≥ 6 per group representing at ≥2 [range; 2 to 4] independent experiments depending on the dose). (E) Representative immunofluorescence images noted increased cleaved-Caspase 3 and decreased Ki67 positive cells in mice treated with lethal (14.5 Gy) and sublethal (12 Gy) WAI at 24 and 48 h, respectively. Error bars, mean ± 95% confidence interval (CI). ns, not significant. *P < 0.01. C, Log-rank comparison; D, two-sided t test.
Escalating doses of WAI resulted in increased mortality with a threshold dose of 14.5 Gy (Fig. 2C). There was a significant difference in weight loss between mice subjected to lethal and sublethal WAI (Fig. 2D and SI Appendix, Fig. S3C). Mice treated with sublethal WAI had less crypt apoptosis at 24 h and increased crypt proliferation at 48 h as compared to mice treated with lethal WAI (Fig. 2E). Altogether, this system enables the study of and recovery from RIGS, following doses of IR that would otherwise induce complete bone marrow ablation after WBI.
Transient Apc Suppression Accelerates Intestinal Regeneration and Mitigates Radiation-Induced Gastrointestinal Lethality following Lethal Whole-Abdomen Irradiation.
To develop a platform to test concepts for ameliorating RIGS, we took advantage of an inducible short-hairpin RNA (shRNA) transgenic platform that involves introduction of a GFP-coupled shRNA expressed from a tetracycline response element (TRE) into a defined genomic locus (28–30). Upon crossing the resulting mice to strains harboring a ubiquitous tet-transactivator, it is possible to achieve broad and inducible suppression of the shRNA target in the mouse following addition of doxycycline (dox), a tetracycline analog (28–30). Since shRNAs suppress gene expression in trans, they do not disrupt the target locus, and, as such, their effects are reversible. Hence, upon dox withdrawal, the endogenous protein is reexpressed. Of note, the shRNAs used in this system are based in the miR30 background and optimized to have high potency and minimal off-target effects. Furthermore, owing to their transcriptional linkage to GFP, cells expressing shRNAs can be readily detected by fluorescence (28, 30).
We took advantage of this system to evaluate the role of Wnt signaling in mitigating pathologies associated with RIGS. The Adenomatous polyposis coli (APC) tumor suppressor controls Wnt signaling output by regulating the degradation of β-catenin, a key transcriptional regulator that drives Wnt-mediated target gene transcription (31–35). Previously, we engineered mice harboring a TRE–GFP–shApc transgene and showed that dox addition could efficiently suppress APC protein expression leading to Wnt activation, intestinal stem cell hyperproliferation, differentiation block, weight loss, and death within 8 to 10 d (36). Restoration of Apc prior to crypt disintegration resulted in normalization of endogenous Wnt signaling and rapid cellular differentiation reestablishing normal tissue homeostasis (36). As the ability to temporally control our genetic perturbation in this system can, in principle, mimic transient pharmacological Wnt activation and thereby avoid lethality, we reasoned that this model would be an ideal context to assess whether transient Wnt pathway activation could ameliorate RIGS after WAI.
Mice harboring the Apc shRNA transgene (shApc) or those harboring a neutral control shRNA (shControl) were subjected to escalating WAI doses and subsequent daily doxycycline gavage for 5 d immediately after WAI to transiently activate the shRNA of interest (Fig. 3A). shControl mice treated with lethal WAI doses (>14.5 Gy) had progressive and irreversible weight loss and had to be killed 6 to 12 d following IR, as expected based on historical experience (37). By contrast, shApc mice treated with these same doses displayed transient weight loss followed by a full recovery within 12 d (Fig. 3B). Histologically, there was a significantly greater number of regenerative crypts per small-intestinal cross section in shApc mice as compared to shControl mice at 48 h after WAI (Fig. 3C). Remarkably, transient Apc suppression resulted in intestinal hyperproliferation and accelerated intestinal epithelial regeneration after ablative WAI (Fig. 3D).
Fig. 3.
Intestinal Wnt activation after whole-abdomen irradiation accelerates intestinal stem cell repopulation and promotes gut epithelial regeneration. (A) Experimental schematic. (B) Serial weight loss after escalating doses of whole-abdomen irradiation (n ≥ 3 per group). (C) Hematoxylin and eosin staining of shApc and shControl intestinal crypts at 6, 24, and 48 h after 14.5 Gy WAI and dox gavage. (D) Quantification of regenerating crypts per cross-sectional area at 6, 24, and 48 h after 14.5 Gy WAI and dox gavage (n ≥ 3 per group). Error bars, mean ± 95% confidence interval (CI).
We also examined the kinetics of biological and molecular changes following transient Wnt pathway activation. There was no difference in intestinal crypt proliferative indices at 6 and 24 h after lethal WAI and dox-dependent shRNA expression, but a marked difference was noted by 48 h (Fig. 4 A and B). As expected, shRNA expression induced by dox gavage resulted in an increase in the expression of GFP in both shControl and shApc mice (Fig. 4C). We have previously demonstrated that the GFP transcript is near-normalized (i.e., >100-fold lower) by 48 h, but that protein levels persist and are detectable for up to 4 to 6 d after dox withdrawal (36). Nevertheless, while GFP marks cells that express the shRNA, the kinetics of target knockdown are not necessarily the same, owing to the potency of the shRNA and stability of the target protein. Thus, to more precisely assess the kinetics of Wnt pathway activation, we examined the expression of canonical Wnt targets (Myc, Axin 2) and intestinal stem cell markers (Lgr5 and Ascl2) in bulk small-intestinal tissue following WAI of shControl and shApc mice at different times after dox exposure. These targets were induced in the small intestine and associated microenvironment at 48 h, but not at 6 or 24 h in shApc mice (Fig. 4 D–G). By contrast, no induction of these targets was observed in shControl mice. Lgr5 in situ hybridization noted rapid loss of Lgr5+ cells in the intestinal crypts at 6 and 24 h, but recovery of Lgr5+ ISCs in the shApc mice by 48 h continuing through 7 d (Fig. 4H). Together these results suggest that intestinal recovery after ablative IR seems to be partially driven by Lgr5 intestinal stem cell repopulation.
Fig. 4.
Wnt activation after lethal whole-abdomen irradiation stimulates intestinal regeneration. (A) Serial immunofluorescence images (GFP, Ki67, DAPI) of shApc and shControl mice after 14.5 Gy WAI and dox gavage (representative images shown). (B) Quantification of Ki67+ IHC cells per cross-sectional area at 6, 24, and 48 h after WAI and dox gavage (n ≥ 4 per group). (C–G) qRT-PCR analysis of gene expression in bulk intestinal isolates following 14.5 WAI and dox gavage. Markers of transgene induction (GFP) (C), Wnt activation Myc (D) and Axin2 (E), and stem cells Lgr5 (F) and Ascl2 (G) are shown for shApc and shControl mice at 6, 24, and 48 h after WAI and dox gavage. Relative gene expression is normalized to unirradiated dox naïve shControl mice. Plots represent the mean ± SEM and are based on 3 biological and 3 technical replicates for each condition at each timepoint. (H) Serial Lgr5 ISH images after 14.5 Gy WAI and dox gavage.
Transient Wnt activation significantly reduced the lethality associated with 14.5 Gy WAI (70% vs. 0% at 14 d after WAI, P = 0.012) (Fig. 5A). The lethal dose to 50% of the mice at 14 d (LD50/14) increased from 12 to 15 Gy after WAI and 5 d of Wnt agonism via dox-driven shApc expression (Fig. 5B). These results demonstrate that transient Wnt activation can mitigate the lethal effects of ablative radiation intestinal injury.
Fig. 5.
Wnt activation after lethal whole-abdomen irradiation mitigates radiation-induced gastrointestinal lethality. (A) Kaplan–Meier survival curve comparing shControl and shApc hairpin induction after 14.5 Gy WAI (n ≥ 2 per group, with ≥2 experimental replicates). (B) Fourteen-day mortality with escalating WAI RT dose in shControl and shApc mice (n ≥ 3 per group). A, Log-rank comparison.
Apc Suppression Accelerates Intestinal Stem Cell Regeneration after RT in a Cell-Autonomous Manner.
We sought to evaluate whether the enhanced intestinal recovery we observed by transient Wnt pathway activation resulted from cell-autonomous effects by examining epithelial regeneration in ex vivo intestinal organoid cultures following irradiation. Small-intestinal organoids were generated from shApc or shControl mice, irradiated (4 Gy), and subsequently cultured in dox-supplemented media (SI Appendix, Fig. S4A). Previously, we have demonstrated that dox-induced Apc suppression in intestinal organoids resulted in increased proliferation and block in differentiation (36). Compared to controls, postirradiation suppression of Apc stimulated an increase in organoid regeneration (SI Appendix, Fig. S4 B and C), demonstrating that the regenerative effects of transient Wnt activation postirradiation can result at least in part from a cell-autonomous effect. In principle, intestinal specific delivery systems may provide an opportunity to stimulate intestinal epithelial regeneration while abrogating any unwanted toxicity resulting from systemic Wnt activation.
Apc Suppression Mitigates RIGS Lethality with Minimal Long-Term Toxicity.
While sustained Apc suppression promotes carcinogenesis in multiple organs, the long-term side effects of transient Apc suppression after lethal WAI were minimal. Importantly, inducible and reversible Apc suppression resulted in no appreciable change in the long-term health of the mice with normal healthy proliferating intestinal epithelium appreciated at 1 y after WAI. The only treatment morbidity was the presence of uniform canities (i.e., gray hair) in the irradiated field (Fig. 1D). Importantly, no mice developed abdominopelvic tumors by 1 y after WAI. Thus, transient Wnt activation can ameliorate RIGS without producing unacceptable toxicities.
Discussion
Herein we developed a preclinical platform to evaluate therapeutic countermeasures against RIGS using a mouse model that permits inducible and reversible gene suppression following localized abdominopelvic radiation exposure. We demonstrate that cell-autonomous Wnt activation, produced in our model by transient Apc suppression, stimulates regeneration and mitigates lethality after ablative ionizing radiation intestinal injury. More generally, our flexible genetic model enables the suppression of any gene after ablative radiation injury, thus providing an important and adaptable model to study the biology and treatment of RIGS.
Our platform for interrogating RIGS incorporates 2 components. The first involves abdominopelvic focused irradiation that enables localized delivery of escalating radiation doses to the intestinal luminal organs while sparing the majority of the mouse bone marrow, producing RIGS without inducing concomitant hematopoietic syndrome using an orthovoltage linear accelerator rather than a Cs-137 gamma-irradiator. The radiation dose necessary to induce RIGS is well below the threshold dose for radiation-induced liver disease [whole-liver dose of 30 Gy (38)], which has a latency period of 1 to 3 mo, and below the threshold dose for radiation-induced kidney disease [15 Gy in a single fraction to the whole kidney (39)] in C57BL/6 mice, which has a prolonged latency period of ∼4 to 5 mo. The second involves the incorporation of shRNA transgenic mice to inducibly and reversibly suppress endogenous gene function in vivo. In contrast to traditional gene-targeting approaches, validated shRNAs used in our approach are targeted to the same genomic locus using recombination-mediated cassette exchange (28–30). This obviates the need for complicated gene-targeting strategies to produce conditional alleles and enables the reversible suppression of an endogenous gene by the simple addition or withdrawal of dox from the food or drinking water.
This transient suppression capability is a critical element of the platform, as persistent and irreversible suppression of Apc throughout the intestine results in intestinal hyperproliferation, organ failure, and rapid death (36), whereas constitutive focal Apc suppression leads to cancer (25). By contrast, transient Apc suppression provides a beneficial effect upon tissue damage, enabling mice to regenerate a functional intestine while remaining tumor-free. In principle, the transient and incomplete nature of shRNA-mediated target inhibition mimics aspects of pharmacological target inhibition, which is rarely continuous or complete.
Collectively, our system provides a highly reproducible in vivo model to study genetic factors influencing intestinal stem cell regeneration while considering dose, fractionation, volume of organ irradiated, radiation modifiers, and intrinsic biology. We used this platform to test whether transient Wnt pathway activation could ameliorate pathologies associated with RIGS. In addition to validating our platform, our results build on work suggesting Wnt agonism via Rspol can improve intestinal regeneration following whole-body irradiation (11, 12). However, these studies were unable to demonstrate that the improved outcomes were acting by directly targeting the intestine, and, moreover, Rspol has recently been shown to bind receptors in addition to Lgr5 that may send Wnt-independent signals (18–21). Our results imply that the effect of Wnt signaling on regeneration and survival is due, at least in part, to its action in the intestinal epithelium, producing a substantial survival advantage at otherwise lethal doses of radiation in the absence of significant side effects, including cancer.
The above observations support the use of small-molecule inhibitors that activate Wnt signaling for RIGS, many of which are in clinical trials for other indications (40–42). Such existing agents include GSK-3 inhibitors (e.g., Tideglusib, CHIR 99021, and Ly2090314) that can activate Wnt signaling by destabilizing the β-catenin destruction complex (41–43). Given that Wnt agonism can act in a cell-autonomous manner, localized drug delivery might be promising to reduce systemic on target toxicities. While our data suggest a cell-autonomous effect on the intestinal epithelium, as demonstrated in our organoid assay, we cannot rule out a cell-nonautonomous effect, as in vivo APC inhibition is not isolated to the intestinal epithelium.
Our platform may be useful for studying general factors related to intestinal regeneration following injury. In fact, the US Department of Health and Human Services has put forward a “dual-utility” philosophy aiming to facilitate the development of drugs for nuclear accidents/radiologic terrorism that also have a routine clinical use. We have demonstrated that the effect of Apc inhibition in our system occurs between 24 and 48 h, consistent with the definition of radiation mitigation as put forth by the NIH (44). Importantly, our system permits control of gene inhibition temporally, which is important in determining if a potential target is a candidate for radiation protection and/or mitigation. Beyond RIGS, the high rate of regeneration in the intestine predisposes the intestinal epithelium to common side effects seen in cancer patients receiving radio- or chemotherapy. Indeed, radiation enteritis is a major factor limiting the delivery of effective doses of tumoricidal radiation therapy, occurs in more than 70% of patients who have undergone abdominopelvic RT, and can result in decreased quality of life among cancer survivors. As there are limited options for prevention and mitigation of these pathologies, pharmacological approaches to stimulate intestinal regeneration represent a major unmet clinical need.
Materials and Methods
Production of mice and all treatments described were approved by the Institutional Animal Care and Use Committee (IACUC) at Memorial Sloan Kettering Cancer Center (New York, NY), under protocols 11–06-012 and 11–06-016. The X-RAD 225Cx (Precision X-ray, Inc.; North Branford, CT) orthovoltage small-animal irradiator was used for WAI and WBI. Experimental animals were monitored by a blinded observer. Littermate controls were used for experiments when appropriate and available. Detailed materials and methods are included in SI Appendix.
Supplementary Material
Acknowledgments
We thank Mayerlin Chalarca, Sang Yong Kim, So Young Kim, and Janelle Simon for technical assistance with animal colonies and in vitro fertilization; James Russell, Pat Zanzonico, and Valerie Longo for maintenance and support with the small-animal micro-irradiator; Amanda Kulick for technical assistance with animal studies; Mesruh Turkekul and Katia Manova-Todorova for technical assistance with Lgr5 ISH; Simon Powell, Charles Sherr, and Christopher Crane for mentorship and advice; Francisco Barriga and Michael Del Latto for a critical review of the manuscript; Kevin O’Rourke for thoughtful discussions and experimental techniques; and all members of the Lowe laboratory for advice and discussions. Microscopy was performed at the Memorial Sloan Kettering Cancer Center Molecular Cytology Core Facility. We acknowledge the following funding sources: NIH P30 CA008748 Cancer Center Support Grant, NIH U54 OD020355-01 (to S.W.L.), NIH R01 CA195787 (to S.W.L.), Stand Up to Cancer (GC230112) (to S.W.L.), and Memorial Sloan Kettering Cancer Center Imaging and Radiation Sciences Program (to P.B.R. and S.W.L.). A Geoffrey Beene Research Foundation Shared Resources Grant provided funding support for the purchase of the XRAD225Cx. P.B.R. is supported in part by a K12 Paul Calebresi Career Development Award for Clinical Oncology (K12 CA184746) and NIH loan repayment program award. S.W.L. is the Geoffrey Beene Chair of Cancer Biology and a Howard Hughes Medical Institute investigator.
Footnotes
Conflict of interest statement: L.E.D. and S.W.L. are founders and scientific advisory board members of Mirimus, Inc., a company that incorporates animal modeling technologies similar to those presented in this work. P.B.R. has received an honorarium from Corning to discuss 3D cell culture techniques, has served as a consultant for AstraZeneca, and is a consultant for EMD Serono for work on radiation sensitizers.
This article is a PNAS Direct Submission. D.G.K. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1906611116/-/DCSupplemental.
References
- 1.Haskin F. E., et al. , “Probabilistic accident consequence uncertainty analysis, early health effects uncertainty assessment” (A Joint Report by US Nuclear Regulatory Commission and Commission of European Communities, 1997), vol. 1. [Google Scholar]
- 2.Mettler F. A. Jr, Voelz G. L., Major radiation exposure–What to expect and how to respond. N. Engl. J. Med. 346, 1554–1561 (2002). [DOI] [PubMed] [Google Scholar]
- 3.Citrin D., et al. , Radioprotectors and mitigators of radiation-induced normal tissue injury. Oncologist 15, 360–371 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang C., et al. , Manganese superoxide dismutase gene therapy protects against irradiation-induced intestinal injury. Curr. Gene Ther. 13, 305–314 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Rios C. I., et al. , Building the strategic national stockpile through the NIAID radiation nuclear countermeasures program. Drug Dev. Res. 75, 23–28 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harfouche G., Martin M. T., Response of normal stem cells to ionizing radiation: A balance between homeostasis and genomic stability. Mutat. Res. 704, 167–174 (2010). [DOI] [PubMed] [Google Scholar]
- 7.Potten C. S., Grant H. K., The relationship between ionizing radiation-induced apoptosis and stem cells in the small and large intestine. Br. J. Cancer 78, 993–1003 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barker N., et al. , Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Metcalfe C., Kljavin N. M., Ybarra R., de Sauvage F. J., Lgr5+ stem cells are indispensable for radiation-induced intestinal regeneration. Cell Stem Cell 14, 149–159 (2014). [DOI] [PubMed] [Google Scholar]
- 10.Tian H., et al. , A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature 478, 255–259 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou W. J., Geng Z. H., Spence J. R., Geng J. G., Induction of intestinal stem cells by R-spondin 1 and Slit2 augments chemoradioprotection. Nature 501, 107–111 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhanja P., et al. , Protective role of R-spondin1, an intestinal stem cell growth factor, against radiation-induced gastrointestinal syndrome in mice. PLoS One 4, e8014 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korinek V., et al. , Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat. Genet. 19, 379–383 (1998). [DOI] [PubMed] [Google Scholar]
- 14.Pinto D., Gregorieff A., Begthel H., Clevers H., Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 17, 1709–1713 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fevr T., Robine S., Louvard D., Huelsken J., Wnt/beta-catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells. Mol. Cell. Biol. 27, 7551–7559 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brannon A. R., et al. , Comparative sequencing analysis reveals high genomic concordance between matched primary and metastatic colorectal cancer lesions. Genome Biol. 15, 454 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim K. A., et al. , Mitogenic influence of human R-spondin1 on the intestinal epithelium. Science 309, 1256–1259 (2005). [DOI] [PubMed] [Google Scholar]
- 18.Clevers H., Nusse R., Wnt/β-catenin signaling and disease. Cell 149, 1192–1205 (2012). [DOI] [PubMed] [Google Scholar]
- 19.Chai R., et al. , Wnt signaling induces proliferation of sensory precursors in the postnatal mouse cochlea. Proc. Natl. Acad. Sci. U.S.A. 109, 8167–8172 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohkawara B., Glinka A., Niehrs C., Rspo3 binds syndecan 4 and induces Wnt/PCP signaling via clathrin-mediated endocytosis to promote morphogenesis. Dev. Cell 20, 303–314 (2011). [DOI] [PubMed] [Google Scholar]
- 21.Lebensohn A. M., Rohatgi R., R-spondins can potentiate WNT signaling without LGRs. eLife 7, e33126 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirsch D. G., et al. , p53 controls radiation-induced gastrointestinal syndrome in mice independent of apoptosis. Science 327, 593–596 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castle K. D., Chen M., Wisdom A. J., Kirsch D. G., Genetically engineered mouse models for studying radiation biology. Transl. Cancer Res. 6 (suppl. 5), S900–S913 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paris F., et al. , Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science 293, 293–297 (2001). [DOI] [PubMed] [Google Scholar]
- 25.de la Cruz Bonilla M., et al. , Fasting reduces intestinal radiotoxicity, enabling dose-escalated radiation therapy for pancreatic cancer. Int. J. Radiat. Oncol. Biol. Phys. S0360-3016(19)33421-2 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anonymous , “The 2018 radiation source protection and security task force report” (US Nuclear Regulatory Commission, 2018).
- 27.Scott B. R., Gott K. M., Potter C. A., Wilder J., A comparison of in vivo cellular responses to Cs-137 gamma rays and 320-kV X rays. Dose Response 11, 444–459 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dow L. E., et al. , A pipeline for the generation of shRNA transgenic mice. Nat. Protoc. 7, 374–393 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dow L. E., et al. , Conditional reverse tet-transactivator mouse strains for the efficient induction of TRE-regulated transgenes in mice. PLoS One 9, e95236 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Premsrirut P. K., et al. , A rapid and scalable system for studying gene function in mice using conditional RNA interference. Cell 145, 145–158 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rubinfeld B., et al. , Association of the APC gene product with beta-catenin. Science 262, 1731–1734 (1993). [DOI] [PubMed] [Google Scholar]
- 32.Su L. K., Vogelstein B., Kinzler K. W., Association of the APC tumor suppressor protein with catenins. Science 262, 1734–1737 (1993). [DOI] [PubMed] [Google Scholar]
- 33.Aberle H., Bauer A., Stappert J., Kispert A., Kemler R., beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 16, 3797–3804 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Behrens J., et al. , Functional interaction of beta-catenin with the transcription factor LEF-1. Nature 382, 638–642 (1996). [DOI] [PubMed] [Google Scholar]
- 35.Molenaar M., et al. , XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell 86, 391–399 (1996). [DOI] [PubMed] [Google Scholar]
- 36.Dow L. E., et al. , Apc restoration promotes cellular differentiation and reestablishes crypt homeostasis in colorectal cancer. Cell 161, 1539–1552 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Potten C. S., A comprehensive study of the radiobiological response of the murine (BDF1) small intestine. Int. J. Radiat. Biol. 58, 925–973 (1990). [DOI] [PubMed] [Google Scholar]
- 38.Wu Z. F., et al. , A mouse radiation-induced liver disease model for stereotactic body radiation therapy validated in patients with hepatocellular carcinoma. Med. Phys. 43, 4349–4361 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Williams M. V., Denekamp J., Radiation induced renal damage in mice: Influence of fraction size. Int. J. Radiat. Oncol. Biol. Phys. 10, 885–893 (1984). [DOI] [PubMed] [Google Scholar]
- 40.Tolosa E., et al. ; TAUROS Investigators , A phase 2 trial of the GSK-3 inhibitor tideglusib in progressive supranuclear palsy. Mov. Disord. 29, 470–478 (2014). [DOI] [PubMed] [Google Scholar]
- 41.Eldar-Finkelman H., Martinez A., GSK-3 inhibitors: Preclinical and clinical focus on CNS. Front. Mol. Neurosci. 4, 32 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walz A., et al. , Molecular pathways: Revisiting glycogen synthase kinase-3β as a target for the treatment of cancer. Clin. Cancer Res. 23, 1891–1897 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kunnimalaiyaan S., Schwartz V. K., Jackson I. A., Clark Gamblin T., Kunnimalaiyaan M., Antiproliferative and apoptotic effect of LY2090314, a GSK-3 inhibitor, in neuroblastoma in vitro. BMC Cancer 18, 560 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.National Institutes of Health , “Strategic plan and research agenda for medical countermeasures against radiological and nuclear threats progress report: 2005–2011 and future research directives: 2012–2016” (National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, 2012).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.