Significance
Phgdh is a crucial enzyme in the astrocytic synthesis of l-serine from glucose. Our study uncovers the role of glial-neuronal cross-talk in regulating NMDAR synaptic activation through the astrocytic export of Phgdh-derived l-serine to generate the coagonists d-serine and glycine. We clarify the relative roles of d-serine and glycine by showing that like d-serine, Phgdh-derived glycine mediates NMDAR synaptic activation in the mature hippocampus. We show that glycine is a significant regulator of d-serine synthesis and release, revealing a cross-talk between glycine and d-serine metabolism that fine tunes NMDAR activation. Our data reveal a multifaceted mechanism regulating NMDAR activity, which ultimately depends on a Phgdh-dependent serine shuttle.
Keywords: d-serine, glycine, gliotransmission, tripartite synapse, Phgdh
Abstract
Astrocytes express the 3-phosphoglycerate dehydrogenase (Phgdh) enzyme required for the synthesis of l-serine from glucose. Astrocytic l-serine was proposed to regulate NMDAR activity by shuttling to neurons to sustain d-serine production, but this hypothesis remains untested. We now report that inhibition of astrocytic Phgdh suppressed the de novo synthesis of l-and d-serine and reduced the NMDAR synaptic potentials and long-term potentiation (LTP) at the Schaffer collaterals-CA1 synapse. Likewise, enzymatic removal of extracellular l-serine impaired LTP, supporting an l-serine shuttle mechanism between glia and neurons in generating the NMDAR coagonist d-serine. Moreover, deletion of serine racemase (SR) in glutamatergic neurons abrogated d-serine synthesis to the same extent as Phgdh inhibition, suggesting that neurons are the predominant source of the newly synthesized d-serine. We also found that the synaptic NMDAR activation in adult SR-knockout (KO) mice requires Phgdh-derived glycine, despite the sharp decline in the postnatal glycine levels as a result of the emergence of the glycine cleavage system. Unexpectedly, we also discovered that glycine regulates d-serine metabolism by a dual mechanism. The first consists of tonic inhibition of SR by intracellular glycine observed in vitro, primary cultures, and in vivo microdialysis. The second involves a transient glycine-induce d-serine release through the Asc-1 transporter, an effect abolished in Asc-1 KO mice and diminished by deleting SR in glutamatergic neurons. Our observations suggest that glycine is a multifaceted regulator of d-serine metabolism and implicate both d-serine and glycine in mediating NMDAR synaptic activation at the mature hippocampus through a Phgdh-dependent shuttle mechanism.
A physiological NMDAR coagonist, d-serine regulates several NMDAR-dependent processes, most notably synaptic plasticity (1–4). Synthesis of d-serine is catalyzed by the serine racemase (SR), an enzyme that converts l- into d-serine along with the generation of pyruvate and ammonia (5, 6). Although initially assumed to be exclusively expressed in astrocytes, SR was later shown to be primarily expressed by neurons (7–14). Deletion of SR in neurons reduces the magnitude of NMDAR-dependent long-term potentiation (LTP) at the hippocampal CA1 pyramidal cell in vitro (9) and in vivo (15).
In addition to d-serine, NMDARs also bind glycine, but the relative roles of d-serine and glycine are still unclear. Glycine preferentially activates synaptic NMDARs in young mice, but this preference switches to d-serine in adults depending on the characteristics of the synapses analyzed (16). Although both glycine and d-serine derive from l-serine, their synthesis is thought to be independently regulated, and there is no reported cross-talk between their metabolism.
Astrocytes selectively express the enzyme 3-phosphoglycerate dehydrogenase (Phgdh), the committed step in the pathway for de novo synthesis of l-serine (17). Deletion of the astrocytic Phgdh reduces brain l-serine levels (18) and decreases the neuronal staining of d-serine (10). These observations led to the proposal of the serine shuttle model, whereby astrocytes export l-serine to neurons to sustain neuronal d-serine synthesis by SR (19). The serine shuttle hypothesis, however, remains untested. It is not known whether the serine shuttle regulates NMDAR activity, as there are no studies that attempted to inhibit the Phgdh pathway and monitor NMDAR-dependent synaptic plasticity. Furthermore, it is not known if the serine shuttle pathway regulates glycine actions at NMDARs as well.
We aimed to test the main inferences of the serine shuttle hypothesis (19). We show that acute inhibition of the astrocytic Phgdh decreased the isolated NMDAR synaptic potentials and LTP at the Schaffer collateral (SC)-CA1 synapses. Removal of extracellular l-serine reduced the magnitude of the LTP, compatible with the role of astrocytic l-serine release in this form of synaptic plasticity. Deleting SR in glutamatergic neurons inhibited the de novo d-serine synthesis to the same extent as a Phgdh inhibitor, indicating that neurons are the primary source of newly synthesized d-serine. However, experiments with SR-knockout (KO) controls show that Phgdh-derived glycine also plays a role in the activation of synaptic NMDARs in adult mice. We found that intracellular glycine is a primary regulator of the biosynthesis of d-serine by SR. Glycine also activated the Asc-1 transporter, promoting transient d-serine release, and this effect was abolished in Asc-1 KO mice and diminished by deleting SR in glutamatergic neurons. Our data indicate that d-serine–mediated NMDAR activity regulation depends on the metabolism of l-serine and glycine via the astrocytic Phgdh pathway.
Results
Phgdh-Derived l-Serine Regulates NMDAR Synaptic Activation.
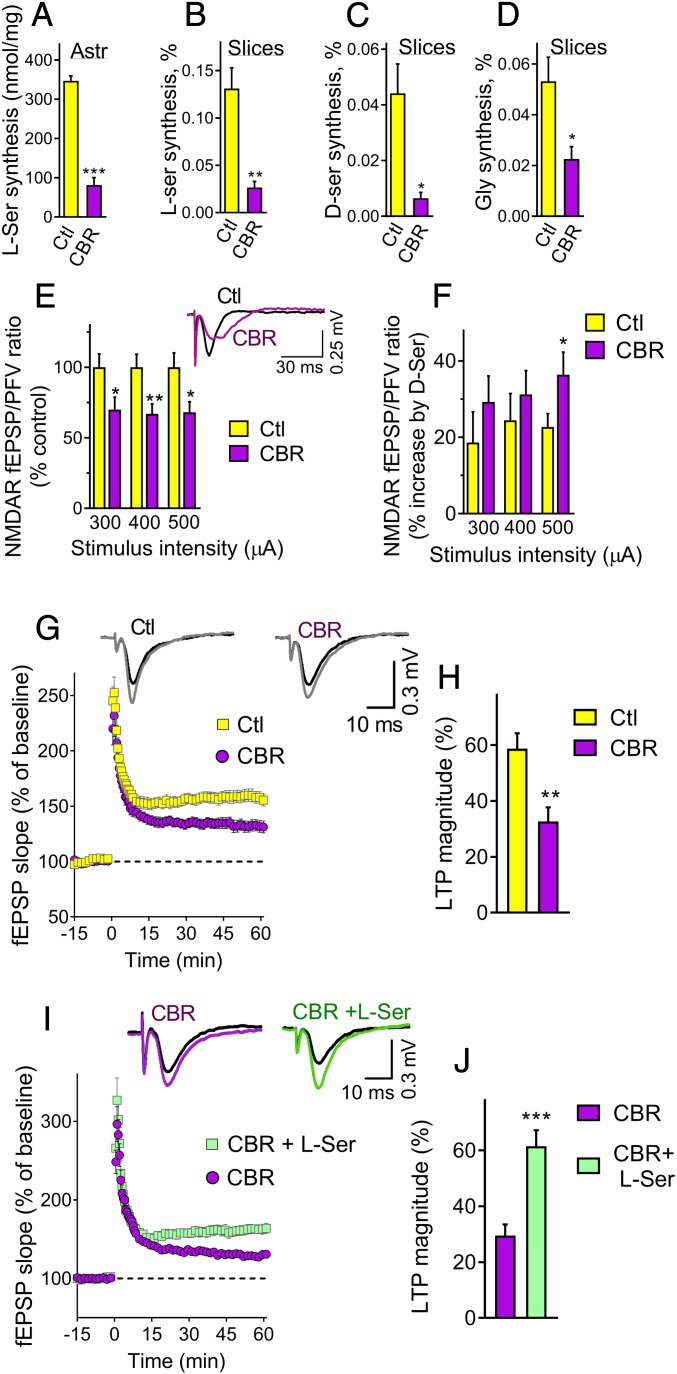
l-serine is produced from glucose in astrocytes (18), but its physiological role in synaptic plasticity is unknown. We investigated the effects of CBR-5884, a selective inhibitor of Phgdh (20), a key enzyme in the synthesis of l-serine from glucose in astrocytes. We found that CBR-5884 inhibited the production of l-serine in primary cortical astrocyte cultures by 75% (Fig. 1A). In acute cortical slices, CBR-5884 decreased the synthesis of l-[14C]serine (Fig. 1B) and d-[14C]serine (Fig. 1C) from [14C]glucose by 70 to 80%. CBR-5884 also inhibited [14C]glycine synthesis by 60% (Fig. 1D), indicating that a substantial fraction of d-serine and glycine derives from l-serine produced via the Phgdh pathway.
Fig. 1.
Phgdh inhibition decreases NMDAR synaptic activation at the SC-CA1 synapses of adult mice. (A) Inhibition of l-serine production by primary astrocytes after 48-h incubation with 40 μM CBR-5884. Paired 2-tailed Student’s t test (P = 0.0001). (B) Inhibition of de novo l-[14C]serine synthesis from [14C]glucose by 40 μM CBR-5884 in acute cortical slices. Unpaired Welch’s 2-tailed t test (P = 0.004). (C) Inhibition of d-[14C]serine synthesis from [14C]glucose by CBR-5884 in acute cortical slices. Unpaired Welch’s 2-tailed t test (P = 0.015). (D) Inhibition of [14C]glycine synthesis from [14C]glucose by CBR-5884 in acute cortical slices. Unpaired Student’s 2-tailed t test (P = 0.017). (E) A 2-h incubation with 40 μM CBR-5884 decreased the NMDAR potentials isolated in a low Mg2+ medium supplemented with NBQX (n = 12 slices). Unpaired 2-tailed Student’s t test of CBR-5884 treatment compared to control (P = 0.029, 0.009, and 0.019 for 300, 400, and 500 mA, respectively). (F) The percent increase in the NMDAR fEPSPs promoted by 100 µM d-serine with and without CBR-5884 (n = 12 to 13 slices). Unpaired 1-tailed Student’s t test (P = 0.032). (G) LTP induced by 1 × 100 Hz in the hippocampal CA1 field following at least 2-h incubation with and without CBR-5884 (n = 15 and 16 slices, respectively). (H) CBR-5884 decreased the LTP magnitude calculated from the last 15-min recording of G. Unpaired 2-tailed Student’s t test (P = 0.0017). (I) Incubation of slices with 2 mM l-serine along with CBR-5884 (n = 11 slices) increased the LTP magnitude when compared to CBR-5884 alone (n = 10 slices), restoring LTP to control levels. (J) l-serine increased the LTP magnitude calculated from the last 15-min recording of I. Unpaired 2-tailed Student’s t test (P = 0.0003). All values are average ± SEM. Data of A–D represent 6 biological replicates. Ctl, control; CBR, CBR-5884. *P < 0.05, **P < 0.01, ***P < 0.001.
To evaluate if Phgdh-derived l-serine is required for NMDAR activity, we monitored the effect of CBR-5884 on the isolated NMDAR potentials at the SC-CA1 synapses. We found that CBR-5884 inhibited the NMDAR responses at different stimuli intensities (Fig. 1E). Perfusion of slices with a saturating dose of d-serine increased the NMDAR field excitatory postsynaptic potentials (fEPSPs), and the percent of stimulation was more pronounced in CBR-5884–treated slices (Fig. 1F), indicating a lower occupancy of the NMDARs coagonist site. CBR-5884 decreased the magnitude of NMDAR-dependent LTP by about 50% (Fig. 1 G and H). Supplementing the artificial cerebrospinal fluid (aCSF) with l-serine prevented CBR-5884 inhibition and restored the LTP levels to control values (Fig. 1 I and J).
The AMPAR fEPSPs were not affected by CBR-5884, confirming a specific effect on NMDAR activity (SI Appendix, Fig. S1A). Likewise, the paired-pulse ratio was unchanged by CBR-5884, suggesting the drug does not affect presynaptic mechanisms of glutamate release (SI Appendix, Fig. S1B). CBR-5884 did not inhibit the NMDAR currents by GluN1a/GluN2a expressed in oocytes, ruling out a nonspecific inhibition of NMDARs (SI Appendix, Fig. S2). Also, CBR-5884 was ineffective against recombinant SR in vitro (SI Appendix, Fig. S3A) or SR ectopically expressed in HEK293 cells (SI Appendix, Fig. S3B). The data are compatible with the notion that CBR-5884 affects NMDAR activity by specifically inhibiting the Phgdh-dependent l-serine synthesis pathway in astrocytes.
To verify the cellular origin of the newly synthesized d-serine pool, we monitored the rate of d-serine synthesis from [14C]glucose in slices of mice with selective deletion of SR in glutamatergic neurons (CAMKIIα-Cre SR-KO). To maximize SR deletion, we employed mice older than the previous study using the same line (9). Similar to Phgdh inhibition, deletion of SR in glutamatergic neurons decreased by almost 80% the de novo synthesis of d-serine in acute cortical slices (SI Appendix, Fig. S4A). There was no cross-contamination among the [14C]-labeled enantiomers as they were completely separated by high-performance liquid chromatography (HPLC) (SI Appendix, Fig. S4B). SR expression levels were reduced by 70% in CAMKIIα-Cre SR-KO when compared to floxed controls (SI Appendix, Fig. S4 C and D). Our data indicate that glutamatergic neurons produce the vast majority of d-serine that ultimately derives from astrocytic l-serine.
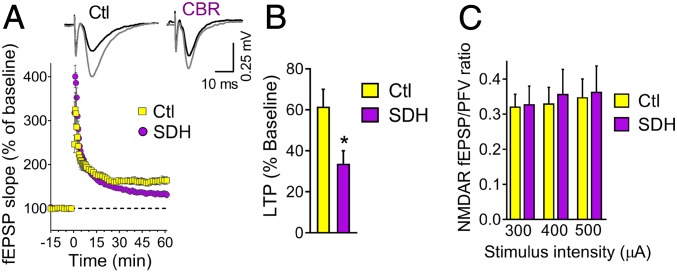
To verify if l-serine export from glia is indeed involved in NMDAR-dependent LTP, we devised an enzymatic method to remove extracellular l-serine in hippocampal slices by using purified recombinant serine dehydratase enzyme (SDH) (SI Appendix, Fig. S5A). In vitro assays indicate that SDH degrades l-serine into pyruvate and ammonia (SI Appendix, Fig. S5B). SDH does not degrade d-serine or glycine even at very high concentration and after overnight incubation (SI Appendix, Fig. S5 C and D).
Incubation of hippocampal slices with SDH reduced the NMDAR-dependent LTP by about 50%, indicating a role of extracellular l-serine in synaptic plasticity (Fig. 2 A and B). There was a trend for a stronger reduction in LTP in slices incubated for longer times with SDH (SI Appendix, Fig. S6 A and B). The isolated NMDAR field potentials were not affected by SDH incubation (Fig. 2C), and neither was the basal neurotransmission (SI Appendix, Fig. S6C) nor the paired-pulse facilitation (SI Appendix, Fig. S6D).
Fig. 2.
Effect of l-serine dehydratase (SDH) treatment on the CA1 neuronal networks of adult mice. (A) LTP induced by 1 × 100 Hz in the hippocampal CA1 field following 3.5- to 8-h incubation without or with 2.6 U/mL SDH (n = 12 and 13 slices, respectively). (B) SDH decreased the LTP magnitude calculated from the average of the last 15-min recording of A. Unpaired 2-tailed Student’s t test (P = 0.014). (C) Isolated NMDAR potentials were unaffected by incubation with SDH (n = 6 and 7 slices). Values are average ± SEM. *P = 0.014.
Phgdh-Derived Glycine also Governs NMDAR Synaptic Activity.
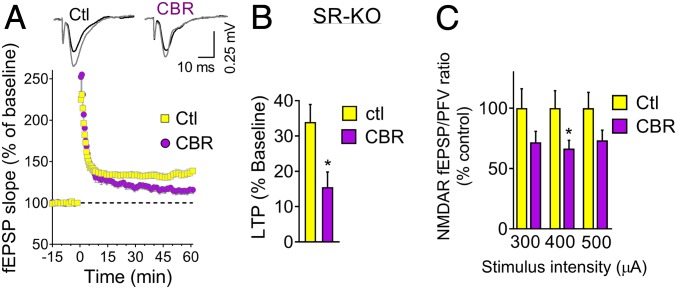
As we observed in SDH-treated slices (Fig. 2C), the isolated NMDAR fEPSPs or EPSCs are often little or not affected in SR-KO mice (4, 21, 22), indicating that additional mechanisms, such as glycine release, sustain NMDAR synaptic activation. Since CBR-5884 also inhibits glycine synthesis (Fig. 1D), we then investigated the roles of Phgdh-derived glycine using SR-KO mice, which mostly lack d-serine (4). We found that CBR-5884 decreased the magnitude of the LTP in SR-KO slices by 50% (Fig. 3 A and B). Likewise, CBR-5884 depressed the isolated NMDAR fEPSPs in slices from SR-KO mice by about 30% (Fig. 3C). Our observations suggest that Phgdh-derived glycine regulates synaptic NMDAR activation in mature SC-CA1 as well.
Fig. 3.
Phgdh inhibition decreases the NMDAR synaptic activation in adult SR-KO mice. (A) LTP induced by 1 × 100 Hz in the hippocampal CA1 field of SR-KO mice following 2-h incubation with and without 40 μM CBR-5884 (n = 12 and 11 slices, respectively). (B) CBR-5884 decreased the LTP magnitude calculated from the average of the last 15-min recording of A. Unpaired 2-tailed Student’s t test (P = 0.010). (C) Isolated NMDAR potentials were decreased by a 2-h incubation with 40 μM CBR-5884 (n = 12 slices). Unpaired 2-tailed Student’s t test comparing control and CBR values at each stimulus (P = 0.14, 0.047, and 0.10 for 300, 400, and 500 mA, respectively). Values are average ± SEM. *P < 0.05.
Postnatal Mechanisms Impacting the Regulation of the Serine Shuttle.
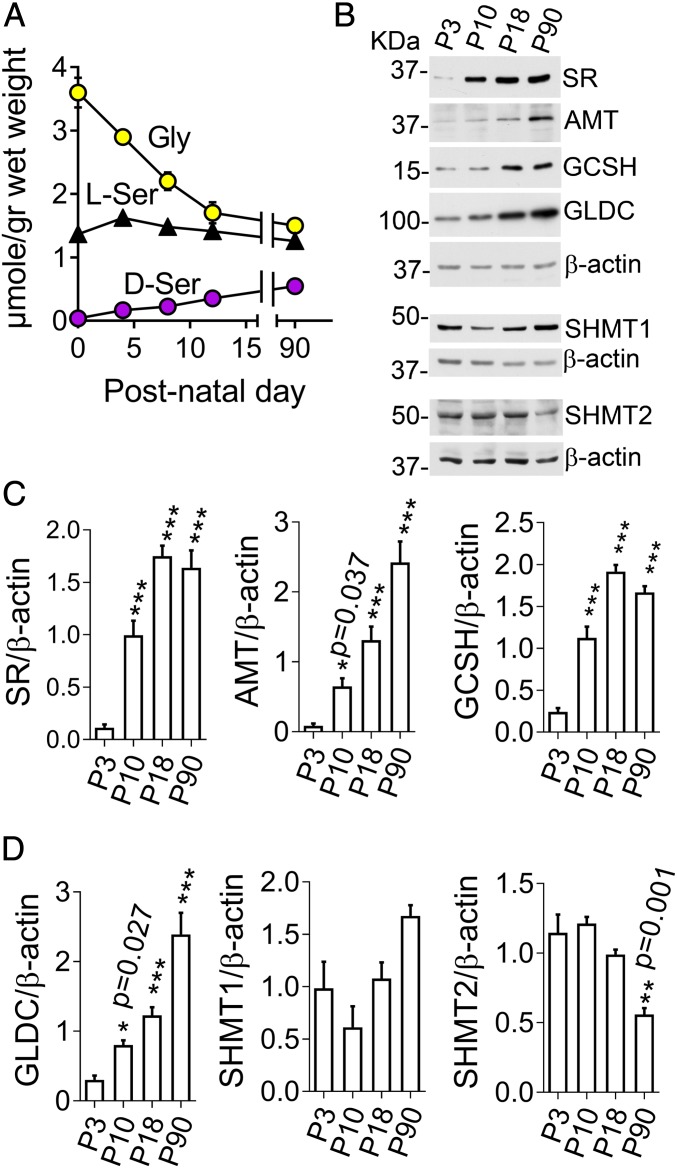
In light of the importance of Phgdh-derived glycine and d-serine in NMDAR activity, we investigated postnatal mechanisms regulating the coagonists’ availability. In agreement with a previous report (16), we found that hippocampal glycine levels progressively decrease in the first 2 postnatal weeks, though it stabilizes in adulthood (Fig. 4A). There is a clear inverse relationship between glycine and d-serine levels. The latter progressively increases during the same period, while levels of l-serine are kept constant (Fig. 4A). Strikingly, SR expression increases more than 1 order of magnitude in the hippocampus (Fig. 4B), explaining the postnatal increase in forebrain d-serine.
Fig. 4.
Postnatal changes in glycine, l-serine, and d-serine levels and their metabolic enzymes in the hippocampus. (A) Hippocampal levels of amino acids in mice. Values are average ± SEM of 5 (P0, P4, P8, and P12) and 10 (P90) animals. (B) Representative Western blot analysis of SR, aminomethyltransferase (AMT), glycine cleavage system protein H (GCSH), glycine decarboxylase (GLDC), serine hydroxymethyltransferase 1 (SHMT1) and 2 (SHMT2) and β-actin loading controls. (C and D) Densitometric analysis of Western blots as in B (4 to 7 mice). One-way ANOVA and Dunnet’s post hoc test of P10, P18, and P90 values compared to P3. Values are average ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
Mechanisms underlying the postnatal decrease in brain glycine levels have not been investigated so far. We found that the expression of the 3 intrinsic subunits of the glycine cleavage enzyme system, AMT, GCSH, and GLDC, increased several times in the hippocampus between postnatal day (P) P3 and P90 (Fig. 4 C and D). SHMT enzymes, which interconvert glycine and l-serine, are mostly unchanged in the first 2 postnatal weeks, though SHMT2 levels decrease at P90 (Fig. 4D). The same pattern of expression changes was observed for all tested enzymes in the neocortex (SI Appendix, Fig. S7). Our results indicate that increased glycine catabolism via the glycine cleavage system and the progressive increase in SR expression accounts for the emergence of d-serine as an additional regulator of NMDARs in the late postnatal period.
Glycine Regulates d-Serine Metabolism.
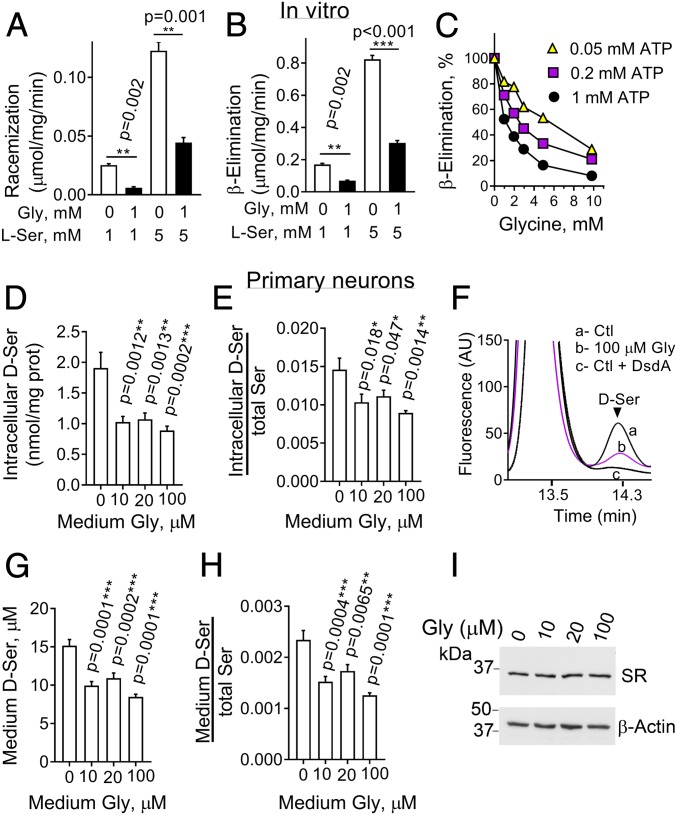
Although d-serine and glycine share a common target receptor, no cross-talk between their metabolic pathways is known. Previous studies found that glycine inhibits the purified SR enzyme in vitro by a competitive-like mechanism (23). However, these experiments were carried out only in vitro and at supraphysiologic l-serine and glycine concentrations. We then monitored the racemization and β-elimination activities of SR under physiological-like intracellular glycine and l-serine levels (both at 1 mM). We found that glycine inhibits the synthesis of d-serine by purified SR by 70 to 80% under our conditions (Fig. 5A). Similarly, glycine strongly inhibited the SR β-elimination (Fig. 5B), and this effect was potentiated by raising the ATP levels (Fig. 5C). Notably, at physiological intracellular ATP levels (1 mM), glycine inhibited the SR β-elimination even in the presence of near-saturating supraphysiological l-serine concentrations (25 mM) (Fig. 5C, circles), indicating a robust inhibitory activity toward SR.
Fig. 5.
Glycine inhibits SR activity in vitro and in primary neuronal cultures. (A) Glycine (1 mM) inhibited the racemization of l-serine into d-serine by the recombinant SR in vitro. The reaction was carried out in the presence of 50 mM triethanolamine-HCl (pH 8.0), 2 mM ATP, 2 mM MgCl2, 150 mM NaCl, 50 µM PLP, and either 1 or 5 mM l-serine. Paired 2-tailed Student’s t test. (B) Glycine (1 mM) inhibited the β-elimination by recombinant SR in the presence of either 1 or 5 mM l-serine. Paired 2-tailed Student’s t test. (C) ATP potentiates the inhibitory effect of glycine on the β-elimination. The assays were carried out in duplicates in the presence of 25 mM l-serine and 0.05, 0.2, or 1 mM ATP. (D) Glycine decreased the intracellular d-serine levels in primary neuronal cultures incubated for 48 h in media containing 1 mM l-serine. Samples with glycine were compared to the control (0 glycine) by 1-way ANOVA and Dunnett’s post hoc test. (E) Intracellular d-serine normalized by the total intracellular serine (L+D) from D. (F) Representative HPLC chromatogram showing the virtual disappearance of the d-serine peak after treating the samples with 100 µg/mL recombinant DsdA. (G) d-serine levels in the culture media of the primary cultures analyzed as in D. (H) Extracellular d-serine normalized by the total extracellular serine (L+D) levels in the primary neuronal cultures, and analyzed as in D. (I) Representative Western blot of SR in primary neuronal cultures incubated in the presence of increasing amounts of glycine. Lower corresponds to β-actin loading control. Values are average ± SEM of 4 (A and B), 2 (C), and 6 (D, E, G, and H) experiments.
Next, we examined the effect of glycine on the synthesis of d-serine in primary neuronal cultures. Since the traditional neurobasal culture medium contains exogenous glycine, we produced a glycine-free neurobasal medium. Supplementation of cultures with as little as 10 μM glycine, a concentration that matches the extracellular glycine levels (24), reduced intracellular d-serine levels by almost 50% (Fig. 5D). Glycine also inhibited the synthesis of d-serine normalized for the total intracellular serine (L+D) concentrations, indicating the inhibition is not due to reciprocal changes in l-serine (Fig. 5E). In control experiments, incubation of the cell extracts with recombinant d-serine deaminase (DsdA) (22) to degrade d-serine confirmed the authenticity of the d-serine signal in the HPLC analysis (Fig. 5F, compare a and b with c). Glycine also reduced the levels of d-serine exported to the culture medium, indicating that it causes a global decrease in d-serine production (Fig. 5 G and H). No changes in SR expression were observed upon glycine treatment (Fig. 5I).
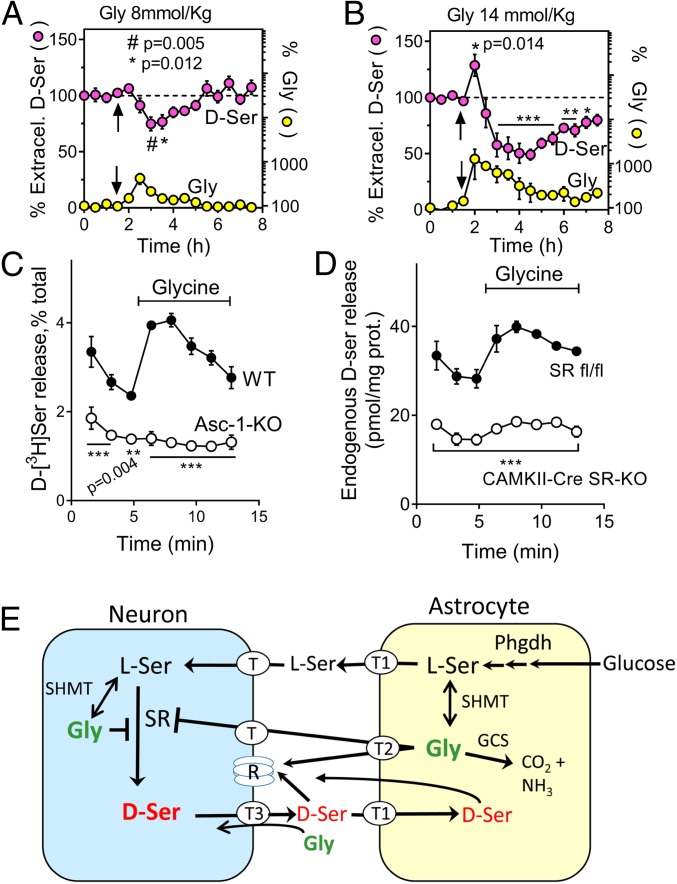
To investigate if glycine affects d-serine metabolism in vivo, we carried out microdialysis of the striatum and monitored extracellular d-serine before and after i.p. injection of glycine. We found that glycine elicited a significant decrease in the extracellular levels of d-serine (Fig. 6A). d-serine levels progressively returned to normal as the extracellular glycine returned to its basal levels (Fig. 6A).
Fig. 6.
Glycine affects d-serine homeostasis by a dual mechanism. (A) In vivo microdialysis of the striatum of 10-wk-old mice shows a decrease in extracellular d-serine following the injection of 8 mmol/kg glycine (i.p.). Arrow denotes the injection time. Values are average ± SEM of 5 experiments. Repeated measures 1-way ANOVA and Dunnett’s post hoc test compare all values with the baseline d-serine value determined 30 min before the glycine administration. Unmarked data points had P > 0.05. (B) In vivo microdialysis of the striatum of 10-wk-old mice upon injection of 14 mmol/kg glycine (i.p.) shows a transient increase in extracellular d-serine followed by a more prolonged decrease. Values are average ± SEM of 4 experiments. **P < 0.01, ***P < 0.001. Other conditions were as in A. (C) Release of preloaded d-[3H]serine from neocortical slices induced by 100 µM glycine (capped line). Slices from Asc-1 KO mice (○) display reduced release when compared to WT littermates (●) before and after stimulation with glycine. ***, different from WT at P < 0.001. To ensure similar levels of preloaded d-serine in WT and Asc-1 KO mice, the slices were preincubated with d-[3H]serine for 15 and 30 min, respectively. (D) The glycine-induced release of endogenous d-serine was reduced in acute cortical slices from CAMKIIα-Cre SR-KO mice (○) when compared to SR fl/fl controls (●). ***, different from SR fl/fl at P < 0.001. Values in C and D are average ± SEM of 3 experiments done in triplicates and analyzed by 2-way repeated measures ANOVA with Bonferroni’s post hoc test. (E) Phgdh-dependent serine shuttle mechanism. The scheme depicts the conversion of glucose into l-serine in astrocytes and the role of Phgdh-derived l-serine in providing d-serine and glycine to activate synaptic NMDARs (R) in adult mice. The model also illustrates the dual effect of glycine on d-serine metabolism. The first is the direct inhibition of the neuronal SR by glycine. The second is the transient increase in d-serine release through a Gly/d-Ser exchange catalyzed by the Asc-1 transporter (T3). Serine and glycine are released from astrocytes through ASCT1 (T1) and GlyT1 (T2), respectively, and taken up in neurons by unidentified transporters (T). SHMT isoforms interconvert l-serine and glycine. The latter is degraded by the glycine cleavage system (GCS), explaining the postnatal decline in forebrain glycine levels.
Surprisingly, the microdialysis experiments also uncovered an additional aspect of glycine/d-serine cross-talk, which was evident only at a higher glycine dose. When administered at 14 nmol/kg, glycine promoted a transient increase in the extracellular levels of d-serine, followed by a stronger and more prolonged decline (Fig. 6B). There was a modest increase in extracellular l-serine levels upon glycine injection (SI Appendix, Fig. S8 A and B). Thus, glycine may transiently increase d-serine release without much affecting the l-serine levels.
To examine a direct effect of glycine on d-serine release, we investigated the role of the Asc-1 transporter, an exchanger that can transport both glycine and d-serine (25) and mediates d-serine release (26). In HEK293 cells cotransfected with the Asc-1 transporter and its ancillary subunit 4F2hc, glycine elicited the release of preloaded d-[3H]serine, compatible with a glycine/d-serine exchange mechanism (SI Appendix, Fig. S9A). No change in d-[3H]serine release was detectable in the absence of Asc-1 (SI Appendix, Fig. S9A), despite the comparable loading of radioactive d-serine between Asc-1/4F2hc- and 4F2hc-expressing cells (SI Appendix, Fig. S9B). In primary neuronal cultures, the addition of glycine at levels as low as 10 µM increased the fractional release of d-serine (SI Appendix, Fig. S9C), and this was abolished by adding the selective Asc-1 nontransportable inhibitor Lu AE00527 (26).
Like that seen in transfected cells and primary cultures, glycine increased the release of d-[3H]serine detected in the perfusate of acute cortical slices (Fig. 6C). The effect of glycine on d-serine release was virtually absent from slices of Asc-1 KO mice (Fig. 6C), despite the comparable levels of preloaded d-[3H]serine in Asc-1 KO and wild-type (WT) slices (0.25 ± 0.08 vs. 0.14 ± 0.05 nmol/mg, respectively, average ± SEM, P = 0.25). These data indicate that Asc-1 is the primary transporter mediating the glycine-induced transient release of d-serine.
Glycine also induced robust release of endogenous d-serine from acute cortical slices (Fig. 6D). This effect was attenuated in slices from glutamatergic neuronal SR-KO mice, which also displayed much lower basal endogenous d-serine release (Fig. 6D), suggesting a neuronal origin for both the basal and glycine-stimulated d-serine release.
Discussion
Our data support the role of a Phgdh-dependent export of l-serine from astroglia to neurons in regulating the NMDAR synaptic activation (Fig. 6E). Inactivation of Phgdh by CBR-5884 depressed the isolated NMDAR synaptic potentials and reduced the hippocampal LTP. The effects were specific, since Phgdh inhibition did not affect the basal synaptic transmission, and adding l-serine prevented the inhibitory activity of CBR-5884 and restored LTP. Moreover, enzymatic degradation of the extracellular l-serine from slices by SDH treatment reduced the LTP magnitude, suggesting an essential role of l-serine release from astrocytes in regulating NMDAR activity. In addition to l-serine, SDH also degrades threonine (27), but the latter has no connection to NMDARs.
We found that inhibition of l-serine synthesis via the Phgdh pathway impaired the synthesis of d-serine to the same extent as the deletion of SR in glutamatergic neurons, supporting a predominant neuronal source of newly synthesized d-serine. Benneyworth et al. (9) detected only a small decrease (30%) in d-serine levels across forebrain regions of CAMKIIα-Cre SR-KO mice, even though the reduction in SR levels was substantially higher. Using the same mouse line, we found that knockout of SR in forebrain glutamatergic neurons decreased the de novo synthesis of d-serine in slices by about 80%. Therefore, direct determination of d-serine synthesis is a better predictor of the cellular sites of d-serine production by avoiding confounding elements that may preserve the local d-serine despite a lower synthesis, such as: 1) retrograde axonal transport of d-serine taken up in other brain areas; 2) higher influx of peripheral d-serine via the blood–brain barrier; 3) diffusion of d-serine from the cerebrospinal fluid into the brain interstitial fluid via transport by ependymal cells; 4) a compensatory decrease in d-serine turnover; or 5) any combination thereof.
Like d-serine, glycine also mediates NMDAR synaptic activation in the hippocampus (16, 22). At the SC-CA1 synapses, glycine stimulates synaptic NMDARs in young mice, but the preference to glycine switches to d-serine in adults. However, there is no consensus on the extent of this effect (16, 28). We found that inhibiting the glycine synthesis by inactivating the Phgdh pathway depresses the isolated NMDAR potentials and reduces the LTP in SR-KO, indicating that Phgdh-derived glycine also governs synaptic NMDARs in the mature hippocampus. Our data suggest that glycine degradation via the glycine cleavage system in the first postnatal weeks ensures the emergence of d-serine as a major coagonist by partially relieving SR inhibition. Nevertheless, the abundance of glycine even in adult mice, still enables its role as a synaptic NMDAR coagonist, as suggested by our experiments with SR-KO mice and Phgdh inhibition.
Our study also demonstrates that glycine regulates d-serine metabolism by a dual mechanism. First, physiological intracellular levels of glycine exert a persistent inhibition of SR activity in vitro and primary neuronal cultures. Glycine also decreased the extracellular d-serine levels at the striatum, suggesting that glycine interferes with d-serine metabolism in vivo. The extent of glycine inhibitory activity makes this amino acid the most robust endogenous regulator of SR activity to date. Second, our study reveals that glycine also transiently increases d-serine release via the Asc-1 transporter, indicating fine tuning of d-serine dynamics in a shorter temporal scale. The specificity of the effects was validated with controls using an Asc-1 inhibitor and Asc-1 KO mice, which abrogated the effects of glycine on d-serine release dynamics. Furthermore, deletion of SR in glutamatergic neurons blunted glycine effects on d-serine release, indicating glycine preferentially affects the neuronal pool of d-serine.
d-serine uptake systems are much less efficient than glycine transporters in removing the coagonist from synapses (29). Therefore, physiological inhibition of SR by glycine may help to prevent NMDAR overactivation and excitotoxicity by excess d-serine at the synapse. On the other hand, pathological suppression of d-serine synthesis by glycine may explain the reduction in cerebral d-serine in patients with nonketotic hyperglycinemia, a condition associated with a massive increase in brain glycine due to mutations in the glycine cleavage system (30).
The connection between glycine and d-serine metabolism clarifies some inconsistencies in the literature. Inhibition of SR by glycine may explain why some studies failed to detect endogenous d-serine in primary neuronal cultures (31), since the traditional neurobasal medium used in cultures contains 400 μM exogenous glycine. Conversely, our data suggest that removing extracellular glycine may affect local d-serine metabolism. Previous studies using glycine oxidase treatment to examine NMDAR coagonist identity (16, 21, 24, 28) may have affected local d-serine dynamics and therefore should be interpreted with caution.
The existence of 2 coagonists allows spatially and temporally restricted regulation of NMDARs. Glycine and d-serine are released from different sites through diverse mechanisms. Glycine is likely released from astrocytes through the reversal of the GlyT1 (32). On the other hand, neurons release d-serine through neutral amino acid antiporters (22, 26). SR is enriched at the dendritic spines (12), suggesting that d-serine may function in an autocrine fashion (2, 3, 7). In addition to different lifetimes and release mechanisms, glycine and d-serine differentially regulate NMDARs, depending on the characteristics of the synaptic connections and receptor types. In the amygdala, the tonic release of d-serine regulates basal NMDAR synaptic activity, while glycine mediates activity-dependent increases in NMDAR activation (21). By contrast, d-serine mediates a large fraction of the activity-dependent NMDAR activation in the mature SC-CA1 synapses (16). However, in the medial performant path-dentate gyrus mature synapses, where the GluN2B NMDARs play a prominent role, glycine remains the major synaptic coagonist (16). We still do not know to what extent the roles of the 2 coagonists differ at synaptic and extrasynaptic NMDARs throughout the brain.
We propose a Phgdh-dependent serine shuttle model that refines the previous untested model (19) by purporting the role of Phgdh in regulating NMDA receptor synaptic activation along with the multifaceted actions of glycine (Fig. 6E). Our working model does not exclude other mechanisms of d-serine signaling, including secondary astroglial d-serine release and subsequent NMDAR activation. Pathological stimuli cause a population of reactive astrocytes to express SR (15), so that astroglial d-serine may be important in pathology as well (3, 14). We assert, however, that under physiological conditions the vast majority of d-serine is of neuronal origin and its production depends on both the astrocytic l-serine export and the intracellular levels of glycine. The astrocytic Phgdh pathway generates the primary regulators of neuronal d-serine, providing multifaceted mechanisms by which astrocyte–neuron metabolic cross-talk regulates synaptic NMDARs.
Materials and Methods
Mice Breeding and Genotyping.
SR fl/fl mice, CAMKIIα-Cre mice, and constitutive SR-KO mice in C57BL/6J background were provided by J. T. Coyle, McLean Hospital, Boston, MA. Genotyping was performed as previously described (9). For obtaining CAMKIIα-Cre SR-KO mice, SR fl/fl were crossed with CAMKIIα-Cre, SR fl/+ and the SR fl/fl littermates were used as controls. Mice were analyzed after 4 to 5 mo. Asc-1 (Slc7a10) KO mice were generated by Deltagen Inc. (San Mateo, CA), backcrossed with C57BL/6 for 5 generations and maintained as Asc-1−/+. Asc-1−/− were obtained by crossing Asc-1+/− and used at postnatal days 10 to 12. All of the procedures used in this study were approved by the Committee of Animal Experimentation of the Technion-Israel Institute of Technology.
Additional methods are described in the SI Appendix, Materials and Methods.
Supplementary Material
Acknowledgments
This work was supported by grants from the Israel Science Foundation (to H.W., S.B., and S.E.) and the Allen and Jewell Prince Center for Neurodegenerative Diseases of the Brain (to H.W. and S.E.), and INSERM and Caen Normandie University (to J.-M.B.). H.W. is the incumbent of the Rebecca and Herman Fineberg Chair in Life Sciences. S.N. is supported by a scholarship from the Ministry of Science and Technology in the name of Zvi Yanai for Arab, Druze, and Circassian students. We thank Drs. Barbara Campanini, Stefano Bruno, and Andrea Mozzarelli (University of Parma) for valuable advice on in vitro SR experiments.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1909458116/-/DCSupplemental.
References
- 1.Mothet J. P., et al. , D-serine is an endogenous ligand for the glycine site of the N-methyl-D-aspartate receptor. Proc. Natl. Acad. Sci. U.S.A. 97, 4926–4931 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolosker H., The neurobiology of d-serine signaling. Adv. Pharmacol. 82, 325–348 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Coyle J. T., Balu D. T., The role of serine racemase in the pathophysiology of brain disorders. Adv. Pharmacol. 82, 35–56 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basu A. C., et al. , Targeted disruption of serine racemase affects glutamatergic neurotransmission and behavior. Mol. Psychiatry 14, 719–727 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Miranda J., Panizzutti R., Foltyn V. N., Wolosker H., Cofactors of serine racemase that physiologically stimulate the synthesis of the N-methyl-D-aspartate (NMDA) receptor coagonist D-serine. Proc. Natl. Acad. Sci. U.S.A. 99, 14542–14547 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marchetti M., et al. , ATP binding to human serine racemase is cooperative and modulated by glycine. FEBS J. 280, 5853–5863 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Kartvelishvily E., Shleper M., Balan L., Dumin E., Wolosker H., Neuron-derived D-serine release provides a novel means to activate N-methyl-D-aspartate receptors. J. Biol. Chem. 281, 14151–14162 (2006). [DOI] [PubMed] [Google Scholar]
- 8.Miya K., et al. , Serine racemase is predominantly localized in neurons in mouse brain. J. Comp. Neurol. 510, 641–654 (2008). [DOI] [PubMed] [Google Scholar]
- 9.Benneyworth M. A., Li Y., Basu A. C., Bolshakov V. Y., Coyle J. T., Cell selective conditional null mutations of serine racemase demonstrate a predominate localization in cortical glutamatergic neurons. Cell. Mol. Neurobiol. 32, 613–624 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ehmsen J. T., et al. , D-serine in glia and neurons derives from 3-phosphoglycerate dehydrogenase. J. Neurosci. 33, 12464–12469 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balu D. T., Takagi S., Puhl M. D., Benneyworth M. A., Coyle J. T., D-serine and serine racemase are localized to neurons in the adult mouse and human forebrain. Cell. Mol. Neurobiol. 34, 419–435 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin H., Jacobi A. A., Anderson S. A., Lynch D. R., D-serine and serine racemase are associated with PSD-95 and glutamatergic synapse stability. Front. Cell. Neurosci. 10, 34 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolosker H., Balu D. T., Coyle J. T., The rise and fall of the d-serine-mediated gliotransmission hypothesis. Trends Neurosci. 39, 712–721 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ivanov A. D., Mothet J. P., The plastic d-serine signaling pathway: Sliding from neurons to glia and vice-versa. Neurosci. Lett. 689, 21–25 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Perez E. J., et al. , Enhanced astrocytic d-serine underlies synaptic damage after traumatic brain injury. J. Clin. Invest. 127, 3114–3125 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le Bail M., et al. , Identity of the NMDA receptor coagonist is synapse specific and developmentally regulated in the hippocampus. Proc. Natl. Acad. Sci. U.S.A. 112, E204–E213 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamasaki M., et al. , 3-Phosphoglycerate dehydrogenase, a key enzyme for l-serine biosynthesis, is preferentially expressed in the radial glia/astrocyte lineage and olfactory ensheathing glia in the mouse brain. J. Neurosci. 21, 7691–7704 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang J. H., et al. , Brain-specific Phgdh deletion reveals a pivotal role for L-serine biosynthesis in controlling the level of D-serine, an N-methyl-D-aspartate receptor co-agonist, in adult brain. J. Biol. Chem. 285, 41380–41390 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolosker H., Radzishevsky I., The serine shuttle between glia and neurons: Implications for neurotransmission and neurodegeneration. Biochem. Soc. Trans. 41, 1546–1550 (2013). [DOI] [PubMed] [Google Scholar]
- 20.Mullarky E., et al. , Identification of a small molecule inhibitor of 3-phosphoglycerate dehydrogenase to target serine biosynthesis in cancers. Proc. Natl. Acad. Sci. U.S.A. 113, 1778–1783 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y., et al. , Identity of endogenous NMDAR glycine site agonist in amygdala is determined by synaptic activity level. Nat. Commun. 4, 1760 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenberg D., et al. , Neuronal D-serine and glycine release via the Asc-1 transporter regulates NMDA receptor-dependent synaptic activity. J. Neurosci. 33, 3533–3544 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dunlop D. S., Neidle A., Regulation of serine racemase activity by amino acids. Brain Res. Mol. Brain Res. 133, 208–214 (2005). [DOI] [PubMed] [Google Scholar]
- 24.Kaplan E., et al. , ASCT1 (Slc1a4) transporter is a physiologic regulator of brain d-serine and neurodevelopment. Proc. Natl. Acad. Sci. U.S.A. 115, 9628–9633 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Safory H., et al. , The alanine-serine-cysteine-1 (Asc-1) transporter controls glycine levels in the brain and is required for glycinergic inhibitory transmission. EMBO Rep. 16, 590–598 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sason H., et al. , Asc-1 transporter regulation of synaptic activity via the tonic release of d-serine in the forebrain. Cereb. Cortex 27, 1573–1587 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Ogawa H., et al. , Rat liver serine dehydratase. Bacterial expression and two folding domains as revealed by limited proteolysis. J. Biol. Chem. 274, 12855–12860 (1999). [DOI] [PubMed] [Google Scholar]
- 28.Ferreira J. S., et al. , Co-agonists differentially tune GluN2B-NMDA receptor trafficking at hippocampal synapses. Elife 6, e25492 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berger A. J., Dieudonné S., Ascher P., Glycine uptake governs glycine site occupancy at NMDA receptors of excitatory synapses. J. Neurophysiol. 80, 3336–3340 (1998). [DOI] [PubMed] [Google Scholar]
- 30.Iwama H., et al. , Depletion of cerebral D-serine in non-ketotic hyperglycinemia: Possible involvement of glycine cleavage system in control of endogenous D-serine. Biochem. Biophys. Res. Commun. 231, 793–796 (1997). [DOI] [PubMed] [Google Scholar]
- 31.Yang Y., et al. , Contribution of astrocytes to hippocampal long-term potentiation through release of D-serine. Proc. Natl. Acad. Sci. U.S.A. 100, 15194–15199 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shibasaki K., Hosoi N., Kaneko R., Tominaga M., Yamada K., Glycine release from astrocytes via functional reversal of GlyT1. J. Neurochem. 140, 395–403 (2017). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.