Abstract
Isonucleosides are rather stable regioisomeric analogs of nucleosides with broad therapeutic potential. We have previously demonstrated the ability of 5′ and 6′-isonucleosides to inhibit the activity of acetylcholinesterase, a major target for Alzheimer’s disease therapy. Continuing with our research on this topic, we report herein on the synthesis and biological evaluation of a variety of novel terminal isonucleosides and theobromine isonucleotide analogs. Xylofuranose-based purine or uracil 5′-isonucleosides and xylofuranos-5′-yl or glucos-6′-yl theobromine derivatives were accessed via Mitsunobu coupling between partially protected xylofuranose or glucofuranose derivatives with a nucleobase using conventional or microwave-assisted heating conditions. Theobromine-containing N-isonucleosidyl sulfonamide and phosphoramidate derivatives were synthesized from isonucleosidyl acetate precursors. The most active compounds in the cholinesterase inhibition assays were a glucopyranose-based theobromine isonucleosidyl acetate, acting as a dual inhibitor of acetylcholinesterase (AChE, Ki = 3.1 µM) and butyrylcholinesterase (BChE, Ki = 5.4 µM), and a 2-O,4-O-bis-xylofuranos-5′-yl uracil derivative, which displayed moderate inhibition of AChE (Ki = 17.5 µM). Docking studies revealed that the active molecules are positioned at the gorge entrance and at the active site of AChE. None of the compounds revealed cytoxic activity to cancer cells as well as to non-malignant mouse fibroblasts.
Keywords: isonucleosides, theobromine, Mitsunobu reaction, cholinesterase inhibitors
1. Introduction
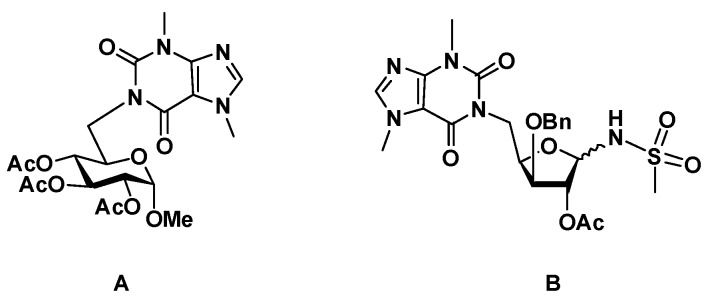
Isonucleosides are regioisomers of nucleosides in which a nucleobase or an analogous nitrogeneous hetereoaromatic motif is linked to the sugar moiety at a non-anomeric position. This group of structures has attracted significant interest in the search of new nucleos(t)ide analogs, owing to the therapeutic potential of these groups of compounds, especially as anticancer and antiviral agents [1,2]. The lack of an N-glycosidic bond in isonucleosides linking the sugar and nucleobase moieties confers them a higher chemical and enzymatic stability than that of nucleosides. Most of the reported isonucleosides possess the nucleobase linked to furanose systems at C-2 or at C-3 [3,4,5,6,7,8,9,10,11], among which some molecules exhibited anticancer [4,5] or antiviral [6,7,8,9,11] activities. Pyranosyl isonucleosides have been relatively less exploited and the few reported examples include 2′-fluoroarabino-2′-yl pyrimidine derivatives [12], which displayed anticancer activities, and pyranos-6′-yl isonucleosides [13,14], which resulted from our previous investigations and showed selective and moderate to good inhibition of acetylcholinesterase (AChE). This enzyme hydrolyses the neurotransmitter acetylcholine and is a main therapeutic target for the symptomatic treatment of Alzheimer’s disease (AD) [15,16]. Isonucleosides comprising 2-acetamido-6-chloropurine or theobromine units linked to methyl glucoside moieties and a bis-glucopyranosid-6-yl thymine derivative were the most significant inhibitors with Ki values ranging from 7.1 µM to 4.3 µM. The good inhibitory effect of the theobromine 6′-isonucleoside (A, Figure 1) motivated the synthesis of furanosyl analogs, which included an N-isonucleosidyl sulfonamide derivative (B, Figure 1) [17]. This isonucleotide analog, in which a sulfonamide is a neutral surrogate and a potential mimetic of a phosphate group, also showed micromolar and selective inhibition of AChE, besides revealing low toxicity in normal fibroblasts and in a neuronal cell, indicating therefore the interest of this type of skeleton in the search for new lead molecules for AD.
Figure 1.
Terminal theobromine isonucleosides, including a glucopyranosid-6′-yl derivative (A) and a furanos-5’-yl-based N-isonucleosidyl sulfonamide (B), as selective AChE inhibitors.
Encouraged by our previous results, we further explored the synthesis and biological potential of terminal isonucleosides, focusing on furanosyl derivatives and giving further emphasis to theobromine derivatives. We report herein on the synthesis of various xylofuranos-5′-yl purine and uracil derivatives, furanosyl or pyranosyl xylo/gluco-configured terminal theobromine isonucleosides and theobromine N-isonucleosidyl analogs of compound B comprising anomeric sulfonamide and phosphoramidate moieties. Molecules possessing a long O-alkyl chain at C-3 (octyl, dodecyl groups), instead of an O-benzyl group present in the previous reported theobromine furanosyl derivatives (such as B), were accessed. The presence of the long hydrocarbon chain may enhance the aptitude of the compounds to cross the blood brain barrier, which is a relevant property to consider when planning anti-AD agents [18], particularly if brain AChE is targeted. The ability of the synthesized compounds to inhibit cholinesterases (ChEs) was further evaluated. Moreover, in view of the anticancer potential of nucleos(t)ide analogs, the cytotoxicity of the new isonucleosides on cancer cells was assessed.
2. Results
2.1. Chemistry
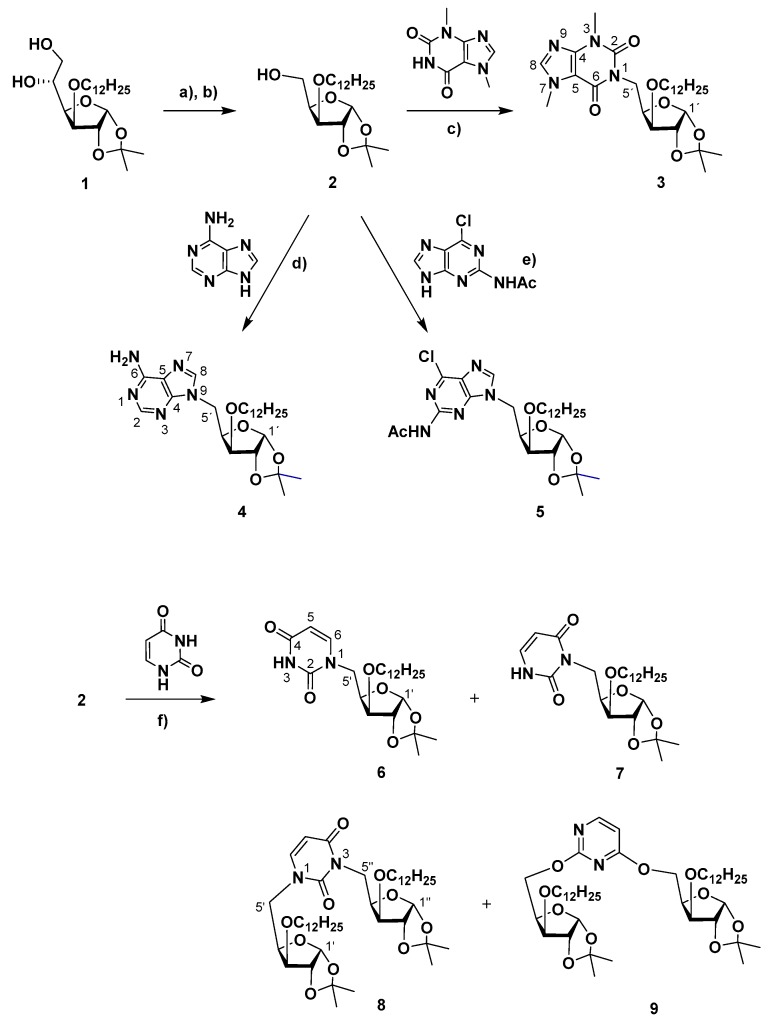
The synthesis of 3′-O-dodecyl xylofuranos-5′-yl isonucleosides was based on the Mitsunobu coupling between the 3′-O-dodecyl-1,2-O-isopropylidene xylofuranose precursor (2), which was prepared from 3′-O-dodecyl-1,2-O-isopropylidene glucofuranose (1) [19], with purine derivatives and uracil in the presence of diethyl azodicarboxylate (DEAD) and triphenylphosphine (Scheme 1). The reaction with the purine alkaloid theobromine in THF at 50 °C during 2 days led to the desired isonucleoside 3 in a low yield (16%). The conversion was significantly improved when performing the reaction under microwave irradiation (MW, 150 W, Pmax 250 Psi) at 65 °C in a mixture of tetrahydrofuran/N,N-dimethylformamide (THF/DMF), conducting to 3 in a satisfactory yield of 44% after only 30 min. The coupling of 2 with adenine was carried out using the above mentioned MW-assisted protocol, since we have previously observed low conversions even after long reaction times (16–96 h) in the Mitsunobu reaction between this nucleobase and partially protected glucosides under conventional heating [14]. The N9-xylofuranos-5′-yl adenine 4 was obtained in moderate yield (33%) within 50 min as the sole heterocoupling product, whose N9‒C-5 linkage was determined by HMBC correlations between protons H-5′ and C-4/C-8 of the purine moiety. N9-regioselectivity also occurred in the coupling between 2 and 2-acetamido-6-chloropurine, which was performed under conventional heating conditions leading to the isonucleoside 5 in 51% yield. Similarly, as for 4, the HMBC spectra of 5 showed the key correlations between H-5′ and both C-4/C-8 which comproved the regiochemistry of the isonucleosidic bond. In contrast with purines, the Mitsunobu reaction involving uracil afforded different regiosomeric products of both mono- and bis-coupling, an outcome previously found when using pyrimidines in analogous reactions performed under conventional heating [14]. The isonucleosides comprising the uracil moiety linked by N1 (6) and by N3 (7) were obtained in identical yields (12%–13%) and were assigned based on the HMBC correlations of the protons H-5′ with C-2/C-6 or with C-2/C-4, respectively. The distinct chemical shifts for C-5′ in the 13C NMR spectra of 6 and 7, at 48.1 ppm and at 40.1 ppm, respectively, were additional indicative spectral features for their regiochemical elucidation. The uracil-linked disaccharides N1,N3- and 2-O,4-O-xylofuran-5-yl uracils (8, 9) were formed in yields of 29% and 5%, respectively. Assignment of the N1,N3-bis-xylofuranos-5-yl uracil (8) was based on its NMR spectral data, which combined the features of both N1- and N3-linked isonucleosides, namely through the HMBC correlations between H-5′ protons with C-2/C-6 and between H-5″ with C-2/C-4 as well as the C-5′ and C-5″ resonances at 49.0 ppm and 40.8 ppm, respectively. In the case of the 2,4-bis-O-xylofuranos-5-yl uracil derivative (9), the 1H NMR and 13C NMR signals of the uracil moiety are deshielded relatively to those of the N1,N3-linked regioisomer 8. The differences between the signals of H-6 and H-5 of the uracil ring are particularly notorious, appearing at 8.18 ppm and 6.41 ppm in 9, whereas in the case of 8 these protons resonate at 7.24 ppm and 5.69 ppm, respectively. In addition, the signals of C-5′ and C-5″ of the sugar moiety at 64.4 and 64.7 ppm further confirmed their connection to the 2-O and 4-O atoms of the uracil unit.
Scheme 1.
Reagents and conditions: (a) NaIO4, THF (60 % aq. soln.), room temp., 2.5 h, 79% [19]; (b) NaBH4, EtOH/H2O, room temp., 1 h, 81% [19]; (c) theobromine, PPh3, diethyl azodicarboxylate (DEAD), THF, 50 °C, 48 h, 16% or THF/DMF, MW, max. 150 W, 65 °C, 30 min, 44%; (d) adenine, PPh3, DEAD, THF/DMF, MW, max. 150 W, 65 °C, 50 min, 31%; (e) 2-NHAc-6-Cl-purine, PPh3, DEAD, THF, 50 °C, 16 h, 51%; (f) uracil, PPh3, DEAD, THF, 50 °C, 18 h, 12% (6), 13% (7), 29% (8), 5% (9).
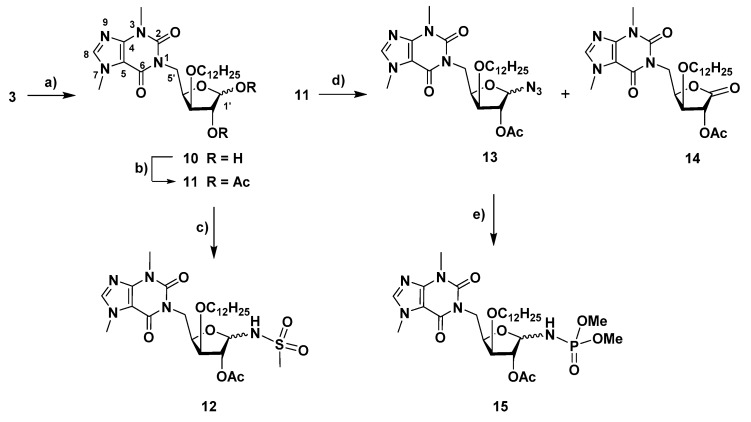
Motivated by our previous studies, which revealed the interest of theobromine isonucleosides as promising lead molecules for AD (Figure 1) [14,17], we further focused our synthetic work on a variety of theobromine derivatives. The theobromine xylofuranosyl isonucleoside 3 was the precursor for the synthesis of isonucleotide analogs via derivatization at the anomeric center (Scheme 2). Thus, 3 was subjected to acid-mediated hydrolysis of the acetonide moiety (aq. trifluoroacetic acid (TFA) 60%), leading to diol 10. Further acetylation (Ac2O/pyridine) afforded the isonucleosidyl acetate 11, which was subsequently converted into N-isonucleosidyl derivatives containing polar anomeric moieties capable of hydrogen bond interactions as potential isosteres of a phosphate group. N-isonucleosidation of methanesulfonamide with 11 in the presence of BF3·Et2O provided the N-isonucleosidyl sulfonamide 12 in 81% yield, as a 3-O-dodecyl analog of isonucleoside B (Figure 1). An anomeric mixture (β/α ratio, 1:0.4) was obtained, which may be a result from the α and β-directing effects exerted by the neighboring acetate group participation and by the remote participation of theobromine carbonyl group, respectively, as well as from anomerization via the acyclic N-sulfonyl imine intermediate, as previously described in the synthesis of B (Figure 1) [17]. On the other hand, the isonucleosidyl acetate 11 was subjected to MW-assisted anomeric azidation with trimethylsilyl azide (TMSN3), mediated by trimethylsilyl triflate (TMSOTf), leading to the α- and β-isonucleosidyl azides 13 in a 1:0.8 ratio and 86% yield. The formation of the α-anomer is likely to arise also from the above mentioned remote group participation in assisting the isonucleosidyl cation intermediate, an effect already described to influence the stereochemical outcome of the anomeric azidation of glucuronamide derivatives [17,20]. Moreover, the xylonolactone-containing theobromine isonucleoside 14 was formed as a secondary product in 14% yield and was obtained in a mixture containing the β-isonucleosyl azide, the separation of which could not be achieved by column chromatography. The structure of lactone 14 was identified based on its NMR and high resolution mass spectrometry (HRMS) data. HMBC experiments showed correlations between H-2′, which appeared at rather low field (δ = 5.59 ppm), H-3′ and H-4′ with C-1′ (δ = 170 ppm). The mechanism leading to the lactone 14 probably involves the formation of an isonucleosidyl imine intermediate, through nitrogen elimination from the anomeric azide moiety, and subsequent hydrolysis during the workup. The azide 13-α was engaged in a Staudinger-type reaction with trimethylphosphite to furnish the N-isonucleosidyl phosphoramidate 15 in 56% yield as a 1:0.3 mixture of α- and β-anomers. Despite the α-anomeric configuration of the isonucleosidyl azide precursor, the β-configured product likely arises from anomerization via acyclic N-phosphoryl imine/iminium intermediates.
Scheme 2.
Reagents and conditions: (a) trifluoroacetic acid (TFA) (60% aq. soln.), room temp., 2 h, 83% (α/β = 1:0.9; (b) Ac2O, py, room temp., 1.5 h, 69% (α/β = 1:0.5); (c) CH3SO2NH2, BF3·Et2O, CH2Cl2/CH3CN, room temp., 2 h, 81% (β/α = 1:0.4); (d) trimethylsilyl azide (TMSN3), trimethylsilyl triflate (TMSOTf), CH3CN, 65 °C, MW, max. 150 W, 1 h 40 min, 86% (13, β/α ratio, 1:0.8) and 14% (14); (e) P(OMe)3, CH2Cl2, room temp., 23 h, 56% (α/β = 1:0.3).
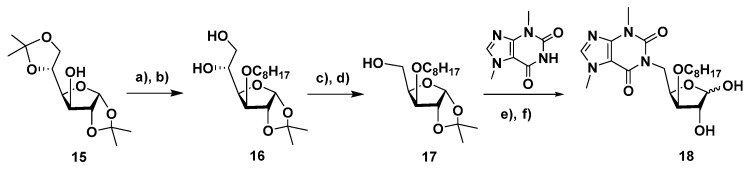
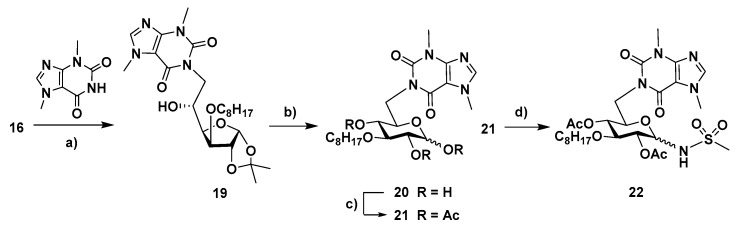
Furanosyl and pyranosyl theobromine isonucleosides containing a 3-O-octyl moiety were also synthesized. The 3-O-octyl-1,2-O-isopropylidene glucofuranose precursor (16, Scheme 3) was accessed through 3-O-octylation of diacetone-D-glucose with octyl bromide in the presence of sodium hydride and subsequent selective hydrolysis of the primary acetonide (aq. acetic acid 70%). The diol 16 was then subjected to oxidative cleavage with sodium periodate and the resulting aldehyde was reduced (NaBH4) to afford the xylofuranose derivative 17. The reaction of 17 with theobromine under the MW-assisted Mitsunobu conditions, which was followed by removal of the isopropylidene moiety, gave the xylofuranos-5′-yl theobromine 18, as an anomeric mixture, in 20% overall yield.
Scheme 3.
Reagents and conditions: (a) C8H17Br, NaH, DMF, room temp., 22 h; (b) AcOH (70% aq. soln.), room temp., 26 h, 88%; 2 steps; (c) NaIO4, THF (60% aq. soln.), room temp., 4 h; (d) NaBH4, EtOH/H2O, room temp., 1.5 h, 65%, 2 steps; (e) theobromine, PPh3, DEAD, DMF, 65 °C, MW, max. 150 W, 30 min; (f) TFA (60% aq. soln.), room temp., 4 h, 20%, 2 steps.
The direct regioselective Mitsunobu coupling of diol 16 with theobromine at C-6 could also be achieved, although in low yield (16%), when carrying out the reaction in refluxing DMF (Scheme 4), while the previously mentioned MW-assisted protocol did not enable significant conversion of 16. Acid hydrolysis of the obtained glucofuranos-6′-yl theobromine 19 led to the pyranos-6′-yl isonucleoside 20, the acetylation of which provided the tri-O-acetylated derivative 21. The regiochemistry of the isonucleosidic linkage (N1-C-6) in 20 was confirmed by the HMBC correlations between the protons H-6′ and both C-2/C-6. The BF3Et2O-mediated reaction of 21 with methanesulfonamide gave the N-isonucleosidyl sulfonamide 22 in 38% yield as an anomeric mixture (β/α ratio, 1:0.4).
Scheme 4.
Reagents and conditions: (a) theobromine, PPh3, DEAD, DMF, reflux, 20 h, 16%; (b) TFA (60% aq. soln.), room temp., 5 h, 87% (α /β = 1:0.9); (c) Ac2O, py, room temp., 2.5 h, 84% (α /β = 1:0.7); (d) CH3SO2NH2, BF3·Et2O, CH2Cl2/CH3CN, room temp., 3.5 h, 38% (β/α = 1:0.4).
2.2. Biological Evaluation
The newly synthesized xylofuranosyl isonucleosides (3–10 and 18), the theobromine-containing isonucleosidyl sulfonamides (12 and 22), azide (13-α) and phosphoramidate (15) and the glucopyranos-6′-yl theobromine derivative 21 were subjected to biological evaluation. Their ability to inhibit the enzymes acetylcholinesterase (AChE, from Electrophorus electricus) and butyrylcholinesterase (BChE, from equine serum) was assessed by the Ellman’s method. The cholinesterase inhibitor galantamine hydrobromide, which is clinically used for the treatment of AD, was used as a standard. The percent inhibition (at 50 µM) determined for the compounds, the inhibition constants, Ki (for competitive inhibition) or Ki′ (for uncompetitive inhibition) for the active molecules, and the respective types of inhibition are compiled in Table 1.
Table 1.
Results of the evaluation of the inhibitory activities of the compounds on cholinesterases.
| AChE | BChE | |||
|---|---|---|---|---|
| Compound | % Inhibition a |
Ki (µM) [Ki′ (µM)] (type of inhibition) |
% Inhibition a |
Ki (µM) [Ki′ (µM)] (type of inhibition) |
| Galantamine hydrobromide | 96.7 | 0.2 ± 0.1 (competitive) | 86.3 | 2.4 ± 0.0 (competitive) |
| Xylofuranos-5′-yl isonucleosides | ||||
| Purine derivatives | ||||
| 4 | 16.3 | n.d. | 11.9 | n.d. |
| 5 | 25.3 | n.d. | 32.5 | n.d. |
| Uracil derivatives | ||||
| 6 | 22.5 | n.d. | 28.5 | n.d. |
| 7 | 17.8 | n.d. | 7.5 | n.d. |
| 8 | 26.1 | n.d. | 32.8 | n.d. |
| 9 | 40.3 | 17.5 ± 0.7 [103.0 ± 4.3] (mixed-type) |
29.8 | n.d. |
| Theobromine derivatives | ||||
| 3 | 13.8 | n.d. | 11.7 | n.d. |
| 10 | 2.6 | n.d. | 22.9 | n.d. |
| 13-α | 18.9 | n.d. | 35.0 | n.d. |
| 18 | 14.5 | n.d. | 19.6 | n.d. |
| Glucopyranos-6′-yl theobromine isonucleoside | ||||
| 21 | 66.5 | 3.1 ± 0.2 [>100] (mixed-type) |
75.1 | 5.4 ± 0.3 [>60] (mixed-type) |
| Theobromine-containing isonucleotide analogs | ||||
| 12 | 1.0 | n.d. | 4.6 | n.d. |
| 15 | 12.4 | n.d. | 35.9 | n.d. |
| 22 | 28.3 | n.d. | 52.6 | n.d. |
a % Inhibition at 50 µM. AChE, acetylcholinesterase; BChE, butyrylcholinesterase.
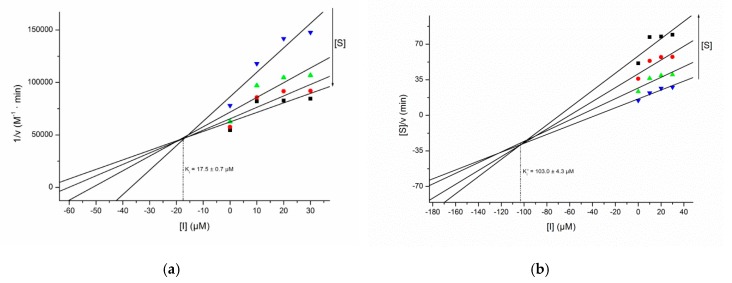
Among the furanosyl isonucleosides, only the 2-O,4-O-uracil-linked pseudodisaccharide 9 showed significant effect on the activity of cholinesterases, exhibiting a moderate mixed-type inhibition of AChE, in which the competitive character is more pronounced than the uncompetitive one (Ki = 17.5 µM, Ki′ = 103.0 µM, Figure 2). The remaining furanosyl isonucleosides were considered inactive, showing, at 50 µM concentration, less than 40% inhibition of the activity of both enzymes. The theobromine-containing furanosyl isonucleotide analogs (12, 15), containing sulfonamide or phosphoramidate groups, as well the isonucleosidyl azide 13-α, were also devoid of any inhibitory effects. Given the fact that the previously reported N-isonucleosidyl sulfonamide B (Figure 1) showed effective inhibition of AChE, the lack of activity of the 3-O-dodecylated analog 12 clearly demonstrates that the presence of the 3-O-benzyl group in B is crucial for its inhibitory activity. In contrast, replacing a 3-O-dodecyl by a 3-O-octyl group appears not to cause a significant change on the activity of xylofuranos-5′-yl theobromine derivatives, as demonstrated by the similar effects of isonucleosides 10 and 18.
Figure 2.
Dixon (a) and Cornish–Bowden (b) plots for the inhibition of AChE by compound 9.
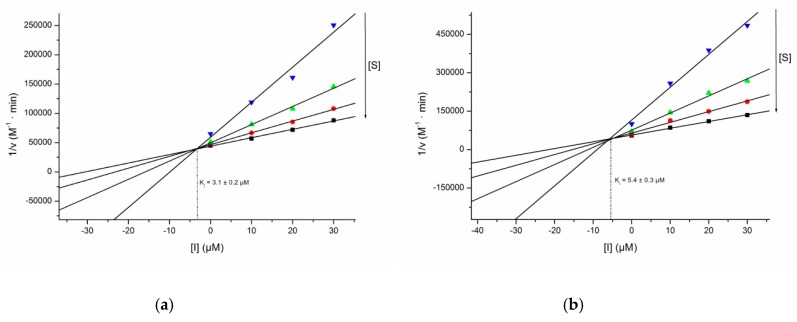
With respect to glucopyranos-6′-yl theobromine isonucleosides, the isonucleosidyl acetate 21 displayed noteworthy inhibitory effects on both cholinesterases. It was the most active compound of the series, exhibiting dual inhibition of AChE and BChE by a mixed-type mechanism with a dominant competitive component, with single digit micromolar Ki values (Figure 3) that are at least ca. 32-fold (Ki = 3.1 µM) and 11-fold lower (Ki = 5.4 µM) than their Ki′ values, respectively. It was only twice less active than the standard galantamine hydrobromide on BChE. The inhibition of BChE may provide therapeutic benefits in AD, since an increasing activity of this enzyme with accompanying decreasing levels of its congener AChE occurs over the course of the disease. Thus, the brain acetylcholine levels became gradually dependent on BChE [21] and a dual AChE/BChE inhibition or a selective BChE inhibition may provide a more effective treatment in advanced stages of AD. The N-isonucleosidyl sulfonamide 22 was a weaker cholinesterase inhibitor than its precursor 21, displaying selective effect towards BChE with 53% inhibition of the enzymatic activity at 50 µM.
Figure 3.
Dixon plots for the inhibition of AChE (a) and BChE (b) by compound 21.
The compounds were tested for their cytotoxicity against human cancer cell lines, namely A375 (epithelial melanoma), A2780 (ovarian carcinoma), HT29 (colorectal adenocarcinoma), MCF7 (breast adenocarcinoma) and SW1736 (thyroid carcinoma), as well as towards non-malignant mouse embryonic fibroblasts (NIH 3T3), using the sulforhodamine-B (SRB) colorimetric assay. None of the molecules revealed cytotoxic effects at concentrations below 30 µM (cut-off of the assay) to either cancer or healthy cells, being therefore considered inactive (EC50 > 30 μM) against the tested cell lines.
2.3. Molecular Docking Studies
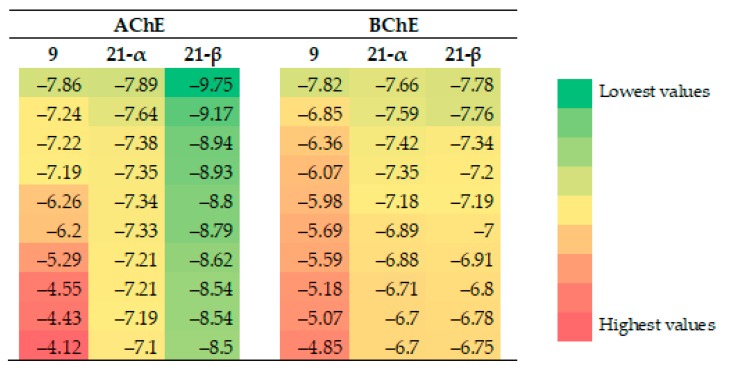
To further understand the binding interactions on a molecular level of compounds 9 and 21, a molecular docking was performed with Autodock 4 [22]. The crystal structures of AChE (PDB: 1C2O) and BChE (PDB: 6EMI) were retrieved from the RCSB Protein Databank. Due to its anomeric mixture, compound 21 was docked as α and β anomers.
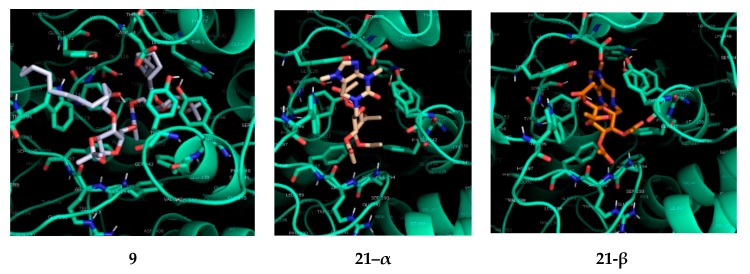
Concerning the AChE docking results, 21-β outperformed compounds 21-α and 9 significantly (Figure 4). With respect to the docking positions of 21-β, this compound seems to fit quite nicely into the entrance of the cavity, where the theobromine moiety is positioned at the entrance of the active site gorge, pointing towards the enzyme’s binding pocket (Figure 5). The main interaction of the theobromine unit is with Tyr124 at the peripheral anionic site (PAS), which appears to be based on an O–H∙∙∙N hydrogen bond with one of the theobromine nitrogen atoms. The tri-O-acetylated glucopyranosyl unit is located in front of the binding pocket, where the acetyl groups bind via N–H∙∙∙O hydrogen bonds to Trp286 at the PAS and Arg296, located between the acyl pocket and the PAS, or Phe295 at the acyl binding pocket, where the N–H∙∙∙O binding cannot be differentiated between these residues.
Figure 4.
Binding energies (kcal/mol) of the top docking poses of compounds 9, 21-α and 21-β into AChE and BChE.
Figure 5.
Lowest-energy binding poses of compounds 9, 21-α and 21-β to AChE.
In compound 21-α, the conformation however hinders the theobromine moiety to enter the active site and give a favorable binding position (Figure 5). Therefore, the theobromine system is mainly located outside of the active site of AChE, establishing as key interaction an O–H∙∙∙Ar π-interaction with Tyr72 at the PAS. Another strong binding occurs with one of the acetyl groups with Phe294.
The docking of compound 9 (Figure 5) shows a strong O–H∙∙∙N interaction of Tyr124 at the PAS with one of the pyrimidines nitrogens. Herein it seems that compound 9 is able to enter the binding pocket where one half fills the cavity of the AChE active site while the other furanosyl moiety sticks out and blocks the entrance of the binding pocket, where Phe295 shows an O–H∙∙∙O binding to the furanosyl oxygen.
The docking of 9, 21-α and 21-β in the structure of BChE resulted in quite similar results, whereas the binding interactions of compound 9 in comparison to 21 fall off significantly (see Figure S20, in the Supplementary Materials). The best docking position however shows a strong binding from π-stacking of the pyrimidine moiety of 9 with Tyr332. This also is a major binding mode of 21-β as this compound also shows a strong π-stacking interaction of the theobromine moiety with Tyr332 and weak interactions of the acetyl groups with sidechains present in the active site of BChE. Compound 21-α shows an interaction of the theobromine nitrogens with the peptide carbonyl of Leu286 and Ser287. However, for the BChE active site, it is difficult to identify strong interactions between the inhibitors and its amino acid residues.
3. Discussion
A variety of novel terminal isonucleosides embodying purine derivatives or uracil were synthesized using the Mitsunobu coupling as a key step. Microwave irradiation proved to be a useful tool in the Mitsunobu reaction between 3-O-dodecyl/octyl-1,2-O-isopropylidene xylofuranose with theobromine or adenine, enabling the access to 5′-isonucleosides in shorter reaction times (30‒50 min) than those needed in conventional stirring for analogous reactions (>16 h) and in moderate yields. In the case of the synthesis of the 3′-O-dodecyl-xylofuranos-5′-yl theobromine derivative 3, a significant increase on the reaction yield (from 16% to 44%) was achieved using MW-assisted heating. Potential nucleotide mimetics, namely theobromine-based N-isonucleosidyl sulfonamides or an N-isonucleosidyl phosphoramidate, which is a previously unreported type of structure, were synthesized through anomeric functionalization of isonucleosidyl acetates, whose stereochemical outcome was influenced by the remote participating effect of the theobromine carbonyl groups.
Biological evaluation revealed the ability of a theobromine isonucleosidyl acetate possessing a glucopyranose moiety (21) to inhibit both AChE and BChE at single-digit micromolar concentrations, reinforcing the potential of theobromine isonucleosides as cholinesterase inhibitors. Since the anomeric mixture was evaluated, it appears that 21-β accounts more for the detected competitive inhibitory effect on AChE, as indicated by the docking simulations. The theobromine unit in 21-β as well as the sugar moiety are important for the binding to the enzyme, establishing interactions at the PAS and at the front of the active site, respectively.
The N-isonucleosidyl sulfonamide derivative (22) showed significantly weaker effects than its precursor 21, a result which is in contrast with our previous findings that suggested that an anomerically linked sulfonamide moiety, namely in compound B (Figure 1), would lead to an increase on the AChE inhibitory ability of the theobromine isonucleoside. This result as well as the lack of activity of the 3-O-dodecyl theobromine N-isonucleosidyl sulfonamide 12 indicate that the 3-O-substituent and the sugar ring system in these types of molecules also account for their ChE inhibitory abilities. A uracil-linked pseudodisaccharide (9) was the only xylofuranose-based isonucleoside which showed significant ChE inhibitory effect, with selective and moderate activity on AChE, which further demonstrate the AChE inhibitory potential of molecules based on this type of skeleton, notwithstanding its large size. Similarly, to a thymine-linked pseudodisaccharide previously described as a competitive AChE inhibitor [14], the interactions between compound 9 and the enzyme seem also to span both the PAS and the active site, with half of the molecule blocking the entrance to the active site gorge and the pyrimidine unit being involved in a key interaction.
The lack of cytotoxicity of the tested molecules to either cancer or non-malignant cells reflects the lower propensity of terminal nucleosides than non-terminal ones to exhibit cytotoxic effects. It is known that 2′-isonucleosides can be, although in a low extend, intracellularly phosphorylated at the primary hydroxyl group by kinases [9,23] and that isonucleoside triphosphates can be recognized by DNA polymerases and be incorporated into the growing DNA chain arresting its elongation [24], similarly to the mechanism of action of the known anticancer nucleosides [1,2]. The absence of a terminal hydroxyl group able for phosphorylation in these newly synthesized 5′-/6′-isonucleosides precludes them to undergo such biological pathway which eventually would lead to cytotoxic effects.
Nevertheless, the non-toxicity of these molecules to healthy cells motivates studies focusing on other biological properties to further explore their therapeutic interest.
4. Materials and Methods
4.1. Chemistry
4.1.1. General Methods
The progress of the reactions was checked by thin layer chromatography (TLC) using silica gel aluminum plates (Merck 60 F254) with visualization under UV light (254 nm) and/or by immersion in a 10% H2SO4 solution in ethanol or in a solution of cerium (IV) sulfate (0.2% w/v) and ammonium molybdate (5% w/v) in H2SO4 (6% aq.) followed by heating (200 °C). Microwave experiments were carried out in a CEM Discover SP Microwave Synthesizer. Flash column chromatography was performed on silica gel 60 G (0.040–0.063 mm, E. Merck). Optical rotations (589 nm, 20 °C) were measured on a Perkin–Elmer 343 polarimeter. NMR spectra were acquired using a BRUKER Avance 400 spectrometer operating at 400.13 MHz for 1H or 100.62 MHz for 13C. The chemical shifts are given in parts per million (ppm). The spectra were calibrated with internal TMS (in the case of 1H NMR spectra in CDCl3) or with the respective solvent residual peak. Coupling constants (J) are given in hertz (Hz). Assignments were made with the help of 2D experiments (COSY, HSQC, HMBC). HRMS spectra were acquired with a QqTOF Impact II mass spectrometer (Bruker Daltonics) equipped with an electrospray ion source (ESI) and were recorded in positive mode.
Synthesis of compound 2 was previously described [19].
4.1.2. General Procedure for the Mitsunobu Coupling of 3-O-Octyl/Dodecyl-1,2-O-isopropylidene-α-D-xylo/glucofuranose (2, 17) with Purine Derivatives/Uracil
To a solution of partially protected 1,2-O-isopropylidene-α-D-xylo/glucofuranose (1 mmol) in THF (28 mL) or DMF (10 mL) under nitrogen atmosphere, PPh3 (2 equiv.), diethyl azodicarboxylate (DEAD, 2 equiv.) and purine derivative/uracil (2 equiv.) were added. The mixture was stirred under the conditions indicated further. The mixture was concentrated under vacuum and the crude residue was subjected to column chromatography.
4.1.3. General Microwave-Assisted Procedure for the Mitsunobu Coupling of 3-O-Octyl/Dodecyl-1,2-O-isopropylidene-α-D-xylofuranose (2, 17) with Theobromine/Adenine
To a solution of partially protected 1,2-O-isopropylidene-α-D-xylofuranose (0.2 mmol) in THF/DMF (1:1, 2 mL) or DMF (2 mL), PPh3 (2 equiv.), diethyl azodicarboxylate (DEAD, 2 equiv.) and theobromine (2 equiv.) were sequentially added. The mixture was stirred under microwave irradiation (150 W max., P max = 250 Psi) at 65 °C for 30–50 min. The solvent was evaporated, and the crude residue was subjected to column chromatography on silica-gel.
4.1.4. 1-(5-Deoxy-3-O-dodecyl-1,2-O-isopropylidene-α-D-xylofuranos-5-yl)-3,7-dimethyl-3,7-dihydro-1H-purine-2,6-dione (3)
Obtained according to the general procedure for Mitsunobu coupling, starting from compound 2 (328 mg, 0.916 mmol), theobromine (340 mg, 1.89 mmol) and using PPh3 (502 mg, 1.91 mmol) and DEAD (40% wt. soln. in toluene, 0.89 mL, 1.95 mmol) in THF (25 mL). The reaction mixture was stirred at 50 °C for 48 h. Purification by column chromatography (from AcOEt/hexane 1:1 to 4:1) afforded 5 (76 mg, 16%) as a yellow oil.
Alternatively, the title compound could be obtained using the MW-assisted procedure, starting from compound 2 (79 mg, 0.219 mmol), theobromine (80 mg, 0.44 mmol) and using PPh3 (115 mg, 0.43 mmol) and DEAD (40% wt. soln. in toluene, 0.2 mL, 0.44 mmol) in THF/DMF (1:1, 2 mL), within a reaction mixture of 30 min and in 44% yield (50 mg). = −5 (c = 1, in CH2Cl2). 1H NMR (CDCl3, 400 MHz): δ 7.49 (s, 1H, H-8), 5.97 (d, 1 H, H-1′, J1′,2′ = 3.9), 4.74 (dd, 1 H, H-5′a, J4′,5′a = 8.8, J5′a,5′b = 14.1), 4.59–4.52 (m, 2 H, H-2’, H-4′), 3.99–3.91 (m, 4 H, CH3, N7, H-5′b, J4′,5′b = 1.8), 3.90 (d, 1 H, H-3′, J3′,4′ = 3.4), 3.69–3.59 (m, 1 H, H-1″a), 3.56 (s, 3 H, CH3-N3), 3.48–3.38 (ddd, H-1″b), 1.62–1.51 (m 2 H, CH2-2″), 1.43 (s, 3 H, CH3, i-Pr), 1.38–1.16 (m, 21 H, CH2-3″ to CH2-11″, CH3, i-Pr), 0.86 (t, 3 H, CH3-12″, J = 6.7). 13C NMR (CDCl3, 100 MHz): δ 155.4 (C-6), 151.7 (C-2), 148.9 (C-4), 141.5 (C-8), 111.5 (Cq, i-Pr), 107.9 (C-5), 105.3 (C-1′), 83.5 (C-3′), 82.5 (C-2′), 78.6 (C-4′), 70.5 (CH2-1″), 40.0 (C-5′), 33.7 (CH3, N7), 32.1 (CH2-2″), 29.8, 29.8, 29.8, 29.8, 29.7, 29.6, 29.5, 26.8 (CH3, N3, CH2-3″ to CH2-10″), 26.3, 26.3 (2 × CH3, i-Pr), 22.8 (CH2-11″), 14.3 (CH3-12″). HRMS: calcd for C27H44N4O6 [M + H]+ 521.3334, found 521.3333; calcd for C27H44N4O6 [M + Na]+ 543.3153, found 543.3149.
4.1.5. 9-(5-Deoxy-3-O-dodecyl-1,2-O-isopropylidene-α-D-xylofuranos-5-yl)adenine (4)
Obtained according to the general MW-assisted procedure for Mitsunobu coupling, starting from compound 2 (80 mg, 0.22 mmol), adenine (60 mg, 0.44 mmol) and using PPh3 (117 mg, 0.44 mmol) and DEAD (40% wt. soln. in toluene, 0.2 mL, 0.44 mmol), in THF/DMF (1:1, 2 mL). The reaction mixture was stirred for 50 min. Purification by column chromatography (from AcOEt/cyclohexane 2:1 to AcOEt) afforded 4 (33 mg, 31%) as a colorless oil. 1H NMR (CDCl3, 400 MHz): δ 8.36 (s, 1 H, H-2), 7.93 (s, 1 H, H-8), 5.96 (d, 1 H, H-1′, J1’,2’ = 3.6), 5.69 (br.s, 2 H, NH2), 4.52 (d, 1 H, H-1′, J1’,2’ = 3.2), 4.64–4.50 (m, 3 H, H-2′, H-5′a, H-4′), 4.35 (dd, 1 H, H-5′b, J5’a,5’b = 15.9, J4’,5’b = 10.0), 3.87 (d, 1 H, H-3′, J2′,3′ = 2.8 ), 3.69–3.60 (m, 1 H, H-1″a), 3.47–3.38 (m, 1 H, H-1″b), 1.62–1.53 (m 2 H, CH2-2″), 1.42–1.20 (m, 26 H, CH2-3″ to CH2-11″, 2 × CH3, i-Pr), 0.87 (t, 4.2 H, CH3-12″, J = 6.7). 13C NMR (CDCl3, 100MHz): δ 155.4 (C-6), 153.0 (C-2), 150.3 (C-4), 141.7 (C-8), 119.6 (C-5), 112.0 (Cq, i-Pr), 105.3 (C-1′), 82.7 (C-3′), 82.2 (C-2′), 78.4 (C-4′), 70.6 (CH2-1″), 42.9 (C-5′), 32.1 (CH2-2″), 29.8, 29.8, 29.8, 29.8, 29.7, 29.6, 29.5, 26.9, 26.3, 26.3, 22.8 (CH2-3″ to CH2-11″, 2 × CH3, i-Pr), 14.3 (CH2-12″). HRMS: calcd for C25H41N5O4 [M + H]+ 476.3231, found 476.3226. calcd for C25H41N5O4 [M + Na]+ 498.3051, found 498.3043.
4.1.6. 2-Acetamido-6-chloro-9-(5-deoxy-3-O-dodecyl-1,2-O-isopropylidene-α-D-xylofuranos-5-yl)purine (5)
Obtained according to the general procedure for Mitsunobu coupling, starting from compound 2 (337 mg, 0.94 mmol), 2-acetamido-6-chloropurine (399 mg, 1.88 mmol) and using PPh3 (504 mg, 1.92 mmol) and DEAD (40% wt. soln. in toluene, 0.82 mL, 1.8 mmol) in THF (25 mL). The reaction mixture was stirred at 50 °C for 16 h. Purification by column chromatography (from AcOEt/petroleum ether 1:2) afforded 5 (265 mg, 51%) as a yellow oil.
= −14 (c = 1, in CH2Cl2). 1H NMR (400 MHz, CDCl3) δ 8.11 (s, 1 H, H-8), 5.95 (d, 1 H, H-1’, J1′,2′ = 3.7), 4.60 (d, 1 H, H-2′), 4.56–4.46 (m, 2 H, H-4′, H-5′a), 4.39 (dd, 1 H, H-5′b, J4′,5′b = 9.0, J5′a,5′b = 15.0), 3.85 (d, 1 H, H-3′, J3′,4′ = 3.0), 3.68–3.58 (m, 1 H, H-1″a), 3.45–3.35 (ddd, H-1″b), 2.54 (s, 3 H, CH3, NHAc), 1.60–1.49 (m 2 H, CH2-2″), 1.40 (s, 3 H, CH3, i-Pr), 1.36–1.16 (m, 21 H, CH2-3″ to CH2-11″, CH3, i-Pr), 0.87 (t, 3 H, CH3-12″, J = 6.7). 13C NMR (100 MHz, CDCl3) δ: 153.0 (C-4), 152.0, 151.3 (C-2, C-6), 145.7 (C-8), 128.0 (C-5), 112.2 (Cq, i-Pr), 105.3 (C-1′), 82.6 (C-3′), 82.0 (C-2′), 77.8 (C-4′), 70.6 (CH2-1″), 43.2 (C-5′), 32.0 (CH2-2″), 29.8, 29.8, 29.8, 29.8, 29.7, 29.6, 29.5, 26.9 (CH2-3″ to CH2-10″), 26.3, 26.2 (2 × CH3, i-Pr), 25.3 (CH3, NHAc), 22.8 (CH2-11″), 14.3 (CH3-12″). HRMS: calcd for C27H42ClN5O5 [M + H]+ 552.2947, found 552.2957; calcd for C27H42ClN5O5 [M + Na]+ 574.2767, found 574.2778.
4.1.7. 1-(5-Deoxy-3-O-dodecyl-1,2-O-isopropylidene-α-D-xylofuranos-5-yl)uracil (6), 3-(5-deoxy-3-O-dodecyl-1,2-O-isopropylidene-α-D-xylofuranos-5-yl)uracil (7), 1,3-bis-(5-deoxy-3-O-dodecyl-1,2-O-isopropylidene-α-D-xylofuranos-5-yl)uracil (8) and 2,4-bis-O-(5-deoxy-3-O-dodecyl-1,2-O-isopropylidene-α-D-xylofuranos-5-yl)uracil (9)
Obtained according to the general procedure for Mitsunobu coupling, starting from compound 2 (286 mg, 0.798 mmol), uracil (182 mg, 1.62 mmol) and using PPh3 (425 mg, 1.62 mmol) and DEAD (40% wt. soln. in toluene, 0.7 mL, 1.54 mmol) in THF (25 mL). The reaction mixture was stirred at 50 °C for 18 h. Purification by column chromatography (from AcOEt/petroleum ether 1:5 to AcOEt) afforded 9 (17 mg, 5%), 8 (91 mg, 29%), 6 (42 mg, 12%) and 7 (46 mg, 13%) as colorless oils.
Compound 6: = −2 (c = 1, in CH2Cl2). 1H NMR (CDCl3, 400 MHz): δ 8.99 (s, 1 H, NH), 7.30 (d, 1 H, H-6, J5,6 = 7.9), 5.91 (d, 1 H, H-1’, J1’,2’ = 3.7), 5.66 (d, 1 H, H-5), 4.56 (d, 1 H, H-2’), 4.41 (dt, 1 H, H-4′, J4′,5′b = 8.8), 4.33 (dd, 1 H, H-5′a, J4′,5′a = 2.6, J5′a,5′b = 14.6), 3.85 (d, 1 H, H-3′, J3′,′ = 3.2), 3.69–3.57 (m, 2 H, H-1″a, H-5′b), 3.44–3.34 (ddd, H-1″b), 1.60–1.50 (m 2 H, CH2-2″), 1.46 (s, 3 H, CH3, i-Pr), 1.34–1.18 (m, 21 H, CH2-3″ to CH2-11″, CH3, i-Pr), 0.87 (t, 3 H, CH3-12″, J = 6.7). 13C NMR (CDCl3, 100MHz): δ 163.8 (C-4), 151.1 (C-2), 145.7 (C-6), 112.1 (Cq, i-Pr), 105.2 (C-1′), 102.1 (C-5), 82.9 (C-3′), 82.2 (C-2′), 78.1 (C-4′), 70.6 (CH2-1″), 48.1 (C-5′), 32.0 (CH2-2″), 29.8, 29.8, 29.8, 29.7, 29.7, 29.5, 29.5, 26.8 (CH2-3″ to CH2-10″) 26.3, 26.2 (2 × CH3, i-Pr), 22.8 (CH2-11″), 14.3 (CH3-12″). HRMS: calcd for C24H40N2O6 [M + Na]+ 475.2779, found 475.2782; calcd for C24H40N2O6 [M + H]+ 453.2959, found 453.2962.
Compound 7: = +6 (c = 1, in CH2Cl2).1H NMR (CDCl3, 400 MHz): δ 9.98 (br.d, 1 H, NH), 7.20 (dd, 1 H, H-6, JNH,6 = 5.8, J5,6 = 7.6), 5.94 (d, 1 H, H-1′, J1′,2′ = 3.8), 5.72 (dd, 1 H, H-5, JNH,5 = 1.2, J5,6 = 7.6 ), 4.59–4.46 (m, 3 H, H-2′, H-4′, H-5′a), 4.00–3.90 (m, 1 H, H-5′b) 3.88 (d, 1 H, H-3′, J3′,4′ = 2.4), 3.68–3.59 (m, 2 H, H-1″a), 3.47–3.37 (ddd, H-1″b), 1.64–1.51 (m 2 H, CH2-2″), 1.45 (s, 3 H, CH3, i-Pr), 1.37–1.16 (m, 21 H, CH2-3″ to CH2-11″, CH3, i-Pr), 0.86 (t, 3 H, CH3-12″, J = 6.7). 13C NMR (CDCl3, 400 MHz): δ 163.6 (C-4), 153.0 (C-2), 139.1 (C-6), 111.7 (Cq, i-Pr), 105.2 (C-1′), 102.0 (C-5), 83.3 (C-3′), 82.4 (C-2′), 78.2 (C-4′), 70.6 (CH2-1″), 40.1 (C-5′), 32.0 (CH2-2″), 29.8, 29.8, 29.8, 29.8, 29.7, 29.6, 29.5, 26.8 (CH2-3″ to CH2-10″), 26.3, 26.2 (2 × CH3, i-Pr), 22.8 (CH2-11″), 14.3 (CH3-12″). HRMS: calcd for C24H40N2O6 [M + Na]+ 475.2779, found 475.2788; calcd for C24H40N2O6 [M + H]+ 453.2959, found 453.2969.
Compound 8: = −4 (c = 1, in CH2Cl2). 1H NMR (CDCl3, 400 MHz): δ 7.24 (d, 1 H, H-6, J5,6 = 7.9), 5.93, 5.90 (2 d, 2 H, H-1′, H-1″, J1′,2′ = 3.8, J1″,2″ = 3.8 ), 5.69 (d, 1 H, H-5), 4.63–4.46 (m, 4 H, H-5″a, H-2′ H-2″, H-4″), 4.44 (ddd, 1 H, H-4′), 4.31 (dd, 1 H, H-5′a, J5′a,5′b = 14.7, J4′,5′a = 2.6), 3.92 (dd, 1 H, H-5″b, J5″a,5″b = 13.8, J4″,5″a = 1.6), 3.86 (d, 1 H, H-3″, J3″,4″ = 3.3), 3.82 (d, 1 H, H-3′, J3′,4′ = 3.1), 3.68–3.55 (m, 3 H, H-5’b, H-1′′′a, H-1″″a), 3.46–3.32 (m, 2 H, H-1′′′b, H-1″″b), 1.60–1.49 (m 4 H, CH2-2′′′, CH2-2″″) 1.45, 1.43 (2 s, 2 × 3 H, 2 × CH3, i-Pr), 1.35–1.16 (m, 42 H, CH2-3′′′ to CH2-11′′′, CH2-3″″ to CH2-11″″, 2 × CH3, i-Pr), 0.86 (t, 3 H, CH3-12′′′, CH3-12″″, J = 6.7). 13C NMR (CDCl3, 100MHz): δ 163.2 (C-4), 151.9 (C-2), 143.4 (C-6), 112.0, 111.5 (Cq, i-Pr), 105.3, 105.3, (C-1′, C-1″), 101.8 (C-5), 83.5 (C-3″), 82.9 (C-3′), 82.4, 82.1 (C-2′, C-2″), 78.3 (C-4′), 78.1 (C-4″), 70.5 (CH2-1′′′, CH2-1″″), 49.0 (C-5′), 40.8 (C-5″), 32.0 (CH2-2′′′, CH2-2″″), 29.8, 29.8, 29.8, 29.7, 29.7, 29.7, 29.7, 29.6, 29.5, 29.5, 29.5, 26.8 (CH2-3′′′ to CH2-10′′′, CH2-3″″ to CH2-10″″), 26.3, 26.3, 26.2, 26.2 (4 × CH3, i-Pr), 22.8 (CH2-11′′′, CH2-11″″), 14.3 (CH3-12′′′, CH3-12″″). HRMS: calcd for C44H76N2O10 [M + Na]+ 815.5392, found 815.5420; calcd for C44H76N2O10 [M + H]+ 793.5573, found 793.5584.
Compound 9: = −31 (c = 1, in CH2Cl2). 1H NMR (CDCl3, 400 MHz): δ = 8.18 (d, 1H, H-6, J = 5.7), 6.41 (d, 1 H, H-5), 5.97–5.92 (m, 2 H, H-1′, H-1″, J = 3.9, J = 4.2), 4.69 (dd, 1H, H-5′a, J5a,5b = 10.5, J4,5a = 3.9), 4.63–4.42 (m, 7 H, H-2′, H-2″, H-4′, H-4″, H-5′b, H-5″a, H-5″b), 3.95, 3.89 (2 d, 2 × 1 H, H-3′, H-3″, J = 2.2 Hz, J = 2.8), 3.66–3.52 (m, 2 H, H-1′′′a, H-1″″a), 3.48–3.36 (m, 2 H, H-1′′′b, H-1″″b), 1.59–1.45 (m, 10 H, 2 × CH3, i-Pr, CH2-2′′′, CH2-2″″); 1.36–1.14 (m, 42 H, 2 × CH3, i-Pr, CH2-3′′′ to CH2-11′′′, CH2-3′′′ to CH2-11″″); 0,87 (t, 6 H, CH3-12′′′, CH3-12″″, J = 6.5) ppm. 13C NMR (CDCl3, 400 MHz): δ 170.9 (C-4), 164.8 (C-2), 158.7 (C-6), 111.9, 111.8 (2 × Cq, i-Pr), 105.4, 105.3 (C-1′, C-1″), 102.54 (C5), 82.7, 82.6, 82.4, 82.4 (C-2′, C-2″, C-3′, C3″), 78.4, 78.3 (C-4′, C4″), 70.8, 70.7 (CH2-1′′′/ CH2-1″″), 64.7, 64.4 (C-5′, C-5″), 32.1, 29.8, 29.8, 29.7, 29.7, 29.5, 27.0, 26.5, 26.4, 26.2, 26.1 (4 × CH3, i-Pr, CH2-2′′′ to CH2-10′′′, CH2-2″″ to CH2-10″″), 22.8 (CH2-11′′′, CH2-11″″), 14.4 (CH3-12′′′, CH3-12″″) ppm. HRMS: calcd for C44H76N2O10 [M + H]+ 793.5573, found 793.5592; calcd for C44H76N2O10 [M + Na]+ 815.5392, found 815.5414.
4.1.8. 1-(5-Deoxy-3-O-dodecyl-α,β-D-xylofuranos-5-yl)-3,7-dimethyl-3,7-dihydro-1H-purine-2,6-dione (10)
A solution of 1-(5-deoxy-3-O-dodecyl-1,2-O-isopropylidene-α-D-xylofuranos-5-yl)-3,7-dimethyl-3,7-dihydro-1H-purine-2,6-dione (3, 39 mg, 0.075 mmol) in aq. trifluoroacetic acid (TFA, 60%, 3 mL) was stirred at room temp. for 2 h. The solvents were co-evaporated with toluene and the residue was subjected to column chromatography (from AcOEt/hexane, 10:1 to AcOEt/MeOH, 12:1) to afford 10 (30 mg, 83%, anomeric mixture, α/β ratio, 1:0.9) as colorless oil. 1H NMR (400 MHz, CDCl3) δ: 7.54 (s, 1.9 H, H-8 α, β), 5.56 (br.s, 1 H, H-1′ α), 5.11 (1, 0.9 H, H-1′ β), 4.67–4.38 (m, 3.8 H, H-4′ α, H-4′ β, H-5′a α, H-5′a β), 4.34–4.19 (m, 1.9 H, H-2′ α, H-2′ β), 4.08–3.90 (m, 9.5 H, H-3′ α, H-3′ β, H-5′b α, H-5’b β, CH3-N7 α, β), 3.71–3.42 (m, 9.5 H, CH2-1″ α, CH2-1″ β, CH3-N3 α, β) 1.70–1.60 (m 3.8 H, CH2-2″ α, CH2-2″ β), 1.37–1.20 (m, 34.2 H, CH2-3″ to CH2-11″), 0.87 (t, 5.7 H, CH3-12″, J = 6.7). 13C NMR (100 MHz, CDCl3) δ: 155.9 (C-6 α, β), 152.0, 151.9 (C-2 α, β), 149.1, 148.9 (C-4 α, β), 142.1 (C-8 α, β), 107.9, 107.8 (C-5 α, β), 103.5 (C-1′ β), 96.4 (C-1′ α), 84.6 (C-3’ β), 84.1 (C-3’ α), 80.1 (C-2′ α), 76.8 (C-4′ α, β), 75.2 (C-2′ β), 71.5 (CH2-1″ α), 70.9 (CH2-1″ β), 43.0 (C-5′, α, β), 33.8, 33.7 (CH3, N7, α, β), 32.1 (CH2-2″, α, β), 30.1, 29.9, 29.9, 29.8, 29.8, 29.8, 29.8, 29.7, 29.6, 29.6, 29.5, 26.2, 22.8, (CH3, N3, CH2-3″ to CH2-11″, α, β), 14.3 (CH2-12″, α, β). HRMS: calcd for C24H40N4O6 [M + Na]+ 503.2840, found 503.2836; calcd for C24H40N4O6 [M + H]+ 481.3021, found 481.3018.
4.1.9. 1-(1,2-Di-O-acetyl-5-deoxy-3-O-dodecyl-α,β-D-xylofuranos-5-yl)-3,7-dimethyl-3,7-dihydro-1H-purine-2,6-dione (11)
A solution of 1-(5-deoxy-3-O-dodecyl-α,β-D-xylofuranos-5-yl)-3,7-dimethyl-3,7-dihydro-1H-purine-2,6-dione (10, 41 mg, 0.078 mmol) in pyridine (3 mL) and acetic anhydride (1.5 mL) was stirred at room temp. for 1.5 h. After co-evaporation of the solvents with toluene, the residue was subjected to column chromatography (AcOEt/hexane, 5:1) to give 11 (33 mg, 69%, anomeric mixture, α/β ratio, 1:0.5) as a colorless oil. 1H NMR (400 MHz, CDCl3) δ: 7.50, 7.48 (2 s, 1.5 H, H-8 α, β), 6.38 (dd, 1 H, H-1′ α, J1’,2’ α = 4.5), 5.99 (1, 0.5 H, H-1′ β), 5.27 (t, 1 H, H-2′ α), 5.23 (s, 0.5 H, H-2′ β), 4.84–4.58 (m, 3 H, H-4′ α, H-4′ β, H-5′a α, H-5′a β), 4.19 (t, 1 H, H-3, J2’,3’ α ~ J2’,4’ α = 5.5), 4.01 (br.d, 0.5 H, H-3′ β, J3’,4’ β = 5.0), 3.99–3.84 (m, 6 H, CH3-N7 α, β, H-5′b α, H-5′b β), 3.74–3.65 (m, 0.5 H, H-1′′a β), 3.65–3.44 (m, 7 H, CH2-1″ α, H-1″b β, CH3-N3 α, β), 2.18 (s, 1.5 H, CH3, Ac, β), 2.05 (s, 4.5 H, CH3, Ac, α, β), 1.99 (s, 3 H, CH3, Ac, α), 1.63–1.50 (m, 3 H, CH2-2″, α, β), 1.41–1.16 (m, 27 H, CH2-3″ to CH2-11″), 0.85 (t, 4.5 H, CH3-12″, J = 6.8). 13C NMR (100 MHz, CDCl3) δ: 170.5, 169.8 (CO, Ac, β), 169.7, 169.4 (CO, Ac, α), 155.3, 155.3 (C-6 α, β), 151.7 (C-2 α, β), 148.9, 148.8 (C-4 α, β), 141.6, 141.4 (C-8 α, β), 107.8 (C-5 α, β), 99.0 (C-1′ β), 84.2 (C-1′ α), 82.0 (C-3’ β), 80.7 (C-3’ α), 80.2 (C-4′ β), 79.8 (C-2′ β), 76.5 (C-2’ α), 76.2 (C-4′ α), 71.2, 71.0 (CH2-1″, α, β), 41.9 (C-5′ β), 41.3 (C-5′ α), 33.7, 33.7 (CH3, N7, α, β), 32.0 (CH2-2″, α, β), 29.9, 29.8, 29.8, 29.8, 29.7, 29.7, 29.6, 29.5, 26.3, 26.2, 22.8, (CH3, N3, CH2-3″ to CH2-11″, α, β), 21.5, 21.0, 20.9, 20.7 (CH3, Ac, α, β), 14.2 (CH2-12″, α, β).
4.1.10. N-[2-O-Acetyl-1,5-dideoxy-5-(3,7-dimethyl-3,7-dihydro-2,6-dioxo-1H-purin-1-yl)-3-O-dodecyl-α,β-D-xylofuranos-1-yl]methanesulfonamide (12)
To a solution of 1-(1,2-di-O-acetyl-5-deoxy-3-O-dodecyl-α,β-D-xylofuranos-5-yl)-3,7-dimethyl-3,7-dihydro-1H-purine-2,6-dione (11, 22 mg, 0.039 mmol) in CH2Cl2/acetonitrile (1.2 mL, 4:1) under nitrogen and at 0 °C, BF3·Et2O (0.03 mL, 0.24 mmol) and methanesulfonamide (21 mg, 0.22 mmol) were added. The mixture was stirred at room temp. for 2 h. Then, it was diluted with CH2Cl2 and washed with a satd. aq. NaHCO3 soln. The aqueous phase was extracted with CH2Cl2 (2×) and the combined organic layers were dried with anhydrous MgSO4. After filtration and evaporation of the solvent, the residue was subjected to column chromatography (AcOEt/hexane, 15:1) to afford 12 (19 mg, 81%, anomeric mixture, β/α ratio, 1:0.4) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ: 7.52, 7.51 (2 s, 1.4 H, H-8 α, β), 5.73 (d, 1 H, NH, β, J1’,NH β = 11.1), 5.61 (dd, 0.4 H, H-1′ α, J1’,2’ α = 3.5, J1’,NH α = 11.3), 5.23 (d, 1 H, H-1′ β), 5.20 (s, 1 H, H-2′ β), 5.14 (dd, 0.4 H, H-2′ α, J1’,2’ α = 3.5, J2’,3’ α = 0.9), 5.07 (d, 0.4 H, NH α), 4.72 (dd, 1 H, H-5′a β, J5’a,5’b β = 13.9, J4’,5’a β = 9.5), 4.63–4.45 (m, 2.2 H, H-5′a α, H-5′b α, H-4′ α, H-4′ β, J5’a,5’b α = 13.8, J4’,5’a α = 8.7), 4.02–3.91 (m, 6.6 H, H-3′ α, H-3′ β, H-5’b β, CH3-N7 α, β), 3.81–3.72 (m, 1.4 H, H-1′′a α, H-1″a β), 3.61–3.49 (m, 5.6 H, H-1″b α, H-1″b β, CH3-N3 α, β), 3.11 (s, 3 H, S-CH3, β), 3.03 (s, 1.2 H, S-CH3, α), 2.11 (s, 1.2 H, CH3, Ac, α), 2.07 (s, 3 H, CH3, Ac, β), 1.70–1.60 (m 2.8 H, CH2-2″ α, CH2-2″ β), 1.37–1.20 (m, 25.2 H, CH2-3″ to CH2-11″, α, β), 0.87 (t, 4.2 H, CH3-12″, α, β, J = 6.7). 13C NMR (100 MHz, CDCl3) δ: 169.6, 168.9 (CO, Ac, α, β) 155.3 (C-6 α, β), 151.7 (C-2 α, β), 149.1, 149.0 (C-4 α, β), 141.8, 141.7 (C-8 α, β), 107.7 (C-5 α, β), 88.0 (C-1′ β), 82.9 (C-1′ α), 81.5 (C-3’ α), 81.0 (C-3’ β), 79.4 (C-4′ β), 78.9 (C-2′ β), 71.8 (CH2-1″, α, β), 43.0 (SCH3, β), 42.3, 41.7 (SCH3, α, C-5′ β), 33.8 (CH3-N7, α, β), 32.0 (CH2-2″, α, β), 29.9, 29.9, 29.8, 29.8, 29.7, 29.7, 29.6, 29.6, 29.5, 26.1, 22.8 (CH3-N3, CH2-3″ to CH2-11″, α, β) 21.0, 21.0 (CH3, Ac, α, β), 14.3 (CH2-12″, α, β). HRMS: calcd for C27H45N5O8S [M + H]+ 600.3062, found 600.3058; calcd for C27H45N5O8S [M + Na]+ 622.2881, found 622.2878.
4.1.11. 1-(2-O-Acetyl-1-azido-1,5-dideoxy-3-O-dodecyl-α-D-xylofuranos-5-yl)-3,7-dimethyl-3,7-dihydro-1H-purine-2,6-dione (13) and 1-(2-O-acetyl-5-deoxy-3-O-dodecyl-D-xylono-1,4-lacton-5-yl)-3,7-dimethyl-3,7-dihydro-1H-purine-2,6-dione (14)
To a solution of 1-(1,2-di-O-acetyl-5-deoxy-3-O-dodecyl-α,β-D-xylofuranos-5-yl)-3,7-dimethyl-3,7-dihydro-1H-purine-2,6-dione (11, 30 mg, 0.053 mmol) in acetonitrile (5 mL), trimethylsilyl azide (TMSN3, 0.06 mL, 0.43 mmol) and trimethylsilyl triflate (TMSOTf, 0.08 mL, 0.044 mmol) were sequentially added. The mixture was stirred at 65 °C under microwave irradion (150 W, Pmax = 250 Psi) for 1 h 40 min. The solution was then diluted with CH2Cl2 and washed with a sat. aq. NaHCO3 soln. The aqueous phase was extracted with CH2Cl2 (3×) and the combined organic phases were dried with anhydrous MgSO4. After filtration and evaporation, the residue was subjected to column chromatography (from AcOEt/cyclohexane, 1:1 to AcOEt) to afford 13-α (11 mg, 38%) and a mixture (18 mg) containing 13-β and the xylonolactone derivative 14 in a 3.3:1 ratio (corresponding to 14 mg of 13-β (48%) and 4 mg of 14 (14%)).
Data for 13-α: = +74 (c = 1, in CH2Cl2). 1H NMR (400 MHz, CDCl3) δ: 7.52 (s, 1 H, H-8), 5.58 (d, 1 H, H′-1, J1′,2′ = 4.7), 5.12 (t, 1 H, H′-2, J1′,2′ ~ J2′,3′), 4.71–4.56 (m, 2 H, H′-4, H′-5a), 4.12 (t, 1 H, H′-3, J2′,3′ ~ J3′,4′ ~ 4.5), 3.99 (s, 3 H, CH3-N7), 3.91 (d, 1 H, H′-5b, J = 12.2), 3.66–3.53 (m, 4 H, H-1″a, CH3-N3), 3.54–3.45 (m, 1 H, H-1″b), 2.13 (s, 3 H, CH3, OAc), 1.62–1.52 (m, 2 H, CH2-2″), 1.38–1.18 (m, 18 H, CH2-3″ to CH2-11″), 0.87 (t, 3 H, CH3-12″, J = 6.7). 13C NMR (100 MHz, CDCl3) δ: 170.1 (CO, OAc), 155.4 (C-6), 151.7 (C-2), 149.0 (C-4), 141.7 (C-8), 107.8 (C-5), 89.6 (C-1′), 81.4 (C-3′), 77.7 (C′-2), 76.5 (C′-4); 71.2 (C-1″), 41.2 (C-5ʹ), 33.8 (CH3-N7), 32.1, 29.9, 29.9, 29.8, 29.8, 29.7, 29.6, 29.5, 26.2, 22.8 (CH3-N3, CH2-2″ to CH2-11″), 20.8 (CH3, OAc), 14.3 (C-12″). HRMS: calcd for C26H41N7O6 [M + H]+ 548.3191, found 548.3187; calcd for C26H41N7O6 [M + Na]+ 570.3011, found 570.3004.
Data for 13-β: 1H NMR (400 MHz, CDCl3) δ: 7.50 (s, 1 H, H-8), 5.11 (s, 1 H, Hʹ-2), 5.02 (s, 1 H, H′-1) 4.83 (dd, 1 H, H-5′a, J5′a,5′b = 14.3, J5′a,4′ = 8.3), 4.59 (ddd, 1 H, H-4′), 4.07 (dd, 1 H, H-5′b, J5′b,4′ = 1.9), 3.99 (s, 3 H, CH3-N7), 3.91 (d, 1 H, H′-3, J3′,4′ = 4.3), 3.79–3.70 (m, 1 H, H-1″a), 3.58 (s, 3 H, CH3-N3), 3.54–3.45 (m, 1 H, H-1″b), 2.07 (s, 3 H, CH3, OAc), 1.65–1.56 (m, 2 H, CH2-2″), 1.44–1.17 (m, 18 H, CH2-3″ to CH2-11″), 0.86 (t, 3 H, CH3-12″, J = 6.7)*. 13C NMR (100 MHz, CDCl3) δ: 169,7 (CO, OAc), 155.3 (C-6), 151.8 (C-2), 149.0 (C-4), 141.6 (C-8), 107.8 (C-5), 93.3 (C-1′), 81.9 (C-4′), 81.4 (C′-3), 79.8 (C′-2); 70.9 (C-1″), 41.2 (C-5′), 33.8 (CH3-N7), 32.1, 29.9, 29.8, 29.8, 29.7, 29.6, 29.5, 26.2, 22.8 (CH3-N3, CH2-2″ to CH2-11″), 20.8 (CH3, OAc), 14.3 (C-12″)*. HRMS: calcd for C26H41N7O6 [M + H]+ 548.3191, found 548.3186.
Data for 14: 1H NMR (400 MHz, CDCl3) δ: 7.51 (s, 1 H, H-8), 5.59 (d, 1 H, H′-2, J2′,3′ = 6.7), 5.07 (ddd, 1 H, H′-4), 4.83 (dd, 1 H, H-5′a, J5′a,5′b = 14.3, J5′a,4′ = 10.9), 4.37 (t, 1 H, H′-3, J2′,3′ ~ J3′,4′), 4.07 (dd, 1 H, H-5′b, J5′b,4′ = 3.0), 3.97 (s, 3 H, CH3-N7), 3.65–3.53 (m, 5 H, CH3-N3, CH2-1″), 2.17 (s, 3 H, CH3, OAc), 1.66–1.54 (m, 2 H, CH2-2″), 1.47–1.15 (m, 18 H, CH2-3″ to CH2-11″), 0.87 (t, 3 H, CH3-12″, J = 6.7)*. 13C NMR (100 MHz, CDCl3) δ: 170.0 (C-1′), 169.4 (CO, Ac), 155.1 (C-6), 151.6 (C-2), 149.1 (C-4), 141.8 (C-8), 107.7 (C-5), 78.7 (C-3′), 75.9 (C-4′), 72.2 (C′-2), 71.3 (C-1″), 40.5 (C-5′), 33.8 (CH3-N7), 32.1, 29.9, 29.8, 29.8, 29.7, 29.5, 29.5, 26.1, 22.8 (CH3-N3, CH2-2″ to CH2-11″), 20.7 (CH3, OAc), 14.3 (C-12″)*. HRMS: calcd for C26H40N4O7 [M + H]+ 521.2970, found 521.2966.
* Data extracted from the spectrum of a mixture containing 13-β/14.
4.1.12. Dimethyl N-[2-O-acetyl-1,5-dideoxy-3-O-dodecyl -5-(3,7-dimethyl-3,7-dihydro-2,6-dioxo-1H-purin-1-yl)-α,β-D-xylofuranos-1-y]phosphoramidate (15)
To a solution of 1-(2-O-acetyl-1-azido-1,5-dideoxy-3-O-dodecyl-α-D-xylofuranos-5-yl)-3,7-dimethyl-3,7-dihydro-1H-purine-2,6-dione (13-α, 11 mg, 0.02 mmol) in CH2Cl2 (2 mL), trimethyl phosphite (0.03 mL, 0.25 mmol) was added. The solution was stirred at room temperature for 23 h. The solution was then concentrated under vacuum and the residue was subjected to column chromatography (from AcOEt/hexane, 15:1 to AcOEt/MeOH, 15:1) to afford 15 (7 mg, 56%, anomeric mixture, α/β ratio, 1:0.3) as a colorless oil. 1H NMR (400 MHz, CDCl3) δ: 7.50 (s, 1.3 H, H-8 α, β), 5.33 (ddd, 1 H, H-1′ α, J1′,2′ α = 3.9 Hz, J1′,P α = 7.5 Hz, J1′,NH α = 11.6), 5.14 (s, 0.3 H, H-2′ β), 5.06 (d, 1 H, H-2′ α, J1′,2′ α = 3.4 Hz), 4.96 (dd, 0.3 H, H-1′ β, J1′,P β = 7,8 Hz, J1′,NH β = 11.2), 4.73–4.57 (m, 1.3 H, H-5′a, α, β, J5′a,5′b α = 13.9, J4′,5′a α = 9.1, J5′a,5′b β = 13.8, J4′,5′a β = 8.9), 4.54–4.42 (m, 1.3 H, H-4′, α, β), 4.15 (t, 0.3 H, NH, β, J1′,NH β ~ JNH,P β = 11.2 Hz), 4.05–3.85 (m, 6.5 H, H-5′b α, β, H-3′ α, β, CH3-N7 α, β), 3.77–3.46 (m, 15.6 H, 2 × OCH3 α, 2 × OCH3 β, CH2-1″ α, β, H-3′ α, β, CH3-N7 α, β), 2.11 (s, 3 H, CH3, Ac, α), 2.06 (s, 0.9 H, Ac, β), 1.66–1.54 (m, 2.6 H, CH2-2″ α, β), 1.38–1.17 (m, 23.4 H, CH2-3″ to CH2-11″ α, β), 0,87 (t, 3.9 H, CH2-12″ α, β, J = 6.7). 13C NMR (100 MHz, CDCl3) δ: 169.9, 169.7 (CO, Ac α, β), 155.4 (C-6 α, β), 151.7 (C-2 α, β); 149.0, 148.9 (C-4 α, β), 141.6, 141.5 (C-8 α, β), 107.8 (C-5 α, β), 87.4 (C-1′ β), 82.4 (C-1ʹ α), 82.2, 81.5 (C-3′ α, β), 79.9 (C-2′ β); 78.4 (C-4′ α or β), 76.2, 76.0, 76.0 (C-2′ α, C-4′ α or β), 71.4, 71.3 (CH2-1″ α, β); 53.4, 53.4, 53.3, 53.2 (4 d, 2 × OCH3 α, β, JC,P = 4.8), 41.7, 40-8 (C-5′ α, β), 33.7 (CH3-N7 α, β), 32.1, 29.8, 29.8, 29.8, 29.6, 29.6, 29.5, 26.2, 26.2, 22.8 (CH3-N3 α, β, CH2-2″ to CH2-11″), 21.1, 21.0 (CH3, Ac, β), 14.3 (CH3-12″ α, β). 31P NMR (162 MHz, CDCl3) δ: 8.49. HRMS: calcd for C28H48N5O9P [M + H]+ 630.3262, found 630.3270; calcd for C28H48N5O9P [M + Na]+ 652.3082, found 652.3090.
4.1.13. 3-O-Octyl-1,2-O-isopropylidene-α-D-glucofuranose (16)
To a solution of 1,2:5,6-di-O-isopropylidene-α-D-glucofuranose (15, 3.0 g, 11.53 mmol) in anhydrous DMF (30 mL) under nitrogen atmosphere and at 0 °C and, NaH (60%, 0.69 g, 17.25 mmol) was added. The suspension was stirred at 0 °C for 10 min. Then, octyl bromide (2.38 mL, 13.79 mmol) was added and the mixture was stirred at room temperature for 22 h. It was then diluted with CH2Cl2 and washed with water and brine solution. The aqueous phase was extracted with CH2Cl2 (3×) and the combined organic layers were dried with anhydrous MgSO4, filtered and concentrated. To the resulting residue, aq. acetic acid (70% soln., 38 mL) was added and the resulting solution was stirred at room temperature for 26 h. After co-evaporation with toluene, the residue was subjected to column chromatography (EtOAc/hexane, 1:3) to afford 16 (3.37 g, 88%, 2 steps) as a colorless oil. 1H NMR (CDCl3, 400 MHz): 5.82 (d, 1 H, H-1, J1,2 = 3.7), 4.47 (d, 1 H, H-2), 4.03 (dd, 1 H, H-4, J3,4 = 3.2, J4,5 = 7.8), 3.95–3.85 (m, 2 H, H-3, H-5), 3.73 (dd, part A of AB system, H-6a, J5,6a = 3.0, J6a,6b = 11.6), 3.63 (dd, part B of AB system, H-6b, J5,6b = 5.6), 3.59–3.49 (m, 1 H, H-1′a), 3.49–3.14 (m, 3 H, H-1′b, OH-5, OH-6), 1.57–1.44 (m, 2 H, CH2-2′), 1.41 (s, 3 H, CH3, i-Pr), 1.31–1.12 (m, 13 H, CH2-3′ to CH2-7′, CH3, i-Pr), 0.81 (t, 3 H, CH3-8′, J = 6.6). 13C NMR (CDCl3, 100 MHz): 111.6 (Cq, i-Pr), 105.0 (C-1), 82.6 (C-3), 82.1 (C-2), 79.7 (C-4), 70.6 (C-1′), 69.3 (C-5), 64.2 (C-6), 31.8, 29.7, 29.3, 29.2, 26.7, 26.2, 26.0, 22.6 (C-2′ to C-7′, 2 × CH3, i-Pr), 14.1 (C-8′). HRMS: calcd for C17H32O6 [M + H]+ 333.2272, found 333.2266; calcd for C17H32O6 [M + Na]+ 355.2091, found 355.2081.
4.1.14. 3-O-Octyl-1,2-O-isopropylidene-α-D-xylofuranose (17)
To a solution of 3-O-octyl-1,2-O-isopropylidene-α-D-glucofuranose (16, 3.14 g, 9.45 mmol) in 60% aq. THF (22 mL), at 0 °C, sodium metaperiodate (4.85 g, 22.68 mmol) was added. The mixture was stirred for 4 h at room temperature. Then, it was diluted with EtOAc and washed with water and brine solution. The aqueous phase was extracted with EtOAc (3×) and the combined organic layers were dried with anhydrous MgSO4. After filtration and concentration under vacuum, the residue was dissolved in EtOH/H2O (57 mL, 2:1). To the resulting solution at 0 °C, NaBH4 (0.461 g, 12 mmol) was added and the mixture was stirred at room temperature for 1.5 h. Then, EtOAc was added. The mixture was washed with brine soln. and the aqueous phase was extracted with EtOAc (2×). The combined organic layers were dried with anhydrous MgSO4, filtered and concentrated. The residue was subjected to column chromatography (EtOAc/petroleum ether, 2:1) to afford 17 (1.85 g, 65%, 2 steps) as a colorless oil. 1H NMR (CDCl3, 400 MHz): 5.98 (d, 1 H, H-1, J1,2 = 3.8), 4.57 (d, 1 H, H-2), 4.27 (br. q, 1 H, H-4), 4.00–3.85 (m, 2 H, H-3, H-5a, H-5b, J3,4 = 3.3, J4,5a = 4.6, J4,5b = 4.4, J5a,5b = 12.3), 3.68–3.58 (m, 1 H, H-1′a), 3.47–3.38 (m, 1 H, H-1′b), 1.61–1.51 (m, 2 H, CH2-2′), 1.49 (s, 3 H, CH3, i-Pr), 1.38–1.19 (m, 13 H, CH2-3′ to CH2-7′, CH3, i-Pr), 0.88 (t, 3 H, CH3-8′, J = 6.6). 13C NMR (CDCl3, 100 MHz): 111.8 (Cq, i-Pr), 105.2 (C-1), 84.5 (C-3), 82.5 (C-2), 79.9 (C-4), 70.6 (C-1′), 61.3 (C-5), 31.9, 29.8, 29.5, 29.3, 27.0, 26.5, 26.2, 22.8 (C-2′ to C-7′, 2 × CH3, i-Pr), 14.2 (C-8′). HRMS: calcd for C16H30O5 [M + H]+ 303.2166, found 303.2156; calcd for C16H30O5 [M + Na]+ 325.1985, found 325.1974.
4.1.15. 1-(5-Deoxy-3-O-dodecyl-α-D-xylofuranos-5-yl)-3,7-dimethyl-3,7-dihydro-1H-purine-2,6-dione (18)
A solution of 3-O-octyl-1,2-O-isopropylidene-α-D-xylofuranose (17, 80 mg, 0.26 mmol) in DMF (2 mL) was subjected to the MW-assisted protocol for Mitsunobu coupling, according to the general procure, using PPh3 (138 mg, 0.526 mmol), DEAD (40 % wt soln in toluene, 0.2 mL, 0.44 mmol) and theobromine (95 mg, 0.527 mmol). The mixture was stirred for 30 min. Column chromatography was performed using (AcOEt/petroleum ether, 2:1 as eluent. To the obtained residue, aq. TFA (60% soln., 3 mL) was added and the resulting solution was stirred at room temperature for 4 h. After co-evaporation of the solvents with toluene, the residue was subjected to column chromatography (from AcOEt/petroleum ether, 20:1 to AcOEt/MeOH, 12:1) to afford 10 (22 mg, 20%, 2 steps, anomeric mixture, α/β ratio, 1:1) as a colorless oil. 1H NMR (400 MHz, CDCl3) δ: 7.54, 7.51 (s, 2 H, H-8 α, β), 5.56 (d, 1 H, H-1′ α, J1′,2′α = 4.0), 5.11 (d, 1 H, H-1′ β, J 1′,2′β = 1.5), 4.68–4.41 (m, 4 H, H-4′ α, H-4′ β, H-5′a α, H-5′a β), 4.30 (dd, 1 H, H-2′ β, J 2′,3′β = 3.6), 4.22 (br.t, 1 H, H-2′ α), 4.08–3.91 (m, 10 H, H-3′ α, H-3′ β, H-5′b α,H-5’b β, CH3-N7 α, β), 3.71–3.61 (m, 2 H, H-1″a α, H-1″a β), 3.60–3.43 (m, 8 H, H-1″b α, H-1″b β, CH3-N3 α, β) 1.66–1.50 (m 4 H, CH2-2″ α, CH2-2″ β), 1.41–1.16 (m, 20 H, CH2-3″ to CH2-7″), 0.87 (t, 6 H, CH3-8″, J = 6.6). 13C NMR (100 MHz, CDCl3) δ: 155.9, 155.5 (C-6 α, β), 152.0, 151.9 (C-2 α, β), 149.2, 149.0 (C-4 α, β), 142.0, 141.8 (C-8 α, β), 107.9, 107.8 (C-5 α, β), 103.4 (C-1′ β), 96.2 (C-1′ α), 84.6 (C-3’ β), 84.2 (C-3’ α), 80.3 (C-2′ β), 77.1 (C-4′ α, β), 75.3 (C-2′ α), 71.5 (CH2-1″ α), 70.9 (CH2-1″ β), 43.1, 41.5 (C-5′, α, β), 33.8, 33.8 (CH3, N7, α, β), 32.0 (CH2-2″, α, β), 30.0, 30.0, 29.9, 29.6, 29.4, 29.4, 26.2, 26.2, 22.8, (CH3, N3, CH2-3″ to CH2-7″, α, β), 14.3 (CH2-8″, α, β). HRMS: calcd for C20H32N4O6 [M + H]+ 425.2395, found 425.2390; calcd for C20H32N4O6 [M + Na]+ 447.2214, found 447.2208.
4.1.16. 1-(6-Deoxy-3-O-dodecyl-1,2-O-isopropylidene-α-D-glucofuranos-6-yl)-3,7-dimethyl-3,7-dihydro-1H-purine-2,6-dione (19)
A solution of 3-O-octyl-1,2-O-isopropylidene-α-D-glucofuranose (16, 80 mg, 0.241 mmol) in DMF (2 mL) was subjected to the protocol for Mitsunobu coupling, according to the general procure, using PPh3 (138 mg, 0.526 mmol), DEAD (40 % wt soln in toluene, 0.24 mL, 0.526 mmol) and theobromine (95 mg, 0.527 mmol). The mixture was stirred under reflux for 20 h. Purification by column chromatography (from AcOEt/petroleum ether 3:1 to 4:1) afforded 19 (19 mg, 16%; isolated yield: 11 mg, 9%) as a colorless oil. 1H NMR (CDCl3, 400 MHz): 7.51 (s, 1 H, H-8), 5.97 (d, 1 H, H-1′, J1,2 = 3.7), 4.54 (d, 1 H, H-2′), 4.43 (dd, part A of AB system, H-6′a, J5′,6′a = 7.3, Ja,b = 17.4), 4.29–4.19 (m, 2 H, H-5′, H-6′b), 4.11 (dd, 1 H, H-4′, J3′,4′ = 3.2, J4′,5′ = 6.3), 4.03 (d, 1 H, H-3′), 3.98 (s, 3 H, CH3-N7), 3.67–3.47 (m, 5 H, CH2-1″, CH3-N3), 1.72–1.52 (m, 2 H, CH2-2″), 1.50 (s, 3 H, CH3, i-Pr), 1.36–1.16 (m, 13 H, CH2-3″ to CH2-7″, CH3, i-Pr), 0.86 (t, 3 H, CH3-8″, J = 6.6). 13C NMR (CDCl3, 100 MHz): 153.4 (C-6), 149.9 (C-4), 141.7 (C-8), 111.7 (Cq, i-Pr), 105.5 (C-1′), 83.1 (C-3′), 82.4 (C-2′), 81.3 (C-4′), 70.9 (C-1″), 69.0 (C-5′), 45.2 (C-6′), 33.8 (CH3, N7), 31.9, 30.0, 29.9, 29.8, 29.6, 29.5, 29.3, 27.0, 26.5, 26.2, 22.8 (CH3, N3, C-2″ to C-7″, 2 × CH3, i-Pr), 14.2 (C-8″). HRMS: calcd for C24H38N4O7 [M + H]+ 495.2813, found 495.2808; calcd for C24H38N4O7 [M + Na]+ 517.2633, found 517.2626.
4.1.17. 1-(6-Deoxy-3-O-dodecyl-α-D-glucopyranos-6-yl)-3,7-dimethyl-3,7-dihydro-1H-purine-2,6-dione (20)
A solution of 1-(6-deoxy-3-O-dodecyl-1,2-O-isopropylidene-α-D-glucofuranos-6-yl)-3,7-dimethyl-3,7-dihydro-1H-purine-2,6-dione (19, 20 mg, 0.04 mmol) in aq. trifluoroacetic acid (TFA, 60%, 3 mL) was stirred at room temp. for 5 h. The solvents were co-evaporated of with toluene and the residue was subjected to column chromatography (from AcOEt/petroleum ether 15:1 to AcOEt/MeOH, 20:1) to afford 20 (16 mg, 87%, anomeric mixture, α/β ratio, 1:0.9) as colorless oil. 1H NMR (CDCl3, 400 MHz): 7.62, 7.61 (2 s, 1.9 H, H-8, α, β), 5.28 (br.s, 1 H, H-1′α), 4.61 (d, 0.9 H, H-1′β, J1′,2′ β = 7.5), 4.52 (dd, 0.9 H, H-6′a β, J5′,6′a β = 2.1, J6a,6b β = 14.0), 4.45 (dd, 1 H, H-6′a α, J5′,6′a α = 3.5, J6a,6b α = 14.4), 4.38–4.28 (m, 1.9 H, H-26′b α, H-6′b β), 4.13 (dt, 1 H, H-5′α, J4′,5′ α = 9.7, J5′,6′a α = J5′,6′b α = 3.5), 4.01, 4.00 (2 s, 5.7 H, CH3-N7), 3.95–3.73 (m, 3.8 H, CH2-1″, α, β), 3.67–3.57 (s, 7.6 H, H-3′ β, H-4′ β, CH3-N3), 3.54–3.49 (m, 2.9 H, H-2′ α, H-3′ α, H-5 β), 3.46–3.01 (m, 7.6 H, H-′ β, H-4′ α, OH-1, OH-2, OH-4), 1.66–1.55 (m, 3.8 H, CH2-2″, α, β), 1.39–1.20 (m, 19 H, CH2-3″ to CH2-7″, α, β), 0.86 (t, 5.7 H, CH3-8″, α, β, J = 6.5). 13C NMR (CDCl3, 100 MHz): 156.0 (C-6 α, β), 152.4 (C-2 α, β), 149.0 (C-4 α, β), 142.1 (C-8 α, β), 107.7 (C-5 α, β), 107.7 (C-5 α, β), 97.2 (C-1′ β), 92.6 (C-1′ α), 83.5 (C-3′ β), 81.2 (C-3′ α), 75.1 (C-4′ β), 74.8 (C-2′ β), 73.8 (C-1″), 73.7 (C-5′ β), 72.2 (C-2′ α), 72.1 (C-2′ β), 72.1 (C-4′ α), 71.3 (C-5′ α), 41.6 (C-6′ α, β), 33.9 (CH3, N7, α, β), 32.0, 31.0, 30.2, 29.9, 29.6, 29.4, 26.2, 22.8 (CH3, N3, C-2″ to C-7″, α, β), 14.3 (C-8″, α, β). HRMS: calcd for C21H34N4O7 [M + H]+ 455.2500, found 455.2494; calcd for C21H34N4O7 [M + Na]+ 477.2320, found 477.2313.
4.1.18. 1-(1,2,4-Tri-O-acetyl-6-deoxy-3-O-dodecyl-α-d-glucopyranos-6-yl)-3,7-dimethyl-3,7-dihydro-1H-purine-2,6-dione (21)
A solution of 1-(6-deoxy-3-O-dodecyl-α-D-glucopyranos-6-yl)-3,7-dimethyl-3,7-dihydro-1H-purine-2,6-dione (20, 27 mg, 0.059 mmol) in pyridine (1.6 mL) and acetic anhydride (1 mL) was stirred at room temp. for 2.5 h. After co-evaporation with toluene, the residue was subjected to column chromatography (AcOEt/hexane, 15:1 to AcOEt) to give 21 (29 mg, 84%, anomeric mixture, β/α ratio, 1:0.7) as a yellow oil. 1H NMR (CDCl3, 400 MHz): 7.53, 7.52 (2 s, 1.7 H, H-8, α, β), 6.24 (br.s, 0.7 H, H-1′α, J1′,2′ α = 3.6), 5.51 (d, 1 H, H-1′β, J1′,2′ β = 8.1), 5.16–4.97 (m, 3.4 H, H-2′α, H-2′β, H-4′α, H-4′β, J2′,3′ α = 9.9, J3′,4′ α = 9.8, J2′,3′ β ~ J3′,4′ β ~ J4′,5′ β ~ 9.5), 4.63–4.49 (m, 1.7 H, H-6′a α, H-6′a β, J6a,6b β = 13.8, J6a,6b α = 14.0, J5,6a α = 9.8, J5,6a β = 9.3), 4.33 (td, 0.7 H, H-5′ α, J4′,5′ α = J5′,6′a α = 9.8, J5′,6′b α = 2.1), 4.04–3.94 (m, 6.1 H, CH3-N7, H-5′ β), 3.90–3.75 (m, 2.4 H, H-6′b α, H-6′b β, H-3 α), 3.66–3.50 (m, 9.5 H, CH2-1″, α, β, H-3′ β, CH3-N3), 2.18, 2.16, 2.08, 2.07, 2.04, 2.04 (6 s, 15.3 H, 3 x CH3, 3 × Ac, α, β), 1.55–1.42 (m, 3.4 H, CH2-2″, α, β), 1.37–1.19 (m, 17 H, CH2-3″ to CH2-7″, α, β), 0.86 (t, 5.1 H, CH3-8″, α, β, J = 6.7). 13C NMR (CDCl3, 100 MHz): 169.9, 169.6, 169.4. 169.1, 169.0 (CO, Ac, α, β), 155.2, 155.1 (C-6 α, β), 151.6, 151.6 (C-2 α, β), 149.0, 148.9 (C-4 α, β), 141.7, 141.7 (C-8 α, β), 107.7, 107.7 (C-5 α, β), 92.3 (C-1′ β), 89.6 (C-1′ α), 80.4 (C-3′ β), 77.0 (C-3′ α), 73.1, 72.9 (C-1″ α, β), 72.6 (C-5′ β), 72.0, 72.0, 71.6, 71.5 (C-2′, C-4′, α, β), 69.6 (C-5′ α), 42.0, 41.9 (C-6′ α, β), 33.8, 33.7 (CH3, N7, α, β), 32.0, 31.1, 30.4, 30.3, 29.9, 29.8, 29.6, 29.5, 29.4, 29.4, 26.1, 26.1, 22.8 (CH3, N3, C-2″ to C-7″, α, β), 21.1, 21.1, 20.9, 20.8 (CH3, Ac, α, β), 14.2 (C-8″, α, β). HRMS: calcd for C27H40N4O10 [M + H]+ 581.2817, found 581.2819; calcd for C27H40N4O10 [M + Na]+ 603.2637, found 603.2633.
4.1.19. N-[2,4-Di-O-acetyl-1,6-dideoxy-6-(3,7-dimethyl-3,7-dihydro-2,6-dioxo-1H-purin-1-yl)-3-O-dodecyl-α,β-D-glucopyranos-1-yl]methanesulfonamide (22)
To a solution of 1-(1,2,4-tri-O-acetyl-6-deoxy-3-O-dodecyl-α-d-glucopyranos-6-yl)-3,7-dimethyl-3,7-dihydro-1H-purine-2,6-dione (21, 20 mg, 0.034 mmol) in CH2Cl2/acetonitrile (1.2 mL, 4:1) under nitrogen and at 0 °C, BF3·Et2O (0.02 mL, 0.16 mmol) and methanesulfonamide (19 mg, 0.19 mmol) were added. The mixture was stirred at room temp. for 3.5 h. Then, it was diluted with CH2Cl2 and washed with a satd. aq. NaHCO3 soln. The aqueous phase was extracted with CH2Cl2 (2×) and the combined organic layers were dried with anhydrous MgSO4. After filtration and evaporation of the solvent, the residue was subjected to column chromatography (AcOEt/petroleum ether, 10:1) to afford 22 (8 mg, 38%, anomeric mixture, β/α ratio, 1:0.4) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ: 7.53 (s, 1.4 H, H-8 α, β), 6.21 (br.s, 0.4 H, NH α), 5.52 (d, 1 H, NH, β, J1’,NH β = 9.6), 5.06–4.89 (m, 1.8 H, H-4′α, H-4′β, H-1 α), 4.82 (t,1 H, H-2 ′ β, J1’,2’ β ~J2’,3’ α ~ 9.3), 4.61–4.51 (m, 1.4 H, H-1′ β, H-2 α), 4.46 (dd, 1 H, H-6′a β, J6a,6b β = 13.8, J5,6a β = 9.8), 4.37–4.25 (m, 0.8 H, H-5′ α, H-6′a α), 3.89 (d, 1 H, H-6′b β), 3.98, 3.95 (2 s, 4.2 H, CH3-N7 α, β), 3.82–3.73 (m, 1.8 H, H-5′ β, H-6′b α, H-3′ α), 3.66–3.47 (m, 8 H, H-3′ β, CH3-N3 α, β, CH2-1″, α, β), 2.85 (s, 4.2 H, S-CH3, α, β), 2.18, 2.11, 2.06, 2.01 (s, 8.4 H, CH3, Ac, α), 1.52–1.42 (m 2.8 H, CH2-2″ α, CH2-2″ β), 1.36–1.14 (m, 14 H, CH2-3″ to CH2-7″, α, β), 0.88 (t, 4.2 H, CH3-8″, α, β, J = 6.6). 13C NMR (100 MHz, CDCl3) δ: 171.0, 170.1, 169.0 (CO, Ac, α, β), 155.1 (C-6 α, β), 151.5 (C-2 α, β), 149.2 (C-4 α, β), 142.1 (C-8 α, β), 107.6 (C-5 α, β), 83.1 (C-1′ β), 80.4 (C-3’ β), 74.3 (C-5′ β), 73.4 (CH2-1′′, α, β), 72.0 (C-2′ β), 71.8 (C-4′ β), 43.2 (SCH3, β), 42.1, 42.1 (C-6′ β, C-6′ α), 42.0 (SCH3, α), 33.7 (CH3, N7, α, β), 32.0, 30.3, 29.9, 29.9, 29.8, 29.6, 29.4, 26.1, 22.8 (CH3, N3, C-2″ to C-7″, α, β), 21.1, 21.0 (CH3, Ac, α), 14.2 (C-8″, α, β). HRMS: calcd for C26H41N5O10S [M + H]+ 616.2647, found 616.2645; calcd for C26H41N5O10S [M + Na]+ 638.2466, found 638.2464.
1H NMR and 13C NMR Spectra for compounds 3–12, 13-α, 15–22 can be found in the Supplementary Materials.
4.2. Biological Assays
4.2.1. Cholinesterase Inhibition Assays
A TECAN Spectra-FluorPlus working on the kinetic mode and measuring the absorbance at λ = 415 nm was used for the enzymatic studies. Acetylcholinesterase (from Electrophorus electricus), 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) and acetylthiocholine iodide were purchased from Fluka. Butyrylcholinesterase (from equine serum) was bought from Sigma. Experimental details for preparation of the solutions and the procedures for the enzyme assays can be found in the Supplementary Materials.
4.2.2. Cytotoxicity Assays
The cytotoxicity of the compounds was evaluated using the sulforhodamine-B (SRB) colorimetric assay. The EC50 values in µM from SRB assays were determined after 96 h of treatment and were averaged from three independent experiments performed each in triplicate; confidence interval CI = 95%, cut-off the assay 30 µM. Compounds with EC50 > 30 µM are considered inactive. The cell lines were kindly provided by Th. Müller (Dep. of Haematology/Oncology, Martin Luther Universität Halle-Wittenberg). Human cancer cell lines: A375 (epithelial melanoma), A2780 (ovarian carcinoma), HT29 (colorectal adenocarcinoma), MCF7 (breast adenocarcinoma), SW1736 (thyroid carcinoma); non-malignant: NIH 3T3 (mouse embryonic fibroblasts).
4.3. Molecular Docking Studies
From the RCSB Protein Databank the crystal structures of AChE (PDB: 1C2O) and BChE (PDB: 6EMI) were selected. The compounds were built using Datawarrior and geometry optimized using MMFF94 force field. For the enzyme preparation, water and co-crystallized ligands were removed, gasteiger charges were added and non-polar hydrogens were merged with into the corresponding heavy atom.
The search space consisted of a 126 × 126 × 126 grid with 0.2 angstrom spacing centered on either the binding site of AChE or BChE. Dockings were executed with Lamarckian GA (200 population size; 25,000,000 evaluations; 15 runs). The pose showing the best binding energy (pose 1, ranked by energy) is shown in the manuscript.
5. Conclusions
In conclusion, purine/uracil 5′-isonucleosides and theobromine-containing 5′/6′-isonucleosides and isonucleotide analogs were synthesized in few steps from easily available xylo- or gluco-configured furanose precursors. A theobromine 6′-isonucleoside was shown to act as a dual AChE/BChE inhibitor at single-digit micromolar concentration range, with a Ki value for BChE being only ca. two-fold lower than that of galantamine hydrobromide. This finding, along with the rather short synthetic pathway for theobromine terminal isonucleosides, encouraging structural optimization, points to the interest of this type of scaffold in the search for stable and non-cytotoxic cholinesterase inhibitor lead compounds.
Acknowledgments
The authors thank the IAESTE student trainee R. Joshi for support in compound synthesis and Lucie Fischer and Sophie Hoenke for collaborating in the cytotoxicity assays.
Supplementary Materials
The following are available online at https://www.mdpi.com/1424-8247/12/3/103/s1.
Author Contributions
Conceptualization, N.M.X.; methodology, N.M.X. (synthesis), R.C. (biological evaluation, docking studies); investigation, N.M.X, E.C.d.S., M.P.P. (synthesis), A.L., R.C. (biological evaluation), I.S., R.C. (docking studies), M.C.O. (HRMS analysis); supervision, N.M.X. (synthesis), R.C. (biological evaluation, docking studies); writing—original draft preparation, N.M.X.; writing—review and editing, N.M.X., R.C.; project administration and funding acquisition, N.M.X.
Funding
This research was funded by ‘Fundacão para a Ciência e Tecnologia’ (FCT), grant number IF/01488/2013, the exploratory project IF/01488/2013/CP1159/CT0006 and the strategic projects UID/MULTI/00612/2013 and UID/MULTI/00612/2019.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Jordheim L.P., Durantel D., Zoulim F., Dumontet C. Advances in the development of nucleoside and nucleotide analogues for cancer and viral diseases. Nat. Rev. Drug Discov. 2013;12:447–464. doi: 10.1038/nrd4010. [DOI] [PubMed] [Google Scholar]
- 2.Shelton J., Lu X., Hollenbaugh J.A., Cho J.H., Amblard F., Schinazi R.F. Metabolism, biochemical actions, and chemical synthesis of anticancer nucleosides, nucleotides, and base analogs. Chem. Rev. 2016;116:14379–14455. doi: 10.1021/acs.chemrev.6b00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montgomery J.A., Clayton S.D., Thomas H.J. Isonucleosides. I. Preparation of methyl 2-deoxy-2-(purin-9-yl) arabinofuranosides and methyl 3-deoxy-3-(purin-9-yl) xylofuranosides. J. Org. Chem. 1975;40:1923–1927. doi: 10.1021/jo00901a012. [DOI] [PubMed] [Google Scholar]
- 4.Yu H.W., Zhang L.R., Zhuo J.C., Ma L.T., Zhang L.H. Studies on the synthesis and biological activities of 4′-(R)-hydroxy-5′-(S)-hydroxymethyl-tetrahydrofuranyl purines and pyrimidines. Bioorg. Med. Chem. 1996;4:609–614. doi: 10.1016/0968-0896(96)00048-X. [DOI] [PubMed] [Google Scholar]
- 5.Yu H.W., Zhang H.Y., Yang Z.J., Min J.M., Ma L.T., Zhang L.H. Studies on the syntheses and biological activities of isonucleosides. Pure Appl. Chem. 1998;70:435–438. doi: 10.1351/pac199870020435. [DOI] [Google Scholar]
- 6.Huryn D.M., Sluboski B.C., Tam S.Y., Weigele M., Sim I., Anderson B.D., Mitsuya H., Broder S. Synthesis and anti-HIV activity of isonucleosides. J. Med. Chem. 1992;35:2347–2354. doi: 10.1021/jm00091a001. [DOI] [PubMed] [Google Scholar]
- 7.Tino J.A., Clark J.M., Field A.K., Jacobs G.A., Lis K.A., Michalik T.L., McGeever-Rubin B., Slusarchyk W.A., Spergel S.H. Synthesis and antiviral activity of novel isonucleoside analogs. J. Med. Chem. 1993;36:1221–1229. doi: 10.1021/jm00061a013. [DOI] [PubMed] [Google Scholar]
- 8.Solke K.F., Huang J.L., Russell J.W., Whiterock V.J., Sundeen J.E., Stratton L.W., Clark J.M. Pharmacokinetics and antiviral activity of a novel isonucleoside, BMS-181165, against simian varicella virus infection in African green monkeys. Antivir. Res. 1994;23:219–224. doi: 10.1016/0166-3542(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 9.Nair V., St. Clair M.H., Reardon J.E., Krasny H.C., Hazen R.J., Paff M.T., Boone L.R., Tisdale M., Najera I., Dornsife R.E., et al. Antiviral, metabolic, and pharmacokinetic properties of the isomeric dideoxynucleoside 4(S)-(6-amino-9H-purin-9-yl) tetrahydro-2(S)-furanmethanol. Antimicrob. Agents Chemother. 1995;39:1993–1999. doi: 10.1128/AAC.39.9.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Purdy D.F., Zintek L.B., Nair V. Synthesis of isonucleosides related to AZT and AZU. Nucleosides Nucleotides. 1994;13:109–126. doi: 10.1080/15257779408013230. [DOI] [Google Scholar]
- 11.Nair V., Piotrowska D.G., Okello M., Vadakkan J. Isonucleosides: Design and synthesis of new isomeric nucleosides with antiviral potential. Nucleosides Nucleotides Nucleic. Acids. 2007;26:687–690. doi: 10.1080/15257770701490639. [DOI] [PubMed] [Google Scholar]
- 12.Bobek M., An S.H., Skrincosky D., De Clercq E., Bernacki R.J. 2’-Fluorinated isonucleosides. 1. Synthesis and biological activity of some methyl 2’-deoxy-2’-fluoro-2’-pyrimidinyl-d-arabinopyranosides. J. Med. Chem. 1989;32:799–807. doi: 10.1021/jm00124a013. [DOI] [PubMed] [Google Scholar]
- 13.Xavier N.M., Lucas S.D., Jorda R., Schwarz S., Loesche A., Csuk R., Oliveira M.C. Synthesis and evaluation of the biological profile of novel analogues of nucleosides and of potential mimetics of sugar phosphates and nucleotides. Synlett. 2015;26:2663–2672. doi: 10.1055/s-0035-1560591. [DOI] [Google Scholar]
- 14.Batista D., Schwarz S., Loesche A., Csuk R., Costa P.J., Oliveira M.C., Xavier N.M. Synthesis of glucopyranos-6′-yl purine and pyrimidine isonucleosides as potential cholinesterase inhibitors. Access to pyrimidine-linked pseudodisaccharides through Mitsunobu reaction. Pure Appl. Chem. 2016;88:363–379. doi: 10.1515/pac-2016-0102. [DOI] [Google Scholar]
- 15.Čolović M.B., Krstić D.Z., Lazarević-Pašti T.D., Bondžić A.M., Vasić V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013;11:315–335. doi: 10.2174/1570159X11311030006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McHardy S.F., Wang H.L., McCowen S.V., Valdez M.C. Recent advances in acetylcholinesterase inhibitors and reactivators: An update on the patent literature (2012–2015) Expert Opin. Ther. Pat. 2017;27:455–476. doi: 10.1080/13543776.2017.1272571. [DOI] [PubMed] [Google Scholar]
- 17.Pereira R.G., Pereira M.P., Serra S.G., Loesche A., Csuk R., Silvestre S., Costa P.J., Oliveira M.C., Xavier N.M. Furanosyl nucleoside analogs embodying triazole or theobromine units as potential lead molecules for Alzheimer’s disease. Eur. J. Org. Chem. 2018;2018:2667–2681. doi: 10.1002/ejoc.201800245. [DOI] [Google Scholar]
- 18.Pardridge W.M. Alzheimer’s disease drug development and the problem of the blood-brain barrier. Alzheimer’s Dement. 2009;5:427–432. doi: 10.1016/j.jalz.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xavier N.M., Goncalves-Pereira R., Jorda R., Hendrychová D., Oliveira M.C. Novel dodecyl-containing azido and glucuronamide-based nucleosides exhibiting anticancer potential. Pure Appl. Chem. 2019 doi: 10.1515/pac-2019-0106. [DOI] [Google Scholar]
- 20.Xavier N.M., Porcheron A., Batista D., Jorda R., Řezníčková E., Kryštof V., Oliveira M.C. Exploitation of new structurally diverse D-glucuronamide-containing N-glycosyl compounds: Synthesis and anticancer potential. Org. Biomol. Chem. 2017;15:4667–4680. doi: 10.1039/C7OB00472A. [DOI] [PubMed] [Google Scholar]
- 21.Mushtaq G., Greig N.H., Khan J.A., Kamal M.A. Status of acetylcholinesterase and butyrylcholinesterase in Alzheimer’s disease and type 2 diabetes mellitus. CNS Neurol. Disord. Drug Targets. 2014;13:1432–1439. doi: 10.2174/1871527313666141023141545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morris G.M., Huey R., Lindstrom W., Sanner M.F., Belew R.K., Goodsell D.S., Olson A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009;30:2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nair V., Sharma P.K. Synthesis of the 5′-phosphonate of 4(S)-(6-amino-9H-purin-9-yl) tetrahydro-2(S)-furanmethanol [S, S-IsoddA] ARKIVOC. 2003;15:10–14. doi: 10.3998/ark.5550190.0004.f02. [DOI] [Google Scholar]
- 24.Jiang C., Li B., Guan Z., Yang Z., Zhang L., Zhang L. Synthesis and recognition of novel isonucleoside triphosphates by DNA polymerases. Bioorganic Med. Chem. 2007;15:3019–3025. doi: 10.1016/j.bmc.2007.02.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.