Abstract
Hyperpolarization-activated cyclic nucleotide-gated (HCN) channels regulate neuronal excitability in both peripheral and central nerve systems. Emerging evidence indicates that HCN channels are involved in the development and maintenance of chronic pain. However, the impact of HCN channel activity in the thalamus on chronic pain has not been examined. In this report, we evaluated the effect on nociceptive behaviors after infusion of a HCN channel blocker ZD7288 into the ventral posterolateral (VPL) nucleus of the thalamus in rats with neuropathic pain or monoarthritis. We show that ZD7288 dose-dependently attenuated mechanical allodynia and thermal hyperalgesia in rats with chronic pain. In the thalamus, immunoreactivity of both HCN1 and HCN2 subunits was increased in both rat models. These results suggest that the increased HCN channel activity in the thalamus of the ascending nociceptive pathway contributes to both chronic neuropathic and inflammatory pain conditions.
Keywords: HCN channel, ZD7288, Thalamus, Neuropathic pain, Monoarthritis
1. Introduction
Chronic pain resulting from nerve injury or inflammation often exhibits mechanical allodynia and thermal hyperalgesia, which can persist for a prolonged period of time after the original injury [1]. Hyperpolarization-activated cyclic nucleotide-gated (HCN) cation channels are widely expressed in both peripheral sensory neurons and neurons in the central nervous system [2,3] besides cardiac tissues [4–8]. HCN channels generate inward current (Ih), a mixed Na+/K+ current when the membrane potential is hyperpolarized. HCN channel activity plays important roles in behavior and physiological process such as sleep and arousal [9], learning and memory [10,11], and anesthesia [12,13]. Misregulation of HCN channel activity has been shown to contribute to neurological and psychological disorders including pain [14,15], epilepsy [16], addiction [17] and anxiety [18].
Considerable evidence suggests that dysfunction of HCN channel activity is associated with the development and maintenance of chronic pain and inhibition of HCN channel activity produces the anti-nociceptive effect [19–21]. HCN protein accumulation as well as up-regulation of Ih current have been observed in neurons of dorsal root ganglion (DRG) [22] and the spinal cord [23] of rodents with peripheral nerve injury. Local or systemic administration of ZD7288, a HCN channel blocker, reduced nociceptive behavior in animals [23]. At the supraspinal level, increased HCN activity appears to be related to chronic pain and comorbidity. For example, HCN1 expression level was increased in the amygdala of rats with chronic constriction of sciatic nerve (CCI) and inhibition of HCN channels was anti-nociceptive [21]. Increased HCN protein expression level and enhanced Ih current were also observed in the periaquaductal gray of CCI rats, whereas infusion of ZD7288 into this brain region attenuated neuropathic pain [14,24]. Moreover, microinfusion of ZD7288 into the medial prefrontal cortex or the anterior cingulate cortex also produced the antinociceptive effect in mice with spared nerve injury [25] or CCI [15]. These data suggest that HCN activities in ascending nociceptive pathways may be an important contributor of chronic pain conditions.
The thalamus is the brain structure that receives the input from multiple ascending pain pathways and connects to the cortex and the limbic system. Human imaging study suggests changes in the thalamus in response to neuropathic pain [26]. The thalamic neuronal firing pattern also became irregular in patients with intractable pain [27,28]. To date, it remains unclear whether the HCN activity in the thalamus has a direct impact on nociceptive behavior and whether HCN protein expression in the thalamus would change under neuropathic and/or inflammatory pain conditions. Both possibilities were examined in this study and we found that HCN immuno-reactivity was increased in the thalamus of rats with chronic pain and inhibition of HCN activity attenuated mechanical allodynia and thermal hyperalgesia in rats.
2. Materials and methods
2.1. Experimental animals
Adult male Sprague–Dawley rats weighing 250–270 g were purchased from Charles River Laboratories (Wilmington, MA). The experimental protocols were approved by the Massachusetts General Hospital Institutional Animal Care and Use Committee.
2.2. Surgical procedures
2.2.1. Chronic constriction injury (CCI) and sham operation
The surgical procedure was performed aseptically on rats anesthetized with pentobarbital (50 mg/kg, i.p.). CCI was performed according to the method of Bennett and Xie [29]. Briefly, one side the rat’s sciatic nerve was exposed in the mid-thigh, four ligatures using 4.0 mm chronic gut sutures were loosely placed around the sciatic nerve with a 1.0–1.5 mm interval between each ligature. Skin incision was closed with wound clips. Rats in sham groups underwent the same procedure but without nerve ligation.
2.2.2. Complete Freud’s adjuvant (CFA)-induced hindpaw monoarthritis
Rats were anesthetized with pentobarbital (50 mg/kg, i.p.). CFA (50 μL) (Sigma F4258) or saline was injected into one side of the ankle articular cavity using a 28 gauge needle [30].
2.2.3. Implantation of a guide cannula for intra-thalamus microinjection
Rats were intraperitoneally anesthetized with pentobarbital (50 mg/kg, i.p.) and placed in a Stoelting stereotaxic instrument. A guide cannula (C315G with an infusion cannula C315I, Plastics One, Roanoke, VA) was implanted in the ventral posterolateral (VPL) nucleus of the thalamus contralateral to the injury or CFA injection side. The implantation of guide cannula was performed according to the following coordinates from the rat brain atlas [31]: 3.4 mm posterior to the Bregma, 3.5 mm lateral to the midline, and 6.5 mm ventral to the skull surface. The guide cannula was fixed to the skull using dental acrylic and jeweler’s screws. A dummy cannula (33 gauge stainless steel wire) was inserted into the guide cannula to reduce the incidence of occlusion. Saline and drug solutions were infused into the thalamus using a microinjection unit (33 gauge cannula) that extended 0.5 mm beyond the tip of the guide cannula. The microinjection unit was attached to a Hamilton microsyringe via polyethylene tubing (PE-10), and an infusion pump was programmed to deliver a volume of 0.5 μL over a period of 1 min into the VPL nucleus of the thalamus. The needle was held for 1 min before retraction, and the injection site was confirmed by visual examination of thalamus sections upon the completion of each experiment. ZD7288 was purchased from Sigma (St Louis, MO, USA) and dissolved in sterile saline.
2.3. Behavioral tests
All behavioral experiments were carried out with the investigators being blinded to treatment conditions. Animals were habituated to the test environment for two consecutive days (30 min per day) before baseline testing.
2.3.1. Mechanical allodynia
A von Frey filament was perpendicularly applied to the plantar surface of each hindpaw. A threshold force of response (in grams) was defined as the first filament that evoked at least two withdrawals out of five applications [32].
2.3.2. Thermal hyperalgesia
Response to radiant heat was assessed using the foot-withdrawal test [33]. Each animal underwent three trials and their results were averaged to yield mean withdrawal latencies. The cutoff was set at 20 s to avoid tissue damage.
2.4. Immunohistochemistry and western blot
Immunostaining and Western blot were carried out as previously reported [21] using the following primary and secondary antibodies: mouse anti-HCN1 antibody (1:800; ab84816, Abcam), mouse anti-HCN2 antibody (1:1000; ab84817, Abcam), Cy3- or FITC conjugated goat anti-mouse antibody (1:300; Jackson ImmunoResearch Laboratories Inc.), and HRP-conjugated donkey anti-mouse antibody (1:10,000; Santa Cruz) Primary antibody omission was used as a control. Sections were examined and captured with an Olympus fluorescence microscope. Western blot was analyzed using ImageJ.
2.5. Statistical analysis of behavioral data
Behavioral data were analyzed using two-way analysis of variance (ANOVA) [21]. Post-hoc Waller-Duncan K-ratio t test was performed to determine the source(s) of differences. GraphPad Prizm5 software was used for the statistical analyses. All data were expressed as mean ± SEM and the statistically significant level was set at P < 0.05.
3. Results
3.1. Intra-thalamus infusion of HCN channel blocker ZD7288 attenuated nociceptive behavior of CCI rats
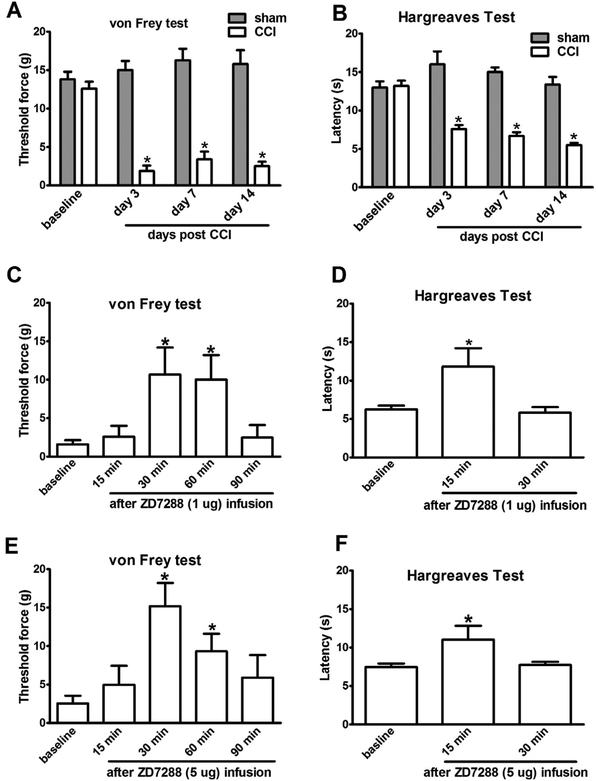
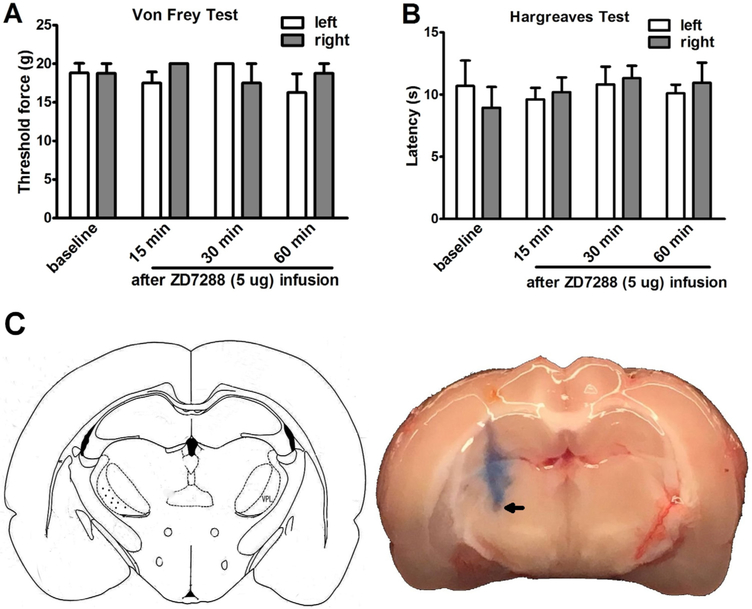
Rats subjected to unilateral CCI exhibited mechanical allodynia and thermal hyperalgesia in the ipsilateral hindpaw as compared to sham controls (Fig. 1A and B). To determine the impact of Ih inhibition in the thalamus on neuropathic pain, nociceptive behavior was assessed in CCI rats after acute infusion of saline or ZD7288 into the VPL nucleus of the thalamus contralateral to the CCI side at fourteen days after injury. Both 1 μg and 5 μg of ZD7288 infusion attenuated mechanical allodynia and the effect of 5 μg of ZD7288 lasted for 90 min (Fig. 1C and E). Measured at 30 min after 5 μg of ZD7288 infusion, the threshold of CCI rats to mechanical stimulus nearly returned to its pre-injury (baseline) level. Thermal hyperalgesia was also reduced after the ZD7288 infusion in CCI rats and the higher ZD7288 dose (5 μg) did not further enhance the effect (Fig. 1D and F). In contrast, the nociceptive behavior in CCI or CFA rats was not affected by saline infusion (Suppl Fig. S1). ZD7288 (5 μg) infusion into the VPL nucleus (Fig. 2C) of the thalamus did not alter either mechanical or thermal sensitivity of sham rats (Fig. 2A and B).
Fig. 1.
Intra-thalamus infusion of ZD7288 reduced nociceptive behavior in CCI rats. (A, B) CCI-induced mechanical allodynia and thermal hyperalgesia lasted for weeks in rats (sham vs CCI. *P < 0.05, n = 6). (C,E) Infusion of ZD7288 into the VPL nucleus contralateral to the CCI side attenuated mechanical allodynia for 90 min. (D,F) Infusion of ZD7288 into the VPL nucleus contralateral to the CCI side transiently reduced thermal hyperalgesia for at least 30 min. (baseline vs after ZD7288 injection * p < 0.05, n = 6–8).
Fig. 2.
Intra-thalamus infusion of ZD7288 did not alter nociceptive threshold in sham rats. (A, B) Infusion of ZD7288 (5 μg) into the right side of the VPL nucleus did not change the hindpaw mechanical and thermal threshold of sham rats. (C,D) Schematic drawing of the injection site in the thalamus and a representative brain section showing ZD7288 or saline injection site.
3.2. Protein expression of HCN channels was increased in the thalamus of CCI rats
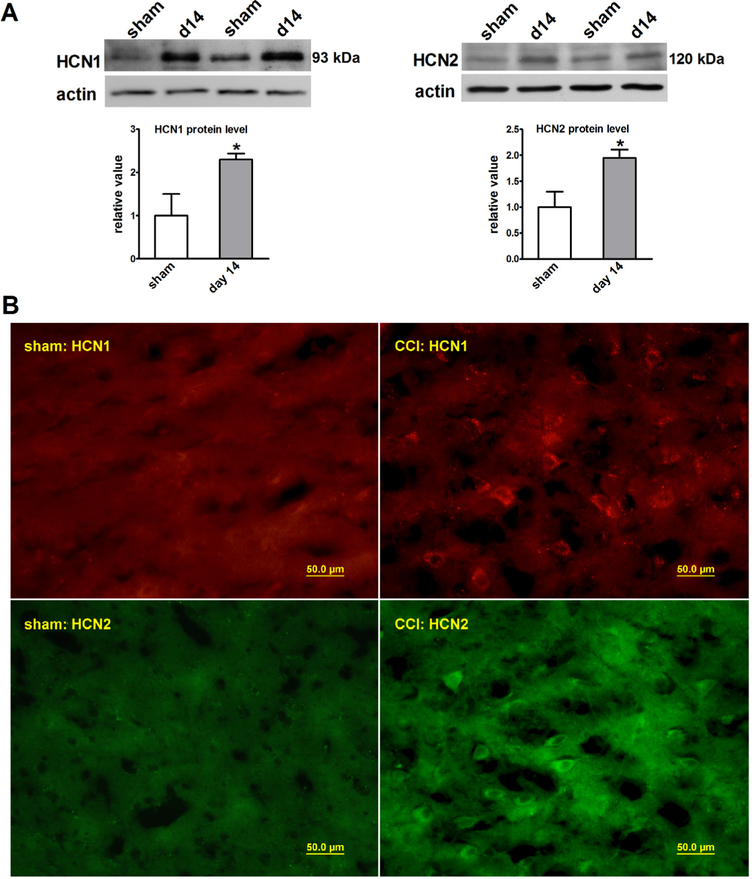
HCN1 and HCN2 proteins are expressed in the thalamus in rodents [5,6,8], which is considered to be relevant to pain signaling in both peripheral and central nerve systems [15,21,22]. Accordingly, we analyzed the HCN1 and HCN2 expression in the thalamus of CCI rats. By fourteen days after CCI, protein expression and immuno-reactivity of both HCN1 and HCN2 were increased in the contralateral thalamus as compared with that of sham rats analyzed by Western blot (Fig. 3A) and immunohistochemistry (Fig. 3B). Since changes in HCN protein expression affects HCN channel activity [34], the increased HCN1 and HCN2 expression in the thalamus may contribute to persistent nociception after CCI.
Fig. 3.
HCN1 and HCN2 protein expression and immunoreactivity were increased in the thalamus of CCI rats. (A) The HCN1 (93 kDa) and HCN2 (120 kDa) protein levels were increased when analyzed at 14 days after CCI (sham vs CCI, n = 5–6, *P < 0.05). (B) HCN1 and HCN2 immunostaining showed the increased number of HCN1 and HCN2 positive cells in the thalamus of CCI rats (40 × magnifications).
3.3. Intra-thalamus infusion of ZD 7288 attenuated nociceptive behavior in rats with inflammatory monoarthritis
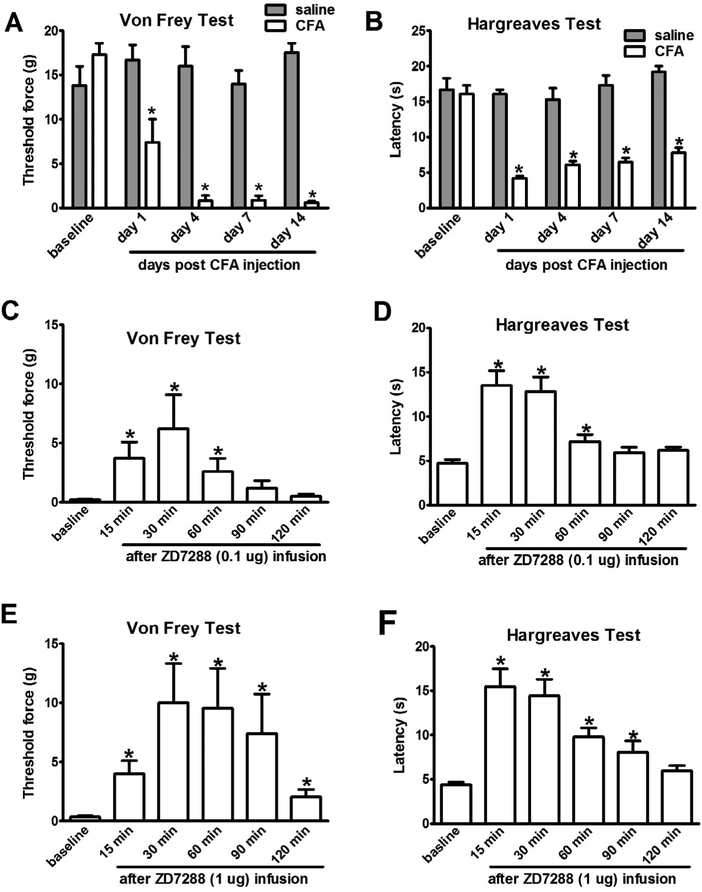
The VPL nucleus of the thalamus is the final relay of the spinothalamic tract. HCN channel activity is increased in both peripheral and central nerve system in chronic pain induced by persistent inflammation [15,35]. To determine whether increased HCN channel activity in the thalamus contributes to the maintenance of inflammatory pain, we tested the effect of ZD7288 in rats with chronic inflammatory monoarthritis. Rats subjected to CFA injection into the ankle joint developed persistent mechanical allodynia and thermal hyperalgesia in the hindpaw compared with sham controls (Fig. 4A and B). Ten days after CFA or saline injection, nociceptive behavior was assessed in the rats after infusion of saline or ZD7288 in the VPL nucleus of the thalamus contralateral to the CFA injection side. Saline infusion did not alter the hindpaw mechanical or thermal withdrawal threshold in CFA rats. However, ZD7288 (1 μg) infusion produced a prolonged anti-nociceptive effect on the hindpaw ipsilateral to CFA injection, which lasted over 120 min (Fig. 4C–F). Mechanical allodynia was significantly attenuated when assessed at 15 min after the ZD7288 infusion and the protective effect peaked at 30 min (Fig. 4C and E), whereas thermal withdrawal threshold returned to the pre-injury level at 15 min after 1 μg of ZD7288 infusion (Fig. 4D and F).
Fig. 4.
Intra-thalamus infusion of ZD7288 reduced nociceptive behavior in rats with CFA-induced inflammatory monoarthritis. (A, B) CFA injection into the ankle joint induced mechanical allodynia and thermal hyperalgesia lasting for weeks in rats (saline vs CFA. *P < 0.05, n = 6). (C,E) Infusion of ZD7288 into the VPL nucleus contralateral to the CFA injection side attenuated mechanical allodynia for up to 120 min. (D,F) At 15 min after infusion of ZD7288 into the VPL nucleus contralateral to the CFA side, thermal hyperalgesia was significantly attenuated (baseline vs after ZD7288 injection * p < 0.05, n = 6–8).
4. Discussion
We report that infusion of a HCN blocker ZD7288 into the VPL nucleus of the thalamus attenuated nociceptive behavior in rats with chronic pain induced by peripheral nerve injury or inflammation. We also observed that HCN1 and HCN2 protein expression was increased in the thalamus of the same rats after nerve injury.
Accumulating evidence suggests that abnormal HCN channel activity contributes to the development and maintenance of chronic pain. In neurons, Ih influences resting membrane potential and input resistance, thereby altering neuronal excitability [36]. Altered Ih induced by nerve injury has been linked to the increased firing of sensory neurons [35,37,38]. Deletion of HCN2 in sensory neurons reduced mechanical hyperalgesia in mice with CFA-induced inflammatory pain [35]. Despite the known presence of HCN channels in the thalamus, the role of the thalamic HCN channel activity in chronic pain condition has not been studied. Our data suggest that HCN channel activity in the thalamus contributes to the behavioral manifestation of chronic pain in two rodent models without changing thermal or mechanical nociceptive threshold in sham rats. These results are in line with a recent finding that HCN channel activity following periphery nerve injury enhanced synaptic transmission between the thalamocortical projection and anterior cingular cortex [15].
The VPL nucleus is a major relay site of the spinothalamic track for pain and temperature sensation [39]. In rats with neuropathic pain, neurons in the VPL nucleus exhibited higher spontaneous firing as well as evoked response [40]. Moreover, increased HCN channel activity may contribute to ectopic firing under chronic pain [20,37]. Our data show that inhibition of HCN channel activity in the VPL nucleus alleviated chronic pain. It has been shown that intrathecal or intra-periaquaductal gray application of HCN channel inhibitor reduced neuropathic pain behaviors [23,24]. These data lend strong support for a critical role of the brain HCN channel activity in chronic pain.
Among four HCN channel subunits (HCN1–4), HCN1 and HCN2 subunits are expressed in neurons of both peripheral and central nerve systems and are highly relevant to pain processing and modulation [41–45]. A recent study using inducible HCN2 gene knockout mice, which showed a dramatic reduction in HCN2 protein expression in the brain including thalamus and cortex, were protected from mechanical allodynia and thermal hyperalgesia after CFA-induced inflammation [35]. In addition, HCN1 gene knockout mice were partially protected from developing cold allodynia [3]. On the other hand, increased HCN1 and HCN2 protein expression has been observed in the PAG and amygdala in rodents with chronic pain [21,24]. In this study, we also found that HCN1 and HCN2 immunoreactivity was increased in the thalamus of rats with chronic neuropathic pain.
While the exact mechanisms by which HCN channel activity in the thalamus regulates chronic pain remains to be examined, the current data suggest that inhibition of HCN subtype channel activity, such as HCN1 and HCN2, may be a method to alleviate chronic pain. Due to bradycardia resulting from blocking the sinoa-trial HCN4 with all known Ih blockers, broad HCN channel blockers may not be useful when applied systemically. The present results suggest that searching for HCN subtype blockers relevant for pain would be beneficial to patients with chronic pain.
Supplementary Material
(A, B) Infusion of saline (0.5 μL) into the contralateral side of the VPL nucleus did not change the mechanical and thermal threshold of the ipsilateral hindpaw in sham or CCI rats at 7 days post surgery. (sham vs CCI. *P<0.05, n = 5). (C, D) Infusion of saline (0.5 μL) into the contralateral side of the VPL nucleus did not change the mechanical and thermal threshold of the ipsilateral hindpaw in sham or CFA rats at 7 days post injury. (sham vs CFA. *P<0.05, n = 5).
HIGHLIGHTS.
Inhibition of HCN channel activity in the VPL nucleus of the thalamus attenuated chronic pain.
HCN1 and HCN2 protein expression levels were increased in the thalamus under chronic pain condition.
Increased HCN channel activity in the thalamus contributed to the maintenance of chronic pain.
Acknowledgements
This work was supported by NIH grants R01 DE022901 and R01 DE018214 (J.M.) and Hangzhou Science and Technology Plan No. 20130633B02 (W.D.) and Zhejiang province’s key project in Medicine & Health #2013ZDA010 (S.Z.).
Footnotes
Conflict of interest
The authors declare no competing financial interests.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.neulet.2016.08.021.
References
- [1].Kuner R, Central mechanisms of pathological pain, Nat. Med 16 (2010) 1258–1266. [DOI] [PubMed] [Google Scholar]
- [2].Gao LL, McMullan S, Djouhri L, Acosta C, Harper AA, Lawson SN , Expression and properties of hyperpolarization-activated current in rat dorsal root ganglion neurons with known sensory function, J. Physiol 590 (2012) 4691–4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Momin A, Cadiou H, Mason A, McNaughton PA, Role of the hyperpolarization-activated current Ih in somatosensory neurons, J. Physiol 586 (2008) 5911–5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ludwig A, Zong X, Jeglitsch M, Hofmann F, Biel M, A family of hyperpolarization-activated mammalian cation channels, Nature 393 (1998) 587–591. [DOI] [PubMed] [Google Scholar]
- [5].Santoro B, Liu DT, Yao H, Bartsch D, Kandel ER, Siegelbaum SA, Tibbs GR, Identification of a gene encoding a hyperpolarization-activated pacemaker channel of brain, Cell 93 (1998) 717–729. [DOI] [PubMed] [Google Scholar]
- [6].Notomi T, Shigemoto R, Immunohistochemical localization of Ih channel subunits HCN1–4, in the rat brain, J. Comp. Neurol 471 (2004) 241–276. [DOI] [PubMed] [Google Scholar]
- [7].Moosmang S, Stieber J, Zong X, Biel M, Hofmann F, Ludwig A, Cellular expression and functional characterization of four hyperpolarization-activated pacemaker channels in cardiac and neuronal tissues, Eur. J. Biochem 268 (2001) 1646–1652. [DOI] [PubMed] [Google Scholar]
- [8].Moosmang S, Biel M, Hofmann F, Ludwig A, Differential distribution of four hyperpolarization-activated cation channels in mouse brain, Biol. Chem 380 (1999) 975–980. [DOI] [PubMed] [Google Scholar]
- [9].Kanyshkova T, Pawlowski M, Meuth P, Dube C, Bender RA, Brewster AL, Baumann A, Baram TZ, Pape HC, Budde T, Postnatal expression pattern of HCN channel isoforms in thalamic neurons: relationship to maturation of thalamocortical oscillations, J. Neurosci 29 (2009) 8847–8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Nolan MF, Malleret G, Lee KH, Gibbs E, Dudman JT, Santoro B, Yin D, Thompson RF, Siegelbaum SA, Kandel ER, Morozov A, The hyperpolarization-activated HCN1 channel is important for motor learning and neuronal integration by cerebellar Purkinje cells, Cell 115 (2003) 551–564. [DOI] [PubMed] [Google Scholar]
- [11].Wang M, Gamo NJ, Yang Y, Jin LE, Wang XJ, Laubach M, Mazer JA, Lee D, Arnsten AF, Neuronal basis of age-related working memory decline, Nature 476 (2011) 210–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ying SW, Abbas SY, Harrison NL, Goldstein PA, Propofol block of I(h) contributes to the suppression of neuronal excitability and rhythmic burst firing in thalamocortical neurons, Eur. J. Neurosci 23 (2006) 465–480. [DOI] [PubMed] [Google Scholar]
- [13].Chen X, Shu S, Bayliss DA, Suppression of ih contributes to propofol-induced inhibition of mouse cortical pyramidal neurons, J. Neurophysiol 94 (2005) 3872–3883. [DOI] [PubMed] [Google Scholar]
- [14].Du L, Wang SJ, Cui J, He WJ, Ruan HZ, The role of HCN channels within the periaqueductal gray in neuropathic pain, Brain Res 1500 (2013) 36–44. [DOI] [PubMed] [Google Scholar]
- [15].Koga K, Descalzi G, Chen T, Ko HG, Lu J, Li S, Son J, Kim T, Kwak C, Huganir RL, Zhao MG, Kaang BK, Collingridge GL, Zhuo M, Coexistence of two forms of LTP in ACC provides a synaptic mechanism for the interactions between anxiety and chronic pain, Neuron 85 (2015) 377–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Shin M, Simkin D, Suyeoka GM, Chetkovich DM, Evaluation of HCN2 abnormalities as a cause of juvenile audiogenic seizures in Black Swiss mice, Brain Res 1083 (2006) 14–20. [DOI] [PubMed] [Google Scholar]
- [17].Okamoto T, Harnett MT, Morikawa H, Hyperpolarization-activated cation current (Ih) is an ethanol target in midbrain dopamine neurons of mice, J. Neurophysiol 95 (2006) 619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kim CS, Chang PY, Johnston D, Enhancement of dorsal hippocampal activity by knockdown of HCN1 channels leads to anxiolytic- and antidepressant-like behaviors, Neuron 75 (2012) 503–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tibbs GR, Rowley TJ, Sanford RL, Herold KF, Proekt A, Hemmings HC Jr., O.S. Andersen, P.A. Goldstein, P.D. Flood, HCN1 channels as targets for anesthetic and nonanesthetic propofol analogs in the amelioration of mechanical and thermal hyperalgesia in a mouse model of neuropathic pain, J. Pharmacol. Exp. Ther 345 (2013) 363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Young GT, Emery EC, Mooney ER, Tsantoulas C, McNaughton PA, Inflammatory and neuropathic pain are rapidly suppressed by peripheral block of hyperpolarisation-activated cyclic nucleotide-gated ion channels, Pain 155 (2014) 1708–1719. [DOI] [PubMed] [Google Scholar]
- [21].Zhang S, You Z, Wang S, Yang J, Yang L, Sun Y, Mi W, Yang L, McCabe MF, Shen S, Chen L, Mao J, Neuropeptide S modulates the amygdaloidal HCN activities (I) in rats: implication in chronic pain, Neuropharmacology (2016). [DOI] [PubMed] [Google Scholar]
- [22].Acosta C, McMullan S, Djouhri L, Gao L, Watkins R, Berry C, Dempsey K, Lawson SN, HCN1 and HCN2 in Rat DRG neurons: levels in nociceptors and non-nociceptors, NT3-dependence and influence of CFA-induced skin inflammation on HCN2 and NT3 expression, PLoS One 7 (2012) e50442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Takasu K, Ono H, Tanabe M, Spinal hyperpolarization-activated cyclic nucleotide-gated cation channels at primary afferent terminals contribute to chronic pain, Pain 151 (2010) 87–96. [DOI] [PubMed] [Google Scholar]
- [24].Du L, Wang SJ, Cui J, He WJ, Ruan HZ, Inhibition of HCN channels within the periaqueductal gray attenuates neuropathic pain in rats, Behav. Neurosci 127 (2013) 325–329. [DOI] [PubMed] [Google Scholar]
- [25].Cordeiro Matos S, Zhang Z, Seguela P, Peripheral neuropathy induces HCN channel dysfunction in pyramidal neurons of the medial prefrontal cortex, J. Neurosci 35 (2015) 13244–13256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Peyron R, Functional brain imaging: what has it brought to our understanding of neuropathic pain? A special focus on allodynic pain mechanisms, Pain 157 (Suppl 1) (2016) S67–71. [DOI] [PubMed] [Google Scholar]
- [27].Jones EG, Thalamocortical dysrhythmia and chronic pain, Pain 150 (2010) 4–5. [DOI] [PubMed] [Google Scholar]
- [28].Lenz FA, Kwan HC, Dostrovsky JO, Tasker RR, Characteristics of the bursting pattern of action potentials that occurs in the thalamus of patients with central pain, Brain Res 496 (1989) 357–360. [DOI] [PubMed] [Google Scholar]
- [29].Bennett GJ, Xie YK, A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man, Pain 33 (1988) 87–107. [DOI] [PubMed] [Google Scholar]
- [30].Butler SH, Godefroy F, Besson JM, Weil-Fugazza J, A limited arthritic model for chronic pain studies in the rat, Pain 48 (1992) 73–81. [DOI] [PubMed] [Google Scholar]
- [31].Paxinos G, Watson C, The Rat Brain in Stereotaxic Coordinates, Seventh Edition (2013). ISBN-13: 978–0123919496. [Google Scholar]
- [32].Tal M, Bennett GJ, Extra-territorial pain in rats with a peripheral mononeuropathy: mechano-hyperalgesia and mechano-allodynia in the territory of an uninjured nerve, Pain 57 (1994) 375–382. [DOI] [PubMed] [Google Scholar]
- [33].Hargreaves K, Dubner R, Brown F, Flores C, Joris J, A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia, Pain 32 (1988) 77–88. [DOI] [PubMed] [Google Scholar]
- [34].Li B, Luo C, Tang W, Chen Z, Li Q, Hu B, Lin J, Zhu G, Zhang JH, Feng H, Role of HCN channels in neuronal hyperexcitability after subarachnoid hemorrhage in rats, J. Neurosci 32 (2012) 3164–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Schnorr S, Eberhardt M, Kistner K, Rajab H, Kasser J, Hess A, Reeh P, Ludwig A, Herrmann S, HCN2 channels account for mechanical (but not heat) hyperalgesia during long-standing inflammation, Pain 155 (2014) 1079–1090. [DOI] [PubMed] [Google Scholar]
- [36].Chen K, Aradi I, Thon N, Eghbal-Ahmadi M, Baram TZ, Soltesz I, Persistently modified h-channels after complex febrile seizures convert the seizure-induced enhancement of inhibition to hyperexcitability, Nat. Med 7 (2001) 331–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Chaplan SR, Guo HQ, Lee DH, Luo L, Liu C, Kuei C, Velumian AA, Butler MP, Brown SM, Dubin AE, Neuronal hyperpolarization-activated pacemaker channels drive neuropathic pain, J. Neurosci 23 (2003) 1169–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wan Y, Involvement of hyperpolarization-activated, cyclic nucleotide-gated cation channels in dorsal root ganglion in neuropathic pain, Sheng Li Xue Bao 60 (2008) 579–580. [PubMed] [Google Scholar]
- [39].Narasimhan TR, Craig A, Arellano L, Harper N, Howie L, Menache M, Birnbaum L, Safe S, Relative sensitivities of 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced Cyp1a-1 and Cyp1a-2 gene expression and immunotoxicity in female B6C3F1 mice, Fundam. Appl. Toxicol 23 (1994) 598–607. [DOI] [PubMed] [Google Scholar]
- [40].Hains BC, Saab CY, Waxman SG, Alterations in burst firing of thalamic VPL neurons and reversal by Na(v)1.3 antisense after spinal cord injury, J. Neurophysiol 95 (2006) 3343–3352. [DOI] [PubMed] [Google Scholar]
- [41].Monteggia LM, Eisch AJ, Tang MD, Kaczmarek LK, Nestler EJ, Cloning and localization of the hyperpolarization-activated cyclic nucleotide-gated channel family in rat brain, Brain Res. Mol. Brain Res 81 (2000) 129–139. [DOI] [PubMed] [Google Scholar]
- [42].Santoro B, Tibbs GR, The HCN gene family: molecular basis of the hyperpolarization-activated pacemaker channels, Ann. N. Y. Acad. Sci 868 (1999) 741–764. [DOI] [PubMed] [Google Scholar]
- [43].Shi W, Wymore R, Yu H, Wu J, Wymore RT, Pan Z, Robinson RB, Dixon JE, McKinnon D, Cohen IS, Distribution and prevalence of hyperpolarization-activated cation channel (HCN) mRNA expression in cardiac tissues, Circ. Res 85 (1999) e1–6. [DOI] [PubMed] [Google Scholar]
- [44].Santoro B, Chen S, Luthi A, Pavlidis P, Shumyatsky GP, Tibbs GR, Siegelbaum SA, Molecular and functional heterogeneity of hyperpolarization-activated pacemaker channels in the mouse CNS, J. Neurosci 20 (2000) 5264–5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ludwig A, Zong X, Stieber J, Hullin R, Hofmann F, Biel M, Two pacemaker channels from human heart with profoundly different activation kinetics, EMBO J 18 (1999) 2323–2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A, B) Infusion of saline (0.5 μL) into the contralateral side of the VPL nucleus did not change the mechanical and thermal threshold of the ipsilateral hindpaw in sham or CCI rats at 7 days post surgery. (sham vs CCI. *P<0.05, n = 5). (C, D) Infusion of saline (0.5 μL) into the contralateral side of the VPL nucleus did not change the mechanical and thermal threshold of the ipsilateral hindpaw in sham or CFA rats at 7 days post injury. (sham vs CFA. *P<0.05, n = 5).