ALIX mediates endosomal trafficking and turnover of abscisic acid receptors through the ESCRT/MVB pathway, modulating abscisic acid–mediated inhibition of plant growth and stomatal aperture.
Abstract
The plant endosomal trafficking pathway controls the abundance of membrane-associated soluble proteins, as shown for abscisic acid (ABA) receptors of the PYRABACTIN RESISTANCE1/PYR1-LIKE/REGULATORY COMPONENTS OF ABA RECEPTORS (PYR/PYL/RCAR) family. ABA receptor targeting for vacuolar degradation occurs through the late endosome route and depends on FYVE DOMAIN PROTEIN REQUIRED FOR ENDOSOMAL SORTING1 (FYVE1) and VACUOLAR PROTEIN SORTING23A (VPS23A), components of the ENDOSOMAL SORTING COMPLEX REQUIRED FOR TRANSPORT-I (ESCRT-I) complexes. FYVE1 and VPS23A interact with ALG-2 INTERACTING PROTEIN-X (ALIX), an ESCRT-III–associated protein, although the functional relevance of such interactions and their consequences in cargo sorting are unknown. In this study we show that Arabidopsis (Arabidopsis thaliana) ALIX directly binds to ABA receptors in late endosomes, promoting their degradation. Impaired ALIX function leads to altered endosomal localization and increased accumulation of ABA receptors. In line with this activity, partial loss-of-function alix-1 mutants display ABA hypersensitivity during growth and stomatal closure, unveiling a role for the ESCRT machinery in the control of water loss through stomata. ABA-hypersensitive responses are suppressed in alix-1 plants impaired in PYR/PYL/RCAR activity, in accordance with ALIX affecting ABA responses primarily by controlling ABA receptor stability. ALIX-1 mutant protein displays reduced interaction with VPS23A and ABA receptors, providing a molecular basis for ABA hypersensitivity in alix-1 mutants. Our findings unveil a negative feedback mechanism triggered by ABA that acts via ALIX to control the accumulation of specific PYR/PYL/RCAR receptors.
INTRODUCTION
The endocytic pathway plays an essential role in eukaryotes, allowing trafficking of protein cargoes from the plasma membrane and the Golgi apparatus to the lysosome/vacuole for subsequent degradation. This process is fundamental to control the abundance, and therefore the activity, of membrane proteins (Winter and Hauser, 2006). Once cargo proteins are sorted into endosomes, they can be transported back to the plasma membrane via recycling endosomes or be targeted to the lysosome/vacuole via multivesicular bodies (MVBs; also termed late endosomes or prevacuolar compartments). The latter requires cargo internalization into intraluminal vesicles (ILVs), which will be released into the vacuolar/lysosomal lumen upon fusion of the MVB with the tonoplast. Sorting of cargo proteins into ILVs is triggered, in most cases, by the attachment of ubiquitin (or polyubiquitin chains with specific configurations) to their cytosolic domains (Gruenberg and Stenmark, 2004). Selective recognition and packaging of ubiquitinated cargoes into ILVs is mediated by ENDOSOMAL SORTING COMPLEXES REQUIRED FOR TRANSPORT (ERCRT) protein complexes (Conibear, 2002; Winter and Hauser, 2006; Nickerson et al., 2007; Henne et al., 2011). Five ESCRT complexes have been described in eukaryotes—ESCRT-0, -I, -II, and -III complexes and the ESCRT-III–associated SKD1/Vps4 complex—although plants seem to lack canonical ESCRT-0 subunits (Winter and Hauser, 2006; Richardson et al., 2011; Paez Valencia et al., 2016). On the contrary, plant-specific ESCRT components have been characterized (Gao et al., 2014, 2015; Reyes et al., 2014; Kolb et al., 2015; Belda-Palazon et al., 2016).
During cargo packaging into ILVs, ESCRT-0, -I and –II complexes contribute to recognition and clustering of ubiquitinated cargo proteins on the MVB surface through their ubiquitin binding activity (Shields and Piper, 2011; MacGurn et al., 2012; Henne et al., 2013). In addition, ESCRT-I and -II complexes associate to trigger membrane deformation and initiate ILV formation (Katzmann et al., 2001; Babst et al., 2002a). This process is further continued by recruitment and polymerization of ESCRT-III subunits (including VPS2 and SNF7), which enable cargo concentration and engulfment inside ILVs, avoiding their diffusion out of ILVs (Babst et al., 2002b; Wemmer et al., 2011). Before MVB luminal sequestration, deubiquitination of cargo proteins by specific deubiquitinases (Doa4/UBPY in yeast [Saccharomyces cerevisiae] and humans and AMSH in animals and plants) occurs (Swaminathan et al., 1999; Reggiori and Pelham, 2001; Kyuuma et al., 2007; McNatt et al., 2007; Isono et al., 2010; Katsiarimpa et al., 2011; Wright et al., 2011). For this activity, Bro1-domain–containing proteins (Bro1 in yeast and ALIX in plants and animals) associate with the ESCRT-III subunit Vps32/SNF7 and recruit the deubiquitinases to the MVBs, which remove ubiquitin from ubiquitinated cargoes (Missotten et al., 1999; Luhtala and Odorizzi, 2004; Cardona-López et al., 2015; Kalinowska et al., 2015; Shen et al., 2016). The last step involves disassembly and dissociation of the ESCRT machinery from the MVB surface by SKD1/Vps4 (Babst, 2005).
Until recently, identified protein targets of the ESCRT machinery in plants mainly corresponded to integral membrane proteins, such as auxin carriers, mineral nutrient transporters, or antigen and hormone receptors (reviewed in Paez Valencia et al., 2016; Dubeaux and Vert, 2017; Isono and Kalinowska, 2017). However, MVB trafficking and vacuolar degradation of soluble proteins have also been reported for members of the PYR/PYL/RCAR (for PYRABACTIN RESISTANCE/PYRABACTIN RESISTANCE–LIKE/REGULATORY COMPONENTS OF THE abscisic acid (ABA) RECEPTOR) family of ABA receptors (Belda-Palazon et al., 2016; Yu et al., 2016). The latter perceive the primary ABA signal and repress type 2C protein phosphatases(PP2Cs), which act as key negative regulators of the ABA pathway (Saez et al., 2006; Rubio et al., 2009; Cutler et al., 2010). PP2C inhibition allows activation of SNF1-related protein kinase 2 (SnRK2s) that, in turn, phosphorylates specific protein targets, including transcription factors, such as ABF family members, and the membrane ion channel protein SLOW ANION CHANNEL-ASSOCIATED1 (SLAC1; Fujii et al., 2009; Fujita et al., 2009; Geiger et al., 2009; Lee et al., 2009). In this way, ABA perception by PYR/PYL/RCAR receptors (hereafter referred to as PYLs for simplicity) triggers different developmental and adaptive responses, including arrest of seed germination and plant growth, and the closure of stomata to limit water loss (Nakashima and Yamaguchi-Shinozaki, 2013).
Protein levels of PYL receptors seem to be tightly controlled by the ubiquitination system at different subcellular compartments by means of specific E3 ubiquitin ligases, as shown by studies performed in Arabidopsis (Arabidopsis thaliana). Thus, in the nucleus, CULLIN4-RING E3 ligases containing either DET1, DDB1-ASSOCIATED1 or DWD protein RNA EXPORT FACTOR1 as the substrate adapter, and RCAR3-INTERACTING F-BOX PROTEIN1 (RIFP1) target PYL receptors for degradation at the 26S proteasome as a way to desensitize plants against ABA (Irigoyen el al., 2014; Li et al., 2016, 2018). PYL receptors can also associate with the plasma membrane by their physical interaction with members of the C2-domain ABA-related (CAR) protein family (Rodriguez et al., 2014). CAR proteins transiently bind to phospholipids in the presence of calcium, increasing PYL abundance at the plasma membrane and favoring ABA signaling. Thus, CAR proteins act as positive regulators of several ABA responses, including ABA-mediated inhibition of seedling establishment and root growth.
Membrane-bound PYL levels are regulated by the RING-type E3 ligase RSL1, which is anchored to the plasma membrane via its C-terminal transmembrane domain. Interaction of RSL1 with PYL receptors (i.e., PYR1 and PYL4) promotes their ubiquitination and subsequent sorting, together with RSL1, through the endosomal pathway for vacuolar degradation (Bueso et al., 2014; Belda-Palazon et al., 2016). Involvement of the ESCRT machinery in this process was shown by physical interaction of PYL receptors with ESCRT-I subunits FYVE1 and VPS23A (also termed FREE1 and ELC, respectively) in vesicle-like structures (Belda-Palazon et al., 2016; Yu et al., 2016). FYVE1 is the only representative of class IV of FYVE-domain–containing proteins in Arabidopsis, whereas VPS23A is a homolog of mammalian TSG101 and yeast Vps23a proteins, which contain a ubiquitin conjugating enzyme variant domain likely used for binding and sorting of ubiquitinated protein cargoes (Spitzer et al., 2006; Gao et al., 2014, 2015; Kolb et al., 2015; Yu et al., 2016). Within ESCRT-I complexes, FYVE1 and VPS23A proteins are known to interact with each other and are required for recognition and internalization of cargo proteins into ILVs (Shen et al., 2016). According to their role in cargo trafficking through MVBs and their interaction with PYL receptors, partial loss of function of FYVE1 and VPS23A led to altered subcellular localization and accumulation of PYL proteins. Consequently, weak Arabidopsis fyve1 and vps23a mutants displayed increased sensitivity to ABA at the seedling stage (Belda-Palazon et al., 2016; Yu et al., 2016). FYVE1 and VPS23A also participate in ILV formation, trafficking to the vacuole, and vacuolar biogenesis (Gao et al., 2014, 2015; Kolb et al., 2015). All these functions also require activity of the ESCRT-III–associated protein ALIX (also termed AtBRO1), the Arabidopsis homolog of mammalian apoptosis-linked gene-2 interacting protein X (ALIX) and yeast Bro1p, because loss-of-function mutants for Arabidopsis ALIX displayed defects in protein cargo trafficking and vacuolar biogenesis (Cardona-López et al., 2015; Kalinowska et al., 2015; Shen et al., 2016). Interestingly, ALIX physically interacts with FYVE1 and VPS23A, suggesting that ALIX might act as a bridge between ESCRT-I and -III complexes and thereby coordinate cargo protein sorting and vacuolar targeting. Such a functional relationship has not been fully demonstrated (Shen et al., 2016).
In this study we show that ALIX physically interacts with and mediates trafficking of PYL ABA receptors for degradation at the vacuole and therefore acts as a negative regulator of ABA responses, including control of stomatal closure. Moreover, a point mutation in the Bro1 domain of ALIX disrupted its interaction with both PYL and VPS23A proteins, manifesting the relevance of ALIX for maintaining the integrity of the cargo–receptor complex and providing a molecular basis for ABA hypersensitivity in alix-1 mutants. Our data support a functional role for ALIX in mediating ESCRT-I and -III complex activity during cargo protein trafficking and highlight the relevance of the endosomal pathway in modulating ABA signaling in plants.
RESULTS
ALIX Physically Interacts with PYL ABA Receptors in Multivesicular Bodies
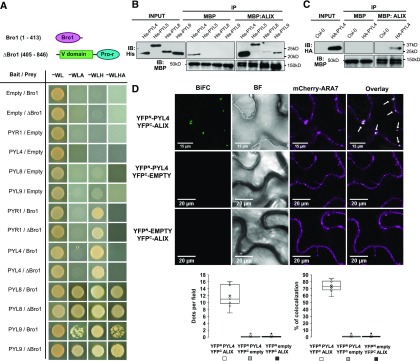
To identify functions for ALIX in plants as well as the regulatory mechanisms that control its activity, we sought proteins that physically interact with ALIX. For this, we performed yeast two hybrid (Y2H) screens, using as bait full-length ALIX and two truncated versions corresponding to its N-terminal Bro1 domain (amino acids 1 to 413) and the C-terminal portion (ΔBro1; amino acids 405 to 846), containing two coiled-coil domains and a proline-rich (Pro-rich) region. After confirming that the three baits were unable to activate reporter gene expression alone, we used them to screen an Arabidopsis cDNA library (Puga et al., 2014). Transformants were grown in media lacking different amino acids (alone or supplemented with 0.5 mM, 3-amino-1,2,4-triazole, 3-AT). Yeast colonies able to grow in the strictest conditions and displaying β-galactosidase activity were selected for DNA sequencing of prey plasmids. A total of 62 different proteins were identified as potential ALIX interactors in our yeast screens (Supplemental Data Set 1). While using the Bro1 domain as bait, we identified one clone corresponding to a truncated version of the ABA receptor PYL9 (amino acids 75 to 188). We aimed to determine whether ALIX interacts with other Arabidopsis PYL family members and whether these interactions are exclusively mediated by the Bro1 domain (Figure 1). Our results showed that both Bro1 and ΔBro1 are able to bind to full-length versions of different PYLs (both dimeric; i.e., PYR1, and monomeric types; PYL4, 8, and 9; Figure 1A). Interaction of ALIX with PYR/PYL receptors was further substantiated by in vitro pull-downs in which recombinant His-PYLs (PYL4, PYL5, PYL8, and PYL9) a maltose binding protein (MBP)-ALIX fusions purified from bacteria were coprecipitated on incubation with an amylose resin, although at different rates for each receptor, showing that His-PYL4 interacts strongly with ALIX (Figure 1B). Semi–in vivo experiments, in which protein extracts from Arabidopsis lines overexpressing HA (hemagglutinin)-tagged PYL4 (oeHA-PYL4; Pizzio et al., 2013) were incubated with amylose resin-bound MBP-ALIX, also showed copurification of both protein fusions, providing additional support to the ALIX-PYL interaction (Figure 1C).
Figure 1.
ALIX Interaction with PYL ABA Receptors.
(A) The structure of ALIX showing the Bro1 domain (Bro1; amino acids 1 to 413) and the coiled coils plus the proline-rich region (∆Bro1; amino acids 405 to 846) are shown in the top panel. Y2H assays showing interaction of PYL ABA receptors with either Bro1 or ∆Bro1 domains. Transformed yeast cells were grown in synthetic drop-out (SD) medium lacking tryptophan and leucine (-WL) as a transformation control and in SD-WL media lacking adenine (–WLA), histidine (–WLH), or both (–WLHA) for interaction assays. Cotransformation with empty plasmids was used as negative control.
(B) Bacteria-purified PYL proteins fused to a 6x His tag (His-PYL) were pulled down on incubation with recombinant MBP-ALIX or MBP bound to amylose resin. Anti-His was used in immunoblots (IBs) to detect His-PYL fusions, and anti-MBP for MBP-ALIX and MBP.
(C) HA-PYL4 was pulled down on incubation of oeHA-PYL4 plant extracts with recombinant MBP-ALIX or MBP bound to amylose resin. Protein extracts from wild-type (Col-0) plants were used as negative control. Anti-HA was used to detect HA-PYL4 and anti-MBP for MBP-ALIX and MBP.
(D) In vivo interaction of ALIX with PYL4 assessed by BiFC. Confocal images were taken of N. benthamiana leaf epidermal cells expressing different BiFC construct combinations together with mCherry-ARA7, a MVB marker. Reconstitution of YFP fluorescence shows that ALIX and PYL4 constructs directly interact in mCherry-ARA7-labeled MVBs (i.e., punctae indicated by arrows in Overlay image). Bright-field (BF) images of the leaf areas are shown. Bars = 15 and 20 µm. The numbers of intracellular punctae per leaf section agroinfiltrated (y axis; dots per 50 × 50 µm field) as well as the percentage of colocalization of YFP-fluorescent and mCherry-ARA7 labeled vesicles (y axis; percentage of colocalization) with each construct combination (x axes) are quantified below the micrographs. Results were obtained from 10 fields from five biological replicates (two fields/replicate).
To confirm that ALIX interacts with PYL ABA receptors in planta, we performed bimolecular fluorescence complementation (BiFC) assays. For this, Nicotiana benthamiana leaves were coinfiltrated with Agrobacterium tumefaciens cells to express ALIX and full-length ABA receptor fusions to the N- or C-terminal portions of the yellow fluorescent protein (YFP). The infiltrated leaves were analyzed by confocal fluorescence microscopy 3 d after agroinfiltration. Physical interaction between ALIX and the five ABA receptors tested was revealed by reconstitution of YFP fluorescence in cells coinfiltrated with constructs corresponding to YFPC-ALIX and YFPN-PYL (PYR1, PYL4, 5, 8, and 9), whereas expression of empty vectors combined with ALIX or receptor constructs did not restore the YFP fluorescence (Figure 1D; Supplemental Movies 1 to 5). Interaction between ALIX and ABA receptors occurred in MVB, as shown by colocalization of the fluorescent signal resulting from the ALIX-PYL4 interaction and the signal from the mCherry-ARA7 MVB marker (Figure 1D; Supplemental Movie 1; Geldner et al., 2009).
alix-1 Mutant Seedlings Display Increased Sensitivity to ABA
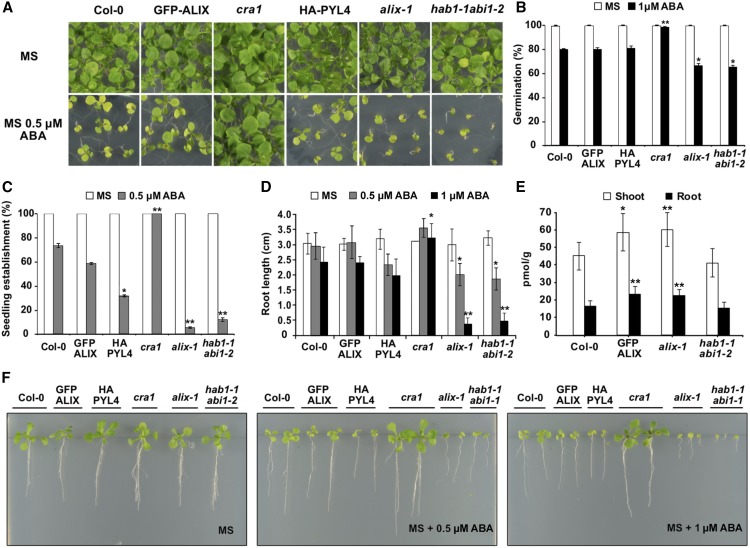
It has been previously shown that protein levels of several PYL receptors are regulated by proteolysis at both the proteasome and the vacuolar compartment (Bueso et al., 2014; Irigoyen et al., 2014). The latter, involving the endosomal trafficking pathway, is mediated by members of the ESCRT machinery ( e.g., FYVE1 and VPS23A) and seems to modulate the abundance of ABA receptors associated with membranes, as is the case for PYL4, as a means to limit ABA responses. Accordingly, weak mutant alleles fyve1 and vps23a showed hypersensitivity to ABA (Belda-Palazon et al., 2016; Yu et al., 2016). Our data indicate that ALIX interacts with PYL ABA receptors, suggesting a negative regulatory role for ALIX in ABA signaling. To test this hypothesis, we characterized several ABA responses in alix-1 mutant plants, including ABA-mediated inhibition of seed germination, seedling establishment, and root growth (Figure 2). As a control, in these experiments we used wild-type (Col-0) plants, a complemented line GFP-ALIX/alix-1 (Cardona-López et al., 2015), cra1 (as ABA-insensitive control; Fernández-Arbaizar et al., 2012), and hab1-1 abi1-2 mutants (as ABA-hypersensitive control; Saez et al., 2006). Compared with wild-type and GFP-ALIX/alix-1 controls, alix-1 plants showed increased responses to ABA in all cases, particularly in the case of ABA-mediated inhibition of seedling establishment and root growth (Figures 2A, 2C, 2D, and 2F). In agreement with previous reports, Arabidopsis transgenic lines overexpressing PYL4 (oeHA-PYL4) showed increased sensitivity to ABA (Pizzio et al., 2013), although not to the same extent as the alix-1 line.
Figure 2.
alix-1 Mutants Show Enhanced Sensitivity to ABA.
(A) Representative photographs of 12-d-old wild-type (Col-0), complemented line GFP-ALIX/alix-1 (labeled as GFP-ALIX), oeHA:PYL4 (HA-PYL4), alix-1, ABA insensitive cra1 and ABA hypersensitive hab1-1 abi1-2 mutants grown in the presence of 0.5 µM ABA.
(B) Percentage of seeds germinated (radicle emergence) in the presence of 1 µM ABA at 3 d after sowing. Genotypes analyzed were as in (A).
(C) ABA-mediated inhibition of seedling establishment (emergence of the first true leaves) of the same genotypes analyzed in (A) that were grown in medium lacking or supplemented with 0.5 µM ABA.
(D) Root length measurements of seedlings germinated in the presence or absence of 0.5 µM ABA after 12 d, or in the presence of 1 µM ABA after 15 d. Genotypes were as in (A).
(E) ABA concentration (pmol/g) in shoot and roots of 14-d-old seedlings for wild-type (Col-0), GFP-ALIX and alix-1 mutant. hab1-1 abi1-2 mutants were used as the ABA hypersensitive control.
(F) Photographs of representative plants analyzed in (D).
*p < 0.05; **p < 0.01 (Student’s t test; Supplemental Data Set 2) with respect to the wild type in the same experimental conditions. In all cases, data are means of three biological replicates (n = 20 in each replicate). Error bars represent sd. MS medium was used as a control in all assays.
To test whether defective ALIX function provokes increased accumulation of ABA that may underlie the ABA hypersensitivity phenotype of alix-1 mutants, the concentration of this hormone was analyzed in roots and shoots of wild-type, GFP-ALIX/alix-1, hab1-1 abi1-2, and alix-1 plants. We detected significantly higher levels of ABA in alix-1 samples, both roots and shoots, compared with wild-type controls (Figure 2E). However, because increased ABA concentration was also observed in the GFP-ALIX/alix-1 line, which behaves as a wild-type control in response to ABA, the higher ABA concentration is not sufficient to explain the ABA hypersensitivity shown by alix-1 mutants. Thus, we concluded that altered ABA perception and/or signaling may also underlie the ABA hypersensitivity of the alix-1 mutants.
Reduced Function of ALIX Decreases Water Loss and Stomatal Aperture
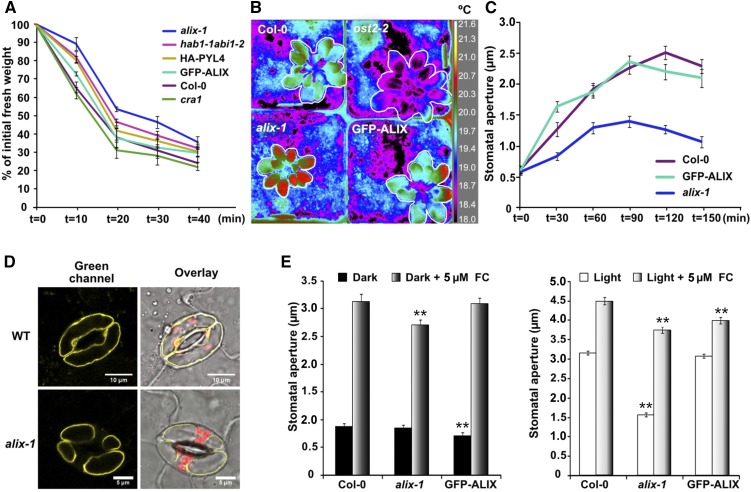
ABA is known to control water loss in plants by inducing stomatal closure (Munemasa et al., 2015). To test whether ALIX contributes to this adaptive response against desiccation, the kinetics of water loss over time were analyzed in alix-1 mutants in comparison to that of wild-type (Col-0), GFP-ALIX/alix-1, oeHA-PYL4, and cra1 (ABA-insensitive), and hab1-1 abi1-2 (ABA-hypersensitive) mutants (Figure 3). As observed in Figure 3A, alix-1 seedlings lost less water than all controls, including the ABA-hypersensitive mutant hab1-1 abi1-2. The rate of water loss in plants positively correlates with that of heat dissipation (Merlot et al., 2002). Accordingly, we observed higher foliar temperature (up to 1°C difference) in alix-1 mutants compared with wild-type and GFP-ALIX/alix-1 controls when plants were analyzed by thermal infrared (IR) imaging (Figure 3B). Conversely, open stomata 2-2 (ost2-2), a mutant with defective stomatal closure, displayed cooler temperature at leaves due to increased water loss through stomata (Merlot et al., 2007; Figure 3B). Thus, we next tested whether reduced water loss and heat dissipation in alix-1 mutants are due to a defect in regulation of stomatal openness. For this, the kinetics of stomatal aperture in response to light were analyzed in leaves of wild-type Col-0, GFP-ALIX/alix-1, and alix-1 plants. The data showed clear differences in stomata responsiveness to light just after 30 min of leaf illumination, with alix-1 stomata being substantially more closed than those of controls (33.8% less open than wild-type control; Figure 3C). Afterward, alix-1 stomata did not open as much as those in controls even after 2 h of light treatment (44.5% less open than the wild-type control), suggesting that incomplete stomata openness in alix-1 mutants leads to reduced water and heat loss.
Figure 3.
Reduced Water Loss and Stomatal Aperture in alix-1 Mutants.
(A) Kinetics of the loss of fresh weight in 15-day-old seedlings of wild-type (Col-0), complemented line GFP-ALIX/alix-1 (labeled as GFP-ALIX), oeHA-PYL4 (HA-PYL4) and alix-1 genotypes. The cra1 and hab1-1 abi1-2 mutants were used as ABA insensitive and hypersensitive controls, respectively. Plants were exposed for 40 min to the drying environment of a laminar flow hood. Values shown are averages ±se from three replicates (n = 15 in each replicate).
(B) IR imaging was used to analyze foliar temperature in wild-type (Col-0), complemented line GFP-ALIX/alix-1, and alix-1 plants. The ost2-2 mutant, which displays incompletely closed stomata, was used as a control. Correspondence between false colors and temperatures (degrees Celsius) in IR images is shown.
(C) Kinetics of stomatal aperture in response to light in leaves of wild-type (Col-0), GFP-ALIX/alix-1, and alix-1 plants. Values shown are averages ±se from three replicates (n = 70 in each replicate).
(D) Confocal imaging was used to visualize vacuolar morphology in guard cells of wild-type (WT, Col-0) and alix-1 plants expressing the tonoplast marker YFP-VAMP711. Plants were grown under short days for 21 d before leaves were collected. Bars = 10 and 5 µm, respectively.
(E) Stomatal aperture measurements in response to FC in dark- and light-treated leaves of wild-type (Col-0), GFP-ALIX/alix-1, and alix-1 plants. Values shown are averages ±se from three replicates (n = 70 in each replicate). *p < 0.05; **p < 0.01 (Student’s t test) with respect to the wild type in the same experimental conditions.
Stomatal aperture is achieved by an increase in vacuolar volume, on rapid fluxes of potassium and other osmolytes, in guard cells (Kim et al., 2010; Santelia and Lawson, 2016). Using confocal microscopy to visualize fluorescence from a tonoplast marker (YFP-VAMP711; Geldner et al., 2009), we observed altered vacuolar morphology in guard cells of light-irradiated alix-1 leaves compared with wild-type controls. Thus, whereas wild-type guard cells were filled with a large vacuole, two or three smaller vacuoles could be visualized in the alix-1 samples (Figure 3D). This alteration should reduce the vacuole volume and pressure required for stomata openness. Previous reports showed that loss of ALIX function leads to defects in vacuole biogenesis (Cardona-López et al., 2015; Kalinowska et al., 2015). To test whether a general mechanical failure occurs in alix-1 guard cells, due to altered vacuolar morphology, we analyzed the effect of fusicoccin (FC; a potent fungal activator of stomatal aperture; Squire and Mansfield, 1972) on their stomata opening. As shown in Figure 3E, FC treatments greatly recovered alix-1 defects in stomatal aperture, although alix-1 stomata still displayed smaller apertures than wild-type controls on FC treatment under both dark and light conditions. Despite this, FC-induced stomata openness in alix-1 plants exceeded that of light-exposed wild-type controls (Figure 3E, right graph), indicating that alix-1 stomata are not mechanically restricted. These results suggest that increased closeness in alix-1 stomata is likely due to constitutive induction of signaling pathways that promote stomatal closure rather than to a major defect in vacuolar dynamics.
Stomata Are Hypersensitive to ABA in alix-1 Mutants
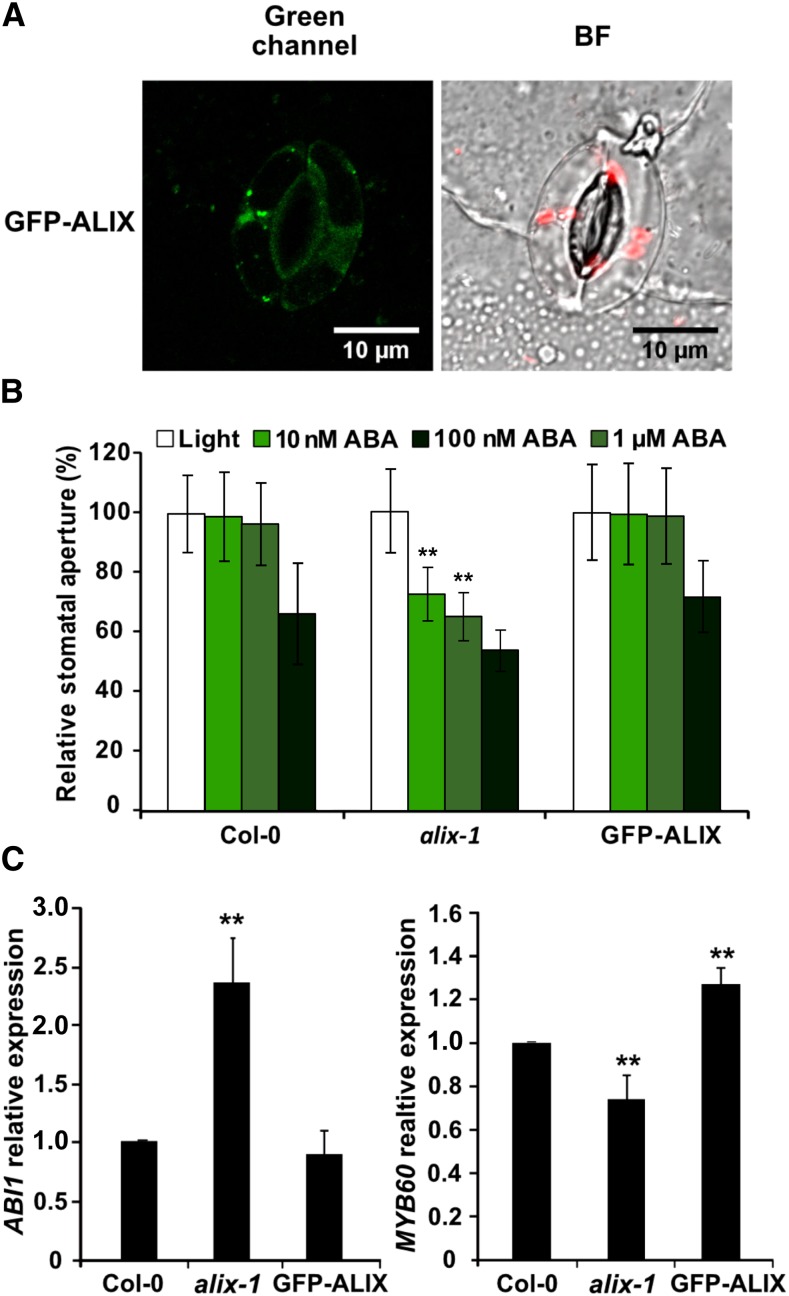
The observation that ALIX acts as a negative regulator of ABA responses prompted us to examine whether ALIX plays a role in controlling stomatal movement in response to ABA in a similar way (Figure 4). Indeed, confocal microscopy imaging showed ALIX localization in the cytosol and vesicle-like compartments of guard cells of plants expressing GFP-ALIX driven by its own promoter, indicating that ALIX function is extended to this specific cell type (Figure 4A). To test this idea, analyses of stomatal closure in response to different concentrations of ABA were performed in alix-1 mutants, wild-type (Col-0), and GFP-ALIX/alix-1 complemented lines. The results showed increased sensitivity of alix-1 stomata to low concentrations of ABA compared with thecontrols (Figure 4B). Thus, at 10 nM ABA, alix-1 stomata already started to close (by 27.5% relative to light-treated alix-1 leaves) whereas wild-type and complemented lines remained open and only started to close when treated with 1 µM ABA, indicating that ALIX function modulates ABA perception and/or signaling for the control of stomatal movement.
Figure 4.
Hypersensitivity to ABA in alix-1 Stomata.
(A) Confocal fluorescence images showing GFP-ALIX localization in the cytoplasm and punctuate structures of wild-type guard cells. Bar=10 µm.
(B) Analysis of stomatal closure in response to different concentrations of ABA in alix-1 mutant, wild-type (Col-0), and GFP-ALIX/alix-1 (labeled as GFP-ALIX) lines. Data are presented as percentage relative to each genotype under light conditions (100% stomatal aperture). Values shown are averages ±se from three replicates (n = 70 in each replicate).
(C) RT-qPCR-analysis of the expression of ABA responsive genes ABI1 and MYB60 in soil-grown alix-1 mutants, wild-type (Col-0), and GFP-ALIX/alix-1 plants.
**p < 0.01 (Student’s t test; Supplemental Data Set 2) with respect to the wild type in the same experimental conditions. Data are means of three biological replicates with two technical replicates per sample. Error bars represent sd.
Because alix-1 mutants show increased sensitivity to ABA and a higher concentration of the ABA hormone, we speculated that alix-1 plants may display constitutive ABA responses at the molecular level. To test this theory, alix-1 seedlings were grown under normal conditions and the transcript levels of ABA-responsive genes were analyzed by RT-qPCR. As a result, we observed increased and decreased expression, respectively, of ABI1 (encoding a PP2C-type phosphatase; Saez et al., 2006) and MYB60 (encoding a guard cell-specific transcription factor that controls stomatal closure; Cominelli et al., 2005) genes in the mutant compared with controls, even under noninductive conditions (Figure 4C), suggesting that alix-1 mutation increases plant sensitivity to endogenous ABA levels.
Trafficking and Vacuolar Degradation of ABA Receptors Are Impaired in alix-1 Mutants
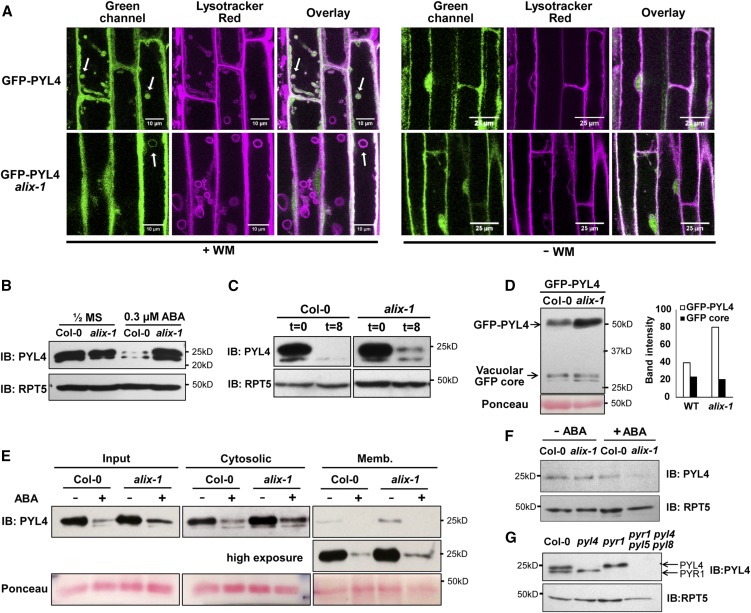
Next we aimed to unravel the molecular basis of defective ABA responses observed in alix-1 mutants. Because ALIX associates with ESCRT-III complexes, enabling protein cargo trafficking, we firstchecked whether ALIX participates in the trafficking of PYL proteins associated with the plasma membrane. For this, we introgressed a 35S:GFP-PYL4 transgene (Belda-Palazon et al., 2016) into the alix-1 mutant background. Localization of the GFP-PYL4 fusion was analyzed by confocal microscopy in wild-type and alix-1 seedlings treated or not with wortmannin (WM), an inhibitor of protein cargo trafficking to vacuoles that causes enlargement of MVBs (Fernandez-Borja et al., 1999; Figure 5). Under normal conditions, GFP-PYL4 fusion mainly localizes in the nucleus, cytosol, and sorting endosomes of wild-type and alix-1 plants (Figure 5A; Bueso et al., 2014). Upon WM treatment of wild-type plants, GFP-PYL4 fluorescence appeared inside enlarged endosomal structures that were stained with LysoTracker Red, an acidophilic dye that allows labeling and tracking of lytic compartments in the late endocytic pathway (Belda-Palazon et al., 2016; Figure 5A). However, in WM-treated alix-1 seedlings, only a small number of LysoTracker Red–stained compartments contained GFP-PYL4 compared with those in wild-type controls, and, occasionally, GFP-PYL4 signal appeared to decorate the membrane of these enlarged vesicles, which could not be appreciated in wild-type controls (Figure 5A). This result suggests that alix-1 mutation interferes with internalization of the ABA receptors into MVB, which should impair their proteolysis at the vacuole. In agreement with this notion, immunoblot analysis of endogenous PYL4 showed increased accumulation of the ABA receptor in the alix-1 mutant background compared with wild-type controls when plants were grown in media containing 0.3 µM ABA (Figure 5B). Increased PYL4 abundance was not observed in complemented GFP-ALIX/alix-1 plants grown in the presence or absence of ABA (Supplemental Figure 1A). These results suggest that ALIX activity toward PYL4 turnover is enhanced in response to ABA, likely reflecting a negative feedback mechanism. RT-qPCR analysis indicated that the changes in PYL4 protein levels between genotypes in each condition were not caused by changes on transcript accumulation (Supplemental Figure 1B). ALIX involvement in the posttranslational control of PYL4 abundance was further substantiated by comparison of the degradation rate of PYL4 in both wild-type and alix-1 backgrounds treated with the protein synthesis inhibitor cycloheximide (CHX), which precludes any effect due to variation in gene expression between samples over time. We observed a delayed decrease of PYL4 levels in alix-1 plants compared with the control upon CHX treatment (Figure 5C). To test whether defective ALIX function impairs sorting and degradation of PYL4 at the vacuole, the levels of a GFP-PYL4 fusion were analyzed in both wild-type and alix-1 plants. Upon delivery of GFP fusion proteins into the vacuole, a secondary degradation product, so-called GFP core, is obtained (daSilva et al., 2005; Scheuring et al., 2012). According to a role for ALIX in vacuolar destabilization of PYL4, the GFP-PYL4/GFP core ratio remained much higher in the alix-1 mutant compared with the wild type (Figure 5D). Noteworthily, alix-1 mutation resulted in increased abundance of PYL4 in both cytosolic and microsomal fractions (Figure 5E) but had no effect on the nuclear pool of PYL4 (Figure 5F). Throughout our immunoblot analyses of endogenous PYL4 abundance, we noticed a secondary band of lower molecular weight, which we confirmed corresponded to PYR1 by using single pyl4 and pyr1 mutants (Figure 5G). Interestingly, alix-1 mutation did not display noticeable defects in the accumulation of PYR1 under the conditions tested, suggesting that, despite its capacity to interact with several ABA receptors, ALIX differentially modulates their abundance, having a larger impact only on a subset of these receptors, including PYL4. Alternatively, partial loss of ALIX function in alix-1 plants might not be sufficient to alter PYR1 homeostasis.
Figure 5.
Impaired Trafficking and Vacuolar Degradation of ABA Receptors in alix-1 Mutants.
(A) Confocal images of wild-type (Col-0) and alix-1 mutant root cells expressing GFP-PYL4 on treatment or not with wortmannin (WM) and stained with LysoTracker Red. Bars = 10 and 25 µm. Arrows indicate representative WM-enlarged vesicles.
(B) Immunoblot (IB) showing accumulation of endogenous PYL4 in wild-type (Col-0) and alix-1 seedlings grown in the presence or absence of 0.3 µM ABAμ. Anti-RPT5 was used as loading control.
(C) Immunoblot (IB) analyses of PYL4 levels in 8-d-old wild-type (Col-0) and alix-1 seedlings treated with 50 µM CHX for 8 h. Anti-RPT5 was used as loading control.
(D) Immunoblot (IB) analysis to track the vacuolar delivery of GFP-PYL4 in the wild-type (WT, Col-0) and alix-1 backgrounds. Anti-GFP was used in immunoblots to detect GFP-PYL4 and the GFP-core signal. Band intensity was quantified using Fiji (ImageJ 1.52i). Ponceau staining was used as loading control as indicated.
(E) Isolation of microsomes from postnuclear fractions (Input) of 8-d-old wild-type (Col-0) or alix-1 seedlings treated or not with ABA for 3 h. Endogenous anti-PYL4 was used to evaluate PYL4 abundance in the cytosolic and microsomal (Memb.) fractions. Ponceau staining was used as loading control as indicated.
(F) Immunoblot (IB) analysis of PYL4 levels in nuclear protein extracts from 8-d-old wild-type (Col-0) or alix-1 seedlings treated or not with ABA for 3 h. Anti-RPT5 was used as loading control.
(G) Immunoblot to test the specificity of anti-PYL4. Protein extracts from 8-d-old wild-type (Col-0), single mutants pyr1 and pyl4 and quadruple mutant pyr1 pyl4 pyl5 pyl8 were used. Arrows indicate the position of endogenous PYR1 and PYL4 proteins in the immunoblot. Anti-RPT5 was used as loading control.
ABA Hypersensitivity of alix-1 Mutants Depends Largely on ABA Receptor Function
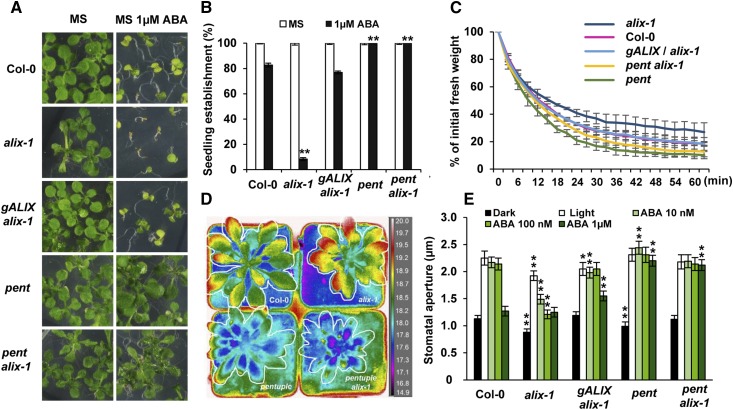
To test whether increased accumulation of PYLs is a main cause for ABA hypersensitivity in alix-1 mutants, we evaluated the effect of combining loss-of-function mutations in both ALIX and ABA receptors. For this, we generated a pentuple pyr1 pyl1 pyl4 pyl5 pyl8 mutant (hereafter pentfor simplicity) in the alix-1 background and analyzed different ABA-related phenotypes compared with the parental and wild-type controls (Figure 6; Supplemental Figure 2). The pentuple mutant we used has been reported to severely impair ABA perception and, therefore, plant responses to this hormone (Antoni et al., 2013). As shown in Figure 6A, increased ABA-mediated inhibition of seedling establishment displayed by alix-1 plants was fully suppressed when combined with the pent mutant (pent alix-1). In these analyses, we included as a control a transgenic line harboring a construct containing the ALIX genomic region (gALIX/alix-1; Cardona-López et al., 2015), which showed complementation of alix-1 phenotypic defects, further supporting a role for ALIX in plant responses to ABA.
Figure 6.
Reduced PYL Function in pent alix-1 Plants Suppresses ABA Defects Caused by alix-1 Mutation.
(A) Representative photographs of 15-d-old wild-type (Col-0), alix-1, complemented line gALIX/alix-1, and pentuple pyr1 pyl1 pyl4 pyl5 pyl8 in wild-type (pent) or alix-1 (pent alix-1) backgrounds grown in the presence of 1 µM ABA.
(B) ABA-mediated inhibition of seedling establishment (emergence of the first true leaves) of the same genotypes analyzed in (A) that were grown in medium lacking or supplemented with 1 µM ABA. Data are means of three biological replicates (n = 20 in each replicate). Error bars represent sd. MS medium was used as a control in all assays.
(C) Kinetics of the loss of fresh weight in 15-d-old seedlings of the genotypes used in (A). Plants were exposed for 60 min to the drying environment of a laminar flow hood. Values shown are averages ±se from three replicates (n = 15 in each replicate).
(D) Analysis of foliar temperature in wild-type (Col-0), alix-1, pent and pent alix-1 plants using IRimaging. Correspondence between false colors and temperatures (°C) in IR images is shown.
(E) Analysis of stomatal closure in response to different concentrations of ABA in the same genotypes analyzed in (A). Values shown are averages ±se from three replicates (n = 100 in each replicate).
In (B) and (E) *p < 0.05; **p < 0.01 (Student’s t test; Supplemental Data Set 2) with respect to the wild type in the same experimental conditions.
The pent alix-1 plants also showed suppression of defects in water loss and heat dissipation (Figures 6C and 6D), suggesting that increased abundance of PYLs largely contributes to enhanced stomatal closure in alix-1 mutants. According to this notion, analyses of stomatal closure in response to different concentrations of ABA, performed in alix-1 mutants, wild-type (Col-0), pent mutants, and pent alix-1 plants, showed that the increased sensitivity of alix-1 stomata to low concentrations of ABA is abolished when ABA perception is hampered (Figure 6E). Of note, reduced PYL function in pent alix-1 mutants also suppressed defects in stomatal aperture found in nontreated alix-1 plants, further supporting the notion that alix-1 stomata are fully operative but highly sensitive to endogenous ABA levels. Overall, our findings indicate that increased abundance of PYLs largely underlies ABA hypersensitivity of alix-1 seedlings and their stomatal behavior.
alix-1 Mutation Limits ALIX Ability to Interact with ABA Receptors and ESCRT Components
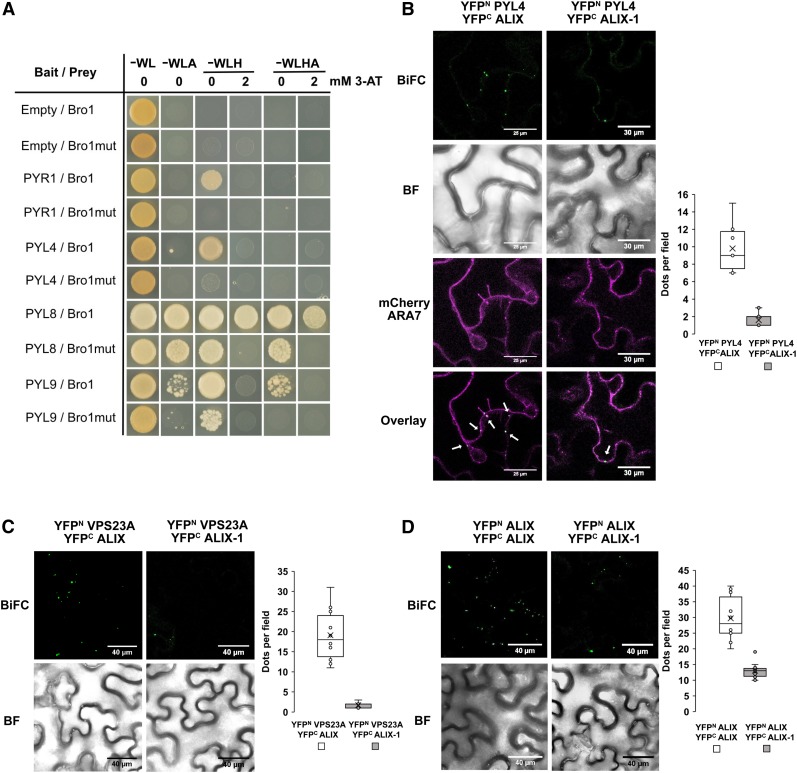
In a previous study, we showed that alix-1 mutation reduces the ability of the resulting mutant protein (ALIX-1) to interact with ESCRT-III component SNF7/VPS32 (Cardona-López et al., 2015). We aimed to test whether alix-1 mutation also affects the interactions of ALIX with ABA receptors (Figure 7). We first performed Y2H assays using as prey either the Bro1 domain or a Bro1 version containing the alix-1 mutation (Bro1mut). As a result, we found that the mutant version displayed reduced interaction with all four PYL receptors tested compared with the wild-type versions (Figure 7A). This reduction in interaction ability was not due to lower accumulation of the mutant protein fusions in these experiments (Supplemental Figure 3). Next we examined whether the impaired ALIX-PYL interaction by the alix-1 mutation could also be observed in planta. To this end, we performed BiFC experiments expressing either wild-type ALIX or ALIX-1 mutant protein together with PYL4, as a representative member of the PYL family. Confirming our previous results (Figure 1D), confocal fluorescence imaging showed interaction between wild-type ALIX and PYL4 in MVBs (Figure 7B). However, such interaction was greatly reduced when the ALIX-1 mutant version was coinfiltrated with PYL4 fusion (16% of that observed for ALIX-PYL4; Figure 7B), as shown by quantitative analysis from Z-stacking sections. This effect could not be explained by lower accumulation of the mutant protein fusion in N. benthamiana leaves (Supplemental Figure 4), further indicating an effect of alix-1 mutation in the interaction ability of ALIX with protein cargoes.
Figure 7.
ALIX-1 Mutant Protein Displays Reduced Interaction with ABA Receptors and the ESCRT Component VPS23A.
(A) Y2H assays for the interaction between the Bro1 domain of ALIX or a variant containing the alix-1 mutation (Bro1mut) and PYL ABA receptors. Transformed yeast cells were grown in synthetic drop-out medium lacking tryptophan and leucine (SD-WL) as a transformation control and in SD-WL media lacking adenine (−WLA), histidine (−WLH), or both (−WLHA) for interaction assays. 3-amino-1,2,4-triazole (3AT; 2mM) was added to better visualize positive interactions. Empty vectors were used as negative controls.
(B) to (D) BiFC assays showing impaired interaction of an ALIX version containing the alix-1 mutation (ALIX-1) with PYL4 (B), VPS23A (C), and wild-type ALIX (D). Bars = 30 µm (A) and 40 µm (C and D). Colocalization of YFP fluorescence by ALIX-PYL4 interaction with mCherry-ARA7-labeled MVBs is indicated by arrows in the Overlay image in (B). Bright-field (BF) images of the corresponding leaf areas are also shown in (B) to (D). The numbers of intracellular punctae per leaf section (y axis; dots per 50 × 50 µm field) agroinfiltrated with each construct combination (x axes) are quantified. Results were obtained from 10 fields from five biological replicates (2 fields/replicate).
Two recent studies have shown that sorting and vacuolar degradation of ABA receptors are mediated by components of the ESCRT machinery, namely, FYVE1 and VPS23A, and that these two proteins interact with each other (Belda-Palazon et al., 2016; Shen et al., 2016; Yu et al., 2016). Moreover, it was found that both FYVE1 and VPS23A physically bind to ALIX in vivo (Shen et al., 2016; Yu et al., 2016). We aimed to test whether alix-1 mutation affects such interactions. We selected VPS23A as a representative component of the ESCRT-I complex. As shown in Figure 7C, interaction with VPS23A was greatly hampered when the ALIX-1 mutant version, instead of wild-type ALIX, was expressed in N. benthamiana epidermal leaf cells, as observed by quantitative analysis from Z-stacking sections of ALIX/VPS23A and ALIX-1/VPS23A agroinfiltrated leaf cells (Figure 7C; Supplemental Figure 5). To evaluate the functionality of the ALIX-1 construct, ALIX/ALIX-1 dimerization levels were also analyzed by BiFC (Figure 7D). Quantitative analysis showed that ALIX-1 interaction with wild-type ALIX is reduced, although to a much lesser extent (43.7% of that shown by ALIX/ALIX constructs) than in the case of the interaction of ALIX-1 with VPS23A and with PYL4 (8.9% and 16% compared with that of ALIX with VPS23A and PYL4, respectively). Reduced physical interaction of ALIX-1 mutant protein with PYL receptors and a known ESCRT-I component mediating their trafficking (i.e., VPS23A), together with decreased ALIX-1 dimerization capability, provides an explanation at the molecular level for defects in sorting and vacuolar degradation of ABA receptors in the Arabidopsis alix-1 mutant.
DISCUSSION
ABA signaling not only involves nuclear events related to the control of transcriptional responses but also regulation of membrane proteins, such as aquaporins and ion channels, that control water and ion fluxes in plants (Cutler et al., 2010; Demir et al., 2013; Brandt et al., 2015). The latter are essential to regulate turgor in guard cells, and therefore stomatal aperture, as a means to limit water loss through the stomatal pore (Kim et al., 2010; Santelia and Lawson, 2016). These processes require desensitization mechanisms that allow plant cells to turn off ABA signaling once water stress is relieved (Antoni et al., 2011; Irigoyen et al., 2014; Castillo et al., 2015; Munemasa et al., 2015). In agreement with this notion, regulatory mechanisms controlling the abundance and/or activity of ABA signaling components associated with membranes have been reported, including ubiquitination of ABA receptors and their trafficking through the endosomal pathway, mediated by components of the ESCRT machinery (i.e., FYVE1 and VPS23A; Bueso et al., 2014; Belda-Palazon et al., 2016; Yu et al., 2016), to the vacuole for proteolysis. Here we propose that ALIX plays an essential role in the control of ABA receptor abundance by facilitating their sorting to the vacuole for degradation. Thus, we showed that alix-1 mutation impairs interaction with PYL4, causing trafficking defects and leading to the increased accumulation of this receptor in different compartments (i.e., cytosol and endosomal vesicles). It has been reported previously that increased PYL4 abundance (as in HA-PYL4–overexpressing plants) is sufficient to increase plant sensitivity to ABA (Pizzio et al., 2013). Thus, increased PYL4 accumulation in alix-1 helps explain the ABA hypersensitivity of this mutant. However, our data showed that alix-1 mutants display a more severe phenotype in the presence of the hormone than HA-PYL4–overexpressing plants (Figure 2), suggesting that ALIX-mediated control of PYL trafficking also spans other PYL family members. In agreement with this, ALIX protein interacts with other members of the PYL family, including PYR1, PYL5, PYL8, and PYL9 in vesicle compartments in planta (Figure 1; Supplemental Movies 2 to 5), pointing to a general role of ALIX in the control of the abundance of PYLs acting in close proximity to membranes.
By modulating ABA receptor abundance at the cytoplasm, ALIX likely participates in the plant desensitization mechanisms against this hormone, repressing ABA-triggered developmental (i.e., seed germination, seedling establishment, and root growth) and water-deficiency responses (stomatal closure). The notion that trafficking of membrane-associated ABA receptors might underlie an ABA-triggered negative feedback mechanism is in line with our observation that PYL4 turnover is enhanced in response to ABA. This negative feedback mechanism may help to temporally restrict PYL4 function, allowing pulsed responses to ABA. Interestingly, ABA seems to regulate differentially the stability of specific PYLs. Thus, contrary to the case of PYL4 for which ABA prompts its destabilization, ABA treatment promotes PYL8 accumulation by inhibiting CRL4 E3 ubiquitin ligases that target it for ubiquitination and proteasomal degradation at the nucleus (Irigoyen et al., 2014; Belda-Palazon et al., 2018). The antagonistic effect of ABA on the abundance of PYLs in separate cell compartments indicates independent modulation of different responses to this hormone.
The involvement of components associated with ESCRT-I (i.e., VPS23A and FYVE1; Shen et al., 2016; Yu et al., 2016) and ESCRT-III (i.e., ALIX; this study) during PYL sorting at MVBs suggests that PYL trafficking to the vacuole is mediated by a canonical ESCRT pathway. It is noteworthy that, VPS23A and FYVE1 physically interacted with ALIX in vivo, as shown by our data and those of others (Shen et al., 2016; Yu et al., 2016). Moreover, a point mutation in the ALIX Bro1 domain disrupted the ALIX-VPS23A interaction, which likely leads to altered trafficking of protein cargoes, including ABA receptors. Consequently, our data support a model in which ALIX contributes to the function of ESCRT-I and ESCRT-III complexes, allowing recognition and clustering of cargo proteins on the MVB surface (Odorizzi, 2006; Belda-Palazon et al., 2016). It has been reported previously that the V-domain (comprising the coiled-coil domains) of ALIX acts as a ubiquitin binding domain that enables ALIX interaction with ubiquitinated protein cargoes, a feature that is conserved in its homologs from other organisms (Fisher et al., 2007; Dowlatshahi et al., 2012; Keren-Kaplan et al., 2013; Pashkova et al., 2013; Kalinowska et al., 2015). In this context, it is conceivable that ALIX, by means of its ubiquitin binding activity, helps transfer ubiquitinated cargoes from ESCRT-I to ESCRT-III complexes, avoiding their diffusion from areas of concentration at the MVB surface. Future studies should test this hypothesis using known targets of the ESCRT machinery, including integral membrane proteins. Notably, however, FYVE1, VPS23A, and ALIX are able to directly interact with unmodified PYL proteins (Belda-Palazon et al., 2016; Yu et al., 2016; this study). These interactions seem to be mediated by additional motifs other than their ubiquitin binding domains, which could reflect roles for FYVE1, VPS23A, and ALIX in ubiquitin-independent sorting of protein cargoes. Indeed, it has been reported previously that sorting into the MVB pathway, particularly in the case of soluble proteins, can occur in the absence of cargo ubiquitination just by physical interaction of cargoes with components of any ESCRT complex (Mageswaran et al., 2014). Indeed, examples of mammalian ALIX-mediated ubiquitin-independent ESCRT-III/MVB sorting have been described (Dores et al., 2012, 2016), a process that might extend to Arabidopsis ALIX when promoting trafficking of PYLs.
Physical interaction of FYVE1 with PYLs was found to be mediated by its N-terminal portion, a 200 amino acid–long intrinsically disordered region (IDR) that contains Pro- and Gln-rich sequences (Belda-Palazon et al., 2016). On binding to partners, IDR regions can adopt metastable conformations that allow molecular recognition of targets with low affinity (Ward et al., 2004). Thus, the IDR region of FYVE1 might fold onto PYL proteins and stabilize their association (Belda-Palazon et al., 2016). Similarly, ALIX contains a Pro-rich IDR region at its C-terminal portion that likely enables interaction of the ALIX ∆Bro1 domain with PYL proteins, as shown by Y2H assays. Of note, in these assays we also identified interaction with PYLs through the Bro1 domain of ALIX, suggesting that different ALIX domains ensure recognition and binding of PYL proteins as a ubiquitin-independent “sorting signal” for entry into the ILVs. Accordingly, Bro1-PYL interaction was severely reduced when a Bro1 version containing the alix-1 mutation was used. The alix-1 mutation leads to substitution of noncharged Gly260 for Asp, a charged amino acid, which likely affects the structure of a tetratricopeptide pocket embedded into the Bro1 domain, resulting in altered Bro1 conformation and impaired protein–protein interaction ability. According to this notion, the ability of ALIX-1 mutant protein to interact with ESCRT-III component SNF7 (Cardona-López et al., 2015); ESCRT-I subunit VPS23A; and, to a lesser extent, with wild-type protein ALIX (this study) is hampered. In this context, according to our findings, weakened interaction of ALIX-1 with PYLs should limit internalization of the latter into ILVs and their subsequent release in the vacuole lumen for degradation (Figure 8). Additional effects due to impaired ALIX function, such as reduced ILV formation at MVBs and defective cargo processing by AMSH proteins (Kalinowska et al., 2015; Shen et al., 2016), cannot be disregarded as they may contribute to the inadequate vacuolar turnover of PYLs in alix-1 mutants. Future work should address the molecular details underlying specific recognition of PYLs, including identification of PYL motifs targeted by ALIX domains. Such studies will help us understand whether similar protein–protein interaction domains mediate binding of ALIX to other ABA pathway components identified in our Y2H screen, such as CAR proteins and VOLTAGE DEPENDENT ANION CHANNEL3 (VDAC3). Although their regulatory relationships have yet to be determined, they might partially underlie the ABA-hypersensitive phenotypes of alix-1 mutants. ALIX-mediated regulation of other ABA pathway components, together with defects in stomatal closure due to altered vacuolar biogenesis and increased ABA levels, might also contribute to the ABA hypersensitive phenotypes of alix-1 mutants. However, the fact that reduced PYL function suppresses ABA defects in pent alix-1 plants, which invokes an effect of ALIX in the control of PYL abundance and function, provides a simpler framework to explain alix-1 hypersensitivity to ABA. Interestingly, additional Bro1-domain proteins might influence trafficking of ABA receptors mediated by the plant ESCRT machineries. It has been recently shown that the plant-specific protein BRO1-DOMAIN PROTEIN AS FREE1 SUPPRESSOR (BRAF) competes with FYVE1 for binding to VPS23A at MVBs. In this way, BRAF1 negatively regulates FYVE1 recruitment to the MVB surface, which should affect internalization of cargoes into ILVs and their vacuolar turnover (Shen et al., 2018). It remains to be tested whether BRAF function affects the accumulation of membrane-associated PYLs and whether it can be counteracted by ALIX to balance ABA receptor abundance according to the environmental conditions.
Figure 8.
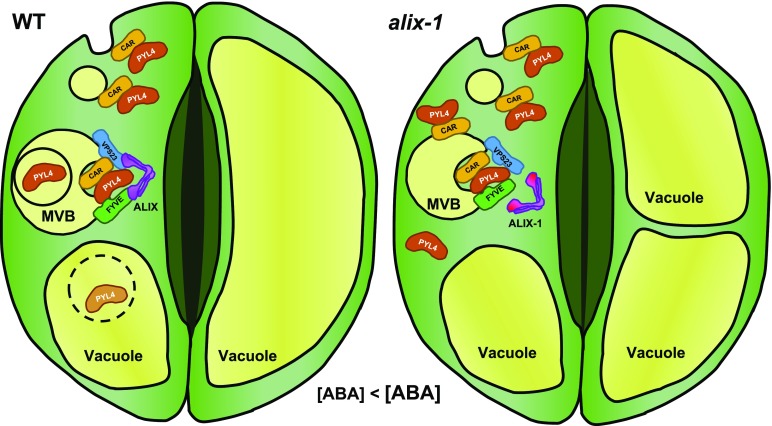
Proposed Model for the Role of ALIX in the Endosomal Trafficking of ABA Receptors.
Soluble PYL4 proteins transiently bind to the plasma membrane via interaction with calcium binding CAR proteins undergoing subsequent endosomal trafficking for vacuolar degradation. MVB sorting of ABA receptors is mediated by the ESCRT machinery aided by ALIX. Vacuolar proteolysis of PYL4 is enhanced in the presence of ABA, likely as a desensitizing mechanism to this hormone. alix-1 mutation impairs the interaction with PYL4 and the ESCRT-I component VPS23A, hampering the internalization of PYL4 into intraluminal vesicles in MVBs. Thus, alix-1 leads to the accumulation of cytosolic and membrane-associated PYL4 that, together with increased ABA content in this mutant, increases sensitivity to ABA. Exacerbated response to ABA, together with vacuolar defects inherent to the alix-1 mutation, causes higher stomatal closure compared with wild-type plants.
In summary, based on our data and current literature, we propose that ALIX, as part of a general ESCRT machinery/MVB pathway, acts as a negative regulator of ABA responses, including stomatal closure. This role involves ALIX-mediated trafficking of membrane-associated ABA receptors for vacuolar degradation.
METHODS
Plant Materials and Growth Conditions.
Arabidopsis (Arabidopsis thaliana) plants used in this study, including mutants and transgenic plants, were of the Col-0 ecotype. Unless otherwise stated, plants were grown in MS media (Murashige and Skoog, 1962) with 1% (w/v)Suc at 21°C under a 16-h-light/8-h-dark cycle using cool white fluorescent light conditions (100 mmol m−2 s−1). Specific treatments were performed as stated in each experiment (see below and figure legends). Mutants alix-1, hab1-1 abi1-2, cra1, pyr1 pyl4 pyl5 pyl8, pyr1 pyl1 pyl4 pyl5 pyl8 (pent), ost2-2, and transgenic lines GFP-ALIX/alix-1, gALIX/alix-1, oeHA-PYL4, and GFP-PYL4 have been described previously by Saez et al. (2006); Merlot et al. (2007); Fernández-Arbaizar et al. (2012); Gonzalez-Guzman et al. (2012); Antoni et al. (2013); Pizzio et al. (2013); Cardona-López et al. (2015); Belda-Palazon et al. (2016).
To generate the pent alix-1 line, pent and alix-1 plants were crossed. The resulting F2 progeny was grown in MS media plates containing 15 μM ABA to select plants that are very insensitive to ABA. Among the latter, plants displaying alix-1 phenotypes were genotyped for pyr/pyl mutations. Primers used to genotype the pent alix-1 mutants are given in Supplemental Table 1.
For BiFC experiments, Nicotiana benthamiana plants were grown in soil in the greenhouse at 22°C under 16-h-light/8-h-dark photoperiod during 3 weeks before agroinfiltration of leaves with the corresponding constructs.
Protein Extraction and Immunoblots
For protein extraction from seedlings, proteins were extracted in buffer containing 50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 10 mM MgCl2, 1 mM phenylmethylsulfonyl fluoride, 0.1% (v/v) Nonidet P-40 and 1x complete protease inhibitor (Roche). After centrifugation at 16,000g at 4°C for 10 min, the supernatants were collected. This step was repeated twice. Protein concentration in the final supernatants was determined using the Bio-Rad Protein Assay kit. In the case of PYL4 immunoblots, proteins were extracted using 50 mM Tris-MES pH 7.4, 0.5 M Suc, 1 mM MgCl2, 10 mM EDTA, 5 mM dithiotreitol, 0.2% (v/v) Nonidet P-40, and 1x complete protease inhibitor (Roche). Subcellular fractionation assays were performed as described (Cardona-López et al., 2015). Nuclear protein extractions were performed as described previously by Liu et al. (2001).
For immunoblots, protein samples were denatured, separated on SDS-PAGE gels, and transferred onto polyvinylidene difluoride membranes (Millipore). Membranes were probed with different antibodies: Living ColorsTM polyclonal anti-GFP at 1:1000 dilution (for detection of BiFC fusions; BD Biosciences Catalog No. 632593), anti-His at 1:10000 dilution (for His-PYL fusions; BD Biosciences Catalog No. 552565), anti-PYL4 at 1:2000 dilution (Yu et al., 2016), anti-MBP at 1:10000 dilution (Abcam Catalog No. ab9084), and anti-MYC-HRP at 1:2000 dilution (Santa Cruz Biotechnology Catalog No. sc-40). For immunodetection of 3HA-PYL fusions, anti-HA-HRP (Roche Catalog No. 11667475001) was used at 1:1000 dilution. To confirm equal protein loading, membranes were immunoblotted using anti-RPT5 at 1:10000 dilution (Abcam Catalog No. ab22676).
Purification of Recombinant Proteins and Pull-Down Experiments
Recombinant His-PYL, MBP alone, and MBP-ALIX fusions were expressed in the Escherichia coli BL21 (DE3) strain carrying pET28-HisT7PYL (Pizzio et al., 2013), empty pMAL-c2X, and pMAL-c2X-ALIX constructs, respectively. The pMAL-c2X-ALIX construct was prepared by transferring the ALIX cDNA from a pDONR207 (Invitrogen) plasmid to a Gateway-adapted pMAL-c2X plasmid (Austin and Waugh, 2012). Bacteria were cultured in Luria-Bertani (LB) medium at 37°C to an optical density at 600 nm of 0.5, at which time protein expression was induced with 0.1 mM isopropyl-D-thio-galactopyranoside for 6 h. Cell lysis was performed using a sonicator (Labsonic U, B. Braun; 5 pulses of 15 s each at low intensity on ice), and lysates were clarified by centrifugation at 16,000g for 30 min at 4°C. His-PYL and MBP-ALIX protein were purified from lysates with Ni-NTA-agarose (Qiagen) or Amylose resin (New England Biolabs) beads, respectively, under native conditions. His-PYLs were eluted with 250 mM imidazole, as described by the manufacturers, whereas MBP-ALIX or MBP were left bound to the amylose resin. Protein concentration in dialyzed final eluates was determined using a Bio-Rad Protein Assay kit.
For in vitro pull-down assay, 5 μg of His-PYLs were incubated with rotation with 10 μg of either MBP-ALIX or MBP alone on amylose resin for 1 h at 4°C in 500 μL of binding buffer (50 mM Tris-HCl, pH 7.5, 100 mM NaCl, 10% (v/v) glycerol, 0.1% (v/v) Tween-20, 1 mM PMSF, 1x EDTA Free-Complete Protease Inhibitor Cocktail [Roche]). After washing three times with binding buffer, the beads were resuspended in 50 μL loading buffer, boiled for 5 min and 40 μL was analyzed by protein gel blotting using anti-His and anti-MBP antibodies. For semi–in vivo pull-down assay, protein extracts from oeHA-PYL4 plants were incubated with rotation with 10 μg of either MBP-ALIX or MBP alone on amylose resin for 1 h at 4°C in 500 μL of above-mentioned binding buffer. Washing and elution were as before. Overall, 30 and 5 μL were analyzed by protein gel blotting using anti-HA (1:1000 dilution; Roche, catalog no 11667475001) and anti-MBP (1:10,000 dilution; Abcam Catalog No. ab9084) antibodies, respectively.
Yeast Two Hybrid Experiments
Different ALIX gene versions, including coding sequences for full-length ALIX (ALIX FL), ALIX Bro1 domain (N-terminal region; amino acids 1 to 413), and ALIX C-terminal region (containing the coiled coils and the proline-rich domain; amino acids 405 to 846) cloned into the pGBKT7 vector using Gateway technology (Gal4 DNA binding domain, BD; Clontech; described by Cardona-López et al. [2015]) were used to screen a whole seedling cDNA library (Puga et al., 2014) prepared in the pGADT7 vector (Gal4 activation domain; Clontech) to detect ALIX-interacting proteins, as described previously by Irigoyen et al. (2014). To confirm potential protein–protein interactions, selected positive clones were cotransformed into Saccharomyces cerevisiae AH109 cells, following standard heat shock protocols (Cardona-López et al., 2015). Successfully transformed colonies were identified on yeast synthetic dropout media lacking Leu and Trp (Clontech). These colonies were resuspended in water and transferred to selective media lacking Ade, His, Leu, and Trp (supplemented or not with 0.5 mM, 3-amino-1,2,4-triazole, 3-AT; Sigma-Aldrich). Yeast cells were incubated at 30°C for 3 d. Empty vectors were cotransformed as negative controls. To test the effect of ALIX-1 mutation on the interaction with PYL proteins, ALIX-1 1-413 (an ALIX 1-413 version containing the alix-1 mutation) was also used. For figure preparation, representative colonies for each bait:prey construct combination from independent plates were used. Protein extracts from yeast cells were prepared using a trichloroacetic acid protein extraction technique (Foiani et al., 1994).
Tracer and Drugs, Microscopy, and Image Processing
Seedlings visualized in live imaging experiments were grown for 3 d and incubated in 0.5x MS liquid medium supplemented or not with specific drugs at concentrations and periods described as follows: For LysoTracker Red (Thermo Fisher Scientific) treatments, seedlings were incubated for 30 min with 1 µM LysoTracker Red. For WM (Invitrogen) treatments, seedlings were incubated with 33 µM WM for 30 min under dark conditions. WM stock was at 50 mM in DMSO.
Confocal laser scanning microscopy was performed on two different inverted microscopes: Leica TCS SP8 (CNB-CSIC), and Zeiss LSM 780 (CEA-Cadarache). Image processing was done with Fiji software from the National Institutes of Health (https://imagej.net/Fiji).
Bimolecular Fluorescence Complementation Assays
The cDNA of ALIX, ALIX-1, PYR1, PYL4, PYL5, PYL8, PYL9, and VPS23A was transferred from pDONR plasmids (Irigoyen et al., 2014; Cardona-López et al., 2015; Yu et al., 2016) to destination vectors of the pBiFP series (To et al., 2006) using the Gateway system (Invitrogen) and transformed into Agrobacterium C58C1 competent cells. Different combinations of Agrobacterium clones expressing fusion proteins as indicated were coinfiltrated into the abaxial surface of leaves from 3-week-old N. benthamiana plants (Sparkes et al., 2006). Leaves were also coinfiltrated with p19, which suppresses gene silencing (Voinnet, 2003). Empty vectors were used as negative controls. Fluorescence was visualized in epidermal cells of leaves 3 d after infiltration using a TCS SP8 confocal microscope (Leica).
ABA Concentration Measurement
ABA levels were analyzed using high-performance liquid chromatography–electrospray ionization–high-resolution accurate mass spectrometry (HPLC-ESI-HRMS). The extraction and purification were performed using the following method: 250 mg of frozen tissue from roots or shoots (previously ground to a powder in a mortar with liquid N2) was homogenized with 2.5 mL of precooled methanol:water:HCOOH (90:9:1, v/v/v with 2.5 mM Na-diethyldithiocarbamate) and 25 μL of a stock solution of 1000 ng/mL of deuterium-labeled internal standard D-ABA. The mixture was shaken for 60 min at room temperature. After extraction, solids were separated by centrifugation at 20,000g for 10 min at room temperature using a Sigma 4-16K Centrifuge (Sigma Laborzentrifugen) and reextracted with an additional 1.25 mL of extraction mixture followed by shaking for 20 min and centrifugation at 20,000g for 10 min at room temperature. A 2-mL aliquot of pooled supernatants was taken and dried at 40°C using a RapidVap Evaporator (Labconco). The residue was dissolved in 500 μL of methanol:0.133% (v/v) acetic acid (40:60, v/v) and centrifuged (20,000g, 10 min at room temperature) before being injected in an HPLC-ESI-HRMS system. ABA was quantified using a Dionex Ultimate 3000 UHPLC device coupled to a Q Exactive Focus Mass Spectrometer (Thermo Fisher Scientific), equipped with an HESI(II) source, a quadrupole mass filter, a C-Trap, a higher-energy collisional dissociation (HCD) collision cell and an Orbitrap mass analyzer (Orbitrap-Focus; Thermo Fisher Scientific). A reverse-phase column (Synergi 4 mm Hydro-RP 80A, 150 2 mm; Phenomenex) was used. A linear gradient of methanol (A), water (B), and 2% (v/v) acetic acid in water (C) was used: 38% (v/v) A for 3 min, 38% (v/v) to 96% (v/v) A for 12 min, 96% (v/v) A for 2 min, and 96% (v/v) to 38% (v/v) A for 1 min, and kept for 4 min; C remains constant at 4% (v/v). The flow rate was 0.30 mL/min, the injection volume was 40 μL and column and sample temperatures were 35°C and 15°C, respectively. The detection and quantification were performed using a Full MS experiment with tandem mass spectrometry (MS/MS) confirmation in the negative-ion mode. Instrument control and data processing were performed using TraceFinder 3.3 EFS software.
Growth Analyses, Water Loss, and Stomatal Aperture Measurements
For seed germination, seedling establishment, and root growth assays, conditions were as described by Irigoyen et al. (2014). Water loss assays in 15-d-old seedlings were performed as described by Belda-Palazon et al. (2016). For this test, seedlings were grown in MS plates, and 15 seedlings per genotype with similar growth, three independent experiments, were submitted to the drying atmosphere of a flow laminar hood. Kinetic analysis of water loss was performed and represented as the percentage of initial fresh weight at each scored time point. Stomatal aperture was measured from epidermal strips of abaxial leaf faces of 4-week-old plants. Epidermal strips were incubated in buffer (50 mM KCl, 10 mM MES-KOH pH 6.0) during 1.5 h in darkness and then transferred to white light (250 100 mmol m−2 s−1) to measure stomatal aperture at different time points as indicated. For ABA treatments, epidermal peels were placed in buffer with different ABA concentrations (ranging from 0 to 1 µM) for 2.5 h under dark or light conditions. For fusicoccin (Sigma-Aldrich) treatment, epidermal peels were maintained for 1.5 h in darkness in the above-mentioned buffer and then transferred to buffer plus 5 µM fusicoccin or an equivalent volume of ethanol and maintained for 2.5 h under dark or light conditions. Stomata were visualized in an optical microscope (Optiphot; Nikon), and apertures were measured with ImageJ freeware. All experiments were performed as blind and repeated at least three times independently.
Thermal Infrared Imaging
Arabidopsis seeds were directly sown on 7 × 7 cm pots containing a mixture of one-third sand (2- to 3-mm particles) and two-thirds horticultural compost (v/v). Pots were incubated in the dark at 4°C for 2 d to break seed dormancy. Plants were then grown in a phytotron under an 8-h light photoperiod and the following conditions. Day conditions: 22°C, 55% relative humidity, every day watering and cool white fluorescent light conditions (100 mmol m−2 s−1). Night conditions: 18°C and 60% relative humidity. Thermal images of 3- to 4-week-old plants were obtained using a FLIR A655sc IR camera (FLIR Systems) equipped with a 24.6 mm focal length lens and FLIR ResearchIR Max software (version 4.20.2.74).
Reverse Transcription Quantitative PCR
RT-qPCR experiments were performed using RNA extracted from Col-0 wild-type, alix-1, and GFP-ALIX/alix-1 plants. To check ABI1 and MYB60 levels, three biological replicates, each consisting of tissue pooled from shoots of three 45-day-old plants growing on soil, were taken. For PYL4 levels analysis, three biological replicates were prepared using seedlings grown in 0.5x MS supplemented or not with 0.3 µM ABA for 6 days. RNA extraction, cleanup and DNase treatment were performed using Direct-zol RNA MiniPrep Plus (Zymo Research). cDNA was synthesized from 400 ng of total RNA using qScript XLT cDNA SuperMix (Quanta Biosciences), and 2 μL from one-twentieth diluted cDNA was used to amplify the genes analyzed using SYBR Green I Master (Roche). ACTIN8 and TUBULIN were used as housekeeping reference genes. The following primers were used (5′-3′): ABI1_F:GAGGCAGAGAGGGTCCTTTT, ABI1_R:AGCCCGGGAAGAAAAATACA, MYB60_F:CCTTCTTGAGGGTTGGATGA, MYB60_R:GTCATCATCCCCGTTAGTTC, TUB_F:GAGCCTTACAACGCTACTCTGTCTGTC, TUB_R: ACACCAGACATAGTAGCAGAAATCAAG, PYL4_F: ATGCTTGCCGTTCACCGTCCT, PYL4_R: CGAGCGGCCGTGGAGTCGCGTG, ACTIN8_F: GACTCAGATCATGTTTGAGACCTTT, ACTIN8_R: ACCGGTTGTACGACCACTGG.
Quantitative PCRs were performed in 384-multiwell optical plates in a Light Cycler 480 Real Time PCR system (Roche). The PCR conditions were as follows: 10 min at 95°C and 40 cycles of 15 s at 95°C and 60 s at 60°C.
Accession numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative database under the following accession numbers: ALIX (At1g15130), FYVE1/ FREE1, (At1g20110), VPS23A/ELC (At3g12400), PYR1 (At4g17870), PYL4 (At2g38310), PYL5 (At5g05440), PYL8 (At5g53160), PYL9 (At1g01360).
Supplemental Data
Supplemental Figure 1. Expression levels of PYL4 and accumulation of its protein upon treatment with ABA. Supports Figure 5.
Supplemental Figure 2. Genotyping of a pentuple pyr1 pyl1 pyl4 pyl5 pyl8 mutant in the alix-1 background (pent alix-1). Supports Figure 6.
Supplemental Figure 3. Expression analysis of bait and prey fusions used in yeast two hybrid assays. Supports Figure 7A.
Supplemental Figure 4. Expression analysis of protein fusions used in BiFC assays. Supports Figure 7B.
Supplemental Figure 5. Expression analysis of protein fusions used in BiFC assays. Supports Figure 7C and D.
Supplemental Table 1. List of primers used in the genotyping of pent alix-1 mutants shown in Supplemental Figure 2.
Supplemental Data Set 1. Lists of proteins identified as interacting with full-length ALIX or ALIX fragments in yeast two hybrid assays.
Supplemental Data Set 2. Statistical analyses.
Supplemental Movie 1. Confocal microscopy movie showing the in vivo interaction of PYL4 with ALIX by BiFC.
Supplemental Movie 2. Confocal microscopy movie showing the in vivo interaction of PYR1 with ALIX by BiFC.
Supplemental Movie 3. Confocal microscopy movie showing the in vivo interaction of PYL5 with ALIX by BiFC.
Supplemental Movie 4. Confocal microscopy movie showing the in vivo interaction of PYL8 with ALIX by BiFC.
Supplemental Movie 5. Confocal microscopy movie showing the in vivo interaction of PYL9 with ALIX by BiFC.
Supplemental Movie Legends. Legends for Supplemental Movies 1 to 5.
Dive Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
Acknowledgments
This research was supported by the Ministerio de Economía y Competitividad (MINECO) and AEI/FEDER/EU (grants BIO2013-46539-R and BIO2016-80551-R to V.R., and BIO2017-82503-R to P.L.R.); by the Spanish Ministry of Education FPI “Severo Ochoa” fellowships (BES2015-071820 to M.G.-L.); by the European Molecular Biology Organization (EMBO) Short-Term programs (ASTF 7678-2016); by the Agence Nationale de Recherche REGLISSE project (ANR-13-ADAP-0008 to L.C.); by an Agencia Española de Cooperación Internacional (AECID) fellowship to D.A.E.-M.; by the Spanish Ministry of Education “Salvador de Madariaga” grant (PRX15/00493 to V.R.); and by Generalitat Valenciana “Programa VALi+d” (APOSTD/2017/039 to B.B.P.). We are thankful to Erika Isono for helpful discussion of the article. We thank Qi Xie for the anti-PYL4 antibody, and Oscar Lorenzo for cra1 seeds. We also thank the confocal microscopy, in vitro plant culture, and greenhouse facilities at the CNB-CSIC for technical support.
AUTHOR CONTRIBUTIONS
M.G.-L., L.C., P.L.R., J.P.-A., N.L. and V.R. conceived the study and designed the experiments. M.G.-L., L.C., D.A.E.-M., L.R., B.B.-P., E.S.-Q., Y.F., A.M.Z., B.R., N.L., and V.R. generated lines and constructs and performed the experiments. M.G.-L., L.C., A.M.Z.; J.M.G.-M. B.R., L.N., P.L.R., J.P.-A., N.L., and V.R. analyzed the data. V.R. wrote the article. All authors revised the article.
References
- Antoni R., Rodriguez L., Gonzalez-Guzman M., Pizzio G.A., Rodriguez P.L. (2011). News on ABA transport, protein degradation, and ABFs/WRKYs in ABA signaling. Curr. Opin. Plant Biol. 14: 547–553. [DOI] [PubMed] [Google Scholar]
- Antoni R., Gonzalez-Guzman M., Rodriguez L., Peirats-Llobet M., Pizzio G.A., Fernandez M.A., De Winne N., De Jaeger G., Dietrich D., Bennett M.J., Rodriguez P.L. (2013). PYRABACTIN RESISTANCE1-LIKE8 plays an important role for the regulation of abscisic acid signaling in root. Plant Physiol. 161: 931–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin B.P., Waugh D.S. (2012). Isolation of Metarhizium anisopliae carboxypeptidase A with native disulfide bonds from the cytosol of Escherichia coli BL21(DE3). Protein Expr. Purif. 82: 116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babst M. (2005). A protein’s final ESCRT. Traffic 6: 2–9. [DOI] [PubMed] [Google Scholar]
- Babst M., Katzmann D.J., Estepa-Sabal E.J., Meerloo T., Emr S.D. (2002a). Escrt-III: An endosome-associated heterooligomeric protein complex required for mvb sorting. Dev. Cell 3: 271–282. [DOI] [PubMed] [Google Scholar]
- Babst M., Katzmann D.J., Snyder W.B., Wendland B., Emr S.D. (2002b). Endosome-associated complex, ESCRT-II, recruits transport machinery for protein sorting at the multivesicular body. Dev. Cell 3: 283–289. [DOI] [PubMed] [Google Scholar]
- Belda-Palazon B., et al. (2018). PYL8 mediates ABA perception in the root through non-cell-autonomous and ligand-stabilization-based mechanisms. Proc. Natl. Acad. Sci. USA 115: E11857–E11863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belda-Palazon B., Rodriguez L., Fernandez M.A., Castillo M.C., Anderson E.M., Gao C., González-Guzmán M., Peirats-Llobet M., Zhao Q., De Winne N., Gevaert K., De Jaeger G., et al. (2016). FYVE1/FREE1 interacts with the PYL4 ABA receptor and mediates its delivery to the vacuolar degradation pathway. Plant Cell 28: 2291–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt B., Munemasa S., Wang C., Nguyen D., Yong T., Yang P.G., Poretsky E., Belknap T.F., Waadt R., Aleman F., Schroeder J.I. (2015). Calcium specificity signaling mechanisms in abscisic acid signal transduction in Arabidopsis guard cells. eLife 4: e03599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueso E., Rodriguez L., Lorenzo-Orts L., Gonzalez-Guzman M., Sayas E., Muñoz-Bertomeu J., Ibañez C., Serrano R., Rodriguez P.L. (2014). The single-subunit RING-type E3 ubiquitin ligase RSL1 targets PYL4 and PYR1 ABA receptors in plasma membrane to modulate abscisic acid signaling. Plant J. 80: 1057–1071. [DOI] [PubMed] [Google Scholar]
- Cardona-López X., Cuyas L., Marín E., Rajulu C., Irigoyen M.L., Gil E., Puga M.I., Bligny R., Nussaume L., Geldner N., Paz-Ares J., Rubio V. (2015). ESCRT-III-associated protein ALIX mediates high-affinity phosphate transporter trafficking to maintain phosphate homeostasis in Arabidopsis. Plant Cell 27: 2560–2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo M.C., Lozano-Juste J., González-Guzmán M., Rodriguez L., Rodriguez P.L., León J. (2015). Inactivation of PYR/PYL/RCAR ABA receptors by tyrosine nitration may enable rapid inhibition of ABA signaling by nitric oxide in plants. Sci. Signal. 8: ra89. [DOI] [PubMed] [Google Scholar]
- Cominelli E., Galbiati M., Vavasseur A., Conti L., Sala T., Vuylsteke M., Leonhardt N., Dellaporta S.L., Tonelli C. (2005). A guard-cell-specific MYB transcription factor regulates stomatal movements and plant drought tolerance. Curr. Biol. 15: 1196–1200. [DOI] [PubMed] [Google Scholar]
- Conibear E. (2002). An ESCRT into the endosome. Mol. Cell 10: 215–216. [DOI] [PubMed] [Google Scholar]
- Cutler S.R., Rodriguez P.L., Finkelstein R.R., Abrams S.R. (2010). Abscisic acid: Emergence of a core signaling network. Annu. Rev. Plant Biol. 61: 651–679. [DOI] [PubMed] [Google Scholar]
- daSilva L.L., Taylor J.P., Hadlington J.L., Hanton S.L., Snowden C.J., Fox S.J., Foresti O., Brandizzi F., Denecke J. (2005). Receptor salvage from the prevacuolar compartment is essential for efficient vacuolar protein targeting. Plant Cell 17: 132–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demir F., Horntrich C., Blachutzik J.O., Scherzer S., Reinders Y., Kierszniowska S., Schulze W.X., Harms G.S., Hedrich R., Geiger D., Kreuzer I. (2013). Arabidopsis nanodomain-delimited ABA signaling pathway regulates the anion channel SLAH3. Proc. Natl. Acad. Sci. USA 110: 8296–8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dores M.R., Chen B., Lin H., Soh U.J., Paing M.M., Montagne W.A., Meerloo T., Trejo J. (2012). ALIX binds a YPX(3)L motif of the GPCR PAR1 and mediates ubiquitin-independent ESCRT-III/MVB sorting. J. Cell Biol. 197: 407–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dores M.R., Grimsey N.J., Mendez F., Trejo J. (2016). ALIX regulates the ubiquitin-independent lysosomal sorting of the P2Y1 purinergic receptor via a YPX3L motif. PLoS One 11: e0157587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlatshahi D.P., Sandrin V., Vivona S., Shaler T.A., Kaiser S.E., Melandri F., Sundquist W.I., Kopito R.R. (2012). ALIX is a Lys63-specific polyubiquitin binding protein that functions in retrovirus budding. Dev. Cell 23: 1247–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubeaux G., Vert G. (2017). Zooming into plant ubiquitin-mediated endocytosis. Curr. Opin. Plant Biol. 40: 56–62. [DOI] [PubMed] [Google Scholar]
- Fernández-Arbaizar A., Regalado J.J., Lorenzo O. (2012). Isolation and characterization of novel mutant loci suppressing the ABA hypersensitivity of the Arabidopsis coronatine insensitive 1-16 (coi1-16) mutant during germination and seedling growth. Plant Cell Physiol. 53: 53–63. [DOI] [PubMed] [Google Scholar]
- Fernandez-Borja M., Wubbolts R., Calafat J., Janssen H., Divecha N., Dusseljee S., Neefjes J. (1999). Multivesicular body morphogenesis requires phosphatidyl-inositol 3-kinase activity. Curr. Biol. 9: 55–58. [DOI] [PubMed] [Google Scholar]
- Fisher R.D., Chung H.Y., Zhai Q., Robinson H., Sundquist W.I., Hill C.P. (2007). Structural and biochemical studies of ALIX/AIP1 and its role in retrovirus budding. Cell 128: 841–852. [DOI] [PubMed] [Google Scholar]
- Foiani M., Marini F., Gamba D., Lucchini G., Plevani P. (1994). The B subunit of the DNA polymerase alpha-primase complex in Saccharomyces cerevisiae executes an essential function at the initial stage of DNA replication. Mol. Cell. Biol. 14: 923–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H., Chinnusamy V., Rodrigues A., Rubio S., Antoni R., Park S.Y., Cutler S.R., Sheen J., Rodriguez P.L., Zhu J.K. (2009). In vitro reconstitution of an abscisic acid signalling pathway. Nature 462: 660–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y., et al. (2009). Three SnRK2 protein kinases are the main positive regulators of abscisic acid signaling in response to water stress in Arabidopsis. Plant Cell Physiol. 50: 2123–2132. [DOI] [PubMed] [Google Scholar]
- Gao C., Luo M., Zhao Q., Yang R., Cui Y., Zeng Y., Xia J., Jiang L. (2014). A unique plant ESCRT component, FREE1, regulates multivesicular body protein sorting and plant growth. Curr. Biol. 24: 2556–2563. [DOI] [PubMed] [Google Scholar]
- Gao C., Zhuang X., Cui Y., Fu X., He Y., Zhao Q., Zeng Y., Shen J., Luo M., Jiang L. (2015). Dual roles of an Arabidopsis ESCRT component FREE1 in regulating vacuolar protein transport and autophagic degradation. Proc. Natl. Acad. Sci. USA 112: 1886–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D., Scherzer S., Mumm P., Stange A., Marten I., Bauer H., Ache P., Matschi S., Liese A., Al-Rasheid K.A., Romeis T., Hedrich R. (2009). Activity of guard cell anion channel SLAC1 is controlled by drought-stress signaling kinase-phosphatase pair. Proc. Natl. Acad. Sci. USA 106: 21425–21430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldner N., Dénervaud-Tendon V., Hyman D.L., Mayer U., Stierhof Y.D., Chory J. (2009). Rapid, combinatorial analysis of membrane compartments in intact plants with a multicolor marker set. Plant J. 59: 169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Guzman M., Pizzio G.A., Antoni R., Vera-Sirera F., Merilo E., Bassel G.W., Fernández M.A., Holdsworth M.J., Perez-Amador M.A., Kollist H., Rodriguez P.L. (2012). Arabidopsis PYR/PYL/RCAR receptors play a major role in quantitative regulation of stomatal aperture and transcriptional response to abscisic acid. Plant Cell 24: 2483–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg J., Stenmark H. (2004). The biogenesis of multivesicular endosomes. Nat. Rev. Mol. Cell Biol. 5: 317–323. [DOI] [PubMed] [Google Scholar]
- Henne W.M., Buchkovich N.J., Emr S.D. (2011). The ESCRT pathway. Dev. Cell 21: 77–91. [DOI] [PubMed] [Google Scholar]
- Henne W.M., Stenmark H., Emr S.D. (2013). Molecular mechanisms of the membrane sculpting ESCRT pathway. Cold Spring Harb. Perspect. Biol. 5: a016766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irigoyen M.L., et al. (2014). Targeted degradation of abscisic acid receptors is mediated by the ubiquitin ligase substrate adaptor DDA1 in Arabidopsis. Plant Cell 26: 712–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isono E., Kalinowska K. (2017). ESCRT-dependent degradation of ubiquitylated plasma membrane proteins in plants. Curr. Opin. Plant Biol. 40: 49–55. [DOI] [PubMed] [Google Scholar]
- Isono E., Katsiarimpa A., Müller I.K., Anzenberger F., Stierhof Y.D., Geldner N., Chory J., Schwechheimer C. (2010). The deubiquitinating enzyme AMSH3 is required for intracellular trafficking and vacuole biogenesis in Arabidopsis thaliana. Plant Cell 22: 1826–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinowska K., Nagel M.K., Goodman K., Cuyas L., Anzenberger F., Alkofer A., Paz-Ares J., Braun P., Rubio V., Otegui M.S., Isono E. (2015). Arabidopsis ALIX is required for the endosomal localization of the deubiquitinating enzyme AMSH3. Proc. Natl. Acad. Sci. USA 112: E5543–E5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsiarimpa A., Anzenberger F., Schlager N., Neubert S., Hauser M.T., Schwechheimer C., Isono E. (2011). The Arabidopsis deubiquitinating enzyme AMSH3 interacts with ESCRT-III subunits and regulates their localization. Plant Cell 23: 3026–3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzmann D.J., Babst M., Emr S.D. (2001). Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell 106: 145–155. [DOI] [PubMed] [Google Scholar]
- Keren-Kaplan T., Attali I., Estrin M., Kuo L.S., Farkash E., Jerabek-Willemsen M., Blutraich N., Artzi S., Peri A., Freed E.O., Wolfson H.J., Prag G. (2013). Structure-based in silico identification of ubiquitin-binding domains provides insights into the ALIX-V:ubiquitin complex and retrovirus budding. EMBO J. 32: 538–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T.H., Böhmer M., Hu H., Nishimura N., Schroeder J.I. (2010). Guard cell signal transduction network: Advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu. Rev. Plant Biol. 61: 561–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb C., Nagel M.K., Kalinowska K., Hagmann J., Ichikawa M., Anzenberger F., Alkofer A., Sato M.H., Braun P., Isono E. (2015). FYVE1 is essential for vacuole biogenesis and intracellular trafficking in Arabidopsis. Plant Physiol. 167: 1361–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyuuma M., Kikuchi K., Kojima K., Sugawara Y., Sato M., Mano N., Goto J., Takeshita T., Yamamoto A., Sugamura K., Tanaka N. (2007). AMSH, an ESCRT-III associated enzyme, deubiquitinates cargo on MVB/late endosomes. Cell Struct. Funct. 31: 159–172. [DOI] [PubMed] [Google Scholar]
- Lee S.C., Lan W., Buchanan B.B., Luan S. (2009). A protein kinase-phosphatase pair interacts with an ion channel to regulate ABA signaling in plant guard cells. Proc. Natl. Acad. Sci. USA 106: 21419–21424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Zhang L., Li X., Kong X., Wang X., Li Y., Liu Z., Wang J., Li X., Yang Y. (2018). AtRAE1 is involved in degradation of ABA receptor RCAR1 and negatively regulates ABA signalling in Arabidopsis. Plant Cell Environ. 41: 231–244. [DOI] [PubMed] [Google Scholar]
- Li Y., Zhang L., Li D., Liu Z., Wang J., Li X., Yang Y. (2016). The Arabidopsis F-box E3 ligase RIFP1 plays a negative role in abscisic acid signalling by facilitating ABA receptor RCAR3 degradation. Plant Cell Environ. 39: 571–582. [DOI] [PubMed] [Google Scholar]
- Liu X.L., Covington M.F., Fankhauser C., Chory J., Wagner D.R. (2001). ELF3 encodes a circadian clock-regulated nuclear protein that functions in an Arabidopsis PHYB signal transduction pathway. Plant Cell 13: 1293–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luhtala N., Odorizzi G. (2004). Bro1 coordinates deubiquitination in the multivesicular body pathway by recruiting Doa4 to endosomes. J. Cell Biol. 166: 717–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGurn J.A., Hsu P.C., Emr S.D. (2012). Ubiquitin and membrane protein turnover: From cradle to grave. Annu. Rev. Biochem. 81: 231–259. [DOI] [PubMed] [Google Scholar]
- Mageswaran S.K., Dixon M.G., Curtiss M., Keener J.P., Babst M. (2014). Binding to any ESCRT can mediate ubiquitin-independent cargo sorting. Traffic 15: 212–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNatt M.W., McKittrick I., West M., Odorizzi G. (2007). Direct binding to Rsp5 mediates ubiquitin-independent sorting of Sna3 via the multivesicular body pathway. Mol. Biol. Cell 18: 697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlot S., Mustilli A.C., Genty B., North H., Lefebvre V., Sotta B., Vavasseur A., Giraudat J. (2002). Use of infrared thermal imaging to isolate Arabidopsis mutants defective in stomatal regulation. Plant J. 30: 601–609. [DOI] [PubMed] [Google Scholar]
- Merlot S., Leonhardt N., Fenzi F., Valon C., Costa M., Piette L., Vavasseur A., Genty B., Boivin K., Müller A., Giraudat J., Leung J. (2007). Constitutive activation of a plasma membrane H(+)-ATPase prevents abscisic acid-mediated stomatal closure. EMBO J. 26: 3216–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missotten M., Nichols A., Rieger K., Sadoul R. (1999). Alix, a novel mouse protein undergoing calcium-dependent interaction with the apoptosis-linked-gene 2 (ALG-2) protein. Cell Death Differ. 6: 124–129. [DOI] [PubMed] [Google Scholar]
- Munemasa S., Hauser F., Park J., Waadt R., Brandt B., Schroeder J.I. (2015). Mechanisms of abscisic acid-mediated control of stomatal aperture. Curr. Opin. Plant Biol. 28: 154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T., Skoog F. (1962). A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 15: 473–497. [Google Scholar]
- Nakashima K., Yamaguchi-Shinozaki K. (2013). ABA signaling in stress-response and seed development. Plant Cell Rep. 32: 959–970. [DOI] [PubMed] [Google Scholar]
- Nickerson D.P., Russell M.R., Odorizzi G. (2007). A concentric circle model of multivesicular body cargo sorting. EMBO Rep. 8: 644–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odorizzi G. (2006). The multiple personalities of Alix. J. Cell Sci. 119: 3025–3032. [DOI] [PubMed] [Google Scholar]
- Paez Valencia J., Goodman K., Otegui M.S. (2016). Endocytosis and endosomal trafficking in plants. Annu. Rev. Plant Biol. 67: 309–335. [DOI] [PubMed] [Google Scholar]
- Pashkova N., Gakhar L., Winistorfer S.C., Sunshine A.B., Rich M., Dunham M.J., Yu L., Piper R.C. (2013). The yeast Alix homolog Bro1 functions as a ubiquitin receptor for protein sorting into multivesicular endosomes. Dev. Cell 25: 520–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzio G.A., Rodriguez L., Antoni R., Gonzalez-Guzman M., Yunta C., Merilo E., Kollist H., Albert A., Rodriguez P.L. (2013). The PYL4 A194T mutant uncovers a key role of PYR1-LIKE4/PROTEIN PHOSPHATASE 2CA interaction for abscisic acid signaling and plant drought resistance. Plant Physiol. 163: 441–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puga M.I., et al. (2014). SPX1 is a phosphate-dependent inhibitor of phosphate starvation response 1 in Arabidopsis. Proc. Natl. Acad. Sci. USA 111: 14947–14952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reggiori F., Pelham H.R. (2001). Sorting of proteins into multivesicular bodies: ubiquitin-dependent and -independent targeting. EMBO J. 20: 5176–5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes F.C., Buono R.A., Roschzttardtz H., Di Rubbo S., Yeun L.H., Russinova E., Otegui M.S. (2014). A novel endosomal sorting complex required for transport (ESCRT) component in Arabidopsis thaliana controls cell expansion and development. J. Biol. Chem. 289: 4980–4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson L.G., Howard A.S., Khuu N., Gidda S.K., McCartney A., Morphy B.J., Mullen R.T. (2011). Protein-protein interaction network and subcellular localization of the Arabidopsis thaliana ESCRT machinery. Front. Plant Sci. 2: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez L., et al. (2014). C2-domain abscisic acid-related proteins mediate the interaction of PYR/PYL/RCAR abscisic acid receptors with the plasma membrane and regulate abscisic acid sensitivity in Arabidopsis. Plant Cell 26: 4802–4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio S., Rodrigues A., Saez A., Dizon M.B., Galle A., Kim T.H., Santiago J., Flexas J., Schroeder J.I., Rodriguez P.L. (2009). Triple loss of function of protein phosphatases type 2C leads to partial constitutive response to endogenous abscisic acid. Plant Physiol. 150: 1345–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez A., Robert N., Maktabi M.H., Schroeder J.I., Serrano R., Rodriguez P.L. (2006). Enhancement of abscisic acid sensitivity and reduction of water consumption in Arabidopsis by combined inactivation of the protein phosphatases type 2C ABI1 and HAB1. Plant Physiol. 141: 1389–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santelia D., Lawson T. (2016). Rethinking guard cell metabolism. Plant Physiol. 172: 1371–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuring D., Künzl F., Viotti C., Yan M.S., Jiang L., Schellmann S., Robinson D.G., Pimpl P. (2012). Ubiquitin initiates sorting of Golgi and plasma membrane proteins into the vacuolar degradation pathway. BMC Plant Biol. 12: 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J., Gao C., Zhao Q., Lin Y., Wang X., Zhuang X., Jiang L. (2016). AtBRO1 functions in ESCRT-I complex to regulate multivesicular body protein sorting. Mol. Plant 9: 760–763. [DOI] [PubMed] [Google Scholar]
- Shen J., Zhao Q., Wang X., Gao C., Zhu Y., Zeng Y., Jiang L. (2018). A plant Bro1 domain protein BRAF regulates multivesicular body biogenesis and membrane protein homeostasis. Nat. Commun. 9: 3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields S.B., Piper R.C. (2011). How ubiquitin functions with ESCRTs. Traffic 12: 1306–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkes I.A., Runions J., Kearns A., Hawes C. (2006). Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nat. Protoc. 1: 2019–2025. [DOI] [PubMed] [Google Scholar]
- Spitzer C., Schellmann S., Sabovljevic A., Shahriari M., Keshavaiah C., Bechtold N., Herzog M., Müller S., Hanisch F.G., Hülskamp M. (2006). The Arabidopsis elch mutant reveals functions of an ESCRT component in cytokinesis. Development 133: 4679–4689. [DOI] [PubMed] [Google Scholar]
- Squire G.R., Mansfield T.A. (1972). Studies of the mechanism of action of fusicoccin, the fungal toxin that induces wilting, and its interaction with abscisic acid. Planta 105: 71–78. [DOI] [PubMed] [Google Scholar]
- Swaminathan S., Amerik A.Y., Hochstrasser M. (1999). The Doa4 deubiquitinating enzyme is required for ubiquitin homeostasis in yeast. Mol. Biol. Cell 10: 2583–2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To A., Valon C., Savino G., Guilleminot J., Devic M., Giraudat J., Parcy F. (2006). A network of local and redundant gene regulation governs Arabidopsis seed maturation. Plant Cell 18: 1642–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voinnet O. (2003). RNA silencing bridging the gaps in wheat extracts. Trends Plant Sci. 8: 307–309. [DOI] [PubMed] [Google Scholar]
- Ward J.J., Sodhi J.S., McGuffin L.J., Buxton B.F., Jones D.T. (2004). Prediction and functional analysis of native disorder in proteins from the three kingdoms of life. J. Mol. Biol. 337: 635–645. [DOI] [PubMed] [Google Scholar]
- Wemmer M., Azmi I., West M., Davies B., Katzmann D., Odorizzi G. (2011). Bro1 binding to Snf7 regulates ESCRT-III membrane scission activity in yeast. J. Cell Biol. 192: 295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter V., Hauser M.T. (2006). Exploring the ESCRTing machinery in eukaryotes. Trends Plant Sci. 11: 115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright M.H., Berlin I., Nash P.D. (2011). Regulation of endocytic sorting by ESCRT-DUB-mediated deubiquitination. Cell Biochem. Biophys. 60: 39–46. [DOI] [PubMed] [Google Scholar]
- Yu F., Lou L., Tian M., Li Q., Ding Y., Cao X., Wu Y., Belda-Palazon B., Rodriguez P.L., Yang S., Xie Q. (2016). ESCRT-I component VPS23A affects ABA signaling by recognizing ABA receptors for endosomal degradation. Mol. Plant 9: 1570–1582. [DOI] [PubMed] [Google Scholar]