Abstract
Introduction
Blood cultures are of limited utility in non-severe community-acquired pneumonia, though routinely recommended for severe community-acquired pneumonia or healthcare-associated pneumonia, due to perceived greater bacteremia risk, particularly with multidrug resistant organisms. The utility of this practice is unknown.
Methods
In this observational cohort study, we abstracted data from medical records for consecutive hospitalizations for pneumonia by adults to an academic medical center from 2014–2015. The primary outcomes included bacteremia, multidrug resistant organism bacteremia, and appropriate management changes attributed to culture results, stratified by pneumonia classification (non-severe community-acquired pneumonia, severe community-acquired pneumonia, or healthcare-associated pneumonia) and likelihood the bacteremia was due to pneumonia versus another infection. We assessed the diagnostic test performance of ≥1 guideline-defined risk factors for bacteremia in non-severe community-acquired pneumonia, for whom cultures are routinely recommended.
Results
Of 456 pneumonia hospitalizations, 30 (6.6%) had bacteremia, with a greater incidence in severe community-acquired pneumonia (14.7%) than non-severe community-acquired pneumonia (7.8%) and healthcare-associated pneumonia (6.6%; p=0.12). Seventeen bacteremia cases were likely due to pneumonia (3.7%). Only 2 (0.4%) had multidrug resistant organisms (both healthcare-associated pneumonia), 1 of whom was due to pneumonia. Appropriate management changes occurred in 8 cases (1.8%; 7 de-escalation and 1 escalation of antibiotics); only 1 with bacteremia likely due to pneumonia (de-escalation). The one case of appropriate antibiotic escalation occurred in a patient with vancomycin-resistant enterococcus unrelated to pneumonia. Having ≥1 guideline-defined risk factors did not identify bacteremia in non-severe community- acquired pneumonia (positive likelihood ratio, 1.10, 95% CI, 0.61–1.99).
Conclusion
Routine blood cultures in pneumonia have extremely low yield and utility irrespective of severity and risk.
Keywords: Hospital Medicine, Pneumonia, Blood Culture, Guideline, Incidence, Diagnostic Test
INTRODUCTION
Pneumonia is a common but heterogeneous disease process with varying risks for resistant pathogens. The Infectious Diseases Society of America (IDSA) and the American Thoracic Society (ATS) have recommended differing initial diagnostic strategies based on pneumonia severity or classification (community-acquired versus healthcare-associated).1,2 One diagnostic approach in pneumonia is the acquisition of blood cultures. Routine blood culture collection was formerly recommended by the IDSA/ATS guidelines issued in 2000 and by the Centers for Medicare and Medicaid Services (CMS) as a core performance measure for community-acquired pneumonia,1,3–5 which led to a rapid rise in obtaining blood cultures in patients hospitalized with pneumonia or suspected pneumonia.6,7 However, the diagnostic yield of routine cultures in community-acquired pneumonia is low (incidence ranges from 0 to 14% across 13 studies) and their utility in identifying resistant pathogens that would warrant broadening antibiotic therapy is even lower (fewer than 1% of patients).8–10 While routine blood cultures in community-acquired pneumonia are now considered low value and no longer recommended by current IDSA/ATS guidelines and CMS, the role of blood cultures in severe community-acquired pneumonia and in healthcare-associated pneumonia is uncertain.
Current IDSA/ATS guidelines recommend obtaining routine blood cultures in severe community-acquired pneumonia, or in non-severe community-acquired pneumonia in the presence of at least one of the following risk factors: intensive care unit admission, cavitary infiltrates, white blood cell count < 4000 cells per microliter, active alcohol use A disorder, chronic severe liver disease, or pleural effusion.1 These recommendations are supported by expert consensus with limited evidence that these explicitly defined risk factors are associated with greater bacteremia risk and that culture data would warrant appropriate management change.11,12 With respect to healthcare-associated pneumonia, there is no consensus whether blood cultures should be routinely collected. While patients with healthcare-associated pneumonia are theorized to be at greater risk for bacteremia with a multidrug-resistant organism, prior healthcare exposure does not accurately identify resistant pathogens.13–15
We designed this study to examine bacteremia incidence in pneumonia by severity and risk (non-severe community-acquired pneumonia, severe community-acquired pneumonia, and healthcare-associated pneumonia), including the incidence of multidrug resistant organisms and whether the culture result led to appropriate management changes. We also evaluated the diagnostic test performance of the aforementioned IDSA/ATS risk factors for identifying bacteremia in non-severe community-acquired pneumonia.
METHODS
Study Design and Subjects
We conducted a retrospective observational cohort study of consecutive adult patients hospitalized for pneumonia to a large, academic medical center in an urban metropolitan area in Texas, from August 2014 to July 2015. Pneumonia hospitalizations were identified by the presence of 1 of following 3 definitions: 1) pneumonia coded as the primary discharge diagnosis (ICD-9-CM 480.XX, 481, 482.XX, 483.X, 485, 486, 487.0); 2) sepsis coded as the primary discharge diagnosis (038, 995.92, 995.91, 785.52) and pneumonia was coded as a secondary diagnosis; and 3) respiratory failure coded as the primary discharge diagnosis (518.81, 518.82, 518.84, 799.1) and pneumonia was coded as a secondary diagnosis16.
We performed chart review to ascertain clinical characteristics upon admission, including demographics, laboratory values, vital signs, imaging findings, IDSA/ATS risk factors for bacteremia, and necessary characteristics to classify pneumonia as severe community-acquired pneumonia or healthcare-associated pneumonia per IDSA/ATS guidelines. Laboratory values and vital signs were defined as the most abnormal value within the first 48 hours of admission. Chest roentgenogram reports were reviewed within 48 hours of admission. healthcare-associated pneumonia was defined as the presence of prior admission within 90 days, nursing home residency, or receipt of active chemotherapy, home infusion therapy, or hemodialysis prior to admission. Severe community-acquired pneumonia was defined as the presence of 3 minor criteria including respiratory rate >30 breaths/minute, PaO2/FiO2 ratio < 250, multi-lobar infiltrates, confusion on admission, blood urea nitrogen >20 mg/dL, white blood cell count < 4000 per microliter, platelets < 100,000 per microliter, core temperature < 36° Celsius, or one major criteria, including use of vasopressors or mechanical ventilation. IDSA/ATS risk factors for which routine blood cultures are recommended in non-severe community-acquired pneumonia include intensive care unit admission, cavitary infiltrates, WBC < 4000 per microliter, active alcohol use disorder, chronic severe liver disease, and pleural effusion. The medical records of patients with positive blood cultures were independently reviewed by two clinicians, a pulmonary and critical care fellow (DZ) and an internal medicine resident (DY), and were stratified by the likelihood that the bacteremia was due to pneumonia. Bacteremia was classified as unlikely and likely pneumonia-related based on the presence or absence of alternate active infections that could better account for the bacteremia, and whether microbiology results matched between blood and other sources such as urine and sputum. Disagreements were adjudicated by negotiated consensus.
Outcomes
The primary outcomes were incidences of true-positive blood cultures (herein, bacteremia) within 48 hours of admission, bacteremia with a multidrug resistant organism,17 and appropriate management changes among patients with bacteremia. Contaminant blood cultures were ascertained by chart review of clinical documentation by infectious disease physicians and the primary treatment team. Appropriate management change was defined as escalation or de-escalation of antibiotics based on pathogen coverage, and was ascertained by reviewing pathogen resistance profile, antibiotics administered, and documentation of clinical decision making. If patients were not initially prescribed appropriate empiric antibiotics, then deescalating to an antibiotic with narrower coverage based on culture results was not considered an appropriate management change (e.g. a patient with non-severe community-acquired pneumonia prescribed vancomycin and piperacillin-tazobactam but narrowed to a respiratory fluoroquinolone after a positive blood culture resulted). Patients without a blood culture during the first 48 hours of admission were assumed to not have bacteremia if a subsequent culture drawn later during admission had no growth or if there was no evidence of bacteremia 30 days following hospital discharge.
Statistical Analysis
We used descriptive analyses to compare bacteremia incidence and appropriate management changes by community-acquired pneumonia, severe community-acquired pneumonia, and healthcare-associated pneumonia, and separately by likelihood the bacteremia was due to pneumonia. We estimated the diagnostic test performance of the presence of ≥1 and ≥2 IDSA/ATS risk factors for bacteremia. We repeated analyses for the most prevalent individual risk factors.
RESULTS
Bacteremia Incidence
Of 517 consecutive hospitalizations with pneumonia, we included 456 (Figure 1). Of these, 434 (95%) had a blood culture result, 30 of whom (6.6%) had bacteremia and 6 (1.3%) had a contaminated culture result. Over half (n=256, 56%) had healthcare-associated pneumonia and 200 (44%) had community-acquired pneumonia, of which 36 (18%) had severe community-acquired pneumonia. Bacteremia incidence was greatest for severe community-acquired pneumonia (13.8%) than for non-severe community-acquired pneumonia (7.9%) and healthcare-associated pneumonia (5.1%), but this difference was not statistically significant (p = 0.12; Table 1).
Figure 1.
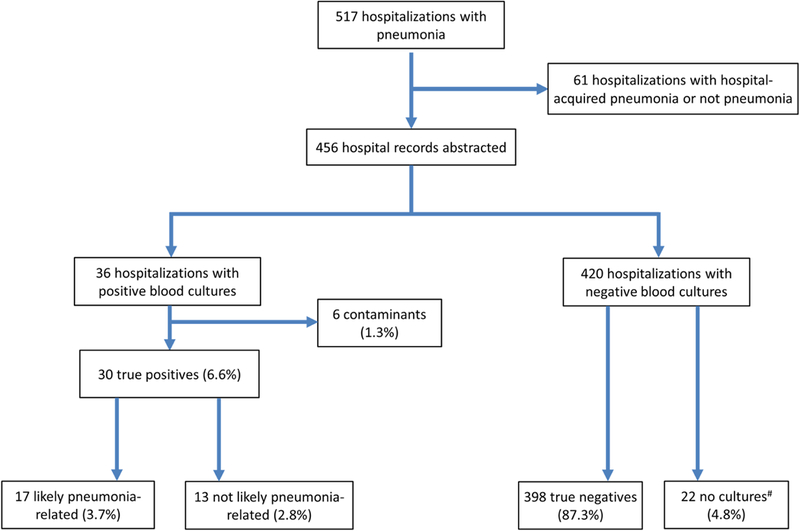
Study Flow Diagram
#All 22 patients without cultures collected did not have subsequent positive blood cultures or hospitalizations for bacteremia within 30 days of hospital discharge.
Table 1.
Incidence of Positive Blood Cultures and Resultant Management Changes by Pneumonia Type
| True-positive blood culture |
Change in management |
|||||
|---|---|---|---|---|---|---|
| Pneumonia classification | All* | Likely pneumonia- related |
Contamina nt |
Escalatio n |
De- escalatio n |
Appropri ate |
| All pneumonia (n = 456) | 30 (6.6%) | 17 (3.7%) | 6 (1.3%) | 1 (0.2%) | 15 (3.3%) | 8 (1.8%) |
| All CAP (n = 200) | 17 (8.5%) | 11 (5.5%) | 2 (1.0%) | 0 | 8 (4.0%) | 2 (1.0%) |
| Non-severe CAP (n= 164) | 12 (7.9%) | 8 (4.9%) | 1 (0.6%) | 0 | 6 (3.7%) | 1 (0.6%) |
| Severe CAP (n =36) | 5 (13.8%) | 3 (8.3%) | 1 (2.8%) | 0 | 2 (5.6%) | 1 (2.8%) |
| HCAP (n = 256) | 13 (5.1%) | 6 (2.3%) | 4 (1.5%) | 1 (0.4%) | 7 (2.7%) | 6 (2.3%) |
| Prior hospital (n =160) | 9 (5.6%) | 2 (1.3%) | 2 (1.2%) | 1 (0.6%) | 6 (3.7%) | 5 (3.1%) |
| NH/LTAC (n = 45) | 4 (8.9%) | 3 (6.7%) | 1 (2.2%) | 0 | 0 | 0 |
Abbreviations: CAP, community-acquired pneumonia; HCAP, healthcare-associated pneumonia; NH, nursing home resident; LTAC, long-term acute care hospital
Of the 30 bacteremia cases, 17 were likely pneumonia-related and 13 were unlikely pneumonia-related. Agreement was 100% between reviewers. Alternate infections existed in all 13 unlikely pneumonia-related cases that better explained the bacteremia, including urinary tract infection (n=5), cholecystitis (n=1), spontaneous bacterial peritonitis (n=1), dental infections (n=1), among other infections. Bacteremia incidence among likely pneumonia-related cases were also greatest for severe community- acquired pneumonia (8.3%) than non-severe community-acquired pneumonia (5.5%) and healthcare-associated pneumonia (2.3%; p=0.13).
Incidence of Bacteremia with a Multidrug Resistant Organism
Only two patients had bacteremia with a multidrug resistant organism (6.7% of patients with bacteremia and 0.4% of the overall cohort). Pseudomonas was identified in three patients but none were multidrug resistant. Of the two cases with a multidrug resistant organism, one had methicillin-resistant Staphylococcus aureus (MRSA) and the other had vancomycin-resistant enterococci (VRE). Both patients had healthcare-associated pneumonia. The MRSA bacteremia was considered likely pneumonia-related, however, the patient with VRE bacteremia was unlikely pneumonia-related since there was a discordance between the sputum culture, which grew Pseudomonas, and the blood and urine cultures, which both grew VRE.
Appropriate Management Changes due to Bacteremia
Of the 30 bacteremia cases, any management change occurred in 16, but were deemed appropriate for only 8 patients (1.8% of the overall cohort; Table 1). All appropriate management changes, except for one instance, were de-escalation from empiric broad spectrum antimicrobial coverage to targeted antibiotic therapy. Appropriate management changes occurred in 6 patients with healthcare-associated pneumonia and 2 patients with community-acquired pneumonia. Among the remaining 8 patients with a management change, changes were not appropriate because the initial empiric antibiotic regimen was too broad and covered for resistant organisms such as MRSA and Pseudomonas without indicated risk factors. Among the 17 bacteremia cases likely due to pneumonia, an appropriate management change occurred in only 1 patient (de-escalation).
The one instance of appropriate escalation of antibiotic therapy occurred in the aforementioned patient with VRE bacteremia that was unlikely related to pneumonia, who was started on daptomycin after the culture resulted. The patient with MRSA bacteremia did not experience an appropriate management change since the patient was already appropriately receiving vancomycin and the patient subsequently chose comfort care with a discontinuation of antibiotics.
Antibiotic duration increased in 15 out of 30 patients with bacteremia. Among the 17 patients with bacteremia likely related to pneumonia, 6 (40%) had their antibiotic duration extended, almost always to 14 days.
Diagnostic Test Performance of IDSA/ATS Risk Factors for Community-Acquired Pneumonia
Among 164 patients with non-severe community-acquired pneumonia, 75 (45.7%) had >1 IDSA/ATS risk factor (Table 2), the most common being pleural effusion, intensive care unit admission, liver disease, and alcohol use disorder. The presence of ≥1 risk factor did not meaningfully rule in or rule out patients at risk for bacteremia since both the positive and negative likelihood ratios were near 1. The 95% confidence intervals suggest that ≥1 risk factor is at best, moderately useful to predict bacteremia. However, ≥2 IDSA/ATS risk factors, which applied to 11% of the non-severe community-acquired pneumonia population, had a better positive likelihood ratio (2.53, 95% CI, 0.62–10.27), but with a positive predictive value of only 16.7%. Pleural effusion and intensive care unit stay were not robust individual risk for bacteremia. Alcohol use disorder or liver disease suggested greater likelihood of bacteremia, but confidence intervals were wide owing to small sample sizes. Negative predictive values for all risk factors were high due to the low incidence of bacteremia.
Table 2.
Diagnostic Test Performance of IDSA/ATS Risk Factors for Non-Severe CAP*
| IDSA Risk factors |
N | Bacteremia | LR+ [95% CI] | LR- [95% CI] | PPV | NPV |
|---|---|---|---|---|---|---|
| ≥ 1 | 57 | 6 (10.5%) | 1.49 [0.81 to 2.74] | 0.75 [0.42 to 1.34] | 10.5% | 94.4% |
| ≥ 2 | 12 | 2 (16.7%) | 2.53 [0.62 to 10.27] | 0.89 [0.69 to 1.15] | 16.7% | 93.4% |
| Selected factors | ||||||
| Pleural effusion | 19 | 1 (5.3%) | 0.70 [0.10 to 4.83] | 1.04 [0.87 to 1.25] | 5.3% | 92.4% |
| ICU stay | 15 | 1 (6.7%) | 0.90 [0.13 to 6.31] | 1.01 [0.85 to 1.21] | 6.7% | 92.6% |
| Liver disease | 7 | 1 (14.3%) | 2.11 [0.28 to 16.14] | 0.95 [0.80 to 1.14] | 14.3% | 92.9% |
| AUD | 4 | 3 (75%) | 12.67 [5.42 to 29.62] | 0.27 [0.05 to 1.45] | 25.0% | 99.3% |
Abbreviations: AUD, alcohol use disorder; ICU, intensive-care unit; LR+, positive likelihood ratio; LR-, negative likelihood ratio; PPV, positive predictive value; NPV, negative predictive value
164 patients had non-severe CAP, 12 of whom had bacteremia
We were unable to estimate the diagnostic test performance of risk factors for cultures leading to appropriate management changes, which is the most clinically meaningful outcome, since this occurred so infrequently.
DISCUSSION
We extend the evidence base for the role and utility of routine blood cultures in the management of adults hospitalized with severe community-acquired pneumonia or healthcare-associated pneumonia. While patients with severe community-acquired pneumonia had double the incidence of bacteremia compared to non-severe community-acquired pneumonia, both groups rarely experienced appropriate management changes (≤2%), most of which were de-escalation of antibiotics. Compared to non-severe community-acquired pneumonia, patients hospitalized with healthcare-associated pneumonia had a higher incidence of bacteremia with a multidrug resistant organism, but the overall incidence was still very low and did not meaningfully change management. Lastly, for community-acquired pneumonia, the presence of at least 1 IDSA/ATS risk factor did not identify patients at greater risk of bacteremia. Taken together, our findings suggest that blood cultures are also of very limited value to guide antibiotic management among patients hospitalized with severe community-acquired pneumonia or healthcare-associated pneumonia, and if confirmed in other cohorts, should no longer be routinely recommended.
Irrespective of pneumonia severity and risk, hospitalized patients with pneumonia had a very low incidence of bacteremia with a multidrug resistant organism and consequently, extremely few patients had appropriate management changes. When appropriate management changes occurred, changes were nearly all de-escalation of the antibiotic regimen. The single instance of antibiotic escalation occurred in a patient with healthcare-associated pneumonia with bacteremia unrelated to pneumonia. Furthermore, only 1 patient in our entire cohort of 456 consecutive pneumonia hospitalizations over a one year time period had bacteremia attributed to pneumonia with an appropriate de-escalation in antibiotic therapy. The most common management change attributed to the blood culture was extending the antibiotic duration to 14 days, regardless of the organism. In the absence of bacteremia with organisms known to result in multisystem complications, such as MRSA,18 the management of bacteremia due to pneumonia is less clear. Shorter duration of antibiotics are equivalent or better for a number of infections among hospitalized patients,21–23 and treating patients with pneumonia until they achieve clinical stability has shown to be effective24 rather than treating for predefined periods of time. Furthermore, unnecessarily prolonged courses of antibiotics can cause numerous complications such as C. difficile diarrhea and antibiotic resistance.19,20
Our findings do not support current IDSA/ATS guidelines to routinely obtain blood cultures for patients hospitalized with non-severe community-acquired pneumonia who have at least one IDSA/ATS risk factor. The presence of a single IDSA/ATS risk factor did not rule in or rule out bacteremia. Even though the presence of two or more IDSA/ATS risk factors was more predictive of bacteremia, given how rare culture results will appropriately inform management changes, blood cultures may only be useful in patients initiated on broad spectrum antibiotics since the results may lead to narrowing of the antibiotic regimen.
Our study was notable for several strengths. First, the vast majority of patients (95%) had a blood culture collected. Though blood cultures were not standard of care for all patients hospitalized with pneumonia, our practice setting permitted nearly universal ascertainment of bacteremia. Second, our cohort had similar bacteremia incidence for community-acquired pneumonia as in previous studies,8,12 as well as a very low rate of bacteremia with a multidrug resistant organism. This implies that our findings are generalizable to non-tertiary care settings. Third, we included consecutive patients hospitalized for pneumonia without exclusions for illness severity or risk factors, such as septic shock or immunocompromised states. Fourth, the contamination rate of blood cultures in our study was lower than previously described, making blood culture interpretation easier for this study.
Our study had certain limitations. First, our cohort was selected from a single academic center in north Texas, so our findings may not be generalizable to other regions with potentially different antibiotic resistance patterns. However, as mentioned above, the bacteremia rate was similar to other cohorts. Second, we identified patients hospitalized with pneumonia using ICD-9-CM discharge diagnoses and classified risk and type of pneumonia by chart review. However, discharge diagnosis codes are an accurate and validated method to identify patients with pneumonia, and any potential misclassification of pneumonia severity and risk would not affect the overall low incidence of bacteremia and appropriate management changes.25,26 Furthermore, we performed structured chart review by two independent reviewers to ascertain the likelihood of pneumonia for patients with bacteremia to ensure that the bacteremia was likely attributable to the pneumonia. Third, we did not ascertain if patients received antibiotic therapy prior to hospitalization. However, previous studies have reported mixed effects of prior antibiotics on bacteremia risk in pneumonia.12,27 Fourth, we were unable to examine the diagnostic test performance for each individual IDSA/ATS risk factor due to low prevalence for certain ones. Lastly, we may have undercoded certain risk factors that may not have been documented in the medical records (i.e. active alcohol use disorder) compared to risk factors with more discrete and objective documentation, such as pleural effusion and intensive care unit admission. Thus, our reported test performance for alcohol use disorder should be cautiously interpreted.
Routine blood cultures are very low yield diagnostic tests with poor utility in patients hospitalized with pneumonia across the spectrum of different healthcare exposures, clinical risk factors, and severity. Not a single patient hospitalized with pneumonia in our cohort, including those with severe community-acquired pneumonia and healthcare-associated pneumonia, had an appropriate escalation of their antibiotic regimen for bacteremia related to pneumonia. While further studies are warranted to confirm our findings in other regions and in community hospital settings, our findings suggest that routine blood culture collection should not be recommended in severe community-acquired pneumonia or healthcare-associated pneumonia, and that current IDSA/ATS guideline recommendations for routine blood culture collection in community-acquired pneumonia with at least one risk factor should be revisited.
CLINICAL SIGNIFICANCE.
Blood culture results rarely change clinical management in adults hospitalized with pneumonia, including for those with severe community-acquired pneumonia or healthcare-associated pneumonia.
The presence of at least 1 guideline-defined risk factor did not meaningful predict the incidence of bacteremia
Routine blood cultures in pneumonia have extremely low yield and utility irrespective of severity and risk.
ACKNOWLEDGEMENTS
Funding: Dr. Makam was supported by the National Institute on Aging (K23AG052603). The study sponsors had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Disclosure and Conflict of Interest: The authors have no conflicts of interest to disclose, financial or otherwise.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007; 44 Suppl 2: S27–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Thoracic S, Infectious Diseases Society of A. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare- associated pneumonia. Am J Respir Crit Care Med 2005; 171(4): 388–416. [DOI] [PubMed] [Google Scholar]
- 3.Fee C, Weber E, Sharpe BA, Nguy M, Quon T, Bookwalter T. JCAHO/CMS core measures for community-acquired pneumonia. Ann Emerg Med 2006; 47(5): 505; author reply 6. [DOI] [PubMed] [Google Scholar]
- 4.Fee C, Weber EJ, Sharpe BA, Quon T. When is a scarlet letter really a red badge of courage?: the paradox of percentage of pneumonia patients receiving antibiotics within 4 hours in accordance with JCAHO and CMS core measures. Ann Emerg Med 2007; 50(2): 205–6. [DOI] [PubMed] [Google Scholar]
- 5.Nicks BA, Manthey DE, Fitch MT. The Centers for Medicare and Medicaid Services (CMS) community-acquired pneumonia core measures lead to unnecessary antibiotic administration by emergency physicians. Acad Emerg Med 2009; 16(2): 184–7. [DOI] [PubMed] [Google Scholar]
- 6.Makam AN, Auerbach AD, Steinman MA. Blood culture use in the emergency department in patients hospitalized for community-acquired pneumonia. JAMA Intern Med 2014; 174(5): 803–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Makam AN, Auerbach AD, Steinman MA. Blood culture use in the emergency department in patients hospitalized with respiratory symptoms due to a nonpneumonia illness. J Hosp Med 2014; 9(8): 521–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Afshar N, Tabas J, Afshar K, Silbergleit R. Blood cultures for community-acquired pneumonia: are they worthy of two quality measures? A systematic review. J Hosp Med 2009; 4(2): 112–23. [DOI] [PubMed] [Google Scholar]
- 9.Chalasani NP, Valdecanas MA, Gopal AK, McGowan JE, Jr., Jurado RL. Clinical utility of blood cultures in adult patients with community-acquired pneumonia without defined underlying risks. Chest 1995; 108(4): 932–6. [DOI] [PubMed] [Google Scholar]
- 10.Campbell SG, Marrie TJ, Anstey R, Ackroyd-Stolarz S, Dickinson G. Utility of blood cultures in the management of adults with community acquired pneumonia discharged from the emergency department. Emerg Med J 2003; 20(6): 521–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Werkhoven CH, Huijts SM, Postma DF, Oosterheert JJ, Bonten MJ. Predictors of Bacteraemia in Patients with Suspected Community-Acquired Pneumonia. PLoS One 2015; 10(11): e0143817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Metersky ML, Ma A, Bratzler DW, Houck PM. Predicting bacteremia in patients with community-acquired pneumonia. Am J Respir Crit Care Med 2004; 169(3): 342–7. [DOI] [PubMed] [Google Scholar]
- 13.Shorr AF, Zilberberg MD, Reichley R, et al. Validation of a clinical score for assessing the risk of resistant pathogens in patients with pneumonia presenting to the emergency department. Clin Infect Dis 2012; 54(2): 193–8. [DOI] [PubMed] [Google Scholar]
- 14.El-Solh AA, Sikka P, Ramadan F, Davies J. Etiology of severe pneumonia in the very elderly. Am J Respir Crit Care Med 2001; 163(3 Pt 1): 645–51. [DOI] [PubMed] [Google Scholar]
- 15.Chalmers JD, Rother C, Salih W, Ewig S. Healthcare-associated pneumonia does not accurately identify potentially resistant pathogens: a systematic review and meta-analysis. Clin Infect Dis 2014; 58(3): 330–9. [DOI] [PubMed] [Google Scholar]
- 16.Lindenauer PK, Lagu T, Shieh MS, Pekow PS, Rothberg MB. Association of diagnostic coding with trends in hospitalizations and mortality of patients with pneumonia, 2003–2009. JAMA 2012; 307(13): 1405–13. [DOI] [PubMed] [Google Scholar]
- 17.Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012; 18(3): 268–81. [DOI] [PubMed] [Google Scholar]
- 18.Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011; 52(3): e18–55. [DOI] [PubMed] [Google Scholar]
- 19.Llor C, Bjerrum L. Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Ther Adv Drug Saf 2014; 5(6): 229–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dingle KE, Didelot X, Quan TP, et al. Effects of control interventions on Clostridium difficile infection in England: an observational study. Lancet Infect Dis 2017; 17(4): 411–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Havey TC, Fowler RA, Daneman N. Duration of antibiotic therapy for bacteremia: a systematic review and meta-analysis. Crit Care 2011; 15(6): R267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spellberg B The Maturing Antibiotic Mantra: “Shorter Is Still Better”. J Hosp Med 2018; 13(5): 361 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Royer S, DeMerle KM, Dickson RP, Prescott HC. Shorter Versus Longer Courses of Antibiotics for Infection in Hospitalized Patients: A Systematic Review and Meta-Analysis. J Hosp Med 2018; 13(5): 336–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uranga A, Espana PP, Bilbao A, et al. Duration of Antibiotic Treatment in Community-Acquired Pneumonia: A Multicenter Randomized Clinical Trial. JAMA Intern Med 2016; 176(9): 1257–65. [DOI] [PubMed] [Google Scholar]
- 25.Drahos J, Vanwormer JJ, Greenlee RT, Landgren O, Koshiol J. Accuracy of ICD- 9-CM codes in identifying infections of pneumonia and herpes simplex virus in administrative data. Ann Epidemiol 2013; 23(5): 291–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mukhopadhyay A, Maliapen M, Ong V, et al. Community-Acquired Pneumonia Case Validation in an Anonymized Electronic Medical Record-Linked Expert System. Clin Infect Dis 2017; 64(suppl_2): S141–S4. [DOI] [PubMed] [Google Scholar]
- 27.Ruhe JJ, Hasbun R. Streptococcus pneumoniae bacteremia: duration of previous antibiotic use and association with penicillin resistance. Clin Infect Dis 2003; 36(9): 1132–8. [DOI] [PubMed] [Google Scholar]


