Abstract
Background.
Human immunodeficiency virus (HIV) prevention interventions for prevention interventions for women include screening, partner notification, promoting condoms, and preexposure prophylaxis (PrEP). Women’s risk of acquiring HIV can help guide recommendations.
Methods.
We used data from Louisiana’s sexually transmitted infection (STI) and HIV registries to study 13- to 59-year-old women following first diagnosis of syphilis, gonorrhea, or chlamydia during 2000–2015. We measured HIV rates reported subsequent to STI (through 2016). Rates for women without STI were estimated by subtracting women with STI from reported cases and from Census estimates for the population. PrEP cost was estimated as $11 000 per year, and effectiveness estimated as 100%.
Results.
STIs were syphilis (6574), gonorrhea (64 995), or chlamydia (140 034). These 211 603 women had 1 865 488 person-years of follow-up and 969 HIV diagnoses. Women with no STI had 5186 HIV diagnoses over 24 359 397 person-years. HIV rates diagnosis (per 100 000 person-years) were higher for women after syphilis (177.3), gonorrhea (73.2), or chlamydia (35.4) compared to women with no STI (22.4). Providing PrEP to all women diagnosed with syphilis or gonorrhea would cost $7 371 111 000 and could have prevented 546 HIV diagnoses. Limiting PrEP to 1 year after syphilis or gonorrhea diagnosis would cost $963 847 334, but only 143 HIV diagnoses were within 2 years after a syphilis or gonorrhea diagnosis.
Conclusions.
Rates of HIV diagnosis were high after women had STI, but not high enough to make PrEP cost-effective for them. Most women diagnosed with HIV did not have previously reported STI.
Keywords: women, STI, HIV, prevention, PrEP
Many interventions are effective for preventing human immunodeficiency virus (HIV) infection including: counseling, HIV testing, condom use, treatment of infected partners, postexposure prophylaxis, and preexposure prophylaxis (PrEP) [1–9]. The costs of these interventions range from a few dollars to thousands of dollars per year. Costly interventions may be appropriate for women at high risk because untreated HIV infection causes high rates of disease and treatment is expensive [9].
Several studies have identified groups of women in Africa who were at high risk for acquiring HIV [10–12]. In the United States, many studies have identified women with high relative risks for having HIV. However, prospectively identifying women who are likely to acquire HIV infection has been much more difficult because the absolute risk for women has been low [13–20]. In 2017, the US Public Health Service updated clinical practice guidance for PrEP usage [21]. The guidance recommended heterosexual adults in the United States consider using PrEP if they were HIV negative, not in a monogamous partnership with a recently tested HIV-negative partner, and if they had been diagnosed with a bacterial sexually transmitted infection (STI) (syphilis or gonorrhea) in the past 6 months. The expected rate of acquiring HIV among women diagnosed with syphilis or gonorrhea was not reported.
Louisiana consistently reports very high rates of STIs [22]. In 2016, Louisiana had the highest rates in the nation for congenital syphilis and for primary and secondary syphilis among women. Women in Louisiana had the second highest rates in the nation for gonorrhea, chlamydia, and HIV [22, 23]. Louisiana therefore provides an important opportunity to examine the risk of new HIV diagnosis after women are diagnosed with an STI.
METHODS
Louisiana maintains separate databases for HIV and STI. At least annually, the databases are matched so that HIV infections among persons in the STI database can be captured in the STI database. First, a deterministic match is conducted in SAS using first name, last name, date of birth, social security number (if available), and aliases. If at least 3 variables match, including social security number, the records are combined. Next, unmatched reports are searched again with the addition of addresses using Link Plus, which provides the probability of a match, and a final decision is made by a staff member during a manual review.
We analyzed all STI database records of 13- to 59-year-old women in Louisiana who had syphilis, gonorrhea, or chlamydia reported between 1 January 2000 and 31 December 2015. Late latent syphilis diagnoses were excluded if nontreponemal antibody titers were <1:32 because they were likely to be old infections. Previous studies have shown that persons with syphilis are more likely to acquire HIV than persons with gonorrhea, and persons with gonorrhea are at higher risk than those with chlamydia [21, 24]. Therefore, we used a hierarchical classification for each woman, selecting her first diagnosis of syphilis, (or if none) first diagnosis of gonorrhea, or (if none) first diagnosis of chlamydia recorded on or after 1 January 2000. Women who were reported as having HIV at their first STI diagnosis (or within 60 days after the visit) were considered to have been HIV infected at the time of their initial visit. Therefore, follow-up for new HIV diagnosis began 60 days after the STI diagnosis and included all HIV infections diagnosed before 31 December 2016 that were reported by 8 December 2017. We also examined women who were ever diagnosed with 2 or more STIs at any visit (coinfected) and women who made >1 visit with an STI (Table 1). Person-years at risk for HIV began 60 days after the date of diagnosis for the STI and ended on the date of HIV diagnosis, or on 31 December 2016 if no HIV was reported.
Table 1.
Rate of Human Immunodeficiency Virus Diagnosis for 13- to 59-Year-Old Women in Louisiana, 2000–2016, Comparing Women With and Without a History of a Preceding Sexually Transmitted Infection in 2000–2015
| No STI Reported, 2000–2015 | STI Reported, 2000–2015 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | No. | PY | Reported With HIV | Characteristic | No. | PY | Reported With HIV | Relative Rateb | ||||
| No. | (%) | Ratea | No. | (%) | Ratea | |||||||
| Total | 1 473 206 | 23 179 014 | 5186 | … | 22.4 | Total | 211 603 | 1 865 488 | 969 | … | 51.9 | 2.3 |
| … | … | … | … | … | … | Syphilis | 6574 | 53 583 | 95 | (9.8) | 177.3 | 7.9 |
| … | … | … | … | … | … | Gonorrhea | 64 995 | 616 518 | 451 | (46.5) | 73.2 | 3.3 |
| … | … | … | … | … | … | Chlamydia | 140 034 | 1 195 387 | 423 | (43.7) | 35.4 | 1.6 |
| Age, yc | Age, yc | |||||||||||
| 13–19 | 221 018 | 2 995 576 | 303 | (5.8) | 10.1 | 13–19 | 86 966 | 761 730 | 120 | (12.4) | 15.8 | 1.6 |
| 20–29 | 335 018 | 4 779 096 | 1530 | (29.5) | 32.0 | 20–29 | 102 584 | 916 210 | 530 | (54.7) | 57.8 | 1.8 |
| 30–39 | 286 323 | 4 723 370 | 1492 | (28.9) | 31.6 | 30–39 | 16 801 | 144 121 | 241 | (24.9) | 58.3 | 1.8 |
| 40–49 | 311 935 | 5 267 390 | 1221 | (23.5) | 23.2 | 40–49 | 4201 | 35 505 | 62 | (6.4) | 174.6 | 7.5 |
| 50–59 | 318 912 | 5 413 582 | 640 | (12.3) | 11.8 | 50–59 | 1051 | 7922 | 16 | (1.6) | 202.0 | 17.1 |
| Raced | Raced | |||||||||||
| Black | 537 339 | 7 922 494 | 4204 | (81.1) | 53.1 | Black | 133 626 | 1 212 269 | 850 | (87.7) | 70.1 | 1.3 |
| White | 830 052 | 13 791 687 | 764 | (14.7) | 5.5 | White | 40 288 | 319 197 | 84 | (8.7) | 26.3 | 4.8 |
| Hispanic | 56 034 | 935 455 | 171 | (3.3) | 18.3 | Hispanic | 3047 | 17 123 | 9 | (0.9) | 52.6 | 2.9 |
| Other | 49 781 | 529 378 | 47 | (0.9) | 8.9 | Other | 2461 | 17 413 | 5 | (0.5) | 28.7 | 3.2 |
| Unknown | 0 | 0 | 0 | … | … | Unknown | 32 181 | 299 486 | 21 | (2.2) | 7.0 | … |
| Areae | Areae | |||||||||||
| High risk | 163 500 | 2 353 168 | 2126 | (41.0) | 90.3 | High risk | 44 697 | 426 332 | 393 | (40.6) | 92.2 | 1.0 |
| Low risk | 1 309 706 | 20 825 846 | 3060 | (59.0) | 14.7 | Low risk | 166 906 | 1 439 156 | 576 | (59.4) | 40.0 | 2.7 |
| … | … | … | … | … | … | Ever coinfection | ||||||
| … | … | … | … | … | … | No | 176 256 | 1 533 281 | 725 | (74.8) | 47.3 | … |
| … | … | … | … | … | … | Yes | 35 347 | 332 207 | 244 | (25.2) | 73.4 | … |
| … | … | … | … | … | … | Visits with infection | ||||||
| … | … | … | … | … | … | 1 | 140 092 | 1 207 693 | 548 | (56.6) | 45.4 | … |
| … | … | … | … | … | … | ≥2 | 71 511 | 657 795 | 421 | (43.4) | 64.0 | … |
Abbreviations: HIV, human immunodeficiency virus; PY, person-years; STI, sexually transmitted infection.
Rate is per 100 000 PY.
Relative rate compares women with an STI to women on the same row who have no STI.
Age is age at diagnosis of HIV for persons with no STI, and age at diagnosis of STI for others.
Race/ethnicity information is available for all women in the census, but unknown for 32 181 women with STI.
Area is missing for 75 HIV-infected women with no history of STI and 11 128 women with STI infection and were distributed to areas (high or low) based on proportion of population.
For comparison, we calculated the rate of newly diagnosed HIV infection among women with no reported preceding STI. To calculate the number of HIV diagnoses, we took all HIV diagnoses for 13- to 59-year-old women in the Louisiana HIV/AIDS Surveillance database from 2000 to 2016 and subtracted the number of HIV diagnoses reported for women following an STI (as previously described). The person-years (PY) at risk for this group includes risk time contributed by women before (and 60 days after) they were reported as having an STI. This estimate was calculated by (1) taking the number of 13- to 59-year-old women living in Louisiana in one year, 2010 (1 473 206 according to the census) [25]; (2) multiplying by 17 years (to account for 2000–2016); and (3) subtracting the PY at risk for the women who had an STI (as described above).
We compared estimates for a variety of subgroups by STI, race/ethnicity, age, location, and year of first STI. Age is not comparable between groups because age was at the time of STI diagnosis for women with STI, and at the time of HIV diagnosis for women without STI. Location was classified as the residence at the time of the STI diagnosis even if the HIV infection was later reported from another location in Louisiana. “High risk” areas were defined as ZIP (postal) codes where there were at least 50 women aged 13–59 years reported with newly diagnosed HIV infection between 2000 and 2016 and their annual rate of new HIV diagnosis was at least 40 per 100 000. Population estimates including ZIP code were only available for 2000 and 2010, so PY at risk was calculated assuming that the age/race proportions of women living in high-risk areas were the same across all years as they were in 2010. Risk factors identified by bivariate analyses were combined to assess the magnitude of the rates for women with multiple risks.
We looked for possible cohort effects on risk of HIV diagnosis by grouping women according to the year of their STI diagnosis (2000–2015), and calculating annual and cumulative rates of HIV diagnoses.
To estimate rates by year of HIV diagnosis, we combined all cohorts of women from the STI database and compared them to women with no STI reported. Infections for women with no STI reported were estimated by taking the yearly number of HIV diagnoses for all women living in Louisiana and subtracting corresponding infections diagnosed among women who had STI. The women at risk were estimated using Census estimates of all women living in Louisiana and subtracting women with a history of STI.
We estimated the annual cost of PrEP to be $11 000 per person, using the midpoint of a range of estimates ($8000–$14 000) [9, 26]. This does not include the costs for the recommended 4 annual medical visits, laboratory costs, costs for efforts to assure medications are taken, or patient expenses associated with attending the clinics. We estimated that effectiveness in preventing HIV was 100% of all diagnoses if all women were treated for the entire time period after their STI.
We also estimated the cost and benefits of treating women for 1 year after a diagnosis of syphilis or gonorrhea. For this we calculated the cost of treating every episode of syphilis or gonorrhea that the women had, and estimated that PrEP would have prevented all infections diagnosed within 2 years after the STI.
Because we included all surveillance data from Louisiana, not a sample, we did not calculate P values for our comparisons or confidence intervals for our estimates. Our numbers are large and most error would be expected to be due to variations in testing and reporting of STI or HIV and not due to chance. Centers for Disease Control and Prevention (CDC) staff did not have access to personal identifiers. Secondary analyses of routinely collected surveillance data do not involve human subjects research and therefore do not require approval by the CDC Institutional Review Boards. This project received a nonresearch determination and approval decision from CDC.
RESULTS
Between January 2000 and December 2015, there were 211 603 women aged 13–59 years living in Louisiana who were reported as having an STI. Using our hierarchy, the first infections for these women were syphilis (6574), gonorrhea (64 995), or chlamydia (140 034) (Table 1). Most of these women were young when they were diagnosed with the STI; 86 966 (41.1%) were 13–19 years old, and 102 584 (48.5%) were 20–29 years old. Race/ethnicity was categorized as black (133 626 [63.1%]), white (40 288 [19.0%]), Hispanic (3047 [1.4%]), other (2461 [1.2%]), and unknown (32 181 [15.2%]). Twenty-one percent (44 697) of the women with STI lived in one of the 21 ZIP code areas considered to be at high risk for HIV. These 21 ZIP codes included 163 500 women aged 13–59 years, 11.1% of all women in the state, and 40.9% of all HIV diagnoses among women in Louisiana.
The 211 603 women with an STI had 1 865 488 PY of follow-up in the database; 969 were subsequently diagnosed as having HIV for 61 days—16.6 years after the STI was diagnosed (mean follow-up, 8.8 years; mean time to HIV, 5.2 years). The 1 473 206 women (aged 13–59) in Louisiana with no STI reported in the interval had 23 179 014 PY of follow-up; 5186 were diagnosed as having HIV between 2000 and 2016. Thus, only 15.7% of new HIV diagnoses (969/6155) were among women who had a previously reported STI. The rate of a subsequent diagnosis of HIV (per 100 000 PY) was higher for women diagnosed with syphilis (177.3), gonorrhea (73.2), or chlamydia (35.4) than for women with no STI reported (22.4) (Table 1). Most STI were diagnosed among women aged 13–29 whereas HIV rates were highest among women aged 20–49. Among all women diagnosed with one of these STI, the rate of subsequent HIV diagnosis (per 100 000 PY) was higher for blacks (70.1) than Hispanics (52.6) or whites (26.3). In contrast, the relative rate for HIV, comparing women with vs without an STI, was higher for white women (4.8) than for black women (1.3) and Hispanic women (2.9). Rates of HIV were high for women who were ever coinfected with ≥2 STIs (73.4 per 100 000 PY) or had ≥2 visits with STI (64.0 per 100 000 PY).
Rates were high for subgroups of women with multiple risk factors, such as black women living in high-risk areas who were diagnosed with syphilis at ages 20–29 (254.8 per 100 000 PY) or at ages 30–59 (207.6 per 100 000 PY) (Table 2). Rates were high for other groups of black women diagnosed with syphilis or gonorrhea. However, the groups with the highest rates were also relatively small groups. For women diagnosed with syphilis, the rate of a subsequent HIV diagnosis was 177.3 per 100 000 PY, 7.3 times the rate for women with no STI (Table 1). Yet, the 95 women who developed HIV after a diagnosis of syphilis comprise only 1.5% of the 6155 women diagnosed with HIV between 2000 and 2016.
Table 2.
Rate of Human Immunodeficiency Virus Diagnosis Between 2000 and 2016 for Select Subgroups of Black Women in Louisiana
| No STI Reported, 2000–2015 | STI Reported, 2000–2015 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Reported With HIV | Characteristic | Reported With HIV | ||||||||
| Age, y | Area | No. | PY | No. | Ratea | STI | No. | PY | No. | Ratea | Relative Rateb |
| 20–29 | High risk | 41 588 | 636 358 | 664 | 104.3 | Syphilis | 805 | 6671 | 17 | 254.8 | 2.4 |
| Gonorrhea | 6409 | 63 967 | 78 | 121.9 | 1.2 | ||||||
| 20–29 | Low risk | 65 618 | 945 419 | 827 | 87.5 | Syphilis | 1860 | 14 270 | 17 | 119.1 | 1.4 |
| Gonorrhea | 16 601 | 155 817 | 108 | 69.3 | 0.8 | ||||||
| 30–59 | High risk | 97 210 | 1 635 027 | 1213 | 74.2 | Syphilis | 532 | 4817 | 10 | 207.6 | 2.8 |
| Gonorrhea | 1287 | 12 726 | 24 | 188.6 | 2.5 | ||||||
| 30–59 | Low risk | 196 284 | 3 300 610 | 1621 | 49.1 | Syphilis | 1105 | 9130 | 17 | 186.2 | 3.8 |
| Gonorrhea | 1737 | 27 088 | 20 | 73.8 | 1.5 | ||||||
Abbreviations: HIV, human immunodeficiency virus; PY, person-years; STI, sexually transmitted infection.
Rate is per 100 000 PY.
Relative rate compares women with an STI to women of the same age and area group who have no STI.
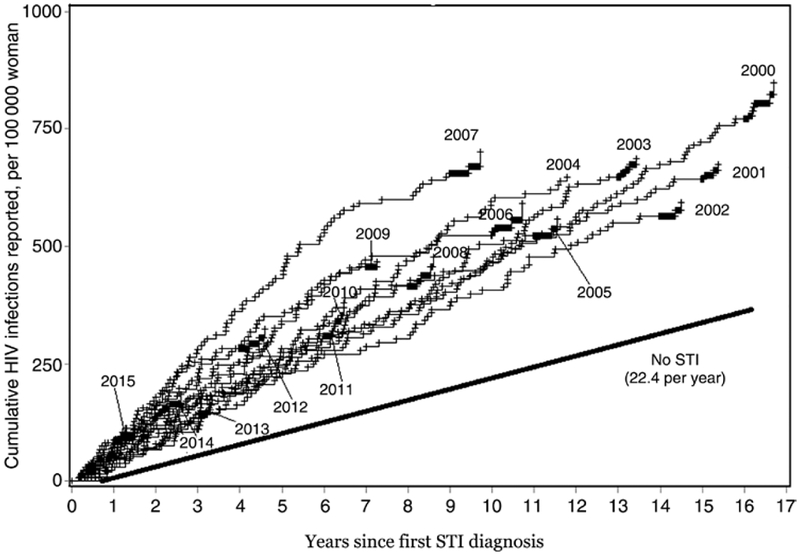
The likelihood of being newly diagnosed with HIV (Figure 1, slopes of the curves) was only slightly higher in the years immediately following the STI diagnosis compared to later years. Even 16 years after their diagnosis, women who had an STI were diagnosed with HIV at a higher rate than women who had not had an STI (Figure 1).
Figure 1.
Cumulative number of human immunodeficiency virus (HIV) infections reported following the diagnosis of a sexually transmitted infection (STI), per 100 000 women, by year of STI diagnosis.
The annual rate of HIV for 13- to 59-year-old women in Louisiana who were not in the STI cohorts fell 23.5% (from 30.2 to 23.1 per 100 000 women) between the years 2001 and 2015 (rates were as low as 15.5 in 2013). The annual rate for women in STI cohorts fell slightly less, 21.6% (from 74.8 to 45.7 per 100 000 women) (data not shown).
If PrEP were 100% effective, providing PrEP to all women diagnosed with syphilis or gonorrhea from the time of their STI diagnosis until 2016, or until they were diagnosed with HIV, would have prevented all 546 new HIV diagnoses (Table 1) at a cost of $7.4 billion for 670 101 PY of treatment. Both the number of HIV diagnoses prevented and the cost of PrEP increased over time as the number of women with past syphilis or gonorrhea increased. In 2016, an estimated 49 diagnoses would have been prevented at a cost of $787 million ($16 million per HIV diagnosis prevented). Alternatively, providing PrEP for 1 year to women who had syphilis or gonorrhea at any time during 2000–2015 would have required treating 71 569 women for a total of 87 622 PY (some were infected multiple times). HIV was diagnosed within 2 years after a syphilis or gonorrhea diagnosis for 143 women. The cost for this approach would have been $963 847 000, which is $6 640 154 per infection diagnosed within 2 years after a diagnosis of syphilis or gonorrhea.
DISCUSSION
Women in Louisiana who had an STI were at increased risk for developing HIV, but that risk was not high enough to make PrEP a cost-effective intervention for all women with STI at current costs. Women with gonorrhea were diagnosed with new HIV infection at a rate 3.3 times that of women with no STI, but at that rate (73.2 per 100 000 PY) 1 out of 1366 was diagnosed with HIV in a year; and it would cost $15 million to prevent one new HIV diagnosis using PrEP. Women with syphilis had the highest rate of new HIV diagnosis (177 per 100 000 PY), but that was one-tenth the estimated diagnosis rate for all men in Louisiana who have had sex with another man within the past 5 years (1760 per 100 000 in 2013) [27]. The rate of new HIV diagnosis among women with an STI was 2.3 times the rate among women with no STI, but only 15.7% of new HIV diagnoses among women were among women with a previously reported STI. Thus, even if PrEP became cost-effective for women with STI, the impact on the population would be small because most of the newly diagnosed HIV infections were among the large group of women who were not at highest risk [24]. Most new HIV diagnoses occurred among women who were not previously diagnosed with an STI.
Our findings are similar to those found in an earlier study in Florida [24]. Rates of HIV diagnosis after any STI were higher in Florida, but decreased by about 75% between 2001 (197.4 per 100 000 PY) and 2011 (49.5 per 100 000 PY). Rates among women with STI in Louisiana did not start out as high, but did not decrease as much, remaining at 45.7 per 100 000 PY in 2015. These rates following STI were much lower than the rates typically seen for men who have sex with men (672–2600 per 100 000 PY) [9, 28] or for women in sub-Saharan Africa (2000–5500 per 100 000 PY) [10–12]. Several cohort studies have regularly tested women in the United States who were at exceptionally high risk for acquiring HIV. These studies included 4738 women followed for 5474 PY, and 29 seroconverted (530 per 100 000 PY) [13–20]. If all of those women could have been on PrEP at a cost of $11 000 per year, and PrEP was 100% effective, then the 29 HIV diagnoses could have been prevented at a cost of $60 214 000.
Other factors were associated with risk for HIV. Among women with no STI reported, the rate for black women (53.1 per 100 000 PY) was 9.7 times the rate for white women (5.5 per 100 000 PY). Women living in high-risk ZIP code areas had rates that were 6.1 times as high as women living elsewhere. Combining risk factors led to higher rates. Black women aged 20–29 years who lived in high-risk areas when they were diagnosed with syphilis had the highest rates (254.8 per 100 000 PY), but this was a relatively small group (805) that accounted for 17 new HIV diagnoses. Thus we were unable to identify any subgroups of women with a risk high enough to make PrEP cost-effective. Other studies have reported that HIV prevention interventions such as HIV testing and partner notification would be cost-effective [9]. Perhaps other cost-effective interventions can be developed. A recent review of available interventions concluded that “interventions focusing on reducing the risk of acquiring HIV, particularly among heterosexuals, are unlikely to result in efficient use of society’s resources” [9].
Our analysis enabled us to study the risk of HIV diagnosis for all women with STI reported by all providers in Louisiana over 16 years. However, our findings have some limitations. We did not actively follow all women to ensure testing for HIV. We measured the risk of being reported with HIV in Louisiana. This was not a measure of true incidence. We know the date of diagnosis, not the date that HIV was acquired. We would have overestimated incidence if women were not tested for HIV at the time of their STI diagnosis. We would underestimate incidence if women acquired HIV that was undetected because they were not tested or if they had moved from Louisiana and were diagnosed elsewhere. Similarly, women with undiagnosed STI or with STI diagnosed in other states were not included in the groups with past STI. Finally, this study aimed to identify women at risk for acquiring HIV in the future, and not to measure how an active STI influences susceptibility to acquiring HIV, so women who that had HIV diagnosed at the same time as their STI diagnosis were not included in the STI group.
In Louisiana, compared to women with no STI, the relative rates of acquiring HIV were higher after women were reported with chlamydia (1.6), gonorrhea (3.3), and syphilis (7.3). Providing PrEP to all women in Louisiana diagnosed with syphilis or gonorrhea since 2000 would have cost more than $787 million in the year 2016, and would have prevented only a small fraction of new HIV infections diagnosed among women. Providing PrEP for a year after infection with syphilis or gonorrhea would reduce costs, but would also reduce the number of infections prevented.
Footnotes
Publisher's Disclaimer: Disclaimer. The findings and conclusions in this work are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Curran K, Baeten JM, Coates TJ, Kurth A, Mugo NR, Celum C. HIV-1 prevention for HIV-1 serodiscordant couples. Curr HIV/AIDS Rep 2012; 9:160–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin JS, Whitlock E, O’Connor E, Bauer V. Behavioral counseling to prevent sexually transmitted infections: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 2008; 149:497–508, W96–9. [DOI] [PubMed] [Google Scholar]
- 3.Marks G, Crepaz N, Senterfitt JW, Janssen RS. Meta-analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States: implications for HIV prevention programs. J Acquir Immune Defic Syndr 2005; 39:446–53. [DOI] [PubMed] [Google Scholar]
- 4.Hughes JP, Baeten JM, Lingappa JR, et al. ; Partners in Prevention HSV/HIV Transmission Study Team. Determinants of per-coital-act HIV-1 infectivity among African HIV-1-serodiscordant couples. J Infect Dis 2012; 205:358–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen MS, Chen YQ, McCauley M, et al. ; HPTN 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young TN, Arens FJ, Kennedy GE, Laurie JW, Rutherford GW. Antiretroviral post-exposure prophylaxis (PEP) for occupational HIV exposure. Cochrane Database Syst Rev 2007; 1:CD002835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. ; TDF2 Study Group. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 2012; 367:423–34. [DOI] [PubMed] [Google Scholar]
- 8.Baeten JM, Donnell D, Ndase P, et al. ; Partners PrEP Study Team. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012; 367:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin F, Farnham PG, Shrestha RK, Mermin J, Sansom SL. Cost effectiveness of HIV prevention interventions in the U.S. Am J Prev Med 2016; 50:699–708. [DOI] [PubMed] [Google Scholar]
- 10.Rehle TM, Hallett TB, Shisana O, et al. A decline in new HIV infections in South Africa: estimating HIV incidence from three national HIV surveys in 2002, 2005 and 2008. PLoS One 2010; 5:e11094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feldblum PJ, Latke MH, Lombaard J, et al. HIV incidence and prevalence among cohorts of women with higher risk behavior in Bloemfontein and Rustenberg, South Africa: a prospective study. BMJ Open 2012; 2:e000626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Damme L, Corneli A, Ahmed K, et al. ; FEM-PrEP Study Group. Preexposure prophylaxis for HIV infection among African women. N Engl J Med 2012; 367: 411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chirgwin KD, Feldman J, Dehovitz JA, Minkoff H, Landesman SH. Incidence and risk factors for heterosexually acquired HIV in an inner-city cohort of women: temporal association with pregnancy. J Acquir Immune Defic Syndr Hum Retrovirol 1999; 20:295–9. [DOI] [PubMed] [Google Scholar]
- 14.Brown-Peterside P, Chiasson MA, Ren L, Koblin BA. Involving women in HIV vaccine efficacy trials: lessons learned from a vaccine preparedness study in New York City. J Urban Health 2000; 77:425–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seage GR 3rd, Holte SE, Metzger D, et al. Are US populations appropriate for trials of human immunodeficiency virus vaccine? The HIVNET Vaccine Preparedness Study. Am J Epidemiol 2001; 153:619–27. [DOI] [PubMed] [Google Scholar]
- 16.Koblin BA, Bonner S, Hoover DR, et al. A randomized trial of enhanced HIV risk-reduction and vaccine trial education interventions among HIV-negative, high-risk women who use noninjection drugs: the UNITY study. J Acquir Immune Defic Syndr 2010; 53:378–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koblin BA, Metch B, Novak RM, et al. ; HVTN 906 Study Team. Feasibility of identifying a cohort of US women at high risk for HIV infection for HIV vaccine efficacy trials: longitudinal results of HVTN 906. J Acquir Immune Defic Syndr 2013; 63:239–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hodder SL, Justman J, Hughes JP, et al. ; HIV Prevention Trials Network 064; Women’s HIV SeroIncidence Study Team. HIV acquisition among women from selected areas of the United States: a cohort study. Ann Intern Med 2013; 158:10–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Novak RM, Metch B, Buchbinder S, et al. Risk behavior among women enrolled in a randomized controlled efficacy trial of an adenoviral vector vaccine to prevent HIV acquisition. AIDS 2013; 27:1763–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adimora AA, Cole SR, Eron JJ. US black women and human immunodeficiency virus prevention: time for new approaches to clinical trials. Clin Infect Dis 2017; 65:324–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention, US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States—2017 update: a clinical practice guideline. Available at: https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf. Accessed 8 November 2018.
- 22.Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2016. Available at: https://www.cdc.gov/std/stats/. Accessed 20 November 2018.
- 23.Centers for Disease Control and Prevention. HIV in the United States by Region. Available at: https://cdc.gov/hiv/statistics/overview/geographicdistribution.html. Accessed 11 December 2018.
- 24.Peterman TA, Newman DR, Maddox L, Schmitt K, Shiver S. Risk for HIV following a diagnosis of syphilis, gonorrhea or chlamydia: 328,456 women in Florida, 2000–2011. Int J STD AIDS 2015; 26:113–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.United States Census Bureau. Community facts. 2010. Available at: https://factfinder.census.gov/faces/nav/jsf/pages/community_facts.xhtml/. Accessed 16 October 2018.
- 26.New York State Department of Health. Pre-exposure prophylaxis to prevent HIV infection: questions and answers. 2012. Available at: https://www.health.ny.gov/publications/0265/. Accessed 27 November 2018.
- 27.Rosenberg ES, Grey JA, Sanchez TH, Sullivan PS. Rates of prevalent HIV infection, prevalent diagnoses, and new diagnoses among men who have sex with men in US states, metropolitan statistical areas, and counties, 2012–2013. JMIR Public Health Surveill 2016; 2:e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Llata E, Braxton J, Asbel L, et al. New human immunodeficiency virus diagnoses among men who have sex with men attending sexually transmitted disease clinics, STD Surveillance Network, January 2010 to June 2013. Sex Transm Dis 2018; 45:577–82. [DOI] [PMC free article] [PubMed] [Google Scholar]