Abstract
Fracture healing is a complex and integrated process that involves mesenchymal progenitor cell (MPC) recruitment, proliferation and differentiation that eventually results in bone regeneration. Prostaglandin E2 (PGE2) is an important regulator of bone metabolism and has an anabolic effect on fracture healing. Prior work from our laboratory showed EP1−/− mice have enhanced fracture healing, stronger cortical bones, higher trabecular bone volume and increased in vivo bone formation. We also showed that bone marrow MSCs from EP1−/− mice exhibit increased osteoblastic differentiation in vitro. In this study we investigate the changes in the periosteal derived mesenchymal progenitor cells (PDMPCs), which are crucial for fracture repair, upon EP1 deletion. EP1−/− PDMPCs exhibit increased numbers of total (CFU-F) and osteoblastic colonies (CFU-O) as well as enhanced osteoblastic and chondrogenic differentiation. Moreover, we tested the possible therapeutic application of a specific EP1 receptor antagonist to accelerate fracture repair. Our findings showed that EP1 antagonist administration to wild type mice in the early stages of repair similarly resulted in enhanced CFU-F, CFUO, and osteoblast differentiation in PDMPCs and resulted in enhanced fracture callus formation at 10 days post fracture and increased bone volume and improved biomechanical healing of femur fractures at 21 days post fracture.
Keywords: EP1, periosteum progenitor cells, osteogenic differentiation and bone fracture
Introduction
In the United States approximately eight million bone fractures occur annually51, 66. Ten to twenty percent of the fractures fail to heal resulting in delayed repair or non-union causing severe disabilities. Non unions often require surgical intervention and results in increased morbidity and health care costs. Currently, non-union treatment involves bone implants with decalcified grafts or autografts5, 20, 57. However, high failure rates with these approaches indicate the pressing need for the development of novel therapies that will increase bone formation, enhance fracture repair rates, and treat non-unions.
Fracture healing is a complex physiological process that includes several defined stages. Healing starts with hematoma formation and an inflammatory response. Additionally, this stage is characterized by activation and proliferation of the periosteal stromal cells also known as periosteal derived mesenchymal progenitor cells (PDMPCs), resulting in a thickened periosteum. PDMPCs further differentiate into osteoblasts and chondrocytes, which eventually form bone via both endochondral and intramembranous ossification. Finally the healing process results in restoration of normal bone architecture and strength through an on-going remodeling process18, 19.
Mesenchymal progenitor cells (MPCs) were first isolated from bone marrow and thought to be connective tissue supportive cells49. Later, these cells were shown to have the potential to differentiate into osteoblastic, chondrogenic and adipogenic lineages9. MPCs are characterized by their ability to form colonies and by expression of specific markers. They are negative for hematopoietic and endothelial markers, CD45 and CD31 respectively, and are positive for markers including CD105, Sca1, CD29, CD90, CD44, and CD73. MPCs from both the periosteum and bone marrow have been shown to participate in the repair process16, 26, 63 and are considered the source of cells responsible for the repair process. Thus, limitation in numbers and functions of MPCs results in impaired bone healing69. Given the importance of MPCs on bone regeneration7, 8, it is important to understand the mechanisms that regulate their cell fate.
The periosteum is a thin layer of osteogenic and fibroblastic cells that surrounds the outside of the bones2. The “cambium layer” of the periosteum is highly cellular and contains PDMPCs, osteoprogenitors, osteoblasts, and fibroblasts as well as micro vessels and sympathetic nerves. Periosteal cells have been shown to be the main cell population to participate in fracture healing, while removal of the periosteum significantly delays the healing process16, 69. Furthermore, lineage-tracing studies have identified periosteal cells as the main source of cells in the callus during healing44.
The inflammatory stage of the repair process is essential for the fracture healing cascade24. Cyclooxogenase 2 (COX2) and its main metabolite, Prostaglandin E2 (PGE2), are major inflammatory mediators required for the healing process to take place52, 53, 56. PGE2 has been shown to play an important role in bone biology; systemic PGE2 administration in mice and rats resulted in increased bone formation and bone resorption55, 64. PGE2 exerts its effect by binding to four receptors: EP1–415, 46, 60. EP2 and EP4 receptors have both bone anabolic and catabolic effects17, 39, 48, 61, 62. Little is known about the role of EP1 receptor in bone biology. In bone cells, EP1 increases osteoblastic cell-line proliferation59. In addition, EP1 is expressed in proliferating chondrocytes, with increased expression during proliferation11. EP1 has been suggested to play a role in osteoclast maturation22. We have previously demonstrated that EP1 knock-out (EP1−/−) mice exhibit increased bone formation in vivo, resulting in stronger bones and reduced bone loss with aging67. Moreover, EP1−/− mice exhibit accelerated fracture healing resulting in faster bone formation and enhanced biomechanics68. These findings suggest that EP1 is a negative regulator of bone formation.
Recently we demonstrated that bone marrow mesenchymal progenitor cells (BM-MPCs) harvested from EP1−/− mice had enhanced osteogenic potential21. In light of the particular importance of PDMSDs in fracture healing and our observations regarding the enhanced repair in the EP1−/− mice, we decided to test whether EP1−/− mice exhibit differences in the functions of PDMPCs. Additionally, as a potential therapeutic target, we tested whether administration of EP1 antagonist following fracture will enhance the healing process by increasing the PDMPC differentiation potential. We found that periosteum of EP1−/− mice consist of more progenitors with increased osteoblastic potential. We also observed that treatment with an EP1 antagonist increases the number of osteoblastic progenitors in the periosteum, and improves the biomechanical properties during fracture healing.
Materials and Methods
Mouse strains:
C57BL/6J mice were purchased from Jackson Laboratory. EP1−/− mice were generously provided by Matthew Breyer27. All animal procedures were approved by the University Committee of Animal Resources (UCAR) at the University of Rochester Medical Center. The EP1−/− mice used in the experiments have global gene deletion of the EP1−/− receptor in all cells and tissues, including bone marrow progenitor cells68.
Bone grafting and periosteal cell isolation:
Periosteal cells were isolated using a protocol described by Zhang et al70. Briefly, wild type C57BL/6J or EP1−/− mice aged 10–14 weeks were anesthetized with Isofluorane and a 4mm femur autograft surgery performed. Briefly a 4mm segment of bone was removed from the femur, the bone marrow was flushed from 4mm autograft segment, and the graft was placed back into the femur and fixed in place with a pin. After five days of in vivo expansion of the periosteal cell population, the autografts are harvested and freed from all muscle attachments. The harvested autografts again have repeated flushing of the marrow cavity and marrow cells are discarded. The periosteal tissue attached to the bone surface is removed and digested with Collagenase D (3mg/ml) for 45 minutes with vigorous shaking at 37°C. The periosteal cells are then strained through a 70mm mesh and plated in 15mm culture dishes. Periosteal progenitor cells were allowed to adhere to the culture dish before media change and grown to 80% confluence. The periosteal progenitor cell isolation typically pools cells from 4–5 mice.
Osteogenic differentiation:
First passage periosteal progenitor cells were plated at 10,000 cells per well of a 12-well plate and grown to confluence. The media then was replaced with alpha MEM with 10% FBS, 1% penicillin streptomycin and supplemented with 100mM beta glycerol-phosphate and 50 μg/ml ascorbic acid. The media was changed every two days and cells were harvested at indicated time points for alkaline phosphatase staining and gene expression analysis.
Adipogenic differentiation:
First passage periosteal progenitor cells were plated at 10,000 cells per well of a 12-well plate and grown to confluence. Adipogenic differentiation was induced with media containing 1μM Dexamethasone, 0.5mM IBMX, 10μg/ml Insulin and 10nM Rosiglitazone for two days. The media then was then replaced with maturation media containing 1μg/ml Insulin and 10nM Rosiglitazone for an additional 6 days. Differentiation was assessed by the appearance of lipid droplets as visualized by staining with Oil Red O and measurement of absorbance at 500nm of eluted Oil red O with 100% isopropanol, as well as adipogenic gene expression by qPCR.
Chondrogenic differentiation:
First passage periosteal progenitor cells were pellet at 2.5×105 cells per 15ml conical tube and supplemented with 0.5ml chondrogenic media (Lonza). The media was changed every two days for 28 days. The cell pellets were either fixed with 10% Neutral Buffer Formalin, embedded in paraffin, cut to 3μm sections, and stained with Alcian Blue, or underwent mRNA expression analysis by qPCR.
Flow cytometry:
Cells were suspended in PBS containing 3% FBS and stained with the following antibodies: CD45-PerCP, CD31-PE-Cy7, Sca1-APC, CD105-PE (BD Pharmingen). Compensation controls were performed using anti-mouse Compensation Standard beads. Gates were determined using Flow Minus One (FMOs) controls.
Colony forming assay:
In low-density cultures, single colonies of fibroblast-like cells are formed, each colony arising from a single precursor cell called a colony forming unit fibroblast (CFU-F)37. Freshly isolated periosteal progenitor cells were plated at 2000 cells per well in a six-well plate and cultured for 10 days. The cells were then fixed and stained with 0.5% Crystal violet in methanol for CFU-F and with ALP substrate NBT/BCIP reagent (Thermo Scientific Pierce) for CFU-O. A colony was considered a cluster of more than 50 cells36, 37.
Gene expression analysis>:
mRNA was extracted at indicated time points using RNA Easy extraction kit (Qiagen). Exactly 0.5 g of RNA was reverse transcribed into cDNA using iScript reagent (Biorad). Quantitative RT-PCR was performed using a RotorGene real-time PCR machine.
Femur open osteotomy model:
12 week-old C57BL/6J mice (males and female) were anesthetized and a 7–8mm-long incision was made in the skin, and the midshaft of the femur was exposed by blunt dissection of the muscle without disturbing the periosteum. The mid-diaphysis of the femur was cut with a dremel saw with diamond blade. The bone was stabilized using 22 gauge metal pin placed through the intramedullary canal. Starting day 1 after surgery the mice were injected i.p. with either vehicle (DMSO) or 10mg/kg EP1 antagonist (SC51089, Cayman chemical) for 5 consecutive days. The 10mg/kg dose of SC51089 used in the experiments was based on prior studies showing that the in vivo effects of SC51089 on analgesia in rats have a Kd of approximately 6mg/kg29,41. In a rat model, 10mg/kg dosing of SC51089 was shown to prevent seizure activity50.
Micro Computed Tomography:
Femurs were harvested at the indicated time points and scanned using VIVA microCT system at a voxel size of 10.5μm. From the 2D slice images generated, an appropriate threshold was chosen for the bone voxels by visually matching thresholds areas to grayscale images. The threshold and the volume of interest were kept constant throughout the analysis for each femur. To measure the new total callus volume and bone volume, contour lines were manually drawn on the key 2D slice images to exclude the cortical bone, with morphing interpolating contours between key slices performed semi-automatically and adjusted manually by the expert operator to ensure the fidelity of callus measurements and the exclusion of cortical bone.
Biomechanical testing:
Femurs were dissected 21 days after fracture from vehicle and EP1 antagonist treated mice. Torsion testing was performed in a Torsional Test Bench (Endura Tech) according to established protocols54. Briefly, muscle and soft tissue were completely removed from the femurs to be tested, and the pins were carefully removed without disrupting the callus. Both ends of the femur were cemented into 6.35mm2 aluminum tube holders using polymethyl-methacrylate (PMMA) in a custom jig to insure axial alignment and to maintain a gage length of ~6.5mm, allowing at least 3mm to be potted to each end. Specimens were then mounted on the EnduraTec TestBench™ system (Bose Corporation, Minnetonka, MN) and tested in torsion at a rate of 10°/s until failure. Ultimate torque, torsional rigidity, ultimate rotation, and energy to failure were determined.
Results
EP1−/− mice periosteum have more osteoblastic progenitors
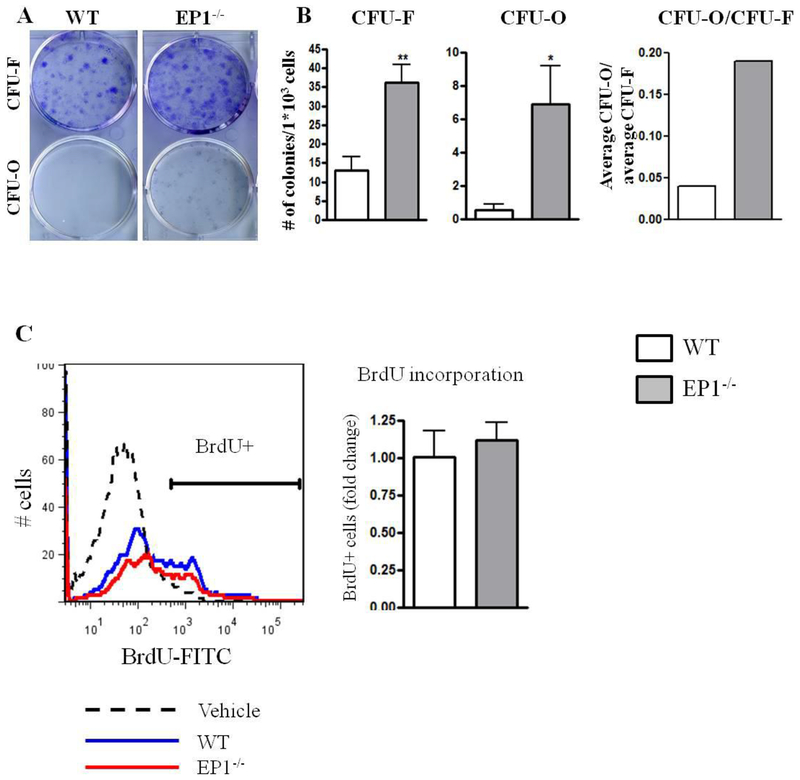
Based on our in vivo observation that EP1−/− mice exhibit accelerated repair, we undertook further analysis of the progenitor population in WT and EP1−/− periosteum. To determine whether there was a difference in the numbers of PDMPCs in EP1−/− mice we analyzed both the colony forming potential by CFU assay and flow cytometric analysis for cell surface markers. We observed that EP1−/− periosteal cells form three times more CFU-Fs than WT (WT: 13 3.5, EP1−/−: 36±4.8, p<0.001). Moreover, EP1−/− periosteal cells formed 7-fold more CFU-Os (WT: 0.5±0.33, EP1−/−: 7±2.27 p<0.05) (Figure 1A–B). To determine if there were differences in periosteal cell proliferation we analyzed the BrdU incorporation rate in the periosteum. No change in BrdU incorporation were observed between WT and EP1−/− cells (WT: 38.8%±19.75, EP1−/− 43.64%±16, p=0.65) (Figure 1C).
Figure 1: EP1−/− periosteal cells form more osteoblastic colonies.
Periosteal cells were isolated from WT or EP1−/− mice and plated at clonal density. Colonies were stained with Crystal Violet for CFU-F and Alkaline Phosphatase for CFU-O (A-B). Mice were injected with BrdU 2 hours before sacrificing. BrdU positive cells were measured using flow cytometry (C).
N=6 replicates per group for each assay. Error bars represent standard error of the mean. Statistical analysis was performed using paired student t-test. (*)=p<0.05 (**)=p<0.01 vs. age-matched WT.
EP1−/− Periosteal cells have increased osteoblastic differentiation
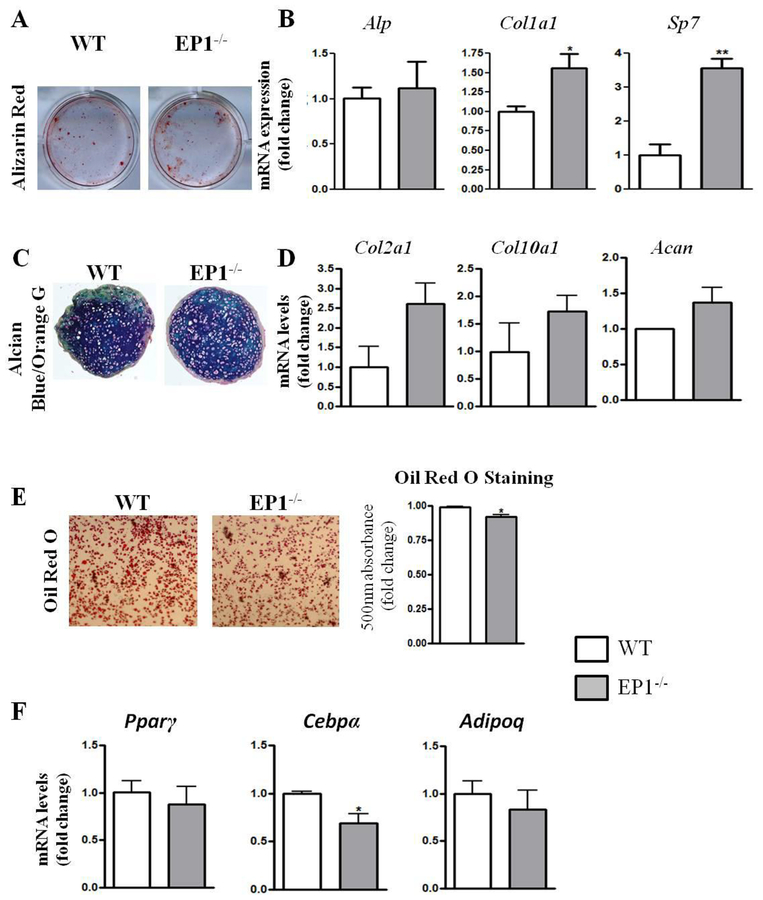
To determine whether EP1 plays a role in PDMPC differentiation, we performed in vitro differentiation assays in the osteoblastic, chondrogenic and adipogenic lineages. We observed that EP1−/− PDMPCs have increased alizarin red staining compared to WT cells (Figure 2A). Gene expression analysis revealed a 1.5 fold increase in Col1a1expression (p=0.019) and 3-fold increase in Sp7 (p=0.007) after 7 days of osteogenic differentiation (Figure 2B). No changes in chondrogenic differentiation were observed by Alcian blue staining between WT and EP1−/− cells (Figure 2C). However, Col2a1 expression was increased 2.5-fold in EP1−/− cells, along with a 1.5 -fold increase in Acan expression, relative to WT (Figure 2D). We observed a slight decrease in adipogenic differentiation potential in EP1−/− cells as indicated by an 8% decrease in Oil Red O staining (p=0.026) and a 35% decrease in Cebpα gene expression (p=0.025), relative to WT cells (Figure 2 E–F).
Figure 2: EP1−/− periosteal cells exhibit enhanced osteoblastic differentiation.
Alizarin Red (A) staining and osteoblastic gene expression (B) following 10 days of induction of osteoblastic medium. Cell pellets sections and stained with Alcian Blue (C) and gene expression analysis for chondrogenic genes (D). Oil Red O staining (E) and adipogenic gene expression (F) following 6 days of induction with adipogenic medium.
N=5 replicates per group for each assay. Error bars represent standard error of the mean. Statistical analysis was performed using paired student t-test. (*)=p<0.05 (**)=p<0.01 vs. age-matched WT.
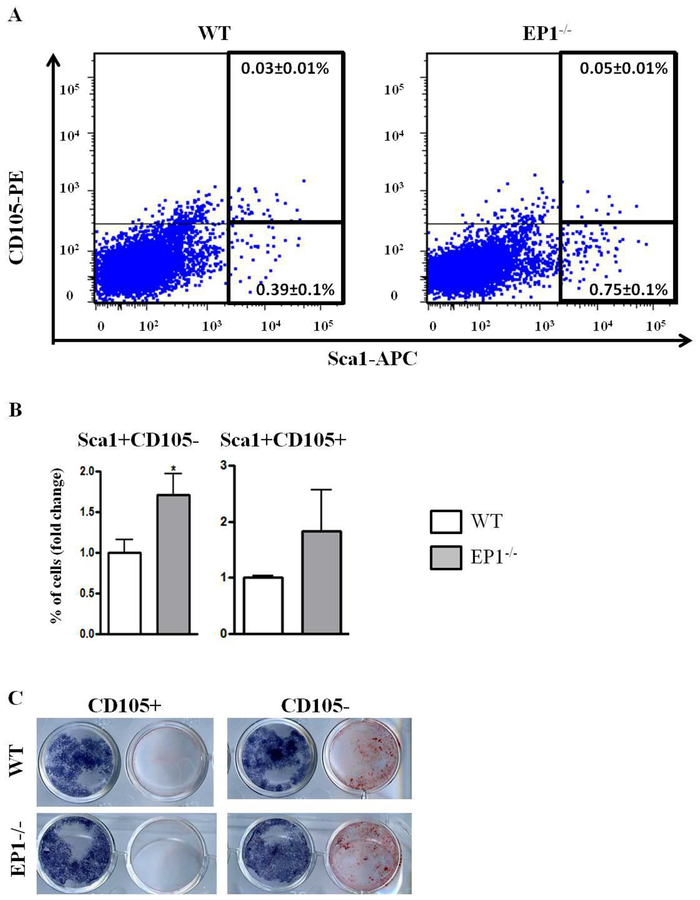
Based on the increased differentiation and increased number of osteoblastic colonies we analyzed the periosteal cells based on mesenchymal cell surface markers. Several previous studies suggested that reduced CD105 expression is associated an increase in osteoblastic differentiation potential3, 4, 6, 38. We sorted and removed CD45−CD31− cells (non-hematopoietic, non-endothelial cells, respectively) and examined expression of the mesenchymal markers: Sca1 and CD105. We did not observe any significant change in the percentage of Sca1+CD105+ cells (WT 0.036%±0.02%, EP1−/− 0.05±0.02, p=0.25). However there was a significant increase in the percentage of the Sca1+CD105− cells in periosteal cells obtained from EP1−/− mice (WT: 0.39%±0.2, EP1−/−: 0.75%±0.26, p=0.04) (Figure 3 A–B). To confirm that these cells have different differentiation potential we sorted Sca1+CD105+ and Sca1+CD105− cells and induced osteoblastic, adipogenic or chondrogenic differentiation. Sorted CD105− cells from WT or EP1−/− mice exhibited increased mineralization relative to matched genotype CD105+ cells after five days of culture in osteogenic medium (Figure 3C). No differences were observed in the differentiation potential of either the CD105+ cells or CD105− from the WT and EP1−/− mice respectively.
Figure 3: EP1−/− periosteum contains more CD105− progenitors.
A-B, freshly isolated periosteal cells were stained with antibodies against: CD45-PrcP, CD31-PEcy7, CD105-PE, Sca1-APC (A-B). The cells were selected for CD45 and CD31 negative cells and the percentage of Sca1 + and CD105+ cells were analyzed. (C), Sca1+CD105+ and Sca1+CD105− cells were sorted and cultured with osteogenic medium for 10 days. In the paired culture wells shown in the figure, the well on the left is stained for alkaline phosphatase and the well on the right is stained with alizarin red to detect mineralization in the cultures.
N=5 replicates per group for each assay. Error bars represent standard error of the mean. Statistical analysis was performed using paired student t-test. (*)=p<0.05 (**)=p<0.01 vs. age-matched WT.
EP1 antagonist treatment during fracture healing changes the periosteal mesenchymal population
Since EP1−/− mice exhibit accelerated fracture healing68 we tested whether systemic administration of an EP1 specific antagonist following fracture will be sufficient to enhance the repair process by inducing PDMPC differentiation.
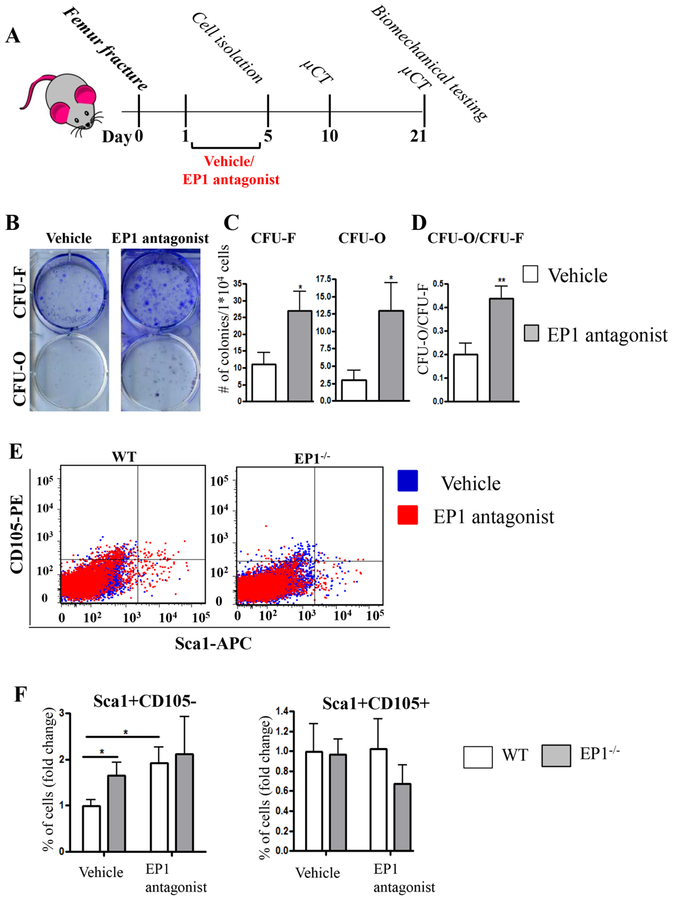
First, we tested whether administration of EP1 antagonist induces changes to PDMPCs (Figure 4A). We observed that following EP1 antagonist treatment PDMSCs form more CFU-Fs (vehicle: 11±3.43, EP1 antagonist: 27±5.8, p=0.039) and CFU-Os (vehicle: 3 ±1.4, EP1 antagonist 12.92±4, p=0.04), resulting in a 2 fold increase in the ratio of CFUO/CFU-F in EP1 antagonist treated PDMPCs (vehicle: 0.2±0.04, EP1 antagonist 0.43±0.05, p=0.0078, Figure 4B–D). Additionally, we observed that EP1 antagonist treatment increased the population of Sca1+CD105− cells (2% versus 6% after treatment, N=3, p<0.05), resulting in a higher proportion of Sca+CD105− than observed in EP1−/− PDMPCs (Figure 4E–F). The increase in the Sca1+CD105+ cells was not significant (0.05% versus 0.08% after treatment, p=0.95).
Figure 4: EP1 antagonist administration increase the number of committed progenitors at the periosteum.
(A) Schematic describing experimental design. (B-D) CFU assays on primary isolated periosteal cells. Colonies were stained with Crystal Violet for CFU-F and Alkaline Phosphatase for CFU-O. (E-F) freshly isolated periosteal cells were stained with antibodies against: CD45-PrcP, CD31-PEcy7, CD105-PE, Sca1-APC. The cells were selected for CD45 and CD31 negative cells and the percentage of Sca1 + and CD105+ cells were analyzed.
N=5–6 replicates per group for each assay. Error bars represent standard error of the mean. Statistical analysis was performed using paired student t-test. (*)=p<0.05 (**)=p<0.01 vs. age-matched vehicle treated animals.
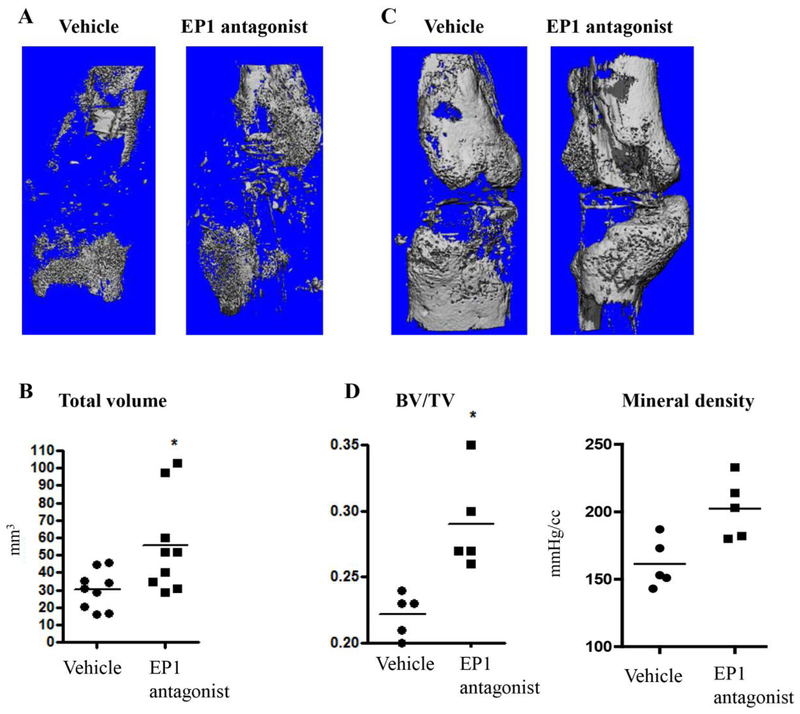
We then analyzed the fractured femurs at day 10 and 21 after fracture. μCT images showed that by day 10 after fracture, the EP1 antagonist treated group had an increase in callus volume (vehicle: 30.14±3.68mm3, EP1 antagonist: 55±9.15, p<0.01) (Figure 5A, 5D). No change in bone volume was observed at this time (vehicle: 5.56±0.96 mm3, EP1 antagonist 4.4±1.27 mm3, p=0.88,). By day 21 the bone volume fraction (BV/TV) was significantly increased in EP1 antagonist treated fractures (vehicle: 0.22±0.007, EP1 antagonist: 0.29±0.036, p= 0.017) (Figure 5B, 5D). Additionally, mineralization density of EP1 antagonist treated fractured bone was higher compared to vehicle (vehicle-161.4±8.4 mgHA/cc, EP1 antagonist-202.4±9.10.0 mgHA/cc) (Figure 5B–D).
Figure 5: EP1 antagonist administration alters fractured femurs properties.
Micro-CT reconstructions of fractured femurs at day 10 (A and B) and day 21 (C and D). Representative images showing callus formation in vehicle treated mice and in EP1 antagonist treated mice at day 10 (A) and day 21 (C). (B) Day 10 BV/TV and mineralized density as determined by Micro-CT (B). Total bone volume at day 21 after fracture as measured by Micro-CT (D).
Day 21 has N=5 per group and Day 10 has 9 mice per group. Error bars represent standard error of the mean. Statistical analysis was performed using paired student t-test. (*)=p<0.05 vs. age-matched vehicle treated animals.
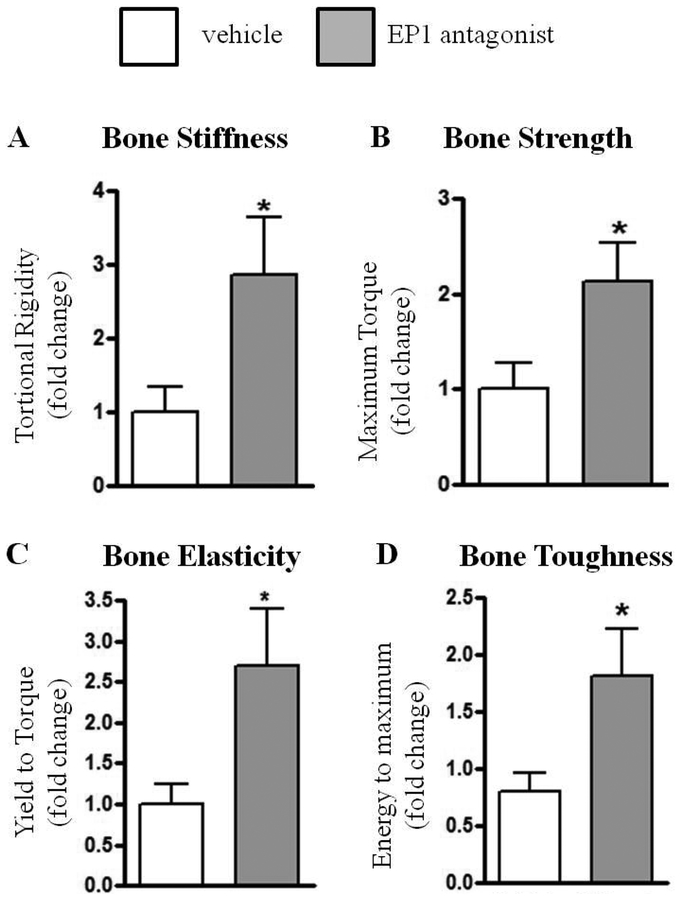
To test the quality of the healed fractured bones torsion testing was conducted 21 days after fracture. A 3-fold increase in torsional rigidity was observed in EP1 antagonist treated fractures, relative to vehicle treated (p=0.04). In addition, EP1 antagonist treatment resulted in increased maximum torque (2-fold increase, p=0.03), energy to maximum (2- fold increase, p=0.04), and higher yield to torque (3- fold increase, p=0.03), relative to vehicle treated fractures (Figure 6).
Figure 6: Femur fractures in EP1 antagonist treated mice heal with increase mechanical strength:
Biomechanical testing on day 21 after fracture. Mice were with either Vehicle or EP1 antagonist for 5 days, starting on post fracture day 1. The experiment is presented as fold differences between paired EP1 antagonist treated mice and Vehicle treated mice. Altogether an N=9 animals per group was examined in two separate experimental cohorts that were combined. Statistical analysis was performed using paired student t-test. (*)=p<0.05 vs. age-matched vehicle treated animals. The mean values of the biomechanical measurements for the combined groups was Torsional rigidity (Vehicle = 125.3 ± 52.92 [N.mm/(rad/mm)]; EP1 antagonist = 261.1 ± 63.7 [N.mm/(rad/mm)]); Maximum torque (Vehicle = 6.46 ± 1.98 (N.mm); EP1 antagonist = 10.62 ± 1.16 (N.mm)); Yield to torque (Vehicle = 5.17 ± 1.67 (N.mm); EP1 antagonist = 8.2 ± 0.8 (N.mm)); and Energy to maximum (Vehicle = 0.35 ± 0.068 (N.mm*(rad/mm) vehicle; EP1 Antagonist = 0.8 ± 0.19 [N.mm*(rad/mm)]).
Discussion
We have previously demonstrated that EP1−/− mice have increased bone formation and accelerated fracture repair in vivo, as well as increased osteogenic differentiation of bone marrow cells in vitro21, 68. In the present study we sought to determine the relationship between the EP1 receptor and periosteal stem cell population and to explore the possible therapeutic potential of an EP1 antagonist to enhance bone regeneration. PGE2 has been shown to induce both anabolic and catabolic effects with overall enhanced bone formation through the activation of the EP2 and EP4 receptors65. While the role of EP2 and EP4 are well described, very little is known about the role of EP1 in bone homeostasis. In the present study, the data shows that EP1 receptor down regulation induces PDMPC differentiation. Furthermore, systemic administration of an EP1 specific antagonist during fracture repair accelerates healing, likely by enhancing differentiation of PDMPCs toward the osteoblastic lineage. Thus, our findings identify EP1 antagonism as a novel therapeutic approach to improve bone regeneration by modulating periosteal progenitor cell function. Using a combination of cellular-based, biomechanical and molecular assays, we provide evidence that EP1 pharmacologic inhibition alters the periosteal derived progenitor cell differentiation potential towards osteogenic lineage.
By analyzing the colony forming ability, we observed a higher number of both CFU-Fs and CFU-Os of EP1−/− mice and a higher ratio of CFU-O/CFU-Fs in periosteal cells from EP1−/− mice compared to WT mice. Considering that the cultures were not stimulated with osteogenic medium, the increased osteogenic differentiation of EP1−/− cells suggests that these cells were already committed to the osteogenic lineage at the time of isolation. We recently had similar findings in wild type and EP1−/− bone marrow progenitor cell populations, suggesting that progenitor cells in bone marrow and in the periosteum are both regulated by EP1 gene deletion21. We characterized the cells by analyzing the cell surface markers Sca1 and CD105 in the PDMPCs isolated from WT and EP1−/− mice. We observed that a higher percentage of cells from the EP1−/− mice are Sca1+/CD105 negative. Moreover, we showed that treatment of WT mice with an EP-1 antagonist similarly resulted in an increase in the percentage of Sca1+/CD105- cells. PDMPCs isolated from EP1 antagonist treated mice also had enhanced in vitro osteoblast differentiation.
While markers specific for different stages of mesenchymal progenitor cell differentiation are not well defined10, 12, 40, there are multiple studies demonstrating that progenitor cells that lose the expression of CD105 (also known as Endoglin) represents a subpopulation of multipotent stromal cells with increased osteogenic and/or chondrogenic differentiation capacities. Differentiation of umbilical cord MSCs into osteogenic and chondrogenic lineage was accompanied by reduction in CD105 expression3, 6. In adipose tissue, two separate studies showed that CD105− cells have increased osteogenic potential. Moreover, down regulation of CD105 expression using shRNA against CD105 resulted in enhanced osteoblastic differentiation of adipose derived MSCs32, 38. A recent study showed that the loss of CD105 in tendon isolated stromal cells was associated with enhanced differentiation into the chondrogenic lineage4. Most importantly, CD105− cells had increased in vivo repair capacity as demonstrated using the calvarial critical size model38. These collective studies suggest that CD105− cells represent a subpopulation of progenitors with increased differentiation potential. In line with this, we observed that in in vitro assays periosteal CD105− cells exhibit increased potential to form mineralized nodules and increased expression of osteoblastic markers compared to CD105+ cells. Considering that we did not observe differences in the nodule potential formation between WT CD105+ and EP1−/− CD105+ cells or between WT CD105− cells and EP1−/− CD105− cells, we can speculate that the enhanced differentiation of EP1−/− periosteal cells is due to the higher number of the CD105− cells compared to WT periosteal cells.
Interestingly, when testing for the differentiation potential, we observed that EP1−/− PDMPCs had increased osteoblastic differentiation with slight but significant decrease in adipogenic differentiation potential. As the osteogenic and adipogenic differentiations have been shown to have reciprocal potential13, 33, it is possible that in EP1−/− mice the enhanced differentiation into the osteoblastic lineage results concomitantly in decrease in the adipogenic differentiation. This potentially can be an important research area as part of the age-related changes in bone is increased differentiation into adipocytes instead of into osteoblasts, resulting in bone loss30. More studies need to be done to test the role of EP1 receptor in adipogenic differentiation.
Based on this observation that down regulation of EP1 increases the number of committed progenitors we tested whether blocking the EP1 receptor pharmacologically can be used as a drug target to enhance repair. The use of a specific EP1 antagonist in animal studies was previously described in other systems1, 23, 50. We used the established dosage in these studies to treat wild type animals and analyze the repair process.29, 41, 50 To direct the effect of the antagonist to the inflammatory stage, we inhibited EP1 receptor activity by EP1 specific antagonist on days 1–5 after fracture. This treatment strategy provides clues for understanding the role of EP1signaling during the early MSC proliferation and differentiation phases of fracture repair.
Our first observation was that following treatment with EP1 antagonist the PDMPCs change their phenotype, as indicated by an increase in the population of CD105− cells. We observed that after 5 days of treatment the percentage of the CD105− cells increased as well as the CFU-O numbers, suggesting that EP1 antagonist treatment promotes the number of committed progenitors at the periosteum, reaching similar percentage as in EP1−/− mice. It is worth noting that we did not observe any changes in flow cytometry analysis of EP1−/− mice following EP1 antagonist administration, suggesting that there was not an off-target effect. This increase in percentage of committed progenitors resulted in increased callus at day 10 after fracture and resulted in bigger bone area at day 21after fracture. The bone volume and the mineralization of the callus was significantly higher at the EP1 antagonist treated group. This supports our in vitro data that EP1receptor down regulation specifically promotes PDMPC differentiation into the osteoblastic lineage. Remarkably, the biomechanical properties of EP1 antagonist treated fractures were significantly increased compared to the vehicle treated animals.
The significance of the findings is enhanced by the fact that EP1 antagonist treatments are in Phase II clinical trials and show limited side effects14, 35. Moreover, efficacy has been demonstrated in the treatment of esophageal pain hypersensitivity in patients with gastric reflux disease, indicating that an EP1 receptor inhibition approach can be translated to clinical trials to improve fracture healing.7,8 An added advantage of EP1 antagonist treatment is that inhibition of EP1 has been shown to reduce pain perception42, 43, 58. Currently, the anti-inflammatory drugs that are used as analgesics are COX2 inhibitors (NSAIDS), which block PGE2 production. However, since PGE2 has an bone anabolic effect mediated by the EP2 and EP4 receptors, NSAIDs used for analgesia can impair fracture healing25, 34, 47. Interestingly, studies suggest the reduction in pain following NSAID mediated by COX-2/PGE2 inhibition may in fact be secondary to decreased EP1 receptor activation28, 31, 45. Thus, pharmacologic agents that selectively inhibit EP1 may have the dual effect to of directly relieving pain and stimulating fracture healing.
Taken together, the studies presented here suggest that treatment with an EP1 antagonist at the first stages of the healing cascade alter the periosteal progenitor population, possibly by increasing the population of more osteoblastic progenitors. These initial changes in the cellular population result in an accelerated healing response, consistent with the healing response in EP1−/− mice. Moreover, the increased mechanical properties in the EP1 antagonist treated group suggest that the accelerated repair process results in functional bone, thus making the EP1 receptor a possible target to improve healing.
In summary, using a genetic model and a translational approach we demonstrated that EP1 is a negative regulator of bone formation, and therefore a novel therapeutic target for bone formation and regeneration. Future studies will determine if EP1 antagonism can stimulate healing in animal models with compromised healing, such as aging and obesity. The ultimate goal is to establish an anabolic for EP1 antagonism in bone regeneration that can be translated to improve fracture healing in humans.
Acknowledgements:
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award number R01AR048681 (RJO).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Abe T, Kunz A, Shimamura M, Zhou P, Anrather J and Iadecola C. The neuroprotective effect of prostaglandin E2 EP1 receptor inhibition has a wide therapeutic window, is sustained in time and is not sexually dimorphic. J Cereb Blood Flow Metab 29(1): 66–72, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen MR, Hock JM and Burr DB. Periosteum: biology, regulation, and response to osteoporosis therapies. Bone 35(5): 1003–1012, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Anderson P, Carrillo-Galvez AB, Garcia-Perez A, Cobo M and Martin F. CD105 (endoglin)-negative murine mesenchymal stromal cells define a new multipotent subpopulation with distinct differentiation and immunomodulatory capacities. PLoS One 8(10): e76979, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asai S, Otsuru S, Candela ME, Cantley L, Uchibe K, Hofmann TJ, Zhang K, Wapner KL, Soslowsky LJ, Horwitz EM and Enomoto-Iwamoto M. Tendon Progenitor Cells in Injured Tendons Have Strong Chondrogenic Potential: The CD105-Negative Subpopulation Induces Chondrogenic Degeneration. Stem Cells 32(12): 3266–3277, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashman O and Phillips AM. Treatment of non-unions with bone defects: which option and why? Injury 44 Suppl 1: S43–45, 2013. [DOI] [PubMed] [Google Scholar]
- 6.Bai XM, Jiang H, Ding JX, Peng T, Ma J, Wang YH, Zhang L, Zhang H and Leng J. Prostaglandin E2 upregulates survivin expression via the EP1 receptor in hepatocellular carcinoma cells. Life Sci 86(5–6): 214–223, 2010. [DOI] [PubMed] [Google Scholar]
- 7.Balakumaran A, Mishra PJ, Pawelczyk E, Yoshizawa S, Sworder BJ, Cherman N, Kuznetsov SA, Bianco P, Giri N, Savage SA, Merlino G, Dumitriu B, Dunbar CE, Young NS, Alter BP and Robey PG. Bone marrow skeletal stem/progenitor cell defects in dyskeratosis congenita and telomere biology disorders. Blood 125(5): 793–802, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bianco P, Cao X, Frenette PS, Mao JJ, Robey PG, Simmons PJ and Wang CY. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med 19(1): 35–42, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bianco P, Kuznetsov SA, Riminucci M and Gehron Robey P. Postnatal skeletal stem cells. Methods Enzymol 419: 117–148, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Bianco P, Robey PG and Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell 2(4): 313–319, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brochhausen C, Neuland P, Kirkpatrick CJ, Nusing RM and Klaus G. Cyclooxygenases and prostaglandin E2 receptors in growth plate chondrocytes in vitro and in situ--prostaglandin E2 dependent proliferation of growth plate chondrocytes. Arthritis Res Ther 8(3): R78, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan CK, Lindau P, Jiang W, Chen JY, Zhang LF, Chen CC, Seita J, Sahoo D, Kim JB, Lee A, Park S, Nag D, Gong Y, Kulkarni S, Luppen CA, Theologis AA, Wan DC, DeBoer A, Seo EY, Vincent-Tompkins JD, Loh K, Walmsley GG, Kraft DL, Wu JC, Longaker MT and Weissman IL. Clonal precursor of bone, cartilage, and hematopoietic niche stromal cells. Proc Natl Acad Sci U S A 110(31): 12643–12648, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan CK, Seo EY, Chen JY, Lo D, McArdle A, Sinha R, Tevlin R, Seita J, Vincent-Tompkins J, Wearda T, Lu WJ, Senarath-Yapa K, Chung MT, Marecic O, Tran M, Yan KS, Upton R, Walmsley GG, Lee AS, Sahoo D, Kuo CJ, Weissman IL and Longaker MT. Identification and specification of the mouse skeletal stem cell. Cell 160(1–2): 285–298, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chapple CR, Abrams P, Andersson KE, Radziszewski P, Masuda T, Small M, Kuwayama T and Deacon S. Phase II study on the efficacy and safety of the EP1 receptor antagonist ONO-8539 for nonneurogenic overactive bladder syndrome. J Urol 191(1): 253–260, 2014. [DOI] [PubMed] [Google Scholar]
- 15.Coleman RA, Smith WL and Narumiya S. International Union of Pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol Rev 46(2): 205–229, 1994. [PubMed] [Google Scholar]
- 16.Colnot C Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. J Bone Miner Res 24(2): 274–282, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Del Toro F Jr., Sylvia VL, Schubkegel SR, Campos R, Dean DD, Boyan BD and Schwartz Z. Characterization of prostaglandin E(2) receptors and their role in 24,25-(OH)(2)D(3)-mediated effects on resting zone chondrocytes. J Cell Physiol 182(2): 196–208, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Einhorn TA. The cell and molecular biology of fracture healing. Clin Orthop Relat Res(355 Suppl): S7–21, 1998. [DOI] [PubMed] [Google Scholar]
- 19.Einhorn TA The science of fracture healing. Journal of Orthopaedic Trauma 19(10 Suppl): S4–6, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Fayaz HC, Giannoudis PV, Vrahas MS, Smith RM, Moran C, Pape HC, Krettek C and Jupiter JB. The role of stem cells in fracture healing and nonunion. Int Orthop 35(11): 1587–1597, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feigenson M, Eliseev RA, Jonason JH, Mills BN and O’Keefe RJ. PGE2 Receptor Subtype 1 (EP1) Regulates Mesenchymal Stromal Cell Osteogenic Differentiation by Modulating Cellular Energy Metabolism. J Cell Biochem 118(12): 4383–4393, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujita D, Yamashita N, Iita S, Amano H, Yamada S and Sakamoto K. Prostaglandin E2 induced the differentiation of osteoclasts in mouse osteoblastdepleted bone marrow cells. Prostaglandins Leukot Essent Fatty Acids 68(5): 351–358, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Fukumoto K, Takagi N, Yamamoto R, Moriyama Y, Takeo S and Tanonaka K. Prostanoid EP1 receptor antagonist reduces blood-brain barrier leakage after cerebral ischemia. Eur J Pharmacol 640(1–3): 82–86, 2010. [DOI] [PubMed] [Google Scholar]
- 24.Gerstenfeld LC, Cullinane DM, Barnes GL, Graves DT and Einhorn TA. Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J Cell Biochem 88(5): 873–884, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Giannoudis PV, MacDonald DA, Matthews SJ, Smith RM, Furlong AJ and De Boer P. Nonunion of the femoral diaphysis. The influence of reaming and non-steroidal anti-inflammatory drugs. J Bone Joint Surg Br 82(5): 655–658, 2000. [DOI] [PubMed] [Google Scholar]
- 26.Granero-Molto F, Weis JA, Miga MI, Landis B, Myers TJ, O’Rear L, Longobardi L, Jansen ED, Mortlock DP and Spagnoli A. Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem Cells 27(8): 1887–1898, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guan Y, Zhang Y, Wu J, Qi Z, Yang G, Dou D, Gao Y, Chen L, Zhang X, Davis LS, Wei M, Fan X, Carmosino M, Hao C, Imig JD, Breyer RM and Breyer MD. Antihypertensive effects of selective prostaglandin E2 receptor subtype 1 targeting. J Clin Invest 117(9): 2496–2505, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hall A, Brown SH, Budd C, Clayton NM, Giblin GM, Goldsmith P, Hayhow TG, Hurst DN, Naylor A, Anthony Rawlings D, Scoccitti T, Wilson AW and Winchester WJ. Discovery of GSK345931A: An EP(1) receptor antagonist with efficacy in preclinical models of inflammatory pain. Bioorg Med Chem Lett 19(2): 497–501, 2009. [DOI] [PubMed] [Google Scholar]
- 29.Hallinan EA, Hagen TJ, Husa RK, Tsymbalov S, Rao SN, vanHoeck JP, Rafferty MF, Stapelfeld A, Savage MA and Reichman M. N-substituted dibenzoxazepines as analgesic PGE2 antagonists. J Med Chem 36(22): 3293–3299, 1993. [DOI] [PubMed] [Google Scholar]
- 30.Holmes C, Khan TS, Owen C, Ciliberti N, Grynpas MD and Stanford WL. Longitudinal analysis of mesenchymal progenitors and bone quality in the stem cell antigen-1-null osteoporotic mouse. J Bone Miner Res 22(9): 1373–1386, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Hori T, Oka T, Hosoi M and Aou S. Pain modulatory actions of cytokines and prostaglandin E2 in the brain. Ann N Y Acad Sci 840: 269–281, 1998. [DOI] [PubMed] [Google Scholar]
- 32.Ishibashi O, Ikegame M, Takizawa F, Yoshizawa T, Moksed MA, Iizawa F, Mera H, Matsuda A and Kawashima H. Endoglin is involved in BMP-2-induced osteogenic differentiation of periodontal ligament cells through a pathway independent of Smad-1/5/8 phosphorylation. J Cell Physiol 222(2): 465–473, 2010. [DOI] [PubMed] [Google Scholar]
- 33.James AW Review of Signaling Pathways Governing MSC Osteogenic and Adipogenic Differentiation. Scientifica (Cairo) 2013: 684736, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeffcoach DR, Sams VG, Lawson CM, Enderson BL, Smith ST, Kline H, Barlow PB, Wylie DR, Krumenacker LA, McMillen JC, Pyda J and Daley BJ. Nonsteroidal anti-inflammatory drugs’ impact on nonunion and infection rates in long-bone fractures. J Trauma Acute Care Surg 76(3): 779–783, 2014. [DOI] [PubMed] [Google Scholar]
- 35.Kondo T, Sei H, Yamasaki T, Tomita T, Ohda Y, Oshima T, Fukui H, Watari J and Miwa H. A novel prostanoid EP1 receptor antagonist, ONO-8539, reduces acid-induced heartburn symptoms in healthy male volunteers: a randomized clinical trial. J Gastroenterol 2017. [DOI] [PubMed] [Google Scholar]
- 36.Kuznetsov SA, Krebsbach PH, Satomura K, Kerr J, Riminucci M, Benayahu D and Robey PG. Single-colony derived strains of human marrow stromal fibroblasts form bone after transplantation in vivo. J Bone Miner Res 12(9): 1335–1347, 1997. [DOI] [PubMed] [Google Scholar]
- 37.Kuznetsov SA, Mankani MH, Bianco P and Robey PG. Enumeration of the colony-forming units-fibroblast from mouse and human bone marrow in normal and pathological conditions. Stem Cell Res 2(1): 83–94, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levi B, Wan DC, Glotzbach JP, Hyun J, Januszyk M, Montoro D, Sorkin M, James AW, Nelson ER, Li S, Quarto N, Lee M, Gurtner GC and Longaker MT. CD105 protein depletion enhances human adipose-derived stromal cell osteogenesis through reduction of transforming growth factor beta1 (TGF-beta1) signaling. J Biol Chem 286(45): 39497–39509, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li X, Pilbeam CC, Pan L, Breyer RM and Raisz LG. Effects of prostaglandin E2 on gene expression in primary osteoblastic cells from prostaglandin receptor knockout mice. Bone 30(4): 567–573, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Mafi R, Hindocha S, Mafi P, Griffin M and Khan WS. Sources of adult mesenchymal stem cells applicable for musculoskeletal applications – a systematic review of the literature. Open Orthop J 5 Suppl 2: 242–248, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malmberg AB, Rafferty MF and Yaksh TL. Antinociceptive effect of spinally delivered prostaglandin E receptor antagonists in the formalin test on the rat. Neurosci Lett 173(1–2): 193–196, 1994. [DOI] [PubMed] [Google Scholar]
- 42.Miki T, Matsunami M, Nakamura S, Okada H, Matsuya H and Kawabata A. ONO-8130, a selective prostanoid EP1 receptor antagonist, relieves bladder pain in mice with cyclophosphamide-induced cystitis. Pain 152(6): 1373–1381, 2011. [DOI] [PubMed] [Google Scholar]
- 43.Minami T, Nakano H, Kobayashi T, Sugimoto Y, Ushikubi F, Ichikawa A, Narumiya S and Ito S. Characterization of EP receptor subtypes responsible for prostaglandin E2-induced pain responses by use of EP1 and EP3 receptor knockout mice. Br J Pharmacol 133(3): 438–444, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murao H, Yamamoto K, Matsuda S and Akiyama H. Periosteal cells are a major source of soft callus in bone fracture. J Bone Miner Metab 31(4): 390–398, 2013. [DOI] [PubMed] [Google Scholar]
- 45.Nakayama Y, Omote K, Kawamata T and Namiki A. Role of prostaglandin receptor subtype EP1 in prostaglandin E2-induced nociceptive transmission in the rat spinal dorsal horn. Brain Res 1010(1–2): 62–68, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Negishi M, Sugimoto Y and Ichikawa A. Molecular mechanisms of diverse actions of prostanoid receptors. Biochim Biophys Acta 1259(1): 109–119, 1995. [DOI] [PubMed] [Google Scholar]
- 47.O’Connor JP, Manigrasso MB, Kim BD and Subramanian S. Fracture healing and lipid mediators. Bonekey Rep 3: 517, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ono K, Akatsu T, Murakami T, Nishikawa M, Yamamoto M, Kugai N, Motoyoshi K and Nagata N. Important role of EP4, a subtype of prostaglandin (PG) E receptor, in osteoclast-like cell formation from mouse bone marrow cells induced by PGE2. J Endocrinol 158(3): R1–5, 1998. [DOI] [PubMed] [Google Scholar]
- 49.Owen M and Friedenstein AJ. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found Symp 136: 42–60, 1988. [DOI] [PubMed] [Google Scholar]
- 50.Pekcec A, Unkruer B, Schlichtiger J, Soerensen J, Hartz AM, Bauer B, van Vliet EA, Gorter JA and Potschka H. Targeting prostaglandin E2 EP1 receptors prevents seizure-associated P-glycoprotein up-regulation. J Pharmacol Exp Ther 330(3): 939–947, 2009. [DOI] [PubMed] [Google Scholar]
- 51.Praemer A, Furner S and Rice DP. Musculoskeletal Conditions in the United States. IL,Rosemont: (1999) [Google Scholar]
- 52.Raisz LG Physiologic and pathologic roles of prostaglandins and other eicosanoids in bone metabolism. J Nutr 125(7 Suppl): 2024S–2027S, 1995. [DOI] [PubMed] [Google Scholar]
- 53.Raisz LG Prostaglandins and bone: physiology and pathophysiology. Osteoarthritis Cartilage 7(4): 419–421, 1999. [DOI] [PubMed] [Google Scholar]
- 54.Reynolds DG, Shaikh S, Papuga MO, Lerner AL, O’Keefe RJ, Schwarz EM and Awad HA. muCT-based measurement of cortical bone graft-tohost union. J Bone Miner Res 24(5): 899–907, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shamir D, Keila S and Weinreb M. A selective EP4 receptor antagonist abrogates the stimulation of osteoblast recruitment from bone marrow stromal cells by prostaglandin E2 in vivo and in vitro. Bone 34(1): 157–162, 2004. [DOI] [PubMed] [Google Scholar]
- 56.Simon AM, Manigrasso MB and O’Connor JP. Cyclo-oxygenase 2 function is essential for bone fracture healing. J Bone Miner Res 17(6): 963–976, 2002. [DOI] [PubMed] [Google Scholar]
- 57.Singh AK and Sinha A. Percutaneous autologous bone marrow injections for delayed or non-union of bones. J Orthop Surg (Hong Kong) 21(2): 267, 2013. [DOI] [PubMed] [Google Scholar]
- 58.Stock JL, Shinjo K, Burkhardt J, Roach M, Taniguchi K, Ishikawa T, Kim HS, Flannery PJ, Coffman TM, McNeish JD and Audoly LP. The prostaglandin E2 EP1 receptor mediates pain perception and regulates blood pressure. J Clin Invest 107(3): 325–331, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suda M, Tanaka K, Natsui K, Usui T, Tanaka I, Fukushima M, Shigeno C, Konishi J, Narumiya S, Ichikawa A and Nakao N. Prostaglandin E receptor subtypes in mouse osteoblastic cell line. Endocrinology 137(5): 1698–1705, 1996. [DOI] [PubMed] [Google Scholar]
- 60.Sugimoto Y and Narumiya S. Prostaglandin E receptors. J Biol Chem 282(16): 11613–11617, 2007. [DOI] [PubMed] [Google Scholar]
- 61.Suzawa T, Miyaura C, Inada M, Maruyama T, Sugimoto Y, Ushikubi F, Ichikawa A, Narumiya S and Suda T. The role of prostaglandin E receptor subtypes (EP1, EP2, EP3, and EP4) in bone resorption: an analysis using specific agonists for the respective EPs. Endocrinology 141(4): 1554–1559, 2000. [DOI] [PubMed] [Google Scholar]
- 62.Tsutsumi R, Xie C, Wei X, Zhang M, Zhang X, Flick LM, Schwarz EM and O’Keefe RJ. PGE2 signaling through the EP4 receptor on fibroblasts upregulates RANKL and stimulates osteolysis. J Bone Miner Res 24(10): 1753–1762, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ueno M, Uchida K, Takaso M, Minehara H, Suto K, Takahira N, Steck R, Schuetz MA and Itoman M. Distribution of bone marrow-derived cells in the fracture callus during plate fixation in a green fluorescent protein-chimeric mouse model. Exp Anim 60(5): 455–462, 2011. [DOI] [PubMed] [Google Scholar]
- 64.Weinreb M, Shamir D, Machwate M, Rodan GA, Harada S and Keila S. Prostaglandin E2 (PGE2) increases the number of rat bone marrow osteogenic stromal cells (BMSC) via binding the EP4 receptor, activating sphingosine kinase and inhibiting caspase activity. Prostaglandins Leukot Essent Fatty Acids 75(2): 81–90, 2006. [DOI] [PubMed] [Google Scholar]
- 65.Xie C, Liang B, Xue M, Lin AS, Loiselle A, Schwarz EM, Guldberg RE, O’Keefe RJ and Zhang X. Rescue of impaired fracture healing in COX-2−/− mice via activation of prostaglandin E2 receptor subtype 4. Am J Pathol 175(2): 772–785, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu HH, Zhao L and Weir MD. Stem cell-calcium phosphate constructs for bone engineering. J Dent Res 89(12): 1482–1488, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang M, Feigenson M, Sheu TJ, Awad HA, Schwarz EM, Jonason JH, Loiselle AE and O’Keefe RJ. Loss of the PGE2 receptor EP1 enhances bone acquisition, which protects against age and ovariectomy-induced impairments in bone strength. Bone 72: 92–100, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang M, Ho HC, Sheu TJ, Breyer MD, Flick LM, Jonason JH, Awad HA, Schwarz EM and O’Keefe RJ. EP1(−/−) mice have enhanced osteoblast differentiation and accelerated fracture repair. J Bone Miner Res 26(4): 792–802, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang X, Naik A, Xie C, Reynolds D, Palmer J, Lin A, Awad H, Guldberg R, Schwarz E and O’Keefe R. Periosteal stem cells are essential for bone revitalization and repair. J Musculoskelet Neuronal Interact 5(4): 360–362, 2005. [PubMed] [Google Scholar]
- 70.Zhang X, Xie C, Lin AS, Ito H, Awad H, Lieberman JR, Rubery PT, Schwarz EM, O’Keefe RJ and Guldberg RE. Periosteal progenitor cell fate in segmental cortical bone graft transplantations: implications for functional tissue engineering. J Bone Miner Res 20(12): 2124–2137, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]