Abstract
Background:
A single dose of IV fish oil (FO) before hepatic ischemia reperfusion injury (HIRI) increases hepatocyte proliferation and reduces necrosis in wild type (WT) mice. It has been suggested that the GPR120 receptor on Kupffer cells mediates FO’s ability to reduce HIRI. The purpose of this study was to determine whether GPR120 is required for FO to reduce HIRI. Methods: Sixty-four (n=8/group) adult male WT (C57BL/6) and GPR120 knockout (KO) mice received IV FO (1 g/kg) or saline 1 hour prior to HIRI or sham operation. Mice were euthanized 24 hours post-operatively for analysis of hepatic histology, NFκB activity, and serum alanine transaminase (ALT) levels.
Results:
FO pre-treated livers had less necrosis after HIRI than saline pre-treated livers in both WT (mean±SEM 25.9±7.3% less, P=0.007) and KO (36.6±7.3% less, P<0.0001) mice. There was no significant difference in percent necrosis between WT-–FO and KO–FO groups. Sham groups demonstrated minimal necrosis (0–1.9%). Mean [95% CI] ALT after HIRI was significantly higher (P=0.04) in WT-Saline mice (1604 U/L [751–3427]) compared to WT–FO (321 U/L [150–686]) but was not significantly higher in KO-Saline mice compared to KO-FO. There were no differences in ALT between WT-FO and KO-FO mice who underwent HIRI or between groups who underwent sham surgery. There were no differences in NFκB or IKKβ activation among groups as measured by Western blot analysis.
Conclusions:
IV FO pretreatment was able to reduce HIRI in GPR120 KO mice, suggesting the hepatoprotective effects of FO are not mediated by GPR120 alone.
Keywords: fish oil, liver, free fatty acid receptor 4, ischemia, reperfusion, GPR120
1. Introduction
Despite the fact that ischemia reperfusion injury and its effect on liver surgery have long been recognized, there are currently no available therapies to reliably prevent or reduce hepatic ischemia reperfusion injury (HIRI). Thus, HIRI continues to be a major barrier to liver surgery and transplantation1–4, particularly in steatotic livers5–9.
Intravenous (IV) fish oil (FO) is an injectable lipid emulsion rich in omega-3 fatty acids (O3FA) and the anti-oxidant α-tocopherol (vitamin E). The administration of a single dose of IV FO one hour before surgically-induced hepatic ischemia has been shown to reduce HIRI in a mouse model10,11.
O3FA, particularly the biochemically active downstream fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are metabolized to anti-inflammatory and pro-resolving mediators12–14. EPA and DHA also directly activate free fatty acid receptors, including the G-protein coupled receptor 120 (GPR120) (also known as free fatty acid receptor 4)15,16. Activation of GPR120 on macrophages, including hepatic Kupffer cells, inhibits pro-inflammatory signaling through the nuclear factor kappa-B (NFκB) and c-Jun N-terminal kinase (JNK) pathways16,17.
A study by Raptis et al. suggested that activation of GPR120 on hepatic Kupffer cells is the mechanism that mediates FO’s ability to reduce HIRI11. However, this study used a synthetic, non-specific free fatty acid receptor agonist (GW9508) and also used clodronate to deplete Kupffer cells to come to this conclusion11. Kupffer cells are critical in initiating HIRI, so their depletion may have additional, GPR120-independent, effects18. Thus, experiments using congenic GPR120 knockout (KO) mice are needed to fully examine its role.
The purpose of this study was to determine whether GPR120 is required for FO to reduce HIRI. Our hypothesis was that pre-treatment with FO would reduce HIRI, as evidenced by a reduction in necrosis, in wild type (WT) mice and this effect would be attenuated in GPR120 KO mice.
2. Methods and Methods
2.1. Animals
All experiments were approved by Boston Children’s Hospital Institutional Animal Care and Use Committee. Sixty-four 6- to 8-week-old male mice were used for this study, including 32 GPR120 KO and 32 WT littermates. KO mice were initially acquired from the UC Davis Knockout Mouse Project Repository and bred with C57BL/6 (Jackson Laboratories, Bar Harbor, ME) mice. Genotypes were confirmed for all mice at 3 weeks of age (Transnetyx, Cordova, TN). Mice were housed 3–5 to a cage in a climate-controlled facility with 12/12 hour light/dark cycles and allowed ad libitum access to standard chow and water. There were 8 mice per group and 8 groups evaluated in a 2 × 2 × 2 factorial experiment (HIRI vs. sham operation × KO vs. WT × FO vs. saline).
2.2. Experimental procedures
One hour prior to surgery, mice received 1 g/kg of IV FO (Omegaven®; Fresenius Kabi, Germany) or isovolumetric saline (0.9% NaCl) via tail vein injection. Mice were then randomized to undergo either HIRI or sham operation under anesthesia with inhaled isoflurane (1–3% continuously via nose cone) as previously described10. Briefly, the HIRI procedure consisted of an upper midline laparotomy with suture ligature occlusion of the portal triads to the cephalad liver lobes for 30 minutes followed by reperfusion and abdominal wall closure. The sham procedure consisted of an upper midline laparotomy, anesthesia for 30 minutes, and abdominal wall closure.
Post-operatively, mice were returned to their cages and allowed ad libitum access to food and water. Analgesia was provided with buprenorphine (0.1 mg/kg subcutaneous every 8–12 hours). Animals were euthanized by carbon dioxide inhalation 24 hours after reperfusion (after closure for sham group). Blood was immediately collected via inferior vena cava puncture and placed on ice. Livers, spleens, and right kidneys were subsequently procured and weighed.
Serum was aspirated from centrifuged whole blood (2,348 g at 4°C for 18 minutes) and stored at ‒80°C. As previously described, liver collection and analysis was standardized to prevent any bias based on gross appearance10. For each mouse, the lateral half of the left lateral lobe (cut diagonally) and the inferior half of the right medial lobe (cut transversely) were marked, fixed in 10% formalin, paraffin embedded, and stained with hematoxylin and eosin (H&E) by the Boston Children’s Hospital clinical pathology department. The medial halves of the left lateral lobes and the superior halves of the right medial lobes were flash frozen in liquid nitrogen and stored at ‒80°C for protein analysis.
2.3. Outcomes and analysis
The primary outcome was percent necrosis, the percentage of hepatic parenchyma that was necrotic at the time of euthanasia (24 hours after reperfusion/closure). Secondary outcomes were serum alanine transaminase (ALT) and liver protein measurements by Western blot analysis to assess the NFκB pathway. Furthermore, pre-operative weights, weight loss after surgery (percent change in weight from pre-operative weight to pre-euthanasia weight), and organ weights (as a percentage of pre-euthanasia body weights) were compared.
H&E slides were reviewed by a masked board-certified pathologist in order to qualitatively compare microscopic anatomy. H&E slides were then used to calculate percent necrosis using ImageJ software (National Institutes of Health, Bethesda, MD). This was performed for each lobe for consecutive fields across the middle of the lobe at 100X magnification (line across) and for the lobe as a whole at 20X magnification (whole lobe).
Serum ALT was measured by the Boston Children’s Hospital clinical laboratory to quantify the degree of hepatic injury.
In order to determine whether O3FA activation of GPR120 protects the liver from HIRI by inhibiting NFκB-mediated inflammation, hepatic NFκB activity was assessed via Western blot analysis. In brief, 24 tissue samples (3 per experimental group) were run for determination of phosphorylated NFκB and inhibitor of nuclear factor kappa-B (IKKβ). The flash frozen liver samples stored at ‒80°C were cut to 25 mg per sample and homogenized in radioimmunoprecipitation assay (RIPA) buffer with protease and phosphate inhibitors (Thermo Fisher Scientific, Waltham, MA). Protein concentrations were determined utilizing a Bradford colorimetric assay (Bio-Rad, Hercules, CA). Proteins (40 μg per sample) were separated on 4–12% Bis-Tris polyacrylamide gels (Thermo Fisher Scientific, Waltham, MA) and transferred to nitrocellulose membranes. Membranes were probed with rabbit anti-NFκB p65, anti-phospho-IKKβ, anti–NFκB, and anti–IKKβ as the primary antibodies at 1:1000 dilution (Cell Signaling Technology, Danvers, MA). For each tested protein, the amount of its phosphorylated state was normalized to its total amount. These results were then normalized to the sham-saline-WT mice to express the results of the other groups in fold-change compared to the control.
2.4. Statistics
There were 64 independent observations for all outcomes except the primary outcome, percent necrosis, which included two observations (one from each liver lobe) from each mouse. The Wilcoxon signed-rank test was used to determine whether there were differences in percent necrosis between the right- and left-lobe pairs, and the results are reported as median and interquartile range (IQR). Regardless of the result of the signed-rank test, repeated measurements within a mouse were expected to be correlated to some degree and to contribute to the overall measurement error. To account for this, percent necrosis was analyzed with a repeated measures mixed model analysis of variance (ANOVA) with a compound symmetric covariance matrix. All other outcomes were analyzed with ordinary least-squares ANOVA. Each ANOVA model contained three independent variables including surgical procedure (HIRI vs. sham), genotype (KO vs. WT) and injection type (FO vs. saline), as well as all higher order interactions.
For each outcome, two preliminary tests of interaction between genotype (KO vs. WT) and injection type (FO vs. saline) were performed, one for the HIRI group and one for the sham group. If either of these tests was statistically significant, then three pairwise comparisons were examined within each surgical group, including WT–FO vs. WT–S (saline), WT–FO vs. KO–FO, and KO–FO vs. KO–S, for a total of six comparisons. Correction for multiple comparisons was made by the step-down Bonferroni method of Holm19.
ALT was analyzed on the log base 10 scale due to extreme skewness and the wide range of ALT observations. Population estimates were back-transformed to the original ALT units (U/L) by applying the antilogarithm and presented as mean (95% confidence interval; CI). The model for percent change in weight from pre-operative to pre-euthanasia assessment was adjusted for pre-operative weight.
ANOVA assumptions of normality and homogeneity of variance were checked by Shapiro-Wilk test and Brown and Forsythe test, respectively. All statistically significant results were corroborated by the nonparametric exact Wilcoxon rank-sum test. Results are reported as mean ± standard error of mean (SEM) unless otherwise noted. All tests of significance are two-sided with P<0.05 considered statistically significant. Data were analyzed using GraphPad Prism 7.0 (La Jolla, CA) and SAS v9.4 (Cary, NC).
3. Results
3.1. Body and organ weights
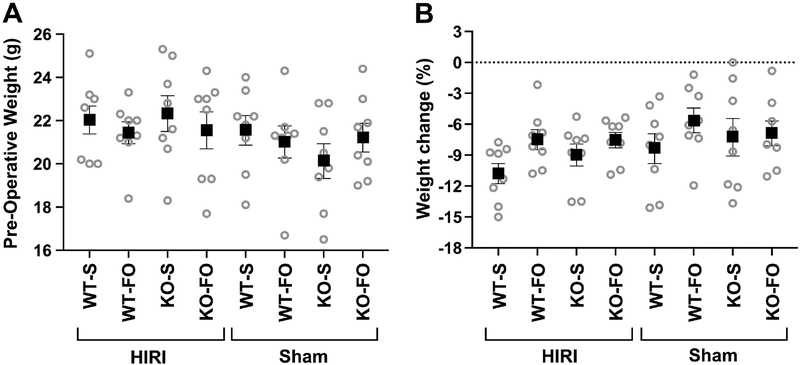
There were no differences in pre-operative body weight (Figure 1A) or in percent change in body weight from pre-operative to pre-euthanasia assessment (i.e. post-operative weight loss, which was mean±SEM 7.9±0.4% over all groups; Figure 1B) among the 8 experimental groups.
Figure 1:
(A) Pre-operative mouse weight and (B) percent change in body weight from pre-operative to pre-euthanasia assessment (i.e. post-operative weight loss) adjusted for pre-operative mouse weight, among experimental groups (n=8/group). There were no significant differences among groups based on 3–way ANOVA. Boxes represent mean and whiskers represent SEM. Abbreviations: FO, fish oil; HIRI, hepatic ischemia reperfusion injury; KO, knockout; S, saline; WT, wild type
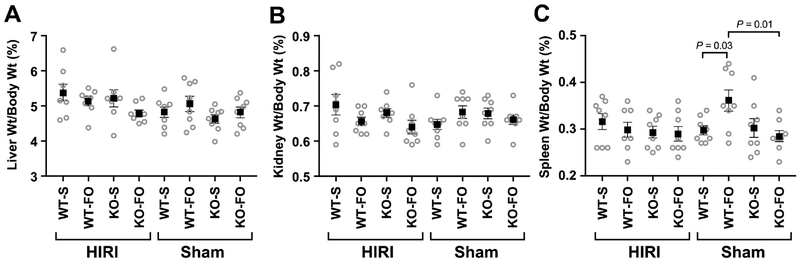
There were no differences in liver (Figure 2A) or kidney (Figure 2B) weights, as a percentage of pre-euthanasia body weights, among experimental groups. Among mice who underwent sham surgery, WT mice who received FO had larger spleens, on average, than WT mice who received saline and KO mice who received FO (Figure 2C).
Figure 2:
(A) Liver, (B) right kidney, and (C) spleen weight as a percentage of body weight at time of euthanasia by experimental group (n=8/group). Boxes represent mean and whiskers represent SEM. P value is from 3–way ANOVA. Abbreviations: FO, fish oil; HIRI, hepatic ischemia reperfusion injury; KO, knockout; S, saline; WT, wild type
3.2. Histologic liver analysis
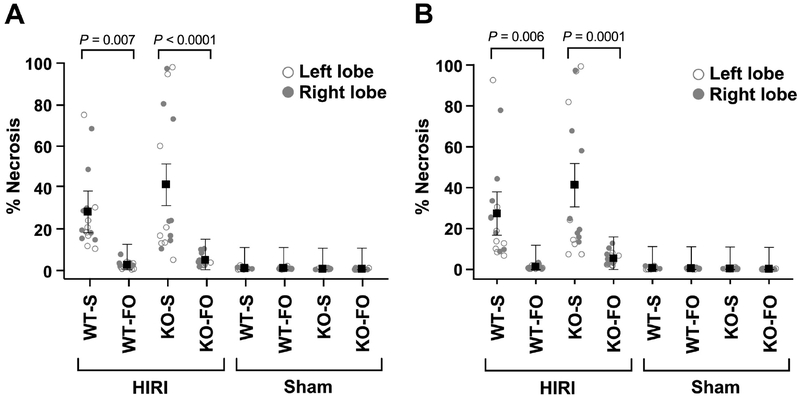
A board-certified pathologist blinded to group assignment was unable to appreciate any morphologic differences between WT and KO livers, kidneys, or spleens. An overall reduction in hepatic injury in mice (WT and KO) who received FO prior to HIRI compared to mice (WT and KO) who received saline prior to HIRI was noted. This was confirmed quantitatively by calculating percent necrosis in the standardized liver specimens.
Calculated for the entire specimen, there was no difference in percent necrosis between right- and left-lobe pairs (median difference‒0.1% (IQR ‒0.6 to 1.0%), P=0.62 by signed-rank test). Livers of mice who received FO prior to HIRI had, on average, 3.4±3.7% necrosis (range across right- and left-lobes 0.2–10.1%) and livers of mice who received saline prior to HIRI had, on average, 34.6±3.7% necrosis (range across right- and left-lobes 4.6–98.2%). FO pre-treated livers had less necrosis than saline pre-treated livers in both WT (25.9±7.3% less) and KO (36.6±7.3% less) mice (P=0.007 and P<0.0001 by mixed model ANOVA, respectively; Figure 3A). There was no significant difference in percent necrosis between WT-FO and KO-FO groups (mean difference 2.5±7.3%, P=1.00). Mice in all groups who underwent sham operation demonstrated minimal necrosis (range across right- and left-lobes 0.0–1.9%) with no differences among groups (P=1.00; Figure 3A).
Figure 3:
Percent area of liver necrosis for (A) the whole specimen at 20X magnification and (B) consecutive fields across the middle of each specimen at 100X magnification by experimental group (n=8/group). Mice were euthanized 24 hours after reperfusion (after closure for shams). Each mouse contributed two observations (right- and left-lobe) for a total of 64 paired observations. Shown are mean ± SEM by group. P values are from a repeated-measures mixed model 3-way ANOVA accounting for within-mouse correlation between right-and left-lobe specimens. Abbreviations: FO, fish oil; HIRI, hepatic ischemia reperfusion injury; KO, knockout; S, saline; WT, wild type
The results when calculated for consecutive fields in a line across the middle of the specimen at higher magnification are similar to the results calculated for the whole specimen (Figure 3B). Again there was no difference in percent necrosis between right- and left-lobe pairs (median difference 0.1% (IQR ‒0.2 to 1.6%), P=0.12 by signed-rank test). For mice who underwent HIRI, FO pre-treated livers had less necrosis than saline pre-treated livers in both WT (26.1±7.7& less, P=0.006) and KO (35.9±7.7% less, P=0.0001) mice and there was no statistical difference in percent necrosis between WT–FO and KO–FO groups (mean difference 4.1±7.7%, P=1.00; Figure 3B). For mice who underwent sham operation, there was minimal necrosis with no differences between groups (P=1.00; Figure 3B).
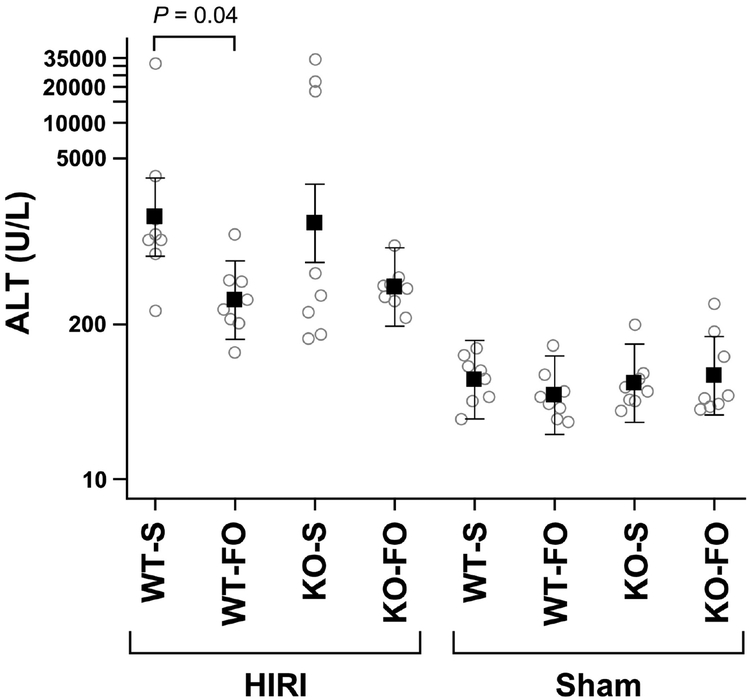
3.3. Serum ALT
Mean serum ALT was higher in WT mice who received saline prior to HIRI compared to those who received FO (1604 U/L (95% CI 751–3427) vs. 321 U/L (150–686), P=0.04; Figure 4). Although serum ALT in KO mice who received saline prior to HIRI had elevated mean ALT compared to those who received FO (1422 U/L (95% CI 666–3037) vs. 414 U/L (194–884)), the difference was not statistically significant after adjustment for multiple comparisons (P=0.23; Figure 4). There was no statistical difference in serum ALT between WT–FO and KO–FO mice who underwent HIRI (P=1.00; Figure 4). There were no differences in serum ALT between groups who underwent sham surgery (P=0.91; Figure 4).
Figure 4:
Serum ALT at time of euthanasia by experimental group (n=8/group). Shown are mean (95% confidence interval). P value is from a 3-way ANOVA.Abbreviations: FO, fish oil; HIRI, hepatic ischemia reperfusion injury; KO, knockout; S, saline; WT, wild type
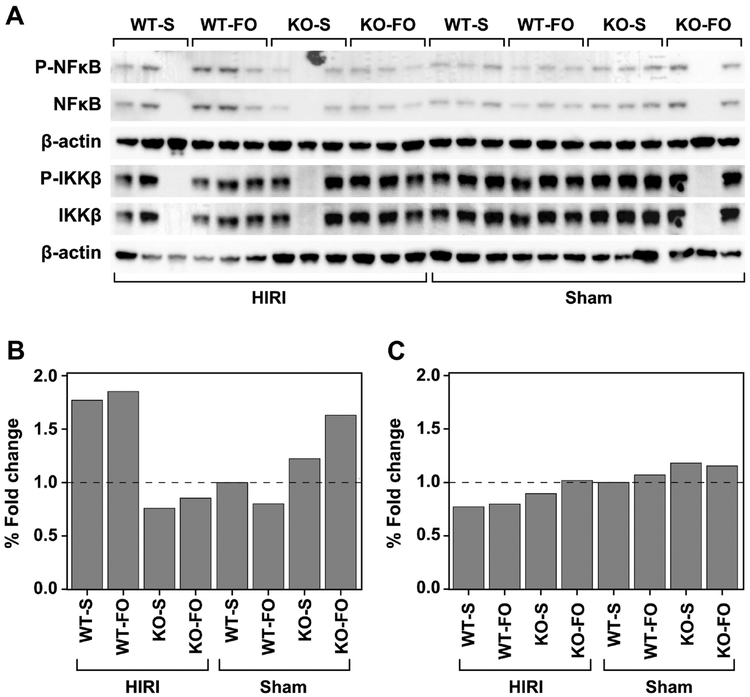
3.4. Protein expression of NFκB pathway
Overall, there was no difference in the level of activation of NFκB or IKKβ among the experimental groups (Figure 5).
Figure 5:
FO compared to saline pre-treatment before HIRI or sham operation does not affect hepatic NFκB expression in WT and KO mice. (A) Western blots. (B) NFκB and (C) IKKβ expression normalized to β-actin. Data are presented as the mean fold change, compared with the WT mice who received saline and underwent sham surgery. Abbreviations: FO, fish oil; HIRI, hepatic ischemia reperfusion injury; KO, knockout; S, saline; WT, wild type
4. Discussion
HIRI remains a barrier to liver transplantation and resection, including partial hepatectomy for donation1–4. HIRI is particularly a concern in steatotic livers, which limits their ability to be used for transplantation5–9,20. Currently, minimizing ischemia time is the only available and utilized preventative measure and there are no available treatment options21. A single bolus of prophylactic IV FO may reduce HIRI10,11. Elucidation of the mechanism by which FO reduces HIRI will accelerate the acceptance of this already available therapeutic option and facilitate the development of targeted therapies with higher potency.
In this study, we measured histologic necrosis in livers of WT and GPR120 KO mice who received a single bolus of IV FO or saline one hour prior to undergoing 70% hepatic ischemia or sham operation followed by 24 hours of reperfusion. We found that FO was able to reduce HIRI in both WT and KO mice, demonstrating that the GPR120 receptor is not required for FO to reduce HIRI.
A previous study suggested that GPR120 agonism is the mechanism by which FO reduces HIRI11. However, our study demonstrates that the hepatoprotective effects of FO are preserved in the absence of GPR120, further strengthening the concept that the mechanism of FO’s hepatoprotection is multifactorial14,22. In the liver, GPR120 is located on Kupffer cells11,16. Upon activation by its principal ligands EPA and DHA, GPR120 associates with β-arrestin2, internalizes into the cytoplasm, and binds to TAB1 (TAK1 binding protein 1)16. TAK1 (TGF–β activated kinase 1) requires TAB1 to become activated in order to activate the pro-inflammatory transcription factors NFκB and JNK. Thus, GPR120 activation prevents pro-inflammatory signaling in Kupffer cells by preventing TAK1 activity16. Although TAK1 is the point at which LPS and TLR signaling converge and affects the NFκB and JNK pathways, macrophages possess several redundant mechanisms for propagating inflammation and it is possible that these redundant pathways (e.g. O3FA’s activation of PPARα, which also inhibits NFκB activation) are able to bypass GPR120’s inhibition14,23–25.
Since GPR120 activation inhibits NFκB activation, we hypothesized that O3FA activation of GPR120 protects the liver from HIRI by inhibiting NFκB-mediated inflammation. We measured NFκB expression in the liver by Western blots and found no differences in IKKβ or NFκB activation between different treatment or genotype groups. Perhaps this should not be surprising given the fact that FO was able to reduce HIRI in KO mice and, again, there are several redundant pathways that affect NFκB activity14,23,25. However, it should be noted that liver tissue contains mostly hepatocytes with only a small population of Kupffer cells and that 24 hours may be too long after reperfusion to capture NFκB activation. Thus, it is possible that the inability to demonstrate differences in NFκB activation was due to methodology rather than physiology.
These findings are consistent with prior reports that FO is able to reduce HIRI. Pre-operative enteral O3FA supplementation has been shown to reduce HIRI in rodents26–30, although the mechanism of this protection is likely different (reduction of steatosis, increased omega-3:omega-6 fatty acid ratio in cell membranes) than that explored here with a single dose of IV FO14,31. Furthermore, pre-operative O3FA supplementation is not feasible for non-elective applications (such as deceased donor liver transplantation, trauma, and shock). Post-operative IV FO administration has been shown to improve outcomes after liver transplantation and hepatic resection in humans32,33, and this is an intriguing therapeutic option for those patients for whom pre-operative planning is not possible and/or in whom hepatic ischemia was unexpected. This study is consistent with previous findings that a single bolus of IV FO given one hour before hepatic ischemia can improve histologic outcomes in mice10,11. These findings support further investigations into the use of FO-based therapies for prevention, and possibly treatment, of HIRI while casting doubt on more targeted GPR120-specific therapies.
A major limitation of this study is the fact that IV FO contains not only O3FA but also α-tocopherol, an antioxidant, which could have confounded our results10. There have been opposing reports as to whether α-tocopherol affects HIRI11,21,34,35. However, even if α-tocopherol is not responsible for FO’s hepatoprotection, it likely contributes to it. Since IV FO cannot be manufactured without α-tocopherol, novel lipid emulsions with varying amounts of α-tocopherol are needed to determine its effect. GPR40 (also known as free fatty acid receptor 1) is also activated by both long-chain fatty acids such as EPA and DHA and by the agonist used by the Raptis group. Although GPR40 is not present on macrophages16 or in the liver at all36, GPR40 KO and GPR40/GPR120 double knockout studies would be necessary to determine whether GPR40 activation plays a role in FO’s hepatoprotection. The fact that we used a global GPR120 KO mouse model could affect our results as well, albeit through indirect and complex metabolic pathways, given that the other major sites of GPR120 expression and activity are intestinal cells, pancreatic islet cells, and adipocytes. However, recent studies have challenged the role of GPR120 in O3FA’s insulin sensitizing and anti-inflammatory effects37,38. Furthermore, Kupffer cell-specific GPR120 KO mice are not available and prior studies that have used siRNA to knockout GPR120 in macrophages (in vitro) and/or clodronate to deplete Kupffer cells fail to account for important cell-cell interactions11,16,39,40.
Our study aimed to determine whether GPR120 is required for FO’s ability to reduce HIRI and found that it is not. Although the rapid action of a single dose of IV FO given one hour prior to ischemia suggests cell surface receptor activation, it is possible that the anti-inflammatory and pro-resolving metabolites of O3FA are primarily responsible for FO’s protection from HIRI. Thus, while ongoing mechanistic studies are undoubtedly important for advancing our understanding of O3FA’s hepatoprotective effects and for designing more specific and efficacious treatment options, we feel they should not delay translational studies investigating the optimal timing and dose of IV FO, which is already commercially available.
5. Conclusions
A single bolus of IV FO given one hour prior to hepatic ischemia reduces HIRI in mice. We used GPR120 KO mice to determine whether this receptor is responsible for FO’s hepatoprotection and found that FO reduces HIRI even in the absence of the GPR120 receptor.
Sources of Support:
Supported by the Boston Children’s Hospital Surgical Foundation, the Joshua Ryan Rappaport Fellowship (PN), the Howard Hughes Medical Institute (BSC), and by the National Institutes of Health [grant numbers 5T32HL007734 (MAB, DTD), F32DK104525-01 (GLF)].
Abbreviations:
- ALT
Alanine transaminase
- ANOVA
Analysis of variance
- CI
Confidence interval
- DHA
Docosahexaenoic acid
- EPA
Eicosapentaenoic acid
- FO
Fish oil
- GPR120
G-protein coupled receptor 120
- H&E
Hematoxylin and eosin
- HIRI
Hepatic ischemia reperfusion injury
- IKKβ
Inhibitor of nuclear factor kappa-B
- IQR
Interquartile range
- IV
Intravenous
- JNK
c-Jun N-terminal kinase
- KO
Knockout
- NFκB
Nuclear factor kappa-B
- O3FA
Omega-3 fatty acids
- RIPA
Radioimmunoprecipitation assay
- S
Saline
- SEM
Standard error of mean
- TAB1
TAK1-binding protein 1
- TAK1
TGF–β activated kinase 1
- WT
Wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of Interest: A license agreement for the use of Omegaven® has been signed by Boston Children’s Hospital and Fresenius Kabi, and a patent has been issued to Dr. Puder; royalties may be forthcoming. Dr. Puder serves as a consultant for Pronova-BASF. All other authors have no declarations of interest relevant to this article to disclose.
References:
- 1.Lemasters JJ, Thurman RG. Reperfusion injury after liver preservation for transplantation. Annu Rev Pharmacol Toxicol 1997;37:327–38. [DOI] [PubMed] [Google Scholar]
- 2.Kupiec-Weglinski JW, Busuttil RW. Ischemia and reperfusion injury in liver transplantation. Transplant Proc 2005;37:1653–6. [DOI] [PubMed] [Google Scholar]
- 3.de Vera ME, Lopez-Solis R, Dvorchik I, Campos S, Morris W, Demetris AJ, et al. Liver transplantation using donation after cardiac death donors: long-term follow-up from a single center. Am J Transplant 2009;9:773–81. [DOI] [PubMed] [Google Scholar]
- 4.Olthoff KM, Kulik L, Samstein B, Kaminski M, Abecassis M, Emond J, et al. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transplant 2010;16:943–9. [DOI] [PubMed] [Google Scholar]
- 5.de Meijer VE, Kalish BT, Puder M, Ijzermans JNM. Systematic review and meta-analysis of steatosis as a risk factor in major hepatic resection. Br J Surg 2010;97:1331–9. [DOI] [PubMed] [Google Scholar]
- 6.Nocito A, El-Badry AM, Clavien PA. When is steatosis too much for transplantation? J Hepatol 2006;45:494–9. [DOI] [PubMed] [Google Scholar]
- 7.Verran D, Kusyk T, Painter D, Fisher J, Koorey D, Strasser S, et al. Clinical experience gained from the use of 120 steatotic donor livers for orthotopic liver transplantation. Liver Transpl 2003;9:500–5. [DOI] [PubMed] [Google Scholar]
- 8.Selzner M, Clavien PA. Fatty liver in liver transplantation and surgery. Semin Liver Dis 2001;21:105–13. [DOI] [PubMed] [Google Scholar]
- 9.Marsman WA, Wiesner RH, Rodriguez L, Batts KP, Porayko MK, Hay JE, et al. Use of fatty donor liver is associated with diminished early patient and graft survival. Transplantation 1996;62:1246–51. [DOI] [PubMed] [Google Scholar]
- 10.Baker MA, Nandivada P, Mitchell PD, Fell GL, Pan A, Anez-Bustillos L, et al. Pretreatment with intravenous fish oil reduces hepatic ischemia reperfusion injury in a murine model. Surgery 2018;163:1035–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raptis DA, Limani P, Jang JH, Ungethüm U, Tschuor C, Graf R, et al. GPR120 on Kupffer cells mediates hepatoprotective effects of ω3-fatty acids. J Hepatol 2014;60:625–32. [DOI] [PubMed] [Google Scholar]
- 12.Lee S, Gura KM, Kim S, Arsenault DA, Bistrian BR, Puder M. Current clinical applications of omega-6 and omega-3 fatty acids. Nutr Clin Pract 2006;21:323–41. [DOI] [PubMed] [Google Scholar]
- 13.Serhan CN, Petasis NA. Resolvins and protectins in inflammation resolution. Chem Rev 2011;111:5922–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calder PC. Mechanisms of Action of (n-3) Fatty Acids. J Nutr 2012;142:592S–9S. [DOI] [PubMed] [Google Scholar]
- 15.Hirasawa A, Hara T, Katsuma S, Adachi T, Tsujimoto G. Free fatty acid receptors and drug discovery. Biol Pharm Bull 2008;31:1847–51. [DOI] [PubMed] [Google Scholar]
- 16.Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010;142:687–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glass CK, Olefsky JM. Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab 2012;15:635–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weigand K, Brost S, Steinebrunner N, Büchler M, Schemmer P, Müller M. Ischemia/Reperfusion injury in liver surgery and transplantation: pathophysiology. HPB Surg 2012;2012:176723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holm S A Simple Sequentially Rejective Multiple Test Procedure. Scand J Stat 1979;6:65–70. [Google Scholar]
- 20.Fan ST, Lo CM, Liu CL, Yong BH, Chan JK, Ng IO. Safety of donors in live donor liver transplantation using right lobe grafts. Arch Surg 2000;135:336–40. [DOI] [PubMed] [Google Scholar]
- 21.Cannistrà M, Ruggiero M, Zullo A, Gallelli G, Serafini S, Maria M, et al. Hepatic ischemia reperfusion injury: A systematic review of literature and the role of current drugs and biomarkers. Int J Surg 2016;33:S57–70. [DOI] [PubMed] [Google Scholar]
- 22.Nandivada P, Cowan E, Carlson SJ, Chang M, Gura KM, Puder M. Mechanisms for the effects of fish oil lipid emulsions in the management of parenteral nutrition-associated liver disease. Prostaglandins Leukot Essent Fatty Acids 2013;89:153–8. [DOI] [PubMed] [Google Scholar]
- 23.Kumar A, Takada Y, Boriek AM, Aggarwal BB. Nuclear factor-kappaB: its role in health and disease. J Mol Med (Berl) 2004;82:434–48. [DOI] [PubMed] [Google Scholar]
- 24.Zúñiga J, Cancino M, Medina F, Varela P, Vargas R, Tapia G, et al. N-3 PUFA Supplementation Triggers PPAR-α Activation and PPAR-α/NF-κB Interaction: Anti-Inflammatory Implications in Liver Ischemia-Reperfusion Injury. PLoS One 2011;6:e28502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nandivada P, Cowan E, Carlson SJ, Chang M, Gura KM, Puder M. Mechanisms for the effects of fish oil lipid emulsions in the management of parenteral nutrition-associated liver disease. Prostaglandins Leukot Essent Fatty Acids 2013;89:153–8. [DOI] [PubMed] [Google Scholar]
- 26.El-Badry AM, Moritz W, Contaldo C, Tian Y, Graf R, Clavien PA. Prevention of reperfusion injury and microcirculatory failure in macrosteatotic mouse liver by omega-3 fatty acids. Hepatology 2007;45:855–63. [DOI] [PubMed] [Google Scholar]
- 27.Zúñiga J, Venegas F, Villarreal M, Núñez D, Chandía M, Valenzuela R, et al. Protection against in vivo liver ischemia-reperfusion injury by n-3 long-chain polyunsaturated fatty acids in the rat. Free Radic Res 2010;44:854–63. [DOI] [PubMed] [Google Scholar]
- 28.Iwasaki W, Kume M, Kudo K, Uchinami H, Kikuchi I, Nakagawa Y, et al. Changes in the fatty acid composition of the liver with the administration of N-3 polyunsaturated fatty acids and the effects on warm ischemia/reperfusion injury in the rat liver. Shock 2010;33:306–14. [DOI] [PubMed] [Google Scholar]
- 29.Marsman HA, Heger M, Kloek JJ, Nienhuis SL, ten Kate FJW, van Gulik TM. Omega-3 fatty acids reduce hepatic steatosis and consequently attenuate ischemia-reperfusion injury following partial hepatectomy in rats. Dig Liver Dis 2011;43:984–90. [DOI] [PubMed] [Google Scholar]
- 30.Linecker M, Limani P, Kambakamba P, Kron P, Tschuor C, Calo N, et al. Omega-3 fatty acids protect fatty and lean mouse livers after major hepatectomy. Ann Surg 2017;266(2):324–32. [DOI] [PubMed] [Google Scholar]
- 31.Nandivada P, Cowan E, Carlson SJ, Chang M, Gura KM, Puder M. Mechanisms for the effects of fish oil lipid emulsions in the management of parenteral nutrition-associated liver disease. Prostaglandins, Leukot Essent Fat Acids 2013;89:153–8. [DOI] [PubMed] [Google Scholar]
- 32.Zhu X-H, Wu Y-F, Qiu Y-D, Jiang C-P, Ding Y-T. Liver-protecting effects of omega-3 fish oil lipid emulsion in liver transplantation. World J Gastroenterol 2012;18:6141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gong Y, Liu Z, Liao Y, Mai C, Chen T, Tang H, et al. Effectiveness of ω−3 polyunsaturated fatty acids based lipid emulsions for treatment of patients after hepatectomy: a prospective clinical trial. Nutrients 2016;8(6). pii: E357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soltys K, Dikdan G, Koneru B. Oxidative stress in fatty livers of obese Zucker rats: rapid amelioration and improved tolerance to warm ischemia with tocopherol. Hepatology 2001;34:13–8. [DOI] [PubMed] [Google Scholar]
- 35.Giakoustidis D, Papageorgiou G, Iliadis S, Kontos N, Kostopoulou E, Papachrestou A, et al. Intramuscular administration of very high dose of a-tocopherol protects liver from severe ischemia/reperfusion injury. World J Surg 2002;26:872–7. [DOI] [PubMed] [Google Scholar]
- 36.Itoh Y, Kawamata Y, Harada M, Kobayashi M, Fujii R, Fukusumi S, et al. Free fatty acids regulate insulin secretion from pancreatic β cells through GPR40. Nature 2003;422:173–6. [DOI] [PubMed] [Google Scholar]
- 37.Bjursell M, Xu X, Admyre T, Böttcher G, Lundin S, Nilsson R, et al. The beneficial effects of n-3 polyunsaturated fatty acids on diet induced obesity and impaired glucose control do not require Gpr120. PLoS One 2014;9:e114942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pærregaard SI, Agerholm M, Serup AK, Ma T, Kiens B, Madsen L, et al. FFAR4 (GPR120) signaling is not required for anti-inflammatory and insulin-sensitizing effects of omega-3 fatty acids. Mediators Inflamm 2016;2016:1536047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li X, Yu Y, Funk CD. Cyclooxygenase-2 induction in macrophages is modulated by docosahexaenoic acid via interactions with free fatty acid receptor 4 (FFA4). FASEB J 2013;27:4987–97. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y, Chen L-Y, Sokolowska M, Eberlein M, Alsaaty S, Martinez-Anton A, et al. The fish oil ingredient, docosahexaenoic acid, activates cytosolic phospholipase A 2 via GPR120 receptor to produce prostaglandin E2 and plays an anti-inflammatory role in macrophages. Immunology 2014;143:81–95. [DOI] [PMC free article] [PubMed] [Google Scholar]