Abstract
Introduction:
Implant-related infections carry a high morbidity. Infectious rates for neuromodulation implants range from 1–9% for DBS, 0–10% for SCS systems, and 3–15% for IT pump systems. Meanwhile, studies on care bundles report infection rate reduction to 1.0% for SCS, and 0.3% for cardiac implants. Herein, we evaluate the effectiveness of an infection prevention bundle (IPB) in minimizing infections after surgeries for neuromodulation implants.
Methods:
An IPB focused on pre-operative checklists, screening questionnaires, MRSA/MSSA decolonization, weight-based antibiotic prophylaxis, strict draping and surgical techniques, and wound care education; was implemented in our Functional Neurosurgery division in April 2015. We retrospectively reviewed all surgeries for implantation or replacement of SCS, DBS, and IT pump system components from March 2013 to October 2017. Subjects were divided into pre-IPB and post-IPB groups. All cases were performed by a single surgeon. Each surgical site was considered a unique surgical case. Infection rates were calculated for pre-IPB and post-IPB groups.
Results:
A total of 688 patients underwent 1,161 unique surgical cases (222 DBS electrodes, 419 IPG, 203 SCS, 317 IT pumps) during the study period. There were 546 pre-IPB and 615 post-IPB surgical cases. Pre-IPB infection rates were: 0%, 1.3%, and 8.7% for SCS, DBS, and IT pumps, respectively. Post-IPB infection rates were: 0%, 0.3%, and 1.8% for SCS, DBS, and IT pumps, respectively.
Conclusions:
Implementation of a standardized IPB approach reduced the number of infections for all studied neuromodulation implants. This approach can be adopted within any specialty to potentially decrease the incidence of implant-related infections.
Keywords: Bundle, checklists, deep brain stimulation (DBS), intrathecal (IT) pumps, spinal cord stimulation (SCS), surgical implant, surgical site infection (SSI)
Introduction:
Implant-related infections carry a high morbidity. Common complications associated with implant-related surgeries include hemorrhage, infection, and hardware failure. Infectious rates for neuromodulation implants range from 1–9% for DBS,1–8 0–10% for SCS systems,9, 10 and 3–15% for IT pump systems.11–13 Meanwhile, studies on care bundles report reduction in infection rates for SCS from 10.4% to 1.0%,9 and rates as low as 0.3% for cardiac implants.14–16
Given the implications associated with implant-related infections, such as requirement for further surgical revisions, possible hardware removal with exacerbation of the underlying disorder, and increased healthcare costs; the standardization of infection prevention strategies should be a top priority of quality improvement initiatives within a Functional Neurosurgery practice.1 Furthermore, the implementation of best practices to minimize implant-related infections is necessary to help ensure cost-effectiveness and continued access to these therapies. In this study, we sought to evaluate the effectiveness of implementing a custom infection prevention bundle (IPB) in minimizing infections after surgeries for implantation of neuromodulation devices.
Methods:
Study Location:
Froedtert Memorial Lutheran Hospital (FMLH) in Milwaukee, Wisconsin, an academic medical center with 804 inpatient beds.
Study Design:
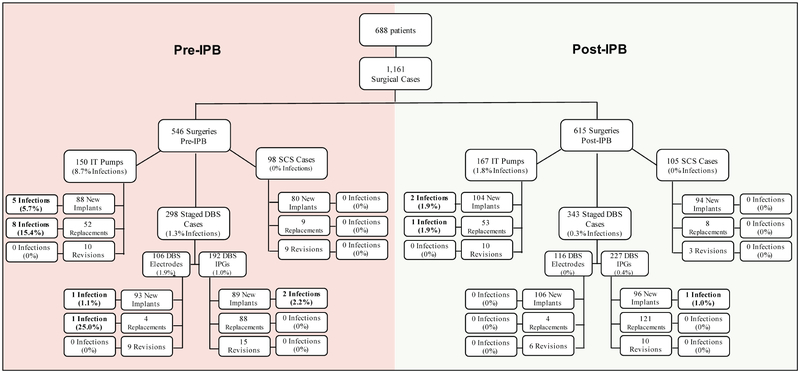
To evaluate the effectiveness of an infection prevention bundle (IPB) instituted in April 2015, we performed a retrospective chart review of adult patients who underwent surgery/surgeries for implantation or replacement of SCS, DBS, and IT drug delivery system components at FMLH during the period of March 2013 to October 2017. The subjects were divided into two groups: the pre-intervention or pre-IPB group, and the post-intervention or post-IPB group. (Figure 1)
Figure 1.
Surgical cases and implant-related infections with 1-year post-operative surveillance
Data collection was focused on demographics (sex, age at time of surgery), length of follow up, risk factors, surgeries for implantation, revision, and/or removal of hardware, indications for surgery, incidence of infections, and the infectious agent(s) identified.
To control for practice-related variabilities, all cases reviewed were performed by a single surgeon (P.P., senior author). Each surgical site was counted as a unique surgical case. Infection rates were calculated for all initial implantation surgeries and all subsequent revision/replacement surgeries before and after implementation of the IPB. The global incidence of post-operative surgical site infection (SSI) when using a 90-day versus a 1-year infection surveillance period was evaluated. Only patients with a minimum follow-up of 12 months were included in subgroup and risk factor comparisons.
Eligibility Criteria:
Inclusion criteria: Males and females older than 18 years of age who underwent elective surgery for implantation or replacement of SCS system components, DBS system components, or IT drug delivery systems at FMLH by a single functional surgeon (P.P., senior author) within the study period of March 2013 to October 2017 and who had a minimum follow-up of 3 months were included.
Exclusion criteria: Patients younger than 18 years of age. Patients who underwent implant placement or replacement surgeries outside of FMLH, by a surgeon other than P.P., and/or outside of the study period of March 2013 to October 2017. Subjects with less than 3 months of follow-up time were not included in the data collection.
Determination of Surgical Site Infection:
The diagnosis of deep surgical site infection followed the guidelines by the Center for Disease Control and Prevention (CDC)17 and included infections occurring within 30 or 90 days after the operative procedure (note: our infection surveillance is extended to a minimum of 1 year given the presence of implantable devices), involving deep soft tissues of the incision (e.g. fascial and muscle layers), and at least one of the following:
Purulent drainage from deep incision.
A deep incision that spontaneously dehisces, or a deep incision deliberately opened/aspirated by a surgeon and organism is identified by a culture or non-culture based microbiologic testing method which is performed for purposes of clinical diagnosis or treatment; or culture or non-culture based microbiologic testing method is not performed AND at least one of the following signs or symptoms: fever (>38°C), localized pain or tenderness. A culture or non-culture based test that has a negative finding does not meet this criterion.
An abscess or other evidence of infection involving the deep incision that is detected on gross anatomical or histopathologic exam, or imaging test.
Only deep surgical site infections requiring surgery for wound debridement/washout and/or hardware removal were considered in this study. Our infection surveillance extends to a minimum of 1 year given the presence of implantable devices.
Ethical Review/Approval:
The study was performed in the context of quality improvement without experimental practices or the need for patient’s identifiable information, therefore it was granted exempt status from our institutional review board.
Infection Prevention Bundle (IPB):
The IPB implemented in April 2015 consisted of the following items:
Pre-operative counseling and questionnaire: patient counseling and preparation instructions, screening questions for signs of infection and presence of open or non-healing wounds.
- Pre-operative check list:
- Nurse call with reminder of pre-operative instructions 2 days prior to surgery.
- Nasal MRSA/MSSA decolonization: Twice daily application of 2% mupirocin ointment to bilateral nares with final application on the morning of surgery.
- Body decolonization: Cleansing with 2% chlorhexidine gluconate cloths the evening before surgery and the morning of surgery.
Pre-operative weight-based antibiotics within 60 minutes of incision
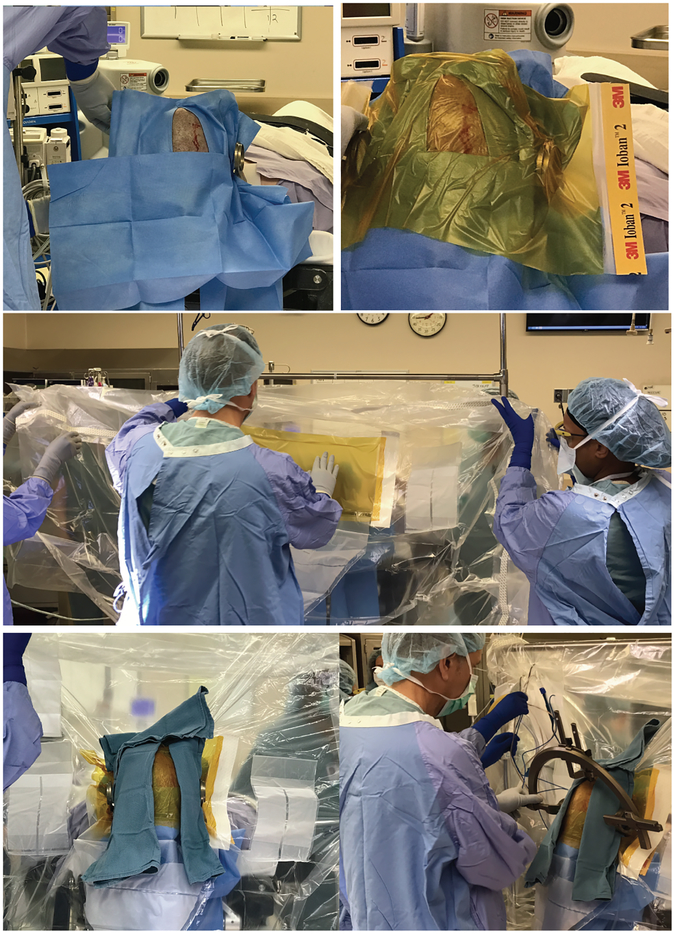
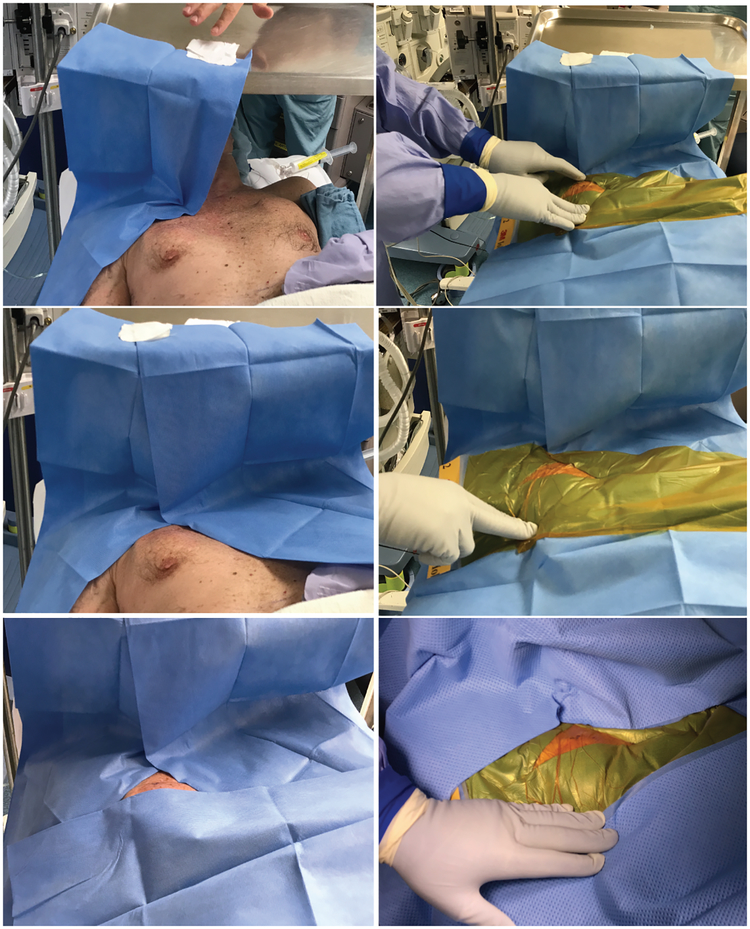
Strict draping and surgical techniques (see Operating room practice section and Figures 2, 3)
Weight-based post-operative antibiotics for 24hr for patients staying overnight vs one dose for outpatients.
Post-operative wound care education to patient and family.
Figure 2.
Draping technique for DBS Stage I surgeries. Top: After a standard skin prep with chlorhexidine/alcohol-based solution, sterile paper drapes are placed surrounding the incision and these are covered by antimicrobial incise drapes. Middle: A second layer of antimicrobial incise drape is first placed over the previously draped incision and the lateral flanks are then tied around IV poles on either side to separate the surgical field from the non-sterile patient field. Bottom: Cuts are made on the incise drape to allow the z-bars through to allow connection to the arc (outer gloves are changed after this step). Exposed non-sterile areas are then covered with sterile towels. Finally, the frame arc is attached and set to pre-determined coordinates.
Figure 3.
Draping technique for DBS IPG replacement surgeries. Left: A mayo tray is placed above the patient’s head with enough height to ensure patient comfort. After a standard skin prep with a chlorhexidine/alcohol-based solution, sterile paper drapes are attached to the top of the mayo tray and then around the patient’s incision without leaving gaps. Right: Antimicrobial incise drapes are placed over the paper drapes creating a water-tight closure. Surgical field drapes are then placed in standard fashion.
Operating Room Practice:
A single functional neurosurgeon (P.P., senior author) performed every case either by himself or with assistance from a single neurosurgical resident. Both surgeon and assistants wore non-paper gowns and double gloves for every case. Exterior gloves were changed after draping for all stage I DBS (electrode placement) procedures. There was no restriction on the number of people allowed in the room at a given time, the frequency of door openings, or the frequency of scrub technician turnover.
DBS surgeries were performed in a staged fashion. Stage I DBS surgeries (electrode implantation) were performed under monitored anesthesia care (MAC) and local anesthesia. Microelectrode recording (MER) was utilized in all Stage I DBS surgeries. Stage II DBS (IPG insertion and connection) were performed under general anesthesia. DBS IPG replacements were performed under MAC and local anesthesia. SCS paddle and battery implantations were performed under MAC and local anesthesia. Initial IT pump system implantations and revisions were performed under general anesthesia. Routine IT pump replacements were performed under MAC and local anesthesia.
Local hair is removed with electric clippers immediately before surgery. Standard surgical site skin preparation is performed by an operating room nurse using a chlorhexidine/alcohol-based solution. Additional skin cleansing with povidone-iodine is performed for all Stage I DBS cases prior to stereotactic headframe pin placement and prior to local anesthetic infiltration to the proposed scalp incision. This is followed by a final skin preparation with a chlorhexidine/alcohol-based solution prior to draping.
Sterile draping is performed by the attending neurosurgeon with or without assistance from a resident or a scrub technician. Two layers of iodophore-impregnated incise drapes are utilized in all Stage I DBS cases (Figure 2). Paper drapes and iodophore-impregnated incise drapes are used to achieve water tight closures in all DBS IPG, SCS, and IT pump placement, and replacement cases (Figure 3). Sterile draping of the C-arm fluoroscopy machine is performed by the scrub technician.
All implants remain closed in their package until immediately needed and are soaked in vancomycin solution (1mg/mL) prior to implantation. All pump implants are anchored using 2–0 silk sutures while the rest of the implants are anchored with 2–0 Vicryl sutures. Wound irrigation with saline is carried out with a bulb syringe prior to wound closure. Wound closures are performed following anatomical layers using 0-Vicryl for fascial layers, 2–0 Vicryl for the intermediate subcutaneous layers, an inverted layer of 3–0 Vicryl for the more superficial subcutaneous layers, and a running 4–0 Vicryl subcuticular suture with Dermabond for the final skin closure. All wounds are dressed with Telfa non-adherent dressing (Covidien Medtronic) and Tegaderm transparent film dressing (3M United States) for 24–48hr.
Statistical Analysis:
Data analysis focused on calculation of infection rates with mean ± standard deviation, and group comparisons via student’s t test and the rate ratio test.18 The Wilson score interval for calculating the binomial proportion confidence limits. Data analysis was performed using statistical software R3.3.1.
Results:
Population:
A total of 688 patients (460 males/224 females; 50 ± 17 years old) underwent a total of 1,161 unique site surgeries during the study period. This included 222 DBS electrode surgeries (199 initial, 8 replacements, 15 revisions), 419 IPG surgeries (185 initial, 209 replacements, 25 revisions), 203 SCS surgeries (174 initial, 17 replacements, 12 revisions), and 317 IT pump system surgeries (192 initial, 105 replacements, 20 revisions). Of these, 546 occurred before the implementation of the IPB (pre-IPB) and 615 occurred after the implementation of the IPB (post-IPB). Tables 1–3, Figure 1.
Table 1.
Baseline patient characteristics for pre-IPB and post-IPB groups with 1-year post operative surveillance
| Surgery Group | Characteristic | Pre-IPB Group | Post-IPB Group | ||
|---|---|---|---|---|---|
| All Patients | Infected Patients | All Patients | Infected Patients | ||
| DBS Systems | No. of patients, (%) | 188 (100%) | 3 (1.6%) | 198 (100%) | 1 (0.5%) |
| Average Age in years | 69 ± 16 | 75 ± 5 | 68 ± 15 | 64 | |
| Sex (Male:Female) | 139:49 | 3:0 | 153:45 | 1:0 | |
| SCS systems | No. of patients, (%) | 51 (100%) | 0 (0%) | 58 (100%) | 0 (0%) |
| Average Age in years | 60 ± 25 | N/A | 66 ± 12 | N/A | |
| Sex (Male:Female) | 29:22 | 0:0 | 32:26 | 0:0 | |
| IT pump systems | No. of patients, (%) | 93 (100%) | 8 (8.6%) | 100 (100%) | 2 (2.0%) |
| Average Age in years | 46 ± 16 | 39 ± 15 | 47 ± 14 | 44 ± 18 | |
| Sex (Male:Female) | 58:35 | 4:4 | 64:36 | 0:2 | |
IPB = Infection prevention bundle
DBS = deep brain stimulation
IPG = implantable pulse generator
IT = intrathecal
SCS = spinal cord stimulator
Table 3.
Incidence of implant-related infections per surgical case type with 1-year post operative surveillance
| Pre-IPB | Post-IPB | Pre-IPB | Post-IPB | |||||
|---|---|---|---|---|---|---|---|---|
| Surgical Cases | Infections (%) | Infections (%) | Infections (%) | Infections (%) | ||||
| All DBS Cases | 188 | 3 (1.6%) | 198 | 1 (0.5%) | 298 | 4 (1.3%) | 343 | 1 (0.3%) |
| Electrode Cases | 76 | 2 (2.6%) | 72 | 0 (0%) | 106 | 2 (1.9%) | 116 | 0 (0%) |
| New implants | 67 | 1 (1.5%) | 70 | 0 (0%) | 93 | 1 (1.1%) | 106 | 0 (0%) |
| Replacements | 4 | 1 (25%) | 4 | 0 (0%) | 4 | 1 (25%) | 4 | 0 (0%) |
| Revisions | 8 | 0 (0%) | 5 | 0 (0%) | 9 | 0 (0%) | 6 | 0 (0%) |
| DBS IPG Cases | 112 | 2 (1.8%) | 126 | 1 (0.8%) | 192 | 2 (1.0%) | 227 | 1 (0.4%) |
| New implants | 54 | 2 (3.7%) | 63 | 1 (1.6%) | 89 | 2 (2.2%) | 96 | 1 (1.0%) |
| Replacements | 53 | 0 (0%) | 68 | 0 (0%) | 88 | 0 (0%) | 121 | 0 (0%) |
| Revisions | 12 | 0 (0%) | 7 | 0 (0%) | 15 | 0 (0%) | 10 | 0 (0%) |
| All SCS Cases | 51 | 0 (0%) | 58 | 0 (0%) | 98 | 0 (0%) | 105 | 0 (0%) |
| New implants | 40 | 0 (0%) | 47 | 0 (0%) | 80 | 0 (0%) | 94 | 0 (0%) |
| Replacements | 9 | 0 (0%) | 8 | 0 (0%) | 9 | 0 (0%) | 8 | 0 (0%) |
| Revisions | 9 | 0 (0%) | 3 | 0 (0%) | 9 | 0 (0%) | 3 | 0 (0%) |
| All IT Pump Cases | 93 | 8 (8.6%) | 100 | 2 (2.0%) | 150 | 13 (8.7%) | 167 | 3 (1.8%) |
| New implants | 44 | 3 (6.8%) | 52 | 1 (1.9%) | 88 | 5 (5.7%) | 104 | 2 (1.9%) |
| Replacements | 52 | 5 (9.6%) | 53 | 1 (1.9%) | 52 | 8 (15.4%) | 53 | 1 (1.9%) |
| Revisions | 10 | 0 (0%) | 10 | 0 (0%) | 10 | 0 (0%) | 10 | 0 (0%) |
IPB = Infection prevention bundle
DBS = deep brain stimulation
IPG = implantable pulse generator
SCS = spinal cord stimulator
IT = intrathecal
Post-operative follow-up:
The mean follow-up time was 32 months (24–53months) in the pre-IPB group and 20 months (12–31months) in the post-IPB group. In patients with IT pump delivery systems for intractable cancer-related pain, follow-up was limited due to cancer-related deaths (9 out of 12 in the pre-IPB group and 1 of 2 in the post-IPB group). As such, follow-up in this subgroup ranged from 4–36 months in the pre-IPB group and 3–15 months in the post-IPB group.
Incidence of infection and infection surveillance periods:
The overall infection rate for all surgical case types (i.e. SCS + DBS+ IT pumps) was 2.9% in the pre-IPB group and 0.2% in the post-IPB group when considering infections occurring within 90 days from surgery and 3.1% in the pre-IPB group and 0.7% in the post-IPB group when considering infections occurring within 1 year from surgery. Table 2. The average time from surgery to surgical site infection (SSI) was 1.3 months in the pre-IPB and 4.2 months in the post-IPB group. Table 4.
Table 2.
Implant-related infections with 90-day versus 1-year post-operative surveillance protocol
| Pre-IPB | Post-IPB | Pre-IPB | Post-IPB | |||||
|---|---|---|---|---|---|---|---|---|
| Surgical Cases | Infectio ns (%) | Infectio ns (%) | Infectio ns (%) | Infectio ns (%) | ||||
| All Case Types | ||||||||
| 90-day surveillance | 332 | 11 (3.3%) | 356 | 1 (0.3%) | 546 | 16 (2.9%) | 615 | 1 (0.2%) |
| 1-year surveillance | 15 (4.5%) | 3 (0.8%) | 17 (3.1%) | 4 (0.7%) | ||||
| All DBS Cases | ||||||||
| 90-day surveillance | 188 | 2 (1.1%) | 198 | 0 (0%) | 298 | 3 (1.0%) | 343 | 0 (0%) |
| 1-year surveillance | 3 (1.6%) | 1 (0.5%) | 4 (1.3%) | 1 (0.3%) | ||||
| Electro de Cases | ||||||||
| 90-day surveillance | 76 | 2 (2.6%) | 72 | 0 (0%) | 106 | 2 (1.9%) | 116 | 0 (0%) |
| 1-year surveillance | 2 (2.6%) | 0 (0%) | 2 (1.9%) | 0 (0%) | ||||
| DBS IPG Cases | ||||||||
| 90-day surveillance | 112 | 1 (0.9%) | 126 | 0 (0%) | 192 | 1 (0.5%) | 227 | 0 (0%) |
| 1-year surveillanc | 2 (1.8%) | 1 (0.8%) | 2 (1.0%) | 1 (0.4%) | ||||
| All SCS Cases | ||||||||
| 90-day surveillance | 51 | 0 (0%) | 58 | 0 (0%) | 98 | 0 (0%) | 105 | 0 (0%) |
| 1-year surveillance | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | ||||
| All IT Pump Cases | ||||||||
| 90-day surveillance | 93 | 8 (8.6%) | 100 | 1 (1.0%) | 150 | 13 (8.7%) | 167 | 1 (0.6%) |
| 1-year surveillance | 8 (8.6%) | 2 (2.0%) | 13 (8.7%) | 3 (1.8%) | ||||
IPB = Infection prevention bundle
DBS = deep brain stimulation
IPG = implantable pulse generator
SCS = spinal cord stimulator
IT = intrathecal
All case types = SCS+DBS+IT pumps
Table 4.
Characteristics of infected patients in pre-IPB and post-IPB groups
| Study Period | Subject | Sex | Age | Implant Type | Infected Surgical site(s) | Time to SSI (months) | Organism(s) |
|---|---|---|---|---|---|---|---|
| Pre-IPB | P1 | F | 48 | ITP | Lumbar wound | 1.0 | Pseudomonas aeruginosas |
| Pre-IPB | P2 | M | 42 | ITP | IT catheter, pump | 0.5 | Pseudomonas aeruginosa, Staphylococcus epidermidis |
| Pre-IPB | P3 | F | 30 | ITP | Lumbar wound | 0.5 | No growth at explant |
| Pre-IPB | P3 | F | 30 | ITP | IT catheter, pump | 1.1 | No growth at explant |
| Pre-IPB | P4 | F | 60 | ITP | IT catheter, pump | 1.3 | Enterobacter aerogenes |
| Pre-IPB | P5 | M | 66 | ITP | IT catheter, pump | 0.3 | Escherichia coli |
| Pre-IPB | P6 | F | 25 | ITP | IT catheter, pump | 0.4 | Escherichia coli |
| Pre-IPB | P7 | M | 37 | ITP | IT pump | 2.1 | Staphylococcus epidermidis |
| Pre-IPB | P8 | M | 24 | ITP | IT pump | 1.5 | No growth at explant |
| Pre-IPB | D1A | M | 73 | DBS | Electrode, IPG | 0.9 | MRSA |
| Pre-IPB | D2A | M | 81 | DBS | Electrode | 0.2 | Morganella morganii |
| Pre-IPB | D3B | M | 72 | DBS | IPG | 5.7 | Staphylococcus aureus, Corynebacterium jeikeium |
| Post-IPB | P9 | F | 57 | ITP | IT catheter, pump | 4.9 | Pseudomonas aeruginosa |
| Post-IPB | P10 | F | 31 | ITP | IT pump | 2.9 | No growth at explant |
| Post-IPB | D4B | M | 64 | DBS | IPG | 4.7 | Staphylococcus epidermidis, Propionibacterium granulosum, Klebsiella oxytoca |
MRSA = Methicillin=resistant staphylococcus aureus
SSI = Surgical site infection
Time in red = time greater than 90 days or 3 months
When using a 90-day infection surveillance period, the incidence of infection in the pre-IPB group was: 0%, 1%, 8.7% for SCS, DBS (Stage I + II), and IT pump cases, respectively. In contrast, post-IPB infection rates were: 0% 0%, 0.6% for SCS, DBS (Stage I + II), and IT pump cases, respectively. When using a 1-year infection surveillance period, the incidence of infection in the pre-IPB group was: 0%, 1.3%, and 8.7% for SCS, DBS (Stage I + II), and IT pump cases, respectively. In contrast, post-IPB infection rates were: 0%, 0.3%, and 1.8% for SCS, DBS (Stage I + II), and IT pump cases, respectively. Table 2.
Surgical site infection-causative organisms:
Under the 1-year infection surveillance period, the infectious agents identified included: Staphylococcus aureus (3), Staphylococcus epidermidis (4), Escherichia coli (2), Enterobacter aerogenes (1), Corynebacterium jeikeium (1), and Morganella morganii (1), and Pseudomonas aeruginosa (3), Propionibacterium granulosum (1). Table 4.
Initial implantations versus replacement surgeries:
Infection rates for initial implantations were: 0% for SCS systems (N=80); 1.1% for DBS for electrodes (N=93), 2.2% for IPGs (N=89); and 5.7% for IT pump systems (N=88) in the pre-IPB group, and 0% for SCS systems (N=94); 0% for DBS electrodes (N=106), 1% for IPGs (N=96); and 1.9% for IT pump systems (N=104) in the post-IPB group (see Table 3, Figure 1).
Infection rates for replacement surgeries were: 0% for SCS systems (N=9); 25% DBS electrodes (N=4), 0% IPGs (N=88); and 15.4% for IT pump system components (N=52) in the pre-IPB group, and 0% for SCS systems (N=8); 0% for DBS electrodes (N=4), 0% for IPGs (N=121); and 1.9% IT pump system components (N=53) in the post-IPB group (see Table 3, Figure 1).
Risk Factors:
There was no significant difference in sex distribution or age between the groups. Diagnoses or indications for surgery included movement disorders (e.g. Parkinson’s disease, essential tremor, dystonia), spasticity (secondary to cerebral palsy, spinal cord injury, stroke, and multiple sclerosis), and intractable cancer-related pain.
Three patients in the pre-IPB DBS group (3 of 188) required wound debridement and hardware removal from four distinct surgical sites. One of these patients suffered an infection after having multiple surgeries for revision, removal, and replacement of a malfunctioning thalamic electrode within a period of 1 month. Given the low incidence of infection in the DBS group, it was not possible to assess the contribution of risk factors (e.g. diagnosis, comorbidities, etc.). Similarly, there were no infections in the SCS group and therefore contribution of risk factors to incidence of infection was not necessary.
The incidence of infection was highest for the IT pump system groups with a total of 13 surgical site infections (8 of 93 patients) in the pre-IPB group and 3 surgical site infections (2 of 100 patients) in the post-IPB group. This difference was significant when comparing between pre-IPB and post-IPB IT pump system cases per unique surgical site (p=0.012) but not when comparing by number of patients (p=0.086). Obesity (BMI >30kg/m2) and a history of previous hardware-related surgical site infection were associated with a higher incidence of infection in both the pre-IPB and post-IPB groups, but this did not reach statistical significance (p=0.65 and 0.72, respectively). Other risk factors associated with a higher incidence of infections include neurogenic bowel/bladder, cerebral palsy, and history of quadriparesis/quadriplegia, although similarly these did not reach statistical significance. Table 5.
Table 5.
Baseline demographics, risk factors, and incidence of SSI with 1-year surveillance protocol for patients with IT pump systems
| Characteristic | Pre-IPB Group | Post-IPB Group | ||
|---|---|---|---|---|
| All Patients | Infected Patients | All Patients | Infected Patients | |
| No. of patients, (%) | 93 (100%) | 8 (8.6%) | 100 (100%) | 2 (2.0%) |
| Average Age in years | 46 ± 16 | 39 ± 15 | 47 ± 14 | 44 ± 18 |
| Sex (Male:Female) | 58:35 | 4:4 | 64:36 | 0:2 |
| Characteristic | Total Patients | Infected Patients, (%) | Total Patients | Infected Patients, (%) |
| Previous Implant revision (non-infectious) | 2 | 0 (0%) | 8 | 0 (0%) |
| Previous implant-related SSI | 1 | 1 (100%) | 4 | 1 (25%) |
| Obesity (BMI > 30kg/m2) | 22 | 4 (18.2%) | 15 | 1 (6.7%) |
| Diabetes | 4 | 0 (0%) | 9 | 0 (0%) |
| Paraplegia | 29 | 2 (6.9%) | 31 | 1 (3.2%) |
| Quadriplegia/Quadriparesis | 51 | 5 (9.8%) | 67 | 1 (1.5%) |
| Neurogenic bowel/bladder | 66 | 8 (12.1%) | 66 | 2 (3.0%) |
| Spinal Cord Injury | 32 | 2 (6.3%) | 44 | 0 (0%) |
| Cerebral Palsy | 24 | 4 (16.7%) | 24 | 1 (4.2%) |
| Demyelinating disorder | 19 | 1 (5.3%) | 26 | 1 (3.8%) |
| History of stroke, ICH | 13 | 1 (7.7%) | 14 | 0 (0%) |
| Cancer | 16 | 1 (6.3%) | 5 | 0 (0%) |
| Statin use | 12 | 0 (0%) | 19 | 0 (0%) |
BMI = body mass index
IPB = infection prevention bundle
ICH = intracerebral hemorrhage
IT = intrathecal
SSI = surgical site infection
No. = number
Discussion:
Infection rates were lower for both pre-IPB and post-IPB groups when using the new 90-day infection surveillance period recommended by the CDC NHSN17 compared to the previous recommendation of a 1 year surveillance for procedures involving implants.19 Consistent with a recent report by Abode-Iyamah et al. 2018,20 our findings suggest that using the shorter infection surveillance period of 90 days may underestimate the incidence of implant-related infections and ultimately lead to loss of opportunities in the identification of risk factors and interventions to prevent or mitigate these infections.
There were no infections in the SCS group in either the pre-IPB or post-IPB periods. This may be related to the fact that several of the items included in the IPB were already being employed in this group prior to April 2015. For the staged DBS cases, only one infection occurred after implementation of the IPB such that the overall infection rate decreased from 1.3% (4/298) to 0.3% (1/343) after its application (p=0.293). The infection rate for the IT pump system group decreased from 8.7% to 1.8% after implementation of the IPB (p=0.012).
There was a higher incidence of infections in the IT pump system replacement group (15.4%, N=52) compared to initial placements (5.7%, N=88) in the pre-IPB study period. This higher incidence of infection for implant replacements is consistent with various reports.2, 5, 6 There was also a higher incidence of infections for electrode replacement surgeries (25%, N=4) compared to initial placements (1.1%, N=93) in the pre-IPB study period, although this large difference is likely related to the small number of electrode replacement surgeries (Figure 1, Table 3). It is also important to note that the one patient who suffered an infection after electrode replacement surgery had undergone multiple surgeries for revision, removal, and replacement of a malfunctioning thalamic electrode within a period of 1 month. All IPG surgical site infections occurred after initial IPG implantation surgeries (2 in the pre-IPB and 1 in the post-IPB study periods) and none after IPG replacement surgeries in contrast to previous reports.2, 5, 6
Overall the infection rates for IT pumps were higher than for other implant groups. It is possible that the higher incidence of infection observed in the IT pump system groups was related to the patients’ comorbidities as there was a trend towards higher incidence of infections in patients with obesity (BMI >30 kg/m2), neurogenic bowel and bladder, history of cerebral palsy, and limited mobility from quadriparesis/quadriplegia, although this did not reach statistical significance.21–24 (Table 4) We also speculate that the higher incidence of infections in the IT pump system groups could be related to the use of silk sutures for anchoring the device since all pump implants were anchored using 2–0 silk sutures, while the rest of the implants were anchored with 2–0 Vicryl sutures. Surgical silk is a braided and black dyed suture derived from the silkworm larva that has been shown to induce a strong host inflammatory response25, 26 and has been associated with late abscess formation.27
Study Limitations:
Although based on a high volume of surgical cases from a single-center and by a single surgeon, thus limiting practice-related variabilities, this study is limited by its retrospective nature. It is possible that some patients may have moved away or sought care by other providers which could lead to underestimation of infection rates in either group. In patients with IT pump delivery systems for intractable cancer-related pain, follow-up was limited due to cancer-related deaths (9 out of 12 in the pre-IPB group and 1 of 2 in the post-IPB group). However, after adjusting for number of deaths in each group, the infection rate in the pre-IPB IT pump group increased to 9.2% whereas the infection rate in the post-IPB group remained unchanged at 1.8%.
The protocols listed in our study were developed prior to the publishing of the updated NACC consensus guidelines and as such some items deviate from the published recommendations. For instance, several of our practice habits including some components of our IPB such as the use of Vancomycin/Gentamicin for pre-operative prophylaxis and the use of vancomycin irrigation, were acquired over the years and originally based on our experience with specific high risk patient populations. Although in our cohort we did not identify any antibiotic-related complications, we realize this practice raises concerns for possible development of antibiotic resistance. We have since modified our protocols to more closely follow the NACC published guidelines.
Finally, the low incidence of infection during both pre-IPB and post-IPB periods precluded the proper evaluation of risk factors in the current study, which emphasizes the need for future prospective large volume studies.
Conclusions:
This study represents the most comprehensive report to date on the use of an infection prevention bundle (IPB) approach for implantable neuromodulation devices. Implementation of a standardized IPB reduced the number of infections after implantation and replacement of DBS system components, SCS system components, and IT drug delivery systems. This is a simple approach that can be easily customized and adopted within any branch of Neurosurgery and across specialties to potentially decrease the incidence of implant-related infections and improve patient outcomes. This work will add to the growing literature on risk factors for infectious complications and infection prevention strategies as applied to neuromodulation therapies with implanted neurological devices. This is of special relevance since implementation of best practices to minimize implant-related infections is necessary to help ensure cost-effectiveness and continued access to these therapies.
Financial/Material Support:
The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, Award Number UL1TR001436. The content is solely the responsibility of the author(s) and does not necessarily represent the official views of the NIH.
List of Abbreviations
- CDC
Centers for Disease Control and Prevention
- DBS
Deep brain stimulation
- FMLH
Froedtert Memorial Lutheran Hospital
- hr
Hour
- IPG
Implantable pulse generator
- IPB
Infection prevention bundle
- IT
Intrathecal
- IV
Intravenous
- MRSA
Methicillin-resistant Staphylococcus aureus
- MSSA
Methicillin-sensitive Staphylococcus aureus
- MER
Microelectrode recording
- MAC
Monitored anesthesia care
- NHSN
National Healthcare Safety Network
- SCS
Spinal cord stimulation
- SSI
Surgical site infection
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of Interest:
Dr. Arocho-Quinones has nothing to disclose. Dr. Pahapill has nothing to disclose. Dr. Chiang-Ching Huang has nothing to disclose. Mr. Barney D. Ward has nothing to disclose.
Publisher's Disclaimer: Disclaimer Note: Portions of this work were presented in abstract/poster form at the International Neuromodulation Society 13th World Congress; Edinburgh, Scotland, UK (May 2017) and the North American Neuromodulation Society 21st Annual Meeting; Las Vegas, NV (January 2018). This work has not been previously published and is not currently under review elsewhere.
References:
- 1.Deer TR, Provenzano DA, Hanes M, et al. The Neurostimulation Appropriateness Consensus Committee (NACC) Recommendations for Infection Prevention and Management. Neuromodulation 2017;20:31–50. [DOI] [PubMed] [Google Scholar]
- 2.Bjerknes S, Skogseid IM, Saehle T, Dietrichs E, Toft M. Surgical site infections after deep brain stimulation surgery: frequency, characteristics and management in a 10-year period. PLoS One 2014;9:e105288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fenoy AJ, Simpson RK Jr. Management of device-related wound complications in deep brain stimulation surgery. J Neurosurg 2012;116:1324–1332. [DOI] [PubMed] [Google Scholar]
- 4.Piacentino M, Pilleri M, Bartolomei L. Hardware-related infections after deep brain stimulation surgery: review of incidence, severity and management in 212 single-center procedures in the first year after implantation. Acta Neurochir (Wien) 2011;153:2337–2341. [DOI] [PubMed] [Google Scholar]
- 5.Thrane JF, Sunde NA, Bergholt B, Rosendal F. Increasing infection rate in multiple implanted pulse generator changes in movement disorder patients treated with deep brain stimulation. Stereotact Funct Neurosurg 2014;92:360–364. [DOI] [PubMed] [Google Scholar]
- 6.Pepper J, Zrinzo L, Mirza B, Foltynie T, Limousin P, Hariz M. The risk of hardware infection in deep brain stimulation surgery is greater at impulse generator replacement than at the primary procedure. Stereotact Funct Neurosurg 2013;91:56–65. [DOI] [PubMed] [Google Scholar]
- 7.Rasouli JJ, Kopell BH. The Adjunctive Use of Vancomycin Powder Appears Safe and May Reduce the Incidence of Surgical-Site Infections After Deep Brain Stimulation Surgery. World Neurosurg 2016;95:9–13. [DOI] [PubMed] [Google Scholar]
- 8.Frizon LA, Hogue O, Wathen C, et al. Subsequent Pulse Generator Replacement Surgery Does Not Increase the Infection Rate in Patients With Deep Brain Stimulator Systems: A Review of 1537 Unique Implants at a Single Center. Neuromodulation 2017;20:444–449. [DOI] [PubMed] [Google Scholar]
- 9.Yusuf E, Bamps S, Thuer B, et al. A Multidisciplinary Infection Control Bundle to Reduce the Number of Spinal Cord Stimulator Infections. Neuromodulation 2017;20:563–566. [DOI] [PubMed] [Google Scholar]
- 10.Pahapill PA. Incidence of Revision Surgery in a Large Cohort of Patients With Thoracic Surgical Three-Column Paddle Leads: A Retrospective Case Review. Neuromodulation 2015;18:367–375. [DOI] [PubMed] [Google Scholar]
- 11.Malheiro L, Gomes A, Barbosa P, Santos L, Sarmento A. Infectious Complications of Intrathecal Drug Administration Systems for Spasticity and Chronic Pain: 145 Patients From a Tertiary Care Center. Neuromodulation 2015;18:421–427. [DOI] [PubMed] [Google Scholar]
- 12.Taira T, Ueta T, Katayama Y, et al. Rate of complications among the recipients of intrathecal baclofen pump in Japan: a multicenter study. Neuromodulation 2013;16:266–272; discussion 272. [DOI] [PubMed] [Google Scholar]
- 13.Ghobrial GM, Thakkar V, Singhal S, et al. Efficacy of intraoperative vancomycin powder use in intrathecal baclofen pump implantation procedures: single institutional series in a high risk population. J Clin Neurosci 2014;21:1786–1789. [DOI] [PubMed] [Google Scholar]
- 14.Manolis AS, Melita H. Prevention of Cardiac Implantable Electronic Device Infections: Single Operator Technique with Use of Povidone-Iodine, Double Gloving, Meticulous Aseptic/Antiseptic Measures and Antibiotic Prophylaxis. Pacing Clin Electrophysiol 2017;40:26–34. [DOI] [PubMed] [Google Scholar]
- 15.Schweizer ML, Chiang HY, Septimus E, et al. Association of a bundled intervention with surgical site infections among patients undergoing cardiac, hip, or knee surgery. JAMA 2015;313:2162–2171. [DOI] [PubMed] [Google Scholar]
- 16.Polyzos KA, Konstantelias AA, Falagas ME. Risk factors for cardiac implantable electronic device infection: a systematic review and meta-analysis. Europace 2015;17:767–777. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. The National Healthcare Safety Network (NHSN) Manual: Procedure associated Module: Division Surgical Site Infection (SSI) [online]. Available at: https://www.cdc.gov/nhsn/pdfs/pscmanual/9pscssicurrent.pdf.
- 18.Fay MP. Two-sided Exact Tests and Matching Confidence Intervals for Discrete Data. R Journal 2010;2:53–58. [Google Scholar]
- 19.Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Am J Infect Control 1992;20:271–274. [DOI] [PubMed] [Google Scholar]
- 20.Abode-Iyamah KO, Chiang HY, Woodroffe RW, et al. Deep brain stimulation hardware-related infections: 10-year experience at a single institution. J Neurosurg 2018:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olsen MA, Nepple JJ, Riew KD, et al. Risk factors for surgical site infection following orthopaedic spinal operations. J Bone Joint Surg Am 2008;90:62–69. [DOI] [PubMed] [Google Scholar]
- 22.Harrop JS, Styliaras JC, Ooi YC, Radcliff KE, Vaccaro AR, Wu C. Contributing factors to surgical site infections. J Am Acad Orthop Surg 2012;20:94–101. [DOI] [PubMed] [Google Scholar]
- 23.Schuster JM, Rechtine G, Norvell DC, Dettori JR. The influence of perioperative risk factors and therapeutic interventions on infection rates after spine surgery: a systematic review. Spine (Phila Pa 1976) 2010;35:S125–137. [DOI] [PubMed] [Google Scholar]
- 24.Korol E, Johnston K, Waser N, et al. A systematic review of risk factors associated with surgical site infections among surgical patients. PLoS One 2013;8:e83743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spelzini F, Konstantinovic ML, Guelinckx I, et al. Tensile strength and host response towards silk and type i polypropylene implants used for augmentation of fascial repair in a rat model. Gynecol Obstet Invest 2007;63:155–162. [DOI] [PubMed] [Google Scholar]
- 26.Meinel L, Hofmann S, Karageorgiou V, et al. The inflammatory responses to silk films in vitro and in vivo. Biomaterials 2005;26:147–155. [DOI] [PubMed] [Google Scholar]
- 27.Calkins CM, St Peter SD, Balcom A, Murphy PJ. Late abscess formation following indirect hernia repair utilizing silk suture. Pediatr Surg Int 2007;23:349–352. [DOI] [PubMed] [Google Scholar]