Abstract
The goal of this research was to explore the preliminary anticancer properties of five plants namely Calotropis procera, Moringa oleifera, Millettia pinnata, Basela alba and Euphorbia neriifolia available in Jharkhand which is used for the medicinal purpose by local tribes. In the present study, plant leaves from five species were collected, dried and extracted with solvents of increasing polarity, followed by assessment of their cytotoxicity in A549 non-small-cell lung cancer cells. In the antimicrobial assay, the methanol extract of the M. pinnata leaves exhibited comparatively higher zone of inhibition of 0.7 ± 0.20 cm against a Salmonella typhi culture than the other extracts. M. pinnata leaves extract also displayed the maximum percentage inhibition in the DPPH, 83.97 ± 0.01 FRAP, 193.14 ± 3.01 mM assays. Furthermore, the cytotoxicity of the chloroform (37.45 ± 1.04) and ethyl acetate extracts (34.20 ± 0.81) of M. pinnata against A549 cells was found relatively higher with respect to another extract. In contrast, a study with the L132 normal epithelial lung cell line revealed less toxicity from the chloroform extract (0.33 ± 0.19) compared to the ethyl acetate extract (6.65 ± 0.59). Based on these findings, phytochemical investigation on chloroform and ethyl acetate extract of M. pinnata was performed using UPLC-ESI–MS/MS analysis revealing the presence of β-sitosterol, lanceolatin B, karanjin, and stigmasterol. Congruently, a complete phytochemical and cytotoxic investigation of the M. pinnata extract constituents might infer the potency of this extract/s as anticancer, antioxidant and antimicrobial agents.
Electronic supplementary material
The online version of this article (10.1007/s13205-019-1927-x) contains supplementary material, which is available to authorized users.
Keywords: Anticancer, Antioxidant assay, Apoptosis, Mass spectroscopy
Introduction
Drug development using natural products has been extensively explored by researchers, and the use of these naturally derived products is frequent in cancer research. Plants play vital role in the medicinal system for the treatment of disease through their herbal compounds, either in the solitary or allied form (Tiwari et al. 2018: Kumar and Sharma 2018). In the Indian and global traditional therapeutic systems, plant-derived substances have been used for the treatment of numerous diseases for a long time (Shamim et al. 2016). Extraction from different portions of plants along with their characterization of functional components and antioxidant, antimicrobial, and anticancer activities are always pursued by scientists. The other important topic that requires extensive work is establishing modern scientific evidence in support of the traditional therapeutic system (Kondhare and Lade 2017). Researchers from all around the world are working on whether antioxidants retrieved from plants are cancer suppressors or enhancers and targeting the possibility of antioxidant activity in cancer treatment (Hawk et al. 2016). Due to its geographical advantages, the state of Jharkhand in India is rich in plant diversity. Forty percent of the total area of Jharkhand is forest that is comprised of more than 160 plant species having medicinal value. These plants are commonly and widely used by local tribes; therefore, it is necessary to explore these plants extensively using efficient scientific facilities (Sharma et al. 2016). In this study, we used five plants, Calotropis procera (L.) (C. procera), Basella alba (L.) (B. alba), Millettia pinnata (L.) (M. pinnata), Moringa oleifera Lam. (M. oleifera), and Euphorbia neriifolia Linn.(E. neriifolia), on the basis of availability and review literature for preliminary analysis, which have already been prescribed comprehensively for the treatment of many diseases, including oncological problems in Indian traditional medicine (Ayurveda) evidenced and summarized in the Charaka Samhita (Kumar et al. 2019). These plants are abundantly present in Jharkhand state and used by local tribe for wound healing properties. The Charaka Samhita is documentation of the Ayurveda of medicine originating from ancient India and is enriched with a history of more than 4000 years. There is documentation in the traditional Indian therapeutic system for the use of plants, animals, and metals in disease treatment (Galib et al. 2011; Kumar et al. 2019).
C. procera, from family Asclepiadaceae and commonly known as ‘akra’ in India, is a 1–3 m height shrub distributed throughout tropical and subtropical Asia and Africa and used in many traditional folk medicines. It has been used for leprosy, eczema, asthma, syphilis, analgesic, antimalarial, cytostatic, piles, cutaneous infections, malaria, low hectic fevers, as an abortifacient in rheumatism, as anti-inflammatory, antimicrobial, and hepatoprotective agents (Bhaskar and Ajay 2009; Mako et al. 2012).
The M. oleifera tree belongs to the family Moringaceae (Drumstick or Sahjan) and is widely present in India. This plant is known for its remarkable range of nutraceutical and pharmaceutical properties, making it versatile and a potential resource for food, drugs and allied uses. Traditional and tribal lore describes the use of these plant parts to treat numerous conditions, such as the dental problems, helminths, fever, ulcers, asthma, endocrine disorders, diabetes, thyroid issues, snakebites, trypanosomes, diarrhoea, rheumatism, typhoid, syphilis, viral disease, and as an antitumour, anti-anaemic, and diuretic agent (Bukar et al. 2010 and Rahman et al. 2009).
The M. pinnata tree, vernacular name Indian Beech or Karanj, belongs to the family Fabaceae, is found abundantly in the Indian forest and is widely used by tribal people on this continent because of its medicinal properties. Whole plants have been used in ayurvedic curative systems to treat several diseases and problems, while the use of crude extracts has also been reported by researchers for the treatment of various conditions, such as tumours, piles, ulcers, gonorrhoea, tooth decay, rheumatism, leucoderma, scabies, and vaginal and skin diseases (Al Muqarrabun et al. 2013). In our previous study, we have reported leaves extact of M. pinnata used in the synthesis of gold nanoparticles with further potential in biomedical applications (Kumar et al. 2019). Due to the successive depletion of fossil fuels and their increasing demand, researchers are focusing on alternative sources of energy, which results in the emergence of biofuels as one of the best available resources, and this in turn has shown the potential of M. pinnata, which has come to forefront in the preceding years (Atabani et al. 2012).
Basella alba/rubra is an edible vine from the Basellaceae and commonly known as Indian/climbing/Chinese spinach or poi sag. It is used in the treatment of intestinal disorders, earache, carminative, itching, scabies, colic, sore throat, liver diseases, diarrhoea, menstrual periods, eczema, ringworm, kidney stones, gonorrhoea, headache, burns, acne, freckles, abscesses, skin diseases, syphilis, tumours, leucorrhoea, malaria, constipation, scabies; as a blood producer, astringent, antipruritic; and to increase weight (Sushila et al. 2010).
Euphorbia neriifolia from the family Euphorbiaceae, commonly known as “Sehund”, is useful for anaemia, bronchitis, cutaneous diseases, dropsy, dyspepsia, delirium, leucoderma, piles, ulcers, jaundice, leprosy, tumours, loss of consciousness, rheumatism and in chronic respiratory troubles found throughout India (Pracheta et al. 2011).
This study provides a detailed report on the antimicrobial, antioxidant and anticancer properties of all five of the abovementioned plants in a novel way. Furthermore, several phytochemicals and antioxidants have been reported to prevent and treat free-radical generated diseases. In contrast to the general belief of antioxidants as disease preventive substances, there are also studies indicating adverse effects or no effect of antioxidants in disease therapy, and it may even enhance disease progression (Li et al. 2014; Sayin et al. 2014; Hawk et al. 2016). Therefore, the present study, which deals with the successive extraction and cytotoxicity assays of extracts from the leaves of the above mentioned plants, contributes significantly to the validation of antioxidant versus anticancer activities.
Materials and methods
Sample procurement, and identification
Plant leaves were collected from the campus of Birla Institute of Technology, Mesra, Ranchi, India (23.4123° N, 85.4399° E). A herbarium sheet was also prepared and deposited for the identification of plants at the Botanical Survey of India, Central National Herbarium, Howrah, India, with specimen numbers: C. procera (Aiton) Dryand(BIT/GK-03), M. oleifera Lam (BIT/GK-01), M. pinnata (L.) Pierre (BIT/GK-02), B. alba L (BIT/GK-04), and E. neriifolia L (BIT/GK-05). (certificate submitted). Bacterial cultures were obtained from the national collection of industrial microorganisms (NCIM), national chemical laboratory, Pune and microbial type culture collection and gene bank (MTCC), Institute of microbial technology, Chandigarh with accession numbers: Bacillus subtilis NCIM 2193, Salmonella typhimurium NCIM 2501, Pseudomonas fluorescens NCIM 2100, E. coli K12 MTCC 1302, Mycobacterium smegmatis. A549 lung cancer cells and L132 normal cells were all obtained from the National centre for cell science (NCCS) Pune, India. Cell culture related chemicals were purchased from GIBCO (Invitrogen, CA, USA). MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide), cell culture grade dimethyl sulfoxide (DMSO) and all analytical grade chemicals were from HiMedia (Mumbai, India), HAuCl4 was purchased from Sigma-Aldrich Corporation.
Extraction of crude phytochemicals
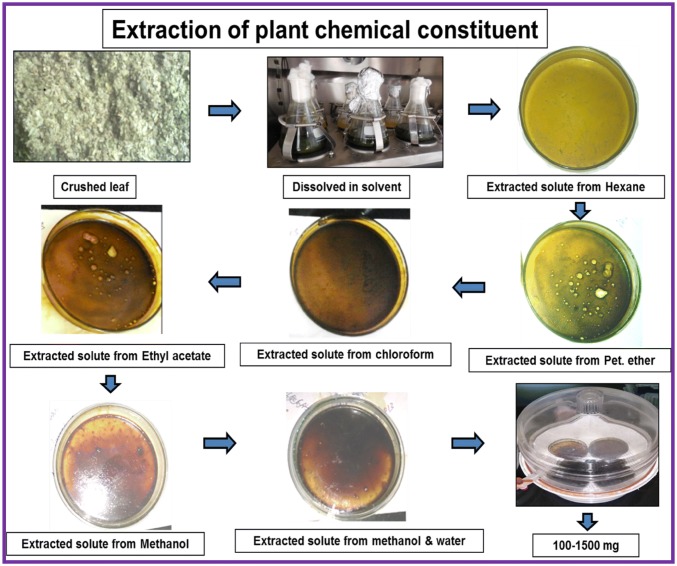
The leaves of all five plants were detached from the whole plant and cleaned with running tap water followed by distilled water. The separated leaves samples were air-dried at normal room temperature and pressure (NTP). Fifty grams of completely dry leaves samples were crushed, and collected in an autoclaved conical flask. The conical flask containing the crushed sample was filled with 250 mL of hexane (a nonpolar extraction solvent, based on the principle of like dissolves like) and kept at NTP for 2–3 h. During the extraction, the sample was shaken repeatedly. Subsequently, the flask was kept in a dark orbital shaker at 37 °C and 150 rpm for 24 h for complete mixing and precipitation of the phyto constituents dissolved in the nonpolar solvent. After plant residues get settled the supernatant was filtered for further processing. Subsequently, the solvent was recovered in rotary evaporator (BUCHI Labotec rotavapor model R-205, Germany) and transferred into pre-weighed, labelled petri dishes. The same process was repeated successively with other solvents of increasing polarity, such as petroleum ether, chloroform, ethyl acetate, methanol and a mixture of methanol and water (1:1, v/v) (Ansil et al. 2014).
Antimicrobial activity
Antimicrobial activity was analysed in agar plate using predescribed method (Perez et al. 1990). Starter cultures of the bacterial strains Salmonella typhi (S. typhi), Bacillus subtilis (B. subtilis), Escherichia coli (E. coli), Pseudomonas fluorescens (P. fluorescens), and Mycobacterium smegmatis (M. smegmatis) were prepared in nutrient broth. 100 μL of the above-prepared inoculums were spread onto nutrient agar plates. A 0.5 cm diameter well was prepared and filled with 30 μL (1 mg/mL) of plant extract. Methanol was used as a negative control. The antibiotics ampicillin, kanamycin, and chloramphenicol were used as standard drugs as well as positive controls. The experiment was conducted in a set of three independent replications. Antibacterial activity was estimated by assessing the diameter of observed zone of inhibition.
DPPH (1, 1-diphenyl-2-picrylhydrazyl) assay
Predescribed method for DPPH assay was used for measuring radical scavenging activity (Brand-Williams et al. 1995). A calibrated ascorbic acid standard curve was generated, and the results were presented in term of percentage inhibition per 100 μg dry sample weight in methanol. The obtained statistical data are expressed as the mean of three independent replications.
ABTS+ (2, 2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid)) free radical assay
In brief, 100 μg of methanol extracts from each plant was used for this analysis. The free radical scavenging activity was evaluated by spectrophotometric method using 2, 2′-azinobis 3-ethylbenzothiazoline-6-sulfonic acid (Re et al. 1999) based decolourization assay.
FRAP (ferric reducing ability of plasma) assay
The working solution for the FRAP was prepared correspond to prereported method with few modifications (Surveswaran et al. 2010). The absorbance was measured at 593 nm using 100 μL of the extract sample in 3 mL of FRAP reagent using Trolox as the standard (Bernardo et al. 2015).
Cyclic voltammetry
The biological oxidation potential was determined from the anodic current waves of cyclic voltammetry (CV). The anodic wave shows specific characteristics of the various low molecular weight antioxidant (LMWA) components, and the amplitude of each wave can be used for the quantitation of a specific component (Roy et al. 2011). An electrochemical analyser (CH Instruments, USA; Model 680B) was used for cyclic voltammetry analysis. The analysis was performed as refer to Roy et al. (2011). A total of 100 μg of dried methanol extract was dissolved in 1 mL of methanol, while 1 M KCl was used as the supporting electrolyte. Ag/AgCl was used as reference electrode, while platinum electrode and a platinum wire as the working electrode and counter electrode respectively. CV tracings were measured in the range of + 1.2 to − 1.2 volts at a normal scan rate of 0.06 V/second.
In vitro cytotoxicity and apoptosis
Cell culture
L132 human embryonic epithelial lung cells were grown in Dulbecco’s modified eagle’s medium (DMEM). Subsequently for A549 adenocarcinoma human alveolar basal epithelial cells culture Ham’s F12 K medium was used. All media are supplemented with 10% FBS (both from Invitrogen, Carlsbad, CA, USA) and 1% antibiotics (HiMedia, Mumbai, India) at 37 °C in CO2 incubator.
MTT assay
Briefly, in 96-well plates 100 µL of cells (1 × 104) were seeded (Greiner, Germany) in F12 cell culture media. Equal concentrations of extract (1 μg/mL; hexane and petroleum ether extracts were dissolved in hexane and petroleum ether, while the other extracts, such as chloroform, ethyl acetate, methanol, and methanol and water mixture, were dissolved in DMSO) from all plants were tested in a monolayer. DMSO, hexane, and petroleum ether were used as a vehicle or negative control. Subsequently, standard drug gefitinib was used as a positive control. The MTT assay for percentage inhibition was done using Nikhil et al. (2014) protocol. IC50 values were calculated using GraphPad Prism software.
Acridine orange staining
To check apoptosis A549 cells were stained with acridine orange/ethidium bromide (AO)/(EtBr) dye following previously described protocol (Nikhil et al. 2014). Imaging of slides was done under a fluorescence microscope (Zeiss, Axiovert 25, and Germany).
DAPI staining
The detection of apoptosis in cells was examined through visualizing changes in the nuclear morphology under a fluorescence microscope through DAPI staining of A549 cells. In brief, 200 µL (0.5 × 105 cells) was seeded in a 24-well plate and incubated for 24 h with equal concentrations of the three best cytotoxic active extracts. After washing cells were incubated for 10 min in the dark with 500 µL of 0.5 mg/mL DAPI and observed under fluorescence microscopy as described in an earlier study by Ansil et al. (2014).
UPLC-ESI–MS/MS-based characterization of active ingredients of medicinal plants
Ultra-performance liquid chromatography-mass spectrometry/mass spectrometry (UPLC-ESI–MS/MS) was used for phytochemical analysis. The LC system (UltiMate 3000 Rapid Separation systems; Thermo Scientific™) coupled with a LTQ XL™ ion trap mass spectrometer Thermo Scientific™ was employed. C18 reversed-phase column with particle size 1.7 µm, internal diameter 2.1 mm; length 50 mm (ACQUITY UPLC BEH C18) was used for separation of extract. For separation and identification, a gradient solvent system comprise of water and methanol (with 0.1% formic acid in each) was optimized for the range of 10–90%, 20 min (the percentage of methanol was changed as 10–40% in 2 min, 40–70% in 3 min, 70–90% in 10 min, 90–70% in 2 min, 70–40% in 1 min, and finally, 40–10 in 2 min) at a flow rate of 600 µL/min. The system operation conditions follow: capillary voltage (− 10 V), source temperature 350 °C, and collision energy of 35 eV. At flow rates of 80 and 40 (arbitrary units) nitrogen gas was used as the sheath and auxiliary gas. The fragmentation ions were detected in full scan positive mode within 100–1000 m/z mass range. The machine was controlled using Xcalibur 2.0.7 software (Thermo Scientific), while the chromatogram was observed at 254 and 310 nm.
Results and discussion
The extraction of the phytochemicals was executed as described in Fig. 1. Significant extraction using the different solvent systems was listed, and an array of compounds with increasing polarity were obtained (Table 1) and stored. The extracted compounds from different solvent systems were a mixture of various compounds with varying nature and behaviour; therefore, the observed effects of each extract is attributed to solitary or synergistic interactions of the phytochemicals (Ansil et al. 2014).
Fig. 1.
Detailed extraction process of compounds from plant leaf sample
Table 1.
Weight of extract (g) obtained from different plant leaves using various solvent along with their percentage
Various plant like: C. procera (L.) is indicated as (A); M. oliefera (Lam.) as (B), M. pinnata (L.) as (C), B. alba (L.) as (D), and E. neriifolia (L.) (E). Similarly various solvent system like Hexane is indicated (1), Petroleum ether as (2), Chloroform as (3), Ethyl acetate as (4), Methanol as (5), Methanol: Water as (6)
Antimicrobial activities
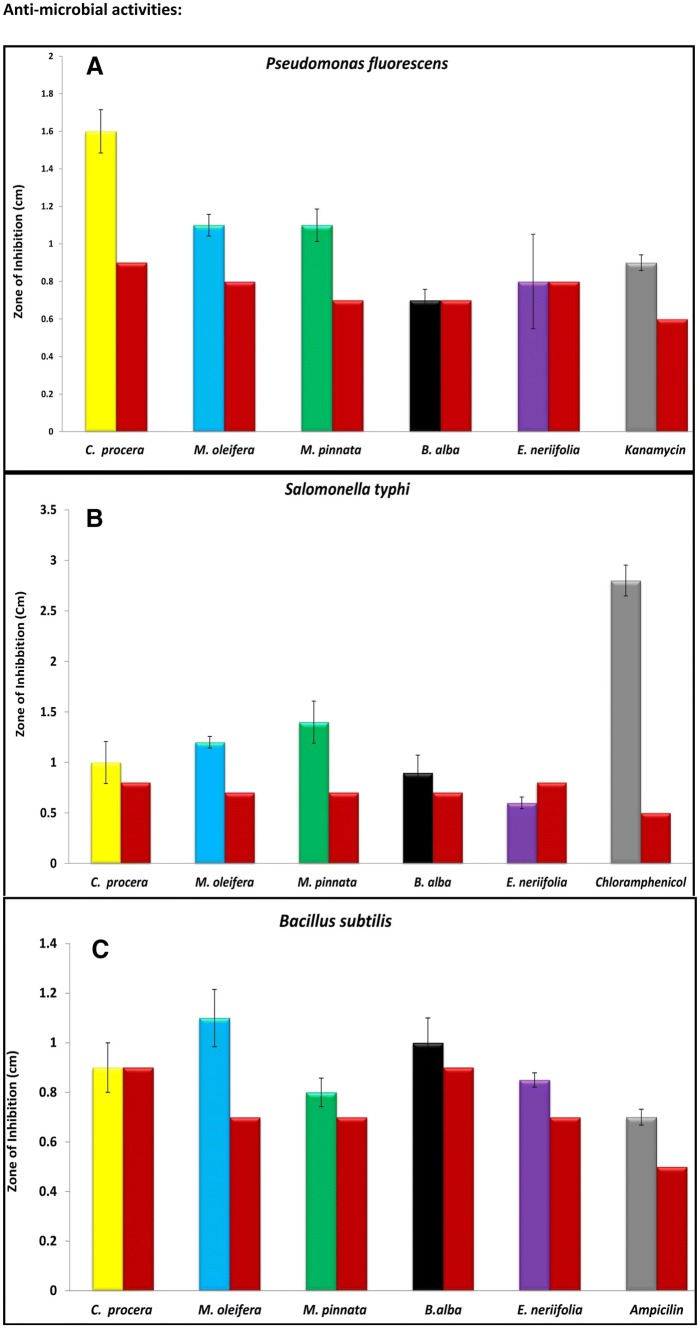
Promising activities were observed for all five plants at 30 μg/mL concentration from the methanol extracts (Fig. 2) with respect to the control against five different pathological and non-pathological bacterial strains, namely, P. fluorescens, M. smegmatis, S. typhi, E. coli and B. subtilis. The zone of inhibition (ZOI) formed by the leaves extract of C. procera in culture medium inoculated with P. Fluorescens (0.7 ± 0.11 cm)was found to be significant compared to the study reported by Mako et al. in 2012. In the M. smegmatis culture, C. procera leaves extract showed a significant ZOI with a diameter of 1.3 ± 0.20 cm. Our results displayed similar activity (1.32 ± 0.01 cm) as reported by Bhaskar and Ajay 2009. These results should be considered a significant finding because of the fair probability of different phytochemical effects from the leaves extract, contrary to the previous report on the seed extract of C. procera (Bhaskar and Ajay 2009).
Fig. 2.
Mean ZOI in terms of centimeter in the culture medium inoculated with different pathological/non pathological strains treated with different plant methanol extract. a Pseudomonas fluorescens; b Salomonella typhi; c Bacillus subtilis; d Escherichia coli; e Mycobacterium smegmatis. Min value for zone of inhibition from plant methanolic extract along with methanol standard presented in bar form
The methanol extract of M. pinnata displayed a significantly higher ZOI of 0.7 ± 0.20 cm against the S. typhi culture compared to an earlier report by Gahlaut and Chhillar (2013). A noteworthy ZOI was observed by M. oleifera in the culture medium inoculated with E. coli (0.5 ± 0.05 cm) and B. subtilis (0.4 ± 0.11 cm). This study is comparatively more substantial than the earlier published works of Bukar et al. (2010) and Rahman et al. (2009). All microbiological activities were found to be expressive and significant in terms of the extraction source or the potentiality of the extract.
Total antioxidant capacity (DPPH, ABTS & FRAP)
The methanol extract of M. pinnata exhibited a maximum percentage inhibition (83.97 ± 0.01, Fig. 3A) and IC50 for the DPPH assay relative to the ascorbic acid standard (54.98 µg), which are significantly higher than those in an earlier report (Sajid et al. 2012). Additionally, M. pinnata leaves also displayed potential activity in terms of standard trolox (Fig. 3B, 19.14 ± 3.09 mM) in the FRAP assay. However, for the ABTS assay, M. oleifera displayed a significant percentage inhibition (98.42 ± 0.08, Fig. 3A) with IC50 (49.01 µg) value of trolox. It is noteworthy that C. procera showed the lowest value, i.e., 52.25 ± 0.03 in DPPH, 95.57 ± 0.28 in ABTS, and 19.35 ± 0.20 mM in FRAP assay. All assays are analysed for 100 µg of crude extract.
Fig. 3.
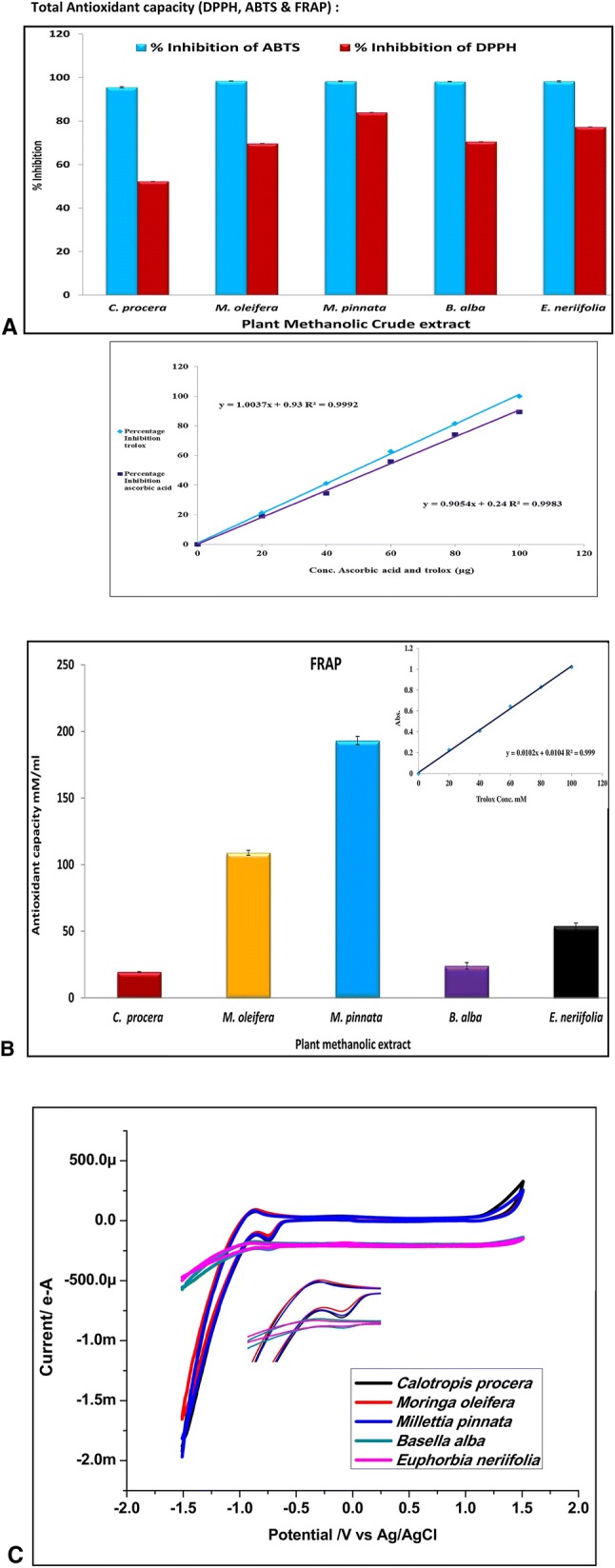
a Percentage inhibition of DPPH and ABTS of methanolic plant extract in terms of ascorbic acid and Trolox. b FRAP of methanolic plant extract in terms of Trolox. c cyclic voltammetry of plant methanolic extract at scan rate 60 mA in terms of ascorbic acid
Electrochemical analysis/cyclic voltammetry
This is the first report for comparative antioxidant potency of the above five plants. The electrochemical analysis (ECA) graph (Fig. 3c) indicates the presence of deflection in the anodic peak, and thus, the maximum scavenging of free radicals. From the above results, it can be inferred that the M. oleifera and M. pinnata extracts have maximum antioxidant activity compared to those of the remaining extracts. The results were compared with a previous work by Panat et al. (2016), and a noteworthy enhancement in the peak was found, indicating significant antioxidant activities present in these plant extracts.
Cell culture and cell viability assay
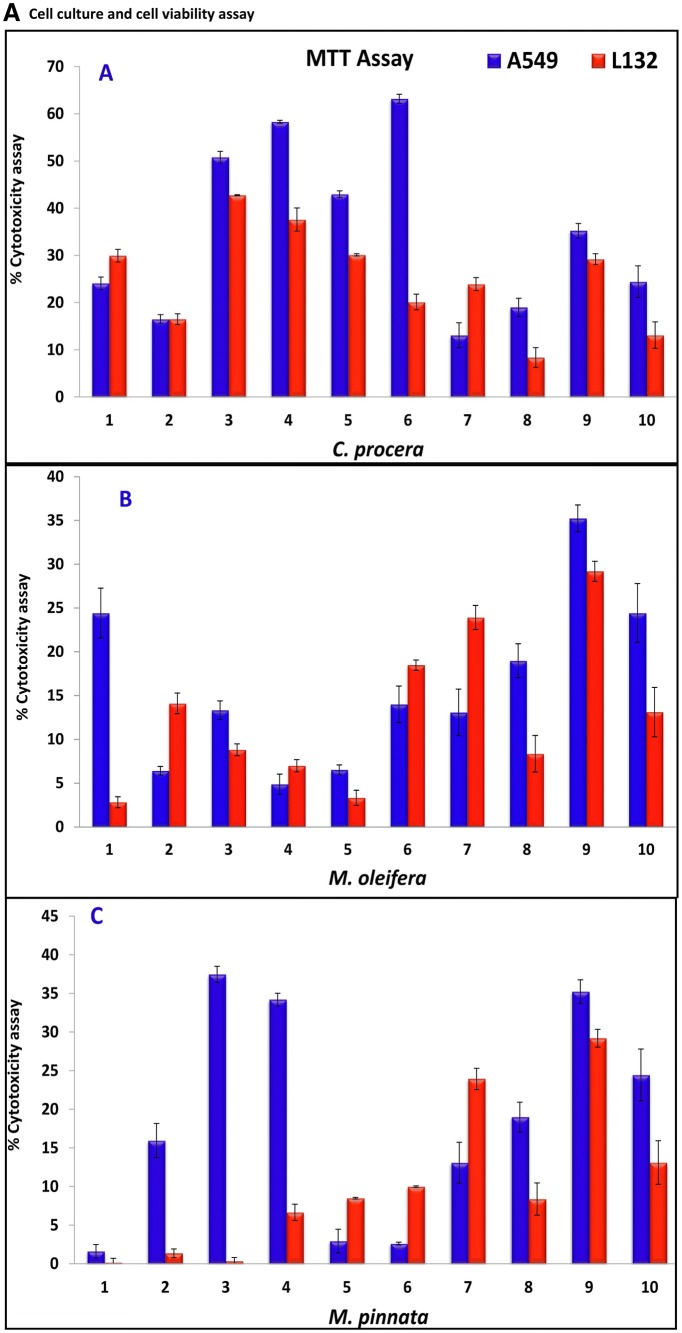
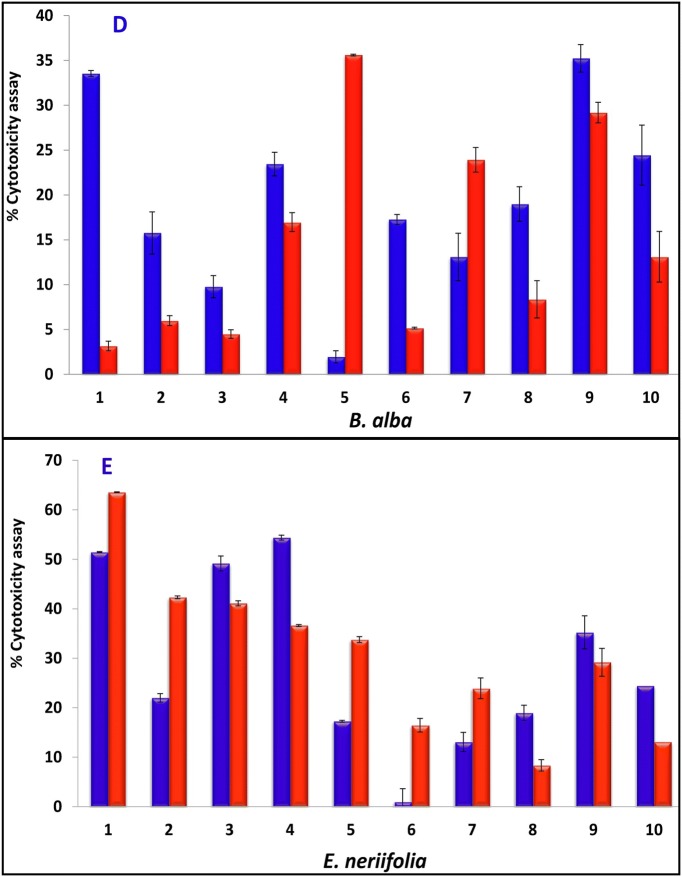
A cytotoxicity assay was performed for all five plant extracts using the MTT assay in the A549 lung cancer cell line, while L132 cells were used as a control (Fig. 4a,b). The cytotoxicity of the C. procera methanol: water extract (Fig. 4b, I) (63.19 ± 0.94), M. pinnata chloroform (Fig. 4b, II) and ethyl acetate extracts (37.45 ± 1.04, 34.20 ± 0.81, respectively) and B. alba hexane extract (Fig. 4b, III) (33.55 ± 0.32) were found to have the greatest potential in the A549 cell line among all the plant extracts. The cytotoxicity exhibited by the standard gefitinib was relatively low (24.44 ± 3.35) compared to that exhibited by these extracts. The results reported here for the leaves of B. alba were found to be greater than those of an earlier report (Sushila et al. 2010) for the whole plant extract, suggesting that the leaves could be a potential source for major cytotoxic activity. Plant leaves extracts of C. procera (Fig. 4b, IV), M. pinnata (Fig. 4 b, V),and B. alba (Fig. 4b, VI) on control L132 cells were comparatively less cytotoxic (20.13 ± 1.66, 0.33 ± 0.19, 6.65 ± 0.59 and 3.16 ± 0.53 respectively) than gefitinib (16.65 ± 0.21). Our results suggest that C. procera, M. pinnata, and B. alba leaves extracts are more cytotoxic to cancer cells than normal cells. Next, IC50 values of the highest potential extracts (C. procera, M. pinnata,and B. alba) were observed to be 2.96 ± 0.01, 4.22 ± 0.02, and 5.6 ± 0.01 μg/mL, respectively, while the IC50 of gefitinib was only 5 ± 3.35 μg/mL (Zhao et al. 2016). It was observed that the M. oleifera leaves extract displayed good potency as an antioxidant but failed to exhibit the same potency on cancer cells, while the C. procera leaves extract was found to be the most cytotoxic but exhibited little antioxidant activity. By comparing the data obtained from this study, one can infer a contradictory deduction for the potency of antioxidants as an anticancer agent, favouring a previous study reported by Sayin et al. (2014). The results of antioxidants assay do not support the notion that all the antioxidants are cytotoxic substances and thus require more comprehensive studies.
Fig. 4.
a Cytotoxicity assay of plant extract in A549, L132 human lung cancer cells and its control, Results represent the mean ± STDEVA. (1) Plant extract of hexane, (2) plant extract of petroleum ethers, (3) plant extract of chloroform, (4) plant extract of ethyl acetate, (5) plant extract of methanol, (6) plant extract of methanol + water, (7) Hexane–negative control, (8) petroleum ethers–negative control, (9) DMSO–negative control, (10) gefitinib–positive control: (final extract was made by dissolving in hexane for hexane extract, petroleum ether for petroleum ether extract while DMSO for the rest of all extracts and standard drug gefitinib). b Cytotoxicity assay on A549 cancer cell line (control, C. procera methanol: water extract–1:1–I, M. pinnata chloroform extract–II, B. alba hexane extract–III) and L132 normal cell line (control, C. procera methanol: water extract–1:1–IV, M. pinnata chloroform extract–V, B. alba hexane extract–VI)
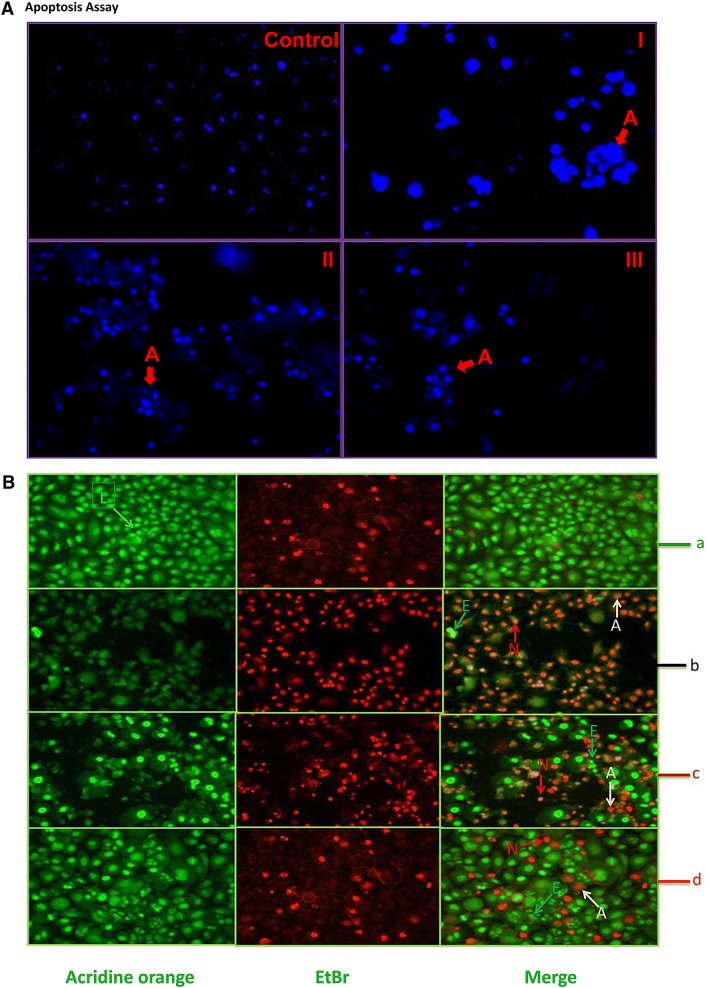
Apoptosis assay
It was clearly visible in treated cells after DAPI staining that (Fig. 5a) the C. procera methanol: water extract-treated cells displayed (Fig. 5a, I) maximum condensation of the nucleus. The M. pinnata chloroform extract (Fig. 5a, II) and B. alba hexane extract (Fig. 5a, III) also indicated the occurrence of apoptosis in treated cells. In DAPI staining, a bright blue condensed or blebbing nucleus is an indication of apoptosis (Ansil et al. 2014). In AO/EtBr staining, condensed but fragmented orange nuclei and an orange nuclear morphology like normal cells lacking condensed chromatin are clear demarcations of late apoptotic and necrotic cells, respectively (Nikhil et al. 2014).The occurrence of apoptosis and necrosis was evidently visible in Fig. 5b after exposure of the active extract to cancer cells followed by staining with AO/EtBr. The uniformly green colour was clearly visible in living cells, while early apoptotic cells contain lively green dots in the nucleus (Nikhil et al. 2014).
Fig. 5.
a DAPI staining of A549 cells (200× magnification), control cells without treatment; apoptotic cells with blebbed nuclei (C. procera methanol: water extract–1:1–I, M. pinnata chloroform extract–II, B. alba hexane extract–III). b acridine orange/ethidium bromide apoptotic assay at 200× magnification using potential plants extract on A549 lung cancer cell lines. a control cells; b cells treated with C. procera methanol: water extract–1:1; c cells treated with M. pinnata chloroform extract; d cells treated with B. alba hexane extract; L living cells, E early apoptotic cells, A apoptotic cells, N necrotic cells
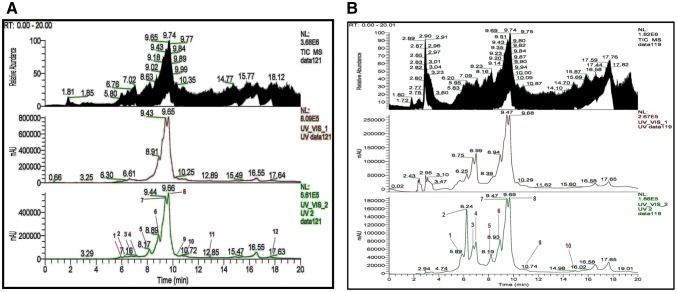
UPLC-ESI–MS/MS analysis of active extracts
The chloroform and ethyl acetate extracts of M. pinnata were found to be effective in A549 and L132 cell lines. Furthermore, UPLC-ESI–MS/MS determination of these extracts revealed the presence of 27 compounds (Table 1 and Figs. 1 to 12 provided in the supplementary data), and the majority of them belong to the class of flavones. The optimized parameters were combined and applied in UPLC–ESI–MS/MS to characterize the phytochemicals in the chloroform and ethyl acetate extracts from the leaves of M. pinnata. The total ion chromatograms (TIC) and linear ion trap mass spectra in positive ion mode of both extracts are shown in Figs. 6a, b, and 7. In the chromatogram and mass spectrum of the chloroform extract (Fig. 6a), 18 compounds were observed with a retention time of 6–18 min. The mass signature of the extract suggests the presence of flavonoids and steroids as major compounds in nature. In the chromatogram of the ethyl acetate extract within the retention time range of 3–16 min (Fig. 6b), 19 compounds were detected. Out of these analysed compounds, our mass analysis confirms presence of 10 similar compounds in the chloroform and ethyl acetate extract.
Fig. 6.
a. Chromatogram from UPLC of chloroform extract. Peak 1-At retaion time of 7.18 min presence of β-sitosterol detected; Peak 7-presence of Karanjin detected at retaion time of 9.44 min. b. Chromatogram from UPLC of ethyl acetate extract. Peak 1-presence of Lanceolatin B detected at 5.89 min; Peak 7-presence of Karanjin detected at 9.47 min; Peak 10-presence of stigmasterol at 10.74 min
Fig. 7.
UPLC-ESI–MS/MS spectrum in positive ion mode for extract is showing the presence of various compounds. Figure 7a indicating Peak having m/z ratio of 262.23 identified as Lanceolatin; Fig. 7b indicating Peak having m/z ratio of 415.13 identified as Karanjin; Fig. 7c indicating Peak having m/z ratio of 293.36 identified as β-sitosterol; and Fig. 7d indicating Peak having m/z ration of 413.41 identified as Stigmasterol
Our mass analysis results (Fig. 7) compared with the available literature confirmed the structural identification of a few of these compounds as well as the presence of β-sitosterol, lanceolatin B, karanjin, and stigmasterol with m/z of 415.13, 262.23, 293.36 and 413.41, respectively. Additionally, comparing the activity of the observed compounds in our extract with the previously reported work of Ding et al. (2009), Chang et al. (1997), Guo et al. (2015), and Shen et al. (2012), we deduce that the potential of the extracts is either due to the presence of β-sitosterol, lanceolatin B, karanjin, and stigmasterol (Fig. 7) autonomously or attributable to their synergistic effects. However, the toxicity displayed by the ethyl acetate extract of M. pinnata in L132 cells remains to be investigated in the future.
Conclusions
The findings of the antioxidant assay indicate that the methanol extract of M. pinnata exhibited a maximum percentage inhibition (83.975 ± 0.0043 and 193.14 ± 3.0943 mM/100 μL) in the DPPH and FRAP assays. The antioxidant properties of M. pinnata were also supported by cyclic voltammetry analysis. Furthermore, the MTT assay in A549 cells showed significant cytotoxicity from the C. procera, M. pinnata, and B. alba extracts. This work is supported by an apoptosis study using AO/EtBr and DAPI staining, which also inferred that the anticancer properties of the M. pinnata extract were the highest and most cytotoxic in all the above mentioned plants of those reported to date. Advancement of this study was achieved through the characterization of compounds by UPLC-ESI–MS/MS, confirming the presence of β-sitosterol, lanceolatin B, karanjin, and stigmasterol in the extracts. Coalescing all the results obtained in this work, we conclude that the phytoconstituents of the M. pinnata leaves extract have potential as anticancer, antioxidant and antimicrobial agents. Thus, isolation and characterization of the chemical constituents present in the M. pinnata leaves extract and exploration of their molecular targets in cancer cells will be the broad goals of this work that may be investigated in future studies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgement
Centre of Excellence (COE) TEQIP-II Grant no- NPIU/TEQIP II/FLN/31/158 is gratefully acknowledged for providing financial assistance to GK in the form of scholarship. Financial assistance provided by BIT Mesra, Ranchi, India to DMP through Seed Money Scheme-2015 (Ref.GO/SMS/DSR-007/2015-2016 dated 13-11-2015) is gratefully acknowledged. Central Instrumentation Facility BIT, Mesra, Ranchi, Jharkhand, India is acknowledged for providing analysis facility of the present study. Prof Shishir Sinha, Department of Chemical Engineering, IIT Roorkee is gratefully acknowledged for his support and suggestions. Authors wish to express a special gratitude to Dr. Stefano Dall’Acqua, Department of Pharmaceutical and Pharmacological Sciences, University of Padova, Italy for proofreading of manuscript and valuable suggestions in removing the technical and grammatical errors.
Abbreviations
- DPPH
1,1-Diphenyl-2-picrilhydrazyl
- AO/EtBr
Acridine orange/ethidium bromide
- DAPI
4′,6-Diamidino-2-phenylindole, dihydro chloride
- FRAP
Ferric reducing ability of plasma
- h
Hour/hours
- m/z
Mass-to-charge ratio
- μL
Microlitre
- mg
Milligram
- mL
Millilitre
- μg
Microgram
- mM
Millimolar
- ABTS+
2,2′-Azinobis (3-ethylbenzothiazoline-6-sulfonic acid)
- TPTZ
Tripyridyltriazine
- DMEM
Dulbecco’s modified eagle’s medium
- FBS
Foetal bovine serum
- ELISA
Enzyme-linked immunosorbent assay
- OD
Optical density
- ZOI
Zone of inhibition
- cm
Centimetre
- STDEVA
Standard deviation
- IC50
50% Inhibitory concentration
- UV–Vis
Ultraviolet–visible
- NADPH
Nicotinamide adenine dinucleotide phosphate
Author contributions
All authors have seen and approved the manuscript and its contents, and that they are aware of the responsibilities connected to authorship. All the work was majorly performed by Mr. Gourav Kumar, under the supervision of Dr. D. M. Pandey, Associate Professor Department of Bio-Engineering, BIT Mesra, and with joint efforts of Ms. Rashmi Gupta, Department of Bio-Engineering, BIT Mesra. All cytotoxicity assays were performed at Indian Institute of Technology Roorkee, India under the guidance of Dr. Shruti Sharan and Dr. Partha Roy, Professor Department of Biotechnology.
Compliance with ethical standards
Conflict of interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Ethical statement
We confirm that this research study does not include either vertebrates or higher invertebrates.
Contributor Information
Gourav Kumar, Email: gourav8490@gmail.com.
Dev Mani Pandey, Phone: 0651-2276223, Email: dmpandey@bitmesra.ac.in.
References
- Al Muqarrabun LMR, Ahmat N, Ruzaina SAS, et al. Medicinal uses, phytochemistry and pharmacology of Pongamia pinnata (L.) Pierre: a review. J Ethnopharmacol. 2013;150(2):395–420. doi: 10.1016/j.jep.2013.08.041. [DOI] [PubMed] [Google Scholar]
- Ansil PN, Wills PJ, Varun R, et al. Cytotoxic and apoptotic activities of Amorphophallus campanulatus (Roxb.) Bl. tuber extracts against human colon carcinoma cell line HCT-15. Saudi J Biol Sci. 2014;21(6):524–531. doi: 10.1016/j.sjbs.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atabani AE, Silitonga AS, Badruddin IA, et al. A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew Sustain Energy Rev. 2012;16(4):2070–2093. doi: 10.1016/j.rser.2012.01.003. [DOI] [Google Scholar]
- Bernardo MA, Silva ML, Santos E, et al. Effect of cinnamon tea on postprandial glucose concentration. J Diabetes Res. 2015;2015:913651. doi: 10.1155/2015/913651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskar VH, Ajay SS. Antimicrobial activity of Calotropis procera seeds. Asian J Chem. 2009;21(7):5788. [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset CLWT. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol. 1995;28(1):25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Bukar A, Uba A, Oyeyi T. Antimicrobial profile of Moringa oleifera Lam. extracts against some food–borne microorganisms. Bayero. Bayero J Pure Appl Sci. 2010;3(1):43–48. [Google Scholar]
- Chang LC, Gerhäuser C, Song L, et al. Activity-guided isolation of constituents of Tephrosia purpurea with the potential to induce the phase II enzyme, quinine reductase. J Nat Prod. 1997;60(9):869–873. doi: 10.1021/np970236p. [DOI] [PubMed] [Google Scholar]
- Ding Y, Nguyen HT, Kim SI, et al. The regulation of inflammatory cytokine secretion in macrophage cell line by the chemical constituents of Rhus sylvestris. Bioorg Med Chem Lett. 2009;19(13):3607–3610. doi: 10.1016/j.bmcl.2009.04.129. [DOI] [PubMed] [Google Scholar]
- Gahlaut A, Chhillar AK. Evaluation of antibacterial potential of plant extracts using resazurin based microtiter dilution assay. Int J Pharm Pharm Sci. 2013;5(2):372–376. [Google Scholar]
- Galib MB, Mashru M, Jagtap C, et al. Therapeutic potentials of metals in ancient India: a review through CharakaSamhita. J Ayurveda Integr Med. 2011;2(2):55. doi: 10.4103/0975-9476.82523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JR, Chen QQ, Lam CWK, et al. Effects of karanjin on cell cycle arrest and apoptosis in human A549, HepG2 and HL-60 cancer cells. Biol Res. 2015;48(1):40. doi: 10.1186/s40659-015-0031-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawk MA, McCallister C, Schafer ZT. Antioxidant activity during tumor progression: a necessity for the survival of cancer cells? Cancers. 2016;8(10):92. doi: 10.1007/s13205-017-1013-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondhare D, Lade H. Phytochemical profile, aldose reductase inhibitory, and antioxidant activities of indian traditional medicinal Coccinia grandis (L.) fruit extract. 3 Biotech. 2017;7:378. doi: 10.1007/s13205-017-1013-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Sharma M. Herbal nanomedicine interactions to enhance pharmacokinetics, pharmaco-dynamics, and therapeutic index for better bioavailability and biocompatibility of herbal formulations. J Mater NanoSci. 2018;5(1):35–58. [Google Scholar]
- Kumar G, Ghosh M, Pandey DM. Method development for optimized green synthesis of gold nanoparticles from Millettia pinnata and their activity in Non-small cell lung cancer cell lines. IET Nanobiotechnol. 2019;13(6):626–633. doi: 10.1049/iet-nbt.2018.5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Chen G, Zhang C, et al. Research progress of natural antioxidants in foods for the treatment of diseases. Food Science Hum Wellness. 2014;3(3):110–116. doi: 10.1016/j.fshw.2014.11.002. [DOI] [Google Scholar]
- Mako GA, Memon AH, Mughal UR, et al. Antibacterial effects of leaves and root extract of Calotropis procera Linn. Pak J Agri Engg Vet Sci. 2012;28:141–149. [Google Scholar]
- Nikhil K, Sharan S, Chakraborty A, et al. Role of isothiocyanate conjugate of pterostilbene on the inhibition of MCF-7 cell proliferation and tumor growth in Ehrlich ascitic cell induced tumor bearing mice. Exp Cell Res. 2014;320(2):311–328. doi: 10.1016/j.yexcr.2013.10.015. [DOI] [PubMed] [Google Scholar]
- Panat NA, Maurya DK, Ghaskadbi SS, et al. Troxerutin, a plant flavonoid, protects cells against oxidative stress-induced cell death through radical scavenging mechanism. Food Chem. 2016;194:32–45. doi: 10.1016/j.foodchem.2015.07.078. [DOI] [PubMed] [Google Scholar]
- Perez C, Pauli M, Bazerque P. An antibiotic assay by the agar well diffusion method. Acta Biol Med Exp. 1990;15(1):113–115. doi: 10.1126/scitranslmed.3007653. [DOI] [Google Scholar]
- Pracheta SV, Paliwal R, Sharma S. In vitro free radical scavenging and antioxidant potential of ethanolic extract of Euphorbia neriifolia Linn. Int J Pharm PharmSci. 2011;3(1):238–242. [Google Scholar]
- Rahman MM, Sheikh MMI, Sharmin SA, et al. Antibacterial activity of leaf juice and extracts of Moringa oleifera Lam against some human pathogenic bacteria. CMU J Nat Sci. 2009;8(2):219. [Google Scholar]
- Re R, Pellegrini N, Proteggente A, et al. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol Med. 1999;26(9):1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Roy N, Laskar RA, Sk I, et al. A detailed study on the antioxidant activity of the stem bark of Dalbergia sissoo Roxb., an Indian medicinal plant. Food Chem. 2011;126(3):1115–1121. doi: 10.1016/j.foodchem.2010.11.143. [DOI] [Google Scholar]
- Sajid ZI, Anwar F, Shabir G, et al. Antioxidant, antimicrobial properties and phenolics of different solvent extracts from bark, leaves and seeds of Pongamia pinnata (L.) Pierre. Molecules. 2012;17(4):3917–3932. doi: 10.3390/molecules17043917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayin VI, Ibrahim MX, Larsson E, et al. Antioxidants accelerate lung cancer progression in mice. Sci Transl Med. 2014;6(221):221ra15–221ra15. doi: 10.1126/scitranslmed.3007653. [DOI] [PubMed] [Google Scholar]
- Shamim G, Ranjan SK, Pandey DM, et al. Lac dye as a potential anti-neoplastic agent. J Cancer Res Ther. 2016;12(2):1033. doi: 10.1155/2015/913651. [DOI] [PubMed] [Google Scholar]
- Sharma S, Sundararajan M, Kumar R (2016) Medicinal plant in Jharkhand State: an overview of current scenario. Global J Eng Sci Res 1–6. ISSN 2348-8034. http://cimfr.csircentral.net/id/eprint/1842
- Shen T, Zhang L, Wang YY, et al. Steroids from Commiphora mukul display antiproliferative effect against human prostate cancer PC3 cells via induction of apoptosis. Bioorg Med Chem Lett. 2012;22(14):4801–4806. doi: 10.1016/j.bmcl.2012.05.052. [DOI] [PubMed] [Google Scholar]
- Surveswaran S, Cai YZ, Xing J, et al. Antioxidant properties and principal phenolic phytochemicals of Indian medicinal plants from Asclepiadoideae and Periplocoideae. Nat Prod Res. 2010;24(3):206–221. doi: 10.1080/14786410802228827. [DOI] [PubMed] [Google Scholar]
- Sushila R, Deepti A, Permender R, et al. Cytotoxic and antibacterial activity of Basella alba whole plant: a relatively unexplored plant. Pharmacologyonline. 2010;3:651–658. [Google Scholar]
- Tiwari R, Latheef SK, Ahmed I, et al. Herbal immunomodulators- a remedial panacea for designing and developing effective drugs and medicines: current scenario and future prospects. Curr Drug Metab. 2018;19(3):264–301. doi: 10.2174/1389200219666180129125436. [DOI] [PubMed] [Google Scholar]
- Zhao ZQ, Yu ZY, Li J, et al. Gefitinib induces lung cancer cell autophagy and apoptosis via blockade of the PI3 K/AKT/mTOR pathway. Oncol Lett. 2016;12(1):63–68. doi: 10.3892/ol.2016.4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.