Abstract
Background
Studies have shown that consumption of high levels of alcohol causes many negative effects on the liver and kidneys where antioxidant ingredients can be a proper solution to reducing the resulting damages. So, the present study investigated the effect of hydroalcoholic extract of Crocus sativus L. (saffron) petal with antioxidant properties on the changes in inflammatory and enzymatic indices resulting from alcohol use in the male rats’ kidney and liver.
Materials and methods
After preparing the extract, LD50 was determined and high-performance liquid chromatography (HPLC) was employed to specify the type and the rate of the active ingredients of the extract. Then, 36 male Wistar rats were randomly assigned into six groups (n=6). The first group was only administered with normal saline and the second group only received ethyl alcohol 6 mL/kg/day·BW. The third and the fourth groups received ethyl alcohol 6 mL/kg/day·BW plus 167.5 and 335 mg/kg/day·BW saffron petal extract for 8 weeks. The fifth and the sixth groups received ethyl alcohol 6 mL/kg/day·BW for the first 8 weeks and were subsequently gavage fed on saffron extract for 167.5 and 335 mg/kg/day·BW, respectively, during the next 8 weeks. In the beginning and after the termination of the treatment, blood samples were collected from all rats.
Results
The LD50 of the extract was about 670 mg/kg. The HPLC results indicated that the extract contains important antioxidant ingredients. At the end of the study, the serum concentration of the inflammatory indices, renal enzymes, and hepatic enzymes experienced a significant reduction in all of the intervened groups compared to the negative control group (minimum significant difference: P<0.05) except for the treatment group 1.
Conclusion
Based on the current results, the extract has a protective effect in a dosage-dependent way and greater protective roles were documented for higher dosages.
Keywords: Crocus sativus L., alcohol, kidney, liver, rat
Introduction
Alcohol use is considered as a substantial general health problem affecting different social, economic, and clinical aspects.1 The epidemiological studies in the US have shown that 29.1% of the 18-year-old or older American adults have alcohol abuse problems and this trend is growing.2 Drinking too much alcohol is an important cause of mortality resulting from hypertension, cardiovascular diseases, cerebral infarction, hepatic cirrhosis, various kinds of cancer and infection, pancreatitis, diabetes type II, and various damages.3 In 2012, alcohol abuse accounted for 5.9% of the total mortality rates worldwide and it has been reported to be the fifth risk factor for early death and disability.4 Furthermore, the high use of alcohol has been found to be associated with a large number of psychiatric disorders along with disruption of interpersonal relationships and malfunctioning. It has also been shown to impose psychological and financial burdens on the families and society through increasing the vehicular accidents, violence, and crime caused by individuals addicted to alcohol.3
Alcohol can infiltrate into almost all body tissues and cause different effects on the target sites and engender various outcomes.5 Liver is the primary site of alcohol metabolism in the body and the hepatic cells are susceptible to the changes resulting from alcohol use where the increase in the frequency of drinking alcohol causes development a vast spectrum of multiple damages from reversible steatosis (fatty liver) to alcoholic hepatitis, fibrosis, and cirrhosis which might even progress to hepatocellular carcinoma.6 The liver becomes large, yellow, fatty, and hard in alcoholic fatty liver. Hepatic cells become expanded by the large macro-vesicular vacuoles containing the fat that exist in the cytoplasm.7 In alcoholic hepatitis, hepatic cells’ destruction and necrosis can be usually seen with cell inflammation and multinuclear white globules and lymphocytes’ infiltration.8 In alcoholic cirrhosis, the hepatic cells’ destruction persists until the appearance of fibroblasts in the lesion site; collagen production is consequently stimulated following which curtain-like walls of connective tissue appear in the periphery of the central and portal vein causing the eventual linkage of port’s triads to the central vein.9 Sensitization of the Kupffer cells is an important event in the initiation of the alcoholic liver diseases developing due to endotoxins. This sensitivity leads to the release of inflammatory mediators (such as cytokines, fat metabolites, and ROS).7–9 In alcoholic fatty liver, enzymes such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma-glutamyl transferase (GGTP) are elevated.8
Prior studies have shown that the alcohol use exerts a substantial effect on the kidneys: alcohol-induced renal damage might become complicated with the interaction between the kidneys and other organs, including liver, intestine, skeletal muscle, and cardiovascular system.10 For example, alcoholic hepatic cirrhosis with its related hepato-renal syndrome is a common side effect of alcohol use and can result in renal deficiencies due to the severe dilation of the visceral veins and constriction of the renal veins.11 Studies have demonstrated that the extreme use of alcohol plays a role in the development of heart diseases which can per se be a major risk factor for the development of chronic renal diseases.12,13 Chronic use of alcohol causes hypertension through various mechanisms, including activation of the renin-angiotensin system, oxidative stress, and increase in the sympathetic activity; the pathological activation of renin-angiotensin system causes an increase in the oxidative stress and expansion of renal damage, i.e., glomerulus, tubules, and vasculators.14–17 In addition, the evidence suggests that a vast array of the electrolytic disorders and acid–base imbalance in alcoholics might be due to the tubular malfunctioning.18,19 The pathophysiological reaction of the kidney leads to the accumulation of liquid in the tissues, ischemic damages, peripheral stimulation, and extreme activation of the hormonal system (renin–angiotensin–aldosterone) which are interconnected with the blood circulation. Additionally, any renal damage can bring about notable increase in the serum creatinine and blood urea nitrogen (BUN).20
The shared pathophysiological tissue mechanisms and the organs’ lesions induced by high use of alcohol include inflammation, increase in oxidative stress, disruption in anabolic signals and regulation of catabolic processes, and disorder in the regulation of lipid metabolism and disruption in signal transmission paths.21
Further, studies have shown that alcohol use can impose irreparable side effects to the cell via formation of free radicals.22 Therefore, use of antioxidant ingredients can be one of the proper ways for reducing the damages resulting from alcohol use. Meanwhile, a tendency has been growing to use natural antioxidants such as those found in the plants. Saffron is one of the medicinal plants under use for many years. It is a small flower-bearing plant belonging to iridaceous family and possesses three to four petals each with three red stigmas.23,24 The antioxidant, antidepressant, and hypertension-curbing effects of saffron petal extracts have been investigated in various studies.25–27 Saffron petal contains strong flavonoid antioxidants, whose different effects on cholesterol reduction and their antiradical characteristics have been repeatedly proven.28
Accordingly, the present study aims at investigating the antioxidant effects of hydroalcoholic extract of saffron petal to evaluate the possible effects of this plant on reducing the damages induced by alcohol use on the liver and kidney. Since no study has so far dealt with the protective effect of saffron petal on the hepatic and renal damages following the use of alcohol, the current research paper intends to examine the effect of alcohol on inflammatory and enzymatic factors’ levels of the liver and kidney. It was assumed that the use of saffron petal extract can reverse these effects. Performing such studies can provide a valuable opportunity for improving the insights into the harmful effects of alcohol use on the body organs and offering useful therapeutic options based on traditional medicine and medicinal herbs for the reduction of such damages. So, the present study has been conducted for investigating the effect of hydroalcoholic extract of Crocus sativus L. petal (HAECSP) on the changes in the inflammatory and enzymatic indices resulting from alcohol use in the liver and kidney of rats.
Materials and methods
Preparation of the plant and extract
Fresh saffron petals were collected from the farms in Mashhad and were dried away from light and moisture. They were then ground and turned into powder by an electrical grinder. Next, a given amount of the samples was measured and mixed in ethanol 80% (for a ratio of 1:20) and the mixture was placed on a shaker for 24 hrs. Afterward, the mixture was filtered using Whatman filter paper No. 1. Next, the filtered solution was condensed in vacuum by the distillation device and dried inside an oven at 40°C for 24 hrs and kept in refrigerator for the later experiments.
Determining the chemical ingredients of the extracts
High-performance liquid chromatography (HPLC) method was done according to the reported procedure.29 A simple and reproducible reversed-phase HPLC with a Knauer liquid chromatography equipped with an ultraviolet detector and a reverse-phase C18 column using isocratic elution with UV absorbance detection was developed and validated for the determination of Safranal, Myricetin, Crocin, Pelargonidin, and Quercetin. The column temperature, mobile phase (0.1%) formic acid in water (B), was maintained within 5–70% and solvent acetonitrile (A), flow rate, injection volume, and detection wavelength were set at 25°C, 1 mL/min, 1 µL, and 255 and 450 nm, respectively. In a similar condition, Safranal, Myricetin, Crocin, Pelargonidin, and Quercetin standard solution (dissolved in methanol) was prepared. For this purpose, 250 mg of dried extracts were dissolved in 10 mL HPLC-grade methanol, sonicated for 15 mins, filtered and further diluted to 5 mg/mL. The peaks obtained from the Crocus sativus L. Then, the petals’ extract of the plant was compared with Safranal, Myricetin, Crocin, Pelargonidin, and Quercetin standard. A stock solution of Safranal, Myricetin, Crocin, Pelargonidin, and Quercetin standard was prepared at 0.1 mg/mL in HPLC-grade methanol, filtered, and further diluted in the same solvent to obtain 15.6, 31.25, 62.5, 125, 250, and 500 µg/mL.
Animals’ preparation and maintenance
The study was conducted on mature male Wistar rats, within 180–220 g in weight and 10 weeks old, which had been procured from the center for raising laboratory animals in Medical Sciences University, Ilam Branch. The animals were kept in standard cages at 25±2°C in a 12-hr light-dark cycle and were allowed to have free access to food and water. The animals were kept under the foresaid conditions and no treatment and intervention were conducted on them so that they could get accustomed to the environment and conditions. All of the studies performed on the animals were in adherence to the instructions for taking care of and using laboratory animals.
LD50 determination
To determine the lethal dosage of the saffron petal hydroalcoholic extract, eight groups, each containing eight mature male Wistar rats, were studied. The first group, as the control group, was administered with 2cc of normal saline and the rest of the groups were gavage-fed on hydroalcoholic extract of saffron petal for 50, 100, 200, 400, 800, 1600, and 3200 mg/kg. Then, the apparent behaviors, physical health, nervous signs, food consumption rate, urine and solid waste excretion status, and the mortality rates of the animals in various groups were evaluated for 24 hrs following which LD50 dosage was calculated using a computer-based technique.30–33
Animal groupings
The present study is an applied research in the field of basic sciences and was conducted on mature male Wistar rats with a mean weight of 200±20 g. To perform the experiment, a sample volume consisting of 36 mature male rats was procured from the center for raising laboratory animals in Medical Sciences University, Ilam Branch. The animals were assigned into six identical groups, each containing six rats which were subjected to oral administration as follows: the first group or the positive control group was only given ordinary daily dietary regime plus distilled water. The second group (negative control group) only received ethyl alcohol for 6 mL/kg/day·BW. The third and the fourth groups were gavage-fed on ethyl alcohol for 6 mL/kg/day·BW34 plus 167.5 and 335 mg/kg/day·BW of hydroalcoholic extract of saffron petal, respectively (prevention phase). The fifth and sixth groups were administered with 6 mL/kg/day·BW of ethyl alcohol during the first 8 weeks and were, subsequently, gavage-administered with 167.5 and 335 mg/kg/day·BW of the extract, respectively (treatment phase). During the study, all of the groups were allowed to have free access to food and all of the treatments were carried out orally on a daily basis.
Measurement of the levels of inflammatory and enzymatic indices of liver and kidney
Before the initiation and after the termination of the period, the animals were kept in fasting state for 12 hrs. Then, blood samples were collected from all rats twice according to all ethical considerations for measuring the levels of kidney (BUN and Creatinine) plus liver’s enzymatic (GGT and ALP, AST, ALT) and inflammatory indices (IL6, CRP, TNF-α and Fibrinogen): once on the first day of treatment and a second time in the end of the intervention (24 hrs after the last treatment). The collected blood samples were kept in serum separation tubes for 20 mins at laboratory temperature for coagulation. Then, they were centrifuged at 25,000 rpm for 15 mins whereby the serums were collected. The prepared serums were used for measuring the levels of inflammatory (IL6, CRP, TNF-α and Fibrinogen) and enzymatic indices of liver performance (alkaline phosphatase (ALP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma-glutamyl transferase (GGT)) as well as kidney’s performance (blood urea nitrogen (BUN) and creatinine). The levels of the inflammatory and enzymatic indices of the kidney and liver were measured according to the standard methods using assay kits based on chromatography in A15 Auto-Analyzer device (Analyzer A15; Bio system, Barcelona, Spain).
Ethics statement
This study was carried out in accordance with the recommendations of International Council for Laboratory Animal Science (ICLAS). This experimental study was approved by the Ethics Committee of Ilam University Medical Sciences (IR.MEDILAM.REC.1397.033).
Data analysis
To perform the statistical data analysis, SPSS16 was used and the obtained quantitative data were presented as mean±SEM. The data were investigated using one-way analysis of variance and Tukey post hoc test. The significance level of all tests was set at P<0.05.
Results
LD50 evaluation
The results obtained from the study during the acute toxicity determination stage of hydroalcoholic extract of Crocus sativus L. petal (HAECSP) indicated that LD50 of the saffron petal hydroalcoholic extract is about 670 mg/kg. Hence, the present study investigated the protective effects of nontoxic dosages (25% and 50% of LD50 dosage) of this extract, i.e., 167.5 mg/kg and 335 mg/kg, on the inflammatory factors and performance parameters of kidney and liver in rats.
Ingredients of the HAECSP
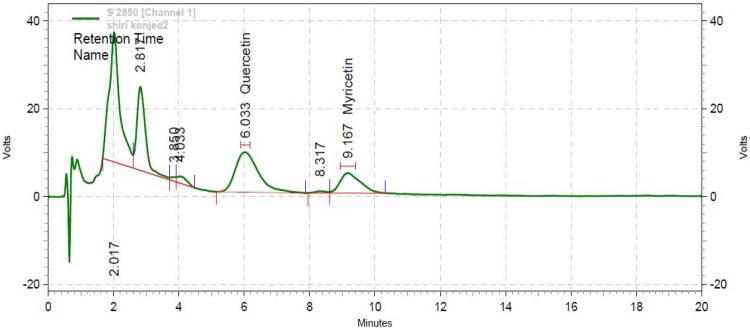
The HPLC results, as summarized in Figure 1, indicated that quercetin and myricetin concentrations were 38.69 μg/mL and 27.34 μg/mL at 255 nm wavelength with retention times (Rt) of 6.033 and 9.167 mins, respectively.
Figure 1.
Chromatogram of Myricetin and Quercetin by HPLC at 255 nm wavelength.
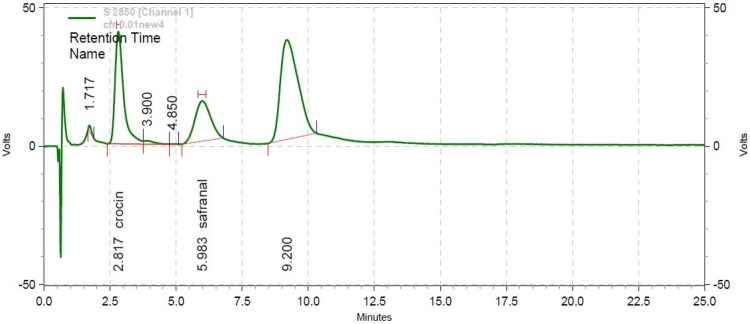
Further, the crocin and safranal concentrations were found as 78.25 μg/mL and 55.65 μg/mL, respectively, at 450 nm wavelength with Rt values of 2.817 mins and 5.980 mins (Figure 2). No peak was observed for pelargonidin ingredient of saffron petal at 255 nm and 450 nm wavelengths.
Figure 2.
Chromatogram of Safranal and Crocin by HPLC at 255 nm wavelength.
Effects of the HAECSP on the inflammatory factors’ levels
According to Table 1, the serum levels of all studied inflammatory indices have been almost identical before the study. The comparison of the mean values of these indices in the studied groups before and after the intervention suggests that the mean values of all four inflammatory indices did not undergo any significant statistical change in the positive control group which had only received an ordinary dietary regime plus normal saline (P>0.05). In the negative control group which had received an ordinary dietary regime plus ethyl alcohol at a dosage of 6 mL/kg/day·BW, the mean value of all inflammatory indices significantly increased as compared to the study initiation (P<0.001). Concerning the groups in the prevention phase, a significant increase was also observed in all of the inflammatory factors following the intervention in the group that had received ethyl alcohol at a dosage of 6 mL/kg/day·BW plus HAECSP for a dosage of 167.5 mg/kg/day·BW (CRP: P<0.05, IL6 and TNF-α: P<0.01, Fib: P<0.001). On the other hand, no significant increase was observed for the group that had received ethyl alcohol at a dosage of 6 mL/kg/day·BW plus HAECSP for a dosage of 335 mg/kg/day·BW in all of the inflammatory indices except for TNF-α (P<0.05) which underwent a significant increase after the intervention in contrast to pre-intervention. This demonstrates the protective effect of the extract, particularly at higher dosages. The treatment effect was far weaker in both of the groups in treatment phases which had been administered with ethyl alcohol for 6 mL/kg/day·BW during the first week and saffron petal extract for dosages of 167.5 and 335 mg/kg/day·BW during the second 8 weeks. Here, a significant effect was observed in all of the inflammatory indices at the end of the study compared to the pre-study (CRP, IL6, and Fib: P<0.001, TNF-α: P<0.01).
Table 1.
Comparison of the mean serum concentrations of the inflammatory indices in the beginning and end of the study in various groups
| Liver enzymes | Control+ | Control− | Prevention phase | Treatment phase | |||
|---|---|---|---|---|---|---|---|
| Normal saline | Alcohol (6 mL/kg/day·BW) | Alcohol (6 mL/kg/day·BW) + HAECSP (167.5 mg/kg/day·BW) | Alcohol (6 mL/kg/day·BW) + HAECSP (335 mg/kg/day·BW) | Alcohol (6 mL/kg/day·BW)/HAECSP (167.5 mg/kg/day·BW) | Alcohol (6 mL/kg/day·BW)/HAECSP (335 mg/kg/day·BW) | ||
| CRP (ng/mL) | Before the intervention | 1.78±0.09 | 1.84±0.13 | 1.89±0.10 | 1.86±0.11 | 1.85±0.07 | 1.75±0.09 |
| After the intervention | 1.81±0.12 | 3.83±0.24 | 2.58±016 | 1.90±0.14 | 3.53±0.26 | 3.28±0.20 | |
| P-value | 0.840 | 0.000*** | 0.041* | 0.117 | 0.000*** | 0.000*** | |
| IL-6 (ng/L) | Before the intervention | 2.64±0.10 | 2.73±0.10 | 2.59±0.11 | 2.60±5.08 | 2.69±0.14 | 2.70±4.09 |
| After the intervention | 2.69±0.12 | 5.98±0.22 | 3.83±0.17 | 2.90±0.18 | 5.50±0.24 | 5.11±0.21 | |
| P-value | 0.732 | 0.000*** | 0.007** | 0.062 | 0.000*** | 0.000*** | |
| TNF-α (pg/mL) | Before the intervention | 11.31±0.29 | 11.16±0.36 | 11.39±0.22 | 11.20±0.17 | 11.26±9.21 | 11.17±0.31 |
| After the intervention | 11.19±0.26 | 19.98±0.74 | 14.10±0.38 | 12.53±0.32 | 18.56±0.36 | 17.39±0.35 | |
| P-value | 0.595 | 0.000*** | 0.006** | 0.046* | 0.000*** | 0.002** | |
| Fibrinogen (mg/mL) | Before the intervention | 2.43±0.10 | 2.28±0.13 | 2.34±0.08 | 2.38±0.09 | 2.39±0.11 | 2.29±0.07 |
| After the intervention | 2.47±0.08 | 4.33±0.34 | 3.79±0.34 | 2.61±0.23 | 4.18±0.24 | 3.94±0.31 | |
| P-value | 0.961 | 0.000*** | 0.000*** | 0.051 | 0.000*** | 0.000*** | |
Notes: Values are given as mean±SEM for six rats in each group (*P<0.05, **P<0.01, ***P<0.001). Significant values are shown in bold.
Abbreviations: HAECSP, hydroalcoholic extract of Crocus sativus petals; CRP, C-reactive protein; IL-6, interleukin 6; TNF-α, tumor necrosis factor-α.
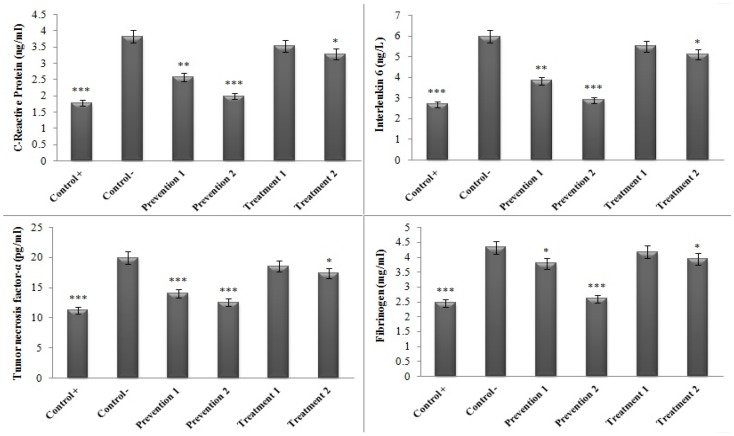
Figure 3 illustrates the results related to the comparison of the serum concentrations of the inflammatory indices between the groups administered with HAECSP and the negative control group (which had only received ethyl alcohol) by the termination of the study. As can be seen, the serum concentration of the inflammatory indicators significantly diminished in all of the studied groups compared to the negative control group (minimum significant difference: P<0.05). The only exception was the treatment group 1 (the group that received alcohol ethanol for 6 mL/kg/day·BW during the first 8 weeks and HAECSP for 167.5 mg/kg/day·BW for the second 8 weeks) which showed no significant difference in this regard compared to the negative control group (Figure 3).
Figure 3.
Comparison of the serum concentrations of the inflammatory indices in various groups in contrast to the negative control group. (*P<0.05, **P<0.01, ***P<0.001).
The comparison of the levels of renal enzymes (ALP, AST, ALT, and GGT) before and after the study across various studied groups has been presented in Table 2. As can be seen, there is no significant difference between the positive control group and the second group of the prevention phase (the group that received ethyl alcohol for a dosage of 6 mL/kg/day·BW plus HAECSP for a dosage of 335 mg/kg/day·BW) in terms of the mean values of these enzymes before and after the intervention (P>0.05). However, the serum levels of these enzymes were found significantly elevated in the studied groups at the end of the intervention as compared to its beginning (P≤0.001).
Table 2.
Comparison of the serum concentrations of hepatic enzymes in various groups at the beginning and end of the study
| Liver enzymes | Control+ | Control− | Prevention phase | Treatment phase | |||
|---|---|---|---|---|---|---|---|
| Normal saline | Alcohol (6 mL/kg/day·BW) | Alcohol (6 mL/kg/day·BW) + HAECSP (167.5 mg/kg/day·BW) | Alcohol (6 mL/kg/day·BW) + HAECSP (335 mg/kg/day·BW) | Alcohol (6 mL/kg/day·BW)/HAECSP (167.5 mg/kg/day·BW) | Alcohol (6 mL/kg/day·BW)/HAECSP (335 mg/kg/day·BW) | ||
| AST (mg/dL) | Before the intervention | 81.16±6.27 | 81.33±5.27 | 79.83±5.94 | 81.83±6.21 | 82.85±5.76 | 81.00±4.42 |
| After the intervention | 82.00±6.41 | 161.66±24.00 | 106.33±6.53 | 89.83±6.91 | 137.28±15.96 | 128.33±8.33 | |
| P-value | 0.341 | 0.000*** | 0.000*** | 0.081 | 0.000*** | 0.000*** | |
| ALT (mg/dL) | Before the intervention | 51.50±1.97 | 52.16±4.95 | 53.50±4.32 | 52.16±5.15 | 52.00±4.85 | 53.66±4.88 |
| After the intervention | 52.16±3.37 | 99.83±13.13 | 64.50±4.27 | 58.50±4.18 | 89.33±9.39 | 79.50±4.50 | |
| P-value | 0.675 | 0.000*** | 0.000*** | 0.073 | 0.000*** | 0.000*** | |
| ALP (mg/dL) | Before the intervention | 594.66±57.25 | 598.50±27.89 | 593.66±27.82 | 592.66±19.63 | 597.16±9.28 | 595.16±15.25 |
| After the intervention | 597.00±50.81 | 1074.83±97.04 | 705.50±33.29 | 662.33±64.50 | 907.16±7.36 | 782.50±22.16 | |
| P-value | 0.595 | 0.000*** | 0.000*** | 0.052 | 0.000*** | 0.000*** | |
| GGT (mg/dL) | Before the intervention | 2.83±0.40 | 3.08±0.60 | 2.91±0.37 | 3.08±0.49 | 2.91±0.31 | 3.00±0.44 |
| After the intervention | 3.00±0.63 | 5.33±0.80 | 3.86±0.44 | 3.21±0.40 | 4.68±0.37 | 4.33±0.65 | |
| P-value | 0.363 | 0.000*** | 0.000*** | 0.077 | 0.000*** | 0.001** | |
Notes: Values are given as mean±SEM for six rats in each group. (**P<0.01, ***P<0.001). Significant values are shown in bold.
Abbreviations: HAECSP, hydroalcoholic extract of Crocus sativus petals; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; GGT, gamma-glutamyl transferase.
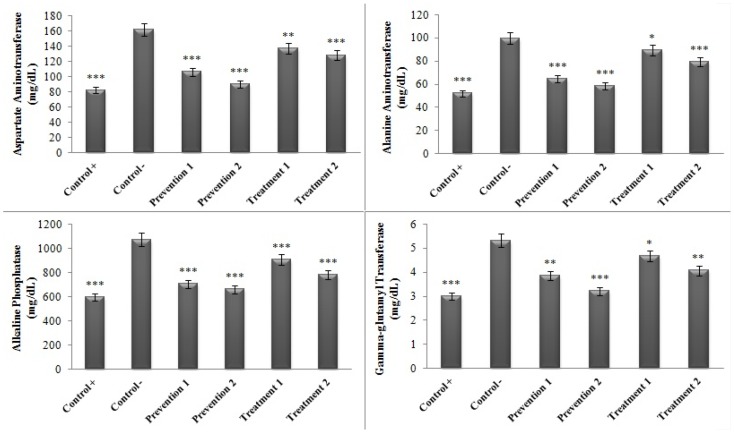
Also the level of the enzymes ALP, AST, ALT, and GGT at the end of the study was compared between the negative control group (which had only received ethyl alcohol) and the other groups. The results showed that the amounts of the hepatic enzymes have undergone considerable reductions in contrast to the negative control group (minimum significant difference: P<0.05) (Figure 4).
Figure 4.
Comparison of the serum concentrations of the hepatic enzymes in various groups in contrast to the negative control group. (*P<0.05, **P<0.01, ***P<0.001).
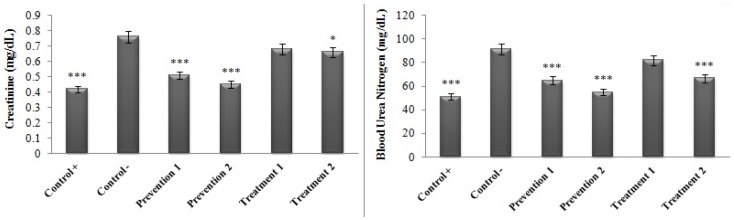
Similar results were also obtained for renal enzymes (creatinine and BUN). Based on Table 3, the comparison of the levels of creatinine and BUN enzymes before and after the study between the positive control group and the second group of the prevention phase (the group that received ethyl alcohol for a dosage of 6 mL/kg/day·BW plus HAECSP for a dosage of 335 mg/kg/day·BW) suggests no significant difference (P>0.05). On the other hand, the serum levels of these enzymes significantly increased in the other studied groups at the end of the test in contrast to its beginning (P≤0.001).
Table 3.
Comparison of the serum concentrations of renal enzymes in various groups at the beginning and end of the study
| Kidney enzymes | Positive control | Negative control | Prevention phase | Treatment phase | |||
|---|---|---|---|---|---|---|---|
| Normal saline | Alcohol (6 mL/kg/day· BW) | Alcohol (6 mL/kg/day· BW) + HAECSP (167.5 mg/kg/day·BW) | Alcohol (6 mL/kg/day· BW) + HAECSP (335 mg/kg/day· BW) | Alcohol (6 mL/kg/day·BW)/HAECSP (167.5 mg/kg/day· BW) | Alcohol (6 mL/kg/day· BW)/HAECSP (335 mg/kg/day· BW) | ||
| Creatinine (mg/dL) | Before the intervention | 0.42±0.01 | 0.40±0.02 | 0.39±0.02 | 0.40±0.03 | 0.39±0.03 | 0.39±0.03 |
| After the intervention | 0.42±0.01 | 0.76±0.09 | 0.51±0.01 | 0.45± −0.02 | 0.68±0.04 | 0.66±0.03 | |
| P-value | 0.415 | 0.000*** | 0.001** | 0.053 | 0.000*** | 0.000*** | |
| BUN (mg/dL) | Before the intervention | 50.50±5.50 | 51.53±7.72 | 52.91±3.58 | 51.76±2.83 | 51.51±3.16 | 52.61±4.72 |
| After the intervention | 50.25±6.84 | 91.61±5.95 | 64.98±6.99 | 55.08±3.15 | 81.95±6.79 | 66.58±5.20 | |
| P-value | 0.521 | 0.000*** | 0.001** | 0.066 | 0.000*** | 0.000*** | |
Notes: Values are given as mean±SEM for six rats in each group. Significant values are shown in bold. **P<0.01, ***P<0.001.
Abbreviations: HAECSP, hydroalcoholic extract of Crocus sativus petals; BUN, blood urea nitrogen.
The results related to the comparison of the serum concentrations of the renal enzymes between the studied groups and the negative control group (that had only received ethyl alcohol) following the intervention are displayed in Figure 5. As can be observed, a significant reduction has occurred in the levels of the enzymes BUN and creatinine in the positive control group, both of the prevention phase groups (P<0.001) and the second group of the treatment phase (treatment group 2: creatinine (P<0.05) and BUN (P<0.001)) in comparison to the negative control group. However, no significant difference was evidenced in terms of the amounts of these enzymes between the treatment group 1 and the negative control group (P>0.05). In this case, the findings suggested that the protective effect of extracts (prevention phase) is greater than its therapeutic effect (treatment phase). In addition, the protective effect of the extract was also found dose-dependent and more accentuated protective role was evidenced against the damages resulting from alcohol use.
Figure 5.
Comparison of the serum concentrations of the renal enzymes in various groups in contrast to the negative control group. *P<0.05, ***P<0.001.
Discussion
The present study was conducted with the aim of examining the effects of alcohol toxicity on liver and kidney and the use of hydroalcoholic extracts of saffron petals in preventing related damages. The results of the present study revealed an increase in inflammatory factors as well as liver and kidney toxicity in response to alcohol consumption. In this study, administration of hydroalcoholic extract of saffron petals led to a significant decrease in serum levels of inflammatory as well as liver and kidney enzymes in comparison with the negative control group.
The results of this study revealed liver toxicity as a result of alcohol consumption. In the ethanol group, the mean serum levels of hepatic enzymes were significantly higher after the study compared to those at the beginning of the study. These enzymes are found abundantly in the liver, and incidence of necrosis or cell membrane damage results in their release into the blood.35 Measurement of the level of these enzymes has been used in evaluating liver dysfunction; elevated activity of these enzymes reflects liver damage.36 In line with this study, many studies have shown that ethanol consumption increases liver transaminases, alkaline phosphatase, inflammatory markers, and fibrotic markers in the liver.37–39 One of the main causes of liver disorders in Europe and the USA is alcohol ingestion40,41 and alcohol overdose (usually about 40 g daily for women and 60 g daily for men) which increases the risk of severe forms of liver disease.42,43
Oxidative stress and mitochondrial dysfunction are two key elements in the pathogenesis of alcoholic liver disease.44,45 It is believed that reactive nitrogen and oxygen species (ROS/RNS) produced by cytochrome P4502E1 (CYP2E1), NADPH oxidase (NOX), and induced nitric oxide nitrate synthase (iNOS) are a driving force in alcoholic liver disease.46 Alcohol toxicity causes significant biochemical modifications in the function of different mitochondrial enzyme systems including the respiratory chain, the fatty acid oxidation pathway, and the urea cycle. It also results in ultrastructural modifications, mitochondrial DNA injury, and aggregation of fat.47 Ethanol affects mitochondrial function, including respiration, ATP synthesis, and reactive oxygen species (ROS) production.48,49 Alcohol oxidation with alcohol dehydrogenase and acetaldehyde dehydrogenase significantly alters the ratio of cytosolic NADH/NAD to mitochondrial due to accumulation of NADH.50,51 The changes in the balance of NADH/NAD facilitate the production of ROS by damaged respiratory complexes and non-mitochondrial redox systems.52 Studies have indicated that oxidative stress disrupts mitochondria and causes steatosis, inflammation, and ethanol-induced fibrosis.52,53
The results of this study revealed that the hydroalcoholic extracts of saffron petals have a protective effect against the toxic effects of alcohol on the liver and these protective effects are more significant at higher doses. According to the evidence obtained from animal studies, saffron has a high protective power against liver toxicity caused by some chemicals by improving liver structural damages, modifying antioxidant enzymes, reducing liver damage markers, lipid and protein oxidation, reducing apoptosis, and increasing glutathione reduction (GSH).54–66 Iranshahi et al found that the aqueous and ethanolic extracts of stigma and petals of saffron can significantly reduce the degeneration of fat caused by carbon tetrachloride (CCl4) and elevated levels of ALT and AST in plasma. They acknowledged that the protective effects of saffron against liver damage caused by CCl4 might occur through stabilizing the liver cell membrane, supporting cellular defense mechanisms such as antioxidant effects and reducing activation of the metabolic CCl4 by inhibiting cytochrome P450 and interacting with CCl4 free radical receptor.63 The results of the study conducted by Omidi et al also showed that dose-dependent saffron petals extract can restore increased levels of liver enzymes caused by acetaminophen to normal levels. Also, histopathologic results on liver pathology showed cellular swelling, severe inflammation, and necrosis in rats exposed to acetaminophen, while only mild hepatocyte degeneration was observed in rats treated with saffron.64
In the study conducted by Mohajeri et al, saffron petal extract reduced the liver damage induced by cisplatin through antioxidant properties by reducing the levels of AST, ALT, MDA, and bilirubin and increasing the total protein and albumin levels.65 The results of this study are consistent with the mentioned studies in terms of the effects of saffron extract on reducing liver toxicity. Liver toxicity is a common liver damage caused by some natural, industrial, chemical, and pharmaceutical products. Saffron, as herbal medicine, can inhibit liver damages through its properties, essentially antioxidant effects, maintaining a cellular hepatic membrane and offering beneficial effect on liver enzymes. Thus, saffron can significantly reduce the effect of adverse effects that cause liver toxicity.66
Studies have shown that chronic alcohol consumption increases the proportion of liver NADH/NAD+ in hepatocytes, leading to suppressed beta-oxidation of fatty acids and increased lipogenesis. It eventually culminates in fat accumulation in hepatocytes.67,68 Alcohol also promotes the synthesis of free fatty acids and triglycerides, and facilitates the liver penetration of fatty acids from adipose tissue and chylomicrons from the intestinal mucosa. It also inhibits the secretion of VLDL by inhibiting the microsomal triglyceride-transmitting protein.9 Alcohol consumption also reduces the expression of AMPK, which is one of the main regulators of fat metabolism in the liver and responsible for the inactivation of Acetyl-CoA carboxylase (ACC); it increases the oxidation of fatty acids and reduces the synthesis of fatty acids in the liver.69 In addition, alcohol contributes to increasing liver lipogenesis by elevating the levels of SREBP-1c which regulates the synthesis of fatty acids. It also decreases lipolysis by minimizing PPAR-α2, which regulates the oxidation of fatty acids.9 Further, ethanol can affect the secretion of adiponectin, which is an adipokine 30-kD, with effects of reducing the fat power and anti-inflammatory effects.70 Ethanol can also directly accelerate the lipogenesis of white fat tissues, and accordingly, reverse the transmission of fat from adipose to the liver.71 The results of a study conducted by Shi et al indicated the protective role of saffron in alcoholic fat liver. They also showed that saffron increased mitochondrial peroxidation, reduced fatty deposits, prevented lipid peroxidation, and accelerated the elimination of alcohol and aldehyde.72 In another study conducted by Asdaq et al, they observed the effect of saffron on various biochemical agents. They also proved that saffron, as herbal medicine, has hyperlipidemia and antioxidant properties.73 In addition, in a study conducted by Hushyar et al, the hypolipidemic properties of saffron petals were studied. They concluded that the extract of saffron petals reduced total cholesterol, triglyceride and LDL and increased HDL levels and reduced leptin and insulin levels. Therefore, they concluded that saffron extract increases antioxidant levels and decreases the lipid peroxidation.74
Other detrimental effects of alcohol on the toxicity of the affected tissues are that free radicals can overshadow the antioxidant defense system of the cell during the ethanol metabolism.75 Comparison of scientific data suggests that reducing the activity of antioxidant defense system enzymes (such as superoxide dismutase, catalase, and glutathione peroxidase) in the animal tissues is due to the oxidative stress conditions induced by ethanol.76–78 Antioxidant agents can reduce the harmful effects of oxidative stress. Many studies have examined the antioxidant properties of ethanolic extract of saffron.79–84 The study conducted by Ardalan et al revealed the antioxidant activity of saffron petals using the free radical 2,2-diphenyl-1-picrylhydrazyl (DPPH) method. They also proved that saffron petals increase the antioxidant content at all doses.85 Studies have suggested that the antioxidant capacity of saffron petals depends on the presence of flavonoids.86 Saffron petals contain many flavonoids. Flavonoids can prevent the enzyme peroxidation of fatty acids and have free radical withdrawal characteristics. Hence, it acts as an antioxidant agent. Thus, it was suggested that the major protective effects of saffron petals are due to the presence of such compounds.63
The results of the current research also showed that damages are associated with systemic inflammation; the serum level of all of the inflammatory parameters studied at the end of the study in the ethanol alcohol receiving groups was significantly higher than that at the beginning of the study. It has been proven that ethanol can stimulate hemorrhagic injuries and cause changes in the structure of enterocytes in the small intestine of animals and humans. It can also change the intestinal microflora, which reflects the excessive growth of gram-negative bacteria and an increase in the lipopolysaccharide source (LPS).87 Alcohol-related inflammatory response is the result of an increase in the transmission of intestine-derived LPS (endotoxins derived from Gram-negative bacteria) to the blood portal, which leads to induction of the expression of TLR-4 and the activation of NF-κB. Accordingly, Kupffer cells (KCs) and macrophages in the liver can be activated by the intestinal LPS source through TLR4 receptor binding, thereby producing large amounts of pro-inflammatory cytokines (TNF-α and IL-1β), nitric oxide (NO), and ROS. This culminates in dysfunction of hepatocytes, necrosis, apoptosis, and the production of extracellular matrix (ECM) as well as fibrosis/cirrhosis.88–90 The findings of the current investigation indicated that injection of hydroalcoholic extracts of saffron petals could mitigate the increased mean value of inflammatory factors (IL6, TNF-α, CRP, and fibrinogen) in response to alcohol consumption.
Recently, the inhibitory roles of this extract have been shown in tissue inflammation using various animal models. Studies have reported that ethanolic and aqueous saffron extracts can mitigate neuropathic pain in severe damages through decreasing anti-inflammatory agents such as TNF-α, IL-6, and IL-1β.91 The results of a study conducted by Hosseinzadeh and Younesi showed that ethanolic and aqueous saffron extracts have anti-inflammatory effects against pain caused by chemicals.92 In a study conducted by Zhang et al, it was found that safranal reduces the neural activity and the expression of inflammatory cytokines TNF-α, IL-1β, while increasing the expression of IL-10 after spinal cord damage. It also reduces edema by reducing the expression of aquaporin-4.93 In another study conducted by Hariri et al, the results showed that crocin and safranal prevent elevation of the levels of AST, ALT, ALP, LDH, creatine phosphokinase (CPK), creatine kinase MB, gamma-glutamyl transferase (GGT), and other inflammatory markers including TNF -α, 8-iso-prostaglandin F2a, and soluble protein-100 beta in rats. These results suggested that safranal inhibits biochemical enzyme changes, as well as inflammatory and neural effects caused by exposure to diazinon.94 A number of other studies have shown the preventive effects of saffron and its safranal on serum inflammatory markers in sea pigs.95–97 The results of the research carried out by Xu et al showed that they could alter the inflammatory pathways and found dual inhibitor activity against COX-1 and COX-2 enzymes. They also stopped the production of PGE2 by inhibiting the production of NF-κB subunits.98 Further, NF-κB plays a major role in regulating the genes involved in cell survival and coordinating the expression of pre-inflammatory enzymes.99,100 Kim et al reported that crocin reduces the formation of nitric oxide synthase stimulating LPS (iNOS) by overexpression of HO-1 through the Ca2+/calmodulin-dependent kinase 4-PI3K/Akt-Nrf2 protein signaling cascade in the 264.7 RAW macrophages.101 This can be another reason for the effect of saffron on inflammatory pathways. Another research revealed that α-crocin had a protective effect on the complications induced by ethanol in the stomach of rats. Crocin also restored the low levels of gastric mucosa, mucus PGE2, and ethanol-induced IL-6. Additionally, α-crocin significantly reduced TNF-α, myeloperoxidase, and protein levels.102 Review of the results of these studies and the present study suggest that saffron has the potential for reducing inflammation factors. In addition, many of these studies have reported different compounds such as flavonoids, tannins, anthocyanins, alkaloids, and saponins in the petals of saffron. The presence of compounds such as crocin and safranal belonging to carotenoids and quercetin groups as well as myricetin belonging to flavonoids group was also observed in this study. Thus, it can be stated that the observed effects may be due to the presence of compounds in saffron petals. In this regard, the results of the research conducted by Calixto et al showed that the analgesic and anti-inflammatory effects of saffron petals were due to the content of flavonoids, tannins, and anthocyanins.103 Other studies have also shown that various flavonoids, such as quercetin, luteolin, hesperidin, and bioflavonoid show anticonvulsant and/or anti-inflammatory activities.103–105
Conclusion
The results of the present study indicated an increase in inflammatory factors as well as liver and kidney toxicity in response to alcohol consumption. The present study revealed that saffron can prevent liver damage through its properties, especially its antioxidant effects, protect the liver cell membrane, increase the level of antioxidants, and decrease the lipid peroxidation. Due to the antioxidant properties of this extract, it also has the potential to reduce kidney damages as well as inflammation and inflammatory factors. The present study proved the presence of compounds such as crocin and safranal belonging to carotenoids and quercetin groups along with myricetin belonging to flavonoids group in saffron petal extract. As these compounds have the potential to prevent enzymatic peroxidation of fatty acids and withdraw the free radicals, they can act as antioxidant agents. Indeed, the effects observed in this study seem to be due to the presence of these compounds in the petals of saffron.
Acknowledgment
The authors of the study appreciate the Deputy Director of Research and Technology at Ilam University of Medical Sciences for supporting this study.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380(9859):2224–2260. doi: 10.1016/S0140-6736(12)61766-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: results from the national epidemiologic survey on alcohol and related conditions III. JAMA Psychiatry. 2015;72(8):757–766. doi: 10.1001/jamapsychiatry.2015.0584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grant B, Chou SP, Saha TD, et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-iv alcohol use disorder in the United States, 2001-2002 to 2012-2013: results from the national epidemiologic survey on alcohol and related conditions. JAMA Psychiatry. 2017;74(9):911–923. doi: 10.1001/jamapsychiatry.2017.2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization (WHO). Global Status Report on Alcohol and Health 2014. Geneva: WHO; 2016. Available from: http://www.who.int/substance_abuse/publications/global_alcohol_report/msb_gsr_2014_1.pdf. Accessed December 31, 2016. [Google Scholar]
- 5.Molina PE, Gardner JD, Souza-Smith FM, Whitaker AM. Alcohol abuse: critical pathophysiological processes and contribution to disease burden. Physiology (Bethesda). 2014;29(3):203–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirpich IA, McClain CJ, Vatsalya V, et al. Liver injury and endotoxemia in male and female alcohol-dependent individuals admitted to an alcohol treatment program. Alcohol Clin Exp Res. 2017;41(4):747–757. doi: 10.1111/acer.13346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao B, Tsukamoto H. Inflammation in alcoholic and nonalcoholic fatty liver disease: friend or foe? Gastroenterology. 2016;150(8):1704–1709. doi: 10.1053/j.gastro.2016.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Testino G, Leone S. Acute alcoholic hepatitis: a literature review and proposal of treatment. Minerva Med. 2018;109(4):290–299. doi: 10.23736/S0026-4806.17.05431-3 [DOI] [PubMed] [Google Scholar]
- 9.Stickel F, Datz C, Hampe J, Bataller R. Pathophysiology and management of alcoholic liver disease: update 2016. Gut Liver. 2017;11(12):173–188. doi: 10.5009/gnl16477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varga ZV, Matyas C, Paloczi J, Pacher P. Alcohol misuse and kidney injury: epidemiological evidence and potential mechanisms. Alcohol Res. 2017;38(2):283–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egerod Israelsen M, Gluud LL, Krag A. Acute kidney injury and hepatorenal syndrome in cirrhosis. J Gastroenterol Hepatol. 2015;30(2):236–243. doi: 10.1111/jgh.12709 [DOI] [PubMed] [Google Scholar]
- 12.Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ. 2011;342:d671. doi: 10.1136/bmj.d671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schefold JC, Filippatos G, Hasenfuss G, Anker SD, von Haehling S. Heart failure and kidney dysfunction: epidemiology, mechanisms and management. Nat Rev Nephrol. 2016;12(10):610–623. doi: 10.1038/nrneph.2016.113 [DOI] [PubMed] [Google Scholar]
- 14.Husain K, Ansari RA, Ferder L. Alcohol-induced hypertension: mechanism and prevention. World J Cardiol. 2014;6(5):245–252. doi: 10.4330/wjc.v6.i5.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Da Silva AL, Ruginsk SG, Uchoa ET, et al. Time-course of neuroendocrine changes and its correlation with hypertension induced by ethanol consumption. Alcohol Alcohol. 2013;48(8):495–504. doi: 10.1093/alcalc/agt040 [DOI] [PubMed] [Google Scholar]
- 16.Chow BS, Allen TJ. Angiotensin II type 2 receptor (AT2R) in renal and cardiovascular disease. Clin Sci (Lond). 2016;130(5):1307–1326. doi: 10.1042/CS20160243 [DOI] [PubMed] [Google Scholar]
- 17.Mennuni S, Rubattu S, Pierelli G, Tocci G, Fofi C, Volpe M. Hypertension and kidneys: unraveling complex molecular mechanisms underlying hypertensive renal damage. J Hum Hypertens. 2014;28(2):74–79. doi: 10.1038/jhh.2013.55 [DOI] [PubMed] [Google Scholar]
- 18.Liamis GL, Milionis HJ, Rizos EC, Siamopoulos KC, Elisaf MS. Mechanisms of hyponatraemia in alcohol patients. Alcohol Alcohol. 2000;35(6):612–616. doi: 10.1093/alcalc/35.6.612 [DOI] [PubMed] [Google Scholar]
- 19.Ragland G. Electrolyte abnormalities in the alcoholic patient. Emerg Med Clin North Am. 1990;8(4):761–773. [PubMed] [Google Scholar]
- 20.Naranjo M, Lerma EV, Rangaswami J. Cardio-renal syndrome: a double edged sword. Dis Mon. 2017;63(4):92–100. doi: 10.1016/j.disamonth.2017.02.001 [DOI] [PubMed] [Google Scholar]
- 21.Souza-Smith FM, Lang CH, Nagy LE, Bailey SM, Parsons LH, Murray GJ. Physiological processes underlying organ injury in alcohol abuse. Am J Physiol Endocrinol Metab. 2016;311(3):605–619. doi: 10.1152/ajpendo.00270.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steiner JL, Lang CH. Etiology of alcoholic cardiomyopathy: mitochondria, oxidative stress and apoptosis. Int J Biochem Cell Biol. 2017;89:125–135. doi: 10.1016/j.biocel.2017.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christodoulou E, Kadoglou NP, Kostomitsopoulos N, Valsami G. Saffron: a natural product with potential pharmaceutical applications. J Pharm Pharmacol. 2015;67(12):1634–1649. doi: 10.1111/jphp.12456 [DOI] [PubMed] [Google Scholar]
- 24.Hosseini A, Razavi BM, Hosseinzadeh H. Saffron (Crocus sativus) petal as a new pharmacological target: a review. Iran J Basic Med Sci. 2018;21(11):1091–1099. doi: 10.22038/IJBMS.2018.31243.7529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeka K, Ruparelia KC, Continenza MA, Stagos D, Vegliò F, Arroo RJ. Petals of Crocus sativus L. as a potential source of the antioxidants crocin and kaempferol. Fitoterapia. 2015;107:128–134. doi: 10.1016/j.fitote.2015.05.014 [DOI] [PubMed] [Google Scholar]
- 26.Akhondzadeh Basti A, Moshiri E, Noorbala AA, Jamshidi AH, Abbasi SH, Akhondzadeh S. Comparison of petal of Crocus sativus L. and fluoxetine in the treatment of depressed outpatients: a pilot double-blind randomized trial. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(2):439–442. doi: 10.1016/j.pnpbp.2006.11.010 [DOI] [PubMed] [Google Scholar]
- 27.Fatehi M, Rashidabady T, Fatehi- Hassanabad Z. Effects of Crocus sativus petals’ extract on rat blood pressure and on responses induced by electrical field stimulation in the rat isolated vas deferens and guinea-pig ileum. J Ethnopharmacol. 2003;84(2–3):199–203. [DOI] [PubMed] [Google Scholar]
- 28.Chen K, Wang XM, Chen F, Bai J. In vitro antimicrobial and free radical scavenging activities of the total flavonoid in petal and stamen of Crocus sativus. Indian J Pharm Sci. 2017;79(3):482–487. doi: 10.4172/pharmaceutical-sciences.1000254 [DOI] [Google Scholar]
- 29.D’Archivio AA, Di Donato F, Foschi M, Maggi MA, Ruggieri F. UHPLC analysis of saffron (Crocus sativus L.): optimization of separation using chemometrics and detection of minor crocetin esters. Molecules. 2018;23(8):E1851. doi: 10.3390/molecules23081851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khleifat K, Shakhanbeh J, Tarawneh K. The chronic effects of Teucrium polium on some blood parameters and histopathology of liver and kidney in the rat. Turk J Biol. 2002;26:65–71. [Google Scholar]
- 31.Amraei M, Ghorbani A, Seifinejad Y, Mousavi SF, Mohamadpour M, Shirzadpour E. The effect of hydroalcoholic extract of Teucrium polium L. on the inflammatory markers and lipid profile in hypercholesterolemic rats. J Inflamm Res. 2018;11:265–272. doi: 10.2147/JIR.S165172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abu Sitta KH, Shomah MS, Salhab AS. Hepatotoxicity of Teucrium polium L tea: supporting evidence in mice models. Austr J Med Herb. 2009;21(4):106–108. [Google Scholar]
- 33.Krache I, Boussoualim N, Charef N, et al. Evaluation of acute and chronic toxic effects of Algerian germander in Swiss albino mice. J App Pharm Sci. 2015;5(Suppl 3):027–032. [Google Scholar]
- 34.Habib-ur-Rehman M, Tahir M, Lone KP, Sami W. Ethanol induced hepatotoxicity in albino rats. J Coll Physicians Surg Pak. 2011;21(10):642–643. doi: 10.2011/JCPSP.642643 [DOI] [PubMed] [Google Scholar]
- 35.Stockham SL, Scott MA. Fundamentals of Veterinary Clinical Pathology. Ames: Iowa State University Press; 2002:434–459. [Google Scholar]
- 36.Contreras-Zentella ML, Hernández-Muñoz R. Is liver enzyme release really associated with cell necrosis induced by oxidant stress? Oxid Med Cell Longev. 2016;2016:3529149. doi: 10.1155/2016/3529149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Afshar M, Richards S, Mann D, et al. Acute immunomodulatory effects of binge alcohol ingestion. Alcohol. 2015;49(1):57–64. doi: 10.1016/j.alcohol.2014.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ki SH, Park O, Zheng M, et al. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology. 2010;52(4):1291–1300. doi: 10.1002/hep.23837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marmier S, Dentin R, Daujat-Chavanieu M, et al. Novel role for carbohydrate responsive element binding protein in the control of ethanol metabolism and susceptibility to binge drinking. Hepatology. 2015;62(4):1086–1100. doi: 10.1002/hep.27778 [DOI] [PubMed] [Google Scholar]
- 40.Masarone M, Rosato V, Dallio M, et al. Epidemiology and natural history of alcoholic liver disease. Rev Recent Clin Trials. 2016;11(3):167–174. [DOI] [PubMed] [Google Scholar]
- 41.Sheron N. Alcohol and liver disease in Europe–simple measures have the potential to prevent tens of thousands of premature deaths. J Hepatol. 2016;64:957–967. doi: 10.1016/j.jhep.2015.11.006 [DOI] [PubMed] [Google Scholar]
- 42.Livero FA, Acco A. Molecular basis of alcoholic fatty liver disease: from incidence to treatment. Hepatol Res. 2016;46:111–123. doi: 10.1111/hepr.12594 [DOI] [PubMed] [Google Scholar]
- 43.Rehm J, Taylor B, Mohapatra S, et al. Alcohol as a risk factor for liver cirrhosis: a systematic review and metaanalysis. Drug Alcohol Rev. 2010;29(4):437–445. doi: 10.1111/j.1465-3362.2009.00153.x [DOI] [PubMed] [Google Scholar]
- 44.Sid B, Verrax J, Calderon PB. Role of oxidative stress in the pathogenesis of alcohol-induced liver disease. Free Radic Res. 2013;47(11):894–904. doi: 10.3109/10715762.2013.828836 [DOI] [PubMed] [Google Scholar]
- 45.Teplova VV, Belosludtsev KN, Belosludtseva NV, Holmuhamedov EL. Role of mitochondria in hepatotoxicity of ethanol. Biophysics (Russia). 2010;55(6):1038–1047. [PubMed] [Google Scholar]
- 46.Tilg H, Moschen AR, Kaneider NC. Pathways of liver injury in alcoholic liver disease. J Hepatol. 2011;55(5):1159–1161. doi: 10.1016/j.jhep.2011.05.015 [DOI] [PubMed] [Google Scholar]
- 47.Auger C, Alhasawi A, Contavadoo M, Appanna VD. Dysfunctional mitochondrial bioenergetics and the pathogenesis of hepatic disorders. Front Cell Dev Biol. 2015;3:40. doi: 10.3389/fcell.2015.00040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song BJ, Abdelmegeed MA, Henderson LE, et al. Increased nitroxidative stress promotes mitochondrial dysfunction in alcoholic and nonalcoholic fatty liver disease. Oxid Med Cell Longev. 2013;2013:781050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mantena KS, King AL, Andringa KK, Eccleston HB, Bailey SM. Mitochondrial dysfunction and oxidative stress in the pathogenesis of alcohol and obesity induced fatty liver diseases. Free Radic Biol Med. 2008;44(7):1259–1272. doi: 10.1016/j.freeradbiomed.2007.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Manzo-Avalos S, Saavedra-Molina A. Cellular and mitochondrial effects of alcohol consumption. Int J Environ Res Public Health. 2010;7(12):4281–4304. doi: 10.3390/ijerph7124281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhong Z, Ramshesh VK, Rehman H, et al. Acute ethanol causes hepatic mitochondrial depolarization in mice: role of ethanol metabolism. PLoS One. 2014;9(3):e91308. doi: 10.1371/journal.pone.0091308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bailey SM, Cunningham CC. Acute and chronic ethanol increases reactive oxygen species generation and decreases viability in fresh, isolated rat hepatocytes. Hepatology. 1998;28(5):1318–1326. doi: 10.1002/hep.510280624 [DOI] [PubMed] [Google Scholar]
- 53.Hoyt LR, Randall MJ, Ather JL, DePuccio DP, Landry CC, Qian X. Mitochondrial ROS induced by chronic ethanol exposure promotes hyper-activation of the NLRP3 inflammasome. Redox Biol. 2017;12:883–896. doi: 10.1016/j.redox.2017.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.El-Beshbishy HA, Hassan MH, Aly H, Doghish A, Alghaithy A. Crocin “saffron” protects against beryllium chloride toxicity in rats through diminution of oxidative stress and enhancing gene expression of antioxidant enzymes. Ecotoxicol Environ Saf. 2012;83:47–54. doi: 10.1016/j.ecoenv.2012.06.003 [DOI] [PubMed] [Google Scholar]
- 55.Lari P, Abnous K, Imenshahidi M, Rashedinia M, Razavi M, Hosseinzadeh H. Evaluation of diazinon-induced hepatotoxicity and protective effects of crocin. Toxicol Ind Health. 2015;31(4):367–376. doi: 10.1177/0748233713475519 [DOI] [PubMed] [Google Scholar]
- 56.Shati A, Alamri S. Role of saffron (Crocus sativus L.) and honey syrup on aluminium induced hepatotoxicity. Saudi Med J. 2010;31(10):1106–1113. [PubMed] [Google Scholar]
- 57.Jnaneshwaria S, Hemshekhara M, Santhosha S, et al. Crocin, a dietary colorant mitigates cyclophosphamide-induced organ toxicity by modulating antioxidant status and inflammatory cytokines. J Pharm Pharmacol. 2013;65(4):604–614. doi: 10.1111/jphp.12016 [DOI] [PubMed] [Google Scholar]
- 58.Nair SC, Panikkar KR, Parthod RK. Protective effects of crocetin on the bladder toxicity induced by cyclophosphamide. Cancer Biother. 1993;8(4):339–344. doi: 10.1089/cbr.1993.8.339 [DOI] [PubMed] [Google Scholar]
- 59.Lin JK, Wang CJ. Protection of crocin dyes on the acute hepatic damage induced by aflatoxin B1 and dimethylnitrosamine in rats. Carcinogenesis. 1986;7(4):595–599. doi: 10.1093/carcin/7.4.595 [DOI] [PubMed] [Google Scholar]
- 60.Wang CJ, Shiow SJ, Lin JK. Effects of crocetin on the hepatotoxicity and hepatic DNA binding of aflatoxin B1 in rats. Carcinogenesis. 1991;12(3):459–462. doi: 10.1093/carcin/12.3.459 [DOI] [PubMed] [Google Scholar]
- 61.EL-Maraghy SA, Rizk SM, El-Sawalhi MM. Hepatoprotective potential of crocin and curcumin against iron overload-induced biochemical alterations in rat. Afr J Biochem Res. 2009;3(5):215–221. [Google Scholar]
- 62.Yousefsani B, Mehri S, Pourahmad J, Hosseinzadeh H. Crocin prevents sub-cellular organelle damage, proteolysis and apoptosis in rat hepatocytes: a justification for its hepatoprotection. Iranian J Pharm Res. 2018;17(2):553–562. [PMC free article] [PubMed] [Google Scholar]
- 63.Iranshahi M, Khoshangosht M, Mohammadkhani Z, Karimi G. Protective effects of aqueous and ethanolic of saffron stigma and petal on liver toxicity induced by carbon tetrachloride in mice. Pharmacologyonline. 2011;1:203–212. [Google Scholar]
- 64.Omidi A, Riahinia N, Montazer Torbati M, Behdani M. Hepatoprotective effect of Crocus sativus (saffron) petals extract against acetaminophen toxicity in male Wistar rats. Avicenna J Phytome. 2014;4(5):330–336. [PMC free article] [PubMed] [Google Scholar]
- 65.Mohajeri Dariush DY. Protective effects of Crocus sativus petal against cisplatin-induced hepatotoxicity in rats. Med Sci J Islamic Azad Univ. 2011;21(4):251–261. [Google Scholar]
- 66.Rezaee Khorasany AR, Hosseinzadeh H. Therapeutic effects of saffron (Crocus sativus L.) in digestive disorders: a review. Iran J Basic Med Sci. 2016;19(5):455–469. [PMC free article] [PubMed] [Google Scholar]
- 67.Donohue TM. Alcohol-induced steatosis in liver cells. World J Gastroenterol. 2007;13(37):4974–4978. doi: 10.3748/wjg.v13.i37.4974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sozio M, Crabb DW. Alcohol and lipid metabolism. Am J Physiol Endocrinol Metabol. 2008;295(1):10–16. doi: 10.1152/ajpendo.00011.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.You M, Matsumoto M, Pacold CM, Cho WK, Crabb DW. The role of AMP-activated protein kinase in the action of ethanol in the liver. Gastroenterology. 2004;127(6):1798–1808. doi: 10.1053/j.gastro.2004.09.049 [DOI] [PubMed] [Google Scholar]
- 70.You M, Rogers CQ. Adiponectin: a key adipokine in alcoholic fatty liver. Exp Biol Med (Maywood). 2009;234(8):850–859. doi: 10.3181/0902-MR-61 [DOI] [PubMed] [Google Scholar]
- 71.Zhong W, Zhao Y, Tang Y, et al. Chronic alcohol exposure stimulates adipose tissue lipolysis in mice: role of reverse triglyceride transport in the pathogenesis of alcoholic steatosis. Am J Pathol. 2012;180(3):998–1007. doi: 10.1016/j.ajpath.2011.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shi Y, Sheng L, Qian ZY, Chen Z. Beneficial effects of crocetin on alcoholic fatty liver in rats and the mechanism. Chin J New Drugs. 2008;17:2115–2118. [Google Scholar]
- 73.Asdaq SMB, Inamdar MN. Potential of Crocus sativus (saffron) and its constituent, crocin, as hypolipidemic and antioxidant in rats. Appl Biochem Biotech. 2010;162(2):358–372. doi: 10.1007/s12010-009-8740-7 [DOI] [PubMed] [Google Scholar]
- 74.Hoshyar R, Hosseinian M, Naghandar MR, et al. Anti-dyslipidemic properties of saffron: reduction in the associated risks of atherosclerosis and insulin resistance. Iran Red Cres Med J. 2016;18(2):e36266. doi: 10.5812/ircmj.36226 [DOI] [Google Scholar]
- 75.Nordman R. Alcohol and antioxidant system. Alcohol Alcohol. 1994;29(5):513–522. [PubMed] [Google Scholar]
- 76.Wu D, Cederbaum I. Alcohol, oxidative stress, and free radical damage. Alcohol Res Health. 2003;27(4):277–284. [PMC free article] [PubMed] [Google Scholar]
- 77.Wilce MC, Parker MW. Structure and function of glutathione Stransferases. Biochim Biophys Acta. 1994;1205(1):1–18. doi: 10.1016/0167-4838(94)90086-8 [DOI] [PubMed] [Google Scholar]
- 78.Alin P, Danielson HU, Mannervik B. 4- Hydroxyalk-2-enals are substrates for glutathione transferase. FEBS Lett. 1985;179(2):267–270. doi: 10.1016/0014-5793(85)80532-9 [DOI] [PubMed] [Google Scholar]
- 79.Rahbani M, Mohajeri D, Rezaie A, Nazeri M. Protective effect of ethanolic extract of saffron (dried stigmas of Crocus sativus L.) on hepatic tissue injury in streptozotocin-induced diabetic rats. J Anim Vet Adv. 2012;11(12):1985–1994. doi: 10.3923/javaa.2012.1985.1994 [DOI] [Google Scholar]
- 80.Farahmand SK, Samini F, Samini M, Samarghandian S. Safranal ameliorates antioxidant enzymes and suppresses lipid peroxidation and nitric oxide formation in aged male rat liver. Biogerontology. 2013;14(1):63–71. doi: 10.1007/s10522-012-9409-0 [DOI] [PubMed] [Google Scholar]
- 81.Ramadan A, Soliman G, Mahmoud SS, Nofal SM, Abdel-Rahman RF. Evaluation of the safety and antioxidant activities of Crocus sativus and Propolis ethanolic extracts. J Saudi Chem Soc. 2012;16(1):13–21. doi: 10.1016/j.jscs.2010.10.012 [DOI] [Google Scholar]
- 82.Chen Y, Yang T, Huang J, et al. Comparative evaluation of the antioxidant capacity of crocetin and crocin in vivo. Chin Pharm Bull. 2010;26:248–251. [Google Scholar]
- 83.Bandegi AR, Vafaei Abbas A, Ghaderdoost B, Rashidy-Pour A. Protective effects of Crocus sativus L. extract and crocin against chronic-stress induced oxidative damage of brain, liver and kidneys in rats. Adv Pharm Bull. 2014;4(Suppl 2):493–499. doi: 10.5681/apb.2014.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lari P, Rashedinia M, Abnous K, Hosseinzadeh H. Crocin improves lipid dysregulation in subacute diazinon exposure through ERK1/2 pathway in rat liver. Drug Res (Stuttg). 2014;64(6):301–305. doi: 10.1055/s-0033-1357196 [DOI] [PubMed] [Google Scholar]
- 85.Ardalan T, Ardalan P, Heravi M. Kinetic study of free radicals scavenging by saffron petal extracts. J Chem Health Risks. 2012;2(4):29–36. [Google Scholar]
- 86.Termentzi A, Kokkalou E. LC-DAD-MS (ESI+) analysis and antioxidant capacity of Crocus sativus petal extracts. Planta Med. 2008;74(5):573–581. doi: 10.1055/s-0028-1088319 [DOI] [PubMed] [Google Scholar]
- 87.Zeng T, Zhang CL, Xiao M, Yang R, Xie KQ. Critical roles of Kupffer cells in the pathogenesis of alcoholic liver disease: from basic science to clinical trials. Front Immunol. 2016;7:538. doi: 10.3389/fimmu.2016.00538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.An L, Wang X, Cederbaum AI. Cytokines in alcoholic liver disease. Arch Toxicol. 2012;86(9):1337–1348. doi: 10.1007/s00204-011-0763-5 [DOI] [PubMed] [Google Scholar]
- 89.Kawaratani H, Tsujimoto T, Douhara A, et al. The effect of inflammatory cytokines in alcoholic liver disease. Mediators Inflamm. 2013;2013:495156. doi: 10.1155/2013/495156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Louvet A, Mathurin P. Alcoholic liver disease: mechanisms of injury and targeted treatment. Nat Rev Gastroenterol Hepatol. 2015;12(4):231–242. doi: 10.1038/nrgastro.2015.35 [DOI] [PubMed] [Google Scholar]
- 91.Amin B, Abnous K, Motamedshariaty V, Hosseinzadeh H. Attenuation of oxidative stress, inflammation and apoptosis by ethanolic and aqueous extracts of Crocus sativus L. stigma after chronic constriction injury of rats. An Acad Bras Cienc. 2014;86(4):1821–1832. [DOI] [PubMed] [Google Scholar]
- 92.Hosseinzadeh H, Younesi HM. Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC Pharmacol. 2002;2:7. doi: 10.1186/1471-2210-2-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang C, Ma J, Fan L, et al. Neuroprotective effects of safranal in a rat model of traumatic injury to the spinal cord by anti-apoptotic, anti-inflammatory and edema-attenuating. Tissue Cell. 2015;47(3):291–300. doi: 10.1016/j.tice.2015.03.007 [DOI] [PubMed] [Google Scholar]
- 94.Hariri AT, Moallem SA, Mahmoudi M, Memar B, Hosseinzadeh H. Sub-acute effects of diazinon on biochemical indices and specific biomarkers in rats: protective effects of crocin and safranal. Food Chem Toxicol. 2010;48(10):2803–2808. doi: 10.1016/j.fct.2010.07.010 [DOI] [PubMed] [Google Scholar]
- 95.Gholamnezhad Z, Koushyar H, Byrami G, Boskabady MH. The extract of Crocus sativus and its constituent safranal, affect serum levels of endothelin and total protein in sensitized guinea pigs. Iran J Basic Med Sci. 2013;16(9):1022–1026. [PMC free article] [PubMed] [Google Scholar]
- 96.Boskabady MH, Farkhondeh T. Anti-inflammatory, antioxidant, and immunomodulatory effects of Crocus sativus L. and its main constituents. Phytother Res. 2016;30(7):1072–1094. doi: 10.1002/ptr.5622 [DOI] [PubMed] [Google Scholar]
- 97.Boskabady MH, Rahbardar MG, Jafari Z. The effect of safranal on histamine (H1) receptors of guinea pig tracheal chains. Fitoterapia. 2011;82(2):162–167. doi: 10.1016/j.fitote.2010.08.017 [DOI] [PubMed] [Google Scholar]
- 98.Xu GL, Li G, Ma HP, Zhong H, Liu F, Ao GZ. Preventive effect of crocin in inflamed animals and in LPS-challenged RAW 264.7 cells. J Agric Food Chem. 2009;57(18):8325–8330. doi: 10.1021/jf901752f [DOI] [PubMed] [Google Scholar]
- 99.Surh YJ, Chun KS, Cha HH, Han SS, Keum YS, Park KK. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF kappa B activation. Mutat Res. 2001;480–481:243–268. doi: 10.1016/S0027-5107(01)00183-X [DOI] [PubMed] [Google Scholar]
- 100.Lappas M, Permezel M, Georgiou HM, Rice GE. Nuclear factor kappa B regulation of proinflammatory cytokines in human gestational tissues in vitro. Biol Reprod. 2002;67(2):668–673. doi: 10.1095/biolreprod67.2.668 [DOI] [PubMed] [Google Scholar]
- 101.Kim JH, Park GY, Bang SY, Park SY, Bae SK, Kim Y. Crocin suppresses LPS-stimulated expression of inducible nitric oxide synthase by upregulation of heme oxygenase-1 via calcium/calmodulin-dependent protein kinase 4. Mediators Inflamm. 2014;2014:728709. doi: 10.1155/2014/728709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.El-Maraghy SA, Rizk SM, Shahin NN. Gastroprotective effect of crocin in ethanol-induced gastric injury in rats. Chem Biol Interact. 2015;229:26–35. doi: 10.1016/j.cbi.2015.01.015 [DOI] [PubMed] [Google Scholar]
- 103.Calixto JB, Beirith A, Ferreira J, Santos AR, Cechinel Filho V, Yunes RA. Naturally occurring antinociceptive substances from plants. Phytother Res. 2000;14(6):401–418. [DOI] [PubMed] [Google Scholar]
- 104.Galati EM, Monforte MT, Kirjavainen S, Forestieri AM, Trovato A, Tripodo MM. Biological effects of hesperidin, a citrus flavonoid. (Note I): anti-inflammatory and analgesic activity. Farmaco. 1994;40(11):709–712. [PubMed] [Google Scholar]
- 105.Ramesh M, Rao YN, Rao AV, et al. Antinociceptive and anti-inflammatory activity of a flavonoid isolated from Caralluma attenuata. J Ethnopharmacol. 1998;62(1):63–66. [DOI] [PubMed] [Google Scholar]