Abstract
Purpose
Metabolic syndrome (MetS) and aortic arterial stiffness (AS) are risk factors for future cardiovascular events. We evaluated their roles in first hospitalization or all-cause mortality prediction in coronary artery disease (CAD) patients.
Patients and methods
From January to December 2012, 115 CAD patients were enrolled from a single center and followed up for 5.5 years. The composite endpoint included hospitalization for unstable angina, myocardial infarction, revascularization, or heart failure and all-cause mortality. Patients with carotid-femoral pulse wave velocity > 10 m/s (measured using applanation tonometry) constituted the high AS group.
Results
During a median 54-month follow-up, there were 43 (37.4%) and 11 (9.6%) hospitalization and mortality events, respectively. Overall, 41 (35.7%) and 70 (60.9%) patients were diagnosed with AS and MetS, respectively. CAD patients with high AS had higher diabetes and MetS percentages, were older, and had higher waist circumference and systolic blood pressure (SBP) but lower glomerular filtration rate than those with low AS. Multivariate logistic regression analysis revealed old age (P < 0.001), diabetes (P = 0.003), and high waist circumference (P = 0.044) and SBP (P = 0.007) as independent predictors of AS in CAD patients. Kaplan–Meier analysis showed that CAD patients with concurrent MetS and high AS had a higher risk for hospitalization (log rank test, P = 0.005) or developing all-cause mortality (log rank test, P = 0.002). Compared with CAD patients without MetS or AS, composite outcome development risk in those with both the conditions was 10.2-fold higher (P < 0.001); this risk was 6.54-fold higher in those with AS alone (P = 0.007).
Conclusion
In CAD patients, age, diabetes, and high waist circumference and SBP are the independent predictors of AS. Additionally, CAD patients with AS with and without MetS have a high first hospitalization or all-cause mortality development risk.
Keywords: aortic arterial stiffness, carotid-femoral pulse wave velocity, coronary artery disease, hospitalization, metabolic syndrome, mortality
Introduction
Metabolic syndrome (MetS) is defined as central obesity accompanied by features such as hypertriglyceridemia, low high-density lipoprotein cholesterol (HDL-C) levels, hyperglycemia, and hypertension (HTN); this condition adversely affects cardiovascular (CV) outcomes.1,2 Evidence had shown that there is correlation between adverse CV events such as ischemic stroke and immune-inflammatory activation.3–5 Also, the features characterizing MetS are reportedly associated with a proinflammatory state through a combination of atherogenic dyslipidemia, dysglycemia, and a prothrombotic state.1,2 Earlier longitudinal studies have shown that men with at least three features of MetS have an increased risk of coronary artery disease (CAD) and diabetes mellitus (DM) development.6,7 Evidence has shown that compared with patients without MetS, those with MetS have a significantly increased adjusted risk of mortality from CAD and cardiovascular disease (CVD) and all-cause mortality.8
Arterial stiffness (AS) due to loss of arterial compliance caused by phenomena such as elastin fiber and collagen deregulation, cellular element re-organization, and low-grade inflammation results in a more rapid travel time and hence a high pulse wave velocity (PWV). Carotid-femoral pulse wave velocity (cfPWV) is a non-invasive and simple clinical method to evaluate AS, and AS is an emerging CVD marker.9 Studies have suggested a relationship of increased PWV with coronary artery calcium score and subclinical CAD and suggested that AS could be a predictor for CVD.10,11 Moreover, a meta-analysis has shown that AS, represented as aortic PWV, is a strong predictor of future CV events and all-cause mortality; additionally, the study has reported that AS’s predictive ability is higher in subjects with a higher baseline CV risk.9
In a study conducted in Chinese HTN patients, increasing levels of cfPWV with an increasing number of MetS components independent of age or gender were observed.12 In an earlier cross-sectional study on DM patients, AS, defined by cfPWV, positively correlated with MetS.13 Moreover, MetS components including obesity, HTN, hypertriglyceridemia, and low HDL-C levels have been shown to be significantly associated with higher annual medical costs.14 However, while increased PWV has been shown to predict a greater risk of CV morbidity and mortality in HTN and DM patients,15 the association between AS and CAD patients with or without MetS remains unclear. To overcome this limitation, the present study aimed to examine the risk factors for AS in CAD patients and the influences of MetS and AS on first hospitalization or all-cause mortality in these patients.
Materials And Methods
Patients
In total, 115 CAD participants were enrolled in this longitudinal study; the participants were found to have a history of CAD between January and December 2012 at the Buddhist Tzu Chi General Hospital, Hualien, Taiwan. In this study, CAD was defined as any segment of coronary arteries with >50% stenosis observed on angiography following medical record review. Blood pressure (BP) for all participants was measured with automatic sphygmomanometer by trained staff in the morning after >10-min rest. Systolic BP (SBP) and diastolic BP (DBP) were recorded three times at 5-min intervals; the measurements were averaged for analysis. Patients with an SBP ≥140 mmHg and/or DBP ≥90 mmHg or those who had received any anti-HTN medication over the past 2 weeks were defined as having HTN. Patients were diagnosed with DM if either their fasting plasma glucose levels were ≥126 mg/dL or they were using oral hypoglycemic medications or insulin. Exclusion criteria were as follows: presence of acute inflammatory diseases, acute CAD, pulmonary edema at the time of blood sampling, or refusal to provide informed consent for the study. An informed consent was obtained from all patients prior to study participation. The study was approved by The Protection of the Human Subjects Institutional Review Board of Tzu-Chi University and Hospital.
Anthropometric Analysis
As done in our previous studies, body weight and body height were measured and waist circumference was measured at the midpoint between the lowest ribs and the iliac crest with the participants in light clothing without shoes. Body mass index (BMI) was calculated as the weight in kilograms divided by the height in meters squared.16–18
Biochemical Examinations
After 8-h overnight fasting, blood samples (approximately 5 mL) of all participants were collected and sent for examination. Laboratory tests performed were as follows: serum levels of blood urea nitrogen (BUN), creatinine (Cre), fasting glucose, total cholesterol, triglyceride (TG), HDL-C, low-density lipoprotein cholesterol, total calcium, and phosphorus. An autoanalyzer (COBAS Integra 800, Roche Diagnostics, Basel, Switzerland) was used for performing the tests. Serum levels of intact parathyroid hormone (iPTH) (Diagnostic Systems Laboratories, Texas, USA) were measured using a commercially available enzyme-linked immunosorbent assay. Finally, the estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation.16–18
Metabolic Syndrome
MetS was defined as per the International Diabetes Federation definition, which includes central obesity with a waist circumference of ≥90 cm (men) or ≥80 cm (women) (Chinese criteria) and meeting at least two of the following criteria: fasting glucose levels ≥ 100 mg/dL, TG levels ≥ 150mg/dL, HDL-C levels of <40mg/dL (men) or <50mg/dL (women), or BP ≥ 130/85 mmHg.19 Serum insulin levels were measured using the microparticle enzyme immunosorbent assay method with an autoanalyzer (Abbott Laboratories, Abbott Park, IL, USA). Insulin resistance was calculated using a homeostasis model assessment of insulin resistance (HOMA-IR) as follows: HOMA-IR = fasting plasma glucose (mg/dL) × fasting serum insulin (μU/mL)/405.17
Carotid-Femoral Pulse Wave Velocity Measurements
After at least 10-min rest in a quiet and temperature-controlled room in the morning, cfPWV measurements were performed once for all patients by one trained staff using pressure applanation tonometry (SphygmoCor system, AtCor Medical, Australia) in the supine position, as done in previous studies.16,18 Pulse wave recordings were performed electrocardiogram (ECG)-gated simultaneously with an R-timing reference and consecutively at the carotid and femoral arteries (the carotid-femoral segment). Integral software was used to process each pulse wave and ECG data set to calculate the mean time difference between R-wave and pulse wave on a beat-to-beat basis, with an average of 10 consecutive cardiac cycles. Subsequently, cfPWV was calculated using distance and the mean time difference between the two recorded points. As previously defined, patients with cfPWV values > 10 m/s were defined as the high AS group.15,16,18
Follow-Up And Endpoints
Patients’ conditions were traced by regular outpatient services or via telephone (in cases of physical immobility) until June 30, 2017. The endpoints were defined as follows: first hospitalization for unstable angina, myocardial infarction, revascularization, and heart failure or all-cause mortality. The event-free survival was defined as the interval between the date of cfPWV evaluation and the date of occurrence of endpoints or the end of follow-up.
Statistical Analysis
Kolmogorov–Smirnov test was used to analyze continuous variables for normal distribution. Normally distributed variables were expressed as means ± standard deviations, and Student’s t-test (two-tailed) was used to compare the patients. Non-normally distributed variables were expressed as medians and interquartile ranges (IQRs), and patients were compared using Mann–Whitney U-test. Categorical variables were expressed as patient numbers and percentages and were analyzed using chi-square test. For further analysis, patients were sub-grouped into four groups as follows: no MetS + no AS, MetS + no AS, no MetS + AS, and MetS + AS. Comparisons among the groups were performed using Kruskal–Wallis analysis or one-way analysis of variance for parameters that presented without or with normal distribution, respectively. Multivariate logistic regression analysis was used to analyze variables associated with the presence of high AS in CAD patients. Kaplan–Meier survival curves with a log rank test were used for comparing the cumulative proportion of CAD patients free from first hospitalization or mortality among these four groups. Multivariate Cox regression models were used to analyze variables associated with the risk of first hospitalization. Data were analyzed using SPSS for Windows (version 19.0; SPSS Inc., Chicago, IL, USA). P-values < 0.05 were considered significant.
Results
The baseline characteristics of the 115 CAD patients, divided into low and high (n = 41, 35.7%) AS, are described in Table 1. Among the 115 patients, 54 (48.7%), 92 (80.0%), and 70 (60.9%) patients were defined as having DM, HTN, and MetS, respectively.
Table 1.
Clinical Variables Of The 115 Coronary Artery Disease Patients With Or Without Aortic Arterial Stiffness
| Variables | All Participants(n = 115) | No Arterial StiffnessGroup (n = 74) | High Arterial StiffnessGroup (n = 41) | P value |
|---|---|---|---|---|
| Age (years) | 65.61 ± 8.97 | 63.38 ± 8.57 | 69.63 ± 8.32 | < 0.001* |
| Height (cm) | 161.21 ± 8.08 | 161.53 ± 7.41 | 160.63 ± 9.23 | 0.572 |
| Body weight (kg) | 68.31 ± 11.84 | 67.41 ± 11.63 | 69.94 ± 12.17 | 0.273 |
| Waist circumference (cm) | 93.08 ± 9.75 | 91.50 ± 9.41 | 95.93 ± 9.82 | 0.019* |
| Body mass index (kg/m2) | 26.19 ± 3.45 | 25.73 ± 3.34 | 27.03 ± 3.53 | 0.053 |
| cfPWV (m/s) | 9.52 ± 2.60 | 8.11 ± 1.59 | 12.06 ± 2.09 | < 0.001* |
| Systolic blood pressure (mmHg) | 132.04 ± 18.14 | 127.72 ± 15.50 | 139.85 ± 20.05 | < 0.001* |
| Diastolic blood pressure (mmHg) | 72.55 ± 10.28 | 71.74 ± 10.32 | 74.00 ± 10.18 | 0.261 |
| Total cholesterol (mg/dL) | 165.67 ± 35.19 | 137.32 ± 35.87 | 162.68 ± 34.17 | 0.501 |
| Triglycerides (mg/dL) | 121.00 (90.00–166.00) | 115.00 (89.00–184.25) | 127.00 (90.50–152.50) | 0.829 |
| HDL-C (mg/dL) | 44.92 ± 11.95 | 46.41 ± 11.79 | 42.24 ± 11.92 | 0.074 |
| LDL-C (mg/dL) | 95.83 ± 26.53 | 96.08 ± 25.31 | 95.37 ± 28.93 | 0.891 |
| Fasting glucose (mg/dL) | 111.00 (96.00–137.00) | 106.00 (96.75–129.50) | 118.00 (95.00–188.50) | 0.140 |
| Blood urea nitrogen (mg/dL) | 16.00 (13.00–19.00) | 16.00 (13.00–18.00) | 17.00 (13.00–21.50) | 0.136 |
| Creatinine (mg/dL) | 1.10 (0.90–1.30) | 1.00 (0.90–1.20) | 1.20 (0.90–1.45) | 0.054 |
| Glomerular filtration rate (mL/min) | 68.73 ± 19.43 | 73.32 ± 17.35 | 62.26 ± 21.45 | 0.007* |
| Total calcium (mg/dL) | 9.12 ± 0.35 | 9.14 ± 0.36 | 9.09 ± 0.35 | 0.503 |
| Phosphorus (mg/dL) | 3.52 ± 0.54 | 3.53 ± 0.56 | 3.51 ± 0.51 | 0.804 |
| Calcium-phosphorous product (mg2/dL2) | 32.16 ± 5.28 | 32.32 ± 5.55 | 31.89 ± 4.82 | 0.678 |
| Intact parathyroid hormone (pg/mL) | 52.87 ± 26.81 | 52.73 ± 29.41 | 53.10 ± 21.70 | 0.944 |
| Insulin (uIU/mL) | 11.90 (7.18–21.11) | 11.06 (6.69–19.85) | 13.02 (8.51–23.12) | 0.434 |
| HOMA-IR | 3.67 (2.11–7.27) | 3.47 (1.81–7.06) | 4.00 (2.73–7.33) | 0.250 |
| Female (%) | 28 (24.3) | 20 (27.0) | 8 (19.5) | 0.368 |
| Diabetes (%) | 56 (48.7) | 27 (36.5) | 29 (70.7) | < 0.001* |
| Hypertension (%) | 92 (80.0) | 56 (75.7) | 36 (87.8) | 0.119 |
| Metabolic syndrome | 70 (60.9) | 40 (54.1) | 30 (73.2) | 0.044* |
Notes: Values for continuous variables given as means ± standard deviation and compared by Student’s t-test; variables not normally distributed given as medians and interquartile range and compared by Mann–Whitney U-test; values are presented as number (%), and analysis was performed using the chi-square test. *P < 0.05 was considered statistically significant.
Abbreviations: cfPWV, carotid-femoral pulse wave velocity; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment of insulin resistance; ARB, angiotensin-receptor blocker; ACE, angiotensin-converting enzyme; CCB, calcium-channel blocker.
On sub-grouping the CAD patients, 34 (29.5%), 11 (9.6%), 40 (34.8%), and 30 (26.1%) patients were categorized into the no MetS + no AS, MetS + no AS, no MetS + AS, and MetS + AS groups, respectively (Table 2). Interestingly, significant differences were identified among these groups in age; waist circumference; BMI; cfPWV values; SBP; eGFR; TG, HDL-C, fasting glucose, BUN, total calcium, phosphorous, calcium-phosphorous product, and insulin levels; and HOMA-IR. After adjusting the risk factors including age, waist circumference, SBP, eGFR, DM, and MetS, old age (P < 0.001), DM (P = 0.003), high waist circumference (P = 0.044), and SBP (P = 0.007) were identified as the independent predictors of AS in CAD patients (Table 3).
Table 2.
Clinical Variables With Aortic Arterial Stiffness In The Coronary Artery Disease Patients With Or Without Metabolic Syndrome
| Variables | No metabolic Syndrome (n = 45) | Metabolic Syndrome (n = 70) | P value | ||
|---|---|---|---|---|---|
| No AS Group (n = 34) | AS Group (n = 11) | No AS Group (n = 40) | AS Group (n = 30) | ||
| Age (years) | 64.82 ± 8.63 | 71.45 ± 5.96 | 62.15 ± 8.45 | 68.97 ± 9.03 | 0.001* |
| Height (cm) | 161.85 ± 6.23 | 161.09 ± 5.91 | 161.25 ± 8.35 | 160.47 ± 10.26 | 0.927 |
| Body weight (kg) | 65.03 ± 10.11 | 65.09 ± 6.25 | 69.43 ± 12.55 | 71.72 ± 13.37 | 0.098 |
| Waist circumference (cm) | 88.50 ± 8.52 | 87.001 ± 8.33 | 94.05 ± 9.47 | 99.20 ± 8.23 | < 0.001* |
| Body mass index (kg/m2) | 24.77 ± 3.15 | 25.15 ± 2.81 | 26.54 ± 3.33 | 27.72 ± 3.55 | 0.004* |
| cfPWV (m/s) | 7.70 ± 1.30 | 11.83 ± 1.02 | 8.46 ± 1.75 | 12.15 ± 2.37 | < 0.001* |
| Systolic blood pressure (mmHg) | 123.15 ± 12.02 | 130.73 ± 17.96 | 131.60 ± 17.13 | 132.04 ± 18.14 | < 0.001* |
| Diastolic blood pressure (mmHg) | 70.15 ± 9.47 | 72.45 ± 7.12 | 73.10 ± 10.93 | 74.57 ± 11.14 | 0.376 |
| Total cholesterol (mg/dL) | 170.26 ± 32.81 | 161.45 ± 38.87 | 164.83 ± 38.51 | 163.13 ± 33.00 | 0.825 |
| Triglycerides (mg/dL) | 103.50 (84.00–130.25) | 95.00 (67.00–127.00) | 157.50 (101.75–233.00) | 138.50(91.75–163.00) | 0.002* |
| HDL-C (mg/dL) | 51.79 ± 12.27 | 46.27 ± 15.16 | 41.83 ± 9.27 | 40.77 ± 10.41 | < 0.001* |
| LDL-C (mg/dL) | 98.18 ± 25.61 | 94.36 ± 31.92 | 94.30 ± 25.23 | 95.73 ± 28.32 | 0.935 |
| Fasting glucose (mg/dL) | 101.00 (95.00–117.25) | 93.00 (89.00–125.00) | 118.50 (101.25–133.75) | 132.50 (107.25–184.00) | 0.001* |
| Blood urea nitrogen (mg/dL) | 15.00 (12.00–17.00) | 13.00 (12.00–19.00) | 16.00 (13.00–19.75) | 19.00 (14.00–23.25) | 0.022* |
| Creatinine (mg/dL) | 1.00 (0.90–1.20) | 1.00 (0.80–1.20) | 1.05 (0.90–1.30) | 1.25 (0.98–1.55) | 0.058 |
| Glomerular filtration rate (mL/min) | 75.06 ± 13.67 | 72.16 ± 14.72 | 69.99 ± 19.829 | 58.62 ± 22.56 | 0.005* |
| Total calcium (mg/dL) | 9.00 ± 0.33 | 9.01 ± 0.33 | 9.26 ± 0.35 | 9.13 ± 0.35 | 0.012* |
| Phosphorus (mg/dL) | 3.43 ± 0.53 | 3.16 ± 0.39 | 3.62 ± 0.58 | 3.63 ± 0.49 | 0.034* |
| Ca X IP product(mg2/dL2) | 30.92 ± 5.03 | 28.49 ± 4.31 | 33.50 ± 5.75 | 33.13 ± 4.43 | 0.011* |
| iPTH (pg/mL) | 54.47 ± 25.86 | 55.50 ± 21.96 | 51.26 ± 32.37 | 52.22 ± 21.91 | 0.944 |
| Insulin (uIU/mL) | 9.33 (4.80–16.14) | 11.11 (6.55–17.79) | 14.63 (9.57–26.33) | 14.50 (9.18–25.59) | 0.026* |
| HOMA-IR | 2.23 (1.31–4.80) | 2.86 (1.36–5.84) | 4.29 (2.92–8.19) | 4.75 (2.91–8.55) | 0.003* |
Notes: Values for continuous variables given as means ± standard deviation and are test by one-way analysis of variance; variables not normally distributed given as medians and interquartile range and are test by Kruskal-Wallis analysis. *P < 0.05 was considered statistically significant.
Abbreviations: AS, arterial stiffness; cfPWV, carotid-femoral pulse wave velocity; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; Ca X IP product, calcium-phosphorous product; iPTH, intact parathyroid hormone; HOMA-IR, homeostasis model assessment of insulin resistance.
Table 3.
Multivariate Logistic Regression Analysis Of The Factors Correlated With Aortic Stiffness Among The 115 Patients With Coronary Artery Disease
| Variables | Odds Ratio | 95% Confidence Interval | P value |
|---|---|---|---|
| Age, 1 year | 1.13 | 1.06−1.21 | < 0.001* |
| Diabetes, present | 4.76 | 1.68−13.47 | 0.003* |
| Waist circumference, 1 cm | 1.06 | 1.00−1.11 | 0.044* |
| Systolic blood pressure, 1 mmHg | 1.04 | 1.01−1.08 | 0.007* |
Notes: Analysis of data was done using the multivariate logistic regression analysis (adapted factors were diabetes, age, waist circumference, systolic blood pressure, glomerular filtration rate and metabolic syndrome). *P < 0.05 was considered statistically significant.
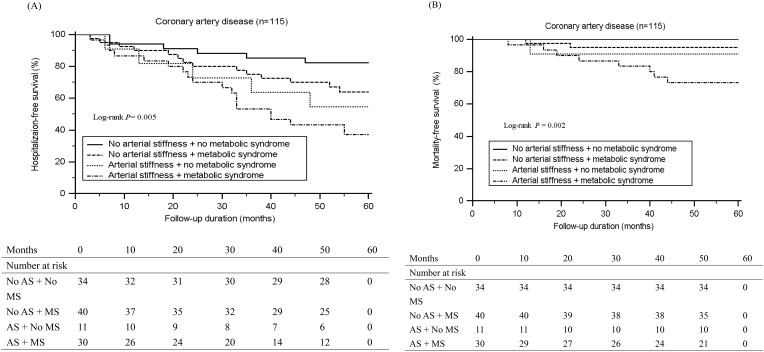
During a median 54-month follow-up, 43 (37.4%) first hospitalization and 11 (9.6%) mortality events were observed. Kaplan–Meier analysis showed that CAD patients with both MetS and AS had the worst free-survival from first hospitalization (log rank test, P = 0.005) (Figure 1A) or all-cause mortality (log rank test, P = 0.002) (Figure 1B). For further analyzing risk factors for developing endpoints using multivariate Cox regression analysis, variables including age, waist circumference, and BMI were adjusted in model 1. Additionally, variables in model 1 as well as SBP; TG, HDL-C, fasting glucose, and insulin levels; and HOMA-IR were adjusted in model 2. Finally, variables in model 2 as well as BUN, Cre, total calcium, phosphorous, and calcium-phosphorous product levels and eGFR were adjusted in model 3 (Table 4). The results showed that compared with CAD patients without MetS or AS, those with both MetS and AS had a 7.38-fold (P < 0.001), 8.23-fold (P < 0.001), and 10.20-fold (P < 0.001) increased risk for developing endpoints as per Models 1, 2, and 3, respectively; furthermore, CAD patients with only AS had a 3.86-fold (P = 0.032), 5.48-fold (P = 0.010), and 6.54-fold (P = 0.007) increased risk for developing endpoints as per Models 1, 2, and 3, respectively. No significant increase in the risk for developing endpoints was observed in CAD patients with MetS only compared with those without both MetS and AS.
Figure 1.
Kaplan-Meier analysis according to aortic arterial stiffness (AS) with or without metabolic syndrome (MS) for (A) first hospitalization (B) mortality in patients with coronary artery disease.
Table 4.
Adjusted Hazard Ratios For A Composite End-Point Consisting Of First Hospitalization Based On A Multivariate Cox Regression Model For Aortic Arterial Stiffness In The 115 Coronary Artery Disease Patients With Or Without Metabolic Syndrome
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | P value | Hazard Ratio (95% CI) | P value | Hazard Ratio (95% CI) | P value | |
| No AS and no MS | Reference | Reference | Reference | |||
| No AS and MS | 2.48 (0.93–6.60) | 0.069 | 2.17 (0.75–6.28) | 0.151 | 2.38 (0.77–7.38) | 0.134 |
| AS and no MS | 3.86 (1.12–13.28) | 0.032* | 5.48 (1.50–20.05) | 0.010* | 6.54 (1.67–25.68) | 0.007* |
| AS and MS | 7.38 (2.61–20.83) | < 0.001* | 8.23 (2.46–27.86) | < 0.001* | 10.20 (2.77–37.53) | < 0.001* |
Notes: Model 1 is adjusted for age, waist circumference, and body mass index. Model 2 is adjusted for the model 1 variables and for systolic blood pressure, triglycerides, high-density lipoprotein cholesterol, fasting glucose, insulin, and homeostasis model assessment of insulin resistance. Model 3 is adjusted for the model 2 variables and for blood urea nitrogen, creatinine, glomerular filtration rate, total calcium, phosphorus, and calcium-phosphorous product. *P < 0.05 was considered statistically significant.
Abbreviations: AS, arterial stiffness; MS, metabolic syndrome; CI, confidence interval.
Discussion
The present study demonstrated that old age, DM, a high waist circumference, and SBP were the predictors of high AS in CAD patients. Additionally, CAD patients with or without MetS but with high AS had a significantly higher risk for first hospitalization and all-cause mortality events than those without MetS and high AS.
Classic CV risk factors such as DM, dyslipidemia, elevated BMI, MetS, and smoking have been implicated in accelerating AS.10,12,13,20–22 Aging is characterized by dysregulated remodeling of elastin and collagen, causing loss of arterial elasticity and thickening of the vascular wall and increased arterial stiffening.21 Evidence has shown that AS measured using PWV has a significantly positive correlation with patients’ age in the presence of occult CAD, MetS, DM, or HTN.10,12,13,20 AS and HTN are pathophysiologically correlated, as increased AS reduces the lumen diameter and leads to a premature return of the reflected wave in late systole, resulting in increased pulse pressure and SBP and decreased DBP.20 Studies have shown a positive correlation of cfPWV with SBP as well as with MetS and increased waist circumference in HTN and DM patients.13,23 Moreover, a systemic review has reported that other than classic risk factors such as gender, dyslipidemia, smoking, and BMI, arterial aging and BP elevation are independently associated with cfPWV.24 Schram et al have found that patients with impaired glucose metabolism and DM have lesser total systemic arterial compliance, greater aortic augmentation index, and a lesser carotid-femoral transit time, which indicates an increased central AS compared with that in patients with normal glucose metabolism.25 Additionally, chronic DM has been found to be independently associated with increased cfPWV and macrovascular event risk after adjusting confounders.26 In a longitudinal study, adiposity was found to manifest as BMI and waist circumference and was positively correlated with cfPWV change, indicating that adiposity was also a predictor of AS.27 In line with these reports, our study revealed that CAD patients with high AS with or without MetS, were significantly older, had higher percentages of DM comorbidity with higher fasting blood glucose levels, higher serum insulin levels and HOMA-IR, and a higher waist circumference and SBP than CAD patients with low AS without MetS. Furthermore, old age, DM, waist circumference, and SBP were found to be the possible risk factors for developing high AS after adjusting for covariates in CAD patients.
When evaluating eGFR > 60 mL/min/1.73 m2, Yan et al found that women with eGFR < 60 mL/min/1.73 m2 had higher PWV and that there was a negative association between eGFR and PWV.28 Two studies have been performed to examine the association between AS and renal function: Ilyas et al have found that CAD patients without a known renal disease exhibit a negative association between PWV and eGFR; Ford et al have found that in patients with more advanced chronic kidney disease (CKD) and an eGFR 15–59 mL/min/1.73 m2, AS is independently associated with a decline in renal function.9,29,30 Furthermore, in patients with advanced CKD, increased severity of AS is an independent predictor of CV events and mortality, with AS improving the risk prediction of these events.31,32 In this study, renal function did not appear as a risk factor for AS development after adjusting for covariates; however, still it was found that CAD patients with high AS with or without MetS have lower renal function. Together with the previously reported studies, we believe that renal function decline plays a role in AS development.
MetS, defined as central obesity along with associated factors such as dyslipidemia, hyperglycemia, and HTN, together with DM and chronic inflammation has been implicated in CV complications in urban population over 40 years old and in AS development.21,22 Evidence has shown that MetS and its components (ie, fasting glucose, BP, and waist circumference) are associated with PWV.33 Yokoyama et al have found that as the number of MetS components increase, PWV significantly increases in patients with DM without CAD or renal diseases.34 Our results showed that CAD patients with MetS and AS had a higher waist circumference, SBP, hypertriglyceridemia, fasting glucose levels, cfPWV, and lower HDL-C levels than those without MetS and AS. Taken together, we believe that MetS plays a role in increased AS in CAD patients.
Central AS associated with increased systolic load and decreased myocardial perfusion pressure is an independent predictor of CV morbidity and mortality.29,31 Ilyas et al have found that in patients who have recently undergone coronary angiography, increased PWV is associated with future hospitalization due to CVD and all-cause mortality.29 Other longitudinal studies performed in CKD patients have shown AS, measured using PWV, as a significant predictor for future worse CV outcomes independent of age, gender, BP, DM, or past CVD.31,32 A meta-analysis including patients with HTN, DM, or end-stage renal disease has shown AS, measured using PWV, as an independent predictor of total CV events, CV mortality and all-cause mortality.9 Additionally, studies have shown that patients with MetS, even those with 1–2 components or those without CVD or DM, have an increased risk for future CAD, CVD, all-cause mortality, and medical costs.7,8,14 In line with these reports, this study showed that high AS or MetS were risk factors for first hospitalization and all-cause mortality events in CAD patients. More importantly, we additionally found that CAD patients with high AS had a higher risk for developing these events than those with MetS. We believe that AS may play a more important role than MetS for worse prognosis prediction in CAD patients. However, these results need further investigations for confirmation.
A limitation of the present study was that it was conducted with a limited number of CAD patients at a single center and not all risk factors for AS development, such as smoking, were included. Therefore, more CAD patients evaluated with a more detailed survey of risk factors were required for studying the possible predictors for AS and its role in the development of future CV events or mortality.
Conclusion
This study demonstrated age, DM, waist circumference, and SBP to be the independent predictors for the presence of high AS in CAD patients. Additionally, CAD patients with MetS and especially the presence of high AS exhibited a significantly higher risk for first hospitalization and all-cause mortality events.
Acknowledgments
This study was supported by a grant from the Buddhist Tzu Chi Medical Foundation, Taiwan (TCMF-MP 107-01-01). The authors would like to thank Enago for the English language review.
Ethics Approval And Informed Consent
The study was approved by an independent ethics committee from the Protection of the Human Subjects Institutional Review Board of Tzu-Chi University and Hospital (IRB099-97), and all participants gave written informed consent according to the general recommendations of the Helsinki Declaration.
Data Availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92:347–355. doi: 10.1079/bjn20041213 [DOI] [PubMed] [Google Scholar]
- 2.Rosenson RS. Assessing risk across the spectrum of patients with the metabolic syndrome. Am J Cardiol. 2005;96:8E–10E. doi: 10.1016/j.amjcard.2005.05.007 [DOI] [PubMed] [Google Scholar]
- 3.Licata G, Tuttolomondo A, Corrao S, et al. Immunoinflammatory activation during the acute phase of lacunar and non-lacunar ischemic stroke: association with time of onset and diabetic state. Int J Immunopathol Pharmacol. 2006;19:639–646. doi: 10.1177/039463200601900320 [DOI] [PubMed] [Google Scholar]
- 4.Tuttolomondo A, Di Sciacca R, Di Raimondo D, et al. Effects of clinical and laboratory variables and of pretreatment with cardiovascular drugs in acute ischaemic stroke: a retrospective chart review from the GIFA study. Int J Cardiol. 2011;151:318–322. doi: 10.1016/j.ijcard.2010.06.005 [DOI] [PubMed] [Google Scholar]
- 5.Di Raimondo D, Tuttolomondo A, Buttà C, et al. Effects of ACE-inhibitors and angiotensin receptor blockers on inflammation. Curr Pharm Des. 2012;18:4385–4413. doi: 10.2174/138161212802481282 [DOI] [PubMed] [Google Scholar]
- 6.Sattar N, Gaw A, Scherbakova O, et al. Metabolic syndrome with and without C-reactive protein as a predictor of coronary heart disease and diabetes in the West of Scotland Coronary Prevention Study. Circulation. 2003;108:414–419. doi: 10.1161/01.CIR.0000080897.52664.94 [DOI] [PubMed] [Google Scholar]
- 7.Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–2716. doi: 10.1001/jama.288.21.2709 [DOI] [PubMed] [Google Scholar]
- 8.Malik S, Wong ND, Franklin SS, et al. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004;110:1245–1250. doi: 10.1161/01.CIR.0000140677.20606.0E [DOI] [PubMed] [Google Scholar]
- 9.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–1327. doi: 10.1016/j.jacc.2009.10.061 [DOI] [PubMed] [Google Scholar]
- 10.Nam HJ, Jung IH, Kim J, et al. Association between brachial-ankle pulse wave velocity and occult coronary artery disease detected by multi-detector computed tomography. Int J Cardiol. 2012;157:227–232. doi: 10.1016/j.ijcard.2011.01.045 [DOI] [PubMed] [Google Scholar]
- 11.Kullo IJ, Bielak LF, Turner ST, Sheedy PF, Peyser PA. Aortic pulse wave velocity is associated with the presence and quantity of coronary artery calcium: a community-based study. Hypertension. 2006;47:174–179. doi: 10.1161/01.HYP.0000199605.35173.14 [DOI] [PubMed] [Google Scholar]
- 12.Gong J, Xie Q, Han Y, et al. Relationship between components of metabolic syndrome and arterial stiffness in Chinese hypertensives. Clin Exp Hypertens. 2019;1–7. doi: 10.1080/10641963.2019.1590385 [DOI] [PubMed] [Google Scholar]
- 13.Levisianou D, Melidonis A, Adamopoulou E, et al. Impact of the metabolic syndrome and its components combinations on arterial stiffness in Type 2 diabetic men. Int Angiol. 2009;28:490–495. [PubMed] [Google Scholar]
- 14.Nichols GA, Moler EJ. Metabolic syndrome components are associated with future medical costs independent of cardiovascular hospitalization and incident diabetes. Metab Syndr Relat Disord. 2011;9:127–133. doi: 10.1089/met.2010.0105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31:1281–1357. doi: 10.1097/01.hjh.0000431740.32696.cc [DOI] [PubMed] [Google Scholar]
- 16.Tsai JP, Wang JH, Chen ML, et al. Association of serum leptin levels with central arterial stiffness in coronary artery disease patients. BMC Cardiovasc Disord. 2016;16:80. doi: 10.1186/s12872-016-0371-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen MC, Lee CJ, Yang CF, et al. Low serum adiponectin level is associated with metabolic syndrome and is an independent marker of peripheral arterial stiffness in hypertensive patients. Diabetol Metab Syndr. 2017;9:49. doi: 10.1186/s13098-017-0247-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang JH, Lee CJ, Yang CF, et al. Serum resistin as an independent marker of aortic stiffness in patients with coronary artery disease. PLoS One. 2017;12:e0183123. doi: 10.1371/journal.pone.0183123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome–a new world-wide definition. A consensus statement from the international diabetes federation. Diabet Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x [DOI] [PubMed] [Google Scholar]
- 20.Laurent S, Boutouyrie P. Arterial stiffness: a new surrogate end point for cardiovascular disease? J Nephrol. 2007;20 Suppl 12:S45–S50. [PubMed] [Google Scholar]
- 21.Palombo C, Kozakova M. Arterial stiffness, atherosclerosis and cardiovascular risk: pathophysiologic mechanisms and emerging clinical indications. Vascul Pharmacol. 2016;77:1–7. doi: 10.1016/j.vph.2015.11.083 [DOI] [PubMed] [Google Scholar]
- 22.Moreira GC, Cipullo JP, Ciorlia LA, et al. Prevalence of metabolic syndrome: association with risk factors and cardiovascular complicationss in an urban population. PLoS One. 2014;9:e105056. doi: 10.1371/journal.pone.0105056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramirez AJ, Christen AI, Sanchez RA. Serum uric acid elevation is associated to arterial stiffness in hypertensive patients with metabolic disturbances. Curr Hypertens Rev. 2018;14:154–160. doi: 10.2174/1573402114666180413143312 [DOI] [PubMed] [Google Scholar]
- 24.Cecelja M, Chowienczyk P. Dissociation of aortic pulse wave velocity with risk factors for cardiovascular disease other than hypertension: a systematic review. Hypertension. 2009;54:1328–1336. doi: 10.1161/HYPERTENSIONAHA.109.137653 [DOI] [PubMed] [Google Scholar]
- 25.Schram MT, Henry RM, van Dijk RA, et al. Increased central artery stiffness in impaired glucose metabolism and type 2 diabetes: the Hoorn Study. Hypertension. 2004;43:176–181. doi: 10.1161/01.HYP.0000111829.46090.92 [DOI] [PubMed] [Google Scholar]
- 26.Agnoletti D, Mansour AS, Zhang Y, et al. Clinical interaction between diabetes duration and aortic stiffness in type 2 diabetes mellitus. J Hum Hypertens. 2017;31:189–194. doi: 10.1038/jhh.2016.58 [DOI] [PubMed] [Google Scholar]
- 27.Brunner EJ, Shipley MJ, Ahmadi-Abhari S, et al. Adiposity, obesity, and arterial aging: longitudinal study of aortic stiffness in the Whitehall II cohort. Hypertension. 2015;66:294–300. doi: 10.1161/HYPERTENSIONAHA.115.05494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yun BH, Chon SJ, Cho SH, et al. Decreased renal function is a risk factor for subclinical coronary atherosclerosis in Korean postmenopausal women. J Menopausal Med. 2016;22:167–173. doi: 10.6118/jmm.2016.22.3.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ilyas B, Dhaun N, Markie D, et al. Renal function is associated with arterial stiffness and predicts outcome in patients with coronary artery disease. QJM. 2009;102:183–191. doi: 10.1093/qjmed/hcn171 [DOI] [PubMed] [Google Scholar]
- 30.Ford ML, Tomlinson LA, Chapman TP, Rajkumar C, Holt SG. Aortic stiffness is independently associated with rate of renal function decline in chronic kidney disease stages 3 and 4. Hypertension. 2010;55:1110–1115. doi: 10.1161/HYPERTENSIONAHA.109.143024 [DOI] [PubMed] [Google Scholar]
- 31.Karras A, Haymann JP, Bozec E, et al. Large artery stiffening and remodeling are independently associated with all-cause mortality and cardiovascular events in chronic kidney disease. Hypertension. 2012;60:1451–1457. doi: 10.1161/HYPERTENSIONAHA.112.197210 [DOI] [PubMed] [Google Scholar]
- 32.Zoungas S, Cameron JD, Kerr PG, et al. Association of carotid intima-medial thickness and indices of arterial stiffness with cardiovascular disease outcomes in CKD. Am J Kidney Dis. 2007;50:622–630. doi: 10.1053/j.ajkd.2007.07.012 [DOI] [PubMed] [Google Scholar]
- 33.Sipila K, Koivistoinen T, Moilanen L, et al. Metabolic syndrome and arterial stiffness: the Health 2000 Survey. Metabolism. 2007;56:320–326. doi: 10.1016/j.metabol.2006.10.008 [DOI] [PubMed] [Google Scholar]
- 34.Yokoyama H, Kuramitsu M, Kanno S, Tada J, Yokota Y, Kamikawa F. Relationship between metabolic syndrome components and vascular properties in Japanese type 2 diabetic patients without cardiovascular disease or nephropathy. Diabetes Res Clin Pract. 2007;75:200–206. doi: 10.1016/j.diabres.2006.06.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.