Abstract
Objective:
To evaluate clinical features and prognostic factors in a large single institutional cohort of chromophobe renal cell carcinoma (chRCC) patients for identification of tumors with the highest metastatic potential.
Patients and methods:
Clinicopathological parameters of all patients with chRCC diagnosed and surgically treated at MSKCC between 1990 and 2016 were identified and compared to those patients treated for clear cell renal cell carcinoma (ccRCC) in the same study period using Wilcoxon test for continuous variables and Fisher exact test for categorical variables. Recurrence-free survival (RFS) and overall survival (OS) were analyzed using Kaplan-Meier method, log-rank test and Cox proportional hazards regression.
Results:
496 patients with chRCC (10-year RFS 91.7% and OS 82.1%) and 3312 patients with ccRCC (10-year RFS 79.4% and OS 63.6%) were included in the analysis. Patients with chRCC were younger (median 59 vs. 61 years, P = 0.0015), less frequently male (54.8% vs. 66.3%, P < 0.0001), showed more favorable T stages (T1-2 in 78% vs. 67%, P < 0.0001) and less frequent sarcomatoid differentiation (1.2 % vs. 4%, P = 0.0008) and displayed lower rates of metastatic development compared with ccRCC patients. Larger tumor size, sarcomatoid differentiation, and higher T-stage are significantly associated with adverse RFS and OS in chromophobe tumors.
Conclusion:
ChRCC is more commonly diagnosed in female and younger patients and is associated with a more favorable clinical outcome and a lower propensity for metastatic development than ccRCC. Larger tumors and sarcomatoid differentiation of chRCC may be considered as risk factors for metastatic development.
Keywords: Non-clear cell renal cell carcinoma, chromophobe, retrospective analysis, clinical outcome
MicroAbstract
Chromophobe renal cell carcinoma is usually a relatively indolent form of kidney cancer with low risk of metastases. In our study we show that if initial kidney tumors present with large size, or with sarcomatoid features, closer follow-up is needed to identify and promptly treat patients with higher risk of metastatic disease and consecutively worse prognosis.
Introduction
Renal cell carcinoma (RCC) is a disease composed by multiple histological subtypes. The most prevalent form, clear cell renal cell carcinoma (clear cell RCC) accounts for 65-70% of the cases, while a heterogeneous group of non-clear cell renal cell carcinoma subtypes1–3 is responsible for the remainder of the cases.
Chromophobe renal cell carcinoma (ChRCC) is the second most common form of non-clear cell renal cell carcinoma after papillary RCC and accounts for 5-10% of all kidney cancers4, 5. The pathogenesis of ChRCC suggests that this subtype is derived from cells of the distal convoluted tubules of the nephron6, in contrast to clear cell renal cell carcinoma, which arises from the proximal tubules. Despite the presentation of ChRCC as large tumors, studies from various surgical cohorts have shown more favorable clinical courses than for the other RCC subtypes, suggesting a low metastatic potential7–10.
Nevertheless ChRCC is an uncommon cancer compared to the other urological tumors and the available studies evaluating the cancer-related outcomes have been often limited by small numbers of patients 7,8,11–14. Literature review suggests that ChRCC is often curable by surgery alone, however 5-10% of the patients with ChRCC develop metastases8, 15. Currently there is no standard of care for these patients. Patients with metastases from this uncommon variant are underrepresented in prospective phase III trials for standard and novel targeted drugs, which are conceived for clear cell RCC, and the available data on medical treatment of ChRCC patients is very scarce15–20.
In light of the recent advances in the understanding of the pathogenesis of ChRCC6, 21 there is an unmet need to assess at time of diagnosis which patients may potentially develop metastases and thus an incurable disease. Until personalized biomarkers and treatment algorithms become valid options in the current practice, reliable clinical variables are needed to serve as prognostic markers.
We sought to study the to date largest series of ChRCC patients and evaluate the clinical outcomes in order to determine prognostic factors for recurrence-free-survival (RFS) and overall survival (OS).
Patients and Methods
Informed consent was obtained from all individual participants included in the study according to the Institutional Review Board approval (#WA0395-12). All procedures performed in the study were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments.
We queried our prospectively maintained institutional database containing 3808 patients who underwent radical or partial nephrectomy at Memorial Sloan Kettering Cancer Center (MSKCC) from 1990 to 2016 and identified a total of 496 patients with chromophobe renal cell carcinoma. Baseline clinicopathological characteristics were compared with those of 3312 patients with clear cell renal cell carcinoma. T-stages were clustered into T1-T2 and T3-T4 for comparative analysis.
Histological subtypes were assigned by an expert GU-pathologist based on guidelines of the International Union Against Cancer (UICC) and the American Joint Committee on Cancer (AJCC) and the Heidelberg Classification System22. A re-review of the tumor sildes was not performed, only in specific cases with mixed or doubtful histologies.
The recurrence-free survival (RFS) and overall survival (OS) were assessed during follow-up visits at our institution. Follow-up includes office visits with physical and laboratory exams, abdominal computed tomography or ultrasound, chest radiography or computed tomography in average every 6 months for 3 years followed by annual visits thereafter. In patients without evidence of metastatic disease at time of diagnosis (M0/Mx), identification of distant metastases (recurrence) was based on radiologic findings and or clinical findings. The cause of death was determined in patients who died during the study duration by review of the medical record or the death certificates.
The parameters of both cohorts were compared using Wilcoxon test for continuous variables and Fisher exact test on categorical variables. To calculate the RFS and OS Kaplan-Meier survival analysis was used. A log-rank test was employed to test for differences in RFS or OS. Cox proportional hazards models were used to assess independent predictors of clinical outcome. Final multivariate models were selected using backward selection. Patients with metastases at diagnosis (M1) were excluded from the analyses of RFS. All statistical analyses were computed using SAS version 9.4 SAS institute Inc., Cary, NC, USA. All tests were two-sided and p<0.05 was considered significant.
Results
The clinicopathological characteristics of the 3808 patients (496 patients with ChRCC and 3312 patients with clear cell RCC) included in the present study are summarized in Table 1. Patients were diagnosed und underwent partial or radical nephrectomy between 1990 and 2016. Details on the surgery type performed and the years of the surgery are enlisted in Supplementary Tables 1 and 2. The median follow-up from the time of surgery was 4.91 years (range .02-25.2) for the ChRCC group and 4.66 years (range .01-25.8) for the clear cell RCC group.
Table 1.
Clinical and pathologic characteristics of the chRCC and ccRCC cohorts
| Total (n=3808) | ccRCC (n=3312) | chRCC (n=496) | p-value | |
|---|---|---|---|---|
| Gender (%) | <.0001 | |||
| Male | 2467 (64.8) | 2195 (66.3) | 272 (54.8) | |
| Female | 1341 (35.2) | 1117 (33.7) | 224 (45.2) | |
| Age at nephrectomy | .0015 | |||
| Median | 61 | 61 | 59 | |
| Interquartile range | (17-92) | (17-92) | (23-89) | |
| BMI | <.0001 | |||
| Median | 28.90 | 29.09 | 27.34 | |
| Interquartile range | (0.21-61.57) | (0.21-61.57) | (0.24-50.10) | |
| Procedure type (%) | <.0001 | |||
| Partial nephrectomy | 2016 (52.9) | 1706 (51.5) | 310 (62.5) | |
| Radical nephrectomy | 1792 (47.1) | 1606 (48.5) | 186 (37.5) | |
| Multifocalilty (%) | 0.06 | |||
| Missing | 700 (.) | 697 (.) | 3 (.) | |
| No | 2861 (92.1) | 2397 (91.7) | 464 (94.1) | |
| Yes | 247 (7.9) | 218 (8.3) | 29 (5.9) | |
| Max tumor size (cm) | 0.26 | |||
| Median | 4.00 | 4.00 | 4.10 | |
| Interquartile range | (0.50-34.00) | (0.50-34.00) | (0.60-20.50) | |
| Sarcomatoid differentiation(%) | .0008 | |||
| No | 3669 (96.3) | 3179 (96) | 490 (98.8) | |
| yes | 139 (3.7) | 133 (4) | 6 (1.2) | |
| Necrosis | <.0001 | |||
| Missing | 2515 (.) | 2514 (.) | 1 (.) | |
| 0 | 1053 (81.4) | 594 (74.4) | 459 (92.7) | |
| 1 | 240 (18.6) | 204 (25.6) | 36 (7.3) | |
| 2010 pT stage (%) | <.0001 | |||
| Missing | 884 (.) | 882 (.) | 2 (.) | |
| T1 or T2 | 2006 (68.6) | 1623 (66.8) | 383 (77.5) | |
| T3 or T4 | 918 (31.4) | 807 (33.2) | 111 (22.5) | |
| Overall survival time | 0.19 | |||
| Median | 56.41 | 55.87 | 61.40 | |
| Interquartile range | (0.00-309.51) | (0.00-309.51) | (0.20-302.24) | |
| Metastatic recurrence | <.0001 | |||
| no | 3277 (86.1) | 2806 (84.7) | 471 (95) | |
| yes | 531 (13.9) | 506 (15.3) | 25 (5) | |
| Overall survival status | <.0001 | |||
| alive | 2849 (74.8) | 2419 (73) | 430 (86.7) | |
| deceased | 959 (25.2) | 893 (27) | 66 (13.3) |
Median age at diagnosis in the ChRCC cohort was 59 years, and 61 years in the clear cell RCC cohort (P = 0.0015) (Supplementary Figure 1). In the present study ChRCC patients were less frequently males (54.8%) compared to patients with Clear cell RCC (66.3%, P < 0.0001), and displayed a lower BMI (27.34 vs. 29.09, P < 0.0001), and were more likely to be treated with partial nephrectomy (62.5% vs. 51.5%, P < 0.0001). Furthermore, ChRCC patients presented with more favorable T stages (T1-2 in 78% vs 67%, P < 0.001) and at last follow-up, metastatic disease was detected in only 5% of the ChRCC patients and in 15.3% of the Clear cell RCC patients. The tumor size was not significantly different between the two cohorts (median 4.1 cm for ChRCC and 4 cm for Clear cell RCC, P = 0.3). While Fuhrman nuclear grade significantly impacted RFS and OS in Clear cell RCC, this variable was not included in the ChRCC analysis, given its limited applicability in this histological subtype.
Five-year RFS was 94.9% for ChRCC and 84% for clear cell RCC, while ten-year RFS was 91.7% for ChRCC and 79.4% for clear cell RCC (logrank P < 0.0001) with the median not reached. Five-year OS was 92.3% for ChRCC and 81.7% for clear cell RCC, while ten-year OS was 82.1% for ChRCC and 63.6% for clear cell RCC (logrank P < 0.0001), with median 21.2 and 14 years respectively (Figure 1).
Figure 1.
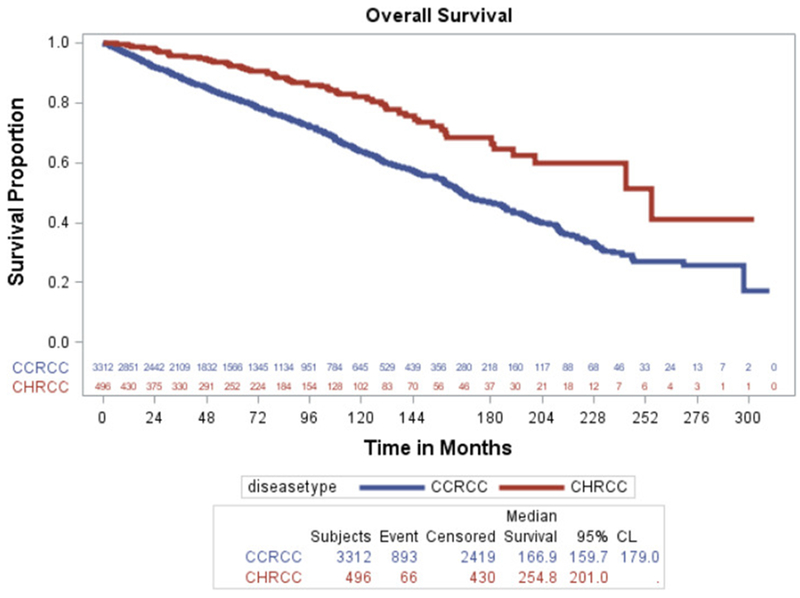
Kaplan-Meier estimates for OS compared for both cohorts.
In univariate analysis of the ChRCC cohort, larger tumor size and sarcomatoid status were significantly associated with adverse RFS and OS. Patient age showed significance for OS and necrosis for RFS only (Supplementary Table 3). In clear cell RCC, tumor size, sarcomatoid status, but also age, gender and BMI showed significant effects on OS and RFS (Supplementary Table 4). In multivariate analyses, when controlled for age, tumor size, sarcomatoid status, procedure type, T stage and BMI, ChRCC patients have better RFS and OS (HR 0.37, 95% CI 0.22,0.63, p=0.002 and HR 0.56, 95% CI 0.42,0.73, p<0.001 respectively) (Tables 2 and 3).
Table 2.
Multivariate analysis for OS. Increased age or larger tumor size, having a sarcomatoid tumor, and a radical nephrectomy and T stage 3 or 4 have a negative effect on overall survival. When all of those are controlled a patient with chRCC has a higher likelihood of survival than a patient with ccRCC.
| Parameter | Hazard Ratio | CI Lower | CI Upper | p-value |
|---|---|---|---|---|
| Histology (chRCC vs ccRCC) | 0.556 | 0.424 | 0.729 | <.0001 |
| Age at Nephrectomy | 1.047 | 1.039 | 1.056 | <.0001 |
| Max Tumor Size | 1.089 | 1.061 | 1.118 | <.0001 |
| Procedure type (Radical vs. Partial Nephrectomy) | 1.603 | 1.269 | 2.025 | <.0001 |
| Sarcomatoid Differentiation | 3.478 | 2.631 | 4.597 | <.0001 |
| 2010 pT Stage (T3 or T4 vs. T1 or T2) | 1.743 | 1.432 | 2.121 | <.0001 |
Table 3.
Multivariate analysis for RFS. Increased age or tumor size, decreased BMI, a sarcomatoid tumor, a radical nephrectomy, and T stages 3 or 4 have a negative effect on overall survival. When all of those are controlled a patient with chRCC has a higher likelihood of no metastatic recurrence than a patient with ccRCC.
| Parameter | Hazard Ratio | CI Lower | CI Upper | p-value |
|---|---|---|---|---|
| Histology (chRCC vs ccRCC) | 0.373 | 0.220 | 0.631 | 0.0002 |
| Age at Nephrectomy | 1.011 | 1.002 | 1.021 | 0.0231 |
| BMI | 0.981 | 0.967 | 0.994 | 0.0058 |
| Max Tumor Size | 1.130 | 1.102 | 1.160 | <.0001 |
| Procedure type (Radical vs. Partial Nephrectomy) | 2.621 | 1.895 | 3.627 | <.0001 |
| Sarcomatoid differentiation | 1.130 | 1.102 | 1.160 | <.0001 |
| 2010 pT Stage (T3 or T4 vs. T1 or T2) | 2.370 | 1.792 | 3.134 | <.0001 |
Discussion
Recent advances in the genomic understanding of chromophobe renal cell carcinoma have highlighted the differences of this uncommon histological variant compared to the other kidney cancer subtypes6, 21. Due to the fact that this is a relatively rare and frequently indolent form of RCC, there are few studies that have examined prognostic features in chromophobe renal cell carcinoma in regards to the risk of developing metastatic disease.
The identification of reliable clinical markers for increased risk for recurrence can improve treatment strategies and necessitate closer follow-up after surgery in selected patients. In fact, given the current lack of systemic treatment options specifically tailored for chromophobe renal cell carcinoma, patients with high-risk features should be carefully monitored to allow e.g. metastasectomy. In future, assessment of underlying molecular drivers of aggressive disease by genomic testing21 of patients with high-risk chromophobe renal cell carcinoma, may allow treatment with novel target agents.
Previous studies have tried to investigate the oncological outcomes and predictive clinical parameters for survival in chromophobe renal cell carcinoma patients. The largest series of 291 cases was presented by the collaborative SATURN dataset group and identified gender, pathological tumor stage and sarcomatoid differentiation as predictors for reduced RFS and CSS8. Similar results concerning the relevance of pathological tumor stage and sarcomatoid differentiation were achieved by several study groups 4, 23, 24, including our own group25; further, this work and our group’s previous results showed that tumor necrosis was associated with unfavourable clinical outcomes4, 15. A direct comparison of OS and CSS in 109 cases of ChRCC and 901 clear cell RCC cases was recently presented by Frees et al11 underscoring the favorable outcome of ChRCC. An interesting characteristic of chromophobe renal cell carcinoma was also demonstrated in an analysis of the Surveillance Epidemiology and End Results (SEER) database: chromophobe renal cell carcinoma was most commonly seen in younger female patients within the non-clear cell RCC group12. A substantial limitation of all previous studies on chromophobe renal cell carcinoma outcomes is the relatively small number of cases, despite multi-institutional efforts. Thus, the limited number of events has likely underpowered most of the previous studies. We therefore intended to analyze the to our knowledge largest dataset on surgically treated chromophobe renal cell carcinoma to confirm the commonly indolent clinical behavior of chromophobe renal cell carcinoma and identify variables associated with decreased survival.
In the present study we confirmed that chromophobe renal cell carcinoma is associated with a more favorable clinical outcome and a lower propensity for metastatic development than clear cell RCC, but we were also able to identify risk factors associated with metastatic development. Increased size and sarcomatoid differentiation of ChRCC tumors lead to lower RFS and OS. Interestingly, in our large series chromophobe renal cell carcinoma was diagnosed more often in females and younger patients compared with clear cell RCC. We further observed that in clear cell RCC, lower BMI and male gender were associated with decreased survival, but this was not the case for chromophobe renal cell carcinoma.
Despite being a single-institutional study, our tertiary care center provides an outstanding expertise of dedicated genitourinary-pathologists for histological subtypization of surgical specimens. Further, we can rely on the experience of multiple surgeons specialized on RCC, minimizing the biases typical of the results from single-center settings. In addition, our study spans over three decades of follow-ups, but the development of the surgical techniques was accounted for, emphasizing the relevance of our results in the current treatment management. However, limiting the assessment of chromophobe renal cell carcinoma outcome to a surgical cohort can potentially underestimate the overall disease aggressiveness.
The major limitation of our work is similar to previous publications on chromophobe renal cell carcinoma. The retrospective nature of the study and the small number of metastatic events limit the power of our statistical analysis. Further, the long span of time of the patient’s inclusion and a large number of missing information regarding the actual cause of death in the chromophobe renal cell carcinoma cohort prevented the evaluation of cancer specific survival.
Our results demonstrate the indolent nature of most chromophobe renal cell carcinoma tumors with important exceptions being large tumors and those with sarcomatoid differentiation. Patients with these risk factors require a more stringent follow-up schedule due to their high-risk of recurrence. Affected patients may also be considered for additional genomic testing and inclusion in clinical studies should be promoted.
Conclusion
Our results demonstrate the indolent nature of most chRCC tumors with important exceptions being large tumors and those with sarcomatoid differentiation. Patients with these risk factors require a more stringent follow-up schedule due to their high-risk of recurrence. Such patient may also be considered for additional genomic testing and consideration for clinical studies of adjuvant therapy or systemic treatment should be promoted.
Supplementary Material
Figure 2.
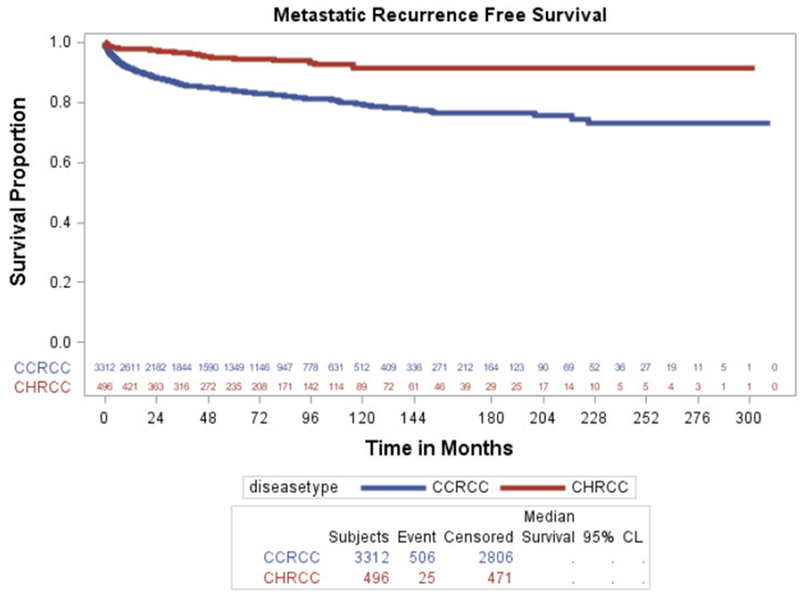
Kaplan-Meier estimates for RFS compared for both cohorts.
Clinical Practice Points.
Chromophobe renal cell carcinoma is a rare and usually a relatively indolent form of kidney cancer with low risk of metastatic development.
We examined the to our knowledge, largest institutional cohort of chRCC patients (n=496) and compared outcomes to a comparable cohort of clear cell RCC patients (n=3312) from our institution.
In our study we show that if chRCC patients initially present with large sized kidney tumors, or with sarcomatoid features the outcome are significantly worse due to metastatic development.
These characteristics support closer follow-up after curative surgery to identify and promptly treat patients with higher risk of metastatic disease.
Acknowledgements
JC was sponsored by the German Research Foundation (DFG) Grant CA1403/1-1. BJM was supported by the Ruth L. Kirschstein National Research Service Award T3to2CA082088. BJM, JAC and AAH were supported by the Sidney Kimmel Center for Prostate and Urologic Cancers and the NIH/NCI Cancer Center Support Grant P30 CA008748. JJH was supported by the Jill and Jeffrey Weiss Fund to the Cure of Kidney Cancer and J. Randall & Kathleen L. MacDonald Kidney Cancer Research Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Eble JN, Sauter G, Epstein JI, Sesterhenn IA. Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs. Lyon, France: IARC Press. 2004. [Google Scholar]
- 2.Hsieh JJ, Purdue MP, Signoretti S, et al. Renal cell carcinoma. Nat Rev Dis Primers. 2017;3:17009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur Urol. 2016;70:93–105. [DOI] [PubMed] [Google Scholar]
- 4.Amin MB, Paner GP, Alvarado-Cabrero I, et al. Chromophobe renal cell carcinoma: histomorphologic characteristics and evaluation of conventional pathologic prognostic parameters in 145 cases. Am J Surg Pathol. 2008;32:1822–1834. [DOI] [PubMed] [Google Scholar]
- 5.Reuter VE, Presti JC Jr. Contemporary approach to the classification of renal epithelial tumors. Semin Oncol. 2000;27:124–137. [PubMed] [Google Scholar]
- 6.Davis CF, Ricketts CJ, Wang M, et al. The somatic genomic landscape of chromophobe renal cell carcinoma. Cancer Cell. 2014;26:319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patard JJ, Leray E, Rioux-Leclercq N, et al. Prognostic value of histologic subtypes in renal cell carcinoma: a multicenter experience. J Clin Oncol. 2005;23:2763–2771. [DOI] [PubMed] [Google Scholar]
- 8.Volpe A, Novara G, Antonelli A, et al. Chromophobe renal cell carcinoma (RCC): oncological outcomes and prognostic factors in a large multicentre series. BJU Int. 2012;110:76–83. [DOI] [PubMed] [Google Scholar]
- 9.Crotty TB, Farrow GM, Lieber MM. Chromophobe cell renal carcinoma: clinicopathological features of 50 cases. J Urol. 1995;154:964–967. [DOI] [PubMed] [Google Scholar]
- 10.Peyromaure M, Misrai V, Thiounn N, et al. Chromophobe renal cell carcinoma: analysis of 61 cases. Cancer. 2004;100:1406–1410. [DOI] [PubMed] [Google Scholar]
- 11.Frees S, Kamal MM, Knoechlein L, et al. Differences in Overall and Cancer-specific Survival of Patients Presenting With Chromophobe Versus Clear Cell Renal Cell Carcinoma: A Propensity Score Matched Analysis. Urology. 2016;98:81–87. [DOI] [PubMed] [Google Scholar]
- 12.Daugherty M, Blakely S, Shapiro O, Vourganti S, Mollapour M, Bratslavsky G. Chromophobe Renal Cell Carcinoma is the Most Common Nonclear Renal Cell Carcinoma in Young Women: Results from the SEER Database. J Urol. 2016;195:847–851. [DOI] [PubMed] [Google Scholar]
- 13.Zhao PJ, Chen XP, Li XS, et al. Chromophobe renal cell carcinoma: analysis of 53 cases. J Cancer Res Clin Oncol. 2012;138:451–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cindolo L, de la Taille A, Schips L, et al. Chromophobe renal cell carcinoma: comprehensive analysis of 104 cases from multicenter European database. Urology. 2005;65:681–686. [DOI] [PubMed] [Google Scholar]
- 15.Przybycin CG, Cronin AM, Darvishian F, et al. Chromophobe renal cell carcinoma: a clinicopathologic study of 203 tumors in 200 patients with primary resection at a single institution. Am J Surg Pathol. 2011;35:962–970. [DOI] [PubMed] [Google Scholar]
- 16.Keizman D, Sarid D, Lee JL, et al. Outcome of Patients With Metastatic Chromophobe Renal Cell Carcinoma Treated With Sunitinib. Oncologist. 2016;21:1212–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maiti A, Brown RE, Corn PG, et al. Antitumor Response to Combined Antiangiogenic and Cytotoxic Chemotherapy in Recurrent Metastatic Chromophobe Renal Cell Carcinoma: Response Signatures and Proteomic Correlates. Clin Genitourin Cancer. 2016;14:e187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rouvinov K, Osyntsov L, Shaco-Levy R, Baram N, Ariad S, Mermershtain W. Rapid Response to Nivolumab in a Patient With Sarcomatoid Transformation of Chromophobe Renal Cell Carcinoma. Clin Genitourin Cancer. 2017. [DOI] [PubMed] [Google Scholar]
- 19.Kroeger N, Xie W, Lee JL, et al. Metastatic non-clear cell renal cell carcinoma treated with targeted therapy agents: characterization of survival outcome and application of the International mRCC Database Consortium criteria. Cancer. 2013;119:2999–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voss MH, Molina AM, Chen YB, et al. Phase II Trial and Correlative Genomic Analysis of Everolimus Plus Bevacizumab in Advanced Non-Clear Cell Renal Cell Carcinoma. J Clin Oncol. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Casuscelli J, Weinhold N, Gundem G, et al. Genomic landscape and evolution of metastatic chromophobe renal cell carcinoma. JCI Insight. 2017;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kovacs G, Akhtar M, Beckwith BJ, et al. The Heidelberg classification of renal cell tumours. J Pathol. 1997;183:131–133. [DOI] [PubMed] [Google Scholar]
- 23.Sharma R, Kaushal V. Sarcomatoid chromophobe renal cell carcinoma: A rare entity with prognostic significance. J Cancer Res Ther. 2015;11:1030. [DOI] [PubMed] [Google Scholar]
- 24.Lauer SR, Zhou M, Master VA, Osunkoya AO. Chromophobe renal cell carcinoma with sarcomatoid differentiation: a clinicopathologic study of 14 cases. Anal Quant Cytopathol Histpathol. 2013;35:77–84. [PubMed] [Google Scholar]
- 25.Ged Y, Chen YB, Knezevic A, et al. Metastatic Chromophobe Renal Cell Carcinoma: Presence or Absence of Sarcomatoid Differentiation Determines Clinical Course and Treatment Outcomes. Clin Genitourin Cancer. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


