Abstract
Desmetramadol is an investigational analgesic consisting of (+) and (−) enantiomers of the tramadol metabolite O-desmethyltramadol (M1). Tramadol is racemic and exerts analgesia by monoaminergic effects of (−)-tramadol and (−)-M1, and by the opioid (+)-M1. Tramadol labeling indicates cytochrome P450 (CYP) isozyme 2D6 ultrarapid metabolizer can produce dangerous (+)-M1 levels, and CYP2D6 poor metabolizers insufficient (+)-M1 for analgesia. We hypothesized that desmetramadol could provide the safety and analgesia of tramadol without its metabolic liabilities. We conducted consecutive double-blind, randomized, placebo-controlled, 3 segment cross-over trials A and B to investigate the steady-state pharmacokinetics and analgesia of 20 mg desmetramadol and 50 mg tramadol in 103 healthy participants without (n = 43) and with (n = 60) cotreatment with the CYP inhibitor paroxetine. In the absence of CYP inhibition (trial A), 20 mg desmetramadol and 50 mg tramadol dosed every 6 hours gave equivalent steady-state (+)-M1, similar adverse events, and analgesia significantly greater than placebo, but equal to each other. In trial B, CYP inhibition significantly depressed tramadol steady-state (+)-M1, reduced its adverse events, and led to insignificant analgesia comparable with placebo. In contrast, CYP inhibition in trial B had no deleterious effect on desmetramadol (+)-M1 or (−)-M1, which gave significant analgesia as in trial A and superior to tramadol (P = .003). Desmetramadol has the safety and efficacy of tramadol without its metabolic liabilities.
Keywords: Desmetramadol, tramadol, poor metabolizer, ultrarapid metabolizer, metabolism
It has been recommended that the morphine milligram equivalent dose in patients receiving opioid therapy be decreased to reduce opioid-related overdose and death.15,20,30 There are unfortunately limited pharmacologic options for patients seeking an alternative to schedule II opioids who still require effective analgesia. A critical challenge, therefore, exists to identify analgesic options for those suffering from pain that are safer and decrease the risk of treatment-related death.
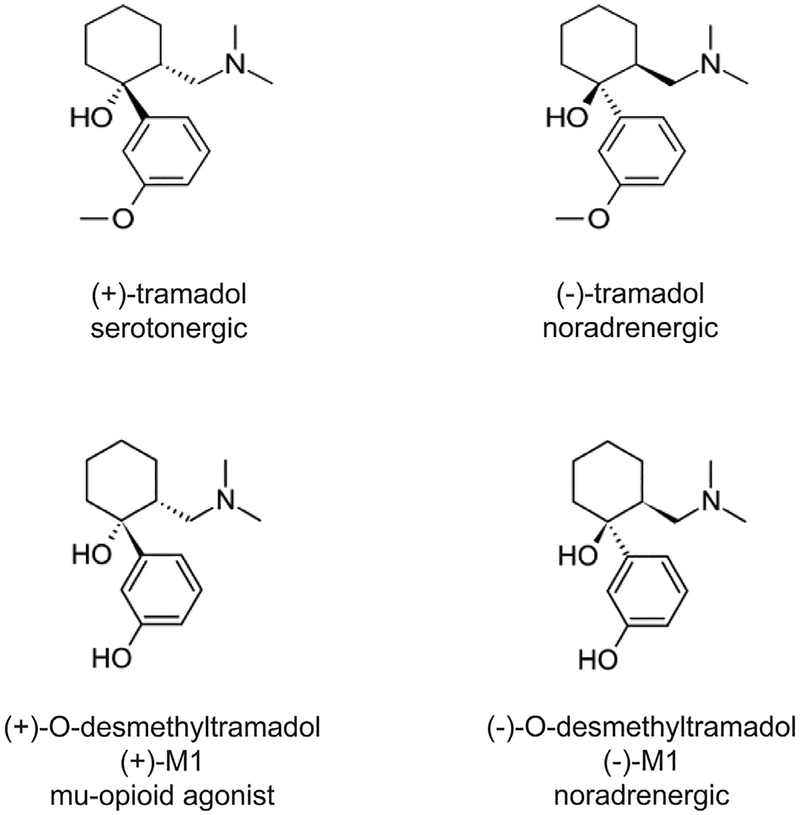
Tramadol is approved in the United States for moderate to moderately severe pain and, as a schedule IV analgesic, is less prone to abuse than schedule II opioids, as well as being safer.10,86,87 Tramadol is extensively metabolized by CYP enzymes,88 and its analgesic activity in humans is thought to be due to a mixed mechanism of 1) norepinephrine reuptake inhibition by the negative enantiomers of tramadol and the O-desmethyltra-madol metabolite ((−)-M1), and 2) agonism at the m-opioid receptor by the positive M1 enantiomer (+)-M1 (Fig 1).29,58 The (+)-tramadol enantiomer inhibits uptake by the serotonin transporter.16,58 It is thought to have a limited role in analgesia,1,58,73 but increases the potential for adverse effects and the serotonin syndrome when taken with serotonergic antidepressants that are also CYP2D6 inhibitors.53
Figure 1.
Chemical structure and dominant pharmacology of each enantiomer of tramadol and the M1 metabolite. An enantiomer is each of a pair of molecules that are mirror images of each other.
Schedule II opioid lethality is caused by a cessation in the drive to breathe mediated by agonism of m-opioid receptors in the brain stem.63 Tramadol is safer than schedule II opioids because it does not cause clinically significant respiratory depression at either therapeutic or supratherapeutic doses.3,29,34,39,48,66,67,75,76,83 Whereas a lethal oral dose of fentanyl, oxycodone, and hydrocodone in opioid-naÿve and nontolerant participants can be 2, 40, and 90 mg, respectively,13,57,81 a lethal tramadol dose is >5 g.12,62,64 Reports of lethal overdoses owing to tramadol alone are, therefore, rare.11,12,62,64,69 It is not understood why tramadol spares respiration. The (+)-M1 metabolite has nearly the in vitro affinity of morphine at the human m-opioid receptor.29,58 Tapentadol is the only other marketed analgesic with the same mixed mechanism pharmacology as tramadol, but embodied in a single parent molecule. Tapentadol has no activity at the serotonin reuptake transporter, but has similar functional potency in vitro to the (+)-M1 and (−)-M1 enantiomers at the μ-opioid receptor (median effective concentration of .67 μmol/L vs .86 μmol/L) and norepinephrine transporter (Ki of .48 μmol/L vs .86 μmol/L), respectively.58 However, clinically tapentadol causes similar respiratory depression to oxycodone and other schedule II agents.80,82 Metabolism acting as a possible protective saturable ceiling mechanism does not serve as an explanation for tramadol’s safety either, because systemic M1 is dose-proportional throughout the range from therapeutic to lethal, where lethal M1 blood concentrations have been found to exceed therapeutic levels (50−100 ng/mL) by ≤50-fold.11,35
The U.S. Food and Drug Administration (FDA) recently amended the tramadol label to alert prescribers to the metabolic liabilities that can arise from unsafe M1 levels in patients who are CYP2D6 ultrarapid metabolizer,35,38,50 the lack of efficacy that can arise in patients who are metabolically deficient owing to either coprescribed inhibitors of CYP2D6 or suboptimal CYP2D6 genetics, and the excess adverse opioid effects that can arise if a coprescribed CYP2D6 inhibitor is discontinued without lowering the tramadol dose.35,55,70,71,84 The prevalence of the ultrarapid metabolizer genotype ranges from 4% in Caucasians to 11% in Middle Easterners.46 An analysis of >9,000 prescription claims found tramadol and CYP2D6 inhibitors coprescribed 21% of the time.65 In patients not coprescribed a CYP2D6 inhibitor, 5−10% are poor metabolizers and carry no functional CYP2D6 alleles, and another 2−11% are intermediate metabolizers who carry 1 reduced-function and 1 nonfunctional allele.9 Abnormal tramadol metabolism is, therefore, a common occurrence, likely affecting more than one-third of patients. The clinical impact is significant; tramadol was the second most prescribed opioid in the United States, with 41 million prescriptions dispensed in 2017.18,28 Eliminating the metabolic liabilities of tramadol could broadly expand its usefulness as a safer alternative to schedule II opioids.
Desmetramadol (Syntrix Pharmaceuticals, Auburn, Washington) is the racemic M1 tramadol metabolite formulated to orally deliver (+)-M1 and (−)-M1 into the systemic circulation with kinetics that replicate ideal tramadol metabolism but without requiring CYP enzymes.93 We hypothesized that desmetramadol could provide the safety and analgesic profile of tramadol without its metabolic liabilities. It was unknown if desmetramadol could provide this profile in metabolically unselected participants (ie, participants having any possible CYP2D6 genotype) and in metabolically deficient participants. The objectives of this first-in-man study were, therefore, to demonstrate that i) desmetramadol and tramadol doses giving equal plasma M1 yield equal analgesia in metabolically unselected participants, but that ii) the same doses in participants made metabolically deficient by the CYP enzyme inhibitor paroxetine yield greater plasma M1 and greater analgesia for desmetramadol than for tramadol. Paroxetine is a strong Zebala et al inhibitor of CYP2D6 and CYP2B6.33,79 The present study, is to our knowledge, the first human assessment of desmetramadol and the first to report its steady-state pharmacokinetics, efficacy, and safety versus placebo and versus tramadol in metabolically unselected and deficient populations.
Methods
Study Design
The study design consisted of 2 consecutive randomized, double-blind, 3-period cross-over, placebo-and active comparator-controlled, single-center trials A and B performed between August 2014 and October 2014 and between October 2017 and December 2017 and conducted in a clinical research unit in Salt Lake City, Utah (ClinicalTrials.gov Identifiers: and ). The study was approved by an independent ethics committee and was conducted in accordance with the Declaration of Helsinki and other applicable guidelines, laws, and regulations. Written informed consent was obtained from all participants.
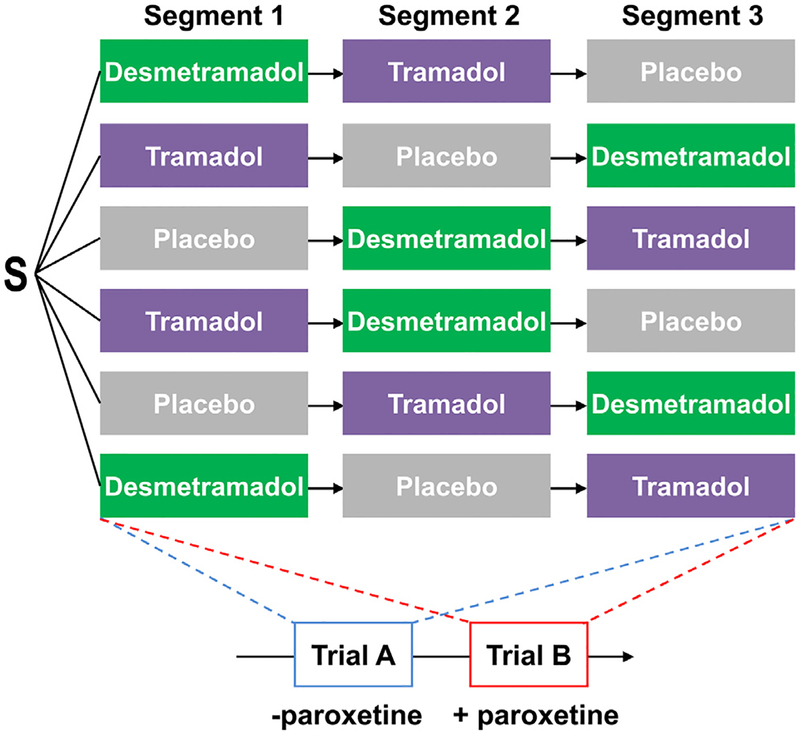
After screening, participants in both trials A and B were randomized to 1 of 6 possible treatment sequences of placebo, 50 mg tramadol (Ultram; Janssen Ortho, LLC, Gurabo, Puerto Rico) and 20 mg desmetramadol (Syntrix Pharmaceuticals; Fig 2). Nine doses of each study drug were given every 6 hours in each of the 3 treatment segments, with segments separated by 1 week in trial A and 2 weeks in trial B. Participants stayed at the clinical research unit during the entirety of each treatment segment and were discharged during the time between segments and at the end of the third segment. For 1 hour before and 1 hour after doses 8 and 9, the participant’s diet (oral intake) was limited to clear liquids only. Participants in trial B also received 3 consecutive 20 mg daily doses of paroxetine beginning 1 day before each treatment segment. Paroxetine levels were quantified by sampling blood immediately before the ninth dose of study drug in each treatment segment (Quest Diagnostics, West Valley City, Utah). Blood was collected to test the CYP2D6 genotype after the ninth dose of study drug of the first segment in trial A and at screening in trial B. The end of the study in both trials consisted of a telephone follow-up 1 week after the end of the third segment.
Figure 2.
Study design. Participants were randomized in trials A and B to all 6 possible treatment sequences with each segment separated by 1 week (trial A) or 2 weeks (trial B). Nine doses of each study drug were given every 6 hours in each segment to reach steady-state levels and then cold-induced pain was assessed after the ninth dose. All participants in trial B additionally received daily paroxetine beginning 1 day before each treatment segment. S, participants randomized.
Randomization to the 6 treatment sequences was in a ratio of 1:1:1:1:1:1 using a computer-generated random list of permuted blocks of 6. Blinding of study drug was by overencapsulation.
Measurements were made to quantify steady-state plasma M1 and tramadol enantiomers by sampling blood immediately before and after the ninth dose of study drug. Cold-induced pain was measured in the cold pressor test after the ninth dose of study drug. Pupil diameter and abuse liability measures were assessed after the seventh dose of study drug in trial A. Adverse events (AEs) and vital signs were collected throughout each trial.
End Points and Formal Study Hypothesis
Trial A
The primary end point consisted of the steady-state minimum (Cssmin) and maximum (Cssmax) plasma concentrations of (+)-tramadol, (−)-tramadol, (+)-M1, and (−)-M1. Secondary end points consisted of cold-induced pain, safety, abuse liability, pupil diameter, and CYP2D6 genotype.
Trial B
The primary end point consisted of cold-induced pain perception or tolerance. Secondary end points consisted of Cssmin and Cssmax, and safety.
The formal study hypothesis was that bioequivalent and equianalgesic doses of tramadol and desmetramadol in trial A will produce significantly greater plasma (+)-M1 and superior analgesia for desmetramadol compared with tramadol in trial B, where participants are metabolically deficient.
Participants
Eligible participants were aged 18 to 55 years, of general good health, had a tolerance to cold-induced pain of ≥20 seconds and ≤120 seconds, and in trial B had a CYP2D6 genotype consistent with an intermediate metabolizer phenotype or normal metabolizer phenotype. Each participant’s CYP2D6 genotype was determined using a multiplex PCR and allele-specific primer extension assay (xTAG Mutation Detection, Luminex Molecular Diagnostics, Austin, Texas) that identifies 17 variants (*1, *2, *3, *4, *5, *6, *7, *8, *9, *10, *11, *12, *14, *15, *17, *41, and gene duplication) and 2 gene rearrangements (Genelex, Inc., Seattle, Washington). The assay covers >93−97% of poor metabolizer phenotypes and has an analytical specificity and sensitivity for detection of these mutations of >99%. Trial A conserved statistical power to detect tramadol and desmetramadol analgesia by enrolling only males because females exhibit large variation and temporal instability to repeated cold-induced pain.41 Further criteria for key inclusion and exclusion criteria are presented in Table 1.
Table 1.
Key Inclusion and Exclusion Criteria
| Key Inclusion Criteria | Key Exclusion Criteria |
|---|---|
| Healthy male and female (trial B) adults ≤55 years old with normal blood pressure, pulse, and respiration | History of seizures, epilepsy, or recognized increase risk of seizure |
| Tolerance to cold-induced pain of ≥20 and ≤120 seconds | History of cirrhosis or laboratory evidence of liver disease |
| Negative urine for substances of abuse | Known or suspected alcohol or drug abuse within the past 6 months |
| Normal or intermediate CYP2D6 metabolizer (trial B) | Inhibitors of monoamine oxidase, serotonin and/or norepinephrine reuptake, and other medications or supplements known to induce or inhibit drug metabolism or that may affect the serotonergic neurotransmitter system |
| Adequate hematologic and liver function per predefined limits | Ethanol, grapefruit, grapefruit-related citrus fruits (eg, Seville oranges, pomelos), grapefruit-related juices or other new medication |
| Cockcroft-Gault glomerular filtration rate of ≥60 mL/min and urinalysis with ≤+1 glucose, +1 ketones, and +1 protein | Pregnant or breast feeding (trial B) |
| Body mass index of 18.0–32 kg/m2 | |
| If female of childbearing potential, must use adequate contraception | |
| Electrocardiogram without clinically significant changes | |
| Negative serology for HIV, hepatitis B surface antigen, and hepatitis C virus antibody |
Designation of CYP2D6 Phenotype
Each participant’s CYP2D6 genotype was reported using the star (*) allele nomenclature and used to predict metabolizer phenotype.9 Each star (*) allele or haplotype is defined by the presence of a specific combination of single-nucleotide polymorphisms and/ or other sequence alterations within the CYP2D6 gene locus. The *1 allele is defined as wild type (see www.pharmvar.org for other alleles). Each CYP2D6 genotype is reported as a diplotype, which includes 1 maternal and 1 paternal allele (eg, *1/*4). Participants with >2 copies of the CYP2D6 gene are denoted by an “xN” after the allele designation; for example, a *2 × 2 haplotype is a duplication of the *2 allele. Each CYP2D6 allele designation was translated into an activity score, that is, 0 for nonfunctional (eg, *3, *4, or *5), .5 for reduced function (eg, *9, *10, or *17), or 1.0 for fully functional (eg, *1, *2, or *27).25 The sum of the activity scores for each allele in the diplotype determines the participant’s overall CYP2D6 activity score; for example, a *1/*1 genotype has an activity score of 2.0, a *3/*9 genotype has an activity score of .5, and a *3/*5 genotype has an activity score of .0 (www.pharmgkb.org/vip/PA166170264). Participants with an activity score of 0 were designated poor metabolizers (individuals carrying no functional alleles), those with a score of .5 or 1.0 were designated intermediate metabolizers, those with a score of 1.5 or 2.0 were designated normal metabolizers (individuals carrying 2 alleles with full function or 1 full function and 1 reduced function allele), and those participants with a score of >2.0 were designated as ultrarapid metabolizers (individuals carrying >2 copies of functional alleles).
Assessments
Steady-State Pharmacokinetics
The Cssmin and Cssmax of (+)-tramadol, (−)-tramadol, (+)-M1, and (−)-M1 were measured by collecting blood immediately before the ninth study drug dose and at 1.0, 1.5, 2.0, 2.5, and 4.0 hours afterward. An additional sample was taken at 8.0 hours in trial B to measure the half-life (t1/2). The tramadol and M1 enantiomers were quantified using a chiral liquid chromatography mass spectroscopy method using a Lux Cellulose-2 (Phenomenex, California) chromatographic column and positive atmospheric pressure chemical ionization mode while operating the instrument in the multiple reaction monitoring mode. The assay was validated in accordance with FDA guidance. The lower limit of quantification for each enantiomer was 5.0 ng/mL. The calibration range was 5−1,000 ng/mL for each enantiomer. Assay accuracy for (+)-M1 was 99.3−103% and the precision (relative standard deviation [SD]) was .8−3.6%. Assays were conducted in the bioanalytical laboratories of IITRI Life Sciences Group (Chicago, Illinois).
Pupillometry
Pupillary contraction measured by pupillometry is a pharmacodynamic marker of target engagement by opioids including tramadol.24 Pupil diameter was measured with a NeurOptic VIP 200 pupillometer (Laguna Hills, California) before the first dose and after the seventh dose of each treatment segment. Pupillometry was not performed in trial B because paroxetine causes pupil dilation that confounds the contractionary effect of opioids.49
Abuse Liability
Opioids induce positive responses in subjective measures of abuse in healthy participants who are not abusing drugs.6–8,54,78,90–92 Abuse liability assessments were performed in trial A after the seventh dose that consisted of a 100-mm visual analog scale (VAS) for each of drug liking−disliking, take drug again, and strength of drug effect measures.
Analgesia
The cold pressor test is an established test model for evaluating opioid induced analgesia including tramadol.37,40,43 Pain intensity (0−10 VAS) at 30 seconds and at first perception, and time (seconds) to hand withdrawal and first pain were determined at 1, 2, and 3 hours after the ninth dose of each study drug and averaged.
Safety and Tolerability
Assessments of the safety and tolerability of desmetramadol and tramadol included 1) AEs and serious AEs, 2) vital signs, 3) laboratory analyses, and 4) study drug discontinuation. AEs were allocated to a study drug if they occurred after its first dose and before either the first dose of the next study drug or the end of the study. The AE relationship to blinded study drug was assessed by the investigator as either not related, unlikely related, possibly related, probably related, or definitely related. An AE was drug-related if it was designated as possibly, probably, or definitely related. The severity of AEs were graded on an FDA-specified scale for healthy adult and adolescent volunteers.23 Vital signs included systolic and diastolic arterial blood pressures, pulse, and respiratory rate. Vital signs were obtained at screening baseline and before and after each study drug administration in trial A. In trial B, baseline vital signs were obtained once for each segment before paroxetine administration and then once after each paroxetine and study drug administration. Vital signs were obtained in trials A and B at the end of each treatment segment and before discharge.
Statistical and Computational Methods
The reported peak mean pain perception to cold before and after a single 50 mg dose of tramadol was used to power the first-in-man trial A (mean [SD] pain intensity before and after tramadol of 6.3 [2.0] and 5.0 [2.3] cm on a VAS, respectively).31 To provide ≥80% power in trial A to detect a −1.3-cm change in pain perception between desmetramadol and placebo, a sample size of 39 participants was planned. To provide ≥97% power in trial B to detect a −.5-cm change in pain perception between desmetramadol and tramadol at 30 seconds, a sample size of 60 participants was planned, as informed by trial A data. Formal statistical analysis plans were developed before unblinding trials A and B. All descriptive and inferential statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, North Carolina) or R version 3.0 (The R Foundation, Vienna, Austria). Continuous end points were analyzed using mixed-effects linear models. The appropriate covariance structure was selected using graphical tools and information criteria. In trial B, the overall analgesic analysis used a backward selection approach to determine the significant effects with treatment, segment, sequence, and gender as fixed effects and participant as a random effect nested within sequence. Segment was added as a fixed effect to find any significant first-order crossover effects. Otherwise, analyses used mixed effects linear models with treatment, segment, and sequence as fixed effects and participant as a random effect nested within sequence. Segment was again added as a fixed effect to find any significant first-order crossover effects. If significant treatment effects were present, least-squares means were compared between desmetramadol and placebo, or tramadol and placebo, using Dunnett’s procedure, and between desmetramadol and tramadol using a paired t-test. In addition to overall analyses, separate analyses for measures of analgesia were performed for males and females. The Cssmin was specified as the smallest and the Cssmax as the largest value for each analyte in a segment. Bioequivalence was computed using the log-transformed Cssmin and Cssmax values for each enantiomer and claimed when the 90% confidence interval (CI) of their ratio was .8 to 1.25.22,59 The t1/2 in trial B was computed using the elimination rate constant (ke) and analyte concentrations C4 and C8 at hour 4 (t4) and hour 8 (t8), respectively, where ke = (ln C4 − ln C8)/(t8 − t4) and t1/2 = .693/ke.
Missing data were confirmed to be missing completely at random and excluded without imputation. The hypothesized superior analgesia of desmetramadol to tramadol in trial B was tested using a 1-sided test at the 5% significance level. All other statistical comparisons were made using 2-sided tests at the 5% significance level and all CIs for bioequivalence were calculated with a 2-sided 90% confidence level.
The intention-to-treat (ITT) population included all participants who were randomized to treatment, and was stipulated to be the primary dataset for analysis and statistical conclusions of significance. The per-protocol population was composed of a subset of participants from the ITT population who completed the study with no major protocol deviations. A major protocol deviation was one that could adversely affect the rights, safety, or well-being of the participants and/or the quality and integrity of data. Protocol deviations were assigned as being major or minor before unblinding. The efficacy population in trial B constituted participants who received all drug doses and had cold pressor efficacy data from all 3 segments. A sensitivity analysis was performed by conducting the analyses for the ITT, per-protocol, and efficacy populations as defined. All results presented are for the ITT population; unless otherwise specified, results from the per-protocol and efficacy population analyses supported those for the ITT population. The safety population included all patients who received study drug. All safety analyses were performed on the safety population.
Results
Participant Disposition and Demographics
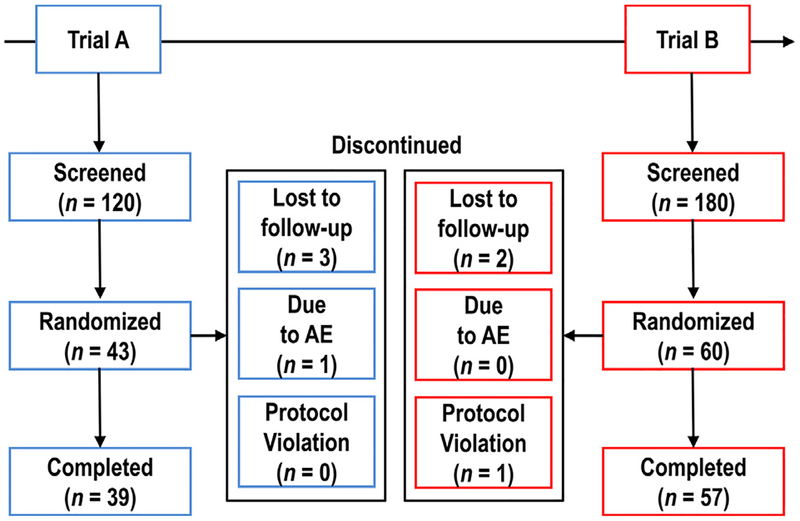
Of the 300 participants screened, 103 participants were randomized in consecutive trials A and B at 1 clinical research unit in the United States (Fig 3). All participants who were randomized (the ITT population) received treatment with study drug and 96 participants (93%) completed the study. A total of 7 of the 103 participants discontinued from the study after treatment was initiated. In the trial A cohort, 4 participants discontinued; 1 discontinued owing to AEs (grade 1 nausea, vomiting, and dizziness after 4 doses of desmetramadol in segment 1; no blood samples were collected and the participant did not advance to the remaining segments with placebo and tramadol) and 3 were lost to follow-up. In the trial B cohort, 1 participant was withdrawn because of a major protocol violation involving a urine test positive for a substance of abuse (cocaine) and 2 participants were lost to follow-up. Participants received 1,111 doses (96%) of 1,161 possible study drug doses in trial A, and received 1,575 doses (97%) of 1,620 possible study drug doses in trial B. Participants with missing study drug doses were evenly distributed across placebo (A, B = 2, 2), desmetramadol (A, B = 1, 1), and tramadol (A, B = 3, 2). All participants in trial B received all planned paroxetine doses while on study, or 525 of 540 (97%) possible doses. Most participants were Caucasian (92%) and baseline demographic characteristics such as age and body mass index were similar in both trial A and B cohorts (Table 2). Normal and intermediate CYP2D6 metabolizers constituted 96% and 100% of the trial A and B cohorts, respectively (for individual CYP2D6 genotypes, see Supplementary Table 1).
Figure 3.
Participant flow and disposition.
Table 2.
Demographics and CYP2D6 Phenotype
| Characteristics | Trial A (n = 43) |
Trial B (n = 60) |
|---|---|---|
| Age, years | 28.4 ± 8.0 | 28.0 ± 6.8 |
| Age range, years | ||
| Minimum | 21 | 18 |
| Maximum | 53 | 45 |
| Sex | ||
| Male | 43 (100) | 42 (70) |
| Female | 0(0) | 18(30) |
| Race | ||
| Caucasian | 39(91) | 56 (93) |
| Asian | 3(7) | 0 (0) |
| Black or African American | 0 (0) | 3(5) |
| American Indian or Alaska Native | 1 (2) | 1 (2) |
| Ethnicity | ||
| Hispanic or Latino | 6(14) | 7(12) |
| Not Hispanic or Latino | 37 (86) | 53 (88) |
| BMI, kg/m2 | 25.5 ± 3.1 | 24.8 ± 3.4 |
| BMI range, kg/m2 | ||
| Minimum | 19.5 | 18.9 |
| Maximum | 31.8 | 31.7 |
| CYP2D6 activity score, phenotype* | ||
| .0, Poor metabolizer | 1 (2) | 0 (0) |
| .5, Intermediate metabolizer | 0 (0) | 3 (5) |
| 1.0, Intermediate metabolizer | 17(41) | 19(32) |
| 1.5, Normal metabolizer | 7(17) | 9(15) |
| 2.0, Normal metabolizer | 16 (38) | 29 (48) |
| 3.0, Ultrarapid metabolizer | 1 (2) | 0 (0) |
NOTE: values are mean ± SD or number (%) unless otherwise noted.
Predicted from genotype. See Supplementary Table 1 for participant genotypes.
Steady-State Pharmacokinetics
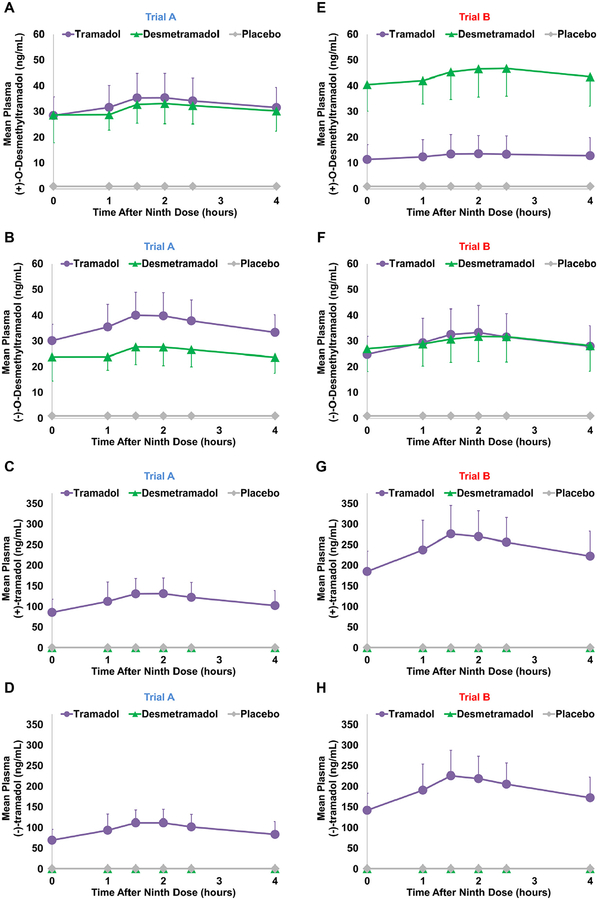
In the absence of paroxetine (trial A), 20 mg desmetramadol dosed every 6 hours replicated the mean steady-state plasma profile of (+)-M1 produced by 50 mg tramadol dosed at the same frequency (Fig 4A). The desmetramadol and tramadol mean Cssmin and Cssmax for (+)-M1 were statistically bioequivalent (mean [SD] = 28 [7] ng/mL vs 26 [6] ng/mL and 37 [10] ng/mL vs 36 [10] ng/mL; 90% CIs, .85−1.08 and .88 −1.13, respectively; Table 3). The Cssmin and Cssmax for (−)-M1 were 30% lower for desmetramadol compared with tramadol and just outside statistical bioequivalence (mean [SD] = 30 [6] ng/mL vs 21 [5] ng/mL and 42 [9] ng/mL vs 30 [9] ng/mL; 90% CIs, .69−.76 and .67 −.76, respectively; Fig 4B and Table 3). Desmetramadol produced no circulating tramadol enantiomers, as expected (Fig 4C and 4D).
Figure 4.
Mean steady-state plasma levels of (+)-M1 (A, E), (−)-M1 (B, F), (+)-tramadol (C, G), and (−)-tramadol (D, H) in trials A (n = 43) and B (n = 60). Bars are for SD and shown in 1 direction. Baseline points all below the quantitation limit.
Table 3.
Steady-State Pharmacokinetics and Paroxetine Levels
| Analyte | Trial A (n = 43) |
Trial B (n=60) |
||
|---|---|---|---|---|
| Tramadol | Desmetramadol | Tramadol | Desmetramadol | |
| (+)-M1 | ||||
| Cssmin, ng/mL, M (SD) | 28(7) | 26 (6) | 11 (6) | 38 (9) |
| 90% CI* | .85–1.08 | 3.4–4.3 | ||
| P value | <.001 | |||
| Cssmax, ng/mL, M (SD) | 37 (10) | 36(10) | 14 (8) | 51 (11) |
| 90% CI | .88–1.13 | 3.4–4.3 | ||
| P value | <.001 | |||
| t1/2, h, M (SD) | 18 (39) | 8 (6) | ||
| P value | .065 | |||
| (−)-M1 | ||||
| Cssmin, ng/mL, M (SD) | 30(6) | 21 (5) | 25 (7) | 25 (8) |
| 90% CI | .69-.76 | .95–1.09 | ||
| P value | .64 | |||
| Cssmax, ng/mL, M (SD) | 42 (9) | 30 (9) | 35(10) | 35(10) |
| 90% CI | .67-.76 | .93–1.07 | ||
| P value | .79 | |||
| t1/2, h, M (SD) | 12(8) | 7(5) | ||
| P value | <.001 | |||
| (+)-tramadol | ||||
| Cssmin, ng/mL, M (SD) | 85(33) | ND | 183 (49) | ND |
| Cssmax, ng/mL, M (SD) | 143 (36) | ND | 295 (62) | ND |
| t1/2, h, M (SD) | 8.6 (2.5) | ND | ||
| (−)-tramadol | ||||
| Cssmin, ng/mL, M (SD) | 68 (27) | ND | 140 (41) | ND |
| Cssmax, ng/mL, M (SD) | 122(32) | ND | 242 (56) | ND |
| t1/2, h, M (SD) | 7.2 (2.3) | ND | ||
| Paroxetine, ng/mL, M (SD) | NA | NA | 11 (8) | 12(9) |
Abbreviations: M, mean; NA, not applicable; ND, not detected.
The pharmacokinetic parameter is statistically bioequivalent if the 90% CI is within the range of .80 to 1.25.
Paroxetine given in 3 daily 20-mg doses in trial B produced a similar level of circulating paroxetine in tramadol and desmetramadol dosed segments (mean [SD] = 11 [8] ng/mL vs 12 [9] ng/mL, respectively; Table 3). Compared with trial A, paroxetine in trial B depressed tramadol (+)-M1 Cssmin (−61%) and Cssmax (−62%), but increased desmetramadol (+)-M1 Cssmin (46%) and Cssmax (41%; Fig 4E vs Fig 4A and Table 3). The paroxetine-induced changes in trial B caused desmetramadol Cssmin and Cssmax for (+)-M1 to each significantly exceed by 3.5-fold the corresponding tramadol Cssmin and Cssmax (mean [SD] = 38 [9] ng/mL vs 11 [6] a ng/mL and 51 [11] ng/mL vs 14 [8] ng/mL, respectively; P < .001; Table 3). The (+)-M1 t1/2 after tramadol dosing was double the t1/2 after desmetramadol dosing (mean [SD] = 18 [39] hours vs 8 [6] hours; P = .065).
Paroxetine resulted in comparatively smaller changes for (−)-M1 (Fig 4F vs Fig 4B). Compared with trial A, the (−)-M1 Cssmin and Cssmax were decreased 17% for tramadol and increased 14−16% for desmetramadol in the presence of paroxetine (Table 3). The paroxetineinduced changes in trial B had the net effect of making desmetramadol and tramadol Cssmin and Cssmax for (−)-M1 statistically bioequivalent (mean [SD] = 25 [7] ng/ mL vs 25 [8] ng/mL and 35 [10] vs 35 [10] ng/mL; 90% CIs, .95−1.09 and .93−1.07; Table 3). Like the positive enantiomer, the t1/2 of (−)-M1 in trial B was greater for tramadol compared with desmetramadol (mean [SD] = 12 [8] hours vs 7 [5] hours; P < .001).
As in trial A, desmetramadol produced no circulating tramadol enantiomers in trial B (Fig 4G and 4H). However, compared with trial A, the mean steady-state (+)-tramadol and (−)-tramadol plasma concentration profiles after tramadol dosing were increased 2-fold in the presence of paroxetine, as were the Cssmin and Cssmax (Fig 4G vs Fig 4C and Fig 4H vs Fig 4D; Table 3).
The Cssmin, Cssmax and t1/2 had no statistically significant sequence or segment effects in either trial A or trial B. There was no carryover from segment to segment in either trial A or trial B, as evidenced by no detectable M1 or tramadol enantiomers in placebo-treated segments, and no detectable tramadol enantiomers in desmetramadol treated segments.
Compared with mean tramadol (+)-M1 in the poor metabolizer and ultrarapid metabolizer of trial A, the mean desmetramadol (+)-M1 was increased 650% (41 ng/mL vs 6.3 ng/mL) and decreased 40% (22 ng/mL vs 36 ng/mL), respectively (Supplementary Fig 1).
Pupillometry and Abuse Measures
The mean predose pupil diameter in trial A was similar in the placebo, tramadol, and desmetramadol dosed segments (mean [SD] =6.1 [.9], 6.1 [.9], and 6.0 [1.1] mm, respectively; Table 4). After dosing, tramadol and desmetramadol each caused a significant decrease in pupil diameter compared with placebo (mean paired reduction [SD] = −.7 (.6) and −1.1 (.8) mm; each < .0001).
Table 4.
Pupil Diameter and Abuse Measures
| Trial A (n = 43) |
|||
|---|---|---|---|
| Placebo | Tramadol | Desmetramadol | |
| Pupil diameter, mm, M (SD) | |||
| Predose | 6.1 (.9) | 6.1 (.9) | 6.0 (1.1) |
| After seventh dose | 6.2 (1.0) | 5.5 (1.1) | 5.1 (1.2) |
| P value vs placebo | <.0001 | <.0001 | |
| Abuse Measures, 0–100 mm, M (SD) | |||
| Drug liking-disliking | 49(14) | 48(15) | 47 (25) |
| Pvalue vs placebo | NS | NS | |
| Take drug again | 49 (19) | 47(18) | 43 (26) |
| Pvalue vs placebo | NS | NS | |
| Strength of drug effect | 12(18) | 32 (29) | 29 (28) |
| Pvalue vs placebo | <.0001 | .0004 |
Abbreviations: M, mean; NS, not significant.
Tramadol and desmetramadol dosing did not cause mean responses in the drug liking−disliking and take drug again VASs to differentiate from placebo (Table 4). There was a significant treatment effect (P < .0001) in the strength of drug effect VAS and mean responses after tramadol and desmetramadol dosing were significantly elevated compared with placebo (mean [SD] = 32 [29] mm vs 12 [8] mm and 29 [28] mm vs 12 [8] mm; P < .001 and P = .0004, respectively). There were also significant segment (P = .004) and sequence (P = .034) effects in the strength of drug effect VAS (Supplementary Table 2).
Analgesia
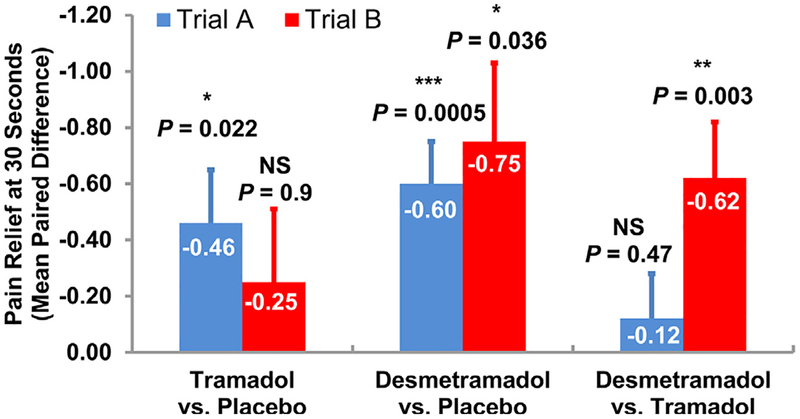
In the male population of trial A (n = 43), there was a similar and statistically significant decrease in average cold-induced pain perception at 30 seconds in participants treated with tramadol and desmetramadol compared with placebo (mean and standard error [SE] = −.46 [.19] and −.60 [.15]; P = .022 and P = .0005, respectively; Fig 5). There was no significant difference between tramadol and desmetramadol in trial A (P = .47). In the male population of trial B (n = 42) treated identically as trial A, except for the inclusion of paroxetine, tramadol failed to statistically differentiate from placebo (P = .90). In contrast, desmetramadol provided pain relief that was statistically superior to both placebo (P = .036) and tramadol (P = .003). The average change in paired pain scores between desmetramadol and placebo, and between desmetramadol and tramadol were similar (mean [SE] = −.75 [.28] and −.62 [.20], respectively; Fig 5). There was no significant treatment effect in the intensity of pain at first perception by participants in either trial A or trial B (Supplementary Table 3).
Figure 5.
Cold-induced pain at 30 seconds in trial A and trial B. Bars are SE of the mean. Abbreviation: NS, not statistically significant. *P < .05; **P < .01; ***P < .001.
The average duration of tolerance to pain in trial A was similar for desmetramadol (63 seconds) and tramadol (62 seconds), and each was significantly greater than placebo (mean paired increase relative to placebo [SE] = 12.6 [3.8] and 12.4 [4.4] seconds; P = .006 and P = .0076, respectively; Supplementary Table 3). In the presence of paroxetine, male participants in trial B tolerated pain 44% longer after desmetramadol than after tramadol (mean paired increase relative to placebo [SE] = 9.9 [1.9] seconds vs 6.9 [1.6] seconds; P < .001 and P = .001, respectively). In both trials, the average time to the first perception of pain was similar for tramadol and desmetramadol, and each was significantly greater than placebo.
There was no significant treatment effect in the trial B female population (see Discussion and Supplementary Table 3). There was no significant sequence or segment effect in any pain measure in either trial A or trial B. Results from the sensitivity analyses were consistent with the primary analgesic analyses.
Safety and Tolerability
AEs
One participant in trial A discontinued owing to AEs after administration of desmetramadol in the first segment. No participants discontinued from trial B owing to AEs.
After dosing with tramadol and desmetramadol in trial A, participants reported a similar qualitative and quantitative profile of drug-related AEs (Table 5). AEs were reported in 49% and 44% of participants after tramadol and desmetramadol, respectively, compared with 24% of participants after placebo. The 5 most common drug-related AEs after desmetramadol and tramadol in trial A were nausea, dizziness, headache, somnolence, and pruritus. The severity of drug-related AEs in trial A were all grade 1 except for a single participant who reported grade 2 headache and dizziness after tramadol.
Table 5.
Summary of Drug-Related AEs (Safety Population)
| Event, n (%) |
Trial A (n = 43; M = 43) |
Trial B (n=60; M = 42, F=18) |
||||
|---|---|---|---|---|---|---|
| Placebo | Tramadol | Desmetramadol | Placebo | Tramadol | Desmetramadol | |
| All AEs | 10 (24) | 20 (49) | 19 (44) | 16(27) | 30 (50) | 40 (67) |
| Specified AEs† | ||||||
| Nausea | 3(7) | 5(12) | 8(19) | 7(12) | 8(13) | 16(27) |
| M | 3(7) | 5(12) | 8(19) | 4(10) | 6(14) | 11 (26) |
| F | — | — | — | 3(17) | 2(11) | 5 (28) |
| Dizziness | 1 (2) | 5(12) | 6(14) | 1 (2) | 8(13) | 12 (20) |
| Male | 1 (2) | 5(12) | 6(14) | 1 (2) | 4(10) | 7(17) |
| Female | — | — | — | 0 (0) | 4(22) | 5 (28) |
| Somnolence | 0 (0) | 2 (5) | 3 (7) | 2 (3) | 4(7) | 10(17) |
| Male | 0 (0) | 2 (5) | 3 (7) | 1 (2) | 2 (5) | 4(10) |
| Female | — | — | — | 1 (6) | 2(11) | 6(33) |
| Headache | 0 (0) | 7(17) | 3 (7) | 3 (5) | 6(10) | 10(17) |
| Male | 0 (0) | 7(17) | 3 (7) | 0 (0) | 0 (0) | 4(10) |
| Female | — | — | — | 3(17) | 6 (33) | 6(33) |
| Vomiting | 0 (0) | 1 (2) | 1 (2) | 1 (2) | 2 (3) | 9(15) |
| Male | 0 (0) | 1 (2) | 1 (2) | 1 (2) | 2 (5) | 6(14) |
| Female | — | — | — | 0 (0) | 0 (0) | 3(17) |
| Presyncope | 0 (0) | 0 (0) | 0 (0) | 1 (2) | 6(10) | |
| Male | 1 (2) | 0 (0) | 0 (0) | 0 (0) | 1 (2) | 3 (7) |
| Female | — | — | — | 0 (0) | 0 (0) | 3(17) |
| Pruritus | 0 (0) | 4(10) | 3 (7) | 1 (2) | 1 (2) | 4 (7) |
| Male | 0 (0) | 4(10) | 3 (7) | 0 (0) | 0 (0) | 1 (2) |
| Female | — | — | — | 1 (6) | 1 (6) | 3(17) |
| Spasticity | 0 (0) | 1 (2) | 0 (0) | 0 (0) | 3 (5) | 3 (5) |
| Male | 0 (0) | 1 (2) | 0 (0) | 0 (0) | 3 (7) | 3 (7) |
| Female | — | — | — | 0 (0) | 0 (0) | 0 (0) |
| Feel abnormal | 0 (0) | 1 (2) | 0 (0) | 0 (0) | 2 (3) | 3 (5) |
| Male | 0 (0) | 1 (2) | 0 (0) | 0 (0) | 2 (5) | 2 (5) |
| Female | — | — | — | 0 (0) | 0 (0) | 1 (6) |
| Feel hot | 0 (0) | 0 (0) | 0 (0) | 1 (2) | 0 (0) | 3 (5) |
| Male | 0 (0) | 0 (0) | 0 (0) | 1 (2) | 0 (0) | 2 (5) |
| Female | — | — | — | 0 (0) | 0 (0) | 1 (6) |
| Euphoria | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2) | 2 (3) |
| Male | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2) | 1 (2) |
| Female | — | — | — | 0 (0) | 0 (0) | 1 (6) |
| Sweating | 0 (0) | 0 (0) | 0 (0) | 1 (2) | 1 (2) | 2 (3) |
| Male | 0 (0) | 0 (0) | 0 (0) | 1 (2) | 1 (2) | 2 (5) |
| Female | — | — | — | 0 (0) | 0 (0) | 0 (0) |
| Severe AEs | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Deaths | 0 (0) | 0 (0) | 0(0) | 0 (0) | 0 (0) | 0 (0) |
AE incidence of ≥3% of participants dosed with desmetramadol in trial B. See Supplementary Table 4 for drug-related AEs of lower incidence.
Drug-related AEs were reported in 27%, 50%, and 67% of participants in trial B after placebo, tramadol and desmetramadol, respectively (Table 5). Compared with the desmetramadol AE profile in trial B, the tramadol AE profile in the same participants featured less nausea (−50%), somnolence (−60%), headache (−40%), vomiting (−78%), presyncope (−83%), and pruritus (−75%). The tramadol AE profile resembled placebo except for an increased incidence of dizziness (8-fold placebo) and muscle spasticity (absent from placebo). The incidence of muscle spasticity after tramadol was the same as after desmetramadol. Female participants in trial B made up 30% of the safety population and 43% of the specified AEs. Drug-related AE severity in trial B was all grade 1 except for 5 participants who had grade 2 drug-related AEs after placebo (somnolence), tramadol (asthenia), and desmetramadol (nausea/vomiting, feeling hot, hypotension).
Less common drug-related AEs reported by participants in trial A and trial B are provided in Supplementary Table 4. No deaths or serious AEs were reported in either trial A or trial B.
Respiration and Other Vital Signs
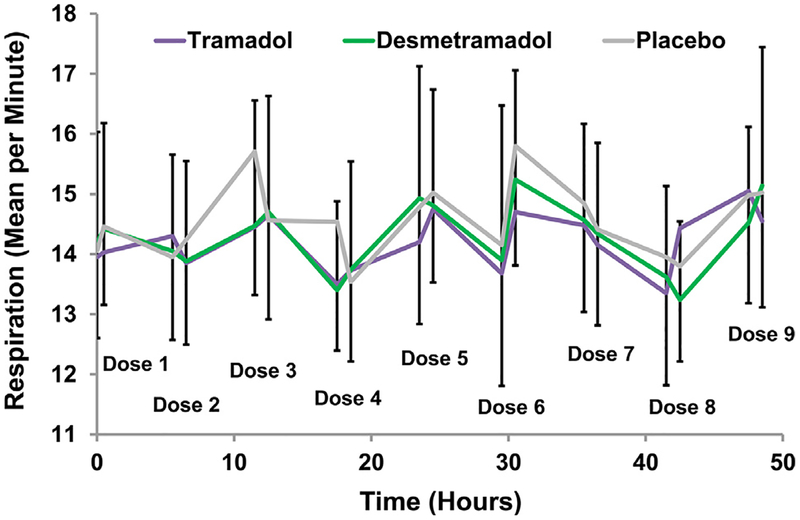
Respiration was assessed 1,744 times in trial A and 2,510 times in trial B. In trial A, respiratory rate was assessed before and after each of the 9 study drug doses in each of the 3 treatment segments (Fig 6). Desmetramadol and tramadol had no discernable predose versus postdose effect on respiratory rate compared with placebo, or compared with each other. Compared with baseline screening assessments in trial A, there was no effect of placebo, tramadol, or desmetramadol on the systolic or diastolic blood pressure, pulse, or respiration at the end of each treatment segment (Supplementary Table 5). Paired comparisons of respiration were made between tramadol and placebo, desmetramadol and placebo, and desmetramadol and tramadol with respect to the average postdose respiration. Average respiration after tramadol and desmetramadol were minimally reduced compared with placebo, and this decrease was statistically significant in the presence of paroxetine, but not in its absence (trial B mean paired difference [SD] = −.34 [.99] and −.30 [.90] breaths per minute; P = .004 and P = .012, respectively; Table 6). In addition to a significant treatment effect (P = .004), there was a significant segment effect (P < .001). There was no significant difference in respiration between desmetramadol and tramadol.
Figure 6.
Mean respiratory rate before and after each study drug administration in trial A. Bars are for SD for desmetramadol (up bars) and placebo (down bars). The horizontal separation of data points at each dose (ie, before and after dosing) are exaggerated to allow adequate visualization of the respiration rate before and after each dose.
Table 6.
Average Postdose Respiratory Rate
| Tramadol Versus Placebo |
Desmetramadol Versus Placebo |
Desmetramadol Versus Tramadol |
|
|---|---|---|---|
| Trial A, n = 43 | |||
| Respiratory rate, min−1, MPD (SD) | − .24 (.97) | − .14(.87) | .11 (.91) |
| NS | NS | NS | |
| Trial B, n = 60 | |||
| Respiratory rate, min−1, MPD (SD) | − .34 (.99) | − .30 (.90) | .05 (.85) |
| P = .004 | P = .012 | P = .345 |
Abbreviations: MPD, mean paired difference; NS, no significant treatment effect.
Discussion
Key Findings and Clinical Significance
Desmetramadol provided superior analgesia to tramadol in metabolically deficient participants, the same group in which tramadol efficacy was lost. Desmetramadol provided the same qualitative and quantitative safety profile as tramadol in metabolically unselected participants and the same as described in the FDA-approved tramadol label.35 Desmetramadol thus obviates the metabolic liabilities of tramadol while preserving its safety profile, because it does not rely on the activity of CYP enzymes for its activity. This property of desmetramadol is significant because tramadol is widely used globally with 41 million prescriptions dispensed in 2017 in the United States alone,18,28 and an estimated one-third or more of patients treated with tramadol fail to metabolize it to its active metabolite with optimal kinetics.9,35,46,50,56,65 The metabolic liabilities of tramadol could explain the misalignment between U.S. prescriber perceptions in the United Sates of modest efficacy and its approved indication for treating moderate to moderately severe pain.35,87
Pharmacologic Underpinnings
Tramadol is racemic, and the negative and positive enantiomers are metabolized in vivo to (−)-M1 and (+)-M1, respectively.29,58 The in vitro μ-opioid receptor binding affinity (Ki) is dominated by (+)-M1 (.0034 μmol/L), compared with substantially weaker affinities for (−)-tramadol (25 μmol/L), (+)-tramadol (1.3 μmol/L), and (−)-M1 (.24 μmol/L). The in vitro inhibition (Ki) of serotonin uptake is dominated by (+)-tramadol (.5 μmol/L), whereas the inhibition of norepinephrine uptake is mediated by the similarly potent (−)-tramadol (.5–1.6 μmol/L) and (−)-M1 (.9–1.4 μmol/L).16,58 The analgesia of tramadol is thought to arise from a combination of μ-opioid receptor binding and inhibition of norepinephrine uptake in the descending pain inhibitory system.58 Serotonin is a transmitter in descending inhibitory and excitatory projections and causes antinociceptive and pronociceptive effects, respectively, leading some investigators to question its role in mediating tramadol analgesia.1,58,73
The role played by the enantiomers of tramadol and M1 in human analgesia has been the subject of investigation. Controlled trials in metabolically deficient patients demonstrated that M1 is necessary for analgesia in both experimental and surgical pain.43,55,70,71,84 Other trials used opioid and α2-adrenoceptor antagonists to demonstrate that human tramadol analgesia is mediated by both opioid receptor agonism and monoaminergic modulation.14 The specific contribution of the tramadol enantiomers to human tramadol analgesia has not been investigated. To our knowledge, this is the first human study to ask whether the tramadol parent enantiomers can be discarded and tramadol analgesia replicated by (−)-M1 and (+)-M1 alone; that is, whether M1 is not only necessary, but sufficient, to replicate tramadol analgesia in both metabolically unselected and deficient participants.
Trial A and Trial B End Points
A distinguishing feature of this study from single-dose trial designs was that participants were on the study drug for >2 days (54 hours, 9 doses). This duration allowed systemic concentrations, including central nervous system concentrations, to equilibrate before key assessments were made. It also allowed for the collection of safety information that more faithfully reflects actual clinical use.
In trial A, 50 mg tramadol and 20 mg desmetramadol dosed every 6 hours gave systemic (+)-M1 levels that were bioequivalent and (−)-M1 levels there were nearly bioequivalent. In the absence of circulating tramadol enantiomers, desmetramadol produced similar responses as tramadol with respect to analgesia, pupil constriction, abuse measures, AE profile, and vital signs. If tramadol enantiomers contributed to analgesia, participants should have experienced greater analgesia after tramadol, but they did not. Serotonergic agents have been reported to cause mydriasis,49 and both pronociceptive and antinociceptive effects.1,58,73 Compared with desmetramadol, tramadol exhibited relative pupil dilation (−.4 mm; post hoc P = .0017) and muted analgesia, possibly suggestive of the serotonergic effects of (+)-tramadol. The most straightforward interpretation of these findings is that circulating M1 enantiomers as provided by desmetramadol are not only necessary, but are also sufficient to replicate the therapeutic pharmacology of tramadol. In this interpretation, tramadol provides superfluous enantiomers with (+)-tramadol contributing unwanted metabolic liabilities related to the under or over production of the (+)-M1 opioid, and unwanted serotonergic activity that may negatively influence analgesia and potentially contribute to the risk of seizure and serotonin syndrome (discussed elsewhere in this article).
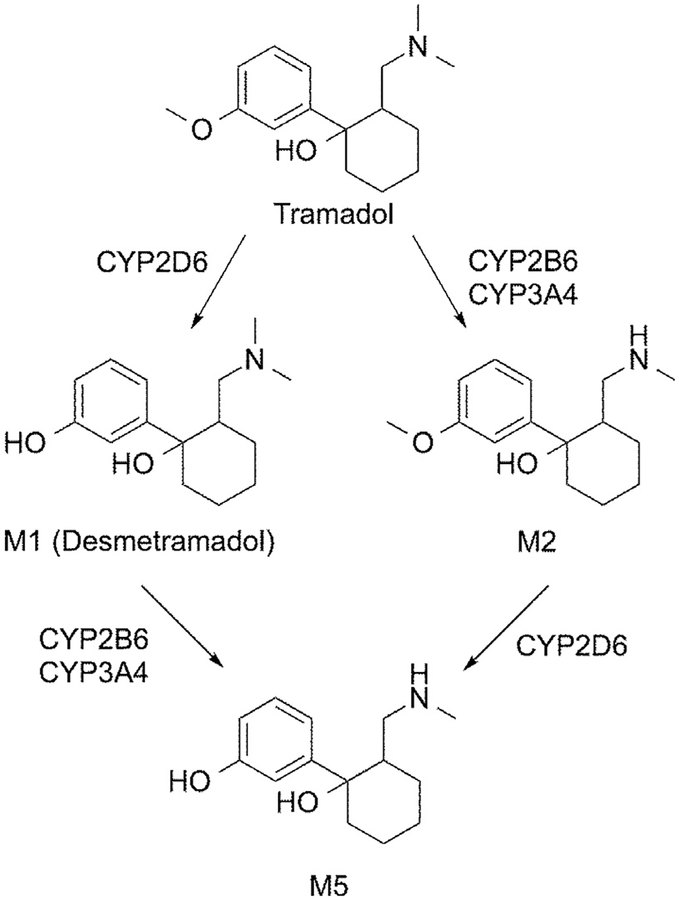
The doses of tramadol and desmetramadol in trial A were advanced into trial B, where participants were made metabolically deficient by coadministration of paroxetine, a strong inhibitor of CYP2D6 and CYP2B6.33,79 Studies in human liver microsomes indicate that tramadol is metabolized to M1 by CYP2D6 and to N-desmethyltramadol by CYP2B6 and CYP3A4; M1 is metabolized to O,N-didesmethyltramadol (M5) by CYP2B6 and CYP3A4 (Fig 7).72,88 Although racemic M5 does bind to the μ-opioid receptor with substantial affinity in vitro (Ki = .10 μmol/L), it is highly polar and neither crosses the blood−brain barrier nor contributes to analgesia or centrally mediated AEs in vivo.29 Metabolism of tramadol to M1 by CYP2D6 favors the positive enantiomer.55 Consistent with these transformations, the presence of paroxetine in trial B depressed tramadol plasma (+)-M1 by approximately 60% and increased desmetramadol plasma (+)-M1 by approximately 40%. The effect of paroxetine on (−)-M1 levels was less pronounced, with tramadol plasma (−)-M1 decreased and desmetramadol plasma (−)-M1 increased by approximately the same amount. The net paroxetine effect in trial B caused tramadol and desmetramadol (−)-M1 to assume bioequivalent levels, and for tramadol (+)-M1 to be depressed to less than one-third of the desmetramadol (+)-M1 level. Consistent with a paroxetine block on tramadol metabolism by CYP2D6 and CYP2B6, (−)-tramadol and (+)-tramadol levels in trial B increased to 200% of their levels in trial A in the absence of paroxetine. Despite the elevated levels of tramadol enantiomers and bioequivalent (−)-M1, the depression of (+)-M1 in trial B was sufficient to cause the analgesic activity of tramadol to collapse to that of placebo. This finding is consistent with prior studies that demonstrated that M1 is necessary for tramadol analgesia in both experimental and surgical pain.43,55,70,71,84 The finding underscores the actual role tramadol enantiomers play in mediating analgesia, because even elevated levels could not compensate for the loss of (+)-M1. In contrast, desmetramadol had no corresponding metabolic liability; in metabolically deficient participants of trial B, it produced therapeutic levels of both M1 enantiomers and analgesia as effective as in the metabolically unselected participants of trial A. Desmetramadol also normalized the abnormal levels of tramadol M1 seen in genetic poor metabolizers and ultrarapid metabolizers. As seen in trial A, desmetramadol returned M1 to therapeutic levels in a poor metabolizer and reduced M1 exposure in an ultrarapid metabolizer. Mechanistically, because desmetramadol does not depend on CYP2D6 for its plasma level, it obviates the metabolic liabilities of tramadol, regardless of whether the metabolic defect is due to inhibition of CYP2D6 (eg, by paroxetine in trial B) or CYP2D6 genetics.
Figure 7.
Tramadol and desmetramadol metabolism catalyzed by CYPs in vitro.
The lack of statistically significant analgesia in the trial B female population dosed with either tramadol or desmetramadol was expected a priori, because normally menstruating women exhibit a variable and increasing cold-induced pain tolerance and threshold over repeated stimulation.41 Females were enrolled in trial B to collect data for the secondary safety and pharmacokinetic end points in both sexes. To ensure sufficient males would be enrolled to test the formal hypothesis and primary pain end point, trial B was intentionally overpowered to 97%.
Desmetramadol had the same safety profile in trial B as in the approved tramadol label.35 Consistent with selective reduction of the (+)-M1 opioid, participants in trial B dosed with tramadol exhibited a safety profile that resembled placebo except for dizziness and muscle spasticity. The latter AEs likely resulted from persistent monoaminergic activity. Desmetramadol had the same incidence of muscle spasticity in trial B as tramadol. Muscle spasticity was more common in trial B than trial A, possibly owing to the additive effect of paroxetine.
Role of Metabolism in Desmetramadol Elimination
The major route of excretion for tramadol and its metabolites is through the kidneys, with >90% of a tramadol dose appearing in the urine.29,45 Inhibition of CYP2B6 by paroxetine in this study increased steady-state desmetramadol levels—approximately 40% for (+)-M1 and approximately 15% for (−)-M1—consistent with a role for CYP2B6 in desmetramadol elimination by its transformation to M5 (Fig 7). A cross-over study in 12 participants administered tramadol with either placebo, ticlopidine (CYP2B6 and CYP2D6 inhibitor), or ticlopidine with itraconazole (CYP3A4 inhibitor) demonstrated the following.31 Ticlopidine alone decreased the formation rate of M1 consistent with inhibition of CYP2D6. The addition of itraconazole had no effect on tramadol pharmacokinetics or the rate of M1 formation rate compared with ticlopidine alone, suggesting that CYP3A4 is of limited importance in the metabolism and elimination of tramadol or desmetramadol in vivo. Another crossover study pretreated 12 participants for 5 days with placebo or rifampicin, an inducer of CYP2B6 and CYP3A4, before the administration of 100 mg oral tramadol.61 Induction decreased the tramadol and M1 AUC by nearly the same amount (59% and 54%) and increased the M1 formation rate by only 12%, consistent with less available CYP2D6 substrate proportionally forming less M1 as the major cause of decreased plasma M1 and to a lesser extent enhancement of the M1 to M5 reaction.
M1 is glucuronidated in vitro most actively by the UDP-glucuronosyltransferases 2B7 and 1A8, with 2B7 having a slight preference for (−)-M1 over (+)-M1 and 1A8 exhibiting strict stereoselectivity for (+)-M1.44 Previous human studies have reported that an oral dose of tramadol is excreted in the urine in the following forms and approximate quantitative ranges: unchanged tramadol (12−32%), unchanged M1 (>10%), M1 glucuronide (2−5%, 24%, 30%, 31%, and 48%), M1 sulphate (2 −5%), unchanged N-desmethyltramadol (>10%), unchanged M5 (5−10%), M5 sulphate (5−10%), and M5 glucuronide (2−5%, 10%, 15%, and 16%).31,45,51,52,68,88 These data collectively suggest that the glucuronidation of desmetramadol, and conversion of desmetramadol to M5 by CYP2B6 are metabolic transformations involved in the in vivo elimination of desmetramadol in the urine, together with unchanged desmetramadol.
Seizures and Serotonin Syndrome
Impact on Respiration
Seizures and serotonin syndrome after normal doses of tramadol alone are exceedingly rare.26,27,36,53 The risk for seizure and serotonin syndrome increases with the concomitant use of serotonergic drugs, although on an absolute basis the risk remains rare and it is common clinical practice to coprescribe tramadol and serotonergic antidepressants in pain disorders.53 Coprescribing antidepressants that are also CYP2D6 inhibitors (eg, bupropion, duloxetine, fluoxetine, or paroxetine) was among several factors associated with enhanced risk of tramadol-induced serotonin syndrome.53 As shown in this study, CYP2D6 inhibition decreased tramadol clearance and exposed a participant to the combined serotonergic effect of the antidepressant and markedly elevated levels of the serotonergic (+)-tramadol enantiomer, which may reach supratherapeutic levels. Desmetramadol may have a lower risk of serotonin syndrome when combined with antidepressants because the serotonergic (+)-tramadol enantiomer is absent, and because plasma levels of its active enantiomers undergo clinically insignificant changes in response to CYP2D6 and CYP2B6 inhibition.
A major cause of schedule II opioid lethality is respiratory depression mediated by agonism of μ-opioid receptors.63 Participants with respiratory depression (oxygen saturation of <94%) after tramadol overdose had ingested a median dose of 2,500 mg (range = 500−4,000 mg), or 25 times the maximum approved therapeutic dose, compared with participants with no respiratory depression who had ingested a median dose of 1,000 mg (range = 450−6,000 mg).60 These properties explain why lethal overdoses owing to tramadol alone are rare.11,12,62,64,69 Achiral analyses of blood from fatal
intoxications with tramadol and M1 found mean blood M1 levels of 1,900 and 1,300 ng/mL, or 38-fold and 26-fold the mean M1 level in this study, respectively.11,42 Even at therapeutic doses, schedule II opioids and the biased opioid receptor ligand TRV130 caused clinically significant respiratory depression, whereas tramadol does not.3,29,34,39,48,66,67,75,76,83 Tramadol is reported to cause a minimal and clinically insignificant decrease in respiration in healthy participants and patients at therapeutic doses.3,48,83 In 1 study, the decrease in respiration was associated with an increase in plasma epinephrine.48 It is unknown whether this respiratory depression by tramadol is mediated by its opioid or monoaminergic mechanism, because adrenergic agents also cause respiratory depression and naloxone failed to fully reverse it.4,5,47,77,85 The effect of serotonin on ventilatory control is more uncertain and depends on the types of respiratory neuron and 5-hydroxytryptophan receptor.2 In this study, mean respiration after tramadol and desmetramadol were minimally decreased compared with placebo, and this decrease was statistically significant in trial B, but not in trial A. To the extent this represents a bone fide phenomenon in this study, it is most likely attributable to the monoaminergic activities of tramadol and desmetramadol rather than their opioid activities, because the paroxetine-induced depression of the (+)-M1 opioid in trial B had no effect on its magnitude.
Abuse Potential
Consistent with its mixed mechanism pharmacology, tramadol is less prone to abuse and diversion than schedule II opioid analgesics and was made a schedule IV controlled substance in 2014 in the United States.10,17,21,32 The abuse potential of tramadol is attributed to the (+)-M1 opioid, which exhibits rate-limited and delayed transport into the central nervous system.32,74 It has been suggested that experienced drug abusers are the most sensitive clinical population for assessing abuse liability.7 However, studies have consistently shown that opioids elicit similar signals of abuse-related subjective effects in non−drug abusers and drug abusers.6,8,19,78,89–92 Morphine, oxycodone, and hydrocodone all elicit robust and statistically significant responses in non−drug abusers in one or all of the measures for drug liking−disliking, take drug again, and strength of drug effect (Table 7). In trial A of this study, 50 mg tramadol and 20 mg desmetramadol exhibited similar and statistically significant responses for strength of drug effect, but were indistinguishable from placebo for drug liking−disliking and take drug again. Strength of drug effect seems to be the more sensitive of the 3 measures in other studies of opioids as well. Absent signals for drug liking−disliking and take drug again are unlikely to be due to insufficient statistical power, because the present study (trial A) distinguishes itself with a sample size that is 2−5 times larger than the size of a typical human abuse liability study. The lack of responses for these abuse measures is likely the result of dose. In recreational drug users (n = 22) who were not opioid experienced, 50 mg tramadol caused no significant subjective effects, but 100 mg caused significant responses in all 3 abuse measures.89
Table 7.
Abuse Measures in Opioid Studies in Drug Abusers and Non-Drug Abusers
| Abuse Measure Versus Placebo* | |||||
|---|---|---|---|---|---|
| Opioid, Oral Route | N | Drug Liking- Disliking | Take Drug Again | Strength of Drug Effect | Study |
| Drug abusers | |||||
| Morphine | |||||
| 25 mg | 12 | 8 | 6 | 35† | Zacny 2005 |
| Oxycodone | |||||
| 15 mg | 9 | 21† | 35† | 43† | Comer 2010 |
| 30 mg | 9 | 19† | 33† | 50† | Comer 2010 |
| Hydromorphone | |||||
| 4 mg | 8 | 29 | — | 19 | Duke 2011 |
| 8 mg | 8 | 44† | — | 32† | Duke 2011 |
| Tramadol | |||||
| 50 mg | 22 | 3 | 5 | 5 | Zacny 2005 |
| 100 mg | 22 | 10† | 9† | 20† | Zacny 2005 |
| 50 mg | 8 | 1 | — | 0 | Duke 2011 |
| 100 mg | 8 | 6 | — | 4 | Duke 2011 |
| 200 mg | 8 | 15 | — | 19 | Duke 2011 |
| 400 mg | 8 | 24 | — | 19 | Duke 2011 |
| Non-drug abusers | |||||
| Morphine | |||||
| 30 mg | 20 | 12† | 12† | 28† | Zacny 2008 |
| 40 mg | 18 | 12† | 12† | 33† | Zacny 2003 |
| Oxycodone | |||||
| 10 mg | 18 | 13† | 15† | 30† | Zacny 2003 |
| 15 mg | 9 | 19† | 18 | 45† | Comer 2010 |
| 20 mg | 18 | 20† | 21† | 48† | Zacny 2003 |
| 20 mg | 20 | 16† | 17† | 53† | Zacny 2008 |
| 30 mg | 9 | 18† | 15 | 53† | Comer 2010 |
| 30 mg | 18 | 21† | 20† | 58† | Zacny 2003 |
| Hydrocodone | |||||
| 15 mg‡ | 20 | 4 | 4 | 33† | Zacny 2009 |
| 30 mg‡ | 20 | 8 | 6 | 50† | Zacny 2009 |
| Tramadol | |||||
| 50 mg | 43 | -1 | -2 | 20† | This study |
| Desmetramadol | |||||
| 20 mg | 43 | -2 | -6 | 17† | This study |
In nondependent recreational opioid users (n = 8) trained to discriminate hydromorphone and methylphenidate, tramadol exhibited a positive dose-dependent trend in drug liking−disliking and strength of drug effect between 100 mg and 400 mg.19 No dose attained statistical significance, likely because of the small sample size. Lower doses of tramadol (50 mg and 100 mg) were identified as placebo, whereas 200 mg and 400 mg doses were identified as hydromorphone. The 400 mg dose also increased scores on a stimulant scale, consistent with the monoaminergic activity of tramadol.
Conclusions
For prescribers seeking to decrease the morphine milligram equivalents in their patients who require effective analgesia, tramadol is a viable option to the schedule II opioids. Tramadol provides analgesia for moderate to moderately severe pain but, compared with the schedule II opioids, has a lower abuse potential and a substantially wider margin of safety with respect to respiratory depression and lethality in overdose. Critical shortcomings of tramadol relate to its metabolic liabilities. The findings from this study indicate that desmetramadol offers the safety and analgesia of tramadol, but without its metabolic liabilities and related drug−drug interactions. Desmetramadol could, therefore, offer expanded safety and usefulness for clinicians who prescribe tramadol as an alternative to schedule II opioids.
Supplementary Material
Perspective:
To our knowledge, this is the first study of desmetramadol in humans and the first to show it provides the same safety and analgesia as tramadol, but without tramadol’s metabolic liabilities and related drug−drug interactions. Desmetramadol could potentially offer expanded safety and usefulness to clinicians seeking an alternative to schedule II opioids.
Acknowledgments
The authors thank Nathaniel Katz of Analgesic Solutions, Natick, Massachusetts, for his valuable comments and suggestions. The authors also thank the patients who participated in this study.
Supported by a National Institute on Drug Abuse grant DA027304 to Syntrix. Syntrix developed the protocols in conjunction with the clinical investigators and provided the study drug. The article was prepared by Syntrix. The authors are fully responsible for all content and editorial decisions for this report. There was no editorial support from other parties. J.A.Z. was an employee of Syntrix during the execution of the reported study, and an owner of Syntrix stock. S.L.S. and L.R.W. were employees of PRA Health Sciences during the conduct of the study. M.S. J was an employee of DF/Net Research during the study and its analysis and received standard compensation. A.D.S, D.Y.M. and S.J.K. were employees of Syntrix during the execution of the study and its analysis.
Footnotes
ClinicalTrials.gov registrations: ,
Supplementary data
Supplementary data related to this article can be found at https://doi:10.1016/j.jpain.2019.04.005.
References
- 1.Bannister K, Bee LA, Dickenson AH: Preclinical and early clinical investigations related to monoaminergic pain modulation. Neurotherapeutics 6:703–712, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bianchi AL, Denavit-Saubie M, Champagnat J: Central control of breathing in mammals: Neuronal circuitry, membrane properties, and neurotransmitters. Physiol Rev 75:1–45, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Bloch MB, Dyer RA, Heijke SA, James MF: Tramadol infusion for postthoracotomy pain relief: A placebo-controlled comparison with epidural morphine. Anesthes Analges 94:523–528, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Bolme P, Corrodi H, Fuxe K, Hokfelt T, Lidbrink P, Goldstein M: Possible involvement of central adrenaline neurons in vasomotor and respiratory control. Studies with clonidine and its interactions with piperoxane and yohimbine. Eur J Pharmacol 28:89–94, 1974 [DOI] [PubMed] [Google Scholar]
- 5.Champagnat J, Denavit-Saubie M, Henry JL, Leviel V: Catecholaminergic depressant effects on bulbar respiratory mechanisms. Brain Res 160:57–68, 1979 [DOI] [PubMed] [Google Scholar]
- 6.Comer SD, Sullivan MA, Vosburg SK, Kowalczyk WJ, Houser J: Abuse liability of oxycodone as a function of pain and drug use history. Drug Alcohol Depend 109:130–138, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Comer SD, Zacny JP, Dworkin RH, Turk DC, Bigelow GE, Foltin RW, Jasinski DR, Sellers EM, Adams EH, Balster R, Burke LB, Cerny I, Colucci RD, Cone E, Cowan P, Farrar JT, Haddox JD, Haythornthwaite JA, Hertz S, Jay GW, Johanson CE, Junor R, Katz NP, Klein M, Kopecky EA, Leiderman DB, McDermott MP, O’Brien C, O’Connor AB, Palmer PP, Raja SN, Rappaport BA, Rauschkolb C, Rowbotham MC, Sampaio C, Setnik B, Sokolowska M, Stauffer JW, Walsh SL: Core outcome measures for opioid abuse liability laboratory assessment studies in humans: IMMPACT recommendations. Pain 153:2315–2324, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper ZD, Sullivan MA, Vosburg SK, Manubay JM, Haney M, Foltin RW, Evans SM, Kowalczyk WJ, Saccone PA, Comer SD: Effects of repeated oxycodone administration on its analgesic and subjective effects in normal, healthy volunteers. Behav Pharmacol 23:271–279, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crews KR, Gaedigk A, Dunnenberger HM, Leeder JS, Klein TE, Caudle KE, Haidar CE, Shen DD, Callaghan JT, Sadhasivam S, Prows CA, Kharasch ED, Skaar TC, Clinical Pharmacogenetics Implementation Consortium: Clinical Pharmacogenetics Implementation Consortium guidelines for cytochrome P450 2D6 genotype and codeine therapy: 2014 update. Clin Pharmacol Ther 95:376–382, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dart RC: RADARS SYSTEM: The evolution of the opioid abuse epidemic in North America In: Lisbon Addictions 2017. in Second European Conference on Addictive Behaviors and Dependencies, Lisboa Congress Centre; 2017 [Google Scholar]
- 11.De Backer B, Renardy F, Denooz R, Charlier C: Quantification in postmortem blood and identification in urine of tramadol and its two main metabolites in two cases of lethal tramadol intoxication. J Anal Toxicol 34:599–604, 2010 [DOI] [PubMed] [Google Scholar]
- 12.De Decker K, Cordonnier J, Jacobs W, Coucke V, Schepens P, Jorens PG: Fatal intoxication due to tramadol alone: Case report and review of the literature. Forensic Sci Int 175:79–82, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Dean RL, Bilsky EJ, Negus SS: Opiate Receptors and Antagonists: From Bench to Clinic. Totowa, NJ, Humana Press, 2009 [Google Scholar]
- 14.Desmeules JA, Piguet V, Collart L, Dayer P: Contribution of monoaminergic modulation to the analgesic effect of tramadol. Br J Clin Pharmacol 41:7–12, 1996 [DOI] [PubMed] [Google Scholar]
- 15.Dowell D, Haegerich TM, Chou R: CDC guideline for prescribing opioids for chronic pain−United States, 2016. JAMA 315:1624–1645, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Driessen B, Reimann W, Giertz H: Effects of the central analgesic tramadol on the uptake and release of noradrenaline and dopamine in vitro. Br J Pharmacol 108:806–811, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drug Enforcement Administration: Schedules of controlled substances: Placement of tramadol into schedule IV. Fed Regist 79:37623–37630. [PubMed] [Google Scholar]
- 18.Drug Enforcement Administration: Diversion Control Division. Drug & Chemical Evaluation Section: Tramadol (Trade Names: Ultram®, Ultracet®). Available at: www.deadiversion.usdoj.gov/drug_chem_info/tramadol.pdf. Accessed November, 5, 2018
- 19.Duke AN, Bigelow GE, Lanier RK, Strain EC: Discriminative stimulus effects of tramadol in humans. J Pharmacol Exp Ther 338:255–262, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dunn KM, Saunders KW, Rutter CM, Banta-Green CJ, Merrill JO, Sullivan MD, Weisner CM, Silverberg MJ, Campbell CI, Psaty BM, Von Korff M: Opioid prescriptions for chronic pain and overdose: A cohort study. Ann Intern Med 152:85–92, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Epstein DH, Preston KL, Jasinski DR: Abuse liability, behavioral pharmacology, and physical-dependence potential of opioids in humans and laboratory animals: Lessons from tramadol. Biol Psychol 73:90–99, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.FDA: FDA Guidance for Industry: Statistical approaches to establishing bioequivalence. Available at: www.fda.gov/downloads/drugs/guidances/ucm070244.pdf. Accessed October 30, 2018
- 23.FDA: Guidance for Industry: Toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. Available at: www.fda.gov/downloads/BiologicsBloodVaccines/ucm091977. Accessed October 30, 2018 [DOI] [PubMed]
- 24.Fliegert F, Kurth B, Gohler K: The effects of tramadol on static and dynamic pupillometry in healthy subjects−the relationship between pharmacodynamics, pharmacokinetics and CYP2D6 metaboliser status. Eur J Clin Pharmacol 61:257–266, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Gaedigk A, Simon SD, Pearce RE, Bradford LD, Kennedy MJ, Leeder JS: The CYP2D6 activity score: Translating genotype information into a qualitative measure of phenotype. Clin Pharmacol Ther 83:234–242, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Gardner JS, Blough D, Drinkard CR, Shatin D, Anderson G, Graham D, Alderfer R: Tramadol and seizures: A surveillance study in a managed care population. Pharmacotherapy 20:1423–1431, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Gasse C, Derby L, Vasilakis-Scaramozza C, Jick H: Incidence of first-time idiopathic seizures in users of tramadol. Pharmacotherapy 20:629–634, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Greenblatt DJ: Opioid prescribing: What are the numbers? Clin Pharmacol Drug Dev 7:6–8, 2018 [DOI] [PubMed] [Google Scholar]
- 29.Grond S, Sablotzki A: Clinical pharmacology of tramadol. Clin Pharmacokinet 43:879–923, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Guy GP Jr. , Zhang K, Bohm MK, Losby J, Lewis B, Young R, Murphy LB, Dowell D: Vital signs: Changes in opioid prescribing in the United States, 2006–2015. MMWR 66:697–704, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hagelberg NM, Saarikoski T, Saari TI, Neuvonen M, Neuvonen PJ, Turpeinen M, Scheinin M, Laine K, Olkkola KT: Ticlopidine inhibits both O-demethylation and renal clearance of tramadol, increasing the exposure to it, but itraconazole has no marked effect on the ticlopidine-tramadol interaction. Eur J Clin Pharmacol 69:867–875, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Health and Human Services: Basis for the recommendation to schedule tramadol in schedule IV of the Controlled Substances Act. Available at: www.regulations.gov/docu-ment?D=DEA-2013-0010-0001. Accessed November 7, 2018
- 33.Hesse LM, Venkatakrishnan K, Court MH, von Moltke LL, Duan SX, Shader RI, Greenblatt DJ: CYP2B6 mediates the in vitro hydroxylation of bupropion: Potential drug interactions with other antidepressants. Drug Metab Dispos 28:1176–1183, 2000 [PubMed] [Google Scholar]
- 34.Houmes RJ, Voets MA, Verkaaik A, Erdmann W, Lachmann B: Efficacy and safety of tramadol versus morphine for moderate and severe postoperative pain with special regard to respiratory depression. Anesthes Analges 74:510–514, 1992 [DOI] [PubMed] [Google Scholar]
- 35.Janssen Pharmaceuticals: FDA Labeling: ULTRAM®-Tramadol hydrochloride tablet. Beerse, Belgium, Janssen Pharmaceuticals, 2017 [Google Scholar]
- 36.Jick H, Derby LE, Vasilakis C, Fife D: The risk of seizures associated with tramadol. Pharmacotherapy 18:607–611, 1998 [PubMed] [Google Scholar]
- 37.Jones SF, McQuay HJ, Moore RA, Hand CW: Morphine and ibuprofen compared using the cold pressor test. Pain 34:117–122, 1988 [DOI] [PubMed] [Google Scholar]
- 38.Kirchheiner J, Keulen JT, Bauer S, Roots I, Brockmoller J: Effects of the CYP2D6 gene duplication on the pharmacokinetics and pharmacodynamics of tramadol. J Clin Psychopharmacol 28:78–83, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Klose R, Ehrhart A, Jung R: [The influence of buprenorphine and tramadol on the postoperative CO2 response after general anaesthesia (author’s transl)]. Anasth Intensivther Notfallmed 17:29–34, 1982 [PubMed] [Google Scholar]
- 40.Koltzenburg M, Pokorny R, Gasser UE, Richarz U: Differential sensitivity of three experimental pain models in detecting the analgesic effects of transdermal fentanyl and buprenorphine. Pain 126:165–174, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Kowalczyk WJ, Evans SM, Bisaga AM, Sullivan MA, Comer SD: Sex differences and hormonal influences on response to cold pressor pain in humans. J Pain 7:151–160, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Kronstrand R, Roman M, Thelander G, Eriksson A: Unintentional fatal intoxications with mitragynine and O-desmethyltramadol from the herbal blend Krypton. J Anal Toxicol 35:242–247, 2011 [DOI] [PubMed] [Google Scholar]
- 43.Laugesen S, Enggaard TP, Pedersen RS, Sindrup SH, Brosen K: Paroxetine, a cytochrome P450 2D6 inhibitor, diminishes the stereoselective O-demethylation and reduces the hypoalgesic effect of tramadol. Clin Pharmacol Ther 77:312–323, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Lehtonen P, Sten T, Aitio O, Kurkela M, Vuorensola K, Finel M, Kostiainen R: Glucuronidation of racemic O-desmethyltramadol, the active metabolite of tramadol. Eur J Pharmaceut Sci 41:523–530, 2010 [DOI] [PubMed] [Google Scholar]
- 45.Lintz W, Erlacin S, Frankus E, Uragg H: [Biotransformation of tramadol in man and animal (author’s transl)]. Arzneimittelforschung 31:1932–1943, 1981 [PubMed] [Google Scholar]
- 46.LLerena A, Naranjo ME, Rodrigues-Soares F, Penas LEM, Farinas H, Tarazona-Santos E: Interethnic variability of CYP2D6 alleles and of predicted and measured metabolic phenotypes across world populations. Expert Opin Drug Metab Toxicol 10:1569–1583, 2014 [DOI] [PubMed] [Google Scholar]
- 47.McCrimmon DR, Lalley PM: Inhibition of respiratory neural discharges by clonidine and 5-hydroxytryptophan. J Pharmacol Exp Ther 222:771–777, 1982 [PubMed] [Google Scholar]
- 48.Mildh LH, Leino KA, Kirvela OA: Effects of tramadol and meperidine on respiration, plasma catecholamine concentrations, and hemodynamics. J Clin Anesthes 11:310–316, 1999 [DOI] [PubMed] [Google Scholar]
- 49.Nielsen AG, Pedersen RS, Noehr-Jensen L, Damkier P, Brosen K: Two separate dose-dependent effects of paroxetine: Mydriasis and inhibition of tramadol’s O-demethylation via CYP2D6. Eur J Clin Pharmacol 66:655–660, 2010 [DOI] [PubMed] [Google Scholar]
- 50.Orliaguet G, Hamza J, Couloigner V, Denoyelle F, Loriot MA, Broly F, Garabedian EN: A case of respiratory depression in a child with ultrarapid CYP2D6 metabolism after tramadol. Pediatrics 135:e753–e755, 2015 [DOI] [PubMed] [Google Scholar]
- 51.Overbeck P, Blaschke G: Direct determination of tramadol glucuronides in human urine by high-performance liquid chromatography with fluorescence detection. J Chromatogr B Biomed Sci Appl 732:185–192, 1999 [DOI] [PubMed] [Google Scholar]
- 52.Paar WD, Poche S, Gerloff J, Dengler HJ: Polymorphic CYP2D6 mediates O-demethylation of the opioid analgesic tramadol. Eur J Clin Pharmacol 53:235–239, 1997 [DOI] [PubMed] [Google Scholar]
- 53.Park SH, Wackernah RC, Stimmel GL: Serotonin syndrome: Is it a reason to avoid the use of tramadol with antidepressants? J Pharmacy Pract 27:71–78, 2014 [DOI] [PubMed] [Google Scholar]
- 54.Petry NM, Bickel WK, Huddleston J, Tzanis E, Badger GJ: A comparison of subjective, psychomotor and physiological effects of a novel muscarinic analgesic, LY297802 tartrate, and oral morphine in occasional drug users. Drug Alcohol Depend 50:129–136, 1998 [DOI] [PubMed] [Google Scholar]
- 55.Poulsen L, Arendt-Nielsen L, Brosen K, Sindrup SH: The hypoalgesic effect of tramadol in relation to CYP2D6. Clin Pharmacol Ther 60:636–644, 1996 [DOI] [PubMed] [Google Scholar]
- 56.Preskorn SH, Kane CP, Lobello K, Nichols AI, Fayyad R, Buckley G, Focht K, Guico-Pabia CJ: Cytochrome P450 2D6 phenoconversion is common in patients being treated for depression: Implications for personalized medicine. J Clin Psychiatry 74:614–621, 2013 [DOI] [PubMed] [Google Scholar]
- 57.Purdue Pharma LP: FDA Labeling: OXYCONTINÒ Oxycodone hydrochloride tablet, film coated, extended release. Stamford, CT, Purdue Pharma LP, 2016 [Google Scholar]
- 58.Raffa RB, Buschmann H, Christoph T, Eichenbaum G, Englberger W, Flores CM, Hertrampf T, Kogel B, Schiene K, Strassburger W, Terlinden R, Tzschentke TM: Mechanistic and functional differentiation of tapentadol and tramadol. Expert Opin Pharmacother 13:1437–1449, 2012 [DOI] [PubMed] [Google Scholar]
- 59.Rani S: Bioequivalence: Issues and perspectives. Indian J Pharmacol 39:218–225, 2007 [Google Scholar]
- 60.Ryan NM, Isbister GK: Tramadol overdose causes seizures and respiratory depression but serotonin toxicity appears unlikely. Clin Toxicol 53:545–550, 2015 [DOI] [PubMed] [Google Scholar]
- 61.Saarikoski T, Saari TI, Hagelberg NM, Neuvonen M, Neuvonen PJ, Scheinin M, Olkkola KT, Laine K: Rifampicin markedly decreases the exposure to oral and intravenous tramadol. Eur J Clin Pharmacol 69:1293–1301, 2013 [DOI] [PubMed] [Google Scholar]
- 62.Sachdeva DK, Jolly BT: Tramadol overdose requiring prolonged opioid antagonism. Am J Emerg Med 15:217–218, 1997 [DOI] [PubMed] [Google Scholar]
- 63.Santiago TV, Edelman NH: Opioids and breathing. J Appl Physiol 59:1675–1685, 1985 [DOI] [PubMed] [Google Scholar]
- 64.Shadnia S, Soltaninejad K, Heydari K, Sasanian G, Abdollahi M: Tramadol intoxication: A review of 114 cases. Human Exp Toxicol 27:201–205, 2008 [DOI] [PubMed] [Google Scholar]
- 65.Shatin D, Gardner JS, Stergachis A, Blough D, Graham D: Impact of mailed warning to prescribers on the co-prescription of tramadol and antidepressants. Pharmacoepidemiol Drug Safety 14:149–154, 2005 [DOI] [PubMed] [Google Scholar]
- 66.Singla N, Minkowitz HS, Soergel DG, Burt DA, Subach RA, Salamea MY, Fossler MJ, Skobieranda F: A randomized, phase IIb study investigating oliceridine (TRV130), a novel micro-receptor G-protein pathway selective (mu-GPS) modulator, for the management of moderate to severe acute pain following abdominoplasty. J Pain Res 10:2413–2424, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Soergel DG, Subach RA, Burnham N, Lark MW, James IE, Sadler BM, Skobieranda F, Violin JD, Webster LR: Biased agonism of the mu-opioid receptor by TRV130 increases analgesia and reduces on-target adverse effects versus morphine: A randomized, double-blind, placebo-controlled, crossover study in healthy volunteers. Pain 155:1829–1835, 2014 [DOI] [PubMed] [Google Scholar]
- 68.Soetebeer UB, Schierenberg MO, Schulz H, Andresen P, Blaschke G: Direct chiral assay of tramadol and detection of the phase II metabolite O-demethyl tramadol glucuronide in human urine using capillary electrophoresis with laser-induced native fluorescence detection. J Chromatogr B Biomed Sci Appl 765:3–13, 2001 [DOI] [PubMed] [Google Scholar]
- 69.Spiller HA, Gorman SE, Villalobos D, Benson BE, Ruskosky DR, Stancavage MM, Anderson DL: Prospective multicenter evaluation of tramadol exposure. J Toxicol Clin Toxicol 35:361–364, 1997 [DOI] [PubMed] [Google Scholar]
- 70.Stamer UM, Lehnen K, Hothker F, Bayerer B, Wolf S, Hoeft A, Stuber F: Impact of CYP2D6 genotype on postoperative tramadol analgesia. Pain 105:231–238, 2003 [DOI] [PubMed] [Google Scholar]
- 71.Stamer UM, Musshoff F, Kobilay M, Madea B, Hoeft A, Stuber F: Concentrations of tramadol and O-desmethyltramadol enantiomers in different CYP2D6 genotypes. Clin Pharmacol Ther 82:41–47, 2007 [DOI] [PubMed] [Google Scholar]
- 72.Subrahmanyam V, Renwick AB, Walters DG, Young PJ, Price RJ, Tonelli AP, Lake BG: Identification of cytochrome P-450 isoforms responsible for cis-tramadol metabolism in human liver microsomes. Drug Metab Dispos 29:1146–1155, 2001 [PubMed] [Google Scholar]
- 73.Suzuki R, Rygh LJ, Dickenson AH: Bad news from the brain: Descending 5-HT pathways that control spinal pain processing. Trends Pharmacol Sci 25:613–617, 2004 [DOI] [PubMed] [Google Scholar]
- 74.Tao Q, Stone DJ, Borenstein MR, Codd EE, Coogan TP, Desai-Krieger D, Liao S, Raffa RB: Differential tramadol and O-desmethyl metabolite levels in brain vs. plasma of mice and rats administered tramadol hydrochloride orally. J Clin Pharm Ther 27:99–106, 2002 [DOI] [PubMed] [Google Scholar]
- 75.Tarkkila P, Tuominen M, Lindgren L: Comparison of respiratory effects of tramadol and oxycodone. J Clin Anesthes 9:582–585, 1997 [DOI] [PubMed] [Google Scholar]
- 76.Tarkkila P, Tuominen M, Lindgren L: Comparison of respiratory effects of tramadol and pethidine. Eur J Anaesthesiol 15:64–68, 1998 [DOI] [PubMed] [Google Scholar]
- 77.Teppema LJ, Nieuwenhuijs D, Olievier CN, Dahan A: Respiratory depression by tramadol in the cat: Involvement of opioid receptors. Anesthesiology 98:420–427, 2003 [DOI] [PubMed] [Google Scholar]
- 78.Tompkins DA, Smith MT, Bigelow GE, Moaddel R, Venkata SL, Strain EC: The effect of repeated intramuscular alfentanil injections on experimental pain and abuse liability indices in healthy males. Clin J Pain 30:36–45, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Troost J, Tatami S, Tsuda Y, Mattheus M, Mehlburger L, Wein M, Michel MC: Effects of strong CYP2D6 and 3A4 inhibitors, paroxetine and ketoconazole, on the pharmacokinetics and cardiovascular safety of tamsulosin. Br J Clin Pharmacol 72:247–256, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tsutaoka BT, Ho RY, Fung SM, Kearney TE: Comparative toxicity of tapentadol and tramadol utilizing data reported to the National Poison Data System. Ann Pharmacother 49:1311–1316, 2015 [DOI] [PubMed] [Google Scholar]
- 81.National US Library of Medicine: TOXNET. Toxicology Data Network. Available at: https://toxnet.nlm.nih.gov. Accessed May, 15, 2019
- 82.van der Schrier R, Jonkman K, van Velzen M, Olofsen E, Drewes AM, Dahan A, Niesters M: An experimental study comparing the respiratory effects of tapentadol and oxycodone in healthy volunteers. Br J Anaesthes 119:1169–1177, 2017 [DOI] [PubMed] [Google Scholar]
- 83.Vickers MD, O’Flaherty D, Szekely SM, Read M, Yoshizumi J: Tramadol: Pain relief by an opioid without depression of respiration. Anaesthesia 47:291–296, 1992 [DOI] [PubMed] [Google Scholar]
- 84.Wang G, Zhang H, He F, Fang X: Effect of the CYP2D6*10 C188T polymorphism on postoperative tramadol analgesia in a Chinese population. Eur J Clin Pharmacol 62:927–931, 2006 [DOI] [PubMed] [Google Scholar]
- 85.Warren KA, Solomon IC: Chronic serotonin-norepinephrine reuptake transporter inhibition modifies basal respiratory output in adult mouse in vitro and in vivo. Respir Physiol Neurobiol 184:9–15, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.World Health Organization: in Expert Committee on Drug Dependence 36th Meeting Tramadol Update Review Report, Geneva, Switzerland, World Health Organization, 2014. Agenda Item 6.1 [Google Scholar]
- 87.World Health Organization: in Expert Committee on Drug Dependence 39th Meeting Tramadol Pre-Review Report, Geneva, Switzerland, World Health Organization, 2017. Agenda Item 5.3 [Google Scholar]
- 88.Wu WN, McKown LA, Liao S: Metabolism of the analgesic drug ULTRAM (tramadol hydrochloride) in humans: API-MS and MS/MS characterization of metabolites. Xenobiotica 32:411–425, 2002 [DOI] [PubMed] [Google Scholar]
- 89.Zacny JP: Profiling the subjective, psychomotor, and physiological effects of tramadol in recreational drug users. Drug Alcohol Depend 80:273–278, 2005 [DOI] [PubMed] [Google Scholar]
- 90.Zacny JP, Gutierrez S: Characterizing the subjective, psychomotor, and physiological effects of oral oxycodone in non-drug-abusing volunteers. Psychopharmacology 170:242–254, 2003 [DOI] [PubMed] [Google Scholar]
- 91.Zacny JP, Gutierrez S: Within-subject comparison of the psychopharmacological profiles of oral hydrocodone and oxycodone combination products in non-drug-abusing volunteers. Drug Alcohol Depend 101:107–114, 2009 [DOI] [PubMed] [Google Scholar]
- 92.Zacny JP, Lichtor SA: Within-subject comparison of the psychopharmacological profiles of oral oxycodone and oral morphine in non-drug-abusing volunteers. Psychopharmacology 196:105–116, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zebala JA: Compositions for overcoming resistance to tramadol United States, Patent 9,717,701, Syntrix Biosystems, Inc., 2017 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.