Abstract
Relapse remains the major cause of death in older patients transplanted for Acute Myeloid Leukemia (AML) in first complete remission (CR1) or for patients with advanced Myelodysplastic Syndrome (MDS) at any age. Conventional myeloablative conditioning followed by allogeneic blood or marrow transplantation is associated with significantly less relapse compared with reduced intensity conditioning (RIC) when performed in younger patients with AML or MDS, but the toxicity of this approach in older patients is prohibitive. We hypothesized that pharmacokinetic targeting to optimize busulfan (BU) exposure, combined with the administration of azacitidine (AZA) post transplantation would mitigate the risk of relapse while reducing nonrelapse mortality (NRM) and ultimately improve progression free survival (PFS). On this phase II multicenter study, 63 patients (40 unrelated donors (URD) and 23 matched related donors (MRD)) received a uniform conditioning regimen consisting of fludarabine IV (days −7 to −3), BU targeted to a daily area under the curve (AUC) of 4000uM*min (Days −6 to −3) following administration of a 25 mg/m2 intravenous test dose on one day between Days −14 to −9, and antithymocyte globulin (days −6, −5 and−4 (two doses for MRD and three for MUD only). Beginning day +42–+90, all patients were planned to receive up to six monthly cycles of AZA at 32mg/m2 subcutaneously × 5days. The median age was 62 years (44–74); 13 had AML and 50 had MDS. 87% of patients were within 20% of the target AUC based on a validation sample. A total of 41 patients (65%) started AZA at a median of 61 (range 43–91) days post-transplant, and 17(41%) of patients completed all 6 cycles of AZA. The cumulative incidence of non-relapse mortality (NRM) at 2 years was 33.4% (95% CI, 22% – 45%). The cumulative incidence of relapse was 25% (95% CI, 15%– 37%) at 2 years. With a median follow-up of 58.9 months, the estimated PFS probability at 2 years and 5 years after transplantation was 41.2% (80% CI, 33.9% – 49.9%) and 26.9% (80% CI, 20.4%–35.5%) respectively for the entire group with a median PFS of 15.8 (95% C.I, 6.7–28.3) mo. The OS probability at 2 and 5 years was 45.7% (95% CI, 34.9%–59.9%) and 31.2% (95% CI, 21.3% t- 45.8%) respectively for the entire group with a median OS of 19.2 (95% C.I. 8.7–37.5) mo. In summary, we demonstrated the feasibility of a novel RIC conditioning regimen with test dose BU targeted to an AUC of 4000uM*min. The feasibility of AZA in this setting appears limited if applied to an unselected population of older HSCT recipients.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) remains the only curative treatment for patients with myelodysplastic syndrome (MDS). Although reduced intensity conditioning (RIC) is the preferred approach for the majority of patients with high-risk MDS based on their age and performance status, the problem of relapse remains pressing. Center for International Blood and Marrow Transplant Research (CIBMTR) registry data shows 3-year probabilities of overall survival (OS) were 52% ± 2% and 49% ± 1% for recipients of matched related donor (MRD) and unrelated donor transplants (URD) for early MDS, respectively. Among patients with advanced MDS, corresponding probabilities were 45% ± 1% and 41% ± 1%.1 The prognosis for patients with acute myeloid leukemia (AML) who are age 60 years or older at the time of initial diagnosis is also poor.2–7 Despite first complete remission (CR1) rates of up to 50% to 60%, prospects for long-term survival after chemotherapy are dismal because of the high risk of relapse.8
Several investigators have reported on the feasibility of employing a RIC approach in elderly patients with AML.9, 10,11, 12,13, 14 a prospective multicenter phase II trial study of allogeneic transplantation for older patients with AML in CR1 using RIC (Cancer and Leukemia Group B 100103 (Alliance for Clinical Trials in Oncology)/Blood and Marrow Transplant Clinical Trial Network 0502) showed that disease-free survival and OS at 2 years after transplantation were 42% and 48%, respectively, and the relapse rate was high at 44% at 2 years.15 Strategies to improve outcomes of allogeneic transplants in patients with MDS and elderly AML are needed, and should focus on efforts to mitigate relapse.
Busulfan (BU) is commonly used in conditioning regimens for HSCT. Previous studies have suggested a therapeutic window for BU area under the curve (AUC) during allogeneic transplant, with decreased survival associated with both high and low levels.16 High levels have been strongly associated with the risk of fatal liver toxicity from hepatic sinusoidal obstruction syndrome.17, 18 In the context of the fludarabine (FLU)/BU regimen, an AUC > 6000 uMol*min was found to be associated with increased toxicity and decreased survival.19 Lower levels have been associated with both graft rejection and greater risk for relapse.20. This has led to the exploration of personalized BU dose based conditioning regimen.21. We hypothesized that we could improve outcomes of RIC by improving disease control while controlling the risk of non-relapse mortality (NRM) by using a higher BU dose, aiming for an AUC that is 75% of “standard” rather than the typical 50% used in RIC regimens. Also, DeLima et al. treated 45 high-risk patients with MDS and AML (67% not in CR) on a phase 1 study with post-transplant AZA for 4 cycles.22 They established the maximal tolerated dose (MTD) as 32mg/m2 given for 5 days and reported a 1-yr event-free survival (EFS) and OS of 58% and 77%, respectively. We sought to optimize the administration of BU and combine that with post-transplant AZA maintenance to reduce relapse without a substantial increase in toxicity to improve progression-free survival (PFS) after HSCT. We conducted a phase II multicenter study within the CALGB (now part of the Alliance) and report the final results here.
Methods
Objectives
The primary objective of the study was to determine if this strategy could improve 2-year PFS in patients with high risk MDS and in patients with AML age 60 and older responding to initial therapy. Secondary objectives were to determine the ability to use pharmacokinetic (PK)-directed BU to achieve an AUC of 4000uM*min within 20% of target AUC in > 80% of patients, the safety and feasibility of using post-transplant AZA, rates of grade II-IV and III-IV acute graft versus host disease (GVHD), incidence of extensive chronic GVHD, treatment-related mortality at 100 days, and 2- and 5-year OS.
Patients and Donors
Patients were eligible if they met the following criteria: 1) AML : age ≥ 60 years and < 75 years, morphologic complete remission (leukemia-free state) defined as bone marrow blasts < 5% (as determined by bone marrow within 4 weeks of beginning preparative regimen), but without requirement for normal peripheral blood counts, no extra medullary leukemia and no blasts in peripheral blood. Patients with prior CNS involvement were eligible as long as disease was in remission at transplant. No more than two cycles of induction chemotherapy and no more than two cycles of consolidation therapy were permitted. Patients treated with hypomethylating agents (AZA or decitabine) who achieved a leukemia-free state could have received up to 4 cycles of therapy to reach this status. No more than 6 months could elapse from documentation of morphologic CR to transplant. Patients with AML following blast transformation of prior chronic myeloid leukemia or other myeloproliferative disease were excluded. 2) MDS: age < 75 years and with high-risk features defined as one of the following: International Prognostic Scoring System (IPSS) risk ≥ Int −2, refractory anemia with excess blasts by French–American–British (FAB) classification, high-risk cytogenetics (either complex karyotype or monosomy 7), and < 10% bone marrow blasts determined by bone marrow biopsy within 4 weeks of beginning preparative regimen. Reduction in marrow blast percentage may have been achieved with chemotherapy or other therapy. Patients could have received treatment with AZA or decitabine prior to study enrollment. Patients who progressed from MDS to AML during treatment were not eligible for enrollment.
The donors were either an HLA-identical sibling (6/6) by serologic typing (A, B, DR) or low-resolution molecular HLA tests or an 8/8 locus matched URD using high resolution DNA-based typing. The donors were required to be healthy and acceptable as per institutional standards for stem cell donation with no significant cardiopulmonary, renal, endocrine, or hepatic disease. There was no age restriction for related donors. Syngeneic donors were not eligible.
Conditioning Regimen
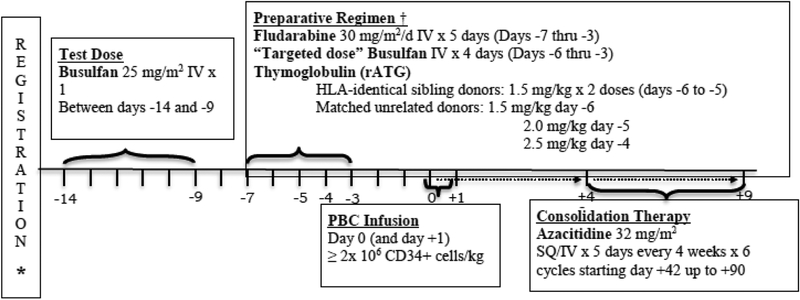
A BU test dose of 25 mg/m2 IV over 45 minutes was administered as a single intravenous (IV) infusion between days −14 and −9. (Figure 1) The test dose was infused over 45 minutes and blood samples were drawn at end of infusion and 1,2,4 and 6 hours after test dose completion. (All samples for pharmacokinetic were sent to Emory Medical laboratories or Seattle Cancer Care Alliance). The PK based targeted treatment dose of BU was calculated as follows: BU* (mg) = test dose (mg) × 4000/test AUC. Initially the treatment dose was originally administered over 3 hours. The protocol was subsequently modified to infuse BU at the same infusion rate as the test dose. BU target level validation samples were obtained at end of infusion and 1,2,4 and 6 hours following the day −6 dose of BU. FLU 30 mg/m2/day was administered IV over 30 minutes for 5 days on Days −7 through −3. Rabbit antithymocyte globulin (thymoglobulin) was administered at 1.5 mg/kg/day IV over 6 hours for 2 doses on Days −6 and −5 in case of related donors. In URD the dose was escalated to 1.5 mg/kg Day −6, 2.0 mg/kg Day −5 and 2.5 mg/kg Day −4.
Figure 1:
Treatment Schema
Post-transplant Azacitidine
Post-transplant AZA was to be started as early as day +42 but not later than day +90 provided the following conditions were met: serum creatinine <2.0 mg/dl, serum bilirubin < 2.0 mg/dl, aspartate amino-transferase AST ≤ 3 X ULN, platelets ≥ 30,000/μl without transfusion for the preceding 72 hours, absolute neutrophil count (ANC) > 500/μl (this may have been achieved with use of growth factors), no acute GVHD grade III or IV and no life-threatening infections or bleeding. The AZA was administered at a dose of 32 mg/m2 subcutaneously (SC) daily for 5 days. Cycles were repeated for up to 6 courses every 4 weeks. If SC administration was not possible, IV administration was permitted. Before the start of cycles 2 to 6 the platelet count needed to be >20,000/μl attained with or without platelet transfusion and ANC > 500/μl with or without the use of myeloid growth factors. If patients were unable to start a subsequent cycle of AZA due to toxicity or other reasons, the start of a subsequent cycle could be delayed up to 4 weeks. If the AZA could not be started after a 4-week delay, the patient could not receive any additional AZA. Patients who developed drug-related grade 3 or 4 renal, hepatic, cardiac, pulmonary or neurologic toxicity had the Aza discontinued permanently. Patients with active acute GVHD grade III-IV were not eligible to receive Aza. If acute GVHD grade III-IV resolved, patients could receive the drug at one lower dose level in subsequent cycles dose level −1, 16mg/m2 and dose level −2 (minimum dose) 8 mg/m2. Patients developing pneumonia or any infection deemed life threatening by the attending physician had the AZA discontinued. If unexplained elevations of creatinine occurred to between 2 and 3 mg/dl, the next cycle was delayed until values returned to normal or baseline and the dose was reduced by one level for the next treatment course AZA was discontinued for any unexplained increase in creatinine > 3 mg/dl.
Donor Mobilization and Target Allograft Composition
Donors received G-CSF 10 mcg/kg SC on Days −5 through −2 (and, if necessary −1). On Days −1 (and 0) donors underwent leukapheresis for 1–2 days to achieve a CD34+ cell dose of ≥ 2 × 106 /kg (actual weight - recipient). If the yield of CD34+ cells was < 2 × 106 /kg on Day −1, an additional apheresis was permitted to be performed on Day 0. If after two apheresis procedures the total CD34+ cell dose was at least 2 × 106 /kg, no further apheresis was required. Target CD34+ cell doses were based on institutional standards for sibling donors, as long as minimum of 2 × 106 /kg was achieved. There was no maximum CD34+ cell dose specified and doses were not capped.
Supportive Care and Patient Assessments
Tacrolimus was targeted to a serum level of 5–10 ng/mL (not to exceed 15 ng/mL). The suggested starting dose was 0.03 mg/kg PO BID beginning on Day −2 tapering between Day +90 to +120 with a goal of stopping by Day +150 to +180. Methotrexate was administered at 5 mg/m2/day IV on Days +1, +3, +6 (and Day +11 in case of URD). Recipients received 5 mcg/kg G-CSF SC daily beginning on Day +12 and continuing until ANC > 1500/μL for two consecutive days or > 5000/μL for one day. Acute GVHD (aGVHD) and chronic GVHD (cGVHD) were graded according to established criteria.16,17Patients were considered evaluable for GVHD if they engrafted. Organ toxicity was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.
Definitions
Neutrophil engraftment was defined as an increase in the absolute neutrophil count to 500/μl or greater after a conditioning regimen–induced nadir. Platelet engraftment was defined as the first day of three consecutive platelet count measurements greater than 20,000/μl without the aid of transfusion. NRM was defined as death in CR. Primary graft failure was defined as failure of neutrophil engraftment by day 30. Cytogenetic risk category was assigned based on the CALGB criteria.8
Statistical Considerations
The primary endpoint was the probability of progression-free survival (PFS) at two years as estimated by the Kaplan-Meier estimator (if there is no censoring prior to 2 years, this is equivalent to a simple proportion of patients alive and progression free at 2 years). Disease progression and death due to any cause was considered an event. The time to PFS was the time interval between transplant and progression, death or last follow-up whichever occurred first. Patients without progression who were lost to follow-up prior to 2 years were censored at the time of their last follow-up. This study was designed as a single arm single-stage trial.
Based on previous studies, a two-year PFS of 25% or lower was considered clinically uninteresting. A two-year PFS of 40% or higher would be considered clinically promising. Assuming a two-year PFS of 40%, a sample size of 64 evaluable patients provides 90% power at the one-sided Type I error of 0.10 to reject the null hypothesis that the two-year PFS was 25%. Based on this design, if at least 21 out of the 64 patients are alive and progression-free for at least 2 years, it would be concluded that the 2-year PFS probability is greater than 25%.
Patients’ demographics and disease and treatment characteristics were summarized with median and range for continuous variables and frequency and percentage for categorical variables. Progression-free survival and overall survival were summarized using the Kaplan-Meier estimator. The cumulative incidence of relapse, non-relapse mortality, acute and chronic GVHD were summarized using the cumulative incidence function treating death as the competing risks. All patients who were lost to follow-up without experiencing the event of interest were censored at the time of their last follow-up. Data quality was ensured by review of data by the Alliance Statistics and Data Center and by the study chairperson following Alliance policies. Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center. All analyses were conducted using the SAS software v. 9.4 on the study database frozen on [July 23rd, 2018
Results
Patients and Donor Characteristics
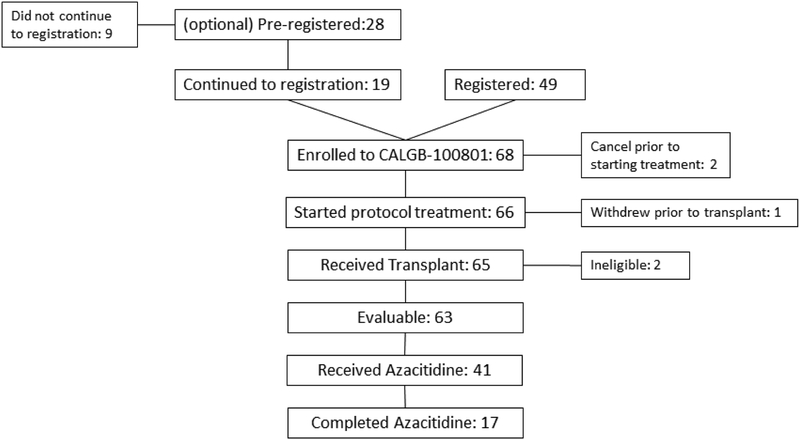
Patient and donor characteristics are provided in Table 1. In all, 68 patients were registered and 65 received transplantations at 10 centers between September 2010 and October 2013. (Figure 2) Each participant signed an institutional review board (IRB)-approved, protocol-specific informed consent document in accordance with federal and institutional guidelines. Five of the patients who were registered (2 received transplantations) were excluded from the primary analysis for the following reasons: two patients were taken off study prior to starting treatment, one patient withdrew consent prior to transplant and two patients who underwent transplantation had chronic myelomonocytic leukemia (CMML) and were deemed ineligible for the study. Thus, the data analysis was limited to the 63 eligible recipients. The median patient age was 62 (range, 44 to 74) years. Fifty patients (79.4%) had high-risk MDS and 13 patients (20.6%) had AML. Twenty-three patients (36.5%) received grafts from matched related donors and 40 patients (63.5%) received unrelated donor grafts. The median time from diagnosis to transplantation was 5.9 (range, 1.8–109.7) months.
Table 1.
Patient Characteristics
| Total (N=63) | |
|---|---|
| Age, years (median, range) | 62 (44–74) |
| Gender | |
| Male | 51 (81.0%) |
| Female | 12 (19.0%) |
| ECOG Performance Status | |
| 0 | 26 (41.3%) |
| 1 | 34 (54.0%) |
| 2 | 3 (4.8%) |
| Disease | |
| AML | 13 (20.6%) |
| MDS | 50 (79.4%) |
| IPSS (MDS Only) | |
| Low | 3 (7.3%) |
| Intermediate-1 | 9 (22.0%) |
| Intermediate-2 | 26 (63.4%) |
| High | 3 (7.3%) |
| Missing | 9 |
| N/A (AML Patients) | 13 |
| Cytogenetics Risk | |
| Normal | 29 (47.5%) |
| Complex | 18 (29.5%) |
| −7, del(7)q | 8 (13.1%) |
| Other | 6 (9.8%) |
| Missing | 2 |
| Induction Regimen | |
| 7+3 | 12 (20.3%) |
| DNA hypomethylating agent | 41 (69.5%) |
| Other | 2 (3.4%) |
| Unknown | 4 (6.8%) |
| No prior chemotherapy | 4 |
| Donor type | |
| MRD | 23 (36.5%) |
| MUD | 40 (63.5%) |
| Months from diagnosis to transplant, median (range) | 5.9 (1.8–109.7) |
| ABO Compatibility | |
| Match | 29 (46%) |
| Major mismatch | 12 (19%) |
| Minor mismatch | 19 (30.2%) |
| Bidirectional | 3 (4.8%) |
| Patient | Donor CMV serology | |
| Negative | Negative | 27 (42.9%) |
| Negative | Positive | 7 (11.1%) |
| Negative | Unknown | 1 (1.6%) |
| Positive | Negative | 8 (12.7%) |
| Positive | Positive | 19 (30.2%) |
| Positive | Unknown | 1 (1.6%) |
| Patient | Donor Gender | |
| Female | Female | 1 (1.6%) |
| Female | Male | 11 (17.5%) |
| Male | Female | 16 (25.4%) |
| Male | Male | 35 (55.6%) |
| Number of Cells Infused, CD34+ ×106/kg, median (range) | 5.0 (2.5–19.3) |
Figure2.
Consort Diagram
Busulfan dosing
Data on BU test dose and therapeutic dosing is shown in Table 2. The median AUC on the validation sample was 4143 (range, 2400–6642) micromol*min. Thus, 87.1 % of patients were within 20% of target AUC based on the validation sample.
Table2.
Busulfan Dosing
| N | Result | |
|---|---|---|
| Test dose AUC (μmol * min), median (range) | 60 | 903.5 (100–1294) |
| BSA, median (range) | 63 | 1.9 (1.5–2.3) |
| Busulfan total dose (mg), median (range) | 63 | 800 (194–1502) |
| AUC on validation sample (μmol * min), median (range) | 62 | 4143 (2400–6642) |
| Validated Sample AUC/Target dose, median (range) | 62 | 1.04 (0.60–1.66) |
| Achieved AUC within 20% of target AUC, N(%) | 62 | 54 (87.1%) |
Engraftment and Chimerism
The median number of CD34+ cells infused was 5.0 × 106 /kg (range: 2.5–19.3). The median time to neutrophil engraftment was 13 days (range, 1 to 166 days) and to platelet engraftment was 11 days (range, 0 to 110 days) post HSCT. Primary graft failure was observed in 4 (6.5%) of patients. Beginning with the first planned sample on day +30, the median proportion of donor cells in samples of peripheral blood analyzed for myeloid chimerism was consistently higher than 64% (range, 64% to 100%) at all time points analyzed. Median CD3+ cell chimerism values gradually increased over time in the surviving patients without relapse and were 93% (range, 0% to 100%) at day +30, 95.5% (range, 68% to 100%) at day +90, 100% (range, 0% to 100%) at day +180, and 100% (range, 0% to 100%) at day +365. (Table 3)
Table3.
Chimerism
| CD3+ (N; Median (Range)) | Total N; Median (range) | |
|---|---|---|
| Day 30 | 35; 93% (0–100) | 40; 100% (64–100) |
| Day 60 | 22; 95% (68–100) | 31; 100% (95–100) |
| Day 90 | 24; 95.5% (0–100) | 31; 100% (92–100) |
| Day 180 | 20; 100% (0–100) | 26; 100% (90–100) |
| Day 365 | 11; 100% (0–100) | 15; 100% (95–100) |
Post-transplant Azacitadine
A total of 41 patients (65%) started AZA at a median of 61 (range 43–91) days post-transplant. Twenty-two patients never started AZA. Seventeen (41% of 41) patients completed all 6 cycles of AZA representing 27% of the study group originally intended to receive 6 cycles of Aza. Details on reasons for not starting Aza and for ending protocol treatment prior to completing AZA are shown in Table 4.
Table 4.
Azacitidine Dosing
| Result | |
|---|---|
| Started Azacitidine | 41 |
| Reasons for ending protocol treatment prior to starting Azacitidine | |
| Death | 8 (36%) |
| Progression | 4 (18%) |
| AE | 5 (23%) |
| Refusal | 2 (9%) |
| Other1 | 3 (14%) |
| Time from transplant to start of Azacitidine (days), median (range) | 61 (43–91) |
| Number of Azacitidine received | |
| 1 | 4 (9.8%) |
| 2 | 4 (9.8%) |
| 3 | 5 (12.2%) |
| 4 | 5 (12.2%) |
| 5 | 5 (12.2%) |
| 6 | 18 (43.9%)2 |
| Completed Azacitidine | 17 (41%) |
| Reasons for ending protocol treatment prior to completing Azacitidine | |
| Death | 4 (17%) |
| Progression | 6 (25%) |
| AE | 5 (21%) |
| Refusal | 8 (33%) |
| Other3 | 1 (4%) |
Patient scheduled to receive bone marrow stem cells not allowed per protocol; could not start AZA due to low ANC on day 90; Pt started on Valcyte for CMV which caused low counts, never met criteria to start AZA
1 patient stated cycle 6, but only received doses 1 and 2; doses 3–5 were missed due to sepsis. Therefore they did not complete treatment per protocol
Treatment delayed greater than 4 weeks due to AE not related to protocol
GVHD and c GVHD
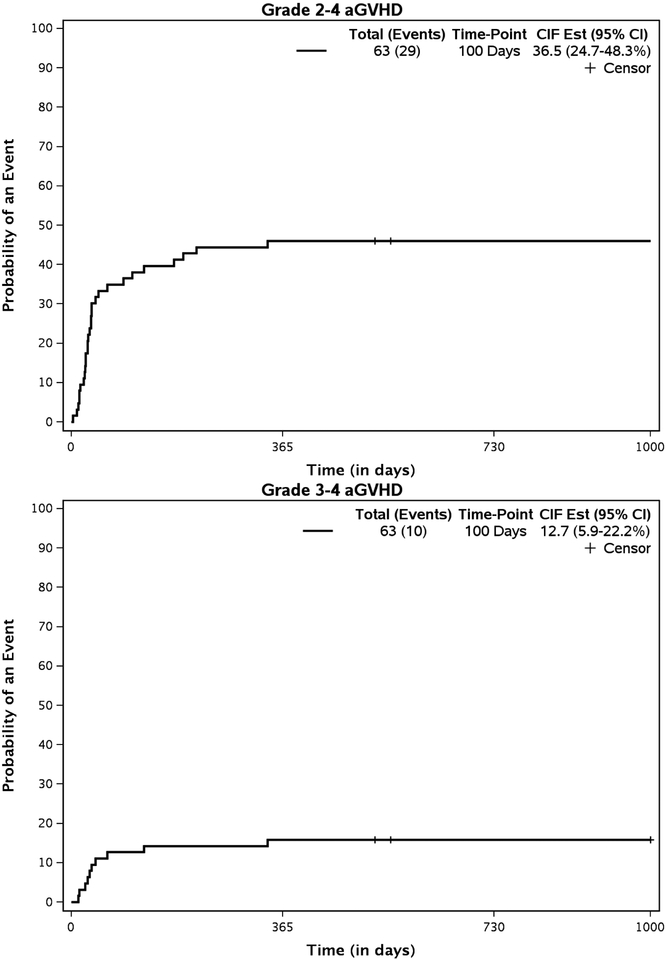
The cumulative incidences of grades II to IV and III to IV aGVHD at 100 days were 36.5% (95% CI, 24.7% to 48.3%) and 12.7% (95% CI, 5.9% to 22.2%), respectively (Figure 3a) . . For all patients experiencing grade II to IV aGVHD, the median time to onset was 31 days (range, 3 to 339 days). The cumulative incidence of any cGVHD by 2 years was 30.2% (95% CI, 19.2% to 41.8%) (Figure 3b); cumulative incidence of extensive cGVHD was 14% (95% CI, 7% to 24.1%) at 2 years. For all patients experiencing limited or extensive cGVHD, the median time to onset of cGVHD was 216 days (range, 50 to 391 days) post-transplantation.
Figure3a.
Cumulative Incidence of acute GVHD
Figure3b.
Cumulative Incidence of any chronic GVHD
Nonhematologic Adverse Events and Opportunistic Infections
The post-transplant rates of > grade 3 adverse events are shown in Table 5. The only grade 3 to 5 organ toxicity seen in >10% of patients was mucositis (31%) and rash (13%). No cases of hepatic sinusoidal obstruction syndrome were observed. Reactivation of cytomegalovirus (viremia) occurred in 14 (41%) of 34 donor/recipient pairs at risk. Three patients developed cytomegalovirus disease (GI 2, CNS 1). No patients died due to cytomegalovirus disease.
Table 5.
Summary of Adverse Events Regardless of Attribution
| Patients with a maximum: | n | (%) |
|---|---|---|
| Grade 1 Event | 0 | (0.0%) |
| Grade 2 Event | 0 | (0.0%) |
| Grade 3 Event | 4 | (6.3%) |
| Grade 4 Event | 42 | (66.7%) |
| Grade 5 Event | 17 | (27.0%) |
| Grade 1 Event | 0 | (0.0%) |
| Grade 2 Event | 1 | (1.6%) |
| Grade 3 Event | 6 | (9.5%) |
| Grade 4 Event | 54 | (85.7%) |
| Grade 5 Event | 0 | (0.0%) |
| Grade 1 Event | 0 | (0.0%) |
| Grade 2 Event | 2 | (3.2%) |
| Grade 3 Event | 37 | (58.7%) |
| Grade 4 Event | 7 | (11.1%) |
| Grade 5 Event | 17 | (27.0%) |
Note: Summaries are based on available patient data
Results for PFS, OS NRM and Relapse,
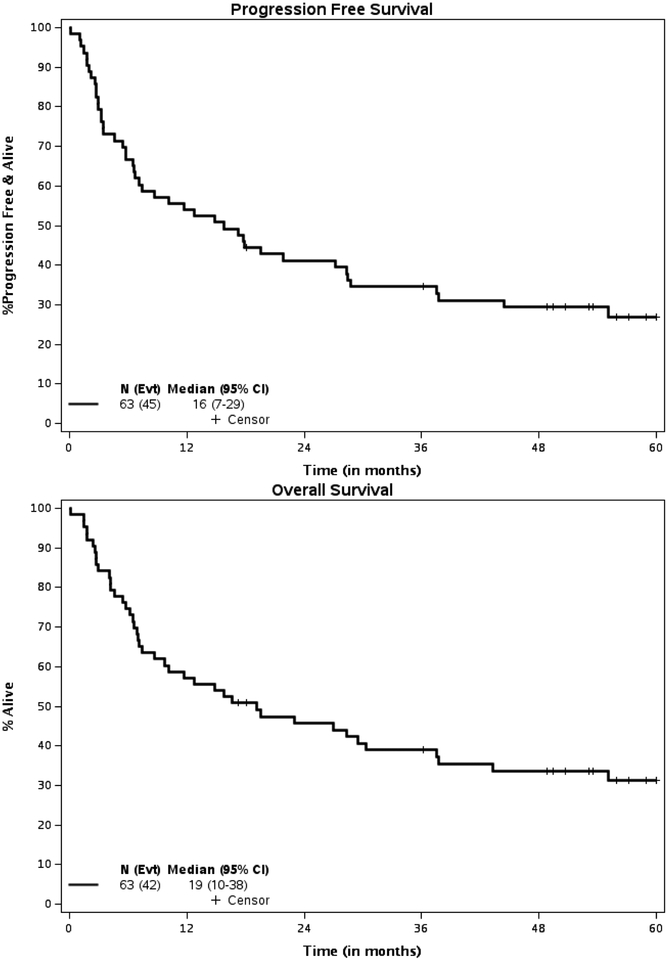
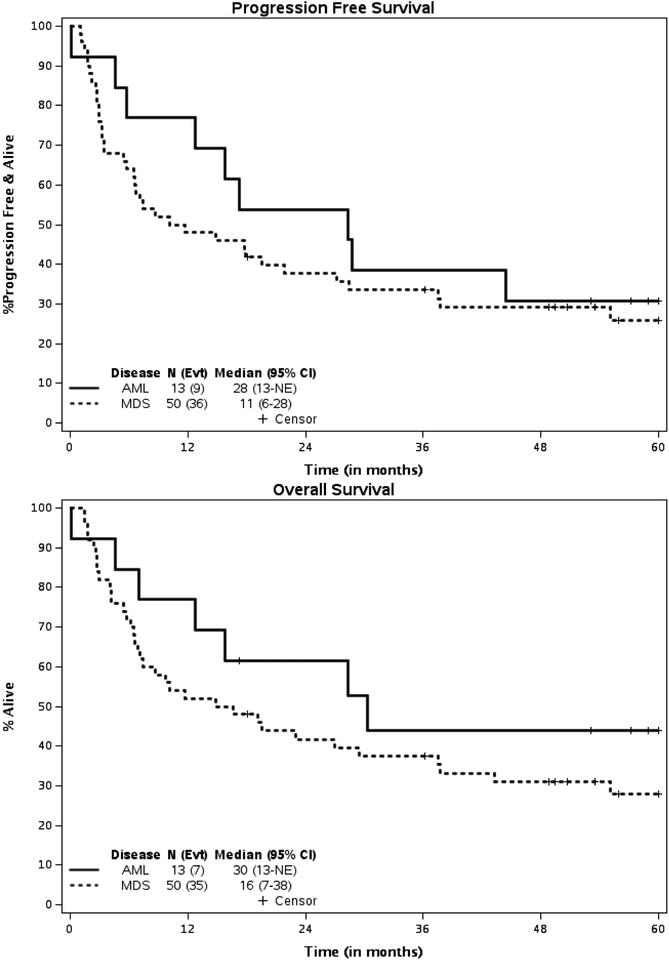
The median follow-up of survivors was 58.9 (95% CI 53.1 to 62.6) months. At 2 years posttransplant, 25 patients (more than the decision threshold of 21 patients) were alive and progression-free. Therefore, per study design, there is sufficient evidence to conclude that PFS probability at 2 years is higher than 25% with a one-sided type I error rate of 10%. Equivalently, the PFS probability at 2 years and 5 yrs. after transplantation was 41.2% (80% CI, 33.9% - 49.9%) and 26.9% (80% CI, 20.4%−35.5%) respectively for the entire group with a median PFS of 15.8 (95% C.I, 6.7–28.3) mos. (Figure 5) The PFS probability at 2 and 5 years years after transplantation for patients with AML was 53.8% (95% CI: 32.6–89.1) and 30.1% (95% CI, 13.6–69.5) respectively with a median PFS of 28.3 months. (95% CI: 5.7-NR) (Figure 6). The PFS probability at 2 years and 5 years after transplantation for patients with MDS was 37.8% (95% CI: 26.5–54) and 25.9% (95%CI, 15.7–42.5%) respectively with a median PFS of 10.9 months. (95% CI: 5.7–27.1).
Figure 5.
Kaplan Meier Survival Curves (All Patients)
Figure 6.
Kaplan Meier Survival Curves (By Disease)
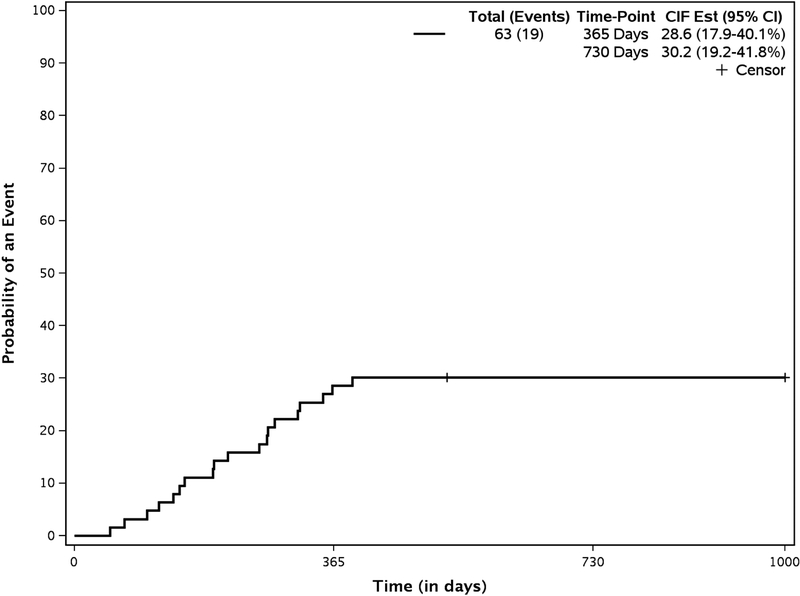
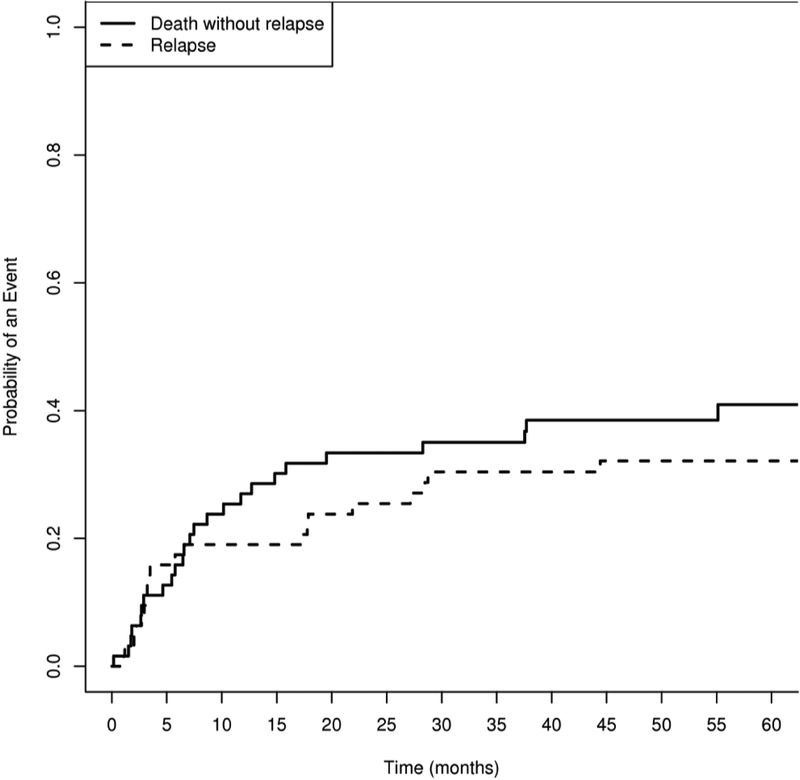
The primary reasons patients came off treatment are shown in Table 6. The 100-day mortality was 16% (N=10). 24 patients (57%) died from causes other than relapse at a median of 222 days (range, 5 to 1678 days) after transplantation. The cumulative incidence of NRM at 2 years was 33.4% (95% CI, 22% to 45%;) (Figure 4). 20 patients relapsed at a median of 140 (range 33–1352) days post HSCT. The cumulative incidence of relapse was 25% (95% CI, 15% to 37%) at 2 years.
Table 6.
Patient disposition
| Total (N=63) | |
|---|---|
| Months of follow-up for survivors, median (95% CI) | 58.9 mo (53.1–62.6) |
| Primary off treatment reason | |
| Treatment completed per protocol | 17 (27.0%) |
| Disease progression | 10 (15.9%) |
| Adverse event | 10 (15.9%) |
| Died during treatment | 12 (19.0%) |
| Patient refused further protocol treatment | 10 (14.9%) |
| Other1 | 4 (6.4%) |
Patient scheduled to receive bone marrow stem cells not allowed per protocol; could not start AZA due to low ANC on day 90; Pt started on Valcyte for CMV which caused low counts, never met criteria to start AZA; Treatment delayed greater than 4 weeks due to AE not related to protocol
Figure 4.
Cumulative Incidence of Non Relapse Mortality and Relapse
Forty-two patients have died. As shown in Table 7, treatment-related death (n=18, 43%) and death from disease (n=18, 43%) were equally likely to be the causes of death, representing 86%% of all deaths. The OS probability at 2 and 5 years was 45.7% (95% CI, 34.9%−59.9%) and 31.2% (95% CI, 21.3% t- 45.8%), respectively, for the entire group with a median OS of 19.2 (95% C.I. 8.7–37.5) months. The OS probability at 2 and 5 years for the patients with AML was 61.5% (95% CI, 40.0–94.6) and 44% (95% CI: 23.3–83), respectively, with a median OS of 30.4 months. (95% CI: 7.1-NR). The OS probability at 2 and 5 years for the patients with MDS was 41.7% (95% CI, 30.0–58.0) and 27.8% (95% CI: 17.4–44.5), respectively, with a median OS of 15.7 (95% CI: 6.9–37.5) months.
Table 7:
Cause of Death
| Cause of death | Total (N=42) |
|---|---|
| Protocol treatment related | 18 (42.9%) |
| Protocol disease related | 18 (42.9%) |
| Not related to protocol treatment or protocol | 5 (11.9%) |
| Unknown | 1 (2.4%) |
Discussion
This prospective study demonstrates the feasibility and challenges of implementing strategies designed to mitigate relapse in patients with high-risk hematological malignancies undergoing HSCT in a multi-center setting. The study met the primary endpoint with the conclusion that PFS probability at 2 year post transplant was greater than 25% with the one-sided type I error of 10% as designed (equivalently, 80% CI was presented above). A test dose of BU to achieve targeted AUC of 4000uM*min is both feasible and effective even in a predominantly older patient population, as the vast majority of patients (87.1%) were within 20% of the targeted value without experiencing grade 4 toxicity. The planned administration of post-transplant maintenance AZA in older patients with AML and high-risk MDS is much more challenging, and one third of our patients could not receive AZA as planned and only 27% of all recipients were able to receive the intended 6 cycles of maintenance therapy. Although we achieved our goal of at least 25% patients who were progression free at 2 or more years post HSCT, we conclude that post-transplant maintenance strategies that are less toxic and more effective than subcutaneous AZA should be sought.
We have also demonstrated the clear value of long-term follow up to achieve a more accurate assessment of the value of any transplant strategy. It is unusual for the first report of a prospective HSCT trial to contain data on a group of patients with a median follow up of nearly 5 years. We demonstrated many adverse relapse and non-relapse events can occur between years 2 to 5 in a high-risk population and caution broad interpretation of studies with less than 2 years median follow up.
Prospective randomized trials and analysis of registry data comparing myeloablative conditioning to RIC in MDS and AML have yielded mixed results.23–27.The CIBMTR registry data shows that between 2000 and 2015 the combination of FLU and BU (6.4mg/kg total dose) was the most commonly used RIC regimen for AML and MDS, accounting for 32% of the transplants..( D′Souza A, Fretham C, 2016) This regimen uses 50% of the standard BU dose without targeting. The toxicity seen with BU-containing RIC regimen using 3.2–6.4mg/kg has been minimal. We aimed to study a PK-based targeted BU preparative regimen with a target AUC 75% of the full dose. We hypothesized that the enhanced antitumor effect of this higher BU exposure would also allow sufficient time for the allo-immune effect to emerge. The target AUC of 4000uM*min was achieved in 87% of patients. Again, as anticipated the toxicity attributable to the conditioning regimen was generally mild to moderate and reversible. Optimizing BU PK through targeted dosing seems rational if the goal is to achieve maximal exposure while limiting toxicity. For instance, the BMT CTN 0901 study demonstrated substantially lower rates of relapse but increased NRM in the recipients of fully myeloablative conditioning. Combining myeloablative conditioning with ex vivo T-cell depletion may also be another useful strategy to prevent relapse while minimizing transplant related toxicity.28, 29
We also aimed to study the use of post-transplant AZA to reduce early post-transplant relapse, directly through the effect of AZA on MDS or alternatively by altering the post-transplant immune environment in a manner that facilitates the graft-versus-leukemia effect. Several authors have reported on the use of DNA hypomethylating agents after allogeneic transplant.22, 30–33 In contrast to our cohort, the majority of patients in these studies had a diagnosis of AML. Patients with MDS accounted for nearly 80% of patients in our cohort and we allowed patients with up to 10% blasts to be eligible. A total of 41 patients (65%) started AZA and 17 (41%) of patients completed all 6 cycles of AZA. This experience is similar to that reported by the other authors. If the goal is to administer maintenance therapy for prolonged periods post-HSCT (up to 12 months or longer) in older patients, subcutaneous AZA may not be an optimal strategy based on our study results, given that less than half of the patients could complete the planned course. Oral maintenance agents or cell/antibody based relapse mitigation strategies may be more attractive alternatives.
We incorporated rabbit ATG (thymoglobulin) into the conditioning regimen in order to reduce the rates of both severe acute and chronic GVHD, as supported by both retrospective and prospective controlled data.34, 35Whether this strategy led to a higher risk of relapse in the patients on this study is a matter of speculation and cannot be addressed with certainty. That said, the overall relapse rate of 25% is in line with reports from high-risk patients reported using non-ATG containing regimens.
Overall, the conditioning regimen was reasonably well tolerated with acceptable and supportable rates of mucositis and cumulative incidences of grades II to IV and III to IV aGVHD at 100 days of 36.5% and 12.7%, respectively. The cumulative incidence of cGVHD at 2 years of 30.2% with extensive c GVHD in 14% is also in line with previous reports, particularly when peripheral blood progenitor cells are used as a graft source. The 100-day cumulative incidence of NRM was 16% and cumulative incidence of NRM at 2 years was 33.4%, which, while appearing high, are also consistent with previous studies that included patients with high risk MDS. The PFS probability at 2 years after transplantation was 41.2% for the entire group (AML 53.8% ,MDS 37.8%) and the OS probability at 5 years was 31.2% (AML 44% MDS 27.8%), with death due to disease accounting for 43% of all deaths. The heterogeneity of the patient cohort and the single arm design of the study make firm conclusions difficult. Also, this study was conducted at a time before incorporation of MRD assessment prior to transplant was routine, and collection of these data will be critical for future prospective trials. However, the results in this elderly group of patients is encouraging.
In a multicenter study of post-transplant AZA maintenance in older AML patients, quantification of circulating tumor-specific CD8+ T cells was evaluated and their presence was associated with freedom from relapse.32 The potential induction of these tumor-specific cells by AZA provides good rationale for its use in this setting. Unfortunately, we did not analyze for the presence of these cells in our study, but this clearly should be incorporated into future studies planning to administer AZA or other hypomethylating agents following HSCT.
In conclusion we have demonstrated the feasibility of a novel RIC conditioning regimen with test dose BU targeted to an AUC of 4000uM*min and report on the largest series of patients to date with both AML and MDS given post-transplant Aza. The feasibility of AZA in this setting appears limited if applied to an unselected population of older HSCT recipients, but may be useful if employed in a more targeted group of patients most likely to benefit. However, the true value of this approach can only be evaluated in a randomized clinical trial. The results of a recently completed randomized study () will be very helpful in determining the ultimate future of this strategy.
Support:
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology), U10CA180833, U10CA180838, U10CA180850, U10CA180854, and UG1CA189819. Also supported in part by funds from Otsuka. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. https://acknowledgments.alliancefound.org
The following institutional networks have participated in this study:
Dartmouth College - Norris Cotton Cancer Center LAPS, Lebanon, NH, Konstantin Dragnev, U10CA180854; Delaware/Christiana Care NCI Community Oncology Research Program, Newark, DE, Gregory Masters, UG1CA189819; Mount Sinai Hospital, New York, NY, Lewis Silverman; The Ohio State University Comprehensive Cancer Center LAPS, Columbus, OH, Claire Verschraegen, U10CA180850; UNC Lineberger Comprehensive Cancer Center LAPS, Chapel Hill, NC, Thomas Shea, U10CA180838; University of Iowa/Holden Comprehensive Cancer Center, Iowa City, IA, Umar Farooq; University of Maryland/Greenebaum Cancer Center, Baltimore, MD, Heather Mannuel; Wake Forest University Health Sciences, Winston-Salem, NC, Heidi Klepin; Washington University - Siteman Cancer Center LAPS, Saint Louis, MO, Nancy Bartlett, U10CA180833; and Weill Medical College of Cornell University, New York, NY, Scott Tagawa.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ClinicalTrials.gov Identifier:
References
- 1.D′Souza A, Fretham C . Current Uses and Outcomes of Hematopoietic Cell Transplantation (HCT): CIBMTR Summary Slides, 2017. Vol 20192017. [Google Scholar]
- 2.Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006;107:3481–3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cancer, Leukemia Group B, Farag SS, et al. Pretreatment cytogenetics add to other prognostic factors predicting complete remission and long-term outcome in patients 60 years of age or older with acute myeloid leukemia: results from Cancer and Leukemia Group B 8461. Blood. 2006;108:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lowenberg B, Ossenkoppele GJ, van Putten W, et al. High-dose daunorubicin in older patients with acute myeloid leukemia. N Engl J Med. 2009;361:1235–1248. [DOI] [PubMed] [Google Scholar]
- 5.Oran B, Weisdorf DJ. Survival for older patients with acute myeloid leukemia: a population-based study. Haematologica. 2012;97:1916–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rowe JM, Tallman MS. How I treat acute myeloid leukemia. Blood. 2010;116:3147–3156. [DOI] [PubMed] [Google Scholar]
- 7.Schoch C, Kern W, Schnittger S, Buchner T, Hiddemann W, Haferlach T. The influence of age on prognosis of de novo acute myeloid leukemia differs according to cytogenetic subgroups. Haematologica. 2004;89:1082–1090. [PubMed] [Google Scholar]
- 8.Vasu S, Kohlschmidt J, Mrozek K, et al. Ten-year outcome of patients with acute myeloid leukemia not treated with allogeneic transplantation in first complete remission. Blood Adv. 2018;2:1645–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farag SS, Maharry K, Zhang MJ, et al. Comparison of reduced-intensity hematopoietic cell transplantation with chemotherapy in patients age 60–70 years with acute myelogenous leukemia in first remission. Biol Blood Marrow Transplant. 2011;17:1796–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McClune BL, Weisdorf DJ, Pedersen TL, et al. Effect of age on outcome of reduced-intensity hematopoietic cell transplantation for older patients with acute myeloid leukemia in first complete remission or with myelodysplastic syndrome. J Clin Oncol. 2010;28:1878–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aoudjhane M, Labopin M, Gorin NC, et al. Comparative outcome of reduced intensity and myeloablative conditioning regimen in HLA identical sibling allogeneic haematopoietic stem cell transplantation for patients older than 50 years of age with acute myeloblastic leukaemia: a retrospective survey from the Acute Leukemia Working Party (ALWP) of the European group for Blood and Marrow Transplantation (EBMT). Leukemia. 2005;19:2304–2312. [DOI] [PubMed] [Google Scholar]
- 12.Bertz H, Potthoff K, Finke J. Allogeneic stem-cell transplantation from related and unrelated donors in older patients with myeloid leukemia. J Clin Oncol. 2003;21:1480–1484. [DOI] [PubMed] [Google Scholar]
- 13.Estey E, de Lima M, Tibes R, et al. Prospective feasibility analysis of reduced-intensity conditioning (RIC) regimens for hematopoietic stem cell transplantation (HSCT) in elderly patients with acute myeloid leukemia (AML) and high-risk myelodysplastic syndrome (MDS). Blood. 2007;109:1395–1400. [DOI] [PubMed] [Google Scholar]
- 14.McSweeney PA, Niederwieser D, Shizuru JA, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97:3390–3400. [DOI] [PubMed] [Google Scholar]
- 15.Devine SM, Owzar K, Blum W, et al. Phase II Study of Allogeneic Transplantation for Older Patients With Acute Myeloid Leukemia in First Complete Remission Using a Reduced-Intensity Conditioning Regimen: Results From Cancer and Leukemia Group B 100103 (Alliance for Clinical Trials in Oncology)/Blood and Marrow Transplant Clinical Trial Network 0502. J Clin Oncol. 2015;33:4167–4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andersson BS, Thall PF, Madden T, et al. Busulfan systemic exposure relative to regimen-related toxicity and acute graft-versus-host disease: defining a therapeutic window for i.v. BuCy2 in chronic myelogenous leukemia. Biol Blood Marrow Transplant. 2002;8:477–485. [DOI] [PubMed] [Google Scholar]
- 17.Dix SP, Wingard JR, Mullins RE, et al. Association of busulfan area under the curve with venoocclusive disease following BMT. Bone Marrow Transplant. 1996;17:225–230. [PubMed] [Google Scholar]
- 18.Grochow LB. Busulfan disposition: the role of therapeutic monitoring in bone marrow transplantation induction regimens. Semin Oncol. 1993;20:18–25; quiz 26. [PubMed] [Google Scholar]
- 19.Geddes M, Kangarloo SB, Naveed F, et al. High busulfan exposure is associated with worse outcomes in a daily i.v. busulfan and fludarabine allogeneic transplant regimen. Biol Blood Marrow Transplant. 2008;14:220–228. [DOI] [PubMed] [Google Scholar]
- 20.Slattery JT, Clift RA, Buckner CD, et al. Marrow transplantation for chronic myeloid leukemia: the influence of plasma busulfan levels on the outcome of transplantation. Blood. 1997;89:3055–3060. [PubMed] [Google Scholar]
- 21.Palmer J, McCune JS, Perales MA, et al. Personalizing Busulfan-Based Conditioning: Considerations from the American Society for Blood and Marrow Transplantation Practice Guidelines Committee. Biol Blood Marrow Transplant. 2016;22:1915–1925. [DOI] [PubMed] [Google Scholar]
- 22.de Lima M, Giralt S, Thall PF, et al. Maintenance therapy with low-dose azacitidine after allogeneic hematopoietic stem cell transplantation for recurrent acute myelogenous leukemia or myelodysplastic syndrome: a dose and schedule finding study. Cancer. 2010;116:5420–5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scott BL, Pasquini MC, Logan BR, et al. Myeloablative Versus Reduced-Intensity Hematopoietic Cell Transplantation for Acute Myeloid Leukemia and Myelodysplastic Syndromes. J Clin Oncol. 2017;35:1154–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kroger N, Iacobelli S, Franke GN, et al. Dose-Reduced Versus Standard Conditioning Followed by Allogeneic Stem-Cell Transplantation for Patients With Myelodysplastic Syndrome: A Prospective Randomized Phase III Study of the EBMT (RICMAC Trial). J Clin Oncol. 2017;35:2157–2164. [DOI] [PubMed] [Google Scholar]
- 25.Bornhauser M, Kienast J, Trenschel R, et al. Reduced-intensity conditioning versus standard conditioning before allogeneic haemopoietic cell transplantation in patients with acute myeloid leukaemia in first complete remission: a prospective, open-label randomised phase 3 trial. Lancet Oncol. 2012;13:1035–1044. [DOI] [PubMed] [Google Scholar]
- 26.Eapen M, Brazauskas R, Hemmer M, et al. Hematopoietic cell transplant for acute myeloid leukemia and myelodysplastic syndrome: conditioning regimen intensity. Blood Adv. 2018;2:2095–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sengsayadeth S, Gatwood KS, Boumendil A, et al. Conditioning intensity in secondary AML with prior myelodysplastic syndrome/myeloproliferative disorders: an EBMT ALWP study. Blood Adv. 2018;2:2127–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malard F, Labopin M, Cho C, et al. Ex vivo and in vivo T cell-depleted allogeneic stem cell transplantation in patients with acute myeloid leukemia in first complete remission resulted in similar overall survival: on behalf of the ALWP of the EBMT and the MSKCC. J Hematol Oncol. 2018;11:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barba P, Martino R, Zhou Q, et al. CD34(+) Cell Selection versus Reduced-Intensity Conditioning and Unmodified Grafts for Allogeneic Hematopoietic Cell Transplantation in Patients Age >50 Years with Acute Myelogenous Leukemia and Myelodysplastic Syndrome. Biol Blood Marrow Transplant. 2018;24:964–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maples KT, Sabo RT, McCarty JM, Toor AA, Hawks KG. Maintenance azacitidine after myeloablative allogeneic hematopoietic cell transplantation for myeloid malignancies. Leuk Lymphoma. 2018;59:2836–2841. [DOI] [PubMed] [Google Scholar]
- 31.Pusic I, Choi J, Fiala MA, et al. Maintenance Therapy with Decitabine after Allogeneic Stem Cell Transplantation for Acute Myelogenous Leukemia and Myelodysplastic Syndrome. Biol Blood Marrow Transplant. 2015;21:1761–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Craddock C, Jilani N, Siddique S, et al. Tolerability and Clinical Activity of Post-Transplantation Azacitidine in Patients Allografted for Acute Myeloid Leukemia Treated on the RICAZA Trial. Biol Blood Marrow Transplant. 2016;22:385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Platzbecker U, Middeke JM, Sockel K, et al. Measurable residual disease-guided treatment with azacitidine to prevent haematological relapse in patients with myelodysplastic syndrome and acute myeloid leukaemia (RELAZA2): an open-label, multicentre, phase 2 trial. Lancet Oncol. 2018;19:1668–1679. [DOI] [PubMed] [Google Scholar]
- 34.Soiffer RJ, Kim HT, McGuirk J, et al. Prospective, Randomized, Double-Blind, Phase III Clinical Trial of Anti-T-Lymphocyte Globulin to Assess Impact on Chronic Graft-Versus-Host Disease-Free Survival in Patients Undergoing HLA-Matched Unrelated Myeloablative Hematopoietic Cell Transplantation. J Clin Oncol. 2017;35:4003–4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soiffer RJ, Lerademacher J, Ho V, et al. Impact of immune modulation with anti-T-cell antibodies on the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Blood. 2011;117:6963–6970. [DOI] [PMC free article] [PubMed] [Google Scholar]