Abstract
Background:
There is a clinical need to be able to reliably detect meaningful changes (0.1 to 0.2 m/s) in usual gait speed (UGS) considering reduced gait speed is associated with morbidity and mortality.
Research question:
What is the impact of tester on UGS assessment, and the influence of test repetition (trial 1 vs. 2), timing method (manual stopwatch vs. automated timing), and starting condition (stationary vs. dynamic start) on the ability to detect changes in UGS and fast gait speed (FGS)?
Methods:
UGS and FGS was assessed in 725 participants on a 8-m course with infrared timing gates positioned at 0, 2, 4 and 6 m. Testing was performed by one of 13 testers trained by a single researcher. Time to walk 4-m from a stationary start (i.e. from 0-m to 4-m) was measured manually using a stopwatch and automatically via the timing gates at 0-m and 4-m. Time taken to walk 4-m with a dynamic start was measured during the same trial by recording the time to walk between the timing gates at 2-m and 6-m (i.e. after 2-m acceleration).
Results:
Testers differed for UGS measured using manual vs. automated timing (p=0.02), with five and two testers recording slower and faster UGS using manual timing, respectively. 95% limits of agreement for trial 1 vs. 2, manual vs. automated timing, and dynamic vs. stationary start ranged from ±0.15 m/s to ±0.20 m/s, coinciding with the range for a clinically meaningful change. Limits of agreement for FGS were larger ranging from ±0.26 m/s to ±0.35 m/s.
Significance:
Repeat testing of UGS should performed by the same tester or using an automated timing method to control for tester effects. Test protocol should remain constant both between and within participants as protocol deviations may result in detection of an artificial clinically meaningful change.
Keywords: activities of daily living, locomotion, physical examination, physical fitness, physical functional performance, walking speed
INTRODUCTION
Gait speed is proposed as the 6th or ‘functional’ vital sign [1]. Reductions in usual gait speed (UGS) below 1.0 m/s are associated with declines in health and life expectancy [2]. To longitudinally monitor an individual’s UGS, there is a need for reliable measures which can detect clinically meaningful changes. Meaningful changes in UGS are reported to range from 0.1 m/s to 0.2 m/s [3,4].
There is no single accepted method of assessing UGS [1]. A common approach is to use a stopwatch to manually time how long an individual takes to walk 4-m from a stationary start [5], although numerous variations have been reported [6-8]. Previous studies have explored the impact of different testing protocols on UGS measurement (see [9] for review); however, analyses were often performed during asynchronous tests where methodological factors were assessed during different trials.
The purpose of this study was to use synchronous testing to explore the impact of tester on UGS assessment, and assess the influence of test repetition (trial 1 vs. 2), timing method (manual stopwatch vs. automated timing), and starting condition (stationary vs. dynamic start) on both UGS and fast gait speed (FGS).
MATERIALS AND METHODS
Individuals aged ≥5 years who could ambulate ≥10-m were invited to participate. There were no exclusion criteria in terms of health status or use of gait assistive devices. Prior Institutional Review Board approval was obtained and all participants provided written informed consent.
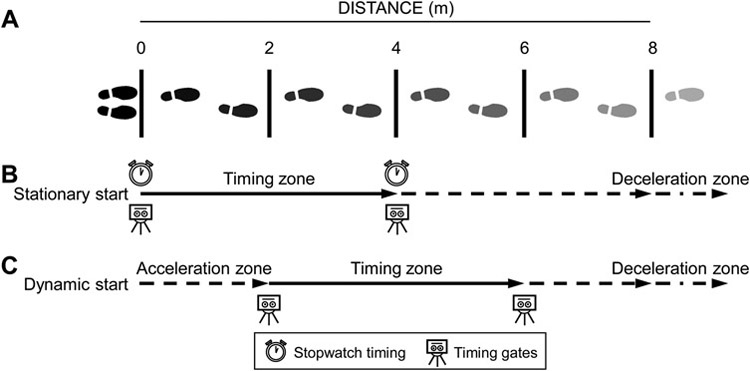
Gait speed was assessed on a permanent 8-m course (Fig. 1A). Tape lines were positioned every 2-m orthogonal to the direction of travel. Infrared timing gates (TF100 multi-beam timing system; TracTronix, Lenexa, KS) positioned at ankle height were centered on the lines at 0, 2, 4 and 6-m and were triggered when any part of the leading leg crossed the plane of the gate. Participants were positioned with their feet together behind the 0-m line, and were instructed to continue walking during each test until passing the 8-m tape line.
Figure 1.
Schematic of testing set-up. Participants performed test on an 8-m course with tape lines positioned every 2-m (A). Time to walk 4-m from a static start was simultaneously measured manually using a stopwatch and automatically via timing gates positioned at 0-m and 4-m (B). Time taken to walk 4-m with a dynamic start was measured during the same trial via timing gates at 2-m and 6-m (i.e. after 2-m acceleration). Participants continued walking during each trial until the tape at 8-m before decelerating.
Testing was performed by one of 13 testers trained by a single experienced researcher (S.J.W.) to follow the same procedure and script. The general procedure and script followed gait speed testing in the Short Physical Performance Battery (SPPB) [10]. For UGS, the instruction was: “Walk at your normal speed, just as if you were walking down the street.” The tester demonstrated the test and participants performed a practice trial before completing two timed trials. The procedure was repeated to measure FGS with the instruction: “I want you to walk as quickly, but as safely as you can without running.”
Time to walk 4-m from a stationary start (i.e. 0-m to 4-m) was simultaneously measured manually using a stopwatch and automatically via the timing gates positioned at 0-m and 4-m (Fig. 1B). The stopwatch was started as soon as the participant’s foot began lifting and stopped when any part of their leading foot crossed the plane of the line at 4-m. Testers stood at the 4-m line during testing to monitor the leading foot. Time taken to walk 4-m with a dynamic start was measured during the same trial via the timing gates at 2-m and 6-m (i.e. after 2-m acceleration) (Fig. 1C).
The influence of tester on UGS from a standing start was determined from the difference in gait speed between manual and automated timing methods, with the latter removing tester effects. A linear mixed model with tester as a random effect was used to assess between testers. Intraclass correlation coefficients (ICC2,1) evaluated reliability between test conditions for both UGS and FGS. Agreement between manually timed trials 1 and 2, manual and automated timing methods, and dynamic and stationary start conditions for both UGS and FGS was evaluated using the Bland-Altman method and calculating 95% limits of agreement (LOA) [11]. The trial with the maximum gait speed was used for analyses exploring the impact of timing method or starting condition, as per the SPPB [10].
RESULTS
There were 725 participants (Table 1). Testers assessed an average of 56±36 participants. There were differences between testers for UGS measured using manual versus automated timing (p=0.02). Five testers recorded slower and two testers recorded faster UGS using manual versus automated timing, respectively (all p<0.05). The largest mean difference between testers was 0.095 m/s (95%CI, 0.050-0.141).
Table 1.
Participant characteristics (n = 725)
| Characteristic | ||
|---|---|---|
| Age (yr) | ||
| Mean ± SD | 45.4 ± 17.7 | |
| Range | 5.6 - 89.9 | |
| Median (IQR) | 46.8 (32.0 - 61.6) | |
| Age frequency (N) | ||
| <18 yrs | 34 | |
| 18 to <35 yrs | 205 | |
| 35 to <50 yrs | 166 | |
| 50 to <65 yrs | 220 | |
| >65 yrs | 100 | |
| Sex (M:F) | ||
| N | 207:518 | |
| % | 29:71 | |
| Height (m) | ||
| Mean ± SD | 1.66 ± 0.11 | |
| Range | 1.10 - 1.97 | |
| Median (IQR) | 1.66 (1.60 - 1.72) | |
| Weight (kg) | ||
| Mean ± SD | 74.8 ± 19.3 | |
| Range | 18.2 - 155.1 | |
| Median (IQR) | 72.2 (61.4 - 85.2) | |
| BMI (kg/m2) | ||
| Mean ± SD | 26.9 ± 6.2 | |
| Range | 13.8 - 49.5 | |
| Median (IQR) | 25.5 (22.6 - 30.3) | |
| Gait assistive device (Yes:No) | ||
| N | 14:711 | |
| % | 2:98 | |
| Usual gait speed (m/s)† | ||
| Mean ± SD | 1.39 ± 0.22 | |
| Range | 0.38 - 2.12 | |
| Median (IQR) | 1.38 (1.27 - 1.54) | |
| Fast gait speed (m/s)† | ||
| Mean ± SD | 1.93 ± 0.32 | |
| Range | 0.45 - 3.64 | |
| Median (IQR) | 1.92 (1.76 - 2.12) | |
SD = standard deviation; IQR = interquartile range
Measured manually (stopwatch) from a static start as the maximum from 2 repeat trials
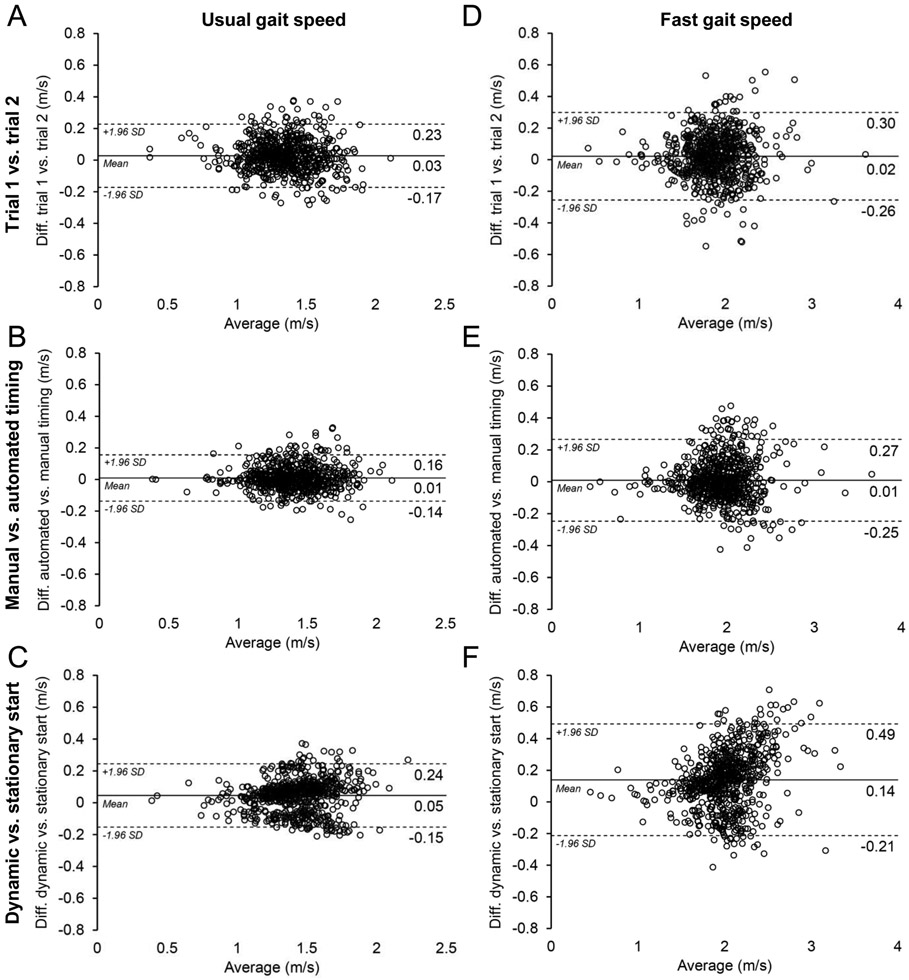
Reliability for manually timed trial 1 vs. 2, manual vs. automated timing, and stationary vs. dynamic start for both UGS and FGS was excellent (ICC2,1=0.93-0.97). Mean differences and 95%LOAs between comparative test conditions are shown in Figure 2. The 95%LOAs for UGS ranged from ±0.15 m/s (Fig. 2B) to ±0.20 m/s (Fig. 2A&C). Sixteen percent of participants had >0.1 m/s difference in UGS between manual and automated timing methods. There was no difference between manual and automated timing methods for participants in the slowest (<1.10 m/s) and fastest (>1.61 m/s) UGS quartiles (0.02 m/s; 95%LOA, ±0.13 m/s and −0.01 m/s; 95%LOA, ±0.15 m/s, respectively).
Figure 2.
Bland-Altman plots of agreement (±95% levels of agreement) for trial 1 vs. 2 (A & D), manual vs. automated timing (B & E), and stationary vs. dynamic start (C & F) for usual and fast gait speed.
The 95%LOAs were wider for FGS ranging from ±0.26 m/s for manual vs. automated timing (Fig. 2D) to ±0.35 m/s for a stationary vs. dynamic start (Fig. 2F). Participant UGS and FGS was 2.9% (95%CI, 2.4 to 3.4%) and 6.3% (95%CI, 5.7 to 6.8%) faster with a dynamic vs. static start, respectively (Fig. 2C&F).
DISCUSSION
These data show both tester and testing protocol influence clinically determined gait speed. Despite testers being identically trained and using explicit instructions, UGS differed between testers when manual vs. automated timing was used. The origin of the discrepancy is not known, but is likely due to subtle differences in stopwatch triggering between testers. The observation raises questions regarding the inter-tester agreement of manually timed UGS and indicates repeat testing should be performed by the same tester. Alternatively, automated timing could be implemented, when finances permit, as it removes tester effects and may improve the questioned reliability of manual timing [12].
Further support for automated timing is provided by the 95%LOA between UGS determined using manual vs. automated timing. The mean difference in UGS between timing methods (0.01 m/s) was in the range of that previously reported during synchronous testing [13], but the 95%LOA (±0.15 m/s) was in the range for a meaningful change (0.1 to 0.2 m/s). If we consider UGS determined from automated timing is the ‘gold standard’ as it removes tester effects, UGS determined via manual timing may not provide acceptable agreement as 16% of participants had a potentially meaningful difference (0.1 m/s) between timing methods.
Previous systematic reviews were unable to conclude whether gait speeds following a dynamic start differed from those following a static start [7,9]. Our data show UGS and FGS are 0.05 m/s (95%CI, ±0.01 m/s) and 0.14 m/s (95%CI, ±0.01 m/s) faster following an initial 2-m acceleration. The benefit of initial acceleration on UGS (+0.05 m/s) in our cohort was less than that reported by Sustakoski et al. [14] (+0.16 m/s) who studied slower (mean speed = 0.97 m/s), older adults (mean age = 77 yrs). It is possible older or slower individuals benefit more from a dynamic start as they can require 2.5-m before steady state walking is achieved [15]. However, the benefit of an acceleration zone on UGS in our cohort did not differ between young (18-35 yrs) and older (>65 yrs) adults (p=0.94, unpaired t-test) or in individuals with the slowest (<1.0 m/s) and fastest (>1.5 m/s) UGS (p=0.82, unpaired t-test).
Overall, our cumulative data suggest limitations in the assessment of UGS using manual timing methods with both tester and testing protocol potentially impacting the ability to detect a clinically meaningful change over time. To improve the assessment of UGS, automated timing can be considered (when finances permit) to control the influence of tester and the testing protocol should remain constant.
HIGHLIGHTS.
Clinically meaningful changes in gait speed range from 0.1 to 0.2 m/s
Assessment of usual gait speed using manual timing differs between testers
95% limits of agreement for protocol deviations coincide with meaningful changes
Repeat testing should be performed by the same tester using a single protocol
Alternatively, automated timing may be considered to control tester effects
Acknowledgements:
This contribution was made possible by support from the National Institutes of Health (P30 AR072581).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement: The authors have no conflicts of interest
REFERENCES
- [1].Middleton A, Fritz SL, Lusardi M. Walking speed: the functional vital sign. J Aging Phys Act 2015;23:314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Studenski S, Perera S, Patel K, Rosano C, Faulkner K, et al. Gait speed and survival in older adults. JAMA 2011;305:50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bohannon RW, Glenney SS. Minimal clinically important difference for change in comfortable gait speed of adults with pathology: a systematic review. J Eval Clin Pract 2014;20:295–300. [DOI] [PubMed] [Google Scholar]
- [4].Chui K, Hood E, Klima D. Meaningful change in walking speed. Top Geriatr Rehabil 2012;28:97–103. [Google Scholar]
- [5].Reuben DB, Magasi S, McCreath HE, Bohannon RW, Wang Y-C, et al. Motor assessment using the NIH Toolbox. Neurology 2013;80:S65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Graham JE, Ostir GV, Fisher SR, Ottenbacher KJ. Assessing walking speed in clinical research: a systematic review. J Eval Clin Pract 2008;14:552–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Peel NM, Kuys SS, Klein K. Gait speed as a measure in geriatric assessment in clinical settings: a systematic review. J Gerontol A Biol Sci Med Sci 2013;68:39–46. [DOI] [PubMed] [Google Scholar]
- [8].Salbach NM, O'Brien KK, Brooks D, Irvin E, Martino R, et al. Reference values for standardized tests of walking speed and distance: a systematic review. Gait Posture 2015;41:341–360. [DOI] [PubMed] [Google Scholar]
- [9].Graham JE, Ostir GV, Kuo YF, Fisher SR, Ottenbacher KJ. Relationship between test methodology and mean velocity in timed walk tests: a review. Arch Phys Med Rehabil 2008;89:865–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994;49:M85–94. [DOI] [PubMed] [Google Scholar]
- [11].Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307–310. [PubMed] [Google Scholar]
- [12].Bohannon RW, Wang YC. Four-meter gait speed: normative values and reliability determined for adults participating in the NIH Toolbox Study. Arch Phys Med Rehabil 2019;100:509–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Peters DM, Fritz SL, Krotish DE. Assessing the reliability and validity of a shorter walk test compared with the 10-Meter Walk Test for measurements of gait speed in healthy, older adults. J Geriatr Phys Ther 2013;36:24–30. [DOI] [PubMed] [Google Scholar]
- [14].Sustakoski A, Perera S, VanSwearingen JM, Studenski SA, Brach JS. The impact of testing protocol on recorded gait speed. Gait Posture 2015;41:329–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lindemann U, Najafi B, Zijlstra W, Hauer K, Muche R, et al. Distance to achieve steady state walking speed in frail elderly persons. Gait Posture 2008;27:91–96. [DOI] [PubMed] [Google Scholar]