Abstract
Objectives
The current study aims to find the differences between glioblastoma multiforme (GBM) and giant cell glioblastoma (GCG) regarding mortality and prognosis among adults and elderly patients in the U.S.
Methods and Materials
This study is a historical cohort type of study and is conducted on adults and elderly individuals with GBM or GCG from the years 1985–2014 in the U.S. Data were collected from the Surveillance, Epidemiology, and End Results Program (SEER) database. The study exposure was GBM or GCG and the outcome was mortality. The potential confounders were age, sex, race, ethnicity, year of diagnosis, primary site, brain overlap, and surgery. A chi‐square test was used for categorical data. A univariate analysis was used for variables having a p‐value <.05. Potential confounders were selected and evaluated using multivariate logistic regression models to calculate the odds ratio with stepwise selection.
Results
The study sample was 25,117. The incidences of GBM and GCG were not similar in relation to age group. Also, Spanish–Hispanic ethnicity was independently protective of GBM and GCG as compared to Non‐Spanish–Hispanic ethnicity patients with GBM have a higher mortality rate than do GCG patients. The mortality rate was higher among patients diagnosed before 2010.
Conclusion
GCG was not statistically significant in association to reduced mortality. Non‐Spanish–Hispanics with GBM or GCG had a higher mortality rate than did Spanish–Hispanics. Factors such as being female, being age 59–65, and having a year of diagnosis before 2010 were independently associated with increased mortality.
Keywords: brain cancer, giant cell glioblastoma, glioblastoma multiforme, mortality, prognosis
When comparing glioblastoma multiforme (GBM) and giant cell glioblastoma (GCG) using SEER database, the researches have found the following: GCG was not statistically significant in association with reduced mortality. Non‐Spanish–Hispanics with GBM or GCG had a higher mortality rate than did Spanish–Hispanics. Factors such as being female, being age 59–65, and having a year of diagnosis before 2010 were independently associated with increased mortality.
1. INTRODUCTION
Brain cancer and other nervous system cancers are the tenth leading cause of death in the U.S. Brain cancer is common among adults and elderly individuals (SEER Program). Giant cell glioblastoma (GCG) is a rare neoplasm characterized by a predominance of bizarre multinucleated giant cells with abundant eosinophilic cytoplasm. (Ohgaki, Peraud, Nakazato, Watanabe, & Deimling, 2000).
Significant efforts to characterize this unusual malignancy have established a glial origin, and it is now considered a subtype of glioblastoma multiforme (GBM; Akslen, Mork, Larsen, & Myrseth, 1988; Becker, Benyo, & Roessmann, 1967; Hadfield & Silverberg, 1972; Katoh et al., 1995; Kawano et al., 1995; Margetts & Kalyan‐Raman, 1989).
GCG has been reported to represent between 2% and 5% of GBM cases (Artico, Cervoni, Celli, Salvati, & Palma, 1993; Palma, Celli, Maleci, Di Lorenzo, & Cantore, 1989; Shinojima et al., 2004).
Importantly, several small series and case reports have suggested that the prognosis of GCG is significantly better than that observed for GBM (Akslen et al., 1988; Becker et al., 1967; Burger & Vollmer, 1980; Chang, Kuwana, Ito, Koike, & Kitamura, 2001; Deb, Sharma, Chander, Mahapatra, & Sarkar, 2006; Gullotta, Casentini, & Neumann, 1980; Klein, Molenkamp, Sorensen, & Roggendorf, 1998; Kroh, Matyja, Marchel, & Bojarski, 2004; Margetts & Kalyan‐Raman, 1989; Sabel, Reifenberger, Weber, Reifenberger, & Schmitt, 2001; Shinojima et al., 2004). The development of GBG is highly related to mutations of the TP53 gene (Kleihues & Glioblastoma,)
MGMT promoter methylation, mutations in the IDH1/2 genes, or BRAF mutations, which are actually used as diagnostic, prognostic, and predictive molecular markers in anaplastic glial tumors (Lohkamp et al., 2016).
Glioblastoma multiforme is a common malignant tumor that originates from astrocytes. It is a rapid‐growing tumor that affects the nervous system, including the brain and the spinal cord (GBM, 2017).
It is estimated that GBM cases in the U.S. account for approximately 20% of all primary CNS tumors in the adult population and almost 75% of all anaplastic gliomas (Nizamutdinov et al., 2018). Glioblastoma multiforme is the most lethal primary malignant central nervous system tumor in adults (Li et al., 2015; Ohgaki & Kleihues, 2005; Stupp, Mason, & Bent, 2005). GBM incidence and prognosis have changed over the past few years. This has been explained by several risk factors, such as sex, age group, race, ethnicity, year of diagnosis, primary site, and surgical removal of the tumor (Pietschmann et al., 2015; Stummer et al., 2008). It has been found that the overall prognosis of patients with GBM is poor, with a median survival of 14.6 months and a five‐year survival rate of <5% (Ostrom et al., 2013; Stupp et al., 2005). A review of the relevant literature, which included a well‐conducted systematic review (Beyer et al., 2016), provided evidence of an association between survival in cases of glioblastoma and several prognostic factors, including age at diagnosis, sex, race/ethnicity, primary site, and treatment (including surgery). However, no information was available about the effect of subtypes of glioblastoma and prognosis, particularly in terms of whether survival in cases of giant cell glioblastoma was different from that in cases of other subtypes of glioblastoma multiforme. Kozak and Moody (2009) conducted a study using the Surveillance, Epidemiology, and End Results (SEER) database from 1988–2004, with which they made a comparison between GCG and GBM and found that GCG had a better prognosis. The present study included samples from 1985 to 2014 to discover the difference in prognosis between glioblastoma subtypes after the evolution of treatment modalities over the past few years. Therefore, the current study aimed to find the differences between GBM and GCG regarding mortality and prognosis among adults and elderly patients in the U.S.
2. MATERIALS AND METHODS
2.1. Study strategy and data source
A historical cohort was assembled using data from the Surveillance, Epidemiology, and End Results (SEER) database in July 2017 (http://www.seer.cancer.gov/). The data were collected via SEER*Stat software from 1985 to 2014. The SEER program was established in 1973 by the U.S. NCI and collects incidences and survival records of patients with malignant tumors from 18 population‐based cancer registries in the U.S. (SEER). The registries represent approximately 28% of the population of the U.S.; registries were selected, in part, for their diverse population subgroups. These surveys have multistage sampling and are considered to be complex, overestimated, and not representative of the entire U.S. population. However, SEER does its own modeling through extrapolation.
2.2. Study population
Patients aged younger than 20 years have a lower incidence rate; frequency rapidly increases starting in the fifth decade of life (Furnari et al., 2007). Therefore, the inclusion criteria for the analysis were patients with a confirmed diagnosis of GBM or GCG at age 18 to 65 from the years 1985–2014. The exclusion criteria included insurance, grading, and tumor size, due to a high percentage (over 25%) of missing data in the SEER database. The SEER database included patients’ insurance data from the years 2007 and onwards. Also, in terms of tumor size, 65% of data was missing in the database. However, glioblastoma has no clear grading system, as it is a type of glioma and is considered the most malignant type (type 4). Therefore, grading was also excluded (http://www.brainlife.org/abstract/2017/Mesfin_F170715.pdf).
2.3. Study variables
The study variables included data of GBM patients (histology codes: ICD‐O‐3:9440/3, 9441/3) with tumors located in several locations: supratentorial (cerebrum, frontal lobe, temporal lobe, parietal lobe, occipital lobe), brain overlap, and infratentorial (cerebellum, ventricle, and brainstem). In addition, primary site codes (C71.0‐C72.0) were extracted from the SEER database. Figure 1 shows the variables that were analyzed.
Figure 1.
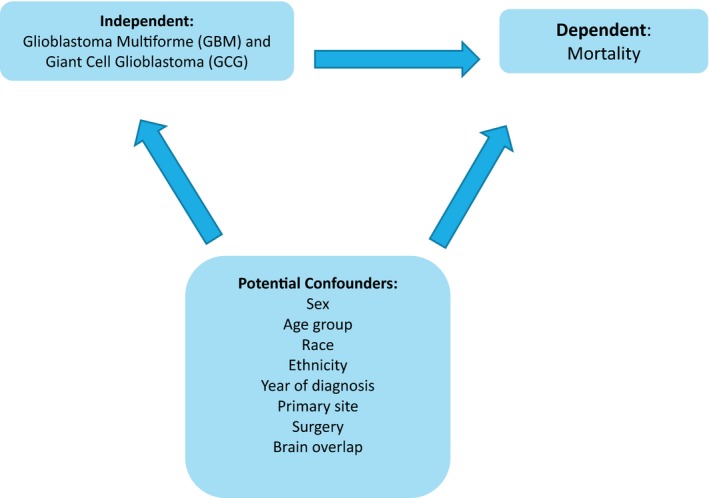
Variables were analyzed using the SEER database and Stata software
In addition, the SEER research data record description was used to categorize other variables such as race, which was categorized into White, Black, and Others. Ethnicity was also categorized into Non‐Spanish–Hispanic–Latino and Spanish–Hispanic–Latino. Year of diagnosis was categorized into years before 2010 and years 2010–2014 due to the approval of Bevacizumab for recurrent glioblastoma in 2010 (Johnson, Leeper, & Uhm, 2013).
2.4. Statistical analysis
First, the population was selected from the SEER database. Then, the characteristics of the population were described. After that, the general distribution of the data was examined. Next, some variables were transformed into appropriate categories (e.g., group was categorized into adults from 18 to 65 years old and elderly individuals 59–65 years old; Li et al., 2017). The primary site was categorized into supratentorial, brain overlap (including the brain ventricles and other unspecified brain locations), and infratentorial regions.
The alpha level was set at 0.2 due to the small sample size of GCG incidences in the SEER database.
A chi‐square test was used for categorical data. Categorical data were expressed by numbers (n) and percentage (%). A univariate analysis was used for variables having a p‐value < .05, while potential confounders (patient's sex, age group, race, ethnicity, year of diagnosis, primary site, brain overlap, and surgery) were selected and evaluated by multivariate logistic regression models to calculate the odds ratio with stepwise selection. A collinearity model was used to determine the relationship between each of the confounders for the exclusion of dependent variables. However, no significant relationship between the confounders was excluded.
2.5. Ethical considerations
Ethical approval was waived, since the analysis was considered nonhuman subjects research by the Florida International University Health Science Institutional Review Board.
3. RESULTS
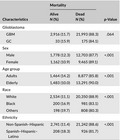
The study sample was 25,117. It included 24,909 patients with GBM and 208 with GCG. However, 88.3% of patients with GBM died within a few years, while 84.1% of GCG patients also died from the tumor. The baseline characteristics of the study sample are explained in Table 1, which shows that gender has a slight variation in GBM and GCG incidences. Males are more likely to develop GBM than GCG; conversely, females are more likely to develop GCG. Table 1 also shows that the incidence of GBM and GCG is not similar in relation to age group. Hence, it is statistically significant that adults have a higher predisposition to developing GCG than GBM.
Table 1.
Baseline characteristics of GBM and GCG patients from 1985–2014 in US
| Characteristics | Type of glioblastoma | ||
|---|---|---|---|
|
GBM NOS N (%) |
GCG N (%) |
p‐Value | |
| Sex | |||
| Male | 14,375 (57.7) | 115 (55.3) | .481 |
| Female | 10,534 (42.3) | 93 (44.7) | |
| Age group | |||
| Adults (18–59) | 10, 221 (41.0) | 120 (57.7) | <.001 |
| Elderly (>60) | 14,686 (59.0) | 88 (42.3) | |
| Race | |||
| White | 22,700 (91.3) | 184 (88.5) | .318 |
| Black | 1,169 (4.7) | 12 (5.8) | |
| Other | 994 (4.0) | 12 (5.8) | |
| Ethnicity | |||
| Non‐Spanish–Hispanic–Latino | 23,791 (95.5) | 192 (92.3) | .027 |
| Spanish–Hispanic–Latino | 1,118 (4.5) | 16 (8) | |
| Year of diagnosis | |||
| Before 2010 | 20,719 (83.3) | 171 (82.2) | .71 |
| 2010–2014 | 4,190 (16.8) | 37 (17.8) | |
| Primary site | |||
| Supratentorial | 17,828 (71.6) | 168 (80.8) | <.001 |
| Brain overlap | 6,767 (27.2) | 33 (15.9) | |
| Infratentorial | 314 (1.3) | 7 (3.4) | |
| Surgery | |||
| None | 3,287 (26.1) | 13 (11.4) | <.001 |
| No GTR | 5,719 (45.5) | 50 (43.9) | |
| GTR | 3,574 (28.4) | 51 (44.7) | |
Abbreviations: GBM, glioblastoma multiforme; GCG, giant cell glioblastoma; GTR, gross total resection; NOS, not otherwise specified.
Race also reveals some variations in terms of the two subtypes of glioblastoma, with individuals who have a white racial background being more prone to GBM, while individuals of other races being more prone to GCG. The Non‐Spanish–Hispanic–Latino ethnicity has a slightly higher incidence of GBM than GCG, while, inversely, Spanish–Hispanic–Latinos have fewer incidences of GBM than GCG. The incidence of GBM was slightly higher than the incidence of GCG before 2010; after 2010, the incidence of GCG was higher. However, incidences of both tumors have decreased considerably since 2010.
The study reveals some statistically significant differences in terms of tumor primary site, with high statistical significance. Both subtypes of tumors originate more often in the supratentorial part of the brain than elsewhere in the central nervous system. However, GCG tumors originate more from the supratentorial site than do GBM tumors. It is also statistically significant that GBM risk is higher in patients with no surgery or no gross total resection, while patients with gross total resection (GTR) have an elevated GCG risk. Table 2 shows that patients with GBM have a higher mortality rate than do GCG patients. Table 3 shows that GCG has an odds ratio [OR] of 0.56 with a confidence interval of 0.53–1.44, which is independently associated with reduced mortality.
Table 2.
Mortality rate of GBM and GCG patients from 1985–2014 in US
| Characteristics | Mortality | p‐Value | |
|---|---|---|---|
|
Alive N (%) |
Dead N (%) |
||
| Glioblastoma | |||
| GBM | 2,916 (11.7) | 21,993 (88.3) | .064 |
| GC | 33 (15.9) | 175 (84.1) | |
| Sex | |||
| Male | 1,778 (12.3) | 12,703 (87.7) | <.001 |
| Female | 1,162 (10.9) | 9,465 (89.1) | |
| Age group | |||
| Adults | 1,464 (14.2) | 8,877 (85.8) | <.001 |
| Elderly | 1,483 (10.0) | 13,291 (90.0) | |
| Race | |||
| White | 2,534 (11.1) | 20,350 (88.9) | <.001 |
| Black | 200 (16.9) | 981 (83.1) | |
| Others | 198 (19.7) | 808 (80.3) | |
| Ethnicity | |||
| Non‐Spanish–Hispanic | 2,741 (11.4) | 21,242 (88.6) | <.001 |
| Spanish–Hispanic–Latino | 208 (18.3) | 926 (81.7) | |
Abbreviations: GBM, glioblastoma multiforme; GCG, giant cell glioblastoma.
Table 3.
Odds ratio of GBM and GCG patients from 1985–2014 in US
| Characteristics | Unadjusted | Adjusteda | ||
|---|---|---|---|---|
| OR (95% CI) | N | OR (95% CI) | N | |
| Glioblastoma | ||||
| GBM | Reference | |||
| GCG | 0.70 (0.5–1.02) | 25,117 | 0.88 (0.53–1.44) | 12,694 |
Abbreviations: CI, confidence interval; GBM, glioblastoma multiforme; GCG, giant cell glioblastoma; OR, odds ratio.
Adjusted for age, sex, race, ethnicity, year of diagnosis, primary site surgery.
Table 2 also shows a slight difference in mortality between age groups in relation to the two glioblastoma subtypes; this difference is statistically significant. It indicates that elderly patients have a worse prognosis than do adults. Glioblastoma patients with a white racial background also face a slightly increased risk of death. The Spanish–Hispanic–Latino ethnicity has a lower mortality rate than do Non‐Spanish–Hispanic–Latinos, as explained in Table 3. The Spanish–Hispanic–Latino ethnicity is independently protective from GBM and GCG (OR 0.63, CI = 0.52–0.77). GBM and GCG tumors with brain overlap have a statistically significant worse outcome than do other primary tumor sites, as shown in Table 2.
Surgery also plays a role in patients’ outcomes. The mortality rate increases in patients with no tumor resection. As shown in Table 3, the factors independently associated with increased mortality are: being female ([OR] 1.12, CI = 1.01–1.25), being age 59 to 65 years (OR 1.64, CI = 1.48–1.82), and being diagnosed earlier than 2010 (OR 5.26, CI = 4.74–5.84). Table 4 shows some secondary findings of the study.
Table 4.
Secondary findings of race/ethnicity and the year of diagnosis
| Characteristics | Unadjusted | Adjusted | ||
|---|---|---|---|---|
| OR (95% CI) | p‐Value | OR (95% CI) | p‐Value | |
| Race | ||||
| White | REF | |||
| Black | 0.61 (0.52–0.71) | <.001 | 0.64 (0.52–0.79) | <.001 |
| Others | 0.50 (0.43–0.60) | <.001 | 0.61 (0.50–0.75) | <.001 |
| Ethnicity | ||||
| Non‐Spanish–Hispanic | REF | |||
| Spanish–Hispanic–Latino | 0.57 (0.49–0.67) | <.001 | 0.63 (0.52–0.77) | <.001 |
| Year of diagnosis | ||||
| Before 2010 | 5.44 (5.01–5.91) | <.001 | 5.26 (4.74–5.84) | <.001 |
| 2010–2014 | REF | |||
Abbreviations: CI, confidence interval; OR, odds ratio.
4. DISCUSSION
To the best of our knowledge, this study is one of the few that address the association of subtype of glioblastoma and mortality in adults in the U.S. after 2010 and that involves a large sample size in GCG and GBM with the utilization of ICD‐0‐3 codes. GBM is more common than GCG and has a higher mortality rate. On the other hand, the current study provides statistically significant data about ethnicity, explaining that the Spanish–Hispanic–Latino ethnicity is independently protective from both glioblastoma subtypes as compared to the Non‐Spanish–Hispanic ethnicity. Furthermore, factors like being female, being age 59 to 65, and having a year of diagnosis before 2010 are independently associated with increased mortality.
This study found that elderly individuals have the highest mortality rate among GBM and GCG patients in comparison to adults (p < .001). Some studies were consistent with the previous findings (Hartmann et al., 2010; Lin & Wagner, 2015; Murthy, Krumholz, & Gross, 2004; Rong et al., 2016). Therefore, age is considered a significant predictor of survival time (Shah, Bista, & Sharma, 2016). This study also demonstrates that elderly individuals are more prone to having GBM than GCG, which explains the rarity of GCG. This finding may indicate that the elderly population is more susceptible to GBM due to an increased chance that cells will mutate into cancer cells. The current study demonstrated that more males are afflicted with GBM than with GCG, while more females are afflicted with GCG (p = .481), consistent with (Colen, Wang, Singh, Gutman, & Zinn, 2015; Matsuda et al., 2011; Nizamutdinov et al., 2018; Ohgaki et al., 2004; Shinojima et al., 2004; Verger et al., 2011). Another study, conducted on Black patients with GBM, showed that Black males were affected by GBM more than were Black females (Loukas, 2014). Therefore, GCG, an uncommon type of glioblastoma multiforme, more often affects females. However, GBM affects males more than females, regardless of race. The previous findings may be explained by genetic factors.
The present study stated that the mortality rate is higher among GBM and GCG patients diagnosed before 2010 (p < .001). Also, one study showed that the prognosis for elderly patients with glioblastoma has improved since the introduction of the Stupp regimen (i.e., radiotherapy plus concomitant and adjuvant temozolomide) in 2005 (Shah et al., 2016). This indicates that year of diagnosis has a significant impact on the prognosis of glioblastoma patients. However, the proportion of patients with GBM is slightly higher than the proportion of GCG patients before 2010. On the other hand, the proportion of GCG incidences is slightly higher than the proportion of GBM incidences after 2010 (p = .71).
Patients who did not have a GTR have a higher mortality rate (p < .001). Moreover, patients who had not undergone surgery or GTR developed GBM more often than they did GCG (p < .001).
Studies like (Koul, Dubey, Torri, Kakumanu, & Goyal, 2012; Pan, Ferguson, & Lam, 2015) had similar findings, stating that GTR has a better survival rate than does partial resection or biopsy. Brain overlap GBM and GCG tumors are associated with higher mortality rates than are supratentorial and infratentorial tumors (p < .001). This finding was similar in one study (Becker et al., 1967).
However, another study showed that the median survival time for both cerebellar GBM (cGBM) and supratentorial GBM (sGBM) patients is 8 months, though sGBM had a worse prognosis as the study progressed (Jeswani et al., 2013). Also, patients with brain overlap tumors have a higher tendency to develop GBM than GCG (p < .001). Because GBM is more common than GCG, it affects brain overlap regions more than supra‐ and infratentorial regions (which are affected more by GCG, p < .001). This accounts for the higher mortality rate. Non‐Spanish–Hispanic people have a higher mortality rate from GBM (88.6%, p < .001). In addition, a study done on Americans with glioblastoma suggested that Latinos tend to have a lower incidence of GBM and present slightly younger than non‐Latino Whites (Shabihkhani et al., 2017).
However, white people were found to have the highest incidence of death from GBM and GCG as compared to individuals of other races (p < .001).
4.1. Limitations
Unfortunately, SEER registry has some unregistered variables, underreported and missing data regarding surgery followed by chemo‐ or radiotherapy. There were also different styles in data coding and reporting, and movement of patients in and out of SEER registry areas.
Furthermore, SEER database may have been affected by the selection bias. In which, prospective studies might be influenced as well. For example, immortal time bias in the assessment of surgery followed by chemo‐ or radiotherapy effectiveness.
5. CONCLUSIONS
GCG was not statistically significant in terms of its association with reduced mortality. Factors such as being female, being age 59 to 65, and having a year of diagnosis before 2010 were independently associated with increased mortality. The Spanish–Hispanic ethnicity was independently protective from GBM and GCG as compared to the Non‐Spanish–Hispanic ethnicity. Additional studies should be conducted on GBM and GCG patients with the inclusion of important factors such as tumor size/activity, disease stage, treatment history, and insurance.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Conceptualization, A.K.B and J.G.R.; methodology, A.K.B; formal analysis, A.K.B; Y.R.B; A.M.F J.G.R and K.A.B; Supervision, K.A.B and J.G.R; writing—original draft preparation, A.K.B; Y.R.B; A.M.F, J.G.R; K.A.B writing—review and editing, A.K.B and K.A.B
ACKNOWLEDGMENTS
We acknowledge Pura Rodríguez for her support in data analysis.
Bin Abdulrahman AK, Bin Abdulrahman KA, Bukhari YR, Faqihi AM, Ruiz JG. Association between giant cell glioblastoma and glioblastoma multiforme in the United States: A retrospective cohort study. Brain Behav. 2019:9;e01402 10.1002/brb3.1402
The work was conducted in Herbert Wertheim College of Medicine, Florida International University, Miami, FL, USA with cooperation with College of Medicine, Imam Mohammad Ibn Saud Islamic University, Riyadh, Saudi Arabia.
DATA AVAILABILITY STATEMENT
The Surveillance, Epidemiology, and End Results (SEER) data used to support the findings of this study were supplied by the National Cancer Institute under license and so cannot be made freely available. Requests for access to these data should be made to the National Cancer Institute (http://www.seer.cancer.gov/).
REFERENCES
- Akslen, L. A. , Mork, S. J. , Larsen, J. L. , & Myrseth, E. (1988). Giant cell glioblastoma: A work‐up of 2 cases with long survival. Acta Neurologica Scandinavica, 79, 194–199. [DOI] [PubMed] [Google Scholar]
- American Brain Tumor Association|Glioblastoma (GBM) (2017). Brain-tumor-information/types-of-tumors/glioblastoma. Retrieved from http://www.abta.org/brain-tumor-information/types-of-tumors/glioblastoma.html
- Artico, M. , Cervoni, L. , Celli, P. , Salvati, M. , & Palma, L. (1993). Supratentorial glioblastoma in children: A series of 27 surgically treated cases. Childs Nervous System, 9, 7–9. [DOI] [PubMed] [Google Scholar]
- Becker, D. P. , Benyo, R. , & Roessmann, U. (1967). Glial origin of monstrocellular tumor: Case report of prolonged survival. Journal of Neurosurgery, 26, 72–77. [DOI] [PubMed] [Google Scholar]
- Beyer, S. , von Bueren, A. O. , Klautke, G. , Guckenberger, M. , Kortmann, R.‐D. , Pietschmann, S. , & Müller, K. (2016). A systematic review on the characteristics, treatments and outcomes of the patients with primary spinal glioblastomas or gliosarcomas reported in literature until March 2015. PLoS ONE, 11(2), e0148312 10.1371/journal.pone.0148312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger, P. C. , & Vollmer, R. T. (1980). Histologic factors of prognostic significance in the glioblastoma multiforme. Cancer, 46, 1179–1186. [DOI] [PubMed] [Google Scholar]
- Chang, C.‐C. , Kuwana, N. , Ito, S. , Koike, Y. U. , & Kitamura, H. (2001). Spinal leptomeningeal metastases of giant cell glioblastoma associated with subarachnoid haemorrhage: Case report. Journal of Clinical Neuroscience, 8, 56–59. 10.1054/jocn.2000.0745 [DOI] [PubMed] [Google Scholar]
- Colen, R. , Wang, J. , Singh, S. , Gutman, D. , & Zinn, P. (2015). Glioblastoma: Imaging genomic mapping reveals sex‐specific Oncogenic Associations of Cell Death. Radiology, 275(1), 215–227. 10.1148/radiol.14141800 [DOI] [PubMed] [Google Scholar]
- Deb, P. , Sharma, M. C. , Chander, B. , Mahapatra, A. K. , & Sarkar, C. (2006). Giant cell glioblastoma multiforme: Report of a case with prolonged survival and transformation to gliosarcoma. Childs Nervous System, 22, 314–319. [DOI] [PubMed] [Google Scholar]
- Furnari, F. B. , Fenton, T. , Bachoo, R. M. , Mukasa, A. , Stommel, J. M. , Stegh, A. , … Cavenee, W. K. (2007). Malignant astrocytic glioma: Genetics, biology, and paths to treatment. Genes & Development, 21(21), 2683–2710. 10.1101/gad.1596707 [DOI] [PubMed] [Google Scholar]
- Gullotta, F. , Casentini, L. , & Neumann, J. (1980). Giant cell gliomas of the temporal lobe. Acta Neurochirurgica, 54, 25–31. 10.1007/BF01401940 [DOI] [PubMed] [Google Scholar]
- Hadfield, M. G. , & Silverberg, S. G. (1972). Light and electron microscopy of giant‐cell glioblastoma. Cancer, 30, 989–996. [DOI] [PubMed] [Google Scholar]
- Hartmann, C. , Hentschel, B. , Wick, W. , Capper, D. , Felsberg, J. , Simon, M. , … von Deimling, A. (2010). Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1‐mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: Implications for classification of gliomas. Acta Neuropathologica, 120(6), 707–718. 10.1007/s00401-010-0781-z [DOI] [PubMed] [Google Scholar]
- Jeswani, S. , Nuño, M. , Folkerts, V. , Mukherjee, D. , Black, K. , & Patil, C. (2013). Comparison of survival between cerebellar and supratentorial glioblastoma patients. Neurosurgery, 73(2), 240–246. 10.1227/01.neu.0000430288.85680.37 [DOI] [PubMed] [Google Scholar]
- Johnson, D. , Leeper, H. , & Uhm, J. (2013). Glioblastoma survival in the United States improved after Food and Drug Administration approval of bevacizumab: A population‐based analysis. Cancer, 119(19), 3489–3495. 10.1002/cncr.28259 [DOI] [PubMed] [Google Scholar]
- Katoh, M. , Aida, T. , Sugimoto, S. , Suwamura, Y. , Abe, H. , Isu, T. , … Nagashima, K. (1995). Immunohistochemical analysis of giant cell glioblastoma. Pathology International, 45, 275–282. [DOI] [PubMed] [Google Scholar]
- Kawano, H. , Kubota, T. , Sato, K. , Goya, T. , Arikawa, S. , & Wakisaka, S. (1995). Immunohistochemical study of giant cell in glioblastoma. Clinical Neuropathology, 14, 118–123. [PubMed] [Google Scholar]
- Kleihues, P. B. P. , Glioblastoma, A. K. D. , et al. (2007) Giant cell glioblastoma In Louis D. N., Ohgaki H., Wiestler O. D., & Cavanee W. K. (Eds.), WHO classification of tumors of the central nervous system (pp. 46–47). Lyon, France: IARCP. [Google Scholar]
- Klein, R. , Molenkamp, G. , Sorensen, N. , & Roggendorf, W. (1998). Favorable outcome of giant cell glioblastoma in a child: Report of an 11‐year survival period. Childs Nervous System, 14, 288–291. [DOI] [PubMed] [Google Scholar]
- Koul, R. , Dubey, A. , Torri, V. , Kakumanu, A. , & Goyal, K. (2012). Glioblastoma multiforme in elderly population. The Internet Journal of Neurosurgery, 8(1), 1–7. [Google Scholar]
- Kozak, K. , & Moody, J. (2009). Giant cell glioblastoma: A glioblastoma subtype with distinct epidemiology and superior prognosis. Neuro‐Oncology., 11(6), 833–841. 10.1215/15228517-2008-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroh, H. , Matyja, E. , Marchel, A. , & Bojarski, P. (2004). Heavily lipidized, calcified giant cell glioblastoma in an 8‐year‐old patient, associated with neurofibromatosis type 1 (NF1): Report of a case with long‐term survival. Clinical Neuropathology, 23, 286–291. [PubMed] [Google Scholar]
- Li, R. , Li, H. , Yan, W. , Yang, P. , Bao, Z. , Zhang, C. , … You, Y. (2015). Genetic and clinical characteristics of primary and secondary glioblastoma is associated with differential molecular subtype distribution. Oncotarget, 6(9), 7318–7324. 10.18632/oncotarget.3440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X. , Li, Y. , Cao, Y. , Li, P. , Liang, B. O. , Sun, J. , & Feng, E. (2017). Risk of subsequent cancer among pediatric, adult and elderly patients following a primary diagnosis of glioblastoma multiforme: A population‐based study of the SEER database. International Journal of Neuroscience, 127(11), 1005–1011. 10.1080/00207454.2017.1288624 [DOI] [PubMed] [Google Scholar]
- Lin, Q. , & Wagner, W. (2015). Epigenetic aging signatures are coherently modified in cancer. PLoS Genetics, 11(6), e1005334 10.1371/journal.pgen.1005334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohkamp, L.‐N. , Schinz, M. , Gehlhaar, C. , Guse, K. , Thomale, U.‐W. , Vajkoczy, P. , … Koch, A. (2016). MGMT promoter methylation and BRAF V600E mutations are helpful markers to discriminate pleomorphic Xanthoastrocytoma from giant cell glioblastoma. PLoS ONE, 11(6), e0156422 10.1371/journal.pone.0156422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loukas, M. (2014). Adult Brain Cancer in the U.S. Black Population: A Surveillance, Epidemiology, and End Results (SEER) analysis of incidence, survival, and trends. Medical Science Monitor, 20, 1510–1517. 10.12659/MSM.890762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margetts, J. C. , & Kalyan‐Raman, U. P. (1989). Giant‐celled glioblastoma of brain: A clinico‐pathological and radiological study of ten cases (including immunohistochemistry and ultrastructure). Cancer, 63, 524–531. [DOI] [PubMed] [Google Scholar]
- Matsuda, M. , Yamamoto, T. , Ishikawa, E. , Nakai, K. , Zaboronok, A. , Takano, S. , & Matsumura, A. (2011). Prognostic factors in glioblastoma multiforme patients receiving high‐dose particle radiotherapy or conventional radiotherapy. British Journal of Radiology, 84(1), S54–S60. 10.1259/bjr/29022270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy, V. , Krumholz, H. , & Gross, C. (2004). Participation in cancer clinical trials. JAMA, 291(22), 2720 10.1001/jama.291.22.2720 [DOI] [PubMed] [Google Scholar]
- Nizamutdinov, D. , Stock, E. M. , Dandashi, J. A. , Vasquez, E. A. , Mao, Y. , Dayawansa, S. , … Huang, J. H. (2018). Prognostication of survival outcomes in patients diagnosed with glioblastoma. World Neurosurgery, 109, 67–74. 10.1016/j.wneu.2017.09.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgaki, H. , Dessen, P. , Jourde, B. , Horstmann, S. , Nishikawa, T. , Di Patre, P.‐L. , … Kleihues, P. (2004). Genetic pathways to glioblastoma. Cancer Research, 64(19), 6892–6899. 10.1158/0008-5472.CAN-04-1337 [DOI] [PubMed] [Google Scholar]
- Ohgaki, H. , & Kleihues, P. (2005). Epidemiology and etiology of gliomas. Acta Neuropathologica, 109(1), 93–108. 10.1007/s00401-005-0991-y [DOI] [PubMed] [Google Scholar]
- Ohgaki, H. , Peraud, A. , Nakazato, Y. , Watanabe, K. , & von Deimling, A. (2000). Giant cell glioblastoma In Kleihues P., & Cavenee W. K. (Eds.), World Health Organization Classification of Tumours: Pathology and genetics of tumours of the nervous system (pp. 40–41). Lyon, France: IARC Press. [Google Scholar]
- Ostrom, Q. T. , Gittleman, H. , Farah, P. , Ondracek, A. , Chen, Y. , Wolinsky, Y. , … Barnholtz‐Sloan, J. S. (2013). CBTRUS Statistical Report: primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro‐Oncology, 15(Suppl. 2), ii1–ii56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma, L. , Celli, P. , Maleci, A. , Di Lorenzo, N. , & Cantore, G. (1989). Malignant monstrocellular brain tumors: A study of 42 surgically treated cases. Acta Neurochirurgica, 97, 17–25. [DOI] [PubMed] [Google Scholar]
- Pan, I. , Ferguson, S. , & Lam, S. (2015). Patient and treatment factors associated with survival among adult glioblastoma patients: A USA population‐based study from 2000–2010. Journal of Clinical Neuroscience, 22(10), 1575–1581. 10.1016/j.jocn.2015.03.032 [DOI] [PubMed] [Google Scholar]
- Pietschmann, S. , von Bueren, A. , Kerber, M. , Baumert, B. , Kortmann, R. , & Müller, K. (2015). An Individual patient data meta‐analysis on characteristics, treatments and outcomes of glioblastoma/ gliosarcoma patients with metastases outside of the central nervous system. PLoS ONE, 10(4), e0121592 10.1371/journal.pone.0121592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong, X. , Yang, W. , Garzon‐Muvdi, T. , Caplan, J. M. , Hui, X. , Lim, M. , & Huang, J. (2016). Influence of insurance status on survival of adults with glioblastoma multiforme: A population‐based study. Cancer, 122(20), 3157–3165. 10.1002/cncr.30160 [DOI] [PubMed] [Google Scholar]
- Sabel, M. , Reifenberger, J. , Weber, R. G. , Reifenberger, G. , & Schmitt, H. P. (2001). Long‐term survival of a patient with giant cell glioblastoma: Case report. Journal of Neurosurgery, 94, 605–611. [DOI] [PubMed] [Google Scholar]
- Surveillance, Epidemiology, and End Results Program . Cancer of the brain and other nervous system – Cancer stat facts. Retrieved from https://seer.cancer.gov/statfacts/html/brain.html [Google Scholar]
- Surveillance, Epidemiology, and End Results Program . Retrieved from https://seer.cancer.gov/
- Shabihkhani, M. , Telesca, D. , Movassaghi, M. , Naeini, Y. B. , Naeini, K. M. , Hojat, S. A. , … Yong, W. H. (2017). Incidence, survival, pathology, and genetics of adult Latino Americans with glioblastoma. Journal of Neuro‐Oncology, 132(2), 351–358. 10.1007/s11060-017-2377-0 [DOI] [PubMed] [Google Scholar]
- Shah, B. , Bista, A. , & Sharma, S. (2016). Survival trends in elderly patients with glioblastoma in the United States: A population‐based study. Anticancer Research, 36(9), 4883–4886. 10.21873/anticanres.11052 [DOI] [PubMed] [Google Scholar]
- Shinojima, N. , Kochi, M. , Hamada, J.‐I. , Nakamura, H. , Yano, S. , Makino, K. , … Ushio, Y. (2004). The influence of sex and the presence of giant cells on postoperative long‐term survival in adult patients with supratentorial glioblastoma multiforme. Journal of Neurosurgery, 101(2), 219–226. 10.3171/jns.2004.101.2.0219 [DOI] [PubMed] [Google Scholar]
- Stummer, W. , Reulen, H.‐J. , Meinel, T. , Pichlmeier, U. , Schumacher, W. , Tonn, J.‐C. , … Pietsch, T. (2008). Extent of resection and survival in glioblastoma multiforme. Neurosurgery, 62(3), 564–576. 10.1227/01.neu.0000317304.31579.17 [DOI] [PubMed] [Google Scholar]
- Stupp, R. , Mason, W. , & van den Bent, M. (2005). Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. Oncology Times, 27(9), 15–16. 10.1097/01.COT.0000289242.47980.f9 [DOI] [PubMed] [Google Scholar]
- Verger, E. , Valduvieco, I. , Caral, L. , Pujol, T. , Ribalta, T. , Viñolas, N. , … Graus, F. (2011). Does gender matter in glioblastoma? Clinical and Translational Oncology, 13(10), 737–741. 10.1007/s12094-011-0725-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The Surveillance, Epidemiology, and End Results (SEER) data used to support the findings of this study were supplied by the National Cancer Institute under license and so cannot be made freely available. Requests for access to these data should be made to the National Cancer Institute (http://www.seer.cancer.gov/).


