Abstract
Background.
Pre-exposure prophylaxis (PrEP) is a well-established biomedical HIV prevention strategy and recommended as a safe strategy to reduce HIV risk during peri-conception, pregnancy and breastfeeding. Efforts are now needed to translate global recommendations into national guidelines and implementation strategies. This article presents the current status of policy guidance for the use of PrEP during periconception, pregnancy and breastfeeding, with a particular focus on high prevalence countries including those in sub-Saharan Africa.
Methods.
We reviewed and summarized PrEP clinical guidelines released by ministries of health or other national-level health bodies that have authored normative guidelines with a particular focus on recommendations for PrEP use during peri-conception, pregnancy, and/or breastfeeding.
Results.
Among countries with PrEP guidelines and/or policy, pregnancy is recognized as a period with increased HIV vulnerability and some specifically recommend PrEP use during pregnancy. Only one country specifically notes that PrEP is contraindicated during pregnancy recognizing a gap in complete safety data from women using PrEP throughout pregnancy. PrEP is not contraindicated as a peri-conception HIV prevention strategy in any country but only three countries have specific guidance for peri-conception HIV prevention. Multiple barriers to the implementation of PrEP during pregnancy and breastfeeding are discussed including barriers at the policy, health systems, social, and personal levels.
Conclusion.
Although pregnancy is a period of heightened risk and fertility rates are high in many settings with high HIV burden, few PrEP policies have included guidance for PrEP use specific to peri-conception, pregnancy and breastfeeding periods. This gap can be overcome by the development or adoption of national clinical guidelines and implementation strategies from exemplary countries.
Keywords: HIV, pre-exposure prophylaxis, PrEP, pregnancy, safer conception, peri-conception, guidelines
Introduction
Tenofovir containing pre-exposure prophylaxis (PrEP) is an effective HIV prevention strategy (2–4) recommended by the World Health Organisation (WHO) as part of a combination prevention package for people with substantial risk of HIV acquisition (5). Providing PrEP to people during periods of greatest HIV vulnerability is likely to prove cost-saving or cost-effective (6, 7). Heterosexual women constitute more than half of all prevalent infections in sub-Saharan Africa and many areas are burdened by HIV incidence rates exceeding 1–2 new HIV infections per year for every 100 women ages 15–49 (8–11). Women in this age group spend a substantial proportion of their lives pregnant and postpartum, periods that constitute a higher risk for HIV (12, 13), and an important consequence of HIV acquisition during pregnancy and breastfeeding (a component of many, but not all, postpartum periods) is high risk of perinatal HIV transmission (14). Thus, providing maximal prevention support to women during periods with greatest vulnerability, including peri-conception, pregnancy, and breastfeeding/postpartum, is a worldwide imperative.
Despite pregnancy being a period of increased HIV vulnerability, it remains important that women and men who are in HIV-affected couples receive support to fulfil their human right of achieving pregnancy safely, including increasing access to comprehensive sexual and reproductive health services and highly effective HIV risk reduction strategies, such as PrEP (1). Many societies and cultures place a high value on childbearing; remaining childless can have significant personal, social, and economic ramifications for men and women (15, 16). Routine provision of PrEP to HIV uninfected women who are attempting to achieve pregnancy (17), during pregnancy, and during breastfeeding has tremendous benefits (6) and takes advantage of PrEP as a time-limited strategy to be discontinued once risk reduces after the postpartum period, either because other HIV prevention strategies can be used instead or because the likelihood of HIV exposure decreases. For women who are actively planning a pregnancy in the near future, PrEP can be integrated into peri-conception (i.e. safer conception) care, particularly for women with a partner of unknown HIV status or not yet virally suppressed on antiretroviral treatment (ART). Most pregnant women engage in routine antenatal care, presenting an ideal opportunity to integrate HIV risk assessment and PrEP provision into an existing health service platform (18). Reaching postpartum women may be more difficult but some programs are considering integration of postpartum services for family planning and HIV prevention into well-child appointments (19, 20).
The policy landscape within different countries varies in terms of PrEP recommendations during peri-conception, pregnancy and breastfeeding. Some countries, including South Africa, Kenya and Canada, have developed safer conception or peri-conception guidelines for people living with, or at risk for, HIV (21–23) but many high prevalence countries lack specific guidance. Unfortunately, until policies, regulatory licensing for PrEP, and program implementation activities are aligned and prioritised within the most affected countries, many ground level providers will either continue holding back from delivering PrEP, or find themselves unable to do so because of policy constraints.
This article summarizes available PrEP guidance from countries experiencing generalized and concentrated HIV epidemics and identifies those that are leading the field of PrEP delivery to women during peri-conception, pregnancy and breastfeeding. Some countries incorporate recommendations specific to PrEP use during breastfeeding, a critical period for perinatal HIV prevention via maternal PrEP use, and we have included those when possible. We propose that these early adopters be considered as benchmarks for other countries to follow, replicating or adapting existing policies and tools to fit local contexts so that more rapid progress can be made to expand PrEP access during peri-conception, pregnancy, and the breastfeeding/postpartum to reach women during a particularly vulnerable period for HIV risk.
Methods
We conducted a desktop review of available policy and guidance documents found on websites for Ministries of Health and other web locations between January and March 2018. Our primary focus was policy documents authored by government ministries or directorates and we also included guidance documents authored by independent clinician societies in order to demonstrate the range of guidelines and recommendations available worldwide. We also sought advice from AVAC and regional experts to confirm our findings.
Discussion
Among the documents examined in depth, few countries had policy or guidance about PrEP use specifically during pregnancy attempts, or peri-conception (Table). Normative guidance from WHO recommends PrEP use during conception attempts (7), a recommendation that is upheld in Kenya’s governmental policy guidelines (24). No other country with a generalized HIV epidemic that was reviewed had any clear policy or guidance about PrEP use during peri-conception or as part of safer conception strategies. In countries with concentrated epidemics, peri-conception guidance often incorporated caveats about considering the viral suppression status in the positive partner and whether PrEP would be an added prevention benefit (23, 25, 26). Australian clinician society guidelines recommend, for example, that “Providers should discuss the available information about the potential risks and benefits of PrEP in circumstances when women desire pregnancy with HIV-positive partners with unsuppressed virus.” Guidance from British, American, and Canadian clinical societies and/or government policy have similar wording.
Table.
Country-based guidelines for PrEP use during peri-conception and pregnancy and breastfeeding
| Description of HIV epidemic and fertility rates |
Year of regulatory approval for PrEP medication |
Policy or recommendation on PrEP used in peri- conception |
Policy or recommendation on PrEP used during pregnancy and breastfeeding |
Documentation (policy or other guidance document) |
|
|---|---|---|---|---|---|
| Normative/International bodies | |||||
| WHO | 2012 | PrEP can be offered to an HIV-negative woman who is trying to conceive if her partner is HIV-positive and not virally suppressed or she does not know his HIV status. | The existing safety data support the use of PrEP in pregnant and breastfeeding women who are at continuing substantial risk of HIV infection. | World Health Organization (2017). WHO Technical Brief: Preventing HIV in Pregnancy and Breastfeeding in the Context of PrEP. (NGR) | |
| Countries with generalized epidemics | |||||
| South Africa |
|
2016 | No specific guidance but there is recognition that HIV-negative women in serodiscordant relationships are at risk of acquiring HIV infection while trying to conceive through unprotected sex | TDF/FTC is contra-indicated for use as PrEP in pregnant or breastfeeding women. However, as the risk of seroconversion during pregnancy is high, the risks and benefits of PrEP should be discussed with potential PrEP users, allowing these women at high risk of HIV acquisition to make an informed decision regarding PrEP use. | South Africa Department of Health. Guidelines for Expanding Combination Prevention and Treatment Options for Sex Workers: Oral Pre-Exposure Prophylaxis (PrEP) and Test and Treat (T&T). May 2016 (GP) |
| Kenya |
|
2016 | PrEP may be offered to the HIV seronegative partner in a sero-discordant relationship during attempts to conceive (as part of a pre-conception care plan for the couple) | Pregnancy and breastfeeding are not a contraindications to PrEP | Ministry of Health. Guidelines on Use of Antiretroviral Drugs for Treating and Preventing HIV 2016. (GP) |
| Uganda |
|
2016 | Special consideration for PrEP should be given to women and adolescents in discordant relations who desire to get pregnant |
During pregnancy, offer PrEP to the negative partner in the discordant couple. PrEP is not contraindicated during pregnancy and breastfeeding |
Technical Guidance on Pre-Exposure Prophylaxis (PrEP) for Persons at High Risk of HIV in Uganda. 2016. Ministry of Health (GP) Consolidated guidelines for prevention and treatment of HIV in Uganda. 2016 Ministry of Health (GP) |
| Lesotho |
|
Not specifically mentioned in ART/PrEP guidance | Not specifically mentioned in ART/PrEP policy guidance | National Guidelines On The Use Of Antiretroviral Therapy For HIV Prevention And Treatment. 2016. Ministry of Health. Government of Lesotho (GP) | |
| Zimbabwe |
|
Not specifically mentioned in ART/PrEP policy guidance | Pregnant women recognized as a group with high HIV risk | Guidelines for Antiretroviral Therapy for the Prevention and Treatment of HIV in Zimbabwe. National Medicine and Therapeutics Policy Advisory Committee and The AIDS and TB Directorate, Ministry of Health and Child Care. Zimbabwe. 2013 (GP) | |
| Botswana |
|
Recommended for HIV serodiscordant couples trying to conceive | Not specifically mentioned in ART/PrEP policy guidance | Handbook of the Botswana 2016 Integrated HIV Clinical Care Guidelines. 2016 Ministry of Health (GP) |
|
| Nigeria |
|
No specific guidance | No specific guidance; Pregnant women are recognized as a group with high HIV risk | National Guidelines for HIV Prevention Treatment and Care. National AIDS and STIs Control Program 2016. Federal Ministry of Health Nigeria (GP) | |
| Swaziland |
|
No specific guidance | PrEP can be used throughout pregnancy and breastfeeding | Implementation guide for PrEP demonstration projects in Swaziland July 2017 (NGR) | |
| Malawi |
|
PrEP use is not supported | PrEP use is not supported | Malawi Guidelines for Clinical Management of HIV in Children and Adults (3rd Edition). Ministry of Health Malawi (GP) | |
| Countries with concentrated epidemics | |||||
| United States |
|
2012 | PrEP is recommended when the partner living with HIV has unsuppressed viral load or when the viral suppression status is not known When the partner living with HIV has achieved viral suppression, it is unclear whether administering PrEP to the partner without HIV further reduces the risk of sexual transmission |
For women using PrEP who become pregnant, providers should discuss available information about potential risks and benefits of beginning or continuing PrEP during pregnancy so that an informed decision can be made | U.S. Public Health Service. Pre-Exposure Prophylaxis for the Prevention of HIV Infection in the United States −2014 (GP) |
| United Kingdom |
|
Recommended if an HIV-positive partner is not on suppressive ART | Suggest continuation of PrEP if a woman is pregnant or breastfeeding when starting or becomes pregnant or initiates breastfeeding while using PrEP | BHIVA/BASHH guidelines on the use of HIV pre-exposure prophylaxis (PrEP) 2017 (NGR) http://www.bhiva.org/documents/Guidelines/PrEP/Consultation/PrEP-guidelines-consultation-2017.pdf |
|
| France |
|
2015 | Not mentioned in guidelines | PrEP may be considered during pregnancy and breastfeeding after the benefits and risks have been discussed with the patient | Truvada check-list pour le médecin prescripteur. http://ansm.sante.fr/content/download/101989/1293117/version/2/file/RTU_Truvada_Check-List-Prescripteurs_20–02-2017.pdf (NGR) |
| Australia and New Zealand | MSM accounted for 73% of new infections in 2015 | Providers should discuss the available information about the potential risks and benefits of PrEP in circumstances when women desire pregnancy with HIV-positive partners with unsuppressed virus | PrEP can be used in pregnancy and may be continued during breastfeeding | Wright, E., et al. (2017). “Australasian Society for HIV, Viral Hepatitis and Sexual Health Medicine HIV pre-exposure prophylaxis: clinical guidelines.” J Virus Erad 3(3): 168–184. (NGR) | |
| Canada | Among people living with HIV, risk factors for HIV include 53% who are MSM, 19% who inject drugs, and 31% heterosexual (22% women) | 2016 | Not specifically mentioned; PrEP is recommended for HIV negative members of serodiscordant couples if the HIV positive partner has a substantial or non-negligible risk of having transmissible HIV | TDF/FTC PrEP may be considered during pregnancy and breastfeeding after the benefits and risks have been discussed with the patient However, in the case of an infected partner who is virally suppressed, PrEP is not recommended. |
Tan, D. H. S., et al. (2017). “Canadian guideline on HIV pre-exposure prophylaxis and nonoccupational postexposure prophylaxis.” CMAJ 189(47): E1448-e1458. (NGR) Loutfy, M., et al. (2018). “No. 354-Canadian HIV Pregnancy Planning Guidelines.” J Obstet Gynaecol Can 40(1): 94–114. (NGR) |
GP: Government policy; NGR: non-governmental recommendation
At the time of publication, numerous other countries were researched including Swaziland, Malawi, India, Peru, Brazil, and Thailand but no PrEP policy documents authored by Ministries of Health or other Directorates nor documents with non-governmental recommendations written in English were found.
Sources: For fertility rates: World Bank Group (2018). “World Bank Open Data.” Retrieved March 15, 2018, from https://data.worldbank.org/; For HIV epidemic descriptions: UNAIDS (2018). “UNAIDS Country Data.” Retrieved March 15, 2018, from http://www.unaids.org/en/regionscountries/countries.
Among the documents examined, nearly all recommend PrEP to be used during pregnancy. Where there is a recommendation or policy for PrEP, use is expected to follow the same clinical guidance with respect to eligibility criteria, screening, duration of use, dosage, and laboratory monitoring as for non-pregnant women and men. Where a woman presents already pregnant, PrEP may be initiated at any gestational age. For countries that have policy or guidance encouraging PrEP use but lack specific recommendations on PrEP use during pregnancy, many recognize increased HIV susceptibility during pregnancy. Only one country – South Africa – lists PrEP as being contraindicated during pregnancy in the Department of Health policy but this is accompanied by a qualifying statement that the risk of HIV seroconversion is high during pregnancy and thus, “the risks and benefits of PrEP should be discussed with potential PrEP users, allowing these women at high risk of HIV acquisition to make an informed decision regarding PrEP use.” Guidance from the Southern African HIV Clinicians Society adds explanation to the government policy with recognition that data related to the safety of PrEP during pregnancy are limited and that there is a need for further review of data available in the Antiretroviral Pregnancy Registry (http://www.apregistry.com/) and within longitudinal cohorts (27). In many ongoing demonstration or scale-up projects, including those for adolescent girls and young women (AGYW), protocols encourage the inclusion of women who express a desire for pregnancy, become pregnant after enrolment or are already pregnant at enrolment to support the accrual of sufficient data to provide definitive safety information. This process would assist where these concerns are contributing to delays in policy and guidance development pertaining to PrEP use specifically in the peri-conception and pregnancy groups (28).
In order for countries to implement widespread PrEP scale-up effectively, barriers need to be overcome at multiple levels, including the overarching political and policy environment, the health systems context, the social and cultural milieu, and personal considerations (Figure).
Figure.
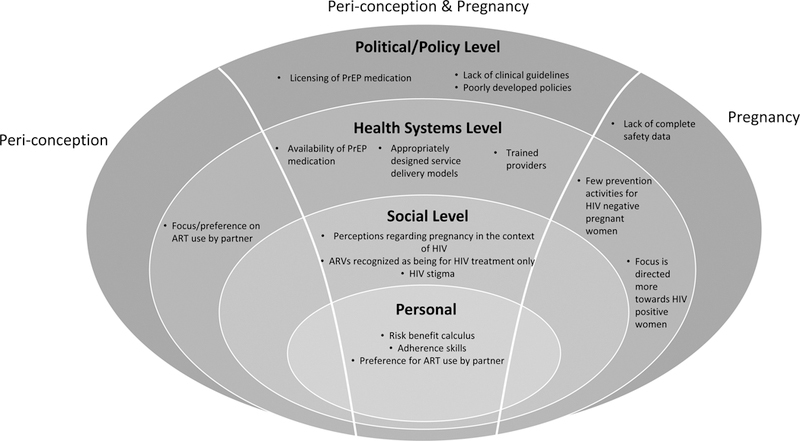
Barriers to the use of PrEP during peri-conception and pregnancy
Political/policy environment
At the political and policy level, the regulatory environment and development and dissemination of policy needs to be urgently addressed through formal documents detailing PrEP availability and clinical considerations. Some countries have inserted PrEP policy and/or clinical guidance into existing ART documents, which may be a less visible location for PrEP information albeit potentially more efficient than creating a novel document. Political will and civil society advocacy have driven these processes forward in some countries. Countries currently lacking policy and/or guidance may benefit from building partnerships with, and learning from, other countries which have already completed PrEP licensing and developed comprehensive guidance and policy documents as this may help to expedite progress and avoid duplication of effort. Next steps for reducing missed opportunities for HIV prevention during pregnancy and breastfeeding include integrating PrEP use into antenatal and post-natal/well mother and baby guidelines so that they are visible to the cadres of clinicians that receive the majority of pregnant and breastfeeding women.
One potential barrier to more rapid integration of PrEP into peri-conception, antenatal and postnatal services, is the gap in full safety data for tenofovir use during pregnancy for women using tenofovir as HIV PrEP. A recent systematic review and WHO guidance state that PrEP is safe to use during pregnancy (7, 29). Considering the transition from peri-conception to pregnancy may go unrecognized for some time, guidance about HIV prevention strategies across these two periods should remain consistent, as it does in the WHO guidance. Indeed, it is counterintuitive to encourage a woman who has mitigated her peri-conception HIV risk with PrEP to discontinue PrEP during pregnancy, a period which has heightened risk for HIV and added consequences for perinatal HIV transmission should seroconversion occur. Permissive policies and clinical guidance can encourage individual decision-making after medical consultation and consideration of risks and benefits.
Health systems
Once documents are released that clearly describe national policies of PrEP use in pregnancy and breastfeeding, practical implementation plans are necessary to create an enabling and supportive health systems environment to facilitate readily and reliably available PrEP medication. This will include ensuring the development of secure PrEP medication supply chains and the establishment of a widespread network of service delivery points, including points that pregnant and breastfeeding/postpartum women access (30). The placement of PrEP services within existing services will be an important early consideration during the design phase. It is likely that these services, in seeking to prevent rather than treat, will be best provided in a non-medicalized setting, integrated with holistic health promotion and disease prevention activities including planned pregnancy services that may engage people who would not ordinarily interact with traditional health (treatment) services. However, to reach negative partners within HIV serodiscordant couples, particularly during the peri-conception period, PrEP can be integrated into ART services which may promote couples-based HIV prevention and support (31). Similarly, PrEP could be readily integrated into existing antenatal services to support access for pregnant women. How best to provide sustained PrEP access for HIV negative women in the post-partum period needs consideration, including whether PrEP might be integrated into well-baby services to support convenient access for new mothers. During the programmatic design phase it will be essential that members of these target groups are engaged so that program developers can hear from potential service users about how they would prefer to access PrEP (32). Failing to engage end-users in early planning may fundamentally undermine program success (33). This early and active engagement approach has been successfully applied with other at-risk populations, including men who have sex with men, and should be replicated to ensure adequate involvement of serodiscordant couples, (including the often overlooked group of heterosexual men) and pregnant and breastfeeding women in service design (34).
As with any new program, in conjunction with demand creation activities, guideline-based provider training will be essential. Providers working in peri-conception, antenatal, and post-natal services need to become more aware of heightened HIV risk during peri-conception, pregnancy, breastfeeding, and postpartum periods, counsel women about this risk, and accurately assess whether PrEP use during these times would be a potential solution for individual patients. Adequate training on PrEP use during pregnancy would seek to encourage and empower providers to undertake open, non-judgemental discussions with HIV affected individuals or couples about their pregnancy desires and different prevention options available to them, including PrEP, and the importance of maintaining adherence to PrEP throughout the period of risk, including continuing through the pregnancy and post-natal periods where necessary. Traditional training models can be costly and time consuming; efforts are needed to develop training techniques, such as certified online learning modules or short in-service training modules, which minimise service disruption, target the correct providers and are cost-efficient (35). Countries may also identify aspects of PrEP delivery that can be conducted by lower cadre workers to optimise efficiency and minimize the workload of already-overstretched clinical providers (35).
Another key health systems related barrier to effective HIV prevention during pregnancy and breastfeeding/postpartum is the continued focus within ANC to identify and initiate ART among HIV positive women, and less focus on repeat HIV testing and PrEP provision to negative women (20). Although HIV re-testing is widely encouraged, this only identifies new infections, it doesn’t prevent them. Despite HIV uninfected women regularly being engaged in services, and perhaps even being identified as at risk of HIV acquisition, they are rarely empowered or enabled to adopt effective HIV prevention strategies to better control their own HIV vulnerability. HIV prevention counselling for pregnant and breastfeeding women needs to be strengthened and diversified beyond condom promotion and male partner testing and linkage to care as part of treatment as prevention (TasP). Although both approaches are essential components to keeping women negative, they will continue to encounter challenges associated with women’s ability to negotiate condom use with male partners and male health seeking behaviours.(36) Consequently, greater advocacy and action is needed across high prevalence countries to expand the focus of the health systems to include providing women with the agency to initiate PrEP as an effective, female driven, biomedical strategy, including taking advantage of opportunities within ANC and post-partum, well-baby services to host PrEP programs.
Particular consideration needs to be given to how peri-conception services might better engage with adolescent girls and young women (AGYW) below the age of 25 who are actively planning pregnancy. Although unintended pregnancies are common, (37) not all pregnancies are unintended in this age group (38). To allow for the diversity of fertility desires seen within this group, PrEP should not only be integrated into traditional family planning (contraceptive) services but also promoted within youth-friendly planned pregnancy/peri-conception services. Furthermore, considering pregnancy, whether intended or not, places these young women at even higher risk of HIV acquisition (39) and other adverse outcomes, such as unsafe abortion (40), depression, and anxiety (41), antenatal services need greater youth friendly focus to facilitate PrEP access as part of comprehensive antenatal care.
Within the field of safer conception, the “U=U” (undetectable equals untransmissable/uninfectious) campaign (42) has raised some interesting questions about the additive benefit of PrEP and other safer conception strategies aside from ART. Although PrEP has long been discussed as an important safer conception strategy ((1, 17), U=U messaging may be contributing to slowed momentum in the implementation of safer conception services, including PrEP, for serodiscordant or serounknown couples. Although, for known, mutually-disclosed serodiscordant couples where the HIV-positive partner is well established on antiretroviral therapy with a confirmed undetectable viral load, there is a strong argument that these couples can conceive without HIV transmission and without the need for any further risk reduction strategies. This viewpoint is discussed in the US, UK and Canadian PrEP recommendations. However, not all couples are in the privileged position where they are able to rely on HIV viral suppression in the positive partner for conception free from HIV transmission. When reviewing the viral suppression estimates of several high burden countries (Table), it is clear that there is space for PrEP and other safer conception strategies since many countries continue to have low rates of viral suppression and many couples likely need additional prevention measures aside from ART and viral suppression (43, 44). Many individuals still do not know their partner’s status (45) or may not be able to support them to engage in care (36). Furthermore, access to viral load monitoring is not yet universal and, even where it is available, providers do not always monitor correctly or respond appropriately to detectable results (43). Within such complex settings, PrEP remains a critical component of safer conception care. Another consideration around U=U messaging is the number of transmissions which occurred via partnerships external to the nuclear couple included in TasP studies (i.e. genetically unlinked infections)(46, 47). It should be acknowledged that, even when attempting pregnancy with a main partner, HIV-uninfected individuals may have more than one partner. Thus, even where their main partner is confirmed virally suppressed on ART, the individual may be exposed to HIV acquisition risks from other partners of whom the provider is unaware. Screening among pregnant and breastfeeding women about other sexual partners, regardless of main pregnancy partner HIV status, should be repeated often to assess whether HIV infection risk may be present due to other partners who may have unknown HIV status or not be engaged in care. Lastly, it remains important to allow for personal preferences. Even in the setting of a positive partner with an undetectable viral load, some HIV uninfected partners may still prefer to use PrEP and their right to choose to do so should be respected. For all these reasons, it is of paramount importance that PrEP continue to be incorporated into policy for serodiscordant and serounknown status couples who wish to plan a pregnancy together.
Social Considerations
With the expansion of PrEP availability, more effort is needed to work with healthcare providers and community members to shift the often negative perceptions held about HIV-affected couples who choose to have children and are therefore perceived to be exposing themselves or their partners to unnecessary HIV risk (48, 49). In this context, the availability of PrEP as a peri-conception prevention strategy may help to reduce stigma and support the normalisation of childbearing for these couples amongst providers and within communities. As with other populations at risk of HIV, another important consideration at the social level will be tackling misconceptions about PrEP that may hinder PrEP uptake or adherence during these seasons of increased risk. Individuals may worry that if someone sees them using PrEP then they will be assumed to be on ARVs and therefore HIV infected (50, 51) or, conversely, that community members or intimate partners will assume that the person is being either promiscuous or unfaithful if they need PrEP to prevent HIV.(30, 52) Education will form a crucial component to help communities understand why PrEP is also useful for stable HIV affected couples who are planning a pregnancy and why PrEP is an invaluable tool for HIV negative women, especially those who may not have any other means to control their HIV risk, during pregnancy and breastfeeding/postpartum.
Personal
Lastly, at the personal level, individuals need to be better equipped to correctly assess their own risk for HIV and weigh the risk-to-benefit ratio of committing to PrEP adherence potentially throughout peri-conception, pregnancy and breastfeeding/postpartum should their HIV risk remain substantial across these different periods.(30, 51) Within this framework, it is also important that HIV uninfected partners understand the key tenets of U=U and the implications of relying on partner ART use and viral suppression as their sole prevention strategy should they choose not to use, or to discontinue, PrEP at any point. In particular, messaging must be very clearly communicated that U=U is only effective if HIV infected individuals remain highly adherent to treatment and are able to access laboratory confirmation of viral suppression. If this is not the case then PrEP remains an important alternative prevention strategy to promote.
Where PrEP is appropriate and the individual chooses to use PrEP, the last remaining, yet perhaps most significant barrier at the personal level will be adherence (50). As seen in a number of pivotal PrEP studies, only individuals who take PrEP consistently enough benefit from its protective effects. More work is needed to develop programs and supportive interventions to assist individuals to sustain sufficient adherence levels, especially during pregnancy when shifts in relationship dynamics, increasing rates of intimate partner violence, economic instability, pregnancy related symptoms and physical limitations may contribute to adherence challenges (53). Perhaps one consideration here is the careful placement of PrEP services so that they are convenient, particularly in terms of integration into antenatal and post-natal services, and that they minimise stigma as much as possible to avoid undermining initiation of, and adherence to, PrEP by those at risk.
Conclusion
There is a paucity of national-level policy and clinical guidance regarding the use of PrEP during peri-conception, pregnancy, and the breastfeeding/postpartum period for high HIV burden countries, despite the growing availability of PrEP and the heightened risk of HIV acquisition during pregnancy and postpartum. Although many countries may have policies which include PrEP use as part of comprehensive HIV prevention packages, far fewer have developed clear policy to implement PrEP during peri-conception, pregnancy and breastfeeding/postpartum periods. For those countries with policy that does not support PrEP use during pregnancy, caveats allow for the consideration of HIV risk during pregnancy and the continuation of PrEP if warranted. Barriers to the delivery of PrEP during peri-conception, pregnancy, and breastfeeding/postpartum can be overcome by the adoption and implementation of existing recommendations from exemplary countries and by including the meaningful involvement of civil society and end-users to adapt PrEP services to suit each country’s specific context. Going forward, clinical guidelines, and tailored training programs need to be developed in parallel with national level policies to ensure the efficient dissemination of information about the value of PrEP use during peri-conception, pregnancy, and breastfeeding/postpartum so that providers gain the knowledge necessary to confidently provide PrEP services to women at risk during these times. Finally, the optimization of pregnancy and postpartum care programs to effectively integrate HIV prevention strategies alongside HIV treatment is an opportunity to seize with utmost enthusiasm.
Key Recommendations:
Benchmark progressive countries; adapting existing policies and recommendations for PrEP use during peri-conception, pregnancy, and postpartum from exemplary countries to implement elsewhere
Encourage countries to sign up to the global consensus statement on safer conception (1), acknowledging there is significant unmet need for periconception care among HIV affected populations in high burden countries
Greater acknowledgement of the overlap between HIV risk and pregnancy rates so that policies, recommendations, and programs better support planned pregnancy as well as preventing unplanned pregnancy
Optimise peri-conception, antenatal and post-natal services to integrate HIV prevention activities that support women to remain HIV-uninfected throughout these three risk periods, including routine, repeated HIV risk screening for all HIV uninfected women, regardless of the HIV status of their main pregnancy partner, including exploring the existence of concurrent partnerships that may represent a significant HIV risk.
Develop provider clinical guidelines and training programs that include specific information about PrEP use during peri-conception, pregnancy and postpartum periods, to equip ground level providers with confidence to identify eligible clients and prescribe PrEP within these specific contexts.
Integrate PrEP policy and clinical guidance into existing antenatal guidance so that it is accessible to key ground level providers of pregnancy care and into well baby/well mother programmes to support postpartum/breastfeeding women
Meaningful engagement of civil society and end-users (including pregnant and breastfeeding women, particularly AGYW, and HIV-affected heterosexual male partners) from the earliest phases of policy, program and service development.
Continue to encourage pharmacovigilance within research studies, demonstration and implementation level projects including collecting and reporting safety data to existing databases such as the Antiretroviral Pregnancy Registry (http://www.apregistry.com/) for TDF/FTC use during pregnancy and breastfeeding to address ongoing safety concerns.
Avoid an overreliance on U=U messaging so that, despite the value of treatment as prevention, PrEP remains an accessible option for those who remain at substantial risk during peri-conception, pregnancy and postpartum/breastfeeding
Promote the provision of PrEP for HIV negative partners in serodiscordant relationships where laboratory confirmation of viral suppression is not available.
Acknowledgements
We are grateful to Emily Bass and Laura Fitch at AVAC as well as Lynn Matthews, Shannon Weber, and Jared Baeten for reviewing the information on PrEP policy and clinician society guidance.
Funding
RH was funded through the Eunice Kennedy Shriver National Institute for Child Health and Human Development R00HD076679.
Footnotes
Conflicts of Interest Statement
The authors declare no conflicts of interest.
References
- 1.Matthews LT, Beyeza-Kashesya J, Cooke I, Davies N, Heffron R, Kaida A, et al. Consensus statement: Supporting Safer Conception and Pregnancy For Men And Women Living with and Affected by HIV. AIDS and behavior. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. The New England journal of medicine. 2012;367(5):399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. The New England journal of medicine. 2010;363(27):2587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. The New England journal of medicine. 2012;367(5):423–34. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Guideline on When to Start Antiretroviral Therapy and on Pre-Exposure Prophylaxis for HIV. Geneva, Switzerland: World Health Organization; 2015. [PubMed] [Google Scholar]
- 6.Price JT, Wheeler SB, Stranix-Chibanda L, Hosek SG, Watts DH, Siberry GK, et al. Cost-Effectiveness of Pre-exposure HIV Prophylaxis During Pregnancy and Breastfeeding in Sub-Saharan Africa. J Acquir Immune Defic Syndr. 2016;72 Suppl 2:S145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organisation. Preventing HIV during pregnancy and breastfeeding in the context of PREP. Geneva: WHO; 2017. [Google Scholar]
- 8.Estimates of global, regional, and national incidence, prevalence, and mortality of HIV, 1980–2015: the Global Burden of Disease Study 2015. Lancet HIV. 2016;3(8):e361–e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.PHIA Project. Swaziland HIV Incidence Measurement Survey 2: A population-based HIV impact assessment (SHIMS2). Accessed 28 March 2018 New York, USA; 2017. [Google Scholar]
- 10.PHIA Project. Lesotho Population-based HIV Impact Assessment LePHIA 2016–2017. Accessed 28 March 2018 New York, USA; 2017. [Google Scholar]
- 11.PHIA Project. Zambia Population-based HIV impact assessment ZAMPHIA 2015–2016. Accessed 28 March 2018 New York, USA: ICAP Columbia University; 2017. [Google Scholar]
- 12.Mugo NR, Heffron R, Donnell D, Wald A, Were EO, Rees H, et al. Increased risk of HIV-1 transmission in pregnancy: a prospective study among African HIV-1-serodiscordant couples. AIDS (London, England). 2011;25(15):1887–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomson KA, Hughes JP, Baeten J, John-Stewart G, et al. Female HIV acquisition per sex act is elevated in late pregnancy and postpartum CROI; 4 March 2018; Boston, USA: 2018. [Google Scholar]
- 14.Drake AL, Wagner A, Richardson B, John-Stewart G. Incident HIV during Pregnancy and Postpartum and Risk of Mother-to-Child HIV Transmission: A Systematic Review and Meta-Analysis. PLOS Medicine. 2014;11(2):e1001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ngure K, Kimemia G, Dew K, Njuguna N, Mugo N, Celum C, et al. Delivering safer conception services to HIV serodiscordant couples in Kenya: perspectives from healthcare providers and HIV serodiscordant couples. Journal of the International AIDS Society. 2017;20(Suppl 1):21309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asemota OA, Klatsky P. Access to infertility care in the developing world: the family promotion gap. Seminars in reproductive medicine. 2015;33(1):17–22. [DOI] [PubMed] [Google Scholar]
- 17.Heffron R, Pintye J, Matthews LT, Weber S, Mugo N. PrEP as Peri-conception HIV Prevention for Women and Men. Current HIV/AIDS reports. 2016;13(3):131–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pintye J, Drake AL, Kinuthia J, Unger JA, Matemo D, Heffron RA, et al. A Risk Assessment Tool for Identifying Pregnant and Postpartum Women Who May Benefit From Preexposure Prophylaxis. Clin Infect Dis. 2017;64(6):751–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brady M, Mullick S, Friedland B, Plagianos M, Du Plessis L, T. M. Learning from women about HIV risk, HIV testing behaviors, and prevention practices in Mpumalanga, South Africa: a descriptive study to inform microbicides introduction. New York: Population Council; 2015. [Google Scholar]
- 20.Chi BH, Rosenberg NE, Mweemba O, Powers KA, Zimba C, Maman S, et al. Involving both parents in HIV prevention during pregnancy and breastfeeding. Bull World Health Organ. 2018;96(1):69–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bekker L-G, Black V, Myer L, Cooper D, Mall S, Mnyami C, et al. Guideline on safer conception in fertile HIV-infected individuals and couples. SAJHIVMed. 2011;June 2011:31–44. [Google Scholar]
- 22.Division of Reproductive Health Ministry of Public Health and Sanitation Kenya Family Planning Guidelines for Service Providers. Nairobi; 2010. [Google Scholar]
- 23.Loutfy M, Kennedy VL, Poliquin V, Dzineku F, Dean NL, Margolese S, et al. No. 354-Canadian HIV Pregnancy Planning Guidelines. Journal of obstetrics and gynaecology Canada : JOGC = Journal d’obstetrique et gynecologie du Canada : JOGC. 2018;40(1):94–114. [DOI] [PubMed] [Google Scholar]
- 24.National AIDS & STI Control Program Ministry of Health Kenya. Guidelines on Use of Antiretroviral Drugs for Treating and Preventing HIV Infections in Kenya. Nairobi; 2016. [Google Scholar]
- 25.Wright E, Grulich A, Roy K, Boyd M, Cornelisse V, Russell D, et al. Australasian Society for HIV, Viral Hepatitis and Sexual Health Medicine HIV pre-exposure prophylaxis: clinical guidelines. Journal of virus eradication. 2017;3(3):168–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brady M, Rodger A, Asboe D, et al. BHIVA/BASHH guidelines on the use of HIV pre-exposure prophylaxis (PrEP) 2017. London, UK: 2017. [DOI] [PubMed] [Google Scholar]
- 27.Bekker LG, Rebe K, Venter F, Maartens G, Moorhouse M, Conradie F, et al. Southern African guidelines on the safe use of pre-exposure prophylaxis in persons at risk of acquiring HIV-1 infection. S Afr J HIV Med. 2016;17(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mugo NR, Hong T, Celum C, Donnell D, Bukusi EA, John-Stewart G, et al. Pregnancy incidence and outcomes among women receiving preexposure prophylaxis for HIV prevention: a randomized clinical trial. JAMA. 2014;312(4):362–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mofenson LM. Tenofovir Pre-exposure Prophylaxis for Pregnant and Breastfeeding Women at Risk of HIV Infection: The Time is Now. PLoS medicine. 2016;13(9):e1002133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mugo NR, Ngure K, Kiragu M, Irungu E, Kilonzo N. PrEP for Africa: What we have learnt and what is needed to move to program implementation. Current opinion in HIV and AIDS. 2016;11(1):80–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heffron R, Ngure K, Odoyo J, Bulya N, Tindimwebwa E, Hong T, et al. Pre-exposure prophylaxis for HIV-negative persons with partners living with HIV: uptake, use, and effectiveness in an open-label demonstration project in East Africa. Gates open research. 2017;1:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bailey JL, Molino ST, Vega AD, Badowski M. A Review of HIV Pre-Exposure Prophylaxis: The Female Perspective. Infectious diseases and therapy. 2017;6(3):363–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geary CW, Bukusi EA. Women and ARV-based HIV prevention-challenges and opportunities. J Int AIDS Soc. 2014;17(3 Suppl 2):19356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koechlin FM, Fonner VA, Dalglish SL, O’Reilly KR, Baggaley R, Grant RM, et al. Values and Preferences on the Use of Oral Pre-exposure Prophylaxis (PrEP) for HIV Prevention Among Multiple Populations: A Systematic Review of the Literature. AIDS Behav. 2017;21(5):1325–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mack N, Wong C, McKenna K, Lemons A, Odhiambo J, Agot K. Human Resource Challenges to Integrating HIV Pre-Exposure Prophylaxis (PrEP) into the Public Health System in Kenya: A Qualitative Study. African journal of reproductive health. 2015;19(1):54–62. [PubMed] [Google Scholar]
- 36.Katirayi L, Chadambuka A, Muchedzi A, Ahimbisibwe A, Musarandega R, Woelk G, et al. Echoes of old HIV paradigms: reassessing the problem of engaging men in HIV testing and treatment through women’s perspectives. Reproductive health. 2017;14(1):124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Althabe F, Moore JL, Gibbons L, Berrueta M, Goudar SS, Chomba E, et al. Adverse maternal and perinatal outcomes in adolescent pregnancies: The Global Network’s Maternal Newborn Health Registry study. Reproductive health. 2015;12 Suppl 2:S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.WHO. Position paper on mainstreaming adolescent pregnancy in efforts to make pregnancy safer. Geneva: WHO; 2010. [Google Scholar]
- 39.Christofides NJ, Jewkes RK, Dunkle KL, Nduna M, Shai NJ, Sterk C. Early adolescent pregnancy increases risk of incident HIV infection in the Eastern Cape, South Africa: a longitudinal study. J Int AIDS Soc. 2014;17(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.WHO. Adolescent Pregnancy Geneva: WHO; 2018. [updated January 2018. Available from: http://www.who.int/mediacentre/factsheets/fs364/en/. [Google Scholar]
- 41.Gavin AR, Lindhorst T, Lohr MJ. The prevalence and correlates of depressive symptoms among adolescent mothers: results from a 17-year longitudinal study. Women & health. 2011;51(6):525–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prevention Access Campaign. Risk of sexual transmission of HIV from a person living with HIV who has an undetectable viral load Messaging Primer and Consensus Statement. United States: Prevention Access Campaign; 2018. [Google Scholar]
- 43.UNAIDS. Ending AIDS: Progress towards the 90–90-90 targets Global AIDS Update 2017. Geneva: UNAIDS; 2017. [Google Scholar]
- 44.UNAIDS. UNAIDS Country Data Geneva, Switzerland: UNAIDS; 2018. [Available from: http://www.unaids.org/en/regionscountries/countries. [Google Scholar]
- 45.Matthews LT, Moore L, Crankshaw TL, Milford C, Mosery FN, Greener R, et al. South Africans with recent pregnancy rarely know partner’s HIV serostatus: implications for serodiscordant couples interventions. BMC Public Health. 2014;14(1):843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Antiretroviral Therapy for the Prevention of HIV-1 Transmission. N Engl J Med. 2016;375(9):830–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Campbell MS, Mullins JI, Hughes JP, Celum C, Wong KG, Raugi DN, et al. Viral Linkage in HIV-1 Seroconverters and Their Partners in an HIV-1 Prevention Clinical Trial. PLoS One. 2011;6(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crankshaw T, Mindry D., Munthree C., Letsoalo T., Maharaj P. Challenges with couples, serodiscordance and HIV disclosure:healthcare provider perspectives on delivering safer conception services for HIV-affected couples, South Africa. JIAS. 2014;17:18832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mindry D, Milford C., Greener L., Greener RM., Maharaj P., Letsoalo T., Munthree C., Crankshaw TL., Smit JA. Client and provider knowledge and views on safer conception for people living with HIV (PLHIV). Sexual & Reproductive Healthcare. 2016:in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koenig LJ, Lyles C, Smith DK. Adherence to antiretroviral medications for HIV pre-exposure prophylaxis: lessons learned from trials and treatment studies. American journal of preventive medicine. 2013;44(1 Suppl 2):S91–8. [DOI] [PubMed] [Google Scholar]
- 51.van der Straten A, Stadler J, Luecke E, Laborde N, Hartmann M, Montgomery ET, et al. Perspectives on use of oral and vaginal antiretrovirals for HIV prevention: the VOICE-C qualitative study in Johannesburg, South Africa. J Int AIDS Soc. 2014;17(3 Suppl 2):19146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Montgomery ET, van der Straten A, Stadler J, Hartmann M, Magazi B, Mathebula F, et al. Male Partner Influence on Women’s HIV Prevention Trial Participation and Use of Pre-exposure Prophylaxis: the Importance of “Understanding”. AIDS Behav. 2015;19(5):784–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bailey BA. Partner violence during pregnancy: prevalence, effects, screening, and management. International journal of women’s health. 2010;2:183–97. [DOI] [PMC free article] [PubMed] [Google Scholar]


