Summary
Red rice contains high levels of proanthocyanidins and anthocyanins, which have been recognized as health‐promoting nutrients. The red coloration of rice grains is controlled by two complementary genes, Rc and Rd. The RcRd genotype produces red pericarp in wild species Oryza rufipogon, whereas most cultivated rice varieties produce white grains resulted from a 14‐bp frame‐shift deletion in the seventh exon of the Rc gene. In the present study, we developed a CRISPR/Cas9‐mediated method to functionally restore the recessive rc allele through reverting the 14‐bp frame‐shift deletion to in‐frame mutations in which the deletions were in multiples of three bases, and successfully converted three elite white pericarp rice varieties into red ones. Rice seeds from T1 in‐frame Rc lines were measured for proanthocyanidins and anthocyanidins, and high accumulation levels of proanthocyanidins and anthocyanidins were observed in red grains from the mutants. Moreover, there was no significant difference between wild‐type and in‐frame Rc mutants in major agronomic traits, indicating that restoration of Rc function had no negative effect on important agronomic traits in rice. Given that most white pericarp rice varieties are resulted from the 14‐bp deletion in Rc, it is conceivable that our method could be applied to most white pericarp rice varieties and would greatly accelerate the breeding of new red rice varieties with elite agronomic traits. In addition, our study demonstrates an effective approach to restore recessive frame‐shift alleles for crop improvement.
Keywords: CRISPR/Cas9, functional recovery, rc, red rice, proanthocyanidins
Introduction
Rice (Oryza sativa L.) is a major staple food crop, feeding more than half the world's population. The majority of rice varieties grown and consumed throughout the world have white pericarp. There are also varieties of rice with brown, red or purple/black pericarp. The pigments of coloured rice contain high levels of proanthocyanidins and anthocyanins which have been recognized as health‐promoting nutrients (Finocchiaro et al., 2007; Gunaratne et al., 2013; Qiu et al., 2010). In China, Japan, Korea and many other countries in South‐East Asia, coloured rice has been widely cultivated and consumed owing to its nutritional properties and health benefits.
Red pericarp is ubiquitous in wild rice species (Oryza rufipogon L.), which are the ancestors of cultivated rice. The red coloration in the grains of wild rice is controlled by two complementary genes, Rc and Rd (Furukawa et al., 2007; Sweeney et al., 2006). Rc encodes a basic helix–loop–helix (bHLH) transcription factor, whereas Rd encodes a dihydroflavonol‐4‐reductase (DFR) protein (Furukawa et al., 2007; Sweeney et al., 2006). The RcRd genotype produces red pericarp in O. rufipogon. In contrast, most cultivated rice varieties produce white grains, and about 97.9% of these are resulted from a 14‐bp frame‐shift deletion in the seventh exon of the Rc gene (Sweeney et al., 2007). These previous studies demonstrated the critical role of Rc in determining the red coloration in rice grains. Thus, the Rc locus could be a genome editing target for developing red rice.
Genome editing technologies, especially the CRISPR/Cas9 system, have emerged as powerful tools to precisely breed crops with improved agronomic traits, such as growth period (Cai et al., 2018), disease resistance (Macovei et al., 2018; Wang et al., 2014, 2016, 2018a, b), genic male sterility (Zhou et al., 2016), nutrition quality (Clasen et al., 2015) and grain yield (Li et al., 2016; Wang et al., 2018a). Currently, the common application of the CRISPR/Cas9 technology in crop breeding is to edit genes that have deleterious effects on agronomic traits. For instance, CRISPR/Cas9‐mediated knockout of the Waxy gene was reported to reduce the amylose content in rice grains and could, thereby, convert common rice into glutinous rice (Zhang et al., 2018). Given the fact that the CRISPR/Cas9 system could produce different types of mutations—deletion, insertion or substitution with lengths from 1 to 3 bases or longer (Zhang et al., 2014, 2016; Zhou et al., 2014)—we hypothesized that this technology could be applied to recover recessive alleles of positive regulatory genes lost during domestication, such as the rc allele, by reverting the frame‐shift mutations to in‐frame versions in which the lengths of deletions or insertions are in multiples of three bases.
In this study, we selected three elite rice varieties with white pericarp, including a japonica variety Xiushui134 (X134), an indica restorer line Shuhui143 (S143) and an indica photo‐/thermo‐sensitive genic male sterile line ZhiNongS (ZNS), to test our hypothesis. A total of 23 T0 plants that contained at least one in‐frame allele of the Rc gene were identified from CRISPR/Cas9 transgenic plants in all three varieties, and rice plants harbouring in‐frame Rc alleles exhibited red coloration. Seeds from T1 in‐frame Rc lines were measured for proanthocyanidins and anthocyanidins, and high accumulation levels of proanthocyanidins and anthocyanidins were observed in red rice grains. Moreover, there was no significant difference between wild‐type and in‐frame Rc mutants in major agronomic traits, indicating that restoration of Rc function had no negative effect on important agronomic traits in rice. Thus, our method could be applied to most white pericarp rice varieties and would greatly accelerate the breeding of elite red rice varieties with high proanthocyanidins and anthocyanins.
Results
Site‐specific mutagenesis of the rc allele mediated by CRISPR/Cas9 system
We first sequenced the Rc and Rd loci in X134, S143 and ZNS, and confirmed that all three varieties contained the same 14‐bp deletion in the seventh exon of the rc allele (Figure S1). There were no mutations in the coding regions of the Rd gene (Figure S2). To achieve functional recovery of the recessive rc allele, two Cas9/sgRNA constructs targeting the flanking sequences of the 14‐bp deletion site were designed (Figure 1a). The two constructs were transformed into Agrobacterium tumefaciens EHA105. Rice calli were co‐cultured with Agrobacterium strains harbouring the two Cas9/sgRNA constructs. A total of 12, 26 and 22 independent T0 transgenic plants were generated from the calli of X134, S143 and ZNS, respectively (Table 1). The genotyping of T0 transgenic plants identified 9, 20 and 17 plants with mutations in the target sites in X134, S143 and ZNS, respectively (Tables 1 and S1). Surprisingly, among the T0 mutant plants, 45.6% (21/46) were putatively homozygous and 52.2% (24/46) were bi‐allelic, whereas only one plant (2.2%) was heterozygous (Tables 1 and S1). The reason for low percentage of the heterozygous genotype could be that the plants were generated by co‐transformation of two sgRNA cassettes.
Figure 1.
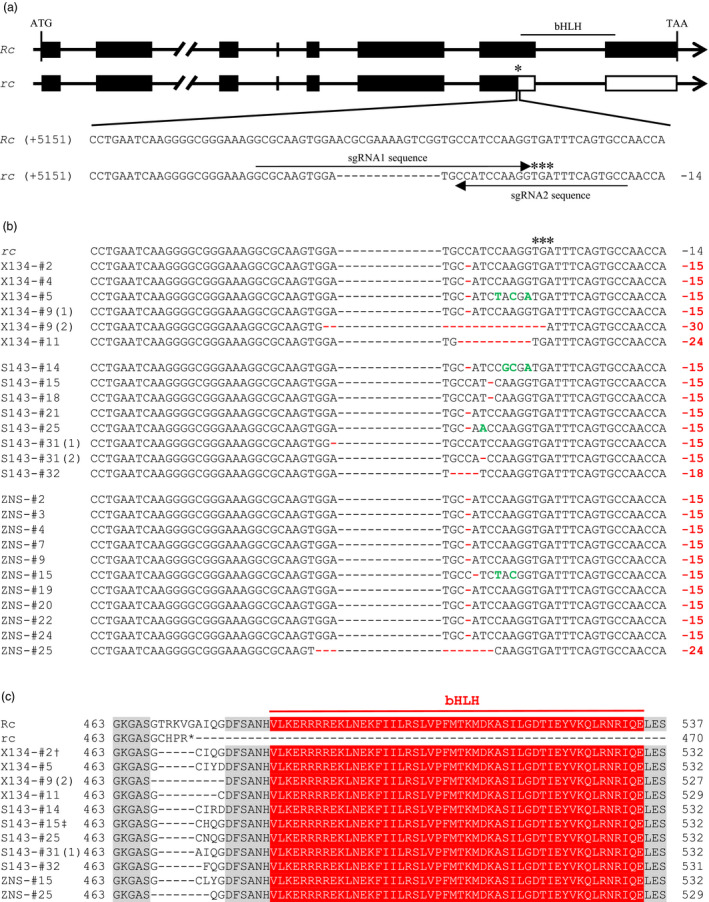
Development of elite red rice varieties through CRISPR/Cas9‐mediated functional recovery of the recessive rc allele. (a) Gene structures of Rc in wild red rice (Oryza rufipogon) and rc in cultivated white pericarp rice. The 14‐bp deletion and the premature stop codon in rc are indicated by black dashes and asterisks, respectively. The two sgRNAs are indicated by arrows. (b) Mutations adjacent to the 14‐bp deletion site in T0 plants harbouring in‐frame Rc variants. Newly introduced deletions and substitutions are indicated by red dashes and green letters, respectively. Numbers on the right side indicate the lengths of deletions compared with wild‐type Rc in O. rufipogon. (c) Multiple alignment of the deduced bHLH coding amino acid sequences of in‐frame Rc variants. †X134‐#2, X134‐#4, X134‐#9(1), S143‐#21, ZNS‐#2, ZNS‐#3, ZNS‐#4, ZNS‐#7, ZNS‐#9, ZNS‐#19, ZNS‐#20, ZNS‐#22 and ZNS‐#24 had an identical amino acid sequence. ‡S143‐#15, S143‐#18 and S143‐#31(2) had an identical amino acid sequence.
Table 1.
T0 plants transformed with Cas9/sgRNA constructs targeting the flanking sequences of the rc 14‐bp deletion site
| Variety | No. of transgenic plants | No. of plants with mutations (%)† | T0 zygosity‡ | No. of plants with in‐frame Rc alleles (%)‡ | ||
|---|---|---|---|---|---|---|
| Homozygous (%) | Bi‐allelic (%) | Heterozygous (%) | ||||
| X134 | 12 | 9 (75.0) | 2 (22.2) | 7 (77.8) | 0 (0.0) | 5 (55.6) |
| S143 | 26 | 20 (76.9) | 10 (50.0) | 9 (45.0) | 1 (5.0) | 7 (35.0) |
| ZNS | 22 | 17 (77.3) | 9 (52.9) | 8 (46.1) | 0 (0.0) | 11 (64.7) |
| Total | 60 | 46 (76.7) | 21 (45.6) | 24 (52.2) | 1 (2.2) | 23 (50.0) |
Percentages were calculated over the total number of transgenic plants.
Percentages were calculated over the total number of plants with mutations.
T0 transgenic rice plants harbouring in‐frame Rc variants exhibited red coloration
We aimed to screen plants with mutations that could revert the 14‐bp deletion to a deletion in multiples of 3 bases. A total of 23 T0 transgenic plants that contained at least one in‐frame allele of the Rc gene were identified in all three varieties (Figure 1b, Tables 1 and S1). Most in‐frame mutants carried a newly introduced 1‐bp deletion adjacent to the 14‐bp deletion site, resulting in deletions of a total of 15 bases (Figure 1b, Table S1). Sequence analysis showed that these in‐frame variants encoded putative full‐length polypeptides. Compared to the wild‐type Rc protein in O. rufipogon, these polypeptides had minor deletions ranging from 5 to 10 amino acids. The polypeptides, however, contained an intact bHLH domain (Figures 1c and S3). Thus, minor deletions of noncritical residues outside the conserved bHLH domain should have no deleterious effects on Rc function.
In T0 X134 and S143 plants harbouring in‐frame Rc variants, the coloration of the grains changed from white to red (Figure 2), indicating that Rc gene function was successfully restored. We also observed that the red pericarp phenotype of the bi‐allelic plants with one dose of in‐frame Rc was similar to that of plants with a homozygous in‐frame Rc genotype. Hence, similar to their wild‐type Rc gene, these in‐frame variants had a dominant effect on the red coloration in rice grains. As for T0 ZNS plants, the mutants displayed an identical male sterility phenotype as the wild‐type ZNS (Figure S4). We failed to harvest seeds from T0 ZNS mutant plants in the summer season in Fuzhou. Instead, the ZNS mutant plants were crossed with a white pericarp restorer line MH86. As expected, the resulting hybrid seeds from the in‐frame Rc ZNS mutants showed red grain pericarp (Figure 2).
Figure 2.
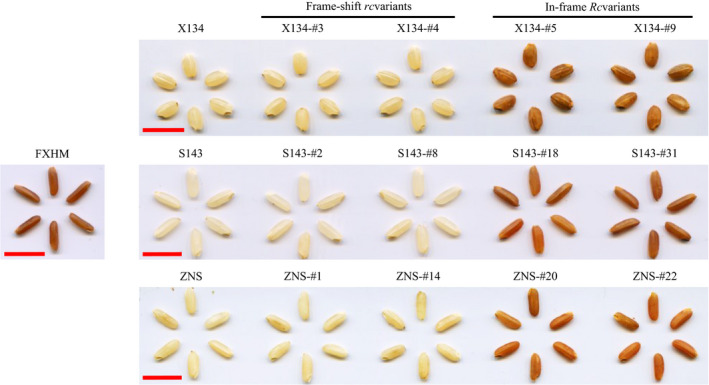
Pericarp phenotypes of T0 mutant plants. FXHM was a commercial red rice variety used as a positive control. Seeds from ZNS mutants were produced by crossing with white rice restorer line MH86. Scale bar: 1 cm.
Inheritance of targeted mutations and putative off‐target analysis in T1 generation
We further investigated six T1 generation lines X134‐#2, X134‐#3, X134‐#9, S143‐#8, S143‐#18 and S143‐#32 for inheritance of the introduced mutations and segregation of the Cas9/sgRNA T‐DNA. We observed that the mutations in the T0 transgenic plants were stably transmitted to the T1 generation (Table 2, Figure 3). Consistently, seeds of T1 in‐frame Rc mutant plants displayed the same red pericarp phenotype as those of T0 plants. Our results also showed that the Cas9/sgRNA T‐DNA could be segregated out in some T1 homozygous mutant plants (Table 2, Figure 3).
Table 2.
Inheritance of the introduced mutations in six T1 lines
| T0 plant | Mutation genotype of T0 plant† | T1 plant | ||||
|---|---|---|---|---|---|---|
| No. of plants tested | No. of plants with mutation phenotype | Hpt and Cas9 positive/Hpt and Cas9 negative§ | No. of transgene‐free plants with homozygous in‐frame Rc allele | |||
| Bi‐allelic | Homozygous‡ | |||||
| X134‐#2 | −15 bp/−15 bp | 8 | 0 | 8 (−15 bp) | 5:3 | 3 |
| X134‐#3 | −13 bp/−19 bp | 9 | 4 | 3 (−19 bp); 2 (−13 bp) | 7:2 | 0 |
| X134‐#9 | −15 bp/−30 bp | 24 | 13 | 6 (−30 bp); 5 (−15 bp) | 17:7 | 3 |
| S143‐#8 | −13 bp/−26 bp | 15 | 9 | 4 (−26 bp); 2 (−13 bp) | 10:5 | 0 |
| S143‐#18 | −15 bp/−20 bp | 16 | 7 | 4 (−20 bp); 5 (−15 bp) | 12:4 | 2 |
| S143‐#32 | −16 bp/−18 bp | 24 | 13 | 6 (−18 bp); 5 (−16 bp) | 18:6 | 2 |
The numbers indicate the lengths of deletions compared with wild‐type Rc in Oryza rufipogon; −: deletion; bi‐allelic mutations are distinguished by ‘/’.
The numbers in brackets indicate the lengths of deletions compared with wild‐type Rc in O. rufipogon.
The presence/absence of Hpt and Cas9 was determined by PCR amplification.
Figure 3.
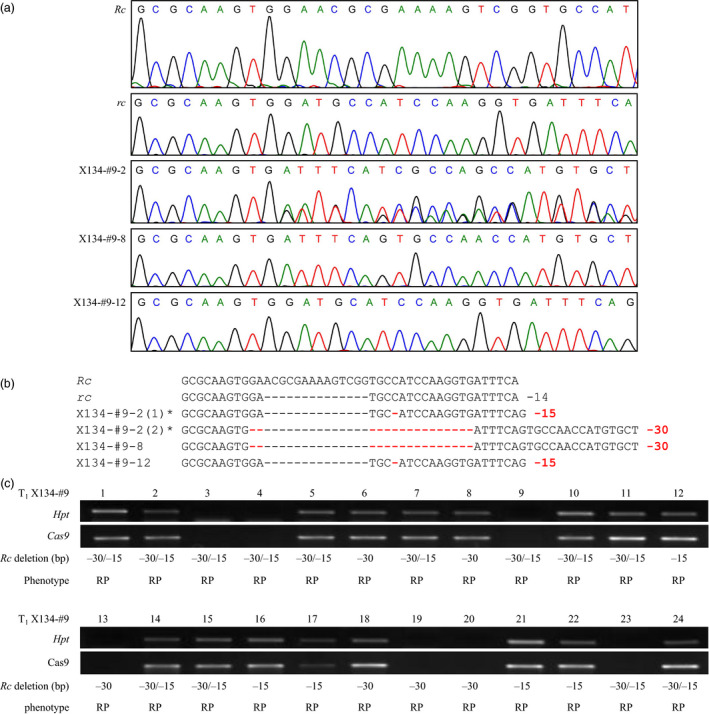
Segregation analysis of T1 progeny of in‐frame Rc line X134‐#9. (a) Examples of sequencing chromatograms of the deletion site in the Rc/rc allele in wild red rice (Rc), white pericarp rice (rc) and T1 progeny of X134‐#9. (b) Alignment of sequences decoded from sequencing chromatograms of the deletion site in the Rc/rc allele in wild red rice (Rc), white pericarp rice (rc) and T1 progeny of X134‐#9. *Bi‐allelic sequences were decoded from sequencing chromatograms using an online program DSDecode (http://dsdecode.scgene.com/). (c) Segregations of the Rc deletion genotypes and the Cas9/sgRNA T‐DNA in T1 progeny of X134‐#9. The presence/absence of the Cas9/sgRNA T‐DNA was determined by PCR amplification for the hygromycin phosphotransferase (Hpt) gene and the Cas9 gene. RP, red pericarp.
The T1 homozygous in‐frame Rc plants were evaluated for potential off‐target mutations. A total of 11 potential off‐target sites (seven for sgRNA1 and four for sgRNA2, respectively) containing three to four mismatched bases were retrieved using the online tools CRISPR‐P (http://skl.scau.edu.cn/offtarget/) and BLASTN (Tables 3 and S2). PCR products amplified from 10 T1 plants (five of X134 lines and five of S143 lines) were sequenced, and no mutation events were detected in all potential off‐target sites (Table 3), indicating that the two sgRNAs had high specificity in inducing mutagenesis of the rc allele.
Table 3.
Evaluation of potential off‐target sites
| Target | Name of putative off‐target sites | Putative off‐target locus | Putative off‐target sequence | No. of mismatch bases | No. of plants examined | No. of indel mutation |
|---|---|---|---|---|---|---|
| sgRNA1 | 1‐OFF1 | ch02: 28556203–28556225 | GCTCAAGTCGACGCCCTCCAAGG | 4 | 10 | 0 |
| 1‐OFF2 | ch03: 21512179–21512201 | CGGCAAGTGGATGCCATCTATGG | 3 | 10 | 0 | |
| 1‐OFF3 | ch04: 26251953–26251975 | GCGCAAGTGGATGGTTTCCTGGG | 4 | 10 | 0 | |
| 1‐OFF4 | ch08: 25739010–25739032 | GCTGAGGTGGAGGCCATCCAAGG | 4 | 10 | 0 | |
| 1‐OFF5 | ch09: 8657855–8657877 | CGGCAAGTGGATGCCATCTATGG | 3 | 10 | 0 | |
| 1‐OFF6 | ch10: 10111987–10112009 | GGGCAAATGGATGGCATGCAAGG | 4 | 10 | 0 | |
| 1‐OFF7 | ch11: 17602327–17602349 | GCGCGACTGGATGGCATCCATGG | 3 | 10 | 0 | |
| sgRNA2 | 2‐OFF1 | ch05: 13717502–13717524 | GGCTCTGAAGTCCCCTTTGATGG | 4 | 10 | 0 |
| 2‐OFF2 | ch05: 20480244–20480266 | GGCTCTGAAGTCCCCTTTGATGG | 4 | 10 | 0 | |
| 2‐OFF3 | ch09: 20085301–20085323 | GGCACTGAAAATTCCTCGGATGG | 4 | 10 | 0 | |
| 2‐OFF4 | ch12: 15994192–15994214 | GGCACCGAAATAATTTTGGATGG | 4 | 10 | 0 |
PAM sequence NGG is indicated in blue. Mismatch nucleotides are marked in red.
Accumulation of proanthocyanidins and anthocyanins in the grains of in‐frame Rc mutant plants
Seeds harvested from T1 in‐frame Rc lines X134‐#2 (−15 bp/−15 bp), X134‐#9 (−15 bp/−30 bp), S143‐#18 (−15 bp/−20 bp) and S143‐#32 (−18 bp/−18 bp) were subjected to measurement of oligomeric proanthocyanidin (OPC) content. We observed that OPC content in the seeds of in‐frame Rc lines was over twofold and fivefold higher than that in the seeds of their corresponding wild types, X134 and S143, respectively (Figure 4a), confirming that red rice grains contain a high level of proanthocyanidins. Remarkably, there was no significant difference between OPC content in heterozygous plant S143‐#18 (−15 bp/−20 bp) and that in homozygous plants S143‐#32 (−18 bp/−18 bp; Figure 4a).
Figure 4.
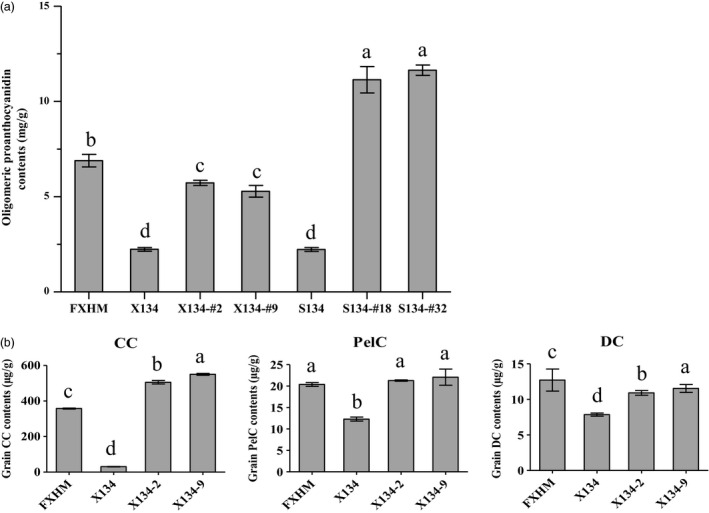
Oligomeric proanthocyanidin and anthocyanidin contents in grains of red rice lines. (a) Oligomeric proanthocyanidin contents in grains of red rice lines in X134 and S143 backgrounds. FXHM was a commercial red rice variety used as a positive control. Data are presented as mean ± SD. Different letters indicate groups with significant differences (ANOVA, P < 0.05). (b) Grain CC, PelC, DC contents (μg/g) of red rice lines in XS134 and corresponding their control lines. Data are presented as mean ± SD. Different letters indicate groups with significant differences (ANOVA, P < 0.05).
The X134‐#2 (−15 bp/−15 bp) and X134‐#9 (−15 bp/−30 bp) were further measured for the accumulation of four main types of anthocyanins: peonidin (PeoC), cyanidin (CC), delphindin (DC) and pelargonidin (PelC). While PeoC was not detected in any of the samples examined (data not shown), CC, PelC and DC in XS134‐2 and XS134‐9 grains were higher than that in wild‐type seeds. The content of CC, which is considered to be the main anthocyanin component in red rice, was about 17‐fold higher than that in the wild type (Figure 4b). The results also showed that restoration of Rc allele could result in accumulation of proanthocyanidins and anthocyanidins in rice grains, to levels comparable to or higher than that in a natural red rice variety.
Major agronomic and grain quality traits in T1 generation
The transgene‐free T1 generation plants with homozygous in‐frame Rc variants, X134‐#2, X134‐#9, S143‐#18 and S143‐#32, were cultured in the field and were investigated for major agronomic traits. The results showed that the in‐frame Rc mutants did not display evident alterations in whole plant and seed morphology (Figure 5a–d). Further investigations on yield‐associated traits showed that there were no significant differences in plant height, panicle number per plant, grain width, grain length, 1000‐grain weight and grain yield per plant between the in‐frame Rc mutants and their corresponding wild types X134 or S143, respectively (Figure 5e–f). The polished grains without red pericarp from T1 homozygous in‐frame Rc variants were also measured for major grain quality traits. The results showed that there were no significant differences in head rice rate, chalkiness rate, chalkiness degree, amylase content, gelatinization temperature of grains and gel consistency between the in‐frame Rc mutants and their corresponding wild types X134 or S143, respectively (Figure S5). Taken together, these results demonstrated that restoration of Rc function had no negative effect on the important agronomic or grain quality traits in rice.
Figure 5.
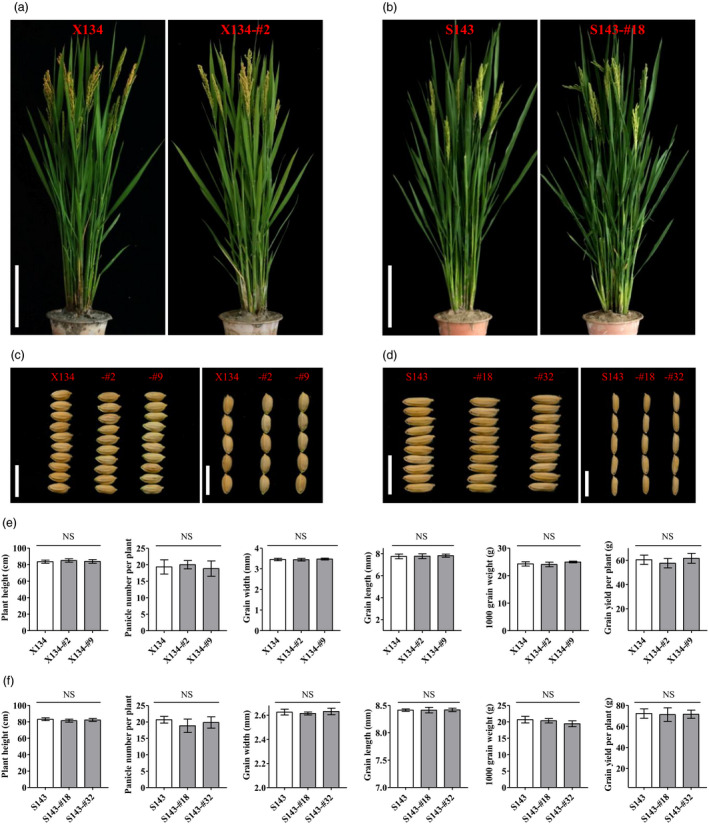
T1 generation homozygous in‐frame Rc variants and their corresponding wild types showed no significant differences in major agronomic traits. (a, b) Morphological phenotypes of in‐frame Rc mutants and their corresponding wild types X134 and S143. Scale bar: 20 cm. (c, d) Phenotypes of seeds with husk of in‐frame Rc mutants and their corresponding wild types X134 and S143. Scale bar: 1 cm. (e, f) Plant height, panicle number per plant, grain width, grain length, 1000‐grain weight and grain yield per plant in in‐frame Rc mutants and their corresponding wild types X134 and S143. NS, no significant difference.
Discussion
Cultivated rice has long been domesticated from the wild rice to meet human needs (Fuller et al., 2009; Huang et al., 2012; Malory et al., 2011). The rice seed is mainly made up of an outer husk layer, a pericarp layer, the starchy endosperm and the embryo. The change from red pericarp colour to white colour was an important hallmark of rice domestication (Sweeney et al., 2007). It was speculated that ancient farmers selected and spread white rice varieties because white grains were easier to cook, easier to dehusk, or had better background for picking out diseases or insects (Sweeney et al., 2007). Most cultivated rice varieties throughout the world today are white pericarp rice. In general, the husk and the pericarp layers are removed from the seeds of white varieties during the milling process, yielding polished white grains for consumption. In contrast, red rice varieties produce red pericarp covered with light‐coloured husk and the seeds are milled only to remove the husk, but the pericarp layer remains for consumption. The red pigment contained high levels of proanthocyanidins and anthocyanins, which have been shown to possess antioxidant properties against free radicals (Finocchiaro et al., 2007; Gunaratne et al., 2013; Qiu et al., 2010). Due to its health‐promoting benefits, red rice has become increasingly popular. In recent decades, although several red rice varieties have been developed based on conventional breeding (Sharma et al., 2014; Zhang et al., 2015), most cultivated red rice varieties suffer from low yield or other poor agronomic traits. Thus, development of elite varieties would be of great significance for production of red rice to meet the growing market.
The CRISPR/Cas9 system is a revolutionary approach that enables precise genome editing in various organisms. The novel biotechnological approach has been widely used in plant breeding for improving various traits through knocking out of the genes with deleterious on agronomic traits. In this study, we demonstrate that the CRISPR/Cas9 system could be an effective approach to restore recessive frame‐shift alleles for crop improvement. We successfully converted three elite white pericarp rice varieties into red ones without compromising yield potential through CRISPR/Cas9‐mediated functional recovery of the recessive rc allele. We generated a total of 60 T0 CRISPR/Cas9 transgenic plants from three varieties and identified 46 (76.7%) plants with mutations in the target sites. Among the 46 plants, 23 (50%) contained at least one in‐frame Rc variant and exhibited red pericarp. Given that about 97.9% of the white pericarp rice varieties are a result of the 14‐bp deletion in Rc (Sweeney et al., 2007), it is conceivable that our method could be applied to most white pericarp rice varieties. In addition, we observed no significant difference between wild‐type and T1 in‐frame Rc plants in major agronomic or grain quality traits. We are now growing T2 in‐frame Rc lines under field conditions. Consistent with results observed in T1 generation, there is no significant difference between wild‐type and T2 in‐frame Rc lines in morphological traits (data not shown). Thus, our approach would greatly accelerate the breeding of new red rice varieties with elite agronomic traits.
The Rc gene has a dominant effect on the red coloration in rice grains (Furukawa et al., 2007; Sweeney et al., 2006). In the present study, we also observed that bi‐allelic plants containing one dose of in‐frame Rc exhibited red pericarp phenotype identical to that of homozygous in‐frame Rc plants. Thus, it is feasible to develop hybrid red rice varieties through developing either red rice restorer lines or red rice sterile lines. In the present study, we successfully generated both the red rice restorer line and sterile line, allowing more flexibility for developing hybrid red rice combinations with elite agronomic traits.
Crop domestication is a process of artificially selecting ancestral wild plants and adapting them for human requirements: yield, taste, storage, etc. However, some beneficial or specialty traits may be lost during the long process. Most recently, genome editing technology has been demonstrated to speed up plant domestication. Editing of domestication‐related genes in ancestral wild plants could mimic changes that occurred in plants during domestication. Using the CRISPR/Cas9 system, Lemmon et al. (2018) generated ground cherry (Physalis pruinosa) plants that yielded more and bigger fruit; Li et al. (2018) generated tomato plants having domesticated phenotypes from ancestral tomato strains with disease resistance and salt tolerance; and Zsögön et al. (2018) generated tomato plants with improved fruit size, fruit number and high accumulation of fruit lycopene from wild tomato (Solanum pimpinellifolium). In this study, we present an alternative strategy for editing of domestication‐related genes for crop improvement. Our results demonstrated that editing of the recessive domestication‐related rc gene allele could recover red pericarp, a specialty trait, in modern cultivated rice varieties. This strategy could be applied to develop new crop varieties with beneficial or specialty traits lost during the domestication process.
Experimental procedures
Plant materials and growth conditions
Three elite white pericarp rice varieties, including a japonica conventional variety Xiushui134 (X134), an indica restorer line Shuhui143 (S143) and an indica two‐line sterile line ZhiNongS (ZNS), and a conventional red rice variety Fengxinhongmi (FXHM) were used in this study. Transgene‐free plants were grown in a standard greenhouse and in the paddy field at Fuzhou experimental station (26.08°N, 119.28°E), Fujian Province, China. Transgenic rice plants were grown in a standard greenhouse.
Construction of CRISPR/Cas9 plant expression vectors and rice transformation
Two single‐guide RNA (sgRNA) sequences targeting the flanking sequences of the 14‐bp deletion site in the rc allele were designed as predicted by the CRISPR‐P program (Lei et al., 2014). The oligonucleotides corresponding to the designed sgRNA sequences were synthesized, and oligonucleotide dimers were cloned into a CRISPR/Cas9 plant expression vector VK005‐01 (ViewSolid Biotech, Beijing, China) according to the manufacturer's instruction. The resulting constructs contained a Cas9 gene driven by the maize ubiquitin promoter and a designed sgRNA sequence under control of the rice U6 promoter.
The Cas9/sgRNA constructs were transfected into Agrobacterium tumefaciens EHA105 by electroporation. Rice calli of X134, S143 and ZNS were transformed with the mixture of Agrobacterium strains harbouring the Cas9/sgRNA constructs. Generation of transgenic rice plants was carried out as previously described (Hiei et al., 1994).
Genotype and agronomic trait analysis
Genomic DNAs were extracted from rice leaves using the hexadecyltrimethylammonium bromide (CTAB) method (Porebski et al., 1997). PCR amplifications were carried out to genotype the seventh exon of the Rc/rc allele, the coding exons of the Rd gene in X134, S143 and ZNS, and mutations in the rc allele in transgenic plants. The PCR products were sequenced by Sanger method. Decoding of sequencing chromatograms was carried out as described (Liu et al., 2015). DNA sequences were aligned using Clustal Omega (Sievers et al., 2011). Segregation of the Cas9/sgRNA T‐DNA was investigated in T1 generation derived from self‐pollination of T0 mutant plants. The presence/absence of the Cas9/sgRNA T‐DNA was evaluated by PCR amplification using specific primers for the hygromycin phosphotransferase (Hpt) gene and the Cas9 gene.
Wild‐type parents and T1 generation plants were investigated for agronomic traits. Rice plants were cultivated at Fuzhou experimental station, Fujian Province, under nature long‐day condition (mid‐May to mid‐September, c. 13.5 h). The management of plants was followed the normal agricultural practice.
All primers used for in this study are listed in Table S2.
Measurement of oligomeric proanthocyanidins and anthocyanins
One gram of rice seeds was de‐hulled and ground enough to pass through a 40‐mesh sieve. The content of oligomeric proanthocyanidins (OPCs) in rice seeds was determined with a Micro Plant Proanthocyanidins Assay Kit (Solarbio Science & Technology, Beijing, China) following the manufacturer's instructions.
Approximately 2 g of rice seeds was powdered after freezing in liquid nitrogen and was homogenized in methanol/hydrochloric acid extraction buffer by sonication according to the method described previously (Abdel‐Aal et al., 2006; Furukawa et al., 2007). The extract was centrifuged at 12 000 g for 10 min. The supernatant was treated with concentrated hydrochloric acid and incubated at 95 °C for 40 min. After cooling, the crude extract containing the anthocyanins was passed through a 0.22‐μm filter and quantified by accurate mass HPLC1290/MS‐Qtrap 6500 (Agilent Technologies, Palo Alto, CA) system packed with a reversed‐phase column (2.1 × 150 mm, 2.7 μm).
Measurements of OPCs and anthocyanins were based on at least three replicates. Data are presented as means ± SD. The statistical significance of the differences in the contents was determined by ANOVA.
Grain quality trait analysis
Polished grains without pericarp were measured for major grain quality traits. The head rice rate, chalkiness rate and chalkiness degree were measured by a rice‐grain appearance analysis system (Wseen, China). The amylose content was obtained by following the ISO‐6647 method. The gelatinization temperature was determined using the alkali digestion test (Little et al., 1958), and the gel consistency of grain was measured as previously described (Cagampang et al., 1973).
Conflicts of interest
The authors declare that they have no conflicts of interest with respect to this work.
Author contributions
F.W., Y.Z. and L.C. conceived the project and designed the experiments. Y.Z., Y.L., S.C., M.F., Z.C., T.H., H.L., F.M. and J.C. performed the experiments. Y.Z, Y.L. and S.C. analysed the data. Y.Z., Y.L. and S.C. wrote the manuscript.
Supporting information
Figure S1 Multiple alignment of the seventh exon of the Rc/rc allele in wild red rice (Oryza rufipogon), and three white pericarp rice varieties X134, S143, and ZNS.
Figure S2 Multiple alignment of the coding regions of the Rd gene in wild red rice (Oryza rufipogon), and three white pericarp rice varieties X134, S143, and ZNS.
Figure S3 Multiple alignment of the deduced amino acid sequences of in‐frame Rc variants.
Figure S4 In‐fame Rc mutants in ZNS background displayed an identical male sterility phenotype as their wild type.
Figure S5 In‐fame Rc mutants and their corresponding wild types showed no significant differences in major grain quality traits.
Table S1 Genotype of T0 mutant plants in three rice varieties.
Table S2 Primers used in this study.
Acknowledgements
This work was supported by Science and Technology Major Project of Fujian Province (2015NZ0002‐3), National Key Research and Development Program of China (2016YFD0100903) and Fujian Provincial Special Fund (2015R1019‐1).
Contributor Information
Liang Chen, Email: chenlg@xmu.edu.cn.
Feng Wang, Email: wf@fjage.org.
References
- Abdel‐Aal, E.‐S.M. , Young, J.C. and Rabalski, I. (2006) Anthocyanin composition in black, blue, pink, purple, and red cereal grains. J. Agric. Food Chem. 54, 4696–4704. [DOI] [PubMed] [Google Scholar]
- Cagampang, G.B. , Perez, C.M. and Juliano, B.O. (1973) A gel consistency test for the eating quality of rice. J. Sci. Food Agric. 24, 1589–1594. [DOI] [PubMed] [Google Scholar]
- Cai, Y. , Chen, L. , Liu, X. , Guo, C. , Sun, S. , Wu, C. , Jiang, B. et al. (2018) CRISPR/Cas9‐mediated targeted mutagenesis of GmFT2a delays flowering time in soya bean. Plant Biotechnol. J. 16, 176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clasen, B.M. , Stoddard, T.J. , Luo, S. , Demorest, Z.L. , Li, J. , Cedrone, F. , Tibebu, R. et al. (2015) Improving cold storage and processing traits in potato through targeted gene knockout. Plant Biotechnol. J. 14, 169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finocchiaro, F. , Ferrari, B. , Gianinetti, A. , Dall'asta, C. , Galaverna, G. , Scazzina, F. and Pellegrini, N. (2007) Characterization of antioxidant compounds of red and white rice and changes in total antioxidant capacity during processing. Mol. Nutr. Food Res. 51, 1006–1019. [DOI] [PubMed] [Google Scholar]
- Fuller, D.Q. , Qin, L. , Zheng, Y. , Zhao, Z. , Chen, X. , Hosoya, L.A. and Sun, G.P. (2009) The domestication process and domestication rate in rice: spikelet bases from the Lower Yangtze. Science, 323, 1607–1610. [DOI] [PubMed] [Google Scholar]
- Furukawa, T. , Maekawa, M. , Oki, T. , Suda, I. , Iida, S. , Shimada, H. , Takamure, I. et al. (2007) The Rc and Rd genes are involved in proanthocyanidin synthesis in rice pericarp. Plant J. 49, 91–102. [DOI] [PubMed] [Google Scholar]
- Gunaratne, A. , Wu, K. , Li, D. , Bentota, A. , Corke, H. and Cai, Y.Z. (2013) Antioxidant activity and nutritional quality of traditional red‐grained rice varieties containing proanthocyanidins. Food Chem. 138, 1153–1161. [DOI] [PubMed] [Google Scholar]
- Hiei, Y. , Ohta, S. , Komari, T. and Kumashiro, T. (1994) Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T‐DNA. Plant J. 6, 271–282. [DOI] [PubMed] [Google Scholar]
- Huang, X. , Kurata, N. , Wei, X. , Wang, Z.X. , Wang, A. , Zhao, Q. , Zhao, Y. et al. (2012) A map of rice genome variation reveals the origin of cultivated rice. Nature, 490, 497–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei, Y. , Lu, L. , Liu, H.Y. , Li, S. , Xing, F. and Chen, L.L. (2014) CRISPR‐P: a web tool for synthetic single‐guide RNA design of CRISPR‐system in plants. Mol. Plant, 7, 1494–1496. [DOI] [PubMed] [Google Scholar]
- Lemmon, Z.H. , Reem, N.T. , Dalrymple, J. , Soyk, S. , Swartwood, K.E. , Rodriguez‐Leal, D. , Van Eck, J. et al. (2018) Rapid improvement of domestication traits in an orphan crop by genome editing. Nat. Plants, 4, 766–770. [DOI] [PubMed] [Google Scholar]
- Li, S. , Gao, F. , Xie, K. , Zeng, X. , Cao, Y. , Zeng, J. , He, Z. et al. (2016) The OsmiR396c‐OsGRF4‐OsGIF1 regulatory module determines grain size and yield in rice. Plant Biotechnol. J. 14, 2134–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, T. , Yang, X. , Yu, Y. , Si, X. , Zhai, X. , Zhang, H. , Dong, W. et al. (2018) Domestication of wild tomato is accelerated by genome editing. Nat. Biotechnol. 36, 1160–1163. [DOI] [PubMed] [Google Scholar]
- Little, R.R. , Hilder, G.B. and Dawson, E.H. (1958) Differential effect of dilute alkali on 25 varieties of milled white rice. Cereal Chem. 35, 111–126. [Google Scholar]
- Liu, W. , Xie, X. , Ma, X. , Li, J. , Chen, J. and Liu, Y.G. (2015) DSDecode: a web‐based tool for decoding of sequencing chromatograms for genotyping of targeted mutations. Mol. Plant, 8, 1431–1433. [DOI] [PubMed] [Google Scholar]
- Macovei, A. , Sevilla, N.R. , Cantos, C. , Jonson, G.B. , Slamet‐Loedin, I. , Cermak, T. , Voytas, D.F. et al. (2018) Novel alleles of rice eIF4G generated by CRISPR/Cas9‐targeted mutagenesis confer resistance to Rice tungro spherical virus . Plant Biotechnol. J. 16, 1918–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malory, S. , Shapter, F.M. , Elphinstone, M.S. , Chivers, I.H. and Henry, R.J. (2011) Characterizing homologues of crop domestication genes in poorly described wild relatives by high‐throughput sequencing of whole genomes. Plant Biotechnol. J. 9, 1131–1140. [DOI] [PubMed] [Google Scholar]
- Porebski, S. , Bailey, L.G. and Baum, B.R. (1997) Modification of a CTAB DNA extraction protocol for plantscontaining high polysaccharide and polyphenol components. Plant Mol. Biol. Rep. 15, 8–15. [Google Scholar]
- Qiu, Y. , Liu, Q. and Beta, T. (2010) Antioxidant properties of commercial wild rice and analysis of soluble and insoluble phenolic acids. Food Chem. 121, 140–147. [Google Scholar]
- Sharma, N. , Kaur, R. , Mangat, G.S. and Singh, K. (2014) Red pericarp introgression lines derived from interspecific crosses of rice: physicochemical characteristics, antioxidative properties and phenolic content. J. Sci. Food Agric. 94, 2912–2920. [DOI] [PubMed] [Google Scholar]
- Sievers, F. , Wilm, A. , Dineen, D. , Gibson, T.J. , Karplus, K. , Li, W. , Lopez, R. et al. (2011) Fast, scalable generation of high‐quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 7, 539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney, M.T. , Thomson, M.J. , Pfeil, B.E. and McCouch, S. (2006) Caught red‐handed: Rc encodes a basic helix‐loop‐helix protein conditioning red pericarp in rice. Plant Cell, 18, 283–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney, M.T. , Thomson, M.J. , Cho, Y.G. , Park, Y.J. , Williamson, S.H. , Bustamante, C.D. and McCouch, S.R. (2007) Global dissemination of a single mutation conferring white pericarp in rice. PLoS Genet. 3, e133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Cheng, X. , Shan, Q. , Zhang, Y. , Liu, J. , Gao, C. and Qiu, J.L. (2014) Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nat. Biotechnol. 32, 947–951. [DOI] [PubMed] [Google Scholar]
- Wang, F. , Wang, C. , Liu, P. , Lei, C. , Hao, W. , Gao, Y. , Liu, Y.G. et al. (2016) Enhanced rice blast resistance by CRISPR/Cas9‐targeted mutagenesis of the ERF transcription factor gene OsERF922 . PLoS ONE, 11, e0154027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, P. , Zhang, J. , Sun, L. , Ma, Y. , Xu, J. , Liang, S. , Deng, J. et al. (2018a) High efficient multisites genome editing in allotetraploid cotton (Gossypium hirsutum) using CRISPR/Cas9 system. Plant Biotechnol. J. 16, 137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X. , Tu, M. , Wang, D. , Liu, J. , Li, Y. , Li, Z. , Wang, Y. et al. (2018b) CRISPR/Cas9‐mediated efficient targeted mutagenesis in grape in the first generation. Plant Biotechnol. J. 16, 844–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. , Zhang, J. , Wei, P. , Zhang, B. , Gou, F. , Feng, Z. , Mao, Y. et al. (2014) The CRISPR/Cas9 system produces specific and homozygous targeted gene editing in rice in one generation. Plant Biotechnol. J. 12, 797–807. [DOI] [PubMed] [Google Scholar]
- Zhang, H. , Shao, Y. , Bao, J. and Beta, T. (2015) Phenolic compounds and antioxidant properties of breeding lines between the white and black rice. Food Chem. 172, 630–639. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Liang, Z. , Zong, Y. , Wang, Y. , Liu, J. , Chen, K. , Qiu, J.L. et al. (2016) Efficient and transgene‐free genome editing in wheat through transient expression of CRISPR/Cas9 DNA or RNA. Nat. Commun. 7, 12617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. , Zhang, H. , Botella, J.R. and Zhu, J.K. (2018) Generation of new glutinous rice by CRISPR/Cas9‐targeted mutagenesis of the Waxy gene in elite rice varieties. J. Integr. Plant Biol. 60, 369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, H. , Liu, B. , Weeks, D.P. , Spalding, M.H. and Yang, B. (2014) Large chromosomal deletions and heritable small genetic changes induced by CRISPR/Cas9 in rice. Nucleic Acids Res. 42, 10903–10914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, H. , He, M. , Li, J. , Chen, L. , Huang, Z. , Zheng, S. , Zhu, L. et al. (2016) Development of commercial thermo‐sensitive genic male sterile rice accelerates hybrid rice breeding using the CRISPR/Cas9‐mediated TMS5 editing system. Sci. Rep. 6, 37395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsögön, A. , Čermák, T. , Naves, E.R. , Notini, M.M. , Edel, K.H. , Weinl, S. , Freschi, L. et al. (2018) De novo domestication of wild tomato using genome editing. Nat. Biotechnol. 36, 1211–1216. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Multiple alignment of the seventh exon of the Rc/rc allele in wild red rice (Oryza rufipogon), and three white pericarp rice varieties X134, S143, and ZNS.
Figure S2 Multiple alignment of the coding regions of the Rd gene in wild red rice (Oryza rufipogon), and three white pericarp rice varieties X134, S143, and ZNS.
Figure S3 Multiple alignment of the deduced amino acid sequences of in‐frame Rc variants.
Figure S4 In‐fame Rc mutants in ZNS background displayed an identical male sterility phenotype as their wild type.
Figure S5 In‐fame Rc mutants and their corresponding wild types showed no significant differences in major grain quality traits.
Table S1 Genotype of T0 mutant plants in three rice varieties.
Table S2 Primers used in this study.


