Abstract
Animals have quick-acting nociceptive reflexes that protect them from tissue damage. Some taxa have also evolved the capacity for pain. Pain appears to be linked to long-term changes in motivation brought about by the aversive nature of the experience. Pain presumably enhances long-term protection through behaviour modification based, in part, on memory. However, crustaceans have long been viewed as responding purely by reflex and thus not experiencing pain. This paper considers behavioural and physiological criteria that distinguish nociception from potential pain in this taxon. These include trade-offs with other motivational systems and prolonged motivational change. Complex, prolonged grooming or rubbing demonstrate the perception of the specific site of stimulus application. Recent evidence of fitness-enhancing, anxiety-like states is also consistent with the idea of pain. Physiological changes in response to noxious stimuli mediate some of the behavioural change. Rapid avoidance learning and prolonged memory indicate central processing rather than mere reflexes. Thus, available data go beyond the idea of just nociception. However, the impossibility of total proof of pain described in ways appropriate for our own species means that pain in crustaceans is still disputed. Pain in animals should be defined in ways that do not depend on human pain experience.
This article is part of the Theo Murphy meeting issue ‘Evolution of mechanisms and behaviour important for pain’.
Keywords: anxiety, avoidance learning, motivation, nociception, pain, trade-off
1. Introduction to pain, nociception and reflexes
Acute pain is thought to comprise two main components. First, there are the neurons that detect tissue damage, called nociceptors [1]. When these fire information about the damage is passed to the central nervous system where it is assessed and, at least in humans, results in an extremely unpleasant experience. This second component is the aspect that we typically consider when we say we are in pain. The feeling is generated in the brain but is often experienced with respect to the part of the body that has been damaged. For example, a finger that has been hit with a hammer is said to hurt as if the pain was in the finger. Some talk of ‘pain perception’ as if the nociceptors transfer the feeling of pain to the brain. It is worth remembering, however, that the pain is in the brain not in the finger.
Nociceptors, however, have another mode of action and enable a reflex response involving relatively few synaptic junctions [1]. The specific fibres involved in this response in vertebrates are larger and myelinated and transmit the information more quickly than those involved specifically in pain. The result is that the reflex movement is initiated before any information gets to the brain. Thus, the reflex does not necessarily result in any level of awareness and is separate from what we would talk of as pain. This reflex response poses difficulty in studies on the possibility of pain in animals because if the animal responds to a noxious stimulus by withdrawing all or just the damaged part of the body that might occur without pain [2]. Some authors have rejected the idea of pain in all but some mammals and have insisted that the responses of crustaceans to tissue damage are purely reflexive [3].
The aim of the current paper is to examine this proposition and to determine if crustaceans react only by nociceptive reflex or if there is evidence of responses that go beyond that expected of reflex. The focus will be on short-term behavioural responses that clearly involve decision-making rather than reflex, or longer-term changes involving motivational change. In addition, physiological changes that mediate these activities will be noted. Further, there is an evolutionary expectation that pain should produce long-term, fitness-enhancing, behavioural modification beyond that achieved with just an immediate reflex [4,5]. After reviewing the evidence on the aspects noted above there is a discussion of non-reflexive behaviour and what that might tell us about pain.
2. Motivational trade-offs
When an animal decides to engage in a specific activity, it is typically influenced by the motivation to engage in other activities. For example, a zebra might approach a water-hole to drink but its tendency to drink might be influenced by the danger posed by the presence of potential predators. This is termed a motivational trade-off [6]. By contrast, a reflex should be the same irrespective of other motivational requirements and not be influenced by information that might affect other motivations. Thus, if immediate responses to a noxious stimulus can be shown to be influenced by information relevant to other requirements then those responses cannot be mere reflexes and must, instead, involve some form of central processing.
For example, when hermit crabs, Pagurus bernhardus, receive electric shock within their shells, some quickly abandon their shells in a manner that superficially looks like a reflex. However, shell evacuation has been shown to be traded-off against other, competing, motivational requirements. Hermit crabs were less likely to evacuate their shells if the odour of a predator was present [7], and those subjected to the increasing intensity of shock abandoned shells of a preferred species at a higher intensity than did those in poor quality shells [8]. If subjected to shocks of a single intensity hermit crabs were more likely to abandon shells of a less preferred species [9]. Thus, although evacuating from a shell might seem a relatively simple behaviour, it is clearly influenced by other motivational requirements, and thus a product of central decision-making. The response to the noxious stimulus is traded-off against the need to avoid predators or the need to maintain a quality shell and is not a pure reflex.
3. Protective behaviour: prolonged rubbing and grooming/wound guarding
Wounded animals often show activities such as rubbing, guarding of wounds and limping, and these activities are interpreted as being consistent with pain [10]. They indicate an awareness of the site of a wound (not necessarily implying consciousness but at least some perception of the afflicted site) and maybe an attempt to alleviate pain. Similar activities have been reported for crustaceans. For example, sodium hydroxide or acetic acid (both known to induce pain in mammals) applied to a single antenna of the glass prawn, Palaemon elegans, resulted in prolonged grooming and rubbing of that specific antenna [11]. The grooming involved repeatedly pulling that specific antenna through the small chelipeds (claws) or through the mouthparts, whereas rubbing was pressing and moving that antenna against the side of the tank. Pinching one antenna with forceps did not affect grooming rate but did increase rubbing. The responses were directed at the treated antenna significantly more than the untreated antenna, indicating an awareness of the specific location of the noxious stimulus. Furthermore, the application of sodium hydroxide to one eye of a glass prawn caused high levels of grooming of that specific eye with either one or both first walking legs. This behaviour was not seen if just seawater was applied [6]. Also, shore crabs, Carcinus maenas, use their claws to scratch at their mouthparts if that is brushed with acetic acid [12] and hermit crabs, shocked on the abdomen, groom at the site of the shock, an activity not seen without the noxious stimulus [8,13]. Furthermore, edible crabs, Cancer pagurus, that have had a clawed appendage twisted off in the manner used in some fisheries [14] held their remaining claw over the wound during a competitive interaction. Manually declawed crabs also touched their wound and picked at the broken exoskeleton with their remaining claw and sometimes showed a ‘shuddering response’. These manually declawed crabs showed a lower motivation to compete for a female and seemed to be more engaged in self-defence than were those induced to autotomize, indicating that the change was owing to the wound rather than the lack of a claw per se [14].
4. Long-term motivational change
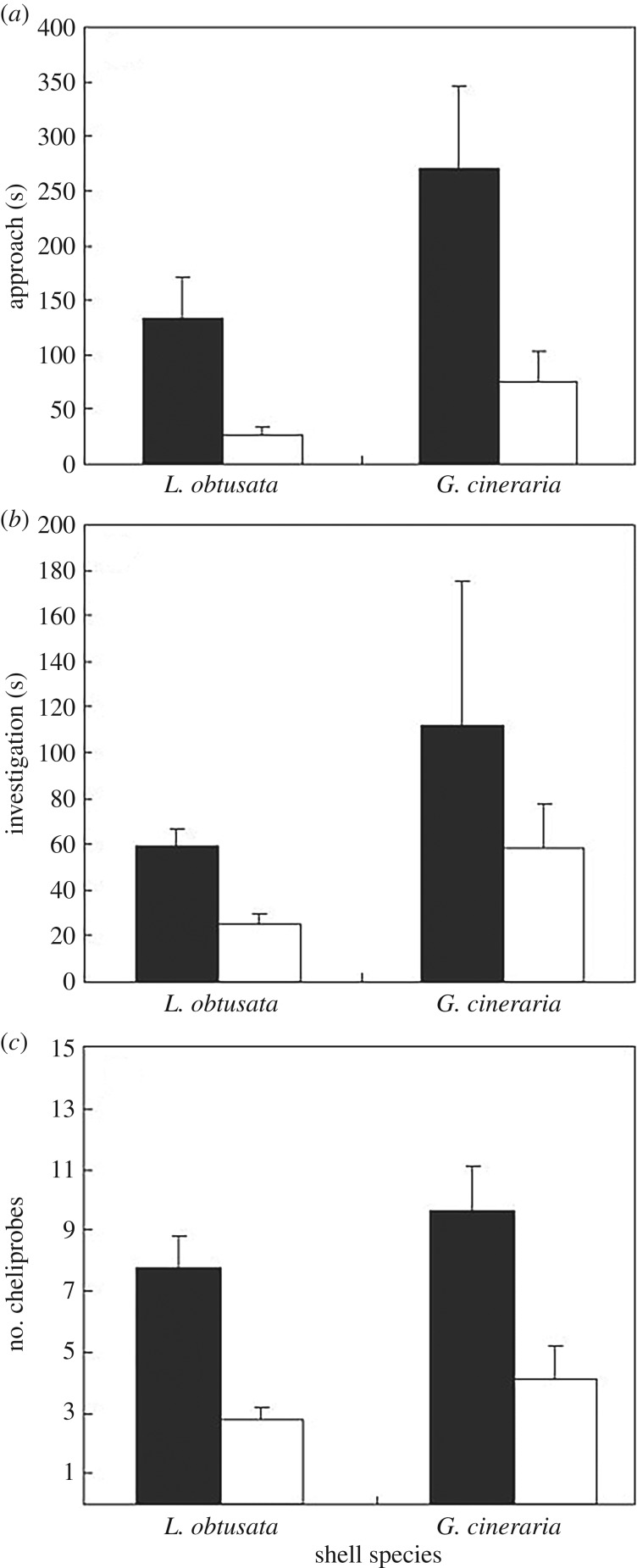
A reflex is a simple, short-term response so any complex long-term response cannot be regarded as reflexive, rather it demonstrates an extended alteration of motivational state. An example is that of hermit crabs shocked within the shells at an intensity below that which typically elicits shell evacuation, compared with crabs that did not receive shock [9]. Crabs were subsequently offered a new shell of the same species that they were inhabiting. Those that had been shocked showed a much higher motivation to obtain a new shell as shown by the probability and time taken to take the new shell [15,16]. They were more likely to contact the offered shell and more likely to move into the new shell. Furthermore, of those that completed these activities, the shocked crabs contacted the shell with a shorter latency, spent less time investigating the new shell before moving in and used their chelipeds (clawed appendages) less often in examining the new shell (figure 1). These data indicate that crabs receiving a shock within the shell subsequently valued that shell less than did crabs that were not shocked [15,16]. In this experiment, the new shell was offered 20 s after the cessation of shock (or an equivalent time for the non-shocked group) and the altered motivation was noted during the sequence of activities after that time. Thus, the altered response cannot be a reflex response to the shock. An additional experiment, however, used longer times between shock and the offering of the shell [13] and this provided evidence of a memory of the aversive shock that lasted at least 1 day. Crabs tested at this time were more likely to approach the shell and used fewer probes of the chelipeds prior to moving in if they had been shocked the previous day. This strengthens the conclusion that the change in behaviour is owing to a long-term motivational change indicating that the perceived value of the original shell is reduced after a shock is received within that shell.
Figure 1.
Mean ± s.e. of (a) time taken to approach (s), (b) duration of investigation (s) and (c) number of cheliprobes by crabs in either L. obtusata or G. cineraria shells that had been shocked (open squares) or not shocked (filled squares) (from [9]).
5. Anxiety and survival
Pain should function to increase survival and we expect animals subjected to noxious, potentially painful stimuli, to become risk averse. This has been termed anxiety [17], which is another long-term motivational change that cannot be described as a reflex. A particularly interesting example of this increased wariness comes from work on crayfish, Procambarus clarkii [17]. These decapods tend to prefer dark to light environments and when placed in a cross maze with two light and two dark arms they prefer the dark arms. However, if they are the first subject to the repeated electric shock that induces escape responses, they show a much stronger avoidance of the light arms. Because a light environment might normally pose a greater risk to crayfish, they appear to become risk averse and are described as showing anxiety. This ‘anxiety’ was accompanied with higher levels of serotonin (5HT) in the brain [17,18]. Furthermore, animals that were not shocked but injected with 5HT showed similar levels of anxiety to those that were shocked. If pre-treated with a 5HT antagonist, however, they did not show the anxiogenic effect of 5HT [18]. There were also close correlations between 5HT levels and behavioural indicators of anxiety thus providing further evidence for a role of 5HT in anxiety. Surprisingly, when shocked crayfish were given chlordiazepoxide (CDZ), a drug that reduces anxiety in humans, it reduced signs of anxiety in crayfish [18]. However, CDZ did not alter 5HT levels in crayfish, suggesting that the effect of the anxiolytic is independent of the biogenic amine [18]. CDZ modulates GABA type A receptors in vertebrates and, in crustaceans, GABA acts as an inhibitory neurotransmitter, the receptors of which are sensitive to anxiolytics [18]. Similarly, the anxiolytic LY354740 given to amphipods, Gammarus fossarum, subject to shock, reduced the time spent hiding in a dark shelter [19].
Crustacean hyperglycaemic hormone (CHH), which elevates haemolymph glucose concentrations, is also released during noxious stimulation [20–22]. CHH is analogous to the stress hormones of vertebrates, in that it mobilizes intracellular glycogen and converts it to glucose [23] but it also causes elevated lactate. Removing one claw of edible crabs by twisting and breaking, but not by induced autotomy, caused a significant increase in lactate and glucose [24], suggesting that the physiological effects were predominantly owing to tissue damage.
There is, however, a potential confounding variable when examining stress hormones following noxious stimulation. Often noxious stimulation, such as electric shock, causes vigorous escape behaviour and this behaviour rather than the shock might cause the physiological change [25]. For example, in the studies of Fossat et al. [17,18], crayfish were given numerous shocks that induced vigorous, tail-flipping escape responses. To investigate if the behavioural responses to shock or the shock per se might cause the apparent physiological stress response, Elwood & Adams [25] shocked shore crabs and compared their behaviour and physiology with non-shocked crabs. As expected, the shocked crabs showed greater activity than non-shocked crabs, with some showing escape and threat responses. By contrast, some of the non-shocked crabs remained still during the experiment. Nevertheless, some crabs in each experimental group simply walked about the observation tank. When just these crabs that showed the same walking behaviour were examined the lactate levels of shocked crabs was significantly higher than that of the non-shocked crabs, indicating that the physiological stress response was caused by the noxious stimulus and not by behavioural differences. These data support the conclusions about physiological stress responses mediating anxiety [17,18].
The stress responses and increased anxiety, however, are anticipated to have some benefit to the animals and this was demonstrated in an experiment on amphipods [19]. These animals showed more hiding in a dark shelter after shock treatment and when a predatory fish was introduced to the observation tank, the shocked animals showed improved survival compared to the non-shocked amphipods [19]. Because the electric shocks occurred 10 min before the predator was introduced, the improved survival could not be owing to a reflex response to the shock.
6. Avoidance learning
Avoidance learning alters future behaviour in a way that reduces future tissue damage. It cannot be regarded as a reflex and is consistent with the idea of pain. The aversive experience is predicted to increase the salience of the stimulus situation and thus results in rapid avoidance learning [26]. This is important because the animal could markedly reduce future tissue damage only if the learning occurred within a few trials. In one example, shore crabs were repeatedly placed into the centre of a brightly lit enclosure that had a dark shelter at each end [27]. On the first trial, each crab moved quickly to one of the shelters and the crab had been preselected to either receive shock every 5 s while in the first choice of shelters or not to receive shock in the first selected shelter. The alternative shelter resulted in no shock or shock, respectively. If the crab received shock it could avoid further shocks in that trial by emerging into the light. After 2 min, the crab was removed from the enclosure and then placed again in the centre so that the effects of having been shocked on the choice of the two shelters could be determined. On the second trial, the choice of the shelter was not affected by having received shock or not in the first trial. Rather, crabs showed a strong preference for the same shelter as selected previously. On the third trial, crabs that had not been shocked in trial two still showed a preference for the previously used shelter. By contrast, those that had received shock in the previous trial were significantly more likely to switch to the alternative shelter. That is, it took only two trials for the initial preference of a shelter to be overcome and for the crabs to avoid the shock shelter. Over subsequent trials, fewer crabs selected the shock shelter and a preference for using the non-shock shelter emerged. Further, over 10 trials, an increasing proportion of crabs that entered the shock shelter emerged during the 2 min, which suggests another form of learning or a sensitization to the shock (sensu [28]). No crab ever emerged from the non-shock shelter showing that the shock is aversive.
During these trials, the crab was always oriented the same way such that it walked either to the left or right to make its choice of shelter. Furthermore, cards with black and white stripes oriented either horizontally or vertically were placed above the shelters. In final trials, these features were reversed for some of the crabs and that demonstrated that crabs used response learning rather than place learning and were not affected by the stripe orientation [27]. That is, if they previously had walked to the left to reach the safe shelter they continued to walk to their left even when turned around by 180° and thus walked to the shelter that had previously resulted in shock.
A second experiment by Magee & Elwood [29] used a different paradigm that represents a more cognitively challenging task [30]. That is, during the trials, a barrier separated the two halves of the tank such that the crab had access to only one shelter at a time. When tested after 10 such trials, they were tested without the barrier, but the animals showed no preference for the non-shock shelter. Rather, they employed different tactics to reduce the number of shocks during the initial trials. Over the five training trials involving shock, there was an increase in the number of crabs exiting that shelter after receiving shock (as happened in Magee & Elwood [27]). Further, they exited the shelter after fewer shocks in later trials. This is consistent with the idea of pain but increased sensitivity might play a role (sensu [28]). However, the main conclusion from Magee & Elwood [29] was that no associations were formed between the location of shock and either egocentric or allocentric cues. In this respect, there is no support for the idea of pain, rather it appears that crabs lack the cognitive skills required for this type of discrimination [26]. However, with less demanding paradigms, rapid avoidance learning is evident [27,31].
A different paradigm involves crabs raising one leg out of shallow water to avoid an electric shock [32–34]. Shocked crabs decrease the number of times they lower their leg compared with non-shocked controls or yolked controls that receive shock whenever a ‘master’ crab is shocked. However, crabs with the brain destroyed also show some capacity casting doubt on pain being involved [33]. Indeed, one might argue that avoidance learning has not occurred in these preparations. The number of shocks per minute declined from 1500 shocks in the first minute but the animal still received 400 shocks per minute after 10 min, which is not a strong example of avoiding shock [33]. Rather than an example of avoidance learning without a brain and without the possibility of pain, this appears to be a different type of process and provides little help in discrimination between reflex and non-reflexive responses to noxious stimuli.
The nature of the response required to avoid shock might also influence the speed of learning. For example, crayfish placed in a shuttle box could avoid shock if they moved from one compartment to another after a light signalled the imminent shock [35]. Some animals were tested while facing towards and others away from the safe compartment. In early trials, all animals showed a tail-flick escape response to the shock, which took those facing away from the safe compartment backwards into the safe area. By contrast, those facing towards the safe compartment darted backwards deeper to the shock compartment. Nevertheless, these animals slowly learned to walk to the safe area when the light came on, thus avoiding the shock. However, those that had trials when facing away rapidly learned to walk to safety when turned to face the safe area. That is the ability to learn was constrained by the response required [35].
7. Are responses purely reflexive or are they more complex?
The examples given here clearly demonstrate that crustacean responses to noxious stimuli frequently go beyond nociceptive reflexes. Of course, some responses are likely to be reflexive such as the tail flick of glass prawns immediately after the antenna is pinched or treated with a noxious chemical [11], or the tail-flick response to shock seen in crayfish [35]. Puri & Faulkes [36] described rapid tail-flick withdrawal from a hot probe by crayfish, P. clarkii, consistent with nociceptive reflex. The crayfish, however, also showed other activities and appeared to engage in brief attacks on the hot probe with coordinated use of both claws, which was also regarded as a reflex response, although it clearly involved visual input as well as the nociceptive input. But, even with short-term responses, there are indications that reflexes may not always account for the nature of the behaviour. We see this with the motivational trade-off between shock avoidance and other motivational requirements, which indicates that the response is owing to a behavioural decision rather than reflex [8,9]. There are also prolonged complex activities involving rubbing, grooming and wound guarding directed to the specific area [11,12,14]. There are long-term behavioural modifications such as hermit crabs showing motivational changes 24 h after being shocked within their shell [9,13]. There are indications of a broad change towards risk aversion, discussed as anxiety [17–19], and improved survival when a predator is present [19]. There are physiological stress responses that mediate some of these behavioural changes [17,18,25]. Further, there may be rapid avoidance learning so that the exposure to noxious stimuli is markedly reduced [27]. Thus, the idea of crustaceans merely responding to noxious stimuli by reflex reactions can be unequivocally rejected.
8. What does this tell us about ‘pain’?
The idea of pain in crustaceans was traditionally rejected because they were thought to respond to noxious stimuli purely by reflex. This is clearly not the case but that does not mean that pain is proved. There may be alternative explanations that do not depend on the idea of negative feelings commonly implied in studies of human pain. We simply do not know, and probably never will know, what crustaceans feel when exposed to noxious stimuli.
What can be said with confidence, however, is that the data for crustaceans are broadly consistent with criteria for pain [4] but again that is not the same as claiming proof. However, some authors have been critical of studies on potential pain in crustaceans and have been confused by the meaning of ‘data being consistent with the idea of pain’. For example, Rose et al. [3] stated that Appel & Elwood [13] claimed ‘that their results proved that the crabs felt pain’. This is despite the repeated comments in Appel & Elwood [13] that proof is not possible, and no proof being claimed. Stevens et al. [37, p. 1] wrote of Elwood & Adams [25] in the following terms ‘We contend that their conclusion that crabs experience pain (as summarized in the title and abstract) is unfounded’. However, Elwood & Adams [25] made no such statements, and instead affirmed the impossibility of proving pain in any animal [38]. Further, Diggles [39] argues that studies on potential pain in crustaceans are of low quality and should not be used as evidence to change the way that crustaceans are used in science or fisheries/aquaculture industries. Diggles [39] also disagrees about which, if any, criteria of pain might be used in evoking the use of a precautionary principle. He disagrees with Birch [40] who suggests that just one fulfilled criterion should be required to change animal welfare policy. He further argues [39, p. 77] that the numerous criteria suggested by Sneddon et al. [4] ‘effectively set ‘the bar’ for pain and sentience so low that it is impossible to have confidence that the behaviours observed in many experiments are even due to nociception, let alone in anyway analogous to how the word pain is used and understood by humans.’ To show pain, Stevens et al. [37, p. 1] state that ‘at the very minimum requires activation of nociceptive pathways, followed by conscious higher level processing’. Nociceptive pathways are evident [36]. However, because it is not possible to demonstrate consciousness, the requirement for it precludes acceptance of pain in any animal. Later in the same commentary, Stevens et al. [37, p. 2] contradictorily note that animal welfare should be addressed pragmatically ‘using stress-related indicators without reference to conscious experiences’. The present author agrees with the latter comment and notes that this has been the approach taken in the empirical studies on crustaceans reviewed in the present paper. Sneddon et al. [41] also note misleading comments and confusion about the potential for pain in fish by this group of authors (e.g. [3]).
These experiments on crustaceans also demonstrate another line of evidence that is consistent with pain. Hermit crabs abandon their shells, which are extremely valuable resources. Some inspect the shell, feeling deep within, but others walk away from the shell and scrabble at the walls of the experimental chamber [8]. Further, in order to escape shock, shore crabs will emerge from a dark shelter and will move into a brightly lit area that normally risks predation [27,42]. While it is true that a reflex response could interrupt an important activity but if without any level of awareness of the stimulus then the animal should immediately return to the shell or shelter and not abandon it altogether. Thus, the shock is aversive and elicits a risky change in behaviour to escape the noxious stimulus.
To conclude, studies of animal pain have been hampered by reference to the experience of our own species. Movement away from an anthropocentric view to a definition based on features that can be observed, rather than those that cannot, would be welcome. Various suggestions for definitions have been made, reviewed in [5], but these tend to become long lists of criteria. One reasonably short definition would be ‘A non-reflexive response to a noxious, potentially tissue-damaging stimulus that alters future behaviour’. It gets away from the idea of feelings or consciousness, which is impossible to access, and avoids arguments that cannot be resolved.
Data accessibility
This article has no additional data.
Competing interests
I declare I have no competing interests.
Funding
The author has not received funding from any commercial organization or any charity or campaign group for animal welfare. Although membership has been offered, he has never been a member of any such charity or group.
References
- 1.Sherrington C. 1906. The integrative action of the nervous system. Oxford, UK: Oxford University Press. [Google Scholar]
- 2.Sherwin CM. 2001. Can invertebrates suffer? Or how robust is argument-by-analogy? Anim. Welfare 10, S104–S118. [Google Scholar]
- 3.Rose JD, Arlinghaus R, Cooke SJ, Diggles BK, Sawynok W, Steven ED, Wynne CDL. 2014. Can fish really feel pain? Fish Fisheries 15, 97–133. ( 10.1111/faf.12010) [DOI] [Google Scholar]
- 4.Sneddon LU, Elwood RW, Adamo SA, Leach MC. 2014. Defining and assessing animal pain. Anim. Behav. 97, 202–212. ( 10.1016/j.anbehav.2014.09.007) [DOI] [Google Scholar]
- 5.Elwood RW. 2019. Assessing the potential for pain in crustaceans and other invertebrates. Berlin, Germany: Springer Books [Google Scholar]
- 6.Elwood RW. 2011. Pain and suffering in invertebrates? ILAR J. 52, 175–184. ( 10.1093/ilar.52.2.175) [DOI] [PubMed] [Google Scholar]
- 7.Magee B, Elwood RW. 2016. Trade-offs between predator avoidance and electric shock avoidance in hermit crabs demonstrate a non-reflexive response to noxious stimuli consistent with prediction of pain. Behav. Processes 130, 31–35. ( 10.1016/j.beproc.2016.06.017) [DOI] [PubMed] [Google Scholar]
- 8.Appel M, Elwood RW. 2009. Motivational trade-offs and the potential for pain experience in hermit crabs. Appl. Anim. Behav. Sci. 119, 120–124. ( 10.1016/j.applanim.2009.03.013) [DOI] [Google Scholar]
- 9.Elwood RW, Appel M. 2009. Pain in hermit crabs? Anim. Behav. 77, 1243–1246. ( 10.1016/j.anbehav.2009.01.028) [DOI] [Google Scholar]
- 10.Weary DM, Neil L, Flower FC, Fraser D. 2006. Identifying and preventing pain in animals. Appl. Anim. Behav. Sci. 100, 64–76. ( 10.1016/j.applanim.2006.04.013) [DOI] [Google Scholar]
- 11.Barr S, Laming PR, Dick JTA, Elwood RW. 2008. Nociception or pain in a decapod crustacean? Anim. Behav. 75, 745–751. ( 10.1016/j.anbehav.2007.07.004) [DOI] [Google Scholar]
- 12.Elwood RW, Dalton N, Riddell G. 2017. Aversive responses by shore crabs to acetic acid but not to capsaicin. Behav. Processes 140, 1–5. ( 10.1016/j.beproc.2017.03.022) [DOI] [PubMed] [Google Scholar]
- 13.Appel M, Elwood RW. 2009. Gender differences, responsiveness and memory of a potentially painful event in hermit crabs. Anim. Behav. 78, 1373–1379. ( 10.1016/j.anbehav.2009.09.008) [DOI] [Google Scholar]
- 14.McCambridge C, Dick JTA, Elwood RW. 2016. Effects of autotomy compared to manual declawing on contests between males for females in the edible crab, Cancer pagurus: implications for fishery practice and animal welfare. Shellfish Res. 35, 1037–1044. ( 10.2983/035.035.0426) [DOI] [Google Scholar]
- 15.Elwood RW, Stewart A. 1985. The timing of decisions during shell investigation by the hermit crab, Pagurus bernhardus. Anim. Behav. 33, 620–627. ( 10.1016/S0003-3472(85)80086-5) [DOI] [Google Scholar]
- 16.Elwood RW. 1995. Motivational change during resource assessment in hermit crabs. J. Exp. Mar. Biol. Ecol. 193, 41–55. ( 10.1016/0022-0981(95)00109-3) [DOI] [Google Scholar]
- 17.Fossat P, Bacque-Cazenave J, De Deurwaerdere P, Delbecque J-P, Cattaert D. 2014. Anxiety-like behavior in crayfish is controlled by serotonin. Science 344, 1293–1297. ( 10.1126/science.1248811) [DOI] [PubMed] [Google Scholar]
- 18.Fossat P, Bacque-Cazenave J, De Deurwaerdere P, Cattaert D, Delbecque J-P. 2015. Serotonin, but not dopamine, controls stress response and anxiety-like behavior in crayfish, Procambarus clarkii. J. Exp. Biol. 218, 2745–2752. ( 10.1242/jeb.120550) [DOI] [PubMed] [Google Scholar]
- 19.Perrot-Minnot M-J, Banchetry L, Cezilly F. 2017. Anxiety-like behaviour increases safety from fish predation in an amphipod crustacea. R. Soc. open sci. 4, 171558 ( 10.1098/rsos.171558) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Webster SG. 1996. Measurement of crustacean hyperglycaemic hormone levels in the edible crab Cancer pagurus during emersion stress. J. Exp. Biol. 199, 1579–1585. [DOI] [PubMed] [Google Scholar]
- 21.Bergmann M, Taylor AC, Moore PG. 2001. Physiological stress in decapod crustaceans (Mundida rugosa and Liocarcinus depurator) discarded in the Clyde Nephrops fishery. J. Exp. Mar. Biol. Ecol. 259, 215–229. ( 10.1016/S0022-0981(01)00231-3) [DOI] [PubMed] [Google Scholar]
- 22.Toullec JY, Vinh J, Le Caer JP, Shillito B, Soyez D. 2002. Structure and phylogeny of the crustacean hyperglycemic hormone and its precursor from a hydrothermal vent crustacean: the crab Bythograea thermydron. Peptides 23, 31–42. ( 10.1016/S0196-9781(01)00576-9) [DOI] [PubMed] [Google Scholar]
- 23.Stentiford GD, Chang ES, Chang SA, Neil DM. 2001. Carbohydrate dynamics and the crustacean hyperglycaemic hormone (CHH): effects of parasitic infection in Norway lobsters (Nephrops norvegicus). Gen. Comp. Endocrinol. 121, 13–22. ( 10.1006/gcen.2000.7575) [DOI] [PubMed] [Google Scholar]
- 24.Patterson L, Dick JTA, Elwood RW. 2007. Physiological stress responses in the edible crab Cancer pagurus to the fishery practice of de-clawing. Mar. Biol. 152, 265–272. ( 10.1007/s00227-007-0681-5) [DOI] [Google Scholar]
- 25.Elwood RW, Adams L. 2015. Electric shock causes physiological stress responses in shore crabs, consistent with prediction of pain. Biol. Lett. 11, 20150800 ( 10.1098/rsbl.2015.0800) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elwood RW. 2017. Assessing negative and positive evidence for animal pain. Anim. Sentience 16, 21. [Google Scholar]
- 27.Magee B, Elwood RW. 2013. Shock avoidance by discrimination learning in the shore crab (Carcinus maenas) is consistent with a key criterion for pain. J. Exp. Biol. 216, 353–358. ( 10.1242/jeb.072041) [DOI] [PubMed] [Google Scholar]
- 28.Crook RJ, Hanlon RT, Walters ET. 2013. Squid have nociceptors that display widespread longterm sensitization and spontaneous activity after bodily injury. J. Neurosci. 33, 10 021–10 026. ( 10.1523/JNEUROSCI.0646-13.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Magee BT, Elwood RW. 2016. No discrimination shock avoidance with sequential presentation of stimuli but shore crabs still reduce shock exposure. Biol. Open 5, 883–888. ( 10.1242/bio.019216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dyer AG, Neumeyer C. 2005. Simultaneous and successive colour discrimination in the honeybee (Apis mellifera). J. Comp. Physiol. A 191, 547–557. ( 10.1007/s00359-005-0622-z) [DOI] [PubMed] [Google Scholar]
- 31.Bhimani R, Huber R. 2016. Operant avoidance learning in crayfish, Orconectes rusticus: computational ethology and the development of an automated learning paradigm. Learn. Behav. 44, 239–249. ( 10.3758/s13420-015-0205-y) [DOI] [PubMed] [Google Scholar]
- 32.Hoyle G. 1976. Learning of leg position by the ghost crab Ocypode ceratophthalma. Behav. Biol. 18, 147–163. ( 10.1016/S0091-6773(76)92038-1) [DOI] [PubMed] [Google Scholar]
- 33.Dunn PDC, Barnes WJP. 1981. Learning of leg position in the shore crab, Carduus maenas. Mar. Behav. Physiol. 8, 67–82. ( 10.1080/10236248109387004) [DOI] [Google Scholar]
- 34.Punzo F. 1983. Localization of brain function and neurochemical correlates of learning in the mud crab, Eurypauopeus depressus (Decapod). Comp. Biochem. Physiol. A 75, 299–305. ( 10.1016/0300-9629(83)90085-3) [DOI] [Google Scholar]
- 35.Kawai N, Kono R, Sugimoto S. 2004. Avoidance learning in the crayfish (Procambarus clarkii) depends on the predatory imminence of the unconditioned stimulus: a behaviour systems approach to learning in invertebrates. Behav. Brain Res. 150, 229–237. ( 10.1016/S0166-4328(03)00261-4) [DOI] [PubMed] [Google Scholar]
- 36.Puri S, Faulkes Z. 2015. Can crayfish take the heat? Procambarus clarkii show nociceptive behaviour to high temperature stimuli, but not low temperature or chemical stimuli. Biol. Open 4, 441–448. ( 10.1242/bio.20149654) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stevens ED, et al. 2016. Stress is not pain. Comment on Elwood and Adams (2015) ‘Electric shock causes physiological stress responses in shore crabs, consistent with prediction of pain’. Biol. Lett. 12, 20151006 ( 10.1098/rsbl.2015.1006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elwood RW. 2016. Stress was never said to be pain: response to Stevens et al. (2016). Biol. Lett. 12, 20160126. ( 10.1098/rsbl.2016.0126) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diggles BK. 2019. Food for thought: review of some scientific issues related to crustacean welfare. ICES J. Mar. Sci. 76, 66–81. ( 10.1093/icesjms/fsy058) [DOI] [Google Scholar]
- 40.Birch J. 2017. Animal sentience and the precautionary principle. Anim. Sentience 16(1). [Google Scholar]
- 41.Sneddon LU, et al. 2018. Fish sentience denial: muddying the waters. Anim. Sentience 3, 1. [Google Scholar]
- 42.Barr S, Elwood RW. 2011. No evidence of morphine analgesia to noxious shock in the shore crab, Carcinus maenas. Behav. Processes 86, 340–344. ( 10.1016/j.beproc.2011.02.002) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.