Abstract
In order to survive, animals must avoid injury and be able to detect potentially damaging stimuli via nociceptive mechanisms. If the injury is accompanied by a negative affective component, future behaviour should be altered and one can conclude the animal experienced the discomfort associated with pain. Fishes are the most successful vertebrate group when considering the number of species that have filled a variety of aquatic niches. The empirical evidence for nociception in fishes from the underlying molecular biology, neurobiology and anatomy of nociceptors through to whole animal behavioural responses is reviewed to demonstrate the evolutionary conservation of nociception and pain from invertebrates to vertebrates. Studies in fish have shown that the biology of the nociceptive system is strikingly similar to that found in mammals. Further, potentially painful events result in behavioural and physiological changes such as reduced activity, guarding behaviour, suspension of normal behaviour, increased ventilation rate and abnormal behaviours which are all prevented by the use of pain-relieving drugs. Fish also perform competing tasks less well when treated with a putative painful stimulus. Therefore, there is ample evidence to demonstrate that it is highly likely that fish experience pain and that pain-related behavioural changes are conserved across vertebrates.
This article is part of the Theo Murphy meeting issue ‘Evolution of mechanisms and behaviour important for pain’.
Keywords: animal behaviour, analgesia, nociceptors, fishes, invertebrates, zebrafish
1. Introduction
Pain is considered a negative affective state associated with tissue injury and we seek to alleviate pain to improve both the human condition and animal welfare [1,2]. Biomedical and basic fundamental studies explore the mechanisms of pain with a view to discovering novel compounds or drugs to reduce pain. In such studies, animal models are often employed and these have been effective in understanding pain and analgesia in humans and other animals [2,3]. A range of animal models has been explored, from invertebrates such as nematodes (Caenorhabditis elegans), leeches (Hirudo medicinalis), fruit flies (Drosophila melanogaster) and molluscs (Aplysia califonica) through to vertebrates [4]. By adopting a comparative approach, an analysis of these studies can yield insights into the evolution of pain. This review will focus on the empirical evidence from fishes since they represent an interesting evolutionary and ecological group. Phylogenetically, fishes are the closest vertebrate group to invertebrates and gave rise to vertebrate tetrapods [5]. Fishes also live a mainly aquatic life-style and thus have different life history and ecological pressures shaping their evolution in comparison with terrestrial invertebrates and vertebrates [6]. An exploration of the research findings from fishes might discover the extent of evolutionary conservation or differences in the underlying mechanisms through to whole animal behavioural responses to pain.
2. Definition of pain
The definition of human pain suggests that there are two components: firstly, a stimulus that could or does cause damage is perceived (termed nociception) and secondly, this leads to a psychological state where an individual experiences suffering or discomfort (termed pain) [1]. Assessment of pain in humans relies on self-report in that we share a common language and we can convey whether we are in pain or not, and further can rate that pain [2]. This becomes problematic in the assessment of pain in animals and infant humans since we cannot communicate with them directly [2]. Instead we identify behavioural and physiological indicators in response to a potentially painful event that are used to indicate pain and in some cases gauge the severity of that pain. For example, grimace scales based on changes in facial expression using Facial Action Coding Schemes have been developed for a variety of mammals including rats [7], mice [8,9], rabbits [10], horses [11] and piglets [12]. In effect, we rely on scientific measures that signify pain in animals and make a judgement based on the animal's responses to make our assessment. Therefore, the modern definition of animal pain relies on a number of testable principles when deciding if an animal can be considered as capable of experiencing pain [2]. All animals are considered capable of nociception, which is the detection of potentially injurious stimuli, and is usually accompanied by a nocifensive withdrawal reflex away from that stimulus. For pain, however, the animal must demonstrate a change in future behavioural decisions and motivational changes [2]. This relies on the fact that pain is an adverse psychological experience resulting in learning, memory formation and altered strategic decision making during and after the event. If an animal has the neural apparatus to detect and experience painful stimuli, their behaviour is altered and this indicates a change from their normal repertoire (e.g. suspension of feeding), which may be detrimental to the animal and all responses are prevented by analgesic drugs, then this evidence confirms pain occurs. Human pain is often referred to when considering pain in animals, but this is flawed thinking in many ways as the nociceptive and pain system will be shaped quite differently in animals which have had a different ecological and evolutionary history [13,14]. Thus, differences or similarities in the molecular, physiological, neurobiological and behavioural changes in response to pain by fishes will be discussed below with a view to understanding the comparative biology of nociception in pain in animals.
3. Molecular biology of nociception
Many molecules and their receptors are involved in nociception and pain. Not all can be discussed here, but examples of those that have been implicated in mammalian pain are acid sensing ion channels (ASICs), which mediate nociceptive responses to acids [15–18], transient receptor potential (TRP) channels, which underpin thermal (heat) nociception [19], and opioid receptors and endogenous substances inherent in opioid-mediated pain-relief or analgesia [20,21].
ASICs are coded by four genes that with alternative splicing result in six isoforms (ASIC1a, ASIC1b, ASIC2a, ASIC2b, ASIC3 and ASIC4) [17] which are expressed in mammalian mechanosensory neurons and are gated by low pH or protons. These membrane-bound receptors are activated by decreased extracellular pH resulting in the opening of an intrinsic membrane-spanning sodium channel. ASICs have been identified in vertebrates [17,22]. However, molecular phylogenetics and electrophysiological approaches have also characterized ASICs from tunicates, lancelets, sea urchins, starfish and acorn worms (belonging to the phyla Hemichordata and Chordata). Therefore, the function of ASICs is conserved across these animal phyla [22]. Prior to this study, it was believed the proton-mediated activation of ASICs (except ASIc2b and ASIC4) originated in teleost (bony) fish since the ASICs identified in a jawless fish, Lampetra fluviatilis, and a cartilaginous fish, Squalus acanthias, formed membrane proteins that were not activated by protons [23]. This means if these species possess nociceptors or other chemosensory receptors they may not respond to acid. Six ASICs have been characterized in zebrafish, Danio rerio, (zASICs; ZASIC1.1, zASIC1.2, zASIC1.3, zASIC2, zASIC4.1 and zASIC4.2) and have similar predicted molecular masses and share 60–75% amino acid similarity with rat and human ASICs [24]. Exposure to or injection of low pH chemicals, and in particular acetic acid, does result in altered behaviour and physiology in zebrafish and rainbow trout [25–33] that is prevented by analgesic drugs and has been shown to be mediated by ASICs [34]. Thus, the function of ASICs does appear to be evolutionarily conserved.
TRP ligand-gated ion channels (TRPV1, TRPV2, TRPV3, TRPV4, TRPM8 and TRPA1) innervate sensations related to temperature and mechanical stimuli and are expressed in mammalian nociceptors [19]. These ion channels form a primary step for detection of pain since they transduce noxious stimuli into currents. TRP ion channels or homologues can be found in invertebrates (e.g. Drosophila [35]; Hirudo [36,37]). Nine thermo-TRP channels have been identified in zebrafish, some of them differing from those present in mammals [38], which may be due to the past genomic duplication in zebrafish. Once activated, the thermo-TRPs of zebrafish (TRPV1/2, TRPV4, TRPM2, TRPM4a, TRPM4b, TRPM4c, TRPM5, TRPA1a and TRPA1b) promote ion influx eliciting changes in the resting membrane potential. They are responsive to a broad range of temperatures, from noxious cold to high temperature, which are potentially tissue damaging [39]. Electrophysiological studies in rainbow trout (Oncorhynchus mykiss) failed to find cold responsive nociceptors [40], but this species lives in very low temperatures (0–25°C), whereas zebrafish are a tropical species inhabiting a smaller range of temperatures (20–30°C). Noxious heat does have a negative effect on goldfish (Carassius auratus) and zebrafish behaviour that is ameliorated by analgesia [41,42], but analgesia did not prevent behavioural responses to cold in larval zebrafish, where it appeared as if cold had an anaesthetizing effect [42].
The endogenous opioid system comprises three G-protein-coupled receptors, the µ, δ and κ-receptors, and their respective ligands β-endorphin, enkephalin and dynorphin [20]. These receptors and substances are found primarily in the nervous system regions involved in nociception and pain in mammals [20], and endogenous substances offer analgesia in both central and peripheral nociceptive systems. Peripheral opioid receptors can be located chiefly in the primary sensory neurons [21], but these receptors are usually inactive until the neurons respond to a noxious stimulus. Opioid drugs exert peripheral anti-nociceptive effects [43–46]. Morphine, a classic opioid drug, exerts influence through μ-opioid receptors and is administered in the case of severe pain [47,48]. In invertebrates, opioid-like receptors and endogenous substances have been found (e.g. C. elegans; Aplysia) although some species lack any discernible opioid receptors or substances (e.g. Drosophila [49]). Nevertheless, opioids can modulate and reduce the nociceptive responses of invertebrates (e.g. Drosophila [50]; Hirudo [51]; Aplysia [52–54]; therefore, the mechanisms are not known [49]. The opioid system in fishes is very similar to that found in mammals [55] and thus fish models are used for testing addiction and withdrawal [56]. Several studies have demonstrated significant changes in the behaviour of zebrafish to potentially painful stimuli, including reduced activity [28–33,57,58], which has also been observed in mammals [2]. Exposure concentration at 0.1% acetic acid or above causes hypoactivity in 5 day post fertilization (dpf) zebrafish, whereas the anaesthetics aspirin (2.5 mg l−1), lidocaine (5 mg l−1) and morphine (48 mg l−1) prevent the change in behaviour [59]. Subcutaneous injection of acetic acid into the frontal lips resulted in a substantial reduction in activity in adult zebrafish and rainbow trout [29,58]. Morphine administration effectively prevents these changes, providing evidence for the conserved role of opioid receptors in fish analgesia [32,59,60]. Although further testing is required, opioids would seem to exert the same function in nociception across the animal kingdom.
4. Nociceptors
Nociceptors are free nerve endings that preferentially detect injurious stimuli that cause tissue damage; in vertebrates they are usually of two fibre types: small myelinated A-delta fibres and smaller unmyelinated C fibres [61]. Nociceptors can be found in both the periphery [17,61] and the viscera and deep tissues [62,63]. Electrophysiological and anatomical investigation can identify which type of fibre nociceptors are, since C fibres lack myelination, are of small diameter and have a slow conduction velocity. Several nociceptor C fibre types have been characterized in mammals. For example, one type is solely mechanically sensitive, whereas another type are also responsive to cold or heat. ‘Silent’ C fibres only respond to heat when sensitized [64]. The majority of cutaneous mammalian fibre types have a nociceptive function (approx. 67% of total fibre type (12% Aδ, 30% C-polymodal' 20% C-mechanothermal or heat alone, and 5% C silent)) leaving 33% as touch and pressure fibres [64–67]. Thus, the mammalian nociception system must be able to differentiate between types of noxious stimuli since they possess a range of stimuli-specific receptors. Terrestrial mammals may have evolved this ability to avoid extremes of heat and cold as well as chemicals and damaging mechanical stimuli.
Aδ-mechanonociceptors conduct rapidly and are believed to signal ‘first pain’ [68]. These receptors have relatively higher mechanical thresholds than touch fibres (5 versus 1–1.5 mN; [66,68]), and a small percentage are heat responsive (12%) and half are stimulated by cold temperatures [66]. C fibres are thought to underpin longer-term pain with humans categorizing Aδ stimulation as ‘pricking’ pain and C-mediated stimulation as ‘pressing’ and ‘dull’ pain [69]. Approximately one-third of C fibres in mammals are polymodal and responsive to noxious chemicals in addition to mechanical and thermal stimuli. Electrophysiological properties of nociceptors have been well investigated (reviews in [15,19,32]); functionally, they encode information on the modality of stimulus, intensity and duration to reflex centres in the central nervous system, they mediate nocifensive withdrawal responses away from the damaging stimulus [70] and they facilitate longer-term behavioural alterations since their continued excitation results in prolonged pain [2].
When considering the watery environment fishes inhabit it is unlikely that their nociception and pain system will be similar to animals that live in a terrestrial environment. The ecological and evolutionary history of fish is likely to present different risks and, in particular, that will affect the risk of encountering potentially painful events or stimuli. Fishes that have a swim bladder can adjust their buoyancy in the water; thus injuries related to gravity (falling) may be reduced. From this perspective, it would be interesting to explore nociception in benthic or bottom-dwelling species that constantly come into contact with possibly hard substrates. Dilution of noxious chemicals entering a large water body may mean they pose a lower risk if dilution results in a sub-threshold concentration. Substantial changes in temperature occur less compared with terrestrial environments; thus the risk of heat or cold damage may be lower. Any differences in the fishes' nociceptive system may reflect these environmental differences.
A jawless fish, the lamprey, possesses receptors that respond to damaging stimuli, along with neuronal opioid-like receptors [71,72]. Studies have yet to conclusively find nociceptors in elasmobranchs [73]. However, in the teleost fishes electrophysiological and anatomical studies have identified nociceptors in the rainbow trout [74,75]. C fibres and Aδ fibres were characterized into three classes of nociceptors including polymodal, mechanothermal and mechanochemical [40,75–77]. These electrophysiology studies confirmed that the trout nociceptors are physiologically identical to mammalian nociceptors [78,79]. However, there are a number of fascinating differences: for example, trout nociceptors are not responsive to noxious cold temperatures below 4°C [40]. In an evolutionary context, this lack of responsiveness to noxious cold (less than 4°C) makes sense, since trout are found at very low temperatures, making it maladaptive to have cold nociceptors. This loss of function is also seen in a mammal: the African naked mole rat (Heterocephalus glaber), which lives in carbon dioxide (CO2)-rich burrows has nociceptors that do not respond to acids. High CO2 levels cause peripheral tissue acidosis, which excites nociceptors in many species, but for the naked mole rat this function has been lost since it would not be adaptive in its natural environment [80,81]. Thus, life history and ecology can shape the nociception and pain system. Zebrafish larvae at 5 dpf perform behavioural changes when exposed to noxious heat, CO2-infused water and acetic acid but no behavioural changes occur when a range of analgesic drugs are provided [42,59,82]. In terms of fibre composition, a comparatively small percentage are innervated by C fibres (4–5%, [83,84]) (terrestrial vertebrates (approx. 50–65%, [84]). Reptiles, including terrestrial species, also have fewer C fibres than mammals [85]. However, in trout, Aδ fibres function as polymodal nociceptors and so have the same functionality as the mammalian C fibres. These fish Aδ polymodal nociceptors are myelinated, so conduction velocity is more rapid and thus signalling of damaging stimuli may be quicker.
Thresholds also differ in fishes compared with mammals. The mechanical and heat thresholds of rainbow trout nociceptors are lower than those found in mammals. The fish mechanical thresholds are comparable to those recorded on the mammalian cornea, where low mechanical thresholds are found compared with mammalian cutaneous nociceptors [86]. If we consider the easily damaged nature of the fish skin, fishes may have evolved nociceptors that respond to relatively lower thresholds to avoid mechanical injury [75]; although scales can provide some protection they can also be lost during abrasion. The thermal threshold of mammalian nociceptors is greater than 40°C yet trout nociceptors are somewhat responsive to temperatures greater than 33°C [40]. If we deliberate on the thermal ecology of rainbow trout, they can be found in temperatures up to 25°C and the risk of coming into contact with higher temperatures is unlikely for this temperate species. By contrast, the behaviour of zebrafish, a tropical species, is not altered by a temperature of 30°C, which is within their natural tolerance range. However, when subject to 40°C, zebrafish activity is significantly reduced, which can be prevented by administering analgesics [42]. Studies using avoidance paradigms suggest that the noxious threshold is 36.5°C in zebrafish [87]. These findings suggest that the heat threshold of fish nociceptors may differ between temperate and tropical species. It would be interesting to test the influence of the thermal environment further by inclusion of thresholds from polar and desert fishes and those that inhabit hydrothermal vent areas. As ectotherms, fishes’ body temperature is dependent upon environmental temperatures (typically 0–30°C), whereas endothermic mammals largely maintain their temperature at 37°C. Temperatures above 30°C could be detrimental to fishes but not to mammals; thus fish nociceptors may have evolved to respond to a relatively lower heat threshold.
Are differences in mechanical and thermal thresholds observed in invertebrates and, as such, are fishes a stepping stone between invertebrates and vertebrates? For mechanical threshold the answer is no; invertebrates including arthropods, nematodes, annelids and molluscs have high mechanical thresholds similar to those found in amphibians, birds and mammals (reviewed in [4]). In the case of heat thresholds, the answer is also no since these invertebrate groups have higher heat thresholds than fishes, which are similar to those found in other vertebrate groups [4]. The only similarity appears to be a lack of cold responsive nociceptors in both invertebrates and fishes, which suggests the development of cold nociceptors evolved in terrestrial vertebrates. To test this hypothesis it would be interesting to investigate nociceptor properties in aquatic mammals, which are exposed to similar environmental conditions to many fishes, to determine whether cold responsiveness has been lost or is conserved. Of course many marine mammals undertake oceanic migrations and would naturally come into contact with a wide range of temperatures from polar regions to the tropics.
5. Behavioural costs of nociception and pain
Many species exhibit nocifensive instantaneous withdrawal responses to damaging stimuli, but to infer an experience of pain, behavioural responses should be lengthier than a short reflex reaction and should be more complicated in nature. For example, normal behaviour should be suspended for a prolonged period and further future decisions should be altered [2]. Nocifensive reflexes have been documented in many taxa and do not necessarily require higher brain areas for processing [2–4]. Prolonged changes in behaviour after a potentially painful stimulus have also been documented in vertebrates and some invertebrates and are indicative of higher central processing [2]. Therefore, behavioural alterations and the performance of abnormal or unusual behaviours often indicate pain. Changes in mammals include modified demeanour (e.g. [88]), responses to handling (e.g. [89]), altered posture (e.g. [90]), reduced activity (e.g. [91]), vocalizations (e.g. [10]), changes in food and water intake (e.g. [10]), gait [92], rearing [93] and many more, dependent upon the species and pain modality. Normal behaviour is affected in fishes during a potentially painful event. Although species dependent, changes in activity, performance of anomalous behaviours (e.g. rubbing affected area; rocking on the substrate; wafting of tail fin; stereotypical swimming), altered posture, suspension of feeding and increased ventilation have been recorded in fishes [25–33,57,58]. Motivational shifts and longer-term behavioural changes have also been recorded in a number of species and reviewed extensively [2–4].
Behaviours are often reduced or not performed during pain, and one can infer that deleterious changes in normal behaviour mean the animal pays a cost whether that be reduced energy intake when feeding is suspended, or time and energy spent on pain-related behaviours. For example, rodents are highly driven to rear up in their cages, but this is reduced after abdominal surgery [94]. Thus, the experience of pain results in a behavioural cost that in some cases may be lethal. Studies in cephalopods have demonstrated that injured squid, Doryteuthis pealeii, altered their escape strategy: injured squid fled from predators at a greater distance than non-injured squid; thus, the response to tissue damage has evolved as a survival tactic. Injury motivated the squid to respond at a greater distance to measurably reduce predation risk [95]. However, when damaged squid were anaesthetized during injury such that they did not experience the associated negative affective component, they did not show an enhanced flight and were more vulnerable to predation. When exposed to a predator, uninjured D. pealeii had 80% survival in contrast to only 19% in anaesthetized injured animals. Anaesthesia administered without injury resulted in a 75% survival rate; thus anaesthesia alone did not significantly affect survival. When D. pealeii were injured without anaesthesia so that they would have experienced the injury, 45% survived, since they responded to predators at a greater distance compared with those that were anaesthetized. Thus, the cost of injury is 35% mortality but if the associated experience of the inury is blocked via anaesthesia, then the mortality cost rises to 61%. Studies investigating responses to electric shock in hermit crabs (Pagurus bernhardus) that occupy mollusc shells, which protect their soft abdomen, have demonstrated 95% of these animals will evacuate a shell when an electric shock is applied [96]. This would leave these crabs vulnerable to predation; however, when this was repeated in the presence of a predator cue only 47% of crabs left their shell, which represents a trade-off between pain and the risk of predation. In fishes, normal feeding behaviour in rainbow trout can be suspended for approximately 3 h in comparison with sham (non-noxious saline injection) treated fish [25–28]. Further, when in pain rainbow trout do not show neophobia to novel objects [26] (2003; figure 1a), nor anti-predator responses in the presence of a predator cue [29]. Obviously failing to respond to predators may have detrimental consequences in a natural habitat where predators may be present. However, this is yet to be explicitly tested. Angling injuries to the mouths of marine shiner perch (Cymatogaster aggregata), which use a suction method of foraging, resulted in reduced feeding compared with fish caught using a non-injurious capture method; thus damage is costly in terms of energy intake [97]. Adult zebrafish demonstrate a substantial reduction in activity and swimming distance resulting in a reduction in complexity of movement (figure 2) as well as space use [58] during pain. The impact of noxious events on activity is also seen in 5 dpf larvae [42,59,82] (figure 1b). These changes will incur a cost in terms of time and energy but the benefits may be to reduce pain, prevent further damage and promote healing. These responses are ameliorated by the use of analgesic or pain-relieving drugs and indeed a range of drugs prevent pain-related changes in fishes (reviewed in [3]; figure 1b), which is also the case in mammals [2,4]. One study explored the impact of an electric shock in goldfish given in an area of their tank where they received food; the fish avoided this area for 3 days before they would enter [98]. These findings clearly show that the fish paid a nutritional cost where their energy intake dropped for 3 days which would negatively affect their physiology. After 3 days of fasting, the hungry goldfish traded off the avoidance of the noxious painful shock with satiating their hunger and meeting their energy requirements.
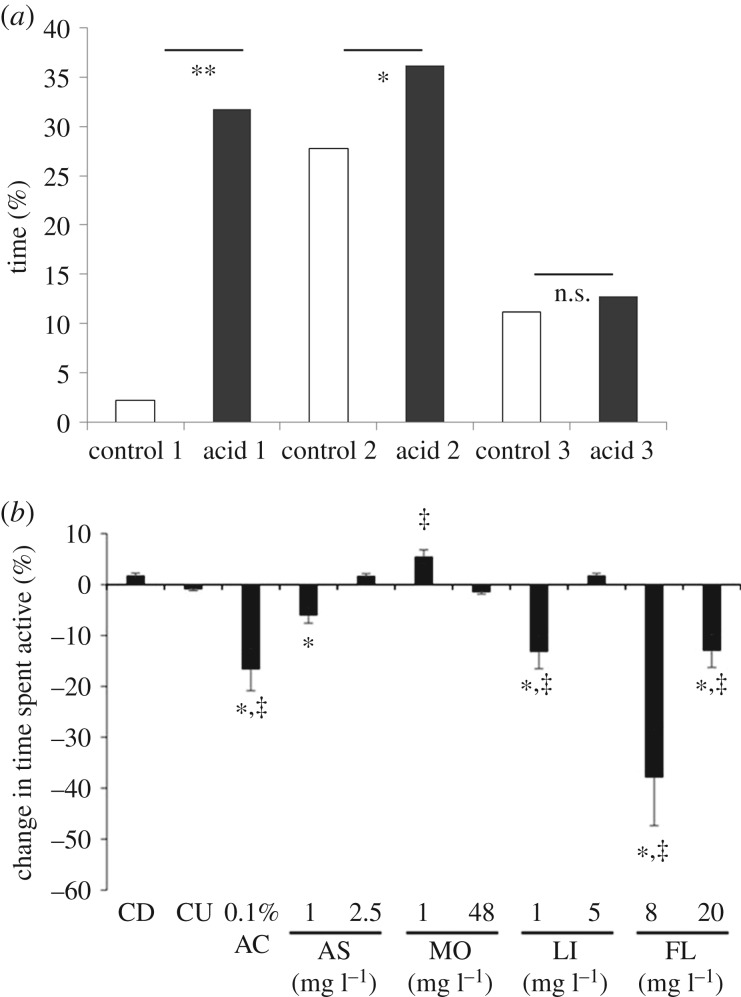
Figure 1.
(a) The mean (+s.d.) proportion of time spent within 5 cm of the object for control rainbow trout or those injected subcutaneously with acetic acid in each of the three test groups: 1. Exposure to a novel object; 2. Exposure to a familiar object or 3. Exposure to a novel object with morphine administered as an analgesic. **p < 0.001; *p < 0.05; n.s., not significant. (Reproduced from [26] with kind permission from Elsevier.) (b) Median percentage (±IQR, interquartile range) change from the pre-stimulation (baseline) values in the time spent active shown by 5 dpf zebrafish exposed to 0.1% acetic acid with 30 min prior exposure to different analgesic substances. CD, control disturbed with addition of water; CU, control undisturbed, with no addition of water; AC, acetic acid; AS, aspirin; MO, morphine; LI, lidocaine; FL, flunixin. ‡Significant difference from the undisturbed control group; *significant difference from the disturbed control group (Mann–Whitney U-test with Bonferroni correction applied, p < 0.0026). Samples sizes: CD, n = 437; CU, n = 429; 0.1% AC, n = 416; 1 mg l−1 AS, n = 416; 2.5 mg l−1 AS, n = 438; 1 mg l−1 MO, n = 435; 48 mg l−1 MO, n = 439; 1 mg l−1 LI, n = 402; 5 mg l−1 LI, n = 391; 8 mg l−1 FL, n = 411; and 20 mg l−1 FL, n = 443. (Reproduced from [59] with kind permission from the Company of Biologists).
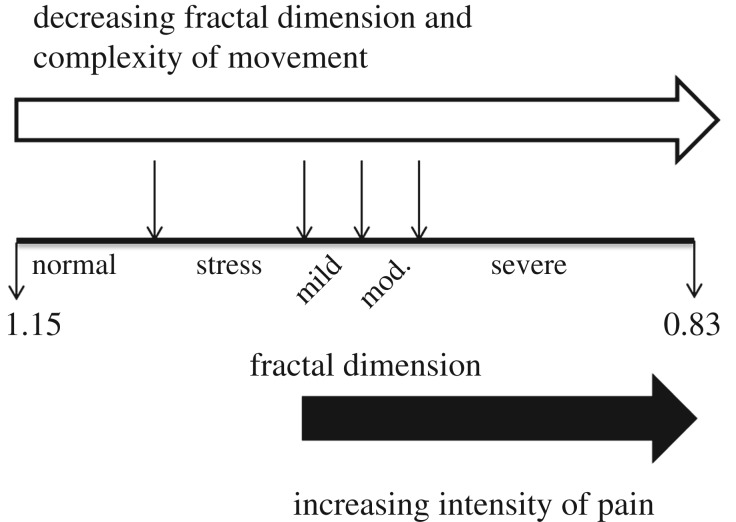
Figure 2.
Fractal dimension analysis of the complex swimming trajectories of adult zebrafish in control undisturbed zebrafish, in zebrafish anaesthetized but given no treatment, and in zebrafish given a fin clip, fin clip with pain-relief (PIT tag inserted in the abdomen), or subcutaneous injection of 1, 5 or 10% acetic acid yield values ranging from normal (1.15) to low (0.83), demonstrating a reduction in complexity in response to stressful or painful treatment, with arbitrary points indicating the impact of stress and mild, moderate (mod.) and severe pain. (Reproduced from [58] under Creative Commons Attribution License CC BY 4.0.)
6. Conclusion
The underlying anatomical, molecular and electrophysiological properties of nociceptors generally appear conserved from invertebrates to vertebrates, with some interesting anomalies. Loss of or lack of evolving a function occurs in fishes where cold nociceptors are yet to be identified. This may be due to the species tested either inhabiting cold waters or never coming into contact with such cold temperatures in their natural habitat; thus they have not evolved cold nociceptors. By contrast, terrestrial vertebrates may suffer tissue damage in extreme cold climactic events, and thus require cold nociception to avoid such injury. Testing of marine mammals may yield interesting insights into whether these aquatic mammals have lost cold responsive nociceptors. Certainly, the African naked mole rat has lost acid responsive nociceptors, possibly owing to the CO2-rich environment it inhabits, showing that pain perception mechanisms are likely shaped to suit the environment that a species inhabits over evolutionary time frames. Nocifensive instantaneous withdrawal reflexes are observed in many taxa, and as such these are evolutionarily conserved as they serve an important function—to prevent and limit contact with damaging stimuli. Behavioural responses that are prolonged and complicated in nature have been recorded in cephalopods and crustaceans whereby costs are incurred. Behavioural costs are also observed in fishes and other vertebrate species. When experiencing a painful stimulus, fish do not show appropriate fear or anti-predator responses, and this may be detrimental in the natural environment if predation risk is a factor. Changes in behaviour are prevented by drugs that provide effective analgesia, providing evidence that these changes are driven by nociception and pain mechanisms. Taken together, this combined evidence suggests a pain experience that dominates attention in fish, and thus it is vital that we seek to minimize and alleviate pain in fish when logistically possible. The empirical evidence in fish demonstrates pain-related behaviours are conserved across vertebrates, with more research needed in invertebrates to investigate prolonged changes in behaviour to discern when these behaviours first arose in evolution.
Acknowledgments
I am grateful to David C. C. Wolfenden for discussions and editorial comments and to the two referees for their useful comments.
Data accessibility
This article has no additional data.
Competing interests
I have no competing interests.
Funding
I received no funding for this study.
References
- 1.IASP. 2019. IASP Terminology. Pain Terms. Washington, DC: International Association for the Study of Pain; See https://www.iasp-pain.org/Education/Content.aspx?ItemNumber=1698&navItemNumber=576 (accessed 8 March 2019). [Google Scholar]
- 2.Sneddon LU, Elwood RW, Adamo S, Leach MC. 2014. Defining and assessing pain in animals. Anim. Behav. 97, 201–212. ( 10.1016/j.anbehav.2014.09.007) [DOI] [Google Scholar]
- 3.Sloman KA, Bouyoucos IA, Brooks EJ, Sneddon LU. 2019. Ethical considerations in fish research. J. Fish Biol. 94, 556–577. ( 10.1111/jfb.13946) [DOI] [PubMed] [Google Scholar]
- 4.Sneddon LU. 2018. Comparative physiology of nociception and pain. Physiology 33, 63–73. ( 10.1152/physiol.00022.2017) [DOI] [PubMed] [Google Scholar]
- 5.Donoghue PCJ, Purnell MA. 2009. The evolutionary emergence of vertebrates from among their spineless relatives. Evol. Educ. Outreach 2, 204–212. ( 10.1007/s12052-009-0134-3) [DOI] [Google Scholar]
- 6.Sneddon LU. 2015. Pain in aquatic animals. J. Exp. Biol. 218, 967–976. ( 10.1242/jeb.088823) [DOI] [PubMed] [Google Scholar]
- 7.Sotocinal SG, Sorge RE, Zaloum A, Tuttle AH, Martin LJ, Wieskopf JS, Mogil JS. 2011. The Rat Grimace Scale: a partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol. Pain 7, 55 ( 10.1186/1744-8069-7-55) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Langford DJ, et al. 2010. Coding of facial expressions of pain in the laboratory mouse. Nat. Methods 7, 447–449. ( 10.1038/nmeth.1455) [DOI] [PubMed] [Google Scholar]
- 9.Leach MC, Klaus K, Miller AL, di Perrotolo MS, Sotocinal SG, Flecknell PA. 2012. The assessment of post-vasectomy pain in mice using behaviour and the Mouse Grimace Scale. PLoS ONE 7, e35656 ( 10.1371/journal.pone.0035656) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leach MC, Allweiler S, Richardson C, Roughan JV, Narbe R, Flecknell PA. 2009. Behavioural effects of ovariohysterectomy and oral administration of meloxicam in laboratory housed rabbits. Res. Vet. Sci. 87, 336–347. ( 10.1016/j.rvsc.2009.02.001) [DOI] [PubMed] [Google Scholar]
- 11.Dalla CE, Minero M, Lebelt D, Stucke D, Canlai E, Leach MC. 2014. Development of the Horse Grimace Scale (HGS) as a pain assessment tool in horses undergoing routine castration. PLoS ONE 9, e92281 ( 10.1371/journal.pone.0092281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Viscardi AV, Hunniford M, Lawlis P, Leach MC, Turner PV. 2017. Development of a Piglet Grimace Scale to evaluate piglet pain using facial expressions following castration and tail docking: a pilot study. Front. Vet. Sci. 4, 51 ( 10.3389/fvets.2017.00051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sneddon LU, Lopez-Luna J, Wolfenden DCC, Leach MC, Valentim AM, Steenbergen PJ, Bardine N, Broom DM, Brown C. 2018. Ample evidence for fish sentience and pain. Anim. Sentience 3, 17. [Google Scholar]
- 14.Birch J. 2018. Degrees of sentience? Anim. Sentience 3, 1. [Google Scholar]
- 15.Burrell BD. 2017. Comparative biology of pain: what invertebrates can tell us about how nociception works. J. Neurophysiol. 117, 1461–1473. ( 10.1152/jn.00600.2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewin GR, Lu Y, Park TJ. 2004. A plethora of painful molecules. Curr. Opin. Neurobiol. 14, 443–449. ( 10.1016/j.conb.2004.07.009) [DOI] [PubMed] [Google Scholar]
- 17.Omerbašića D, Schuhmacher L-N, Bernal Sierra YA, St John Smith E, Lewin G. 2015. ASICs and mammalian mechanoreceptor function. Neuropharmacology 94, 80–86. ( 10.1016/j.neuropharm.2014.12.007) [DOI] [PubMed] [Google Scholar]
- 18.St John Smith E, Lewin GR. 2009. Nociceptors: a phylogenetic view. J. Comp. Physiol. A 195, 1089–1106. ( 10.1007/s00359-009-0482-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marwaha L, Bansal Y, Singh R, Saroj P, Bhandari R, Kuhad A. 2016. TRP channels: potential drug target for neuropathic pain. Inflammopharmacology 24, 305–317. ( 10.1007/s10787-016-0288-x) [DOI] [PubMed] [Google Scholar]
- 20.Rachinger-Adam B, Conzen P, Azad SC. 2011. Pharmacology of peripheral opioid receptors. Curr. Opin. Anesthesiol. 24, 408–413. ( 10.1097/ACO.0b013e32834873e5) [DOI] [PubMed] [Google Scholar]
- 21.Chen JJ, Dymshitz J, Vasko MR. 1997. Regulation of opioid receptors in rat sensory neurons in culture. Mol. Pharmacol. 51, 666–673. ( 10.1124/mol.51.4.666) [DOI] [PubMed] [Google Scholar]
- 22.Lynagh T, Mikhaleva Y, Colding JM, Glover JC, Pless SA. 2018. Acid-sensing ion channels emerged over 600 Mya and are conserved throughout the deuterostomes. Proc. Natl Acad. Sci. USA 115, 8430–8435. ( 10.1073/pnas.1806614115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coric T, Zheng D, Gerstein M, Canessa CM. 2005. Proton sensitivity of ASIC1 appeared with the rise of fishes by changes of residues in the region that follows TM1 in the ectodomain of the channel. J. Physiol. 568, 725–735. ( 10.1113/jphysiol.2005.087734) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Viña E, Parisi V, Sánchez-Ramos C, Cabo R, Guerrera MC, Quirós LM, Germanà A, Vega JA, García-Suárez O. 2015. Acid-sensing ion channels (ASICs) 2 and 4.2 are expressed in the retina of the adult zebrafish. Cell Tissue Res. 360, 223–231. ( 10.1007/s00441-014-2084-5) [DOI] [PubMed] [Google Scholar]
- 25.Sneddon LU. 2003. The evidence for pain in fish: the use of morphine as an analgesic. Appl. Anim. Behav. Sci. 83, 153–162. ( 10.1016/S0168-1591(03)00113-8) [DOI] [Google Scholar]
- 26.Sneddon LU, Braithwaite VA, Gentle MJ. 2003. Novel object test: examining nociception and fear in the rainbow trout. J. Pain 4, 431–440. ( 10.1067/S1526-5900(03)00717-X) [DOI] [PubMed] [Google Scholar]
- 27.Sneddon LU, Braithwaite VA, Gentle MJ. 2003. Do fishes have nociceptors? Evidence for the evolution of a vertebrate sensory system. Proc. R. Soc. Lond. B 270, 1115–1121. ( 10.1098/rspb.2003.2349) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reilly SC, Quinn JP, Cossins AR, Sneddon LU. 2008. Behavioural analysis of a nociceptive event in fish: comparisons between three species demonstrate specific responses. Appl. Anim. Behav. Sci. 114, 248–259. ( 10.1016/j.applanim.2008.01.016) [DOI] [Google Scholar]
- 29.Ashley PJ, Ringrose S, Edwards KL, McCrohan CR, Sneddon LU. 2009. Effect of noxious stimulation upon antipredator responses and dominance status in rainbow trout. Anim. Behav. 77, 403–410. ( 10.1016/j.anbehav.2008.10.015) [DOI] [Google Scholar]
- 30.Newby NC, Wilkie MP, Stevens ED. 2009. Morphine uptake, disposition, and analgesic efficacy in the common goldfish (Carassius auratus). Can. J. Zool. 87, 388–399. ( 10.1139/Z09-023) [DOI] [Google Scholar]
- 31.Mettam JM, Oulton LJ, McCrohan CR, Sneddon LU. 2011. The efficacy of three types of analgesic drug in reducing pain in the rainbow trout, Oncorhynchus mykiss. Appl. Anim. Behav. Sci. 133, 265–274. ( 10.1016/j.applanim.2011.06.009) [DOI] [Google Scholar]
- 32.Taylor JC, Dewberry LS, Totsch SK, Yessick LR, DeBerry JJ, Watts SA, Sorge RE. 2017. A novel zebrafish-based model of nociception. Physiol. Behav. 174, 83–88. ( 10.1016/j.physbeh.2017.03.009) [DOI] [PubMed] [Google Scholar]
- 33.do Nascimento JET, de Morais SM, de Lisboa DS, Sousa MD, Santos SAAR, Magalhaes FEA, Campos AR. 2018. The orofacial antinociceptive effect of Kaempferol-3-O-rutinoside, isolated from the plant Ouratea fieldingiana, on adult zebrafish (Danio rerio). Biomed. Pharmacother. 107, 1030–1036. ( 10.1016/j.biopha.2018.08.089) [DOI] [PubMed] [Google Scholar]
- 34.Batista FLA, et al. 2018. Antinociceptive activity of ethanolic extract of Azadirachta indica A. Juss (neem, Meliaceae) fruit through opioid, glutamatergic and acid-sensitive ion pathways in adult zebrafish (Danio rerio). Biomed. Pharmacother. 108, 408–416. ( 10.1016/j.biopha.2018.08.160) [DOI] [PubMed] [Google Scholar]
- 35.Sokabe T, Tsujiuchi S, Kadowaki T, Tominaga M. 2008. Drosophila Painless is a Ca2+-requiring channel activated by noxious heat. J. Neurosci. 28, 9929–9938. ( 10.1523/JNEUROSCI.2757-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. 1997. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389, 816–824. ( 10.1038/39807) [DOI] [PubMed] [Google Scholar]
- 37.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. 2000. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288, 306–313. ( 10.1126/science.288.5464.306) [DOI] [PubMed] [Google Scholar]
- 38.Saito S, Shingai R. 2006. Evolution of thermoTRP ion homologs in vertebrates. Physiol. Genom. 27, 219–230. ( 10.1152/physiolgenomics.00322.2005) [DOI] [PubMed] [Google Scholar]
- 39.Romanovsky AA. 2007. Thermoregulation: some concepts have changed. Functional architecture of the thermoregulatory system. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, 37–46. ( 10.1152/ajpregu.00668.2006) [DOI] [PubMed] [Google Scholar]
- 40.Ashley PJ, Sneddon LU, McCrohan CR. 2007. Nociception in fish: stimulus–response properties of receptors on the head of trout Oncorhynchus mykiss. Brain Res. 1166, 47–54. ( 10.1016/j.brainres.2007.07.011) [DOI] [PubMed] [Google Scholar]
- 41.Nordgreen J, Garner JP, Janczak AM, Ranheim B, Muir WM, Horsberg TE. 2009. Thermonociception in fish: effects of two different doses of morphine on thermal threshold and post-test behaviour in goldfish (Carassius auratus). Appl. Anim. Behav. Sci. 119, 101–107. ( 10.1016/j.applanim.2009.03.015) [DOI] [Google Scholar]
- 42.Lopez-Luna J, Al-Jubouri Q, Al-Nuaimy W, Sneddon LU. 2017. Impact of analgesic drugs on the behavioural responses of larval zebrafish to potentially noxious temperatures. Appl. Anim. Behav. Sci. 188, 97–105. ( 10.1016/j.applanim.2017.01.002) [DOI] [Google Scholar]
- 43.Ferreira SH, Nakamura M. 1979. II - Prostaglandin hyperalgesia: the peripheral analgesic activity of morphine, enkephalins and opioid antagonists. Prostaglandins 18, 191–200. ( 10.1016/0090-6980(79)90104-7) [DOI] [PubMed] [Google Scholar]
- 44.Jeanjean AP, Moussaoui SM, Maloteaux JM, Laduron PM. 1995. Interleukin-1β induces long-term increase of axonally transported opiate receptors and substance P. Neuroscience 68, 151–157. ( 10.1016/0306-4522(95)00106-S) [DOI] [PubMed] [Google Scholar]
- 45.Stein C, Millan MJ, Shippenberg TS, Peter K, Herz A. 1989. Peripheral opioid receptors mediating antinociception in inflammation. Evidence for involvement of mu, delta and kappa receptors. J. Pharmacol. Exp. Ther. 248, 1269–1275. [PubMed] [Google Scholar]
- 46.Zöllner C, Shaqura MA, Bopaiah CP, Mousa S, Stein C, Schäfer M. 2003. Painful inflammation-induced increase in μ-opioid receptor binding and G-protein coupling in primary afferent neurons. Mol. Pharmacol. 64, 202–210. ( 10.1124/mol.64.2.202) [DOI] [PubMed] [Google Scholar]
- 47.Gutstein HB, Akil H. 2001. Opioid analgesics. In Goodman & Gilman's the pharmacological basis of therapeutics (eds Goodman LS, Hardman JG, Limbird LE, Gilman AG), pp. 569–619. New York, NY: McGraw-Hill. [Google Scholar]
- 48.McNally GP, Akil H. 2002. Opioid peptides and their receptors: overview and function in pain modulation. In Neuropsychopharmacology: the fifth generation of progress (ed. Davis KL.), pp. 35–46. Philadelphia, PA: Lippincott Williams & Wilkins. [Google Scholar]
- 49.Walters ET. 2018. Nociceptive biology of molluscs and arthropods: evolutionary clues about functions and mechanisms potentially related to pain. Front. Physiol. 9, 1049 ( 10.3389/fphys.2018.01049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khuong TM, Neeley GG. 2013. Conserved systems and functional genomic assessment of nociception. FEBS J. 280, 5298–5306. ( 10.1111/febs.12464) [DOI] [PubMed] [Google Scholar]
- 51.Salzet M, Stefano GB. 1997. Invertebrate proenkephalin: δ opioid binding sites in leech ganglia and immunocytes. Brain Res. 768, 224–232. ( 10.1016/S0006-8993(97)00646-X) [DOI] [PubMed] [Google Scholar]
- 52.Dores RM, Lecaude S, Bauer D, Danielson PB. 2002. Analyzing the evolution of the opioid/orphanin gene family. Mass Spectrom. Rev. 21, 220–243. ( 10.1002/mas.10029) [DOI] [PubMed] [Google Scholar]
- 53.Kavaliers M, Hirst M, Teskey GC. 1983. A functional role for an opiate system in snail thermal behavior. Science 220, 99–101. ( 10.1126/science.6298941) [DOI] [PubMed] [Google Scholar]
- 54.Thomas AW, Kavaliers M, Prato FS, Ossenkopp KP. 1997. Pulsed magnetic field induced ‘analgesia’ in the land snail, Cepaea nemoralis, and the effects of μ, δ, and κ opioid receptor agonists/antagonists. Peptides 18, 703–709. ( 10.1016/S0196-9781(97)00004-1) [DOI] [PubMed] [Google Scholar]
- 55.Demin KA, et al. 2018. Zebrafish models relevant to studying central opioid and endocannabinoid systems. Prog. Neuro-Psychopharm. Biol. Psychiat. 86, 301–312. ( 10.1016/j.pnpbp.2018.03.024) [DOI] [PubMed] [Google Scholar]
- 56.Bosse GD, Peterson RT. 2017. Development of an opioid self-administration assay to study drug seeking in zebrafish. Behav. Brain Res. 335, 158–166. ( 10.1016/j.bbr.2017.08.001) [DOI] [PubMed] [Google Scholar]
- 57.Costa FV, Rosa LV, Quadros VA, Santos ARS, Kalueff AV, Rosemberg DB. 2019. Understanding nociception-related phenotypes in adult zebrafish: behavioral and pharmacological characterization using a new acetic acid model. Brain Behav. Res. 359, 570–578. ( 10.1016/j.bbr.2018.10.009) [DOI] [PubMed] [Google Scholar]
- 58.Deakin AG, Cossins AR, Spencer JW, Young IS, Sneddon LU. 2019. Welfare challenges influence the complexity of movement: fractal analysis of behaviour in zebrafish. Fishes 4, 8 ( 10.3390/fishes4010008) [DOI] [Google Scholar]
- 59.Lopez-Luna J, Al-Jubouri Q, Al-Nuaimy W, Sneddon LU. 2017. Activity reduced by noxious chemical stimulation is ameliorated by immersion in analgesic drugs in zebrafish. J. Exp. Biol. 220, 1451–1458. ( 10.1242/jeb.146969) [DOI] [PubMed] [Google Scholar]
- 60.Magalhaes FEA, et al. 2018. Adult zebrafish (Danio rerio) as a model for the study of corneal antinociceptive compounds. Zebrafish 15, 566–574. ( 10.1089/zeb.2018.1633) [DOI] [PubMed] [Google Scholar]
- 61.Lynn B. 1994. The fibre composition of cutaneous nerves and the classification and response properties of cutaneous afferents, with particular reference to nociception. Pain Rev. 1, 172–183. [Google Scholar]
- 62.Mantyh PW. 2014. The neurobiology of skeletal pain. Eur. J. Neurosci. 39, 508–519. ( 10.1111/ejn.12462) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pasricha PJ. 2013. Unraveling the mystery of pain in chronic pancreatitis. Nat. Rev. Gastroenterol. Hepatol. 9, 140–151. ( 10.1038/nrgastro.2011.274) [DOI] [PubMed] [Google Scholar]
- 64.Kress M, Koltzenburg M, Reeh PW, Handwerker HO. 1992. Responsiveness and functional attributes of electrically localized terminals of cutaneous C-fibers in vivo and in vitro. J. Neurophysiol. 68, 581–595. ( 10.1152/jn.1992.68.2.581) [DOI] [PubMed] [Google Scholar]
- 65.Albers KM, Woodbury CJ, Ritter AM, Davis BM, Koerber HR. 2006. Glial cell-line-derived neurotrophic factor expression in skin alters the mechanical sensitivity of cutaneous nociceptors . J. Neurosci. 26, 2981–2990. ( 10.1523/JNEUROSCI.22-10-04057.2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cain DM, Khasabov SG, Simone DA. 2001. Response properties of mechanoreceptors and nociceptors in mouse glabrous skin: an in vivo study. J. Neurophysiol. 85, 1561–1574. ( 10.1152/jn.2001.85.4.1561) [DOI] [PubMed] [Google Scholar]
- 67.Lewin GR, Moshourab R. 2004. Mechanosensation and pain. J. Neurobiol. 61, 30–44. ( 10.1002/neu.20078) [DOI] [PubMed] [Google Scholar]
- 68.Koltzenburg M, Stucky CL, Lewin GR. 1997. Receptive properties of mouse sensory neurons innervating hairy skin. J. Neurophysiol. 78, 1841–1850. ( 10.1152/jn.1997.78.4.1841) [DOI] [PubMed] [Google Scholar]
- 69.Beissner F, Brandau A, Henke C, Felden L, Baumgärtner U, Treede R-D, Oertel BJ, Lötsch J. 2010. Quick discrimination of Adelta and C fiber mediated pain based on three verbal descriptors. PLoS ONE 5, e12944 ( 10.1371/journal.pone.0012944) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Handwerker HO. 2009. Nociceptors and characteristics. In Encyclopedia of neuroscience (eds Binder MD, Hirokawa N, Windhorst U), pp. 2876–2879. Berlin, Germany: Springer; ( 10.1007/978-3-540-29678-2) [DOI] [Google Scholar]
- 71.Matthews G, Wickelgren WO. 1978. Trigeminal sensory neurons of the sea lamprey. J. Comp. Physiol. A 123, 329–333. ( 10.1007/BF00656966) [DOI] [Google Scholar]
- 72.McClendon J, Lecaudé S, Dores R, Dores RM. 2010. Evolution of the opioid/ORL-1 receptor gene family. Ann. NY Acad. Sci. 1200, 85–94. ( 10.1111/j.1749-6632.2010.05515.x) [DOI] [PubMed] [Google Scholar]
- 73.Snow PJ, Renshaw GMC, Hamlin KE. 1996. Localization of enkephalin immunoreactivity in the spinal cord of the long-tailed ray Himantura fai. J. Comp. Neurol. 367, 264–273. () [DOI] [PubMed] [Google Scholar]
- 74.Sneddon LU. 2002. Anatomical and electrophysiological analysis of the trigeminal nerve in a teleost fish, Oncorhynchus mykiss. Neurosci. Lett. 319, 167–171. ( 10.1016/S0304-3940(01)02584-8) [DOI] [PubMed] [Google Scholar]
- 75.Sneddon LU. 2003. Trigeminal somatosensory innervation of the head of a teleost fish with particular reference to nociception. Brain Res. 972, 44–52. ( 10.1016/S0006-8993(03)02483-1) [DOI] [PubMed] [Google Scholar]
- 76.Ashley PJ, Sneddon LU, McCrohan CR. 2006. Properties of corneal receptors in a teleost fish. Neurosci. Lett. 410, 165–168. ( 10.1016/j.neulet.2006.08.047) [DOI] [PubMed] [Google Scholar]
- 77.Mettam JJ, McCrohan CR, Sneddon LU. 2012. Characterisation of chemosensory trigeminal receptors in the rainbow trout (Oncorhynchus mykiss): responses to irritants and carbon dioxide . J. Exp. Biol. 215, 685–693. ( 10.1242/jeb.060350) [DOI] [PubMed] [Google Scholar]
- 78.Sneddon LU. 2004. Evolution of nociception in vertebrates: comparative analysis of lower vertebrates. Brain Res. Rev. 46, 123–130. ( 10.1016/j.brainresrev.2004.07.007) [DOI] [PubMed] [Google Scholar]
- 79.Sneddon LU. 2011. Pain perception in fish. J. Consc. Stud. 18, 209–229. [Google Scholar]
- 80.Park TJ, et al. 2008. Selective inflammatory pain insensitivity in the African naked mole-rat (Heterocephalus glaber). PLoS Biol. 6, e13 ( 10.1371/journal.pbio.0060013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brett R. 1991. The population structure of naked mole-rat colonies. In The biology of the naked mole rat (eds Sherman PW, Jarvis JUM, Alexander RD), pp. 97–136. Princeton, NJ: Princeton University Press. [Google Scholar]
- 82.Lopez-Luna J, Canty MN, Al-Jubouri Q, Al-Nuaimy W, Sneddon LU. 2017. Behavioural responses of fish larvae modulated by analgesic drugs after a stress exposure. Appl. Anim. Behav. Sci. 195, 115–120. ( 10.1016/j.applanim.2017.05.021) [DOI] [Google Scholar]
- 83.Roques JAC, Abbink W, Geurds F, van de Vis H, Flik G.. 2010. Tailfin clipping, a painful procedure: studies on Nile tilapia and common carp. Physiol. Behav. 101, 533–540. ( 10.1016/j.physbeh.2010.08.001) [DOI] [PubMed] [Google Scholar]
- 84.Young RF. 1977. Fiber spectrum of the trigeminal sensory root of frog, cat and man determined by electron microscopy. In Pain in the trigeminal region (eds Anderson DL, Matthews B), pp. 137–160. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 85.Terashima S, Liang YF. 1994. C mechanical nociceptive neurons in the crotaline trigeminal ganglia. Neurosci. Lett. 179, 33–36. ( 10.1016/0304-3940(94)90928-8) [DOI] [PubMed] [Google Scholar]
- 86.Belmonte C, Giraldez F. 1981. Responses of cat corneal sensory receptors to mechanical and thermal stimulation . J. Physiol. 321, 355–368. ( 10.1113/jphysiol.1981.sp013989) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Curtright A, Rosser M, Goh S, Keown B, Wagner E, Sharifi J, Raible DW, Dhaka A. 2015. Modeling nociception in zebrafish: a way forward for unbiased analgesic discovery. PLoS ONE 10, e0116766 ( 10.1371/journal.pone.0116766) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stanway GW, Taylor PM, Brodbelt DC. 1996. A comparison of pre-operative morphine and buprenorphine in cats. J. Vet. Anaesthes. 23, 78 ( 10.1046/j.1467-2987.2001.00062.x) [DOI] [PubMed] [Google Scholar]
- 89.Thornton PD, Waterman-Pearson AE. 1999. Quantification of the pain and distress responses to castration in young lambs. Res. Vet. Sci. 66, 107–118. ( 10.1053/rvsc.1998.0252) [DOI] [PubMed] [Google Scholar]
- 90.Slingsby LS, Waterman-Pearson AE. 1998. Comparison of pethidine, buprenorphine and ketoprofen for postoperative analgesia after ovariohysterectomy in the cat. Vet. Rec. 143, 185–189. ( 10.1136/vr.143.7.185) [DOI] [PubMed] [Google Scholar]
- 91.Roughan JV, Flecknell PA. 2000. Effects of surgery and analgesic administration on spontaneous behavior in singly housed rats. Res. Vet. Sci. 69, 283–288. ( 10.1053/rvsc.2000.0430) [DOI] [PubMed] [Google Scholar]
- 92.Sprecher DJ, Hostetler DE, Kanneene JB. 1997. A lameness-scoring system that uses posture and gait to predict dairy cattle reproductive performance. Theriogenology 47, 1179–1187. ( 10.1016/S0093-691X(97)00098-8) [DOI] [PubMed] [Google Scholar]
- 93.Matson DJ, Broom DC, Carson SR, Baldassari J, Kehne J, Cortright DN. 2007. Inflammation-induced reduction of spontaneous activity by adjuvant: a novel model to study the effect of analgesics in rats . J. Pharmacol. Exp. Therap. 320, 194–201. ( 10.1124/jpet.106.109736) [DOI] [PubMed] [Google Scholar]
- 94.Roughan JV, Flecknell PA. 2001. Behavioural effects of laparotomy and analgesic effects of ketoprofen and carprofen in rats. Pain 90, 65–74. ( 10.1016/S0304-3959(00)00387-0) [DOI] [PubMed] [Google Scholar]
- 95.Crook RJ, Dickson K, Hanlon RT, Walters ET. 2014. Nociceptive sensitization reduces predation risk. Curr. Biol. 24, 1121–1125. ( 10.1016/j.cub.2014.03.043) [DOI] [PubMed] [Google Scholar]
- 96.Magee B, Elwood RW. 2016. Trade-offs between predator avoidance and electric shock avoidance in hermit crabs demonstrate a non-reflexive response to noxious stimuli consistent with prediction of pain. Behav. Processes 130, 31–35. ( 10.1016/j.beproc.2016.06.017) [DOI] [PubMed] [Google Scholar]
- 97.Thompson M, Van Wassenbergh S, Rogers SM, Seamone SG, Higham TE.. 2018. Angling-induced injuries have a negative impact on suction feeding performance and hydrodynamics in marine shiner perch, Cymatogaster aggregata. J. Exp. Biol. 221, jeb180935 ( 10.1242/jeb.180935) [DOI] [PubMed] [Google Scholar]
- 98.Millsopp S, Laming P. 2008. Trade-offs between feeding and shock avoidance in goldfish (Carassius auratus). Appl. Anim. Behav. Sci. 113, 247–254. ( 10.1016/j.applanim.2007.11.004) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.