Abstract
Transient receptor potential (TRP) cation channels are highly conserved, polymodal sensors which respond to a wide variety of stimuli. Perhaps most notably, TRP channels serve critical functions in nociception and pain. A growing body of evidence suggests that transient receptor potential melastatin (TRPM) and transient receptor potential ankyrin (TRPA) thermal and electrophile sensitivities predate the protostome–deuterostome split (greater than 550 Ma). However, TRPM and TRPA channels are also thought to detect modified terpenes (e.g. menthol). Although terpenoids like menthol are thought to be aversive and/or harmful to insects, mechanistic sensitivity studies have been largely restricted to chordates. Furthermore, it is unknown if TRP-menthol sensing is as ancient as thermal and/or electrophile sensitivity. Combining genetic, optical, electrophysiological, behavioural and phylogenetic approaches, we tested the hypothesis that insect TRP channels play a conserved role in menthol sensing. We found that topical application of menthol to Drosophila melanogaster larvae elicits a Trpm- and TrpA1-dependent nocifensive rolling behaviour, which requires activation of Class IV nociceptor neurons. Further, in characterizing the evolution of TRP channels, we put forth the hypotheses that three previously undescribed TRPM channel clades (basal, αTRPM and βTRPM), as well as TRPs with residues critical for menthol sensing, were present in ancestral bilaterians.
This article is part of the Theo Murphy meeting issue ‘Evolution of mechanisms and behaviour important for pain’.
Keywords: TRP channels, Drosophila, nociception, menthol, chemosensation, evolution
1. Introduction
Menthol and icilin are often referred to as ‘cooling agents’—while these chemicals do not physically chill, topical application typically elicits a pleasant cooling sensation in humans [1]. Perhaps owing to its perceived cooling properties, menthol has been used as a topical analgesic, often to reduce the severity of itching and/or burning sensations [2–4]. That said, what is pleasant, harmful, or potentially painful will often be species-dependent. It has been previously reported that menthol affects the behaviour of insects; for example, menthol-infused foods are aversive to Drosophila melanogaster, and there is some evidence that menthol functions as an insecticide [5–11]. However, relatively little is known concerning the mechanisms by which insects sense and respond to the cooling agents, as previous studies have focused largely on deuterostomes, and, with respect to molecular determinants among those species, chiefly on terrestrial chordates [12–20]. By extension, it is unknown if possible shared mechanisms have their origins in a common ancestor.
Like many other compounds, menthol and icilin are thought to be detected by transient receptor potential (TRP) channels—primarily transient receptor potential melastatin (TRPM)8 and transient receptor potential ankyrin (TRPA)1 [17,21,22]. TRP channels are variably selective cation channels which are differentially gated by a wide variety of thermal, chemical and mechanical stimuli. It is the multimodal nature of TRPM8 and TRPA1—in humans constituted by at least menthol, icilin, and cold sensing—which partly underlies the similar phenomenological character (i.e. cooling) associated with these very different stimuli [22–24].
The chordate TRPM family is typically divided into eight distinct paralogues (TRPM1-TRPM8) thought to have emerged sometime prior to the divergence of tetrapods and fishes (although fishes are thought to have lost their TRPM8 orthologue) [25]. TRPM8 is multimodal, responding to cold, menthol, and in mammals, icilin [22,26]. It has been suggested that menthol directly binds with the TRPM8 voltage sensor-like domain (VSLD), and gating requires interactions between this domain, bound menthol molecules, and the highly conserved C-terminal TRP domain [13,20]. TRPM8-menthol gating has been well characterized in mammalian channels, and a number of critical amino acid residues have been identified in both the VSLD and the TRP domain [12,14,16,19,20].
In contrast to the TRPM family, the chordate TRPA family is very small, typically containing only a single member, TRPA1 [27,28]. TRPA1 is a polymodal nociceptor involved in the detection of noxious cold, noxious heat, menthol, icilin and electrophilic chemicals such as allyl isothiocyanate (AITC, found in mustard oil and wasabi) [21,24,29,30]. It has been suggested that TRPA1 menthol sensitivity is linked to specific serine and threonine residues found in transmembrane segment 5, and that several nearby residues are responsible for species-specific TRPA1-menthol interactions [31].
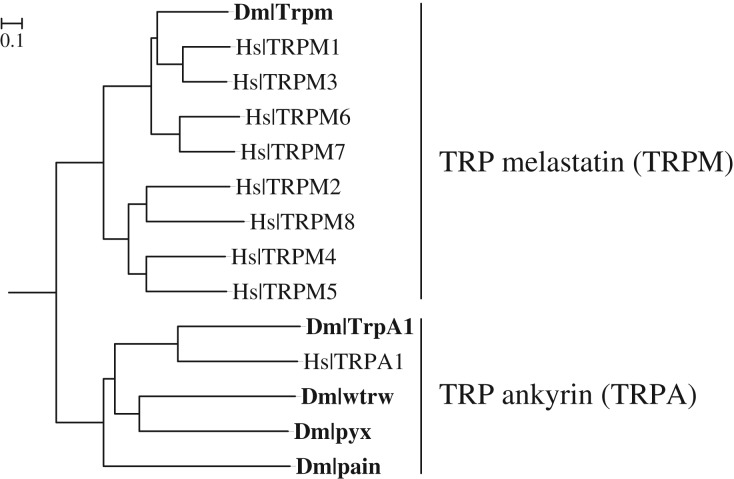
Insects lack a true TRPM8 orthologue. In fact, the Drosophila genome contains only a single TRPM family gene, Trpm (figure 1, top) [32]. While separated by several gene duplication events and more than 550 million years of evolution, Drosophila Trpm and its chordate counterpart, TRPM8, are both involved in cold sensing [33]. With respect to TRPAs, the Drosophila genome encodes four TRPA family genes (figure 1, bottom): TrpA1 (the homologue to chordate TRPA1), painless (pain), water witch (wtrw), and pyrexia (pyx). Like vertebrate TRPA1, Drosophila TrpA1 has been implicated in high-temperature and chemical nociception [27,33–37].
Figure 1.
The Drosophila melanogaster (Dm) genome encodes one TRPM (Trpm) and four TRPA (TrpA1, painless, pyrexia, and water witch) channels. Mid-point rooted tree of Drosophila (Dm) and human (Hs) TRPMs and TRPAs.
TRPA1 high-temperature and electrophile sensitivities are present in both protostomes and deuterostomes [27,36–45]. While current evidence suggests—despite some controversy—that TRPA1 cold sensitivity evolved relatively recently, perhaps among early synapsids (less than 300 Ma) [46–48], TRPM cold sensitivity is conserved in insects [33]. As such, a growing body of evidence suggests that TRP channel thermal and electrophile sensitivity may have a common origin predating the protostome–deuterostome split (greater than 550 Ma). However, it remains unknown if TRPA- and TRPM-dependent menthol sensitivity is equally ancient.
In order to further elucidate the evolutionary history of TRPM and TRPA channels, we explored the mechanisms by which Drosophila senses and responds to cooling agents. Herein, we report that topical menthol application elicits a dose-dependent nocifensive rolling behaviour in Drosophila. Further, we use a suite of genetic, optical, electrophysiological and behavioural approaches to demonstrate that the menthol-evoked response is Trpm- and TrpA1-dependent, and acts via Class IV (CIV) nociceptors. Finally, in light of these findings, we assess the evolutionary histories and sequence homologies of TRPM and TRPA channels. In doing so, we characterize several putative TRPM and TRPA channels in Acropora digitifera (coral), Strongylocentrotus purpuratus (purple sea urchin), Octopus bimaculoides (California two-spot octopus), Priapulus caudatus (penis worm) and Aplysia californica (California sea hare), as well as outline the discovery of three previously undescribed TRPM channel clades (basal TRPMs, αTRPMs and βTRPMs), which we hypothesize were present in the last common bilaterian ancestor, Urbilateria. The results of these studies add to a growing body of evidence suggesting that chemosensation and nociception have functional origins in the Precambrian world [49].
2. Material and methods
(a). Animals
All D. melanogaster stocks were maintained at 24°C under a 12 : 12 light : dark cycle. In order to accelerate development, genetic crosses (and relevant controls) were raised for 5 days at 29°C under a 12 : 12 light : dark cycle. Wandering 3rd instar larvae were used for all experiments. The OregonR (ORR) strain was used to assess wild-type behaviour, and, where appropriate, the w1118 strain and outcrossed parental strains were used as genetic background controls. Transgenic and mutant strains included: Trpm2 and wtrw1 (gifts of K. Venkatachalam); TrpA1W903* and TrpA11 (gifts of W. D. Tracey); pyx3, pain70, and Trpm deficiency Df(2R)XTE-11 (gifts of M. J. Galko); GAL4GMR57C10 (pan-neuronal driver, BDSC no. 39171); GAL4ppk (CIV driver, BDSC no. 32079); UAS-CaMPARI (BDSC no. 58763); UAS-TeTxLC (active tetanus toxin, BDSC no. 28837); UAS-IMP-TNTVI-A (inactive tetanus toxin, BDSC no. 28840); UAS-TrpA1-RNAi 1 (BDSC no. 31384); UAS-TrpA1-RNAi 2 (BDSC no. 66905); UAS-Trpm-RNAi 1 (BDSC no. 31672); and UAS-Trpm-RNAi 2 (BDSC no. 31291).
(b). Behaviour
Crystalline l(-)-menthol (Acros Organics, Geel, Belgium) and icilin (Tocris Bioscience, Bristol, UK) were suspended in liquid dimethyl sulfoxide (DMSO) at various per cent weight/volume concentrations (% w/v): menthol (8%: 512 mM; 6%: 384 mM; 4%: 256 mM; 2%: 128 mM); icilin (0.5%: 16 mM; 1%: 32 mM; 15%: 482 mM). Solutions were discarded after 24 h. Individual larval subjects were placed in a well of a glass 9-well plate, and 10 µl of solution was delivered to the well via micropipette. Subjects were observed for 60 s and their behaviours recorded. In order to increase subject-background contrast in the representative images/videos, animals were left to freely locomote on a moist black arena, and menthol was applied as above.
(c). CaMPARI-based Ca2+ analyses
UAS-CaMPARI expression was driven via GAL4GMR57C10. Live, freely behaving larvae were exposed to 8% menthol or vehicle as described above. Photoconverting light was delivered under a Zeiss AxioZoom V16 microscope as previously described [50,51]. Larvae were subsequently placed in one drop of 1 : 5 diethyl ether : halocarbon oil and secured between a slide and slide cover. CIV neurons were imaged via a Zeiss LSM780 confocal microscope, and the resulting z-stacks were volume rendered as two-dimensional maximum intensity projections. Red and green fluorescence intensity was assessed using the FIJI distribution of ImageJ software.
(d). Electrophysiology
To record single-unit spiking activity of CIV neurons, we first prepared fillet preparations from GAL4ppk>UAS-mCD8::GFP larvae. Larvae were placed in a Petri dish lined with Sylgard® 184 (Dow Corning, Midland, MI, USA) filled with HL-3 saline. The ventral body wall was cut open with fine scissors, and all muscles were carefully removed with a polished tungsten needle and scissors. The preparation was then constantly superfused with HL-3 saline at a rate of 1 ml min−1 at room temperature, and allowed to rest for more than 1 h before recording. Menthol was first dissolved in DMSO at a concentration of 500 mM and then diluted in HL-3 saline to a final concentration of 500 µM. Menthol was bath-applied to the specimen through superfusion. Extracellular recordings were made with a macropatch pipette (tip diameter, 5–10 µm) connected to the headstage of a patch-clamp amplifier (AxoPatch200B, Molecular Devices, Sunnyvale, CA, USA). Gentle suction was applied to draw the soma and a small portion of neurite into the pipette. The amplifier was set to the track mode to record neuronal spikes. The output signals from the amplifier were digitized at a sampling frequency of 10 kHz using a Micro1401 A/D converter (Cambridge Electronic Design, Cambridge, UK) and acquired into a laptop computer running Windows 10 with Spike2 software v. 8 (Cambridge Electric Design, Cambridge, UK). Average spike frequency was measured in a 30 s time window during baseline control conditions, superfusion of vehicle, and superfusion of menthol.
(e). Phylogenetics
Amino acid sequences for previously characterized TRP channels were collected from the following databases: JGI Genome (Monosiga brevicollis, Nematostella vectensis and Daphnia pulex), Ensembl (Danio rerio, Gallus gallus and Strigamia maritima), NCBI (Hydra vulgaris, Homo sapiens, Mus musculus, Apis mellifera and Galendromus occidentalis), WormBase (Caenorhabditis elegans) or FlyBase (D. melanogaster). Panulirus argus sequences were collected from [52]. In order to identify novel TRPM and TRPA channels, publically available protein models based on genomic and/or transcriptomic sequences for Ac. digitifera [53], S. purpuratus [54,55], O. bimaculoides [56], P. caudatus (BioProject PRJNA20497, GenBank AXZU00000000.2) and Ap. californica (BioProject, PRJNA209509, GenBank AASC00000000.3) were pBLASTed [57] against D. melanogaster TRP sequences. Sequences greater than 200 aa in length and with an E-value < 1 × 10−30 were retained and subsequently analysed via InterProScan [58]. Sequences which had predicted transmembrane segments and characteristic Ankyrin repeats (for TRPAs) were retained. Accession numbers are available in the electronic supplementary material, table S1.
Amino acid sequences were MUSCLE [59] aligned in MEGA7 [60]. Poorly aligned regions and spurious sequences were identified and trimmed using automated methods packaged with TrimAl [61]. As TRPA Ankyrin repeats were generally poorly aligned, they were manually removed prior to automated trimming by excluding everything N-terminal of 20 aa before the start of predicted transmembrane segment 1. IQ-Tree was then used to perform a composition chi-squared test in order to assess sequence homogeneity within TRP subfamilies [62]. Owing to extreme divergence, and in order to minimize possible topology disruptions owing to long branch attraction [63–65], P. argus TRPMm, TRPA-like1, and TRPA5-like1, and C. elegans TRPA-2 were excluded from final analyses; each failed the composition chi-squared test and introduced extremely long branches with weak support in a first-pass analysis. Final, trimmed alignments were used to generate phylogenetic trees. Bayesian trees were constructed in MrBayes (version 3.2.6) using a mixed amino acid substitution model and a gamma distributed rate of variation [66,67]. Two independent Markov chain Monte Carlo analyses (initial settings: 1 000 000 chains with sampling every 10) were run until convergence (less than 0.05). Twenty five per cent of the chain was considered burn-in and discarded. Maximum-likelihood trees were constructed in IQ-Tree using an amino acid substitution model automatically selected by ModelFinder [68]. Ultrafast bootstrapping (2000 bootstraps) was also performed in IQ-Tree [69]. Trees were visualized and edited in iTOL [70] and Adobe Illustrator CS6. Branches with low support (posterior probability less than 0.7 or bootstrap less than 70) were considered unresolved, and were collapsed to polytomies in the final trees. Ancestral sequence predictions were made in MEGA7 using the maximum-likelihood approach against previously generated alignments and Bayesian trees [71].
(f). Statistical analyses
Population proportions are presented as % ± standard error of the proportion (s.e.p.); differences in proportion were assessed using a generalized linear model with a logit link and a binomial error distribution, and pairwise comparisons were made using the lsmeans R package [72] and the Tukey method for multiple comparisons. CaMPARI photoconversion, spike frequency and latency measures are presented as mean ± standard error of the mean (s.e.m.); differences were assessed in GraphPad PRISM (GraphPad Software, La Jolla, California, USA) by unpaired t-test or ANOVA with Dunnett's post hoc test. Asterisk (*) indicates statistical significance p < 0.05, with significant p-values (as compared to wild-type or appropriate control) listed in the associated figure legend.
3. Results
(a). Menthol elicits Trpm- and TrpA1-dependent nocifensive rolling in Drosophila larvae
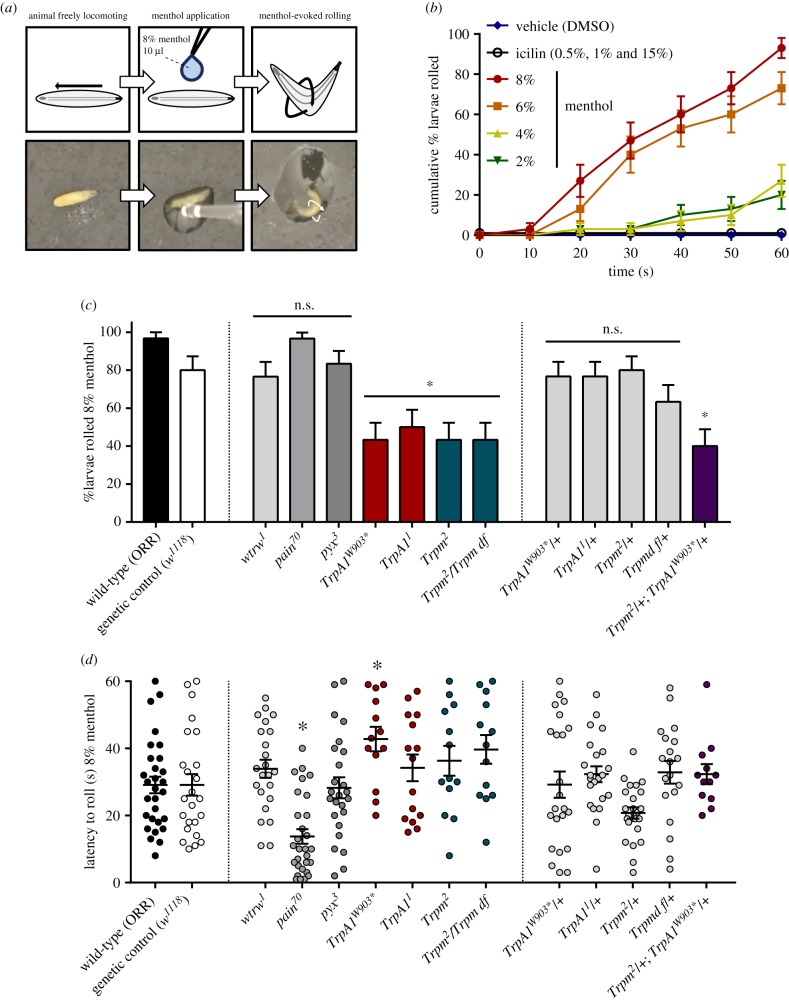
Vehicle (DMSO), menthol or icilin was topically applied to freely behaving D. melanogaster larvae, and their behaviour recorded (10 µl, delivered via micropipette). Mentholated solutions elicited a stereotyped rolling behaviour, identical to a previously described [73,74] nocifensive response (figure 2a; electronic supplementary material, movie S1). The proportion of animals which responded to menthol increased with higher menthol concentrations (figure 2b). By contrast, treatment with DMSO (figure 2b, vehicle; electronic supplementary material, movie S2) or icilin (figure 2b; electronic supplementary material, movie S3) did not elicit rolling.
Figure 2.
Drosophila larvae exhibit nocifensive rolling in response to menthol. (a) Menthol-evoked rolling. Top, cartoon. Bottom, video stills. (b) Cumulative proportion of wild-type rollers in response to menthol. The rolling response increased at higher menthol concentrations (% w/v). Cumulative proportion ± s.e.p. n = 30 for each condition. (c) Proportion of rollers in response to 8% menthol. Compared to wild-type, fewer TrpA1 and Trpm mutants rolled in response to menthol (TrpA1W903*, p = 0.0221; TrpA11, p = 0.0479; Trpm2, p = 0.0221; Trpm2/Trpm df, p = 0.0221). Trpm and TrpA1 are haplosufficient for menthol sensitivity, but fewer transheterozygous mutants rolled in response to menthol (p = 0.0090). Proportions represented as % rollers ± s.e.p. n = 30 for each condition. (d) Latency to roll in response to 8% menthol. painless mutation sensitized rollers (p = 0.0006), while TrpA1W903* mutation desensitized rollers (p = 0.0388). Latency represented as time to roll in seconds ± s.e.m. n proportional to % rollers, where initial sample size was 30. (Online version in colour.)
Given TRP-dependent mechanisms of menthol sensing in vertebrates, we hypothesized that menthol-evoked rolling requires TRPM and TRPA channels. To test this, we assessed the behaviour of whole-animal Trpm, TrpA1, pyx, pain, and wtrw mutants. Compared to the control, a significantly smaller proportion of homozygous TrpA1 and Trpm mutants rolled in response to menthol (figure 2c). Additionally, we observed a significantly increased latency to roll for those TrpA1W903* mutants that did respond (figure 2d). By contrast, pain mutation sensitized larvae to menthol—homozygous pain mutants rolled with severely decreased latency, and many rolled immediately following menthol application (figure 2d). Given that painless mutants could be sensitized to the point of immediately rolling following menthol application, it appears likely that menthol was rapidly delivered across the cuticle.
As TrpA1 and Trpm were both required for the menthol-evoked response, mutations were tested combinatorially. While heterozygous TrpA1 (TrpA1W903*/+ and TrpA11/+) and Trpm (Trpm2/+ and Trpm df/+) mutants had no significant behavioural defects, transheterozygous (TrpA1W903*/+;Trpm2/+) mutants exhibited significantly inhibited menthol-evoked rolling, indicating that these channels genetically interact in menthol sensing (figure 2c).
(b). Class IV nociceptors mediate menthol-evoked rolling
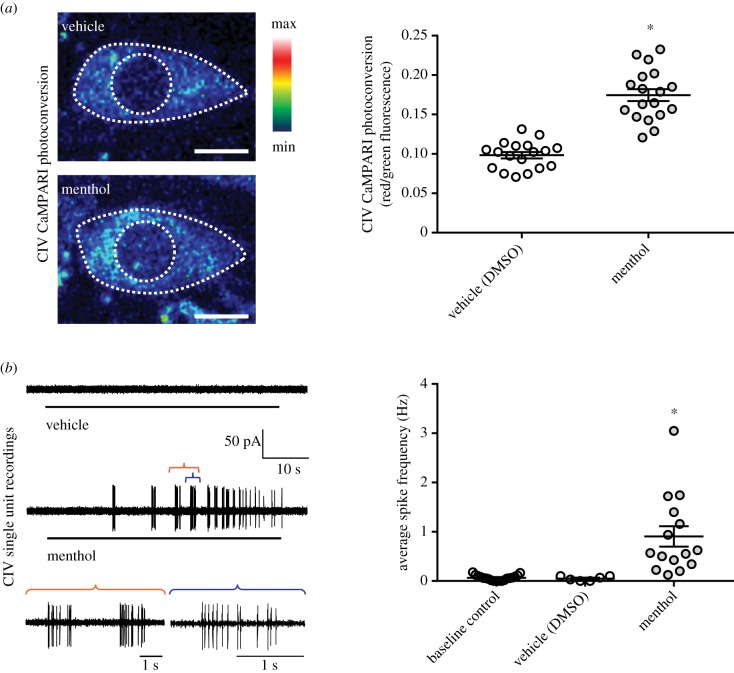
Drosophila larvae have two known classes of peripheral nociceptors: multidendritic (md) Class III neurons are cold nociceptors and innocuous touch mechanosensors, whereas md CIV neurons are polymodal nociceptors which detect noxious heat, mechanical insults and short-wavelength light [33,34,74–78]. As rolling behaviour has been previously associated with CIV nociceptor activation, we hypothesized that menthol-evoked rolling requires CIV activation. In order to assess activation patterns in vivo, the GAL4-UAS system was used to drive neuronal expression of CaMPARI [79], a genetically encoded Ca2+ indicator. Live, freely behaving transgenic animals were exposed to menthol or vehicle, as above. The level of CIV neural activation was assessed post hoc, in live animals, as a green-to-red shift in CaMPARI fluorescence, which occurs in the presence of violet light as a function of increases in intracellular Ca2+ concentration. CaMPARI analyses revealed significant activation of CIV nociceptors in response to menthol, relative to vehicle control (figure 3a). To directly assess menthol-evoked activation of CIV neurons, we performed single-unit electrophysiological recordings of CIV neurons. Larvae were filleted, and mentholated saline solutions were superfused across the preparations. Electrophysiological recordings demonstrate that superfusion of menthol significantly increases spike frequency in CIV neurons relative to controls (figure 3b).
Figure 3.
Menthol exposure activates Class IV (CIV) nociceptors. (a) Topical application of menthol to whole animals resulted in an increased CIV Ca2+ response relative to vehicle control. Left, representative CIV CaMPARI photoconversion visualized using a 16-colour lookup table. Right, CIV neurons were significantly activated by 8% menthol, as compared to vehicle control (p < 0.0001). Quantification of CaMPARI photoconversion by Fred/Fgreen CaMPARI fluorescence intensities. Photoconversion presented as mean ± s.e.m. n = 18 for each condition. (b) Bath application of menthol activated CIV neurons in filleted preparations relative to controls. Left, example CIV single-unit recordings (coloured brackets depict detailed view of strong menthol-evoked spiking activity). Right, menthol superfusion (500 µM) elicited significantly increased average spike frequency in CIV neurons (p = 0.0001). Average frequency in Hz ± s.e.m. Saline control, n = 18; DMSO control, n = 6; menthol, n = 15. (Online version in colour.)
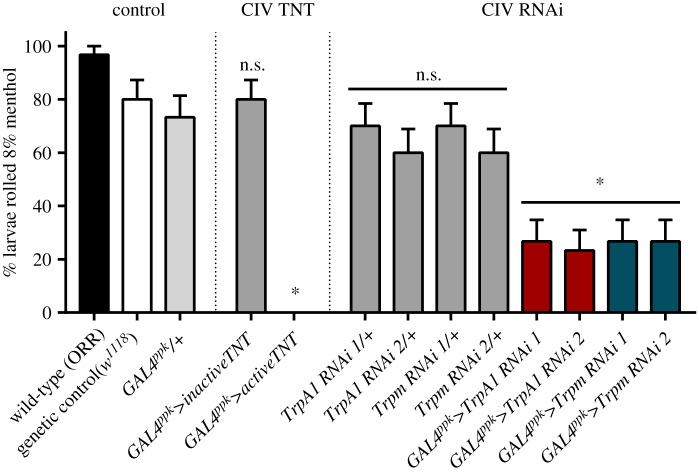
In order to test the necessity of CIV neurons for menthol-evoked rolling, genetically encoded active tetanus toxin (TNT) was used to block synaptic transmission in CIV neurons. GAL4ppk-driven, CIV-specific expression of TNT completely ablated menthol-evoked rolling (figure 4).
Figure 4.
The TRP-dependent menthol response operates via CIV neurons. Silencing neural transmission in CIV neurons with active tetanus toxin (TNT) completely ablated the menthol response, while CIV-driven expression of inactive TNT did not significantly affect the rolling response. CIV-specific, RNAi-mediated knockdown using two independent transgenes for TrpA1 and Trpm resulted in fewer animals rolling in response to menthol, as compared to the control (TrpA1 RNAi 1, p = 0.0032; TrpA1 RNAi 2, p = 0.0018; Trpm RNAi 1, p = 0.0032; and Trpm RNAi 2, p = 0.0032). Proportions represented as % ± s.e.p. n = 30 for each condition. (Online version in colour.)
In light of TRP mutant and CIV TNT phenotypes, we hypothesized that menthol-evoked behaviour may be dependent on TrpA1 and/or Trpm function in CIV nociceptors. We previously demonstrated that TrpA1 and Trpm transcripts are detectibly expressed in CIV nociceptors ([80], GEO: GSE46154). Consistent with these data, CIV-targeted, RNAi-mediated knockdown of Trpm or TrpA1 strongly inhibited menthol-evoked rolling (figure 4), supporting a functional role for these TRP channels in CIV-mediated menthol sensing.
(c). Residues critical to menthol sensing were probably present in ancestral bilaterian channels
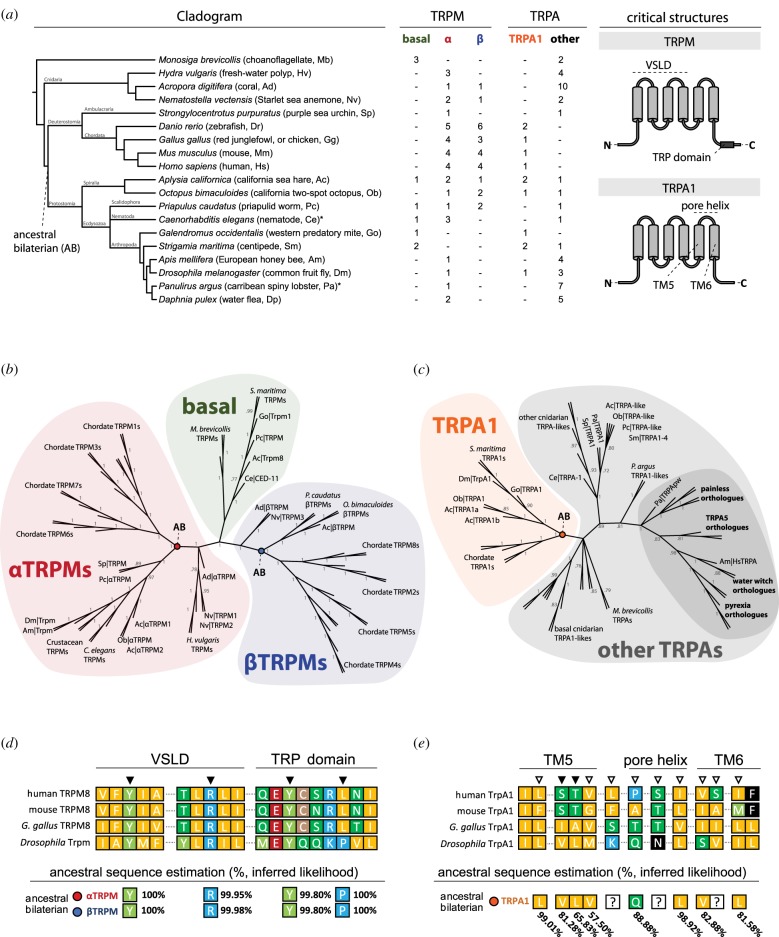
A number of specific amino acid residues have been associated with menthol sensitivity in mammalian TRPM [12,14,16,19,20] and TRPA [31] channels. Phylogenetic and sequence analyses were performed in order to assess how well conserved these residues are across taxa, and to infer their evolutionary history. The amino acid sequences for 69 TRPM channels and 56 TRPA channels (electronic supplementary material, table S1) were used to generate phylogenetic trees by both Bayesian and maximum-likelihood approaches. Trees were constructed using previously and newly characterized TRP sequences across a variety of metazoan species, with a choanoflagellate (M. brevicollis) outgroup (figure 5a). Both methods produced trees with largely consistent topologies (electronic supplementary material, figures S1–S4).
Figure 5.
The evolutionary history of TRPM and TRPA channels, and of residues critical to menthol sensing. (a) Left, cladogram of species used in analysis. Asterisk (*) indicates that one or more previously known sequences were discarded owing to extreme divergence, as described in the Material and methods and electronic supplementary material. All sequence accession numbers are available in the electronic supplementary material. Right, domains previously identified as critical to menthol sensing. (b) Bayesian consensus tree for TRPM channels. Posterior probabilities are indicated for each internal branch. Branches with a posterior probability less than 0.70 were collapsed. For aesthetic purposes, branch lengths were ignored when generating this figure. Trees with branch lengths to scale are available in the electronic supplementary material. Green, basal clade; red, αTRPM clade; blue, βTRPM clade. (c) Bayesian consensus tree for TRPA channels, as in (b). Orange, TRPA1 clade; grey, other TRPAs; dark grey, clade previously described as ‘basal’. (d) Alignment for select TRPM sequences and ancestral sequence estimations. Black arrows indicate previously identified critical residue site. % values represent probability of amino acid at the indicated site, at the colour-coded node. (e) Alignment for select TRPA sequences and ancestral sequence estimations, as in (d). White arrows indicate residues associated with species-specific menthol responses, in mammals. Question mark (?) indicates that no single amino acid was predicted with greater than 50% inferred likelihood.
It has been previously noted that several species express TRPM-like channels which cluster independent of, or differ greatly from, other chordate and/or insect TRPMs [28,52,81]. In accordance with this, the consensus TRPM phylogeny shows a group of protostome TRPMs located basally, near choanoflagellate TRPMs (figure 5b, green). Previously published phylogenies have shown most arthropod TRPMs to be most closely related to chordate TRPMs 1, 3, 6 and 7, yet this relationship has not yet been formally discussed [25,28]. These trees demonstrate that non-basal TRPM channels group into two monophyletic clades (designated αTRPM and βTRPM), which collectively constitute all other analysed TRPM channels (figure 5b, red and blue). Each clade contains both protostome and deuterostome TRPMs and is rooted in cnidarian TRPMs, indicating that these two clades may have existed prior to the protostome–deuterostome split. The topology of these trees is consistent with a hypothesis that at least three distinct TRPM channels (designated basal TRPM, αTRPM and βTRPM) predate the last common bilaterian ancestor, Urbilateria. Further, this hypothesis is compatible with previously formulated hypotheses regarding the independent diversification of chordate TRPM channels [25,28,32].
As both α- and βTRPMs have been implicated in menthol sensing, ancestral sequence predictions were generated for ancestral bilaterian α- and βTRPM. Four TRPM8 residues—Y745, R842, Y1005 and L1009 (figure 5d, black arrows)—have been identified as critical to vertebrate menthol sensing [20]. Three of these residues, Y745, R842 and Y1005, are conserved in Drosophila, and ancestral sequence predictions suggest with high confidence that they are conserved from a common ancestral bilaterian sequence (figure 5d, bottom). The only site which differs from chordate TRPM8 is a predicted ancestral proline in place of L1009; proline, however, is more common at this site across TRPM channels, including in Drosophila, and previous work has shown that L1009P substitution in mouse TRPM8 does not affect menthol sensitivity [12].
For TRPAs, both previously characterized clades—TRPA1 and the ‘basal’ clade (figure 5c, orange and dark grey)—formed as expected, and conformed to other published topologies [7,27,28,32]. Although the term ‘basal’ has been used to describe other TRPAs, Peng et al. have suggested that the TRPA1 clade is the most ancestral [28]. This analysis does not clearly support nor undermine this hypothesis. What is clear, however, is that the TRPA1 clade predates the protostome–deuterostome split.
Previous work has shown that the TRPA1 pore region is important to mammalian menthol sensitivity. Serine and threonine residues in TM5 are thought to be most critical to menthol sensitivity (figure 5e, black arrows), and a variety of others (figure 5e, white arrows) are associated with species-specific differences in menthol sensitivity [31]. These regions are generally poorly conserved between mammals and other species (figure 5e, top), including other chordates (e.g. G. gallus). In contrast to TRPM conservation, fewer critical TRPA1 residues appear to be consistently conserved from a common bilaterian ancestor, and there is substantially less certainty as to the identity of these ancestral sequences (figure 5e, bottom).
4. Discussion
(a). Transient receptor potential-dependent transduction and menthol sensing in Drosophila
We have demonstrated that Drosophila larvae execute a dose-dependent, menthol-evoked, aversive rolling response consistent with the nocifensive behaviour displayed in response to noxious heat and mechanical insult. Cellularly, menthol exposure activates CIV nociceptors, and blocking synaptic transmission in these neurons inhibits menthol-evoked rolling. Molecularly, TrpA1 and Trpm are required for menthol-induced aversive behaviour, and genetically interact in this capacity.
Curiously, it has been previously reported that Drosophila TrpA1 does not directly gate in response to menthol [31]. The study in question, however, used lower concentrations of menthol applied to channels expressed in vitro. These sorts of discrepancies are not uncommon in studies of other TRPA1 modalities, and there are similar conflicting reports concerning TRPA1's role in cold nociception: some groups report that TRPA1 acts as a direct cold sensor, others state it responds to an indirect, cold-associated factor (reactive oxygen species and/or intracellular Ca2+), while others claim it responds only to innocuous cooling, or does not respond to cold at all [47,82–86]; some isoforms of TRPA1 have been shown to directly gate in response to noxious high temperatures, yet high temperature nociception can be rescued by heat-insensitive isoforms [37]; and there is still debate as to whether or not TRPA1 functions similarly in vivo and in vitro [46]. Further, TRPA1s are differentially activated and/or inhibited by menthol, across species, across concentrations [41,47]. It is therefore unsurprising, if no less puzzling, that Drosophila TrpA1 is required for menthol detection in vivo, yet the channel does not gate in response to relatively low concentrations of menthol in vitro. Instead, these and other findings suggest that we may not fully understand how TRPA1 functions, and that it may not be a simple sensor which directly responds to an incredibly wide variety of stimuli.
Moreover, although menthol and TRPA/TRPM interactions are most frequently studied, menthol is likely to be very promiscuous with respect to which channels it activates. Reports suggest that menthol activates, potentiates, or inhibits voltage-gated Na+ channels, L-type voltage-gated Ca2+ channels, the cystic fibrosis transmembrane conductance regulator, mouse TRPV3, TRPL, a wide variety of Ca2+-dependent channels, as well as GABA, serotonin, and acetylcholine receptors [7,87–91]. Another notable player is the hymenopteran specific TRPA (HsTRPA), which is more closely related to Painless than to TRPA1 (figure 5c, dark grey). HsTRPA1 thermal sensitivity is depressed by application of menthol [7]. Given that painless mutants are hypersensitive to menthol (figure 2c), the so-called basal TRPAs may be complex mediators of chemo-sensitivity, rather than simple sensors. Currently, the menthol-specific ligand-binding and gating mechanisms of most receptors are not as well understood as those of chordate TRPA1 and TRPM8. However, these mechanisms are no doubt important, perhaps to species-specific behavioural responses, or to menthol's purported analgesic effect [2–4,88]. Future studies will need to investigate not only how other channels function in menthol sensing, but how they might interact with channels with incompletely understood activation properties (e.g. TRPA1), as these interactions may explain differences in menthol-evoked activity in vivo and in vitro.
(b). Transient receptor potential channels and the evolution of chemical nociception
Phylogenetic analyses have revealed that three TRPM clades—designated basal, αTRPM and βTRPM—and the TRPA1 clade, probably predate the protostome–deuterostome split. Further, many TRPM and TRPA1 residues critical to menthol sensing are conserved from ancestral bilaterian channels (although more variably so for TRPA1s). That residues critical to menthol sensing may predate the protostome–deuterostome split influences how we may understand the evolution of menthol production and avoidance. There is some evidence that menthol may be lethal to several insect species [10,11], including D. melanogaster [9]; as such, the ability to sense and respond to menthol may be adaptive. Given that there is considerable latency to roll in response to menthol (figure 2d), it is unclear if the aversive rolling behaviour is protective. Menthol sensing may be more important for adult females, which rather carefully select egg-laying sites and show aversion towards mentholated food [5]. Yet residues critical to menthol sensitivity substantially predate the emergence of Lamiaceae, the family of angiosperms that most notably produce menthol [92,93]. This appears consistent with early plants evolving menthol (or more broadly, terpene) production in order to repel animals, rather than early animal TRPs evolving menthol sensitivity in order to avoid certain plants. It is possible that animal TRP channels maintained menthol sensitivity since well before terrestrial animals encountered menthol producers, much like how mammalian TRP channels are sensitive to icilin, which is not known to be naturally occurring [1,16,84,94]. Pinpointing the exact origins of these abilities requires additional studies, in particular studies involving more basal (e.g. Xenacoelomorpha) and non-bilaterian species. Yet, it is conceivable that the functional capacity of these channels evolved in, or prior to, a common bilaterian ancestor, which inhabited a very different environment than extant terrestrial animals [95].
The structure of the urbilaterian nervous system is still under considerable debate [96–100]. However, given that TRP channels function in similar ways, in similar subsets of neurons, across taxa, it seems increasingly likely that ancestral TRP channels were expressed in some form of urbilaterian neural tissues, and that these tissues responded to external cues. Still, the function of conserved, menthol-associated residues in ancestral TRPs remains mysterious. However, while menthol production appears to be restricted to plants, terpene production (and more broadly, volatile organic compound production) is far more widespread. For example, volatile organic producers include plants, insects, protists, bacteria and fungi [101], which have been hypothesized to use volatile organic compounds in forms of microbial communication [102]. With this in mind, and concerning terpene sensing by early metazoan TRPs, one plausible hypothesis is that TRP-terpene sensitivity emerged as an early method of communication. Alternatively (or perhaps as sub-hypotheses), TRP-terpene sensing may have been employed as a mechanism by which to identify appropriate food sources, or to signal potential danger, thereby avoiding damage from predation or infection—perhaps an early form of early nociception.
5. Conclusion
Herein, we have described cellular and molecular mechanisms by which D. melanogaster responds to menthol. Phylogenetic analyses revealed that extant TRPM channels are descended from three ancestral clades (basal, αTRPM and βTRPM), and that several residues critical to menthol sensing are conserved from common TRPM and TRPA1 ancestors. Collectively, these findings suggest that bilaterian menthol sensing has its origins in a common ancestor, and more broadly, contribute to a body of evidence suggesting that the mechanisms underlying TRP function and chemical nociception are ancient and highly conserved.
Acknowledgements
We would like to thank the Bloomington Drosophila Stock Center, as well as Drs Michael J. Galko, W. Dan Tracey and Kartik Venkatachalam for providing Drosophila strains.
Data accessibility
Microarray data are available at NCBI Gene Expression Omnibus (accession number GSE46154; https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE46154). All other datasets supporting this article have been provided as part of the electronic supplementary material.
Authors' contributions
Conceptualization and experimental design, N.J.H., J.M.L., A.S. and D.N.C.; genetics, N.J.H. and J.M.L.; behaviour assays, N.J.H., J.M.L., M.N.B.; CaMPARI microscopy, N.J.H. and T.R.G.; electrophysiology, A.S.; phylogenetic and sequence analyses, N.J.H.; statistical analyses, N.J.H.; wrote the original manuscript, N.J.H.; critically analysed and edited the manuscript, N.J.H., J.M.L., A.S., T.R.G., M.N.B. and D.N.C.; approved the final draft, N.J.H., J.M.L., A.S., T.R.G., M.N.B. and D.N.C.; funding acquisition, N.J.H. and D.N.C.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the National Institute of Neurological Disorders and Stroke (NINDS) (R01NS086082; D.N.C.); the National Institute of General Medical Sciences (NIGMS) IMSD (R25GM109442-01A1; T.R.G. and D.N.C.); Georgia State University (GSU) Brains & Behavior Seed Grant (D.N.C.); GSU Brains & Behavior Fellowships (N.J.H. and J.M.L.); and the Kenneth W. and Georganne F. Honeycutt Fellowship (N.J.H.).
References
- 1.Wei ET, Seid DA. 1983. AG-3–5: a chemical producing sensations of cold. J. Pharm. Pharmacol. 35, 110–112. ( 10.1111/j.2042-7158.1983.tb04279.x) [DOI] [PubMed] [Google Scholar]
- 2.Romaneli RS, Boaratti AZ, Rodrigues AT, Queiroz MA, Khan KU, Nascimento TMT, Fernandes JBK, Mansano CFM. 2018. Efficacy of benzocaine, eugenol and menthol as anesthetics for freshwater angelfish (Pterophyllum scalare). J. Aquat. Anim. Health 30, 210–216. ( 10.1002/aah.10030) [DOI] [PubMed] [Google Scholar]
- 3.Liu B, Jordt S-E. 2018. Cooling the itch via TRPM8. J. Invest. Dermatol. 138, 1254–1256. ( 10.1016/j.jid.2018.01.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ton HT, Phan TX, Abramyan AM, Shi L, Ahern GP. 2017. Identification of a putative binding site critical for general anesthetic activation of TRPA1. Proc. Natl Acad. Sci. USA 114, 3762–3767. ( 10.1073/pnas.1618144114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abed-Vieillard D, Cortot J. 2016. When choice makes sense: menthol influence on mating, oviposition and fecundity in Drosophila melanogaster. Front. Integr. Neurosci. 10, 5 ( 10.3389/fnint.2016.00005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abed-Vieillard D, Cortot J, Everaerts C, Ferveur J-F. 2014. Choice alters Drosophila oviposition site preference on menthol. Biol. Open 3, 22–28. ( 10.1242/bio.20136973) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kohno K, Sokabe T, Tominaga M, Kadowaki T. 2010. Honey bee thermal/chemical sensor, AmHsTRPA, reveals neofunctionalization and loss of transient receptor potential channel genes. J. Neurosci. 30, 12 219–12 229. ( 10.1523/JNEUROSCI.2001-10.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maliszewska J, Jankowska M, Kletkiewicz H, Stankiewicz M, Rogalska J. 2018. Effect of capsaicin and other thermo-TRP agonists on thermoregulatory processes in the American cockroach. Molecules 23, 3360 ( 10.3390/molecules23123360). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thorpe WH. 1939. Further studies on pre-imaginal olfactory conditioning in insects. Proc. R. Soc. Lond. B 127, 424–433. ( 10.1098/rspb.1939.0032) [DOI] [Google Scholar]
- 10.Rice PJ, Coats J. 1994. Insecticidal properties of several monoterpenoids to the house fly (Diptera: Muscidae), red flour beetle (Coleoptera: Tenebrionidae), and southern corn rootworm (Coleoptera: Chrysomelidae). J. Econ. Entomol. 87, 1172–1179. ( 10.1093/jee/87.5.1172) [DOI] [PubMed] [Google Scholar]
- 11.Samarasekera R, Weerasinghe Indira S, Hemalal KDP. 2007. Insecticidal activity of menthol derivatives against mosquitoes. Pest Manag. Sci. 64, 290–295. ( 10.1002/ps.1516) [DOI] [PubMed] [Google Scholar]
- 12.Bandell M, Dubin AE, Petrus MJ, Orth A, Mathur J, Hwang SW, Patapoutian A. 2006. High-throughput random mutagenesis screen reveals TRPM8 residues specifically required for activation by menthol. Nat. Neurosci. 9, 493–500. ( 10.1038/nn1665) [DOI] [PubMed] [Google Scholar]
- 13.Janssens A, Voets T. 2011. Ligand stoichiometry of the cold- and menthol-activated channel TRPM8. J. Physiol. 589, 4827–4835. ( 10.1113/jphysiol.2011.216523) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malkia A, Pertusa M, Fernández-Ballester G, Ferrer-Montiel A, Viana F. 2009. Differential role of the menthol-binding residue Y745 in the antagonism of thermally gated TRPM8 channels. Mol. Pain 5, 1744–8069. ( 10.1186/1744-8069-5-62) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Myers BR, Sigal YM, Julius D. 2009. Evolution of thermal response properties in a cold-activated TRP channel. PLoS ONE 4, e5741 ( 10.1371/journal.pone.0005741) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pedretti A, Marconi C, Bettinelli I, Vistoli G. 2009. Comparative modeling of the quaternary structure for the human TRPM8 channel and analysis of its binding features. Biochim. Biophys. Acta Biomembr. 1788, 973–982. ( 10.1016/j.bbamem.2009.02.007) [DOI] [PubMed] [Google Scholar]
- 17.Peier AM, et al. 2002. A TRP channel that senses cold stimuli and menthol. Cell 108, 705–715. ( 10.1016/S0092-8674(02)00652-9) [DOI] [PubMed] [Google Scholar]
- 18.Pertusa M, Rivera B, González A, Ugarte G, Madrid R. 2018. Critical role of the pore domain in the cold response of TRPM8 channels identified by ortholog functional comparison. J. Biol. Chem. 293, 12 454–12 471. ( 10.1074/jbc.RA118.002256) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rath P, Hilton JK, Sisco NJ, Van Horn WD. 2016. Implications of human transient receptor potential melastatin 8 (TRPM8) channel gating from menthol binding studies of the sensing domain. Biochemistry 55, 114–124. ( 10.1021/acs.biochem.5b00931) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin Y, Wu M, Zubcevic L, Borschel WF, Lander GC, Lee S-Y. 2018. Structure of the cold- and menthol-sensing ion channel TRPM8. Science 359, 237–241. ( 10.1126/science.aan4325) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karashima Y, Damann N, Prenen J, Talavera K, Segal A, Voets T, Nilius B. 2007. Bimodal action of menthol on the transient receptor potential channel TRPA1. J. Neurosci. 27, 9874 ( 10.1523/jneurosci.2221-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKemy DD, Neuhausser WM, Julius D. 2002. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416, 52–58. ( 10.1038/nature719) [DOI] [PubMed] [Google Scholar]
- 23.Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, Patapoutian A. 2007. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature 445, 541–545. ( 10.1038/nature05544) [DOI] [PubMed] [Google Scholar]
- 24.Story GM, et al. 2003. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112, 819–829. ( 10.1016/S0092-8674(03)00158-2) [DOI] [PubMed] [Google Scholar]
- 25.Saito S, Shingai R. 2006. Evolution of thermoTRP ion channel homologs in vertebrates. Physiol. Genomics 27, 219–230. ( 10.1152/physiolgenomics.00322.2005) [DOI] [PubMed] [Google Scholar]
- 26.Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A. 2007. TRPM8 is required for cold sensation in mice. Neuron 54, 371–378. ( 10.1016/j.neuron.2007.02.024) [DOI] [PubMed] [Google Scholar]
- 27.Kang K, Pulver SR, Panzano VC, Chang EC, Griffith LC, Theobald DL, Garrity PA. 2010. Analysis of Drosophila TRPA1 reveals an ancient origin for human chemical nociception. Nature 464, 597–600. ( 10.1038/nature08848) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng G, Shi X, Kadowaki T. 2015. Evolution of TRP channels inferred by their classification in diverse animal species. Mol. Phylogenet. Evol. 84, 145–157. ( 10.1016/j.ympev.2014.06.016) [DOI] [PubMed] [Google Scholar]
- 29.Jordt S-E, Bautista DM, Chuang H-H, McKemy DD, Zygmunt PM, Högestätt ED, Meng ID, Julius D. 2004. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 427, 260–265. ( 10.1038/nature02282) [DOI] [PubMed] [Google Scholar]
- 30.Vandewauw I, et al. 2018. A TRP channel trio mediates acute noxious heat sensing. Nature 555, 662–666. ( 10.1038/nature26137) [DOI] [PubMed] [Google Scholar]
- 31.Xiao B, Dubin AE, Bursulaya B, Viswanath V, Jegla TJ, Patapoutian A. 2008. Identification of the transmembrane domain five as a critical molecular determinant of menthol sensitivity in mammalian TRPA1 channels. J. Neurosci. 28, 9640–9651. ( 10.1523/JNEUROSCI.2772-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsuura H, Sokabe T, Kohno K, Tominaga M, Kadowaki T. 2009. Evolutionary conservation and changes in insect TRP channels. BMC Evol. Biol. 9, 228 ( 10.1186/1471-2148-9-228) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turner HN, et al. 2016. The TRP channels Pkd2, NompC, and Trpm act in cold-sensing neurons to mediate unique aversive behaviors to noxious cold in Drosophila. Curr. Biol. 26, 3116–3128. ( 10.1016/j.cub.2016.09.038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Himmel NJ, Patel AA, Cox DN. 2017. Invertebrate nociception. In The Oxford research encyclopedia of neuroscience (ed. Katz PS.). Oxford, UK: Oxford University Press; ( 10.1093/acrefore/9780190264086.013.166) [DOI] [Google Scholar]
- 35.Kim SH, Lee Y, Akitake B, Woodward OM, Guggino WB, Montell C. 2010. Drosophila TRPA1 channel mediates chemical avoidance in gustatory receptor neurons. Proc. Natl Acad. Sci. USA 107, 8440 ( 10.1073/pnas.1001425107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neely GG, et al. 2011. TrpA1 regulates thermal nociception in Drosophila. PLoS ONE 6, e24343 ( 10.1371/journal.pone.0024343) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhong L, Bellemer A, Yan H, Honjo K, Robertson J, Hwang RY, Pitt GS, Tracey WD. 2012. Thermosensory and nonthermosensory isoforms of Drosophila melanogaster TRPA1 reveal heat-sensor domains of a ThermoTRP channel. Cell Rep. 1, 43–55. ( 10.1016/j.celrep.2011.11.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peng G, Kashio M, Li T, Dong X, Tominaga M, Kadowaki T. 2016. TRPA1 channels in Drosophila and honey bee ectoparasitic mites share heat sensitivity and temperature-related physiological functions. Front. Physiol. 7, 447 ( 10.3389/fphys.2016.00447) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang X, Li T, Kashio M, Xu Y, Tominaga M, Kadowaki T. 2018. HsTRPA of the red imported fire ant, Solenopsis invicta, functions as a nocisensor and uncovers the evolutionary plasticity of HsTRPA channels. eNeuro 5, ENEURO.0327-0317.2018 ( 10.1523/ENEURO.0327-17.2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gracheva EO, et al. 2010. Molecular basis of infrared detection by snakes. Nature 464, 1006–1011. ( 10.1038/nature08943) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moparthi L, et al. 2016. Human TRPA1 is a heat sensor displaying intrinsic U-shaped thermosensitivity. Sci. Rep. 6, 28763 ( 10.1038/srep28763) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saito CT, Fukuta N, Tominaga M, Saito S, Ohta T, Takahashi K, Banzawa N, Imagawa T. 2014. Heat and noxious chemical sensor, chicken TRPA1, as a target of bird repellents and identification of its structural determinants by multispecies functional comparison. Mol. Biol. Evol. 31, 708–-722. ( 10.1093/molbev/msu001) [DOI] [PubMed] [Google Scholar]
- 43.Saito S, Nakatsuka K, Takahashi K, Fukuta N, Imagawa T, Ohta T, Tominaga M. 2012. Analysis of transient receptor potential ankyrin 1 (TRPA1) in frogs and lizards illuminates both nociceptive heat and chemical sensitivities and coexpression with TRP vanilloid 1 (TRPV1) in ancestral vertebrates. J. Biol. Chem. 287, 30 743–30 754. ( 10.1074/jbc.M112.362194) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sato A, Sokabe T, Kashio M, Yasukochi Y, Tominaga M, Shiomi K. 2014. Embryonic thermosensitive TRPA1 determines transgenerational diapause phenotype of the silkworm, Bombyx mori. Proc. Natl Acad. Sci. USA 111, E1249–E1255. ( 10.1073/pnas.1322134111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang G, Qiu YT, Lu T, Kwon H-W, Pitts RJ, Van Loon JJA, Takken W, Zwiebel LJ. 2009. Anopheles gambiae TRPA1 is a heat-activated channel expressed in thermosensitive sensilla of female antennae. Eur. J. Neurosci. 30, 967–974. ( 10.1111/j.1460-9568.2009.06901.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caspani O, Heppenstall PA. 2009. TRPA1 and cold transduction: an unresolved issue? J. Gen. Physiol. 133, 245–249. ( 10.1085/jgp.200810136) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen J, Kang D, Xu J, Lake M, Hogan JO, Sun C, Walter K, Yao B, Kim D. 2013. Species differences and molecular determinant of TRPA1 cold sensitivity. Nat. Commun. 4, 2501 ( 10.1038/ncomms3501) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saito S, Tominaga M. 2017. Evolutionary tuning of TRPA1 and TRPV1 thermal and chemical sensitivity in vertebrates. Temperature (Austin) 4, 141–152. ( 10.1080/23328940.2017.1315478) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walters ET. 2018. Nociceptive biology of molluscs and arthropods: evolutionary clues about functions and mechanisms potentially related to pain. Front. Physiol. 9, 1049 ( 10.3389/fphys.2018.01049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Im SH, Patel AA, Cox DN, Galko MJ. 2018. Drosophila insulin receptor regulates the persistence of injury-induced nociceptive sensitization. Dis. Model. Mech. 11, dmm034231 ( 10.1242/dmm.034231) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patel AA, Cox DN. 2017. Behavioral and functional assays for investigating mechanisms of noxious cold detection and multimodal sensory processing in Drosophila larvae. Bio Protoc. 7, e2388 ( 10.21769/BioProtoc.2388) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kozma MT, Schmidt M, Ngo-Vu H, Sparks SD, Senatore A, Derby CD. 2018. Chemoreceptor proteins in the Caribbean spiny lobster, Panulirus argus: expression of ionotropic receptors, gustatory receptors, and TRP channels in two chemosensory organs and brain. PLoS ONE 13, e0203935 ( 10.1371/journal.pone.0203935) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shinzato C, et al. 2011. Using the Acropora digitifera genome to understand coral responses to environmental change. Nature 476, 320 ( 10.1038/nature10249) [DOI] [PubMed] [Google Scholar]
- 54.Kudtarkar P, Cameron RA. 2017. Echinobase: an expanding resource for echinoderm genomic information. Database 2017, bax074 ( 10.1093/database/bax074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yuan A, He D, Davidson E, Samanta M, Cameron RA. 2008. SpBase: the sea urchin genome database and web site. Nucleic Acids Res. 37, D750–D754. ( 10.1093/nar/gkn887) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Albertin CB, Simakov O, Mitros T, Wang ZY, Pungor JR, Edsinger-Gonzales E, Brenner S, Ragsdale CW, Rokhsar DS. 2015. The octopus genome and the evolution of cephalopod neural and morphological novelties. Nature 524, 220–224. ( 10.1038/nature14668) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402. ( 10.1093/nar/25.17.3389) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jones P, et al. 2014. InterProScan 5: genome-scale protein function classification. Bioinformatics 30, 1236–1240. ( 10.1093/bioinformatics/btu031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797. ( 10.1093/nar/gkh340) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stecher G, Kumar S, Tamura K. 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874. ( 10.1093/molbev/msw054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Capella-Gutiérrez S, Silla-Martínez JM, Gabaldón T. 2009. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25, 1972–1973. ( 10.1093/bioinformatics/btp348) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schmidt HA, Minh BQ, von Haeseler A, Nguyen L-T. 2014. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 32, 268–274. ( 10.1093/molbev/msu300) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goldman N, Parks SL. 2014. Maximum likelihood inference of small trees in the presence of long branches. Syst. Biol. 63, 798–811. ( 10.1093/sysbio/syu044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kück P, Mayer C, Wägele J-W, Misof B. 2012. Long branch effects distort maximum likelihood phylogenies in simulations despite selection of the correct model. PLoS ONE 7, e36593 ( 10.1371/journal.pone.0036593) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Philippe H, Zhou Y, Brinkmann H, Rodrigue N, Delsuc F. 2005. Heterotachy and long-branch attraction in phylogenetics. BMC Evol. Biol. 5, 50 ( 10.1186/1471-2148-5-50) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huelsenbeck JP, Ronquist F, Nielsen R, Bollback JP. 2001. Bayesian inference of phylogeny and its impact on evolutionary biology. Science 294, 2310–2314. ( 10.1126/science.1065889) [DOI] [PubMed] [Google Scholar]
- 67.Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572–1574. ( 10.1093/bioinformatics/btg180) [DOI] [PubMed] [Google Scholar]
- 68.Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS. 2017. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat. Methods 14, 587–589. ( 10.1038/nmeth.4285) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hoang DT, Vinh LS, Chernomor O, Minh BQ, von Haeseler A. 2017. UFBoot2: improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 35, 518–522. ( 10.1093/molbev/msx281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Letunic I, Bork P. 2016. Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 44, W242–W245. ( 10.1093/nar/gkw290) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thornton JM, Jones DT, Taylor WR. 1992. The rapid generation of mutation data matrices from protein sequences. Bioinformatics 8, 275–282. ( 10.1093/bioinformatics/8.3.275) [DOI] [PubMed] [Google Scholar]
- 72.Lenth RV. 2016. Least-squares means: the R package lsmeans. J. Stat. Softw. 69, 33 ( 10.18637/jss.v069.i01) [DOI] [Google Scholar]
- 73.Hwang RY, Zhong L, Xu Y, Johnson T, Zhang F, Deisseroth K, Tracey WD. 2007. Nociceptive neurons protect Drosophila larvae from parasitoid wasps. Curr. Biol. 17, 2105–2116. ( 10.1016/j.cub.2007.11.029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tracey WD, Wilson RI, Laurent G, Benzer S. 2003. painless, a Drosophila gene essential for nociception. Cell 113, 261–273. ( 10.1016/S0092-8674(03)00272-1) [DOI] [PubMed] [Google Scholar]
- 75.Im SH, Galko MJ. 2012. Pokes, sunburn, and hot sauce: Drosophila as an emerging model for the biology of nociception. Dev. Dyn. 241, 16–26. ( 10.1002/dvdy.22737) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tsubouchi A, Caldwell JC, Tracey WD. 2012. Dendritic filopodia, Ripped Pocket, NOMPC, and NMDARs contribute to the sense of touch in Drosophila larvae. Curr. Biol. 22, 2124–2134. ( 10.1016/j.cub.2012.09.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xiang Y, Yuan Q, Vogt N, Looger LL, Jan LY, Jan YN. 2010. Light-avoidance-mediating photoreceptors tile the Drosophila larval body wall. Nature 468, 921–926. ( 10.1038/nature09576) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yan Z, Zhang W, He Y, Gorczyca D, Xiang Y, Cheng LE, Meltzer S, Jan LY, Jan YN. 2013. Drosophila NOMPC is a mechanotransduction channel subunit for gentle-touch sensation. Nature 493, 221–225. ( 10.1038/nature11685) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fosque BF, et al. 2015. Labeling of active neural circuits in vivo with designed calcium integrators. Science 347, 755–760. ( 10.1126/science.1260922) [DOI] [PubMed] [Google Scholar]
- 80.Iyer EPR, Iyer SC, Sullivan L, Wang D, Meduri R, Graybeal LL, Cox DN. 2013. Functional genomic analyses of two morphologically distinct classes of Drosophila sensory neurons: post-mitotic roles of transcription factors in dendritic patterning. PLoS ONE 8, e72434 ( 10.1371/journal.pone.0072434) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Driscoll K, Stanfield GM, Droste R, Horvitz HR. 2017. Presumptive TRP channel CED-11 promotes cell volume decrease and facilitates degradation of apoptotic cells in Caenorhabditis elegans. Proc. Natl Acad. Sci. USA 114, 8806–8811. ( 10.1073/pnas.1705084114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Arenas OM, Zaharieva EE, Para A, Vásquez-Doorman C, Petersen CP, Gallio M. 2017. Activation of planarian TRPA1 by reactive oxygen species reveals a conserved mechanism for animal nociception. Nat. Neurosci. 20, 1686–1693. ( 10.1038/s41593-017-0005-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chatzigeorgiou M, et al. 2010. Specific roles for DEG/ENaC and TRP channels in touch and thermosensation in C. elegans nociceptors. Nat. Neurosci. 13, 861–868. ( 10.1038/nn.2581) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Doerner JF, Gisselmann G, Hatt H, Wetzel CH. 2007. Transient receptor potential channel A1 is directly gated by calcium ions. J. Biol. Chem. 282, 13 180–13 189. ( 10.1074/jbc.M607849200) [DOI] [PubMed] [Google Scholar]
- 85.Sawada Y, Hosokawa H, Hori A, Matsumura K, Kobayashi S. 2007. Cold sensitivity of recombinant TRPA1 channels. Brain Res. 1160, 39–46. ( 10.1016/j.brainres.2007.05.047) [DOI] [PubMed] [Google Scholar]
- 86.Zurborg S, Yurgionas B, Jira JA, Caspani O, Heppenstall PA. 2007. Direct activation of the ion channel TRPA1 by Ca2+. Nat. Neurosci. 10, 277–279. ( 10.1038/nn1843) [DOI] [PubMed] [Google Scholar]
- 87.Gaudioso C, Hao J, Martin-Eauclaire M-F, Gabriac M, Delmas P. 2012. Menthol pain relief through cumulative inactivation of voltage-gated sodium channels. Pain 153, 473–484. ( 10.1016/j.pain.2011.11.014) [DOI] [PubMed] [Google Scholar]
- 88.Macpherson LJ, Hwang SW, Miyamoto T, Dubin AE, Patapoutian A, Story GM. 2006. More than cool: promiscuous relationships of menthol and other sensory compounds. Mol. Cell. Neurosci. 32, 335–343. ( 10.1016/j.mcn.2006.05.005) [DOI] [PubMed] [Google Scholar]
- 89.Morise M, et al. 2010. Heterologous regulation of anion transporters by menthol in human airway epithelial cells. Eur. J. Pharmacol. 635, 204–211. ( 10.1016/j.ejphar.2010.03.032) [DOI] [PubMed] [Google Scholar]
- 90.Oz M, El Nebrisi EG, Yang K-HS, Howarth FC, Al Kury LT. 2017. Cellular and molecular targets of menthol actions. Front. Pharmacol. 8, 472 ( 10.3389/fphar.2017.00472) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Parnas M, Peters M, Dadon D, Lev S, Vertkin I, Slutsky I, Minke B. 2009. Carvacrol is a novel inhibitor of Drosophila TRPL and mammalian TRPM7 channels. Cell Calcium 45, 300–309. ( 10.1016/j.ceca.2008.11.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Drew BT, Sytsma KJ. 2012. Phylogenetics, biogeography, and staminal evolution in the tribe Mentheae (Lamiaceae). Am. J. Bot. 99, 933–953. ( 10.3732/ajb.1100549) [DOI] [PubMed] [Google Scholar]
- 93.Singh B, Sharma RA. 2015. Plant terpenes: defense responses, phylogenetic analysis, regulation and clinical applications. 3 Biotech 5, 129–151. ( 10.1007/s13205-014-0220-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kühn FJP, Witschas K, Kühn C, Lückhoff A. 2010. Contribution of the S5-pore-S6 domain to the gating characteristics of the cation channels TRPM2 and TRPM8. J. Biol. Chem. 285, 26 806–26 814. ( 10.1074/jbc.M110.109975) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Valentine JW. 2002. Prelude to the Cambrian explosion. Annu. Rev. Earth Planet Sci. 30, 285–306. ( 10.1146/annurev.earth.30.082901.092917) [DOI] [Google Scholar]
- 96.Bailly X, Reichert H, Hartenstein V. 2013. The urbilaterian brain revisited: novel insights into old questions from new flatworm clades. Dev. Genes Evol. 223, 149–157. ( 10.1007/s00427-012-0423-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hejnol A, Martindale MQ. 2008. Acoel development supports a simple planula-like urbilaterian. Phil. Trans. R. Soc. B 363, 1493–1501. ( 10.1098/rstb.2007.2239) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Moroz LL. 2009. On the independent origins of complex brains and neurons. Brain Behav. Evol. 74, 177–190. ( 10.1159/000258665) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Moroz LL. 2012. Phylogenomics meets neuroscience: how many times might complex brains have evolved? Acta Biol. Hung. 63(Suppl 2), 3–19. ( 10.1556/ABiol.63.2012.Suppl.2.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Northcutt RG. 2010. Cladistic analysis reveals brainless urbilateria. Brain Behav. Evol. 76, 1–2. ( 10.1159/000316443) [DOI] [PubMed] [Google Scholar]
- 101.Schulz-Bohm K, Martín-Sánchez L, Garbeva P. 2017. Microbial volatiles: small molecules with an important role in intra- and inter-kingdom interactions. Front. Microbiol. 8, 2484 ( 10.3389/fmicb.2017.02484) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schmidt R, et al. 2017. Fungal volatile compounds induce production of the secondary metabolite Sodorifen in Serratia plymuthica PRI-2C. Sci. Rep. 7, 862 ( 10.1038/s41598-017-00893-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Microarray data are available at NCBI Gene Expression Omnibus (accession number GSE46154; https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE46154). All other datasets supporting this article have been provided as part of the electronic supplementary material.