Abstract
Chemically induced nociception has not yet been studied intensively in genetically tractable models. Hence, our goal was to establish a Drosophila assay that can be used to study the cellular and molecular/genetic bases of chemically induced nociception. Drosophila larvae exposed to increasing concentrations of hydrochloric acid (HCl) produced an increasingly intense aversive rolling response. HCl (0.5%) was subthreshold and provoked no response. All classes of peripheral multidendritic (md) sensory neurons (classes I–IV) are required for full responsiveness to acid, with class IV making the largest contribution. At the cellular level, classes IV, III and I showed increases in calcium following acid exposure. In the central nervous system, Basin-4 second-order neurons are the key regulators of chemically induced nociception, with a slight contribution from other types. Finally, chemical nociception can be sensitized by tissue damage. Subthreshold HCl provoked chemical allodynia in larvae 4 h after physical puncture wounding. Pinch wounding and UV irradiation, which do not compromise the cuticle, did not cause chemical allodynia. In sum, we developed a novel assay to study chemically induced nociception in Drosophila larvae. This assay, combined with the high genetic resolving power of Drosophila, should improve our basic understanding of fundamental mechanisms of chemical nociception.
This article is part of the Theo Murphy meeting issue ‘Evolution of mechanisms and behaviour important for pain’.
Keywords: Drosophila, chemical nociception, sensory neurons, Basin interneurons, sensitization, allodynia
1. Introduction
All animals can discriminate noxious environmental stimuli that can potentially induce tissue damage. This nociceptive sensory capacity is crucial for health and survival. Noxious thermal, mechanical or chemical stimuli elicit specific escape behaviours designed to avoid the potentially damaging stimulus [1]. Drosophila larvae display different nociceptive responses based on the stimulus presented. Noxious heat and harsh mechanical stimuli induce bending [2], rolling and fast crawling behaviours [3,4], with rolling representing the dominant response. Conversely, noxious cold provokes a different behaviour, contraction of the head and tail towards the middle of the body [5]. Assays that measure aversive behaviours are helping to dissect the fundamental mechanisms of thermal [4–6] and mechanical nociception [7–9].
Chemical nociception has been relatively understudied in most organisms. In adult flies TRPA1, an evolutionarily conserved cation channel expressed in gustatory neurons, mediates chemical avoidance to noxious compounds such as aristolochic acid [10], allyl isothiocyanate (AITC, the noxious compound found in wasabi) and benzyl isothiocyanate (BITC) [11], N-methyl maleimide (NMM) and cinnamaldehyde (CA) [12]. These studies have measured proboscis extension to potential food sources rather than whole-animal aversion to chemicals. As such they examine an intersection between gustation (taste) and chemical nociception. Assays that probe responses that might be evoked by contact of noxious chemicals with the skin barrier have not yet been developed in Drosophila. What kind of aversive response might result from exposure to noxious chemical(s) is currently unknown.
Drosophila larvae have two major types of peripheral sensory neurons, type I and type II [13]. Type I neurons have a single ciliated dendrite that mediates mechanosensory functions such as light touch [14]. Type II neurons, also called multidendritic (md) neurons, exhibit elaborate dendritic projections that cover the barrier epidermis [15]. The md neurons comprise four classes (classes I–IV) based on their peripheral arbour complexity [16]. Class I neurons have been implicated in locomotion and proprioception [17–20]. Both class II and class III have been implicated in light-touch responses, with class III playing the major role [21,22]. Cold nociception is also mediated by class III neurons [5]. Class IV neurons are multimodal nociceptors, implicated in behavioural responses to noxious heat [4], harsh mechanical stimuli [9] and noxious low wavelength light [23]. At present, it is unknown which, if any, peripheral sensory neurons have a role in chemical nociception.
Second-order interneurons located in the ventral nerve cord receive synaptic input from the various classes of md sensory neurons [24]. These second-order interneurons likely allow integration of different sensory stimuli [2,7,25–27]. Basin interneurons (Basins 1–4) were the first identified interneurons implicated in multisensory integration and noxious responses [25,26]. Basin-1 mediates mechanosensory responses (vibration), while Basin-4 regulates thermal nociceptive responses via Goro neurons [26]. Other classes of second-order neurons, including medial clusters of class IV dendrite arborization (C4 da) second-order interneurons (mCSIs) [27], Down-and-Back (DnB) interneurons [2], dorsal pair (DP) insulin-like peptide 7 (ilp7) producing neurons (DP-ilp7 neurons) [7] and A08n neurons [7,28], also regulate nocifensive behavioural responses in Drosophila larvae. The roles, if any, of these different interneurons in chemical nociception have not yet been elucidated.
Tissue injury typically causes nociceptive sensitization [29]. For instance, UV exposure in Drosophila larvae sensitizes nociceptive sensory neurons to an innocuous thermal stimulus (thermal allodynia) [30]. This assay has helped to identify several signalling pathways mediating thermal allodynia, including TNF [30,31], Hedgehog [32] and tachykinin signalling [33]. In vertebrates, tissue damage can also cause hypersensitivity to chemical stimuli. A classic example would be the sting resulting from lemon juice when it encounters an open cut on a cook's hand. Whether chemical nociceptive responses in Drosophila can be sensitized by tissue injury remains an open question.
2. Results
(a). Development of a novel assay for chemical nociception in Drosophila larvae
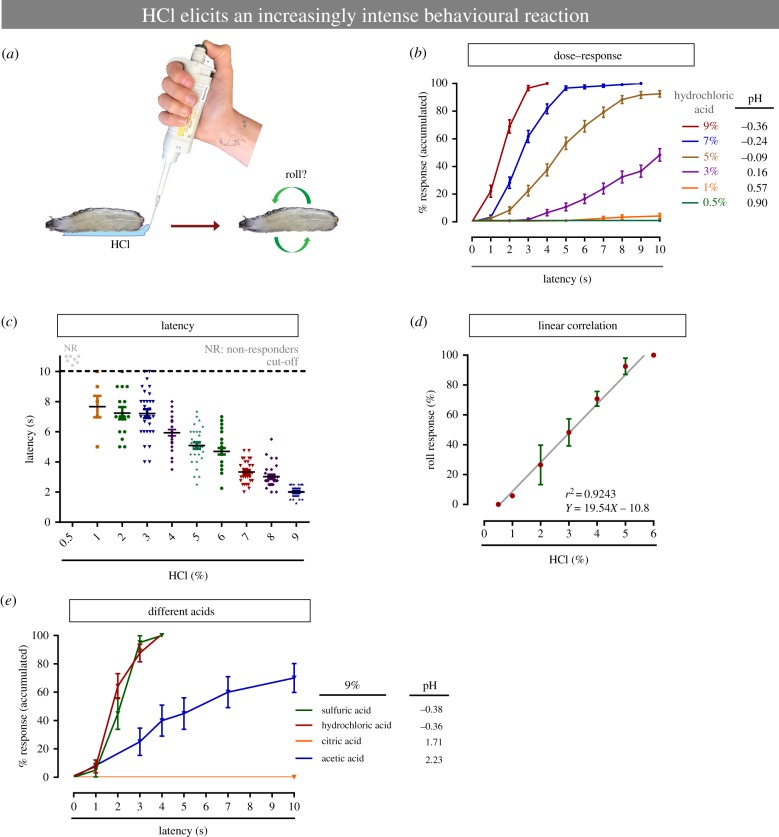
To study chemical nociception in Drosophila larvae we developed a new behavioural assay. In this procedure, Drosophila larvae are briefly exposed to HCl, a presumably noxious acid (figure 1a). Concentrations below 0.5% HCl did not elicit responses that differed from normal locomotion (electronic supplementary material, movies S1 and S2); thus this concentration can be considered as subthreshold. Increasing the concentration of HCl (from 1 to 9%) caused aversive rolling, a behaviour that is also seen with exposure to noxious heat and mechanical stimuli [3,4,9] (electronic supplementary material, movies S3 and S4). This nociceptive behaviour was more prevalent as the acid concentration was increased (figure 1b) and the latency (time to rolling after HCl application) decreased dramatically with increasing acid concentration (figure 1c). The relationship between HCl concentration and behavioural responsiveness is linear (figure 1d). We also asked if other acids induce nociceptive behaviour at similar concentrations to HCl. Sulfuric acid induced a per cent of rolling response (100%) similar to HCl, while acetic acid was less potent (figure 1e). Among acids with slightly higher pH values, citric acid (pH 1.71) did not elicit any nociceptive behavioural responses, whereas acetic acid (pH 2.23) did (figure 1e). Larvae of different developmental stages did not show significant differences in nociceptive responsiveness to acid (p = 0.4667). However, larvae at the latest developmental stage (late third instar) did show a slight delay in the behavioural response when compared with the early developmental stages (electronic supplementary material, figure S1A). Together these data introduce and describe a new assay to measure chemical nociceptive behaviour in Drosophila larvae.
Figure 1.
Development of a novel chemical nociception assay in Drosophila larvae. (a) Schematic cartoon of chemical nociception assay. (b) Behavioural aversive rolling response versus increasing chemical stimulus concentrations. (c) Latency of the behavioural response. (d) Relationship between chemical concentration and nociceptive behaviour. (e) Behavioural aversive rolling response to different acids. (b–d) n = 30 larvae and repeated three times. (e) 20–30 larvae. Error bars represent mean ± s.e.
(b). Noxious chemical stimulation induces cellular stress and tissue damage
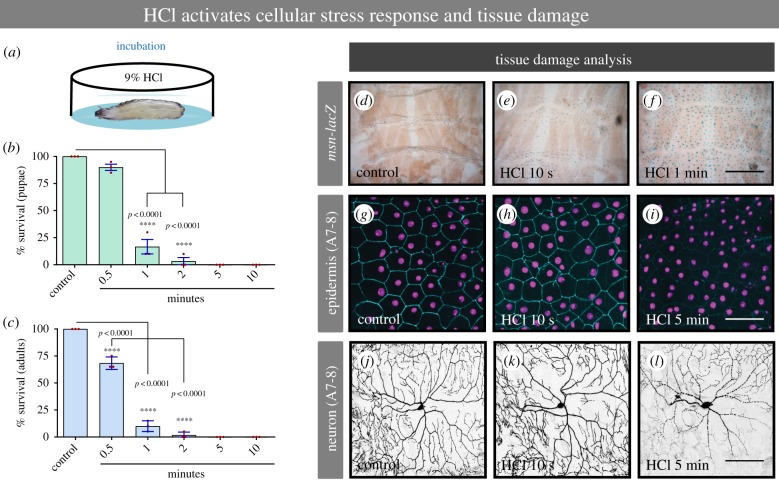
Noxious stimuli evoke an aversive behavioural reaction presumably because they cause tissue damage and/or adversely affect survival. We tested whether exposure to acid is noxious by these criteria. Exposure to a high concentration of HCl (figure 2a, 9%) for increasing times dramatically decreased the survival rate of larvae to the pupal and adult stages (figure 2b,c). We next examined whether HCl exposure caused a cell stress response in third instar larvae. We exposed larvae to 9% HCl for either 10 s or 1 min and examined activation of msn-lacZ, a reporter of Jun N-terminal kinase (JNK) signalling/cellular stress [34]. We observed a modest induction of JNK activity in the barrier epidermal sheet upon brief exposure to 9% HCl when compared with the control larvae (figure 2d–f). At the cellular level, reduction of an epidermal membrane marker (figure 2g–i) and blebbing along the neuronal dendritic branches of class IV nociceptive sensory neurons were also observed (figure 2j–l). These changes were a function of both time of acid exposure and proximity to the posterior location of initial acid exposure. The more posterior abdominal segments (A7-A8) were affected more severely than more anterior segments further from the initial exposure to acid (A4-A5; electronic supplementary material, figure S2A–F). Together, our results suggest that HCl exposure is noxious, as defined by a decrease in survival and qualitative indicators of tissue damage, and that the level of noxiousness is determined by time of exposure and proximity to the source of exposure.
Figure 2.
Noxious chemical stimulus induces cellular stress response and tissue damage. (a) Cartoon depicting the larvae incubation in 9% HCl. (b,c) Larval and adult survival per cent in response to increasing time of incubation with 9% HCl. (d–f) Epidermal cell stress response induced by 9% HCl. (g–i) Epidermal tissue damage analysis in response to noxious acid. (j–l) Class IV sensory neuronal tissue damage in response to noxious acid. A7-8: Abdominal segments 7 or 8. msn-lacZ: reporter of Jun N-terminal kinase (JNK) signalling/cellular stress. (b,c) n = 60 for each condition. Error bars represent mean ± s.e. Statistical significance was determined via one-way ANOVA with a Dunnet's post hoc test. (d–f) n = 4 larvae for each condition; (g–l) n = 9 larvae for each condition and representative images are shown. Bar in d–f, 50 µm. Bar in g–l, 100 µm.
(c). Multiple classes of multidentritic sensory neurons mediate chemical nociception responses
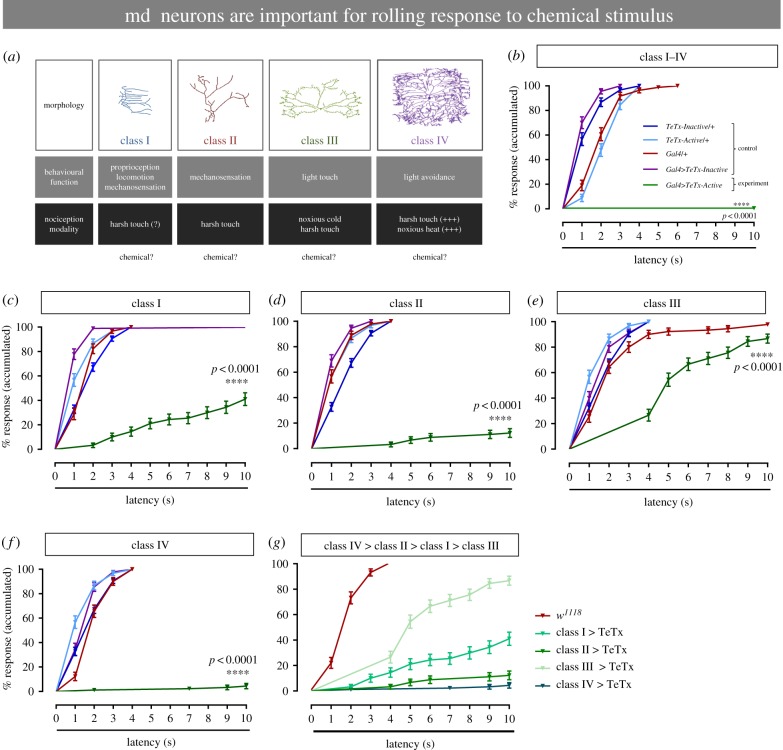
The different classes of md sensory neurons (I–IV) that tile the larval barrier epidermis sense several sensory modalities, including heat, light touch, cold and harsh touch (figure 3a). Using a genetic inactivation strategy, we next asked whether md sensory neurons mediate chemical nociception. We combined a pan-md sensory neuron-specific Gal4 driver (md-Gal4) with a transgene encoding an active tetanus toxin (UAS-TeTxLC = UAS-TeTx-Active) or an inactive toxin control (UAS-IMP TNT VI-A = UAS-TeTx-Inactive) [35]. Expression of tetanus toxin in all md sensory neurons completely abrogated the response to a noxious chemical stimulus compared with relevant genetic controls (figure 3b). This suggests that one or more type of md sensory neuron class mediates chemical nociception.
Figure 3.
Multidentritic sensory neurons mediate chemical behavioural nociception responses. (a) Cartoon depicting the morphology, behavioural function, and nociceptive modality of the different peripheral sensory neurons. (b–g) Behavioural response to noxious chemical stimulus (HCl 9%). (b) Silencing of all multidendritic sensory neurons, by blocking the synaptic output using tetanus neurotoxin. Silencing specific sensory neurons using tetanus neurotoxin in class I (c), class II (d), class III (e) and class IV (f). (g) Comparison of the degree of the silencing effect in the md sensory neurons (classes I–IV). The genotype colour-code used in (b) applies for (b–f). (b–f) n = 90 for each condition, data from three independent replicates of at least 30 larvae each. Error bars represent mean ± s.e. Log-rank (Mantel–Cox) test was used to determine statistical significance between different groups.
We next silenced each class of md sensory neuron (I–IV) individually by combining sensory neuron class-specific Gal4 drivers with the active and inactive tetanus toxins used above. Since aversive rolling is seen with heat, harsh touch and chemical exposure we suspected that class IV sensory neurons might also play a role in chemical nociception. Surprisingly, silencing class I, class II, and to a lesser degree class III sensory neurons substantially attenuated chemical nociception (figure 3c–e). However, the strongest block of chemical nociception, as expected from the rolling behaviour, was seen with silencing class IV neurons (figure 3f). The strength of the nociceptive defect observed upon silencing each sensory neuron was class IV > class II > class I > class III (figure 3g). A separate class of peripheral sensory neurons, chordotonal neurons [16], had a minor effect on chemical nociceptive responses (electronic supplementary material, figure S3). Taken together, these data indicate that there is a distributed requirement for acid-evoked rolling in Drosophila larvae, with multiple md sensory neuron classes playing a role, either directly or indirectly, in the behavioural response.
(d). Acid activates class IV nociceptive neurons
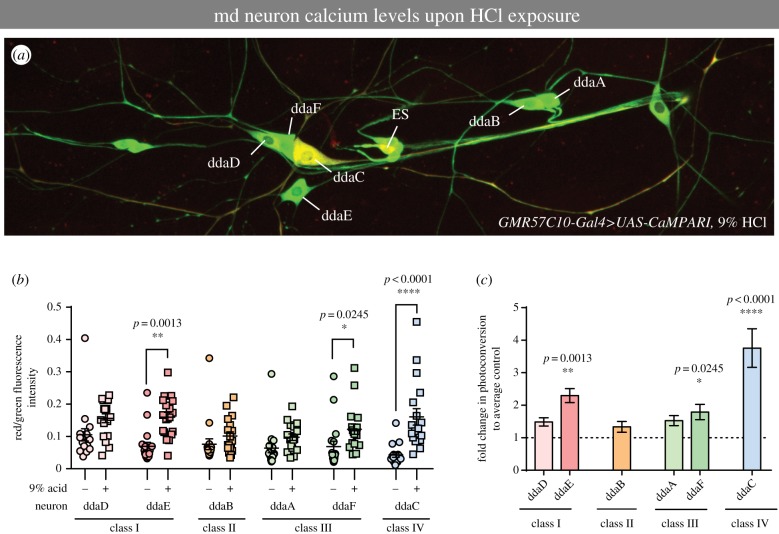
As multiple classes of md sensory neurons exhibit differing contributions to HCl-induced chemical nociception, we next sought to assess how HCl exposure influenced neural activity in these neurons. Freely behaving third instar larvae expressing the genetically encoded calcium indicator CaMPARI [36] were subjected to treatment with 9% HCl and immediately analysed post hoc for neural activation in md neuron subclasses. As inactivation of md neurons was sufficient to inhibit acid-evoked rolling behaviour, we focused our analyses on CaMPARI responses in md neuron subclasses. Visually, this analysis revealed strong HCl-induced activation of class IV (dorsal dendritic arborization neuron C, ddaC) nociceptive neurons relative to mock-treated controls (figure 4a). When we quantified red/green fluorescence ratios in representative images, we observed HCl-induced activation of a class IV neuron (ddaC) and to a lesser extent a subset of class I (ddaE) and class III (ddaF) md neurons (figure 4b,c). No significant activation, relative to controls, was observed for the class II (ddaB) neurons (figure 4b,c), although there was an upward trend in CaMPARI photoconverted signal for all md neurons. These data support a primary sensory role for class IV neurons in chemical nociception.
Figure 4.
Acid activates class IV nociceptive neurons. (a) Representative image of CaMPARI photoconversion in the larval dorsal cluster of md sensory neurons following treatment with 9% HCl and violet light exposure. The class IV ddaC neuron visibly exhibits the highest level of red fluorescence relative to other md neurons subclasses/subtypes (ddaA/B/D/E/F). External sensory neuron (ES) also displayed relatively high fluorescence levels. (b) Red/green CaMPARI fluorescence intensity quantification for each md neuron class/subtype in control mock-treated (no HCl) or 9% HCl-treated third instar larvae. Each circle/square represents an individual neuron of the specified class. (c) Fold difference in green-to-red CaMPARI photoconversion by md neuron class/subtype when compared with mean vehicle control. (b,c) Data are means ± s.e.; n = 18 neurons; asterisks (*) indicates significant difference in CaMPARI photoconversion when compared with mock-treated vehicle control. Statistical significance between vehicle control and acid groups was determined via Kruskal–Wallis with Dunn's multiple comparisons test.
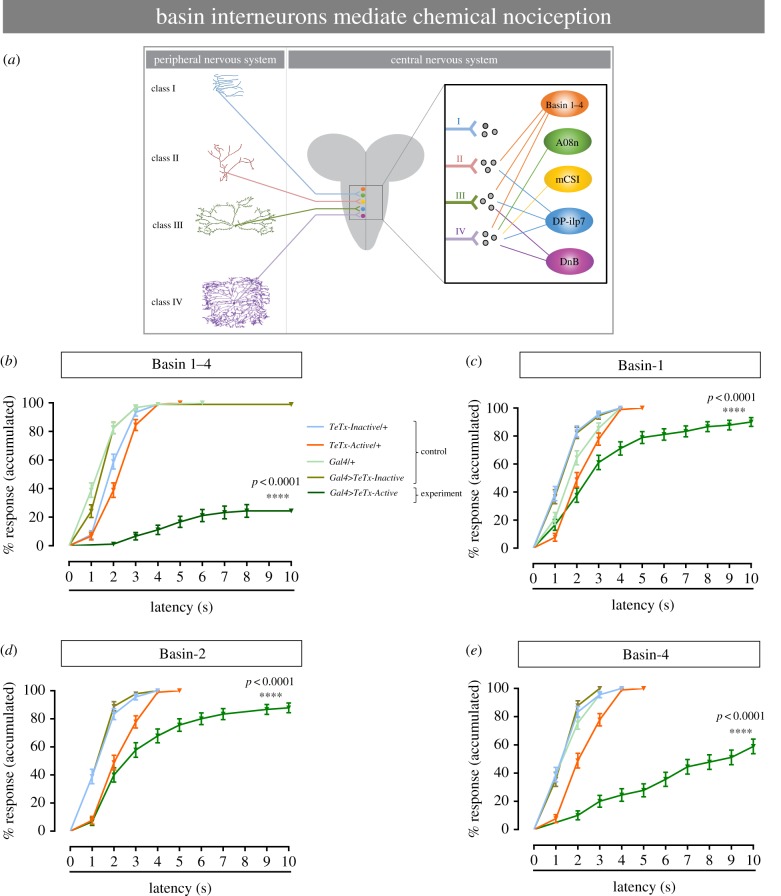
(e). Basin interneurons mediate chemical nociception
Recent investigations have identified multiple interneurons that receive input from class IV sensory neurons and help mediate Drosophila larval nocifensive escape behaviours in response to noxious thermal and mechanical stimuli [2,7,26–28] (figure 5a). We next asked whether any of these interneurons (Basins, Goro, mCSI, DnB and A08n) also play a role in chemical nociception. We silenced each of these interneurons using neuron-specific Gal4 drivers (Basin-Gal4, Goro-Gal4, mCSI-Gal4, DnB-Gal4 and A08n-Gal4) and the same tetanus toxin transgenes used above to silence peripheral sensory neurons. Ectopic expression of the active form of tetanus toxin transgene (UAS-TeTx-Active) in all Basin interneurons drastically reduced Drosophila larvae responses to 9% HCl, compared with the control larvae (figure 5b). This suggests that basin multisensory interneurons are required for the rolling behaviour response to chemical stimulus.
Figure 5.
Second-order interneurons mediate chemical nociception response. (a) Schematic of the different peripheral sensory neurons and interneurons located in the ventral nerve cord. Adapted from Chin & Tracey [24]. (b–e) Behavioural response to noxious chemical stimulus (HCl 9%). (b) Silencing all Basin interneurons (Basin-1, Basin-2, Basin-3 and Basin-4) by blocking the synaptic output using tetanus neurotoxin. Silencing specific Basin second-order interneurons by using tetanus neurotoxin: (c) Basin-1, (d) Basin-2 and (e) Basin-4. The genotype colour-code used in (b) applies for (b–e). (b–e) n = 90 for each group, data from three independent replicates of 30 larvae each. Error bars represent mean ± s.e. Log-rank test was used to determine statistical significance between different groups.
Basin interneurons comprise four neuron types of basin-shaped arbours (Basins 1–4), so we asked which of these interneuron types mediate chemical nociception using the same class-specific strategy we used for the sensory neurons, where class-specific drivers were available. When Basin-1 or Basin-2 interneurons were individually silenced, the nociceptive behavioural response to noxious chemical stimulation was partially decreased (figure 5c,d). A more significant decrease was observed when only Basin-4 neurons were silenced (figure 5e). The other interneuron classes (mCSIs, A08n and DnB) and neurons further in the nociceptive circuit (Goro) all displayed partial contributions to chemical nociception (electronic supplementary material, figures S4A–F), none of which was as strong as that observed by silencing Basins. Taken together, Basin interneurons, especially Basin-4 interneurons, appear to mediate chemical nociception.
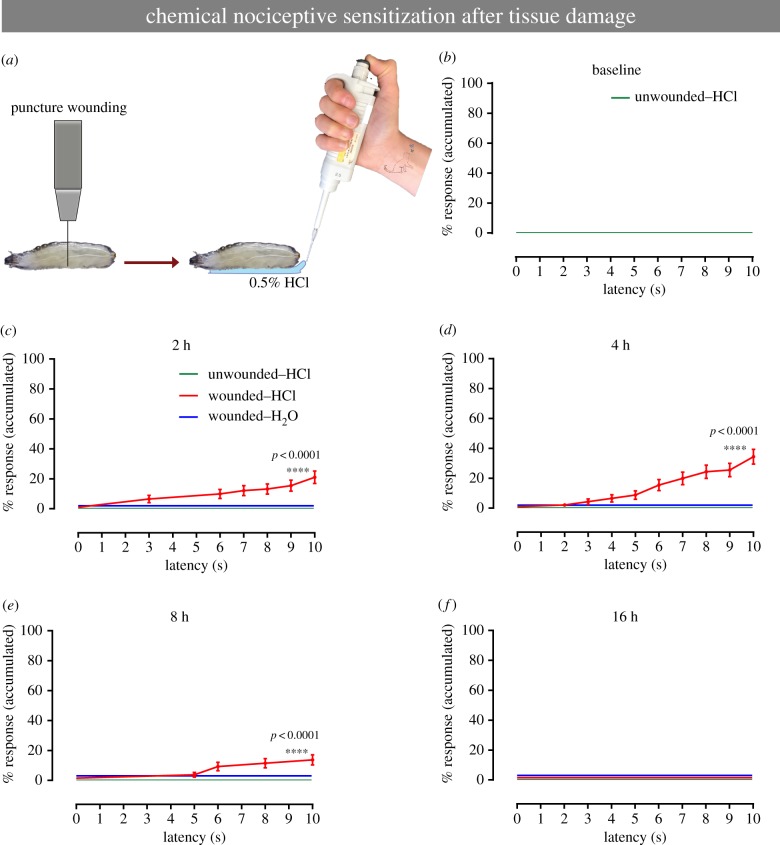
(f). Possible sensitization of chemical nociceptive responses by physical injury
A hallmark of nociceptive behaviours is that they can be sensitized by tissue injury, and this occurs with responses to both noxious heat [30] and noxious cold [37] in Drosophila larvae. Thus, we examined whether aversive rolling to a subthreshold concentration of HCl (0.5%), which would constitute chemical allodynia, could be observed in larvae. The classical sensitizing injury used in larvae to date is UV irradiation, which causes epidermal death and morphological disruption [30]. Very little responsiveness to subthreshold HCl was observed after this injury (electronic supplementary material, figure S5A,B). We reasoned that sensitization to chemical stimuli might require an injury that breaches the cuticle so we tried puncture wounding [34] (figure 6a). Compared with uninjured larvae (figure 6b), wounded larvae exhibited the emergence of an aversive rolling response at 2–4 h after injury (figure 6c,d). This acute hypersensitivity was short-lived, as it faded by 8 h post-injury (figure 6e) and was gone by 16 h post-injury (figure 6f). A breach in the cuticle appears to be important for the emergence of sensitization, as pinch wounding (which creates a larger wound but leaves the cuticle intact) [38] did not elicit chemical allodynia (electronic supplementary material, figure S5C–F). Taken together these results indicate that certain types of physical injuries that breach the cuticle may alter cuticle permeability in a manner that enhances the larval nociceptive response to a normally subthreshold concentration of HCl.
Figure 6.
Chemical sensitization induced by tissue damage. (a) Schematic of chemical sensitization model. After the tissue damage, induced by puncture wound, animals were treated with non-noxious stimulus. (b–f) Behavioural response to non-noxious chemical stimulus (HCl 0.5%). (b) Baseline behavioural response of the animals to a benign solution, MilliQ water. (c–g) Behavioural response of the animals to non-noxious chemical stimulus, at different time points after puncture wounding: (c) 2 h, (d) 4 h, (e) 8 h and (f) 16 h. Colour-coding of groups used in (c) applies for (c–f). (b–f) n = 90 for each group, data from three independent replicates of 30 larvae each. Error bars represent mean ± s.e. Log-rank test was used to determine statistical significance between different groups.
3. Discussion
Chemical nociception is arguably the least-studied noxious sensory modality to date in Drosophila. Ingestion (or potential ingestion) of noxious tastants such as AITC [12,39], aristolochic acid [10], cinnemaldehyde [12], l-canavanine [40,41] and acetic acid [42] reduces proboscis extension in Drosophila adults. Likewise, a volatile repellent, citronellal, induces an avoidance behaviour by activating olfactory receptor neurons [43]. These studies have identified a transient receptor potential (TRP) ion channel (TrpA1) and gustatory receptors that are required for inhibition of the proboscis extension response to potential food [10–12,39].
Our interest here was to develop an assay that challenges the barrier of the organism with a simple noxious chemical (in this case acid) and that does not necessarily have crosstalk with gustatory responses. Below, we discuss some of the implications of our findings with this new assay and their potential utility.
The md neuron(s) required for the full behavioural response to acid are distinct from larval gustatory neurons [44] and have not previously been implicated in taste. That md neurons are required for responses to acid is suggested by the fact that the observed behaviour (aversive rolling) is similar to that observed with noxious heat [4] and noxious mechanical stimuli [4,9]. Indeed, genetic silencing of class IV md neurons, which are also responsible for aversive rolling in response to temperatures above 40°C [4,30] and to harsh touch [4,8,9], led to a near-complete block of aversive rolling behaviour, suggesting that gustatory neurons have no role in the response to acid applied to the larval posterior. Surprisingly, similar class-specific silencing experiments revealed that all peripheral md neurons (class IV > class II > class I > class III) were required to some extent for acid-evoked rolling—not just the expected class IV neurons. Fast-rolling is only observed with optogenetic activation of class IV neurons [3,5], while optogenetic activation of classes I, II and III lead to other behaviours (halting or contraction/C-bend/slow-rolling) [5,7]. Our results add to the diverse array of modalities that can elicit rolling from class IV neurons (heat, harsh touch, noxious light) and raise questions about how the other classes are involved.
Our CaMPARI calcium imaging experiments indicated that class I and class III neurons were also activated by acid exposure, whereas class II was not. Because our genetic silencing experiments indicate that class II and III md neurons are also required for acid-evoked behaviour, these data suggest that class II and III neurons may facilitate rolling behaviour through downstream circuitry, rather than by direct activation by acid exposure. This seems possible as multiple classes of downstream second-order neurons were at least partially required for acid-evoked rolling. Furthermore, recent studies have demonstrated that class II, III and IV neurons share common postsynaptic interneuron partners implicated in rolling behaviour. For example, class II and IV neurons connect to DP-ilp7 interneurons to facilitate rolling in response to mechano-nociceptive stimuli [7], while class II, III and IV neurons connect to Basins [26]. These second-order neurons have been implicated in responses to other noxious sensory modalities [2,7,26], and, as we determined in this work, Basin 1–4 interneurons (mainly Basin-4) also mediate chemical nociception, suggesting these interneurons are multisensory integrators [25,26]. It will be interesting to determine how the full circuit(s) differ when a larva is challenged with particular stimuli.
In Drosophila thermal nociceptive sensitization has thus far been studied most extensively. Following UV irradiation both thermal allodynia (emergent responses to subthreshold stimuli) and thermal hyperalgesia (intensified responses to noxious stimuli) are observed [30]. By contrast, UV irradiation causes a behavioural switch from contraction to head and tail raising in response to noxious cold [37]. Curiously, neither UV irradiation nor physical pinch wounding (an injury that causes extensive damage to underlying sensory neurons) evoked chemical allodynia. Puncture wounding, however, a procedure that creates a breach in the overlying cuticle barrier, caused a transient (2–4 h) and relatively mild hypersensitivity to subthreshold acid. Whether the observed behavioural hypersensitivity represents a change in cuticle permeability that increases exposure to exogenously applied acid or is the result of specific damage-induced signalling pathways that alter neuronal firing is not yet clear. Moving forward, it will be interesting to test the involvement of previously identified thermal acute sensitization pathways [30,32,33], insulin signalling, which regulates the duration of thermal nociceptive sensitization [45], and Pvr signalling, which sets the threshold of mechanical nociception [46].
For humans, chemical nociception may be of less importance than the thermal or mechanical modalities as exposure to relevant environmental stimuli—sharp objects or extreme temperatures—are substantially more common than exposure to noxious acid. That said, local release of protons nearly always accompanies tissue damage, and thus acid-sensing may be a general feature of diverse injury-induced sensory responses [47]. Chemical nociception may be more important for Drosophila larvae as they may encounter relevant concentrations of acids, alcohols and other noxious chemicals as they feed. Although environmental exposure to harsh acids may not be common for fly larvae, the apparent pH threshold for responsiveness may be in the range that larvae experience in rotting or overcrowded fruit. The assay developed here has a simple practical advantage over thermal nociception assays [4,30] developed to date. Both thermal assays (hot and cold) require specialized tools capable of delivering a precise set temperatures to the larva. This can lead to some user-to-user variability in dose–response curves [30,31,33,48] and requires user expertise. Mechanical nociceptive assays developed to date [8,9] require a similar user competence. Chemical nociception is substantially easier to assess as it simply involves pipetting a small volume of solution on the larva and observing the resulting behaviour. Drosophila are well-suited for gene discovery [49] and the development of this chemical nociception assay enables future efforts to unravel the molecular/genetic basis of this understudied sensory modality.
4. Experimental procedures
(a). Drosophila stocks and genetics
All fly stocks used in this work were maintained on cornmeal medium at 18°C. Larval progeny used in behavioural experiments were raised at 25°C. w1118 was used as a control strain. msn-lacZ (l(3)06946) was used to monitor JNK activation [34]. The GAL4/UAS system was used to drive tissue-specific gene expression of transgenes under UAS control [50]. The following Gal4 lines were used: Gal42-21 (class I) [51], GMR37B02-Gal4 (class II) [52], 19-12-Gal4 (class III) [23], ppk1.9-Gal4 (class IV) [53], md-Gal4109(2)80 (class I–IV) [54], chordotonal neurons ch-Gal4 (BL59949) [55], Basin-Gal4 interneuron drivers (Basin 1–4, GMR72F11-Gal4; Basin-1, JRC-R20B01-Gal4; Basin-2, RC-SS00739-Gal4; and Basin-4, JRC-SS00740-Gal4); Goro interneurons (R72F11-Gal4) [26], mCSIs interneurons (R94B10-Gal4, referred to here as mCSI-Gal4-1, and R52F05-Gal4, referred to here as mCSI-Gal4-2): [27], A08n-Gal4 interneurons (GMR82E12-Gal4) [7], DnB interneurons (412-Gal4, referred to here as DnB-Gal4-1, and 4051-Gal4, referred to here as DnB-Gal4-2) [2]. GMR57C10-Gal4 (pan-neuronal) and Goro neuron-Gal4 (GMR69F06-Gal4) were obtained from Bloomington (BL39171 and BL39497, respectively). e22c-Gal4, UAS-DsRed2Nuc(21),FasIII-GFP [56] and ppk-Gal4, UAS-mCD8-GFP were used to label larval epidermal membranes and the class IV sensory neurons, respectively. The following UAS transgenes were used: UAS-TeTxLC (UAS-TeTx-Active) and UAS-IMP TNT VI-A (UAS-TeTx-Inactive) [35]; UAS-CaMPARI [36]. See electronic supplementary material, table S1 for a list of genotypes of flies used in each figure/panel.
(b). Chemical nociception assay
A concentrated HCl stock solution (Sigma: 37.5–38.5%) was first diluted to 10% (assuming the stock concentration was 37%). The 10% stock was further diluted with MilliQ water to generate HCl solutions ranging from 0.5 to 9%. All of the other acids: sulfuric acid (vol/vol %), acetic acid (vol/vol %), and citric acid (wt/vol %) were used at 9% concentration. A pH meter (Accumet Basic, Fisher Scientific) was used to measure the pH, at room temperature, of the different acid solutions. Individual mid-third instar Drosophila larvae, crawling freely on 48-well cell culture plate covers made of polystyrene, were exposed to a particular solution by pipetting 1.5 µl of an HCl dilution (or MilliQ water for control) to the posterior end of the larva. Larvae were allowed to explore the dish for about 10 s before challenging them with the chemical stimulus. Larvae that reached the dish wall were redirected to the centre, before applying the chemical stimulus. If the pipette tip contacted the larva, that particular larva's behavioural response was not included to safeguard against the response being a combination of touch and acid. A complete roll of 360° along the body axis within 10 s of HCl exposure was scored as aversive behaviour. Other responses (fast crawling, turning, and bending) were not categorized as aversive responses for the purpose of this assay. When animals were exposed to a non-noxious or benign solution stimulus, MilliQ water, they did not elicit any reactions distinct from normal locomotion or light touch. Each larva was assessed behaviourally only once. The experimenter was blind to the genotype of the larva being tested. Three independent trials of 30 larvae each were performed unless otherwise indicated.
Behavioural data generated were plotted as the accumulated per cent (%) of response on the Y-axis versus latency (0 to 10 s) on the X-axis. A 100% response means all larvae exhibited an aversive roll (at some latency under the 10 s cut-off) and a 0% response means none exhibited an aversive roll at any latency under the 10 s cut-off. This method of plotting the frequency distribution of responses generates curves that are similar to lifespan curves for survival data and allows comparison of how many total larvae responded, how many did not respond, and how many responded at each particular latency. The log-rank Mantel–Cox test was used to statistically compare any curves plotted in this manner.
(c). Immunofluorescence
To evaluate epidermal and neuronal tissue damage induced by HCl, third instar epidermal (e22c-Gal4, UAS-DsRed2Nuc,FasIII-GFP) and neuronal (ppk-Gal4, UAS-mCD8-GFP) reporter larvae were submerged in 100 µl of 9% HCl, anaesthetized with ether (ethyl ether anhydrous, Fisher Scientific), dissected in ice-cold phosphate-buffered saline (PBS), and then fixed for 1 h in 4% formaldehyde (FA). After several washes in PBS-Tx (1× phosphate-buffered saline with 0.3% Triton X-100) the samples were incubated overnight at 4°C with primary antibodies: mouse anti-GFP (1 : 500) for neurons; mouse anti-FasIII (1 : 50) and rabbit anti-DsRed (1 : 1000) for epidermal cells. Secondary antibodies (applied for 24 h at 4°C) were Alexa 647 anti-mouse (1 : 500) for neuronal samples and Alexa 488 anti-rabbit (1 : 500) and Alexa 647 anti-mouse (1 : 500) for epidermal tissues. After final washes in PBS-Tx, all stained samples were mounted in Vectashield (Vector Laboratories) and observed (see below).
(d). β-Galactosidase histochemistry
Larvae carrying msn-lacZ were submerged completely in 100 µl of 9% HCl or MilliQ water for either 10 s or 1 min, incubated for 4 h at 25°C, dissected in ice-cold PBS, fixed for 30 min at room temperature with cold 2% glutaraldehyde, rinsed with PBS, and then stained at 30°C for 30–45 min with X-Gal (5-bromo-4-chloro-3-indolyl-d-galactopyranoside) as described [34].
(e). Confocal microscopy and stereomicroscopy
Larvae were imaged on an Olympus FV1000 confocal microscope and Fluoview software was used to obtain the images. Laser wavelengths were 488 nm (Green Fluorescent Protein, GFP), and 543 nm (Far Red Fluorescent Protein). Images were captured at a resolution of 1024 × 1024 pixels for tissue damage experiments using a 20× numerical aperture (NA) 0.7 dry objective lens at 1.4× zoom. A Z-series stack, step-size of 1.5 µm, was collected and processed into a single Z-projection. Identical settings for laser intensity and other image capture parameters were applied for comparison of staining in the control and experimental groups. All figures were assembled with Photoshop CS6 and Illustrator CS6 (Adobe).
(f). CaMPARI calcium analyses
For CaMPARI experiments, freely behaving third instar larvae expressing UAS-CaMPARI under the control of GMR57C10-Gal4 were subjected to photoconverting light in the presence or absence (mock) of 9% HCl. Once larval locomotion initiated, 1.5 µl of either vehicle or 9% HCl was applied via micropipette to the posterior portion of the animal, and the subject was simultaneously illuminated with violet–blue, photoconverting light (excitation = 440 nm/short pass, dichroic mirror = 562 nm; Semrock) via a Zeiss AxioZoom V16 microscope, as previously described [5,57]. Larvae were left to behave under photoconverting light (84 000 lux) for 20 s. Dorsal clusters were then immediately imaged, in vivo, under a Zeiss LSM780 confocal microscope. Z-stacks were converted to maximum intensity projections, and Fred : Fgreen CaMPARI fluorescence intensity ratio was calculated using ImageJ software.
(g). Induction of tissue damage
UV: Third-instar larvae were anaesthetized with ether then mounted ventral-side up on glass slides and placed in a Spectrolinker XL-1000 ultraviolet crosslinker (Spectronics Corporation). UV treatment lasted approximately 6 s and was approximately 14 mJ cm−2, at a wavelength of 254 nm. Larvae were returned to regular fly food at 25°C before measuring behaviour at various times after UV irradiation. Pinch and puncture wounding: These were performed as described previously [58].
(h). Statistical analysis
All statistical analyses were performed using GraphPad Prism v. 7 (GraphPad Software, La Jolla, CA, USA, www.graphpad.com). All the data were tested for a normal distribution using Kolmogorov–Smirnov (KS) or Shapiro–Wilk normality test, and were then analysed using one-way ANOVA followed by Dunnet's post hoc test for survival assay. As calcium data were not normally distributed, differences were assessed by Kruskal–Wallis with Dunn's multiple comparisons test. For behavioural data, statistical significance was tested using log-rank (Mantel–Cox) analysis. Asterisks in the graphs indicate the significance of p-values comparing indicated group with controls: *p < 0.05, **p < 0.01, ****p < 0.0001.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Marta Zlatic for Basin (1, 2 and 4)-Gal4 drivers and Wesley B. Grueber, for DnB Gal4 drivers. Stocks obtained from the Bloomington Drosophila Stock Center (NIH P40OD018537) were used in this study. We thank summer undergraduate students Naveen K. Balakrishman and Joel Nelson for their help refining the chemical nociception assay, and Dr Paulucci-Holthauzen of the MD Anderson microscopy core facility for providing technical support, and assistance with the use of the microscope.
Data accessibility
Supplementary data are available in the electronic supplementary material.
Authors' contributions
Writing: original draft, R.L.-B. and M.J.G.; review and editing, R.L.-B., N.J.H., D.N.C., H.B.G. and M.J.G. Conceptualization: R.L.-B., N.J.H., D.N.C. and M.J.G. Investigation: R.L.-B. designed and developed the chemical nociception assay and performed all behavioural analyses; N.J.H. performed CaMPARI imaging. Data analysis: R.L.-B. and N.J.H. All authors critically read and commented on the final version of the manuscript.
Competing interests
We declare we have no competing interests.
Funding
H.B.G. and R.L.-B. were supported by 5R01DA036680. M.J.G. and R.L.B. were supported by R35GM126929. N.J.H. and D.N.C. were supported by 5R01NS086082, a Georgia State University (GSU) Brains & Behavior Seed Grant (DNC); and (GSU) Brains & Behavior Fellowship and Kenneth W. and Georganne F. Honeycutt Fellowship (N.J.H.).
References
- 1.Barrot M. 2012. Tests and models of nociception and pain in rodents. Neuroscience 211, 39–50. ( 10.1016/j.neuroscience.2011.12.041) [DOI] [PubMed] [Google Scholar]
- 2.Burgos A, et al. 2018. Nociceptive interneurons control modular motor pathways to promote escape behavior in Drosophila. eLife 7, e26016 ( 10.7554/eLife.26016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hwang RY, Zhong L, Xu Y, Johnson T, Zhang F, Deisseroth K, Tracey WD. 2007. Nociceptive neurons protect Drosophila larvae from parasitoid wasps. Curr. Biol. 17, 2105–2116. ( 10.1016/j.cub.2007.11.029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tracey WDJ, Wilson RI, Laurent G, Benzer S. 2003. painless, a Drosophila gene essential for nociception. Cell 113, 261–273. ( 10.1016/S0092-8674(03)00272-1) [DOI] [PubMed] [Google Scholar]
- 5.Turner HN, et al. 2016. The TRP channels Pkd2, NompC, and Trpm act in cold-sensing neurons to mediate unique aversive behaviors to noxious cold in Drosophila. Curr. Biol. 26, 3116–3128. ( 10.1016/j.cub.2016.09.038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Honjo K, Mauthner SE, Wang Y, Skene JHP, Tracey WD Jr. 2016. Nociceptor-enriched genes required for normal thermal nociception. Cell Rep. 16, 295–303. ( 10.1016/j.celrep.2016.06.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu C, et al. 2017. Sensory integration and neuromodulatory feedback facilitate Drosophila mechanonociceptive behavior. Nat. Neurosci. 20, 1085–1095. ( 10.1038/nn.4580) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim SE, Coste B, Chadha A, Cook B, Patapoutian A. 2012. The role of Drosophila Piezo in mechanical nociception. Nature 483, 209–212. ( 10.1038/nature10801) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhong L, Hwang RY, Tracey WD. 2010. Pickpocket is a DEG/ENaC protein required for mechanical nociception in Drosophila larvae. Curr. Biol. 20, 429–434. ( 10.1016/j.cub.2009.12.057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim SH, Lee Y, Akitake B, Woodward OM, Guggino WB, Montell C. 2010. Drosophila TRPA1 channel mediates chemical avoidance in gustatory receptor neurons. Proc. Natl Acad. Sci. USA 107, 8440–8445. ( 10.1073/pnas.1001425107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Anzi B, Tracey WD Jr, Benzer S. 2006. Response of Drosophila to wasabi is mediated by painless, the fly homolog of mammalian TRPA1/ANKTM1. Curr. Biol. 16, 1034–1040. ( 10.1016/j.cub.2006.04.002) [DOI] [PubMed] [Google Scholar]
- 12.Kang K, Pulver SR, Panzano VC, Chang EC, Griffith LC, Theobald DL, Garrity PA. 2010. Analysis of Drosophila TRPA1 reveals an ancient origin for human chemical nociception. Nature 464, 597–600. ( 10.1038/nature08848) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orgogozo V, Grueber WB. 2005. FlyPNS, a database of the Drosophila embryonic and larval peripheral nervous system. BMC Dev. Biol. 5, 4 ( 10.1186/1471-213X-5-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kernan M, Cowan D, Zuker C. 1994. Genetic dissection of mechanosensory transduction: mechanoreception-defective mutations of Drosophila. Neuron 12, 1195–1206. ( 10.1016/0896-6273(94)90437-5) [DOI] [PubMed] [Google Scholar]
- 15.Gao FB, Brenman JE, Jan LY, Jan YN. 1999. Genes regulating dendritic outgrowth, branching, and routing in Drosophila. Genes Dev. 13, 2549–2561. ( 10.1101/gad.13.19.2549) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singhania A, Grueber WB. 2014. Development of the embryonic and larval peripheral nervous system of Drosophila. Wiley Interdiscip. Rev. Dev. Biol. 3, 193–210. ( 10.1002/wdev.135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng LE, Song W, Looger LL, Jan LY, Jan YN. 2010. The role of the TRP channel NompC in Drosophila larval and adult locomotion. Neuron 67, 373–380. ( 10.1016/j.neuron.2010.07.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He L, et al. 2019. Direction selectivity in Drosophila proprioceptors requires the mechanosensory channel Tmc. Curr. Biol. 29, 945–956. ( 10.1016/j.cub.2019.02.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hughes CL, Thomas JB. 2007. A sensory feedback circuit coordinates muscle activity in Drosophila. Mol. Cell. Neurosci. 35, 383–396. ( 10.1016/j.mcn.2007.04.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaadia RD, Li W, Voleti V, Singhania A, Hillman EMC, Grueber WB. 2019. Characterization of proprioceptive system dynamics in behaving Drosophila larvae using high-speed volumetric microscopy. Curr. Biol. 29, 935–944. ( 10.1016/j.cub.2019.01.060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsubouchi A, Caldwell JC, Tracey WD. 2012. Dendritic filopodia, Ripped Pocket, NOMPC, and NMDARs contribute to the sense of touch in Drosophila larvae. Curr. Biol. 22, 2124–2134. ( 10.1016/j.cub.2012.09.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan Z, Zhang W, He Y, Gorczyca D, Xiang Y, Cheng LE, Meltzer S, Jan LY, Jan YN. 2013. Drosophila NOMPC is a mechanotransduction channel subunit for gentle-touch sensation. Nature 493, 221–225. ( 10.1038/nature11685) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiang Y, Yuan Q, Vogt N, Looger LL, Jan LY, Jan YN. 2010. Light-avoidance-mediating photoreceptors tile the Drosophila larval body wall. Nature 468, 921–926. ( 10.1038/nature09576) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chin MR, Tracey WD Jr. 2017. Nociceptive circuits: can't escape detection. Curr. Biol. 27, R796–R798. ( 10.1016/j.cub.2017.07.031) [DOI] [PubMed] [Google Scholar]
- 25.Jovanic T, et al. 2016. Competitive disinhibition mediates behavioral choice and sequences in Drosophila. Cell 167, P858–P870. ( 10.1016/j.cell.2016.09.009) [DOI] [PubMed] [Google Scholar]
- 26.Ohyama T, et al. 2015. A multilevel multimodal circuit enhances action selection in Drosophila. Nature 520, 633–639. ( 10.1038/nature14297) [DOI] [PubMed] [Google Scholar]
- 27.Yoshino J, Morikawa RK, Hasegawa E, Emoto K. 2017. Neural circuitry that evokes escape behavior upon activation of nociceptive sensory neurons in Drosophila larvae. Curr. Biol. 27, 2499–2504. ( 10.1016/j.cub.2017.06.068) [DOI] [PubMed] [Google Scholar]
- 28.Kaneko T, et al. 2017. Serotonergic modulation enables pathway-specific plasticity in a developing sensory circuit in Drosophila. Neuron 95, 623–638. ( 10.1016/j.neuron.2017.06.034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gold MS, Gebhart GF. 2010. Nociceptor sensitization in pain pathogenesis. Nat. Med. 16, 1248–1257. ( 10.1038/nm.2235) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Babcock DT, Landry C, Galko MJ. 2009. Cytokine signaling mediates UV-induced nociceptive sensitization in Drosophila larvae. Curr. Biol. 19, 799–806. ( 10.1016/j.cub.2009.03.062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jo J, Im SH, Babcock DT, Iyer SC, Gunawan F, Cox DN, Galko MJ. 2017. Drosophila caspase activity is required independently of apoptosis to produce active TNF/Eiger during nociceptive sensitization. Cell Death Dis. 8, e2786 ( 10.1038/cddis.2016.474) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Babcock DT, Shi S, Jo J, Shaw M, Gutstein HB, Galko MJ. 2011. Hedgehog signaling regulates nociceptive sensitization. Curr. Biol. 21, 1525–1533. ( 10.1016/j.cub.2011.08.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Im SH, et al. 2015. Tachykinin acts upstream of autocrine Hedgehog signaling during nociceptive sensitization in Drosophila. eLife 4, e10735 ( 10.7554/eLife.10735) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galko MJ, Krasnow MA. 2004. Cellular and genetic analysis of wound healing in Drosophila larvae. PLoS Biol. 2, E239 ( 10.1371/journal.pbio.0020239) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sweeney ST, Broadie K, Keane J, Niemann H, O'Kane CJ. 1995. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron 14, 341–351. ( 10.1016/0896-6273(95)90290-2) [DOI] [PubMed] [Google Scholar]
- 36.Fosque BF, et al. 2015. Neural circuits. Labeling of active neural circuits in vivo with designed calcium integrators. Science 347, 755–760. ( 10.1126/science.1260922) [DOI] [PubMed] [Google Scholar]
- 37.Turner HN, Patel AA, Cox DN, Galko MJ. 2018. Injury-induced cold sensitization in Drosophila larvae involves behavioral shifts that require the TRP channel Brv1. PLoS ONE 13, e0209577 ( 10.1371/journal.pone.0209577) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu Y, Brock AR, Wang Y, Fujitani K, Ueda R, Galko MJ. 2009. A blood-borne PDGF/VEGF-like ligand initiates wound-induced epidermal cell migration in Drosophila larvae. Curr. Biol. 19, 1473–1477. ( 10.1016/j.cub.2009.07.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mandel SJ, Shoaf ML, Braco JT, Silver WL, Johnson EC. 2018. Behavioral aversion to AITC requires both painless and dTRPA1 in Drosophila. Front. Neural Circuits 12, 45 ( 10.3389/fncir.2018.00045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee Y, Kang MJ, Shim J, Cheong CU, Moon SJ, Montell C. 2012. Gustatory receptors required for avoiding the insecticide l-canavanine. J. Neurosci. 32, 1429–1435. ( 10.1523/JNEUROSCI.4630-11.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shim J, Lee Y, Jeong YT, Kim Y, Lee MG, Montell C, Moon SJ. 2015. The full repertoire of Drosophila gustatory receptors for detecting an aversive compound. Nat. Commun. 6, 8867 ( 10.1038/ncomms9867) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rimal S, Sang J, Poudel S, Thakur D, Montell C, Lee Y. 2019. Mechanism of acetic acid gustatory repulsion in Drosophila. Cell Rep. 26, 1432–1442. ( 10.1016/j.celrep.2019.01.042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kwon Y, Kim SH, Ronderos DS, Lee Y, Akitake B, Woodward OM, Guggino WB, Smith DP, Montell C. 2010. Drosophila TRPA1 channel is required to avoid the naturally occurring insect repellent citronellal. Curr. Biol. 20, 1672–1678. ( 10.1016/j.cub.2010.08.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Apostolopoulou AA, Rist A, Thum AS. 2015. Taste processing in Drosophila larvae. Front. Integrative Neurosci. 9, 50 ( 10.3389/fnint.2015.00050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Im SH, Patel AA, Cox DN, Galko MJ. 2018. Drosophila insulin receptor regulates the persistence of injury-induced nociceptive sensitization. Dis. Models Mech. 11, dmm034231 ( 10.1242/dmm.034231). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lopez-Bellido R, Puig S, Huang PJ, Tsai CR, Turner HN, Galko MJ, Gutstein HB. 2019. Growth factor signaling regulates mechanical nociception in flies and vertebrates. J. Neurosci. 39, 6012–6030. ( 10.1523/JNEUROSCI.2950-18.2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dube GR, Elagoz A, Mangat H. 2009. Acid sensing ion channels and acid nociception. Curr. Pharm. Design 15, 1750–1766. ( 10.2174/138161209788186263) [DOI] [PubMed] [Google Scholar]
- 48.Follansbee TL, Gjelsvik KJ, Brann CL, McParland AL, Longhurst CA, Galko MJ, Ganter GK. 2017. Drosophila nociceptive sensitization requires BMP signaling via the canonical SMAD pathway. J. Neurosci. 37, 8524–8533. ( 10.1523/JNEUROSCI.3458-16.2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bellen HJ, Tong C, Tsuda H. 2010. 100 years of Drosophila research and its impact on vertebrate neuroscience: a history lesson for the future. Nat. Rev. Neurosci. 11, 514–522. ( 10.1038/nrn2839) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brand AH, Perrimon N. 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401–415. [DOI] [PubMed] [Google Scholar]
- 51.Grueber WB, Jan LY, Jan YN. 2003. Different levels of the homeodomain protein Cut regulate distinct dendrite branching patterns of Drosophila multidendritic neurons. Cell 112, 805–818. ( 10.1016/S0092-8674(03)00160-0) [DOI] [PubMed] [Google Scholar]
- 52.Jenett A, et al. 2012. A GAL4-driver line resource for Drosophila neurobiology. Cell Rep. 2, 991–1001. ( 10.1016/j.celrep.2012.09.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ainsley JA, Pettus JM, Bosenko D, Gerstein CE, Zinkevich N, Anderson MG, Adams CM, Welsh MJ, Johnson WA. 2003. Enhanced locomotion caused by loss of the Drosophila DEG/ENaC protein Pickpocket1. Curr. Biol. 13, 1557–1563. ( 10.1016/S0960-9822(03)00596-7) [DOI] [PubMed] [Google Scholar]
- 54.Song W, Onishi M, Jan LY, Jan YN. 2007. Peripheral multidendritic sensory neurons are necessary for rhythmic locomotion behavior in Drosophila larvae. Proc. Natl Acad. Sci. USA 104, 5199–5204. ( 10.1073/pnas.0700895104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nicholson L, Singh GK, Osterwalder T, Roman GW, Davis RL, Keshishian H. 2008. Spatial and temporal control of gene expression in Drosophila using the inducible GeneSwitch GAL4 system. I. Screen for larval nervous system drivers. Genetics 178, 215–234. ( 10.1534/genetics.107.081968) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Y, Antunes M, Anderson AE, Kadrmas JL, Jacinto A, Galko MJ. 2015. Integrin adhesions suppress syncytium formation in the Drosophila larval epidermis. Curr. Biol. 25, 2215–2227. ( 10.1016/j.cub.2015.07.031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patel AA, Cox DN. 2017. Behavioral and functional assays for investigating mechanisms of noxious cold detection and multimodal sensory processing in Drosophila larvae. Bio-protocol. 7, e2388 ( 10.21769/BioProtoc.2388) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burra S, Wang Y, Brock AR, Galko MJ. 2013. Using Drosophila larvae to study epidermal wound closure and inflammation. Methods Mol. Biol. 1037, 449-461. ( 10.1007/978-1-62703-505-7_26) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Supplementary data are available in the electronic supplementary material.