Abstract
Injury occurring in the neonatal period in mammals is known to induce plasticity in pain pathways that may lead to pain dysfunction in later life. Whether these effects are unique to the mammalian nervous system is not well understood. Here, we investigate whether similar effects of early-life injury are found in a large-brained comparative model, the cephalopod Euprymna scolopes. We show that the peripheral nervous system of E. scolopes undergoes profound and permanent plasticity after injury of peripheral tissue in the early post-hatching period, but not after the same injury given in the later juvenile period. Additionally, both innate defensive behaviour and learning are impaired by injury in early life. We suggest that these similar patterns of nervous system and behavioural remodelling that occur in squid and in mammals indicate an adaptive value for long-lasting plasticity arising from early-life injury, and suggest that injuries inflicted in very early life may signal to the nervous system that the environment is highly dangerous. Thus, neonatal pain plasticity may be a conserved pattern whose purpose is to set the developing nervous system's baseline responsiveness to threat.
This article is part of the Theo Murphy meeting issue ‘Evolution of mechanisms and behaviour important for pain’.
Keywords: behaviour, cephalopod, early-life injury, nociceptive sensitization, pain
1. Introduction
It is widely accepted that injury and painful experiences in the early neonatal period induce plasticity in nociceptive pathways that is expressed as disordered pain experience, particularly the development of chronic or aberrant pain in later life [1–3]. This pattern is well studied both in human neonates and in rodent models, where effects point consistently toward multiple priming effects on developing nervous systems that precipitate chronic pain in adulthood [2]. As is typical in mammalian models of chronic pain, effects are generally considered to be primarily or exclusively pathological, with no obvious function or natural benefit arising from early, experience-dependent re-wiring of pain pathways described.
Although the protective value of acute pain plasticity at all life stages is generally accepted, identifying functional significance of long-term pain that outlasts injury healing has been difficult. In part, this may be due to the reliance on mammalian models (including humans) where many generations of relaxed selection on protective behaviours have obscured highly conserved, naturally selected functions for long-lasting pain plasticity, and a focus on evoked behavioural assays that often do not replicate naturally occurring and spontaneous behaviours. Recently, using a comparative model, the longfin, inshore squid (Doryteuthis pealei), we have shown that long-term nociceptive sensitization in adults serves a vitally important anti-predator function, enhancing survival of animals whose injuries make them preferential targets for predators [4]. Interestingly, this finding has recently been replicated in another non-traditional, outbred invertebrate model, an amphipod [5], suggesting that a broader, comparative perspective on evolution and functions of chronic-type pain might reveal not only additional functional consequences for such pain, but also novel therapeutic targets for its treatment or prevention.
Cephalopods are appealing comparative models of nociceptive sensitization and pain-like behaviours. They are neurologically and behaviourally complex, and we have shown previously that cephalopods display robust short- and long-term sensitization of primary nociceptive afferents after tissue injury [6,7], and that this plasticity produces behavioural changes that are adaptive [4,8]. While these effects persist for at least several days after injury, all previous studies were conducted on adults, and to date there has been no investigation of differences in persistence of effects of injury given at different life stages. Given the extensive evidence for lifelong effects on nociceptive circuits and subsequent pain perception in mammals when injury occurs in early life, here we consider whether similar effects also occur in a wide range of species, including cephalopods.
The small, tropical sepiolid Euprymna scolopes (Hawaiian bobtail squid) is ideally suited to investigating lifelong effects of early-life experiences. It has one of the shortest generation times of all the cephalopods, reaching sexual maturity in 8–10 weeks after hatching [9]. It is a ‘typical’ cephalopod in that it has a large and highly derived nervous system that rivals that of lower vertebrates for cell number and overall complexity, despite its completely separate evolutionary history [10], and previous work has shown that these small squid express experience-dependent plasticity in defensive behaviours [11] and are capable of complex cognitive tasks [12]. Unlike mammals, cephalopods do not engage in parental care; hatchlings emerge from eggs as miniature adults in terms of body form and behaviour, and are independent and solitary from hatchling. Thus, this novel invertebrate model of nociceptive sensitization also permits experimental treatments to be performed without the potentially confounding effects of maternal separation.
In this study, we examine whether injury that occurs in early life permanently changes the squid nervous system in ways that appear analogous, either structurally or functionally, to changes that occur in mammals subject to early-life injury. Additionally, we test whether early-life injury (ELI) produces behavioural plasticity that is likely to have fitness consequences persisting beyond the time period when injuries are healing.
2. Material and methods
(a). Animals
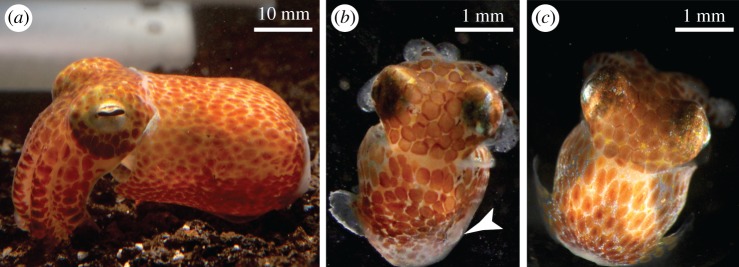
Hawaiian bobtail squid (Euprymna scolopes, N = 68, figure 1a–c) were reared in a recirculating, artificial sweater system at 24°C under a natural light cycle. Squid were fed ad libitum on live mysid shrimp (Amerimysis bahia) until four weeks of age, and then twice daily on live grass shrimp (Paleamontes spp.).
Figure 1.
Hawaiian bobtail squid, Euprymna scolopes. (a) Adult, uninjured. (b) Hatchling (two weeks post-hatch) showing complete erosion of right fin (arrowhead) and minor abrasion on left fin. (c) Age-matched control hatchling showing two normal, uninjured fins. (Online version in colour.)
(b). Injury procedure
Squid received injury or sham treatment at either two weeks, six weeks or 13 weeks post-hatching (figure 2a). For injuries, the fin was gripped tightly at its lateral midpoint with grooved forceps, resulting in a crush injury that was typically accompanied by tissue tearing along the crush boundary. Sham injury was a light touch on the fin with forceps. Injuries were proportionate to the size of the squid. In a small subset of hatchlings, the injury was fin erosion (figure 1b) caused by being washed against a mildly abrasive container wall over the course of 12 h. We found no differences in results between the two injury models. All squid showed complete healing of injuries within two to three weeks, with injured adult fins fully regenerated and indistinguishable from controls on microscopic examination.
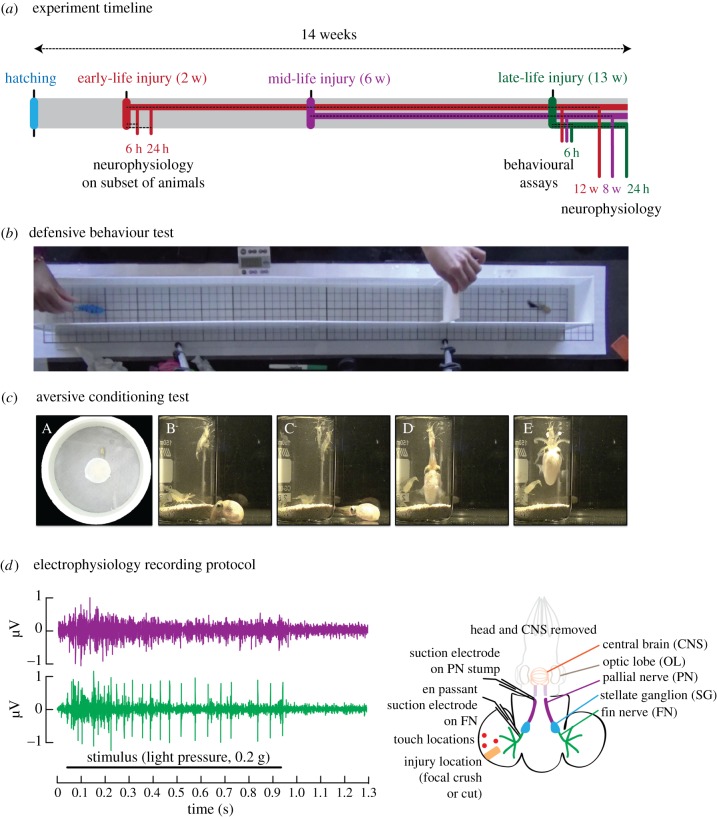
Figure 2.
Experimental timeline and methods. (a) Experimental timeline showing timecourses for the three experimental groups. ELI occurred at two weeks post-hatching. At 6 and 24 h post injury or sham treatment, a subset of animals was euthanized for electrophysiology. Others were left undisturbed until the last experiment week, when they underwent behavioural testing as adults and electrophysiology. An MLI group was injured at six weeks post-hatching, and an LLI group was injured at 13 weeks post-hatching. The late-life timepoint represented acute, adult onset injury, whereas the early- and mid-life groups can be considered chronic. (b) Apparatus used for testing defensive behaviour. A raceway with squid confined at one end was used to test responses to the approach of a simulated fish predator. Distances were measured by a grid below the floor. (c) Apparatus and striking behaviour for the aversive learning assay. The photo sequence shows the arena from above, with the beaker containing shrimp prey located in the centre of the arena. Subsequent images show a strike sequence made by a squid onto the glass. (d) Electrophysiological recording protocol. In adult animals, paired recordings were made from two major peripheral nerves, the fin nerve, which innervates the region of injury (green trace), and the pallial nerve (purple trace), which carries signals from the stellate ganglion to the brain. Fin nerves were recorded en passant, while pallial nerves were cut. In hatchlings, only the pallial nerve was recorded. (Online version in colour.)
(c). Behavioural tests
(i). Defensive behaviour
Squid were placed individually into the end box of a plexiglass raceway (figure 2b), which was separated from the longer section by a clear plexiglass divider and a white opaque shield, and acclimated for 60 s. At the far end of the longer section, a robotic fish was immersed and held in place until the shield was lifted, then released and allowed to swim toward the squid. Total swim time was approximately 30 s. Once the fish reached the clear divider, the shield was replaced and the squid was returned to its home tank. Control trials were conducted in a pilot stage to ensure that squid were unresponsive to other aspects of the environment or to the divider being lifted. Trials were videotaped from overhead for later analysis.
(ii). Aversive conditioning
Squid were food-deprived for 24 h prior to testing. A beaker containing five live shrimp was placed in the centre of a round test arena (22.4 cm diameter, 14 cm water depth, figure 2c). The beaker was obscured with an opaque white shield, then one squid was moved individually into the arena and allowed to acclimate for 60 s. The shield was lifted, and the squid's behaviour was filmed for 10 min. In this well-validated assay, squid make predatory strikes at the shrimp, but instead their tentacle clubs hit the glass of the beaker, thus providing mildly noxious sensory input from the high-speed impact of the tentacle clubs with the glass, and denying the squid the expected food reward for each strike made [13]. Over the course of short, continuous exposure, squid learn to inhibit their striking behaviour [12]. Each squid was tested twice, once in unaltered home tank water and once after 50 ml of predator scent (water taken from a separate tank containing a large rockfish) was added into the arena. Trials were videotaped from overhead for later analysis.
(d). Electrophysiology
Squid were euthanized by immersion in isotonic MgCl2 [14]. Five minutes after cessation of breathing, animals were decapitated and decerebrated, viscera were removed and the pallial nerves and fin nerves were exposed. The preparation was cut down the dorsal midline, and halves were recorded sequentially. In hatchlings, only the pallial nerve was used. In adults, both fin and pallial nerves were dissected and recorded.
Tissue was rinsed in filtered, artificial seawater. Ten minutes after rinsing, the pallial nerve stump and a loop of the fin nerve (in adults) were drawn into separate suction electrodes (figure 2d) attached to an extracellular amplifier (A-M Systems model 1700). Signals were sampled at 10 kHz and digitized (AD Instruments Powerlab 4/35 with LabChart Pro software). Three positions on the fin were selected for stimulation. One touch on each position was given first with a light von Frey filament (0.02 g), then the same positions were touched with a heavy filament (0.16 g). Filaments were pressed until bending then held in place for 1 s. Spikes produced in each nerve were recorded simultaneously, and results from the three touches were averaged. Once these baseline touches were complete, the fin tissue was crushed with grooved forceps for 2 s. von Frey filament stimulations were repeated 10 min after ex vivo crush.
(e). Data analysis and statistical procedures
(i). Defensive behavioural tests
Using recorded video files, distances between the eye of the squid and the nose of the fish, when the squid made the first visible behavioural response (of any kind) were measured. These behaviours included colour changes, changes in arm posture [11], swimming, jetting or inking. We also measured the distance at which the squid performed escape jetting and inking. Each squid was tested once only. Distances were averaged among the treatment groups and were normally distributed. One-way ANOVA followed by post hoc t-tests was performed in GraphPad Prism.
(ii). Aversive conditioning
Recorded video files were analysed by trained observers who counted the number of strikes made by each squid at the glass. Separately, video files were analysed using Ethovision software (Noldus) to track movement of squid. Distances and zone occupancies were averaged for each treatment group and condition (predator present or absent), then analysed with two-way ANOVA followed by planned comparisons made with post hoc t-tests with sequential Bonferroni correction. Only controls, ELI and LLI groups were tested in this assay.
(iii). Electrophysiology data
Counts of evoked spikes were made from 1 s of maximal firing in response to touch of the filaments. The three replicate touches for each filament were averaged, and counts from each half of the same animal's body were averaged. Some counts showed departure from normal distributions and were analysed with non-parametric pairwise comparisons after being excluded from ANOVA screening. All counts that were normally distributed were analysed with one-way ANOVA followed by planned, post hoc, sequential Bonferroni-corrected t-tests.
3. Results
(a). Short-term and long-term nociceptive sensitization occurs in hatchlings after fin injury
In squid injured at two weeks post-hatching, there was a significant increase in evoked spikes produced in response to both innocuous and noxious touch at 6 h post-injury (one-way ANOVA, F3,15 = 6.98, p = 0.0036, figure 3a; pairwise post hoc comparisons, control versus injured: innocuous touch, p = 0.026, noxious touch, p = 0.012). However, there was no evidence for additional, short-term sensitization in response to a second, ex vivo tissue crush after baseline recordings (p = 0.33, figure 3b). This pattern persisted at 24 h post-injury (one-way ANOVA, F3,22 = 17.67, p < 0.0001; pairwise post hoc comparisons, control versus injured: innocuous touch, p < 0.001, noxious touch, p < 0.001). At 24 h, there was evidence for short-term sensitization in the control group after ex vivo crush, in response to the innocuous touch (paired t-test, p = 0.03).
Figure 3.
Hatchling nervous systems are capable of short- and long-term nociceptive sensitization. (a) At 6 h after in vivo fin injury, recordings from the pallial nerve show significant increases in evoked spikes after application of either a light von Frey filament (0.02 g) delivering innocuous pressure to the mantle tissue, or a heavy filament (0.16 g) delivering noxious pressure (unpaired t-tests, *p < 0.05). However, in comparisons within treatment groups, there is no evidence for short-term, ex vivo sensitization in response to tissue crush after baseline recordings. (b) At 24 h after in vivo fin injury, there is significantly higher evoked activity in response to both light and noxious touch on the mantle tissue (unpaired t-tests, **p < 0.01, ***p < 0.001. Additionally, there is short-term, ex vivo sensitization to the light filament apparent 10 min after ex vivo tissue crush (paired t-test, p < 0.05). Numbers inside bars are group sample sizes. Bars show mean + 1 s.e.m.
(b). Defensive behaviour is permanently altered by early-life injury, but not by injury in mid-life
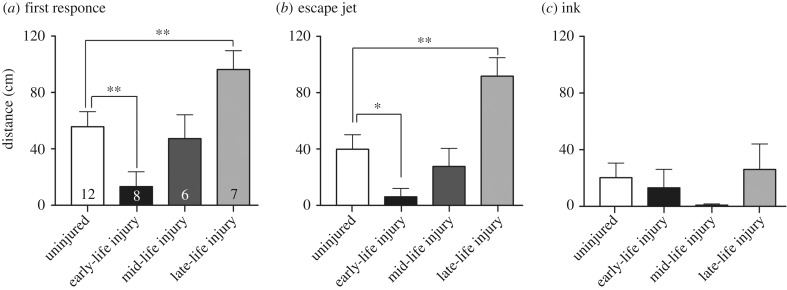
In tests of reactivity to a simulated predator threat, we measured the distance between the focal squid and the predator mimic at three points: where the squid made its first response, distance when escape jetting was initiated and distance between the two when inking occurred. There was a significant difference among the four treatment groups (control, ELI, mid-life injury (MLI) and late-life injury (LLI)) for distance at first response (one-way ANOVA, F3,33 = 5.81, p = 0.0027, figure 4). Squid injured in early life showed significantly shorter response distances compared with controls (unpaired t-test, p = 0.01), but there was no difference between controls and mid-life injured squid. Squid injured as adults and tested at the acute timepoint (6 h post injury) showed significantly greater response distances than controls (p = 0.003). An identical pattern was present for initiation of escape jetting (one-way ANOVA, F3,33 = 8.05, p = 0.004; control versus ELI, p = 0.03, control versus LLI, p = 0.002). Inking was uncommon, and there were no differences among the groups.
Figure 4.
Injury in early life affects adult defensive behaviour. (a) Distance between squid and robotic fish when the first defensive behaviour of any kind was recorded. ELI squid show delayed responses compared with controls. MLI produces no effect on defensive behaviour, while acute injury in adults results in earlier responses compared with controls. (b) Distance between squid and fish when the first high-level defensive behaviour was performed. (c) Inking was rare and there were no differences among the four groups. Comparisons with controls made with unpaired t-tests. *p < 0.05, **p < 0.01. Numbers inside bars are group sample sizes (same for all panels). Bars show mean + 1 s.e.m.
(c). Aversive conditioning and risk assessment is inhibited by both early-life and acute injury
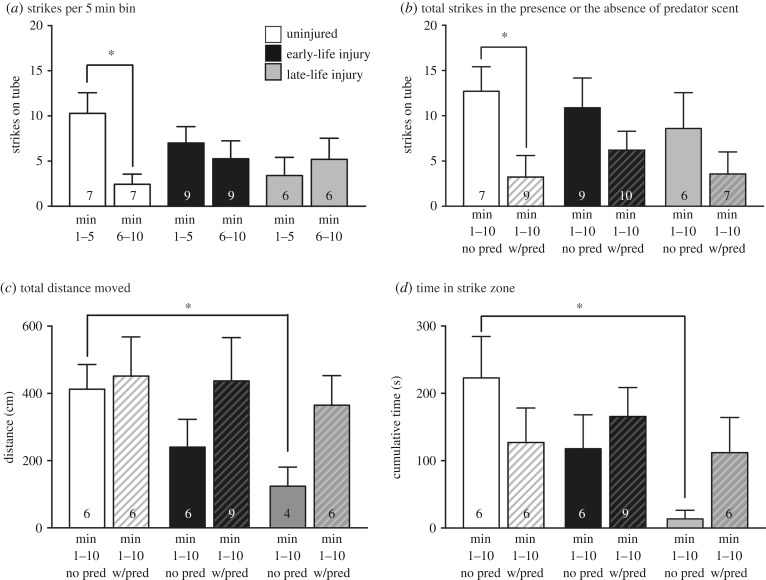
In a single 10 min learning trial, control squid showed a significant decline in strikes made at prey between the first and second 5 min intervals (paired t-test, t = 3.29, d.f. = 6, p = 0.01, figure 5a), but there were no declines in the two injured groups. The difference was not due to fewer learning ‘opportunities’, as there were no differences in strikes made in the first 5 min among the three treatment groups (one-way ANOVA, F2,17 = 2.44, p = 0.11), nor in total strikes made overall (one-way ANOVA, F2,18, p = 0.73). When predator scent was added to the arena, control squid showed a significant decline in the total number of strikes made (unpaired t-test, t = 2.68, d.f. = 14, p = 0.01, figure 5b), but there were no declines in the other two groups. Comparisons of activity and space use during the trial showed lower values only in the acutely injured (LLI) group in the absence of predator scent, compared with controls (distance swum, t = 2.81, d.f. = 8, p = 0.026; time in strike zone, t = 3.33, d.f. = 8, p = 0.01, figures 5c,d and 6).
Figure 5.
Both chronically and acutely injured squid show cognitive deficit and altered risk assessment. (a) In an aversive conditioning task, strikes made at confined prey declined significantly in the second half of a 10 min trial for uninjured squid. Neither squid injured in early life nor squid injured acutely as adults showed significant learning. Paired t-tests, *p < 0.05. (b) When the task was repeated in the presence of a predator olfactory cue, uninjured squid significantly decreased foraging behaviour, but neither injured group did. (c) Only the acutely injured (LLI) squid showed a reduction in the total distance moved during the trial in the absence of a predator. There were no differences when in the presence of predator scent. (d) Similarly, there was no difference in the time squid spent in the ‘strike zone’ around the beaker (see also figure 6) in the presence of predator scent. Acutely injured squid spent less time in the strike zone compared with controls in the absence of predator scent. Unpaired t-tests, *p < 0.05. Numbers inside bars are group sample sizes. Note fewer samples in (c,d) relate to lack of automated tracking data for some individuals. Bars show mean + 1 s.e.m.
Figure 6.
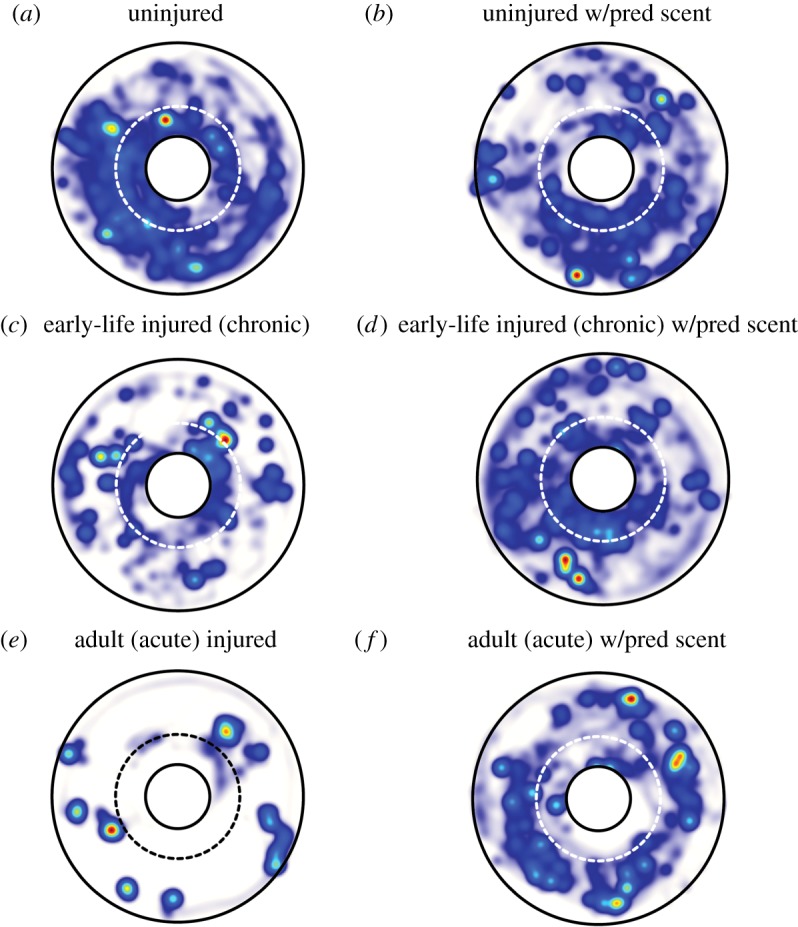
Heatmaps generated by automated video tracking of squid activity during the 10 min trial of the aversive conditioning experiment (Ethovision, Noldus). Colour shows the relative dwell time in a given area (blue, low; red, high), averaged over all animals in a treatment group. Outer arena boundaries are in black. The beaker containing shrimp prey is centred in the arena and shown as a white circle outlined in black. Broken lines (white or black) show the ‘strike zone’ around the beaker. (a,b) In general, uninjured animals were highly active, with limited periods of stasis. No clear difference in space use is apparent in the presence of predator scent. (c,d) Early-life injured squid showed somewhat less activity and reduced use of space, but increased activity and space use in the presence of predator scent. (e,f) Acutely injured (late-life injured) squid showed limited activity in the absence of predator scent, but increased activity when a predator cue was present. (Online version in colour.)
(d). Early-life injury produces permanent nociceptive sensitization in peripheral nerves, but mid-life injury does not
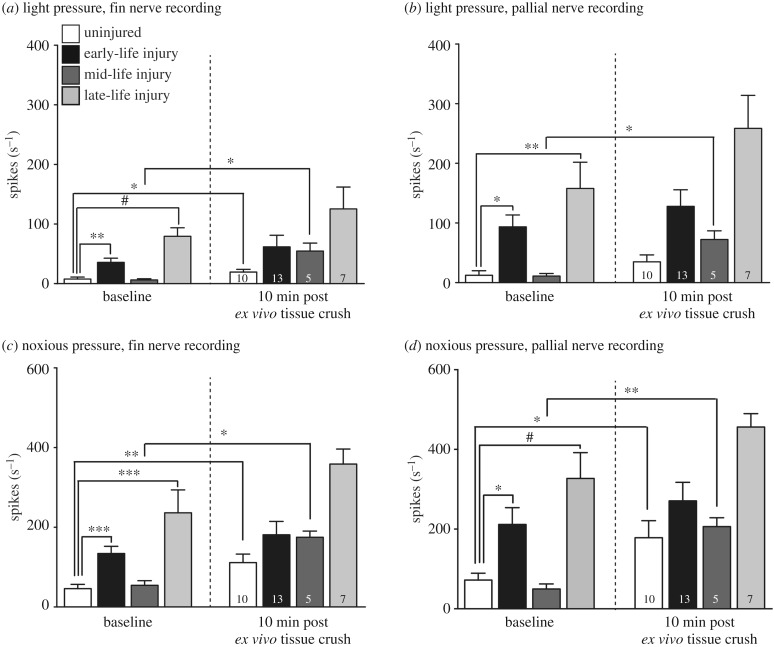
Adult squid injured in early life showed significantly higher rates of firing in the fin nerve in response to innocuous pressure compared with controls (unpaired t-test, t = 3.29, d.f. = 21, p = 0.0034, figure 7a,b), as did adult squid injured in late life (t = 5.69, d.f. = 15, p < 0.0001), but squid injured in mid-life did not. A similar pattern was apparent in recordings from the pallial nerve, with early-life and late-life injured groups showing significantly higher firing rates compared with controls at the baseline timepoint (some data were not normally distributed, Shapiro–Wilk test, W = 0.78, p = 0.02; Mann–Whitney U-tests, ELI versus control, p = 0.0012, LLI versus control, p = 0.0003). In response to applications of a heavy von Frey filament producing noxious pressure, recordings from both fin and pallial nerves showed higher firing rates in the ELI and LLI groups, compared with controls (fin nerve: ELI, Mann–Whitney U-test, p = 0.03, LLI, Mann–Whitney U-test, p = 0.005; pallial nerve: ELI, unpaired t-test, p = 0.01, LLI, p < 0.0001, figure 7c,d).
Figure 7.
Paired fin/pallial electrophysiological recordings of adult squid. (a) Injury in early life produces permanent hyperexcitability in the fin nerve when the fin tissue in stimulated with a light von Frey filament, compared with uninjured squid. This pattern is not present in MLI squid. Squid injured acutely (24 h prior to recording) in late life also show hyperexcitability compared with controls. In tests of short-term, ex vivo sensitization in response to tissue crush, there was an increase in spikes produced in squid that did not receive an in vivo injury (uninjured), and in squid injured in mid-life. No plasticity was evident in the ELI or LLI groups. (b) A similar pattern is apparent in the pallial nerve when fin tissue was stimulated with a light von Frey filament. In general, early-life and LLI groups showed sensitization at baseline recording, while short-term plasticity occurred only in the MLI group. (c) When the fin tissue was stimulated with a heavy von Frey filament producing noxious pressure, the same pattern of long-term and short-term plasticity was seen as in (a). (d) In response to noxious pressure on the fin, pallial nerve excitability was the same as for (b), except that short-term plasticity is also apparent in the uninjured group. Mixed model ANOVA followed by post hoc, sequential Bonferroni-corrected t-tests. Comparisons among groups at baseline made with unpaired t-tests. Comparisons within groups before and after ex vivo tissue crush made with paired t-tests. *p < 0.05, **p < 0.01, ***p < 0.001, #p < 0.0001. Numbers inside bars are group sample sizes (same for baseline and post-crush). Bars show mean + 1 s.e.m.
(e). Early-life injury blocks further sensitization to ex vivo tissue injury, but injury in mid-life does not
We have previously shown that uninjured squid show robust short-term sensitization to ex vivo tissue injury, measured in fin nerve recordings [7]. Paired comparisons of fin nerve firing rates made within treatment groups before and after ex vivo crush showed that both control and mid-life injured squid showed significant short-term sensitization in response to touch with an innocuous filament (control: p = 0.01; MLI: p = 0.03, figure 7a,b), but further short-term sensitization was abolished by either early-life or late-life (acute) injury. In pallial nerve recordings, paired comparisons showed significant short-term sensitization in response to light pressure after ex vivo tissue crush in the MLI group only (paired t-test, p = 0.016, figure 7c,d). Patterns were identical in response to application of the filament producing noxious pressure. In both fin and pallial nerve recordings, both control and MLI groups showed significant short-term ex vivo sensitization (fin nerve: control, paired t-test, p = 0.009, MLI, p = 0.01; pallial nerve: control, p = 0.04, MLI, p = 0.005)
4. Discussion
Here, we show that injury in early life produces permanent changes to defensive behaviour, short-term memory and peripheral nervous system excitability in an invertebrate model, the bobtail squid. By contrast, the same injury given in the late juvenile stage at six weeks post-hatching did not result in lifetime changes to either peripheral nervous system excitability or behaviour. Injury given shortly before testing in adult animals late in their life produced hyperexcitability of the peripheral nervous system and escalated defensive behaviours, as previously described [6–8,15].
Although we had predicted that all injuries, whether given in early or late life, would result in hyper-reactive defensive behaviour, we found this to be the case only with acute, late-life injuries in adults. By contrast, injury in early life produced what appeared to be hypo-reactivity to a simulated predator threat. In rodents subjected to ELI, there is evidence for both increased defensive withdrawal latencies, which would appear similar to the hypo-reactivity we observed in our animals, and also increased vigilance toward unfamiliar conspecifics, which implies heightened outward responses to threats [16]. In the same species of squid, we have shown that there is a clear ontogenetic shift from highly escalated defensive behaviours in response to a simulated predator in juveniles to lower-level and non-responses in adults [11]. Although the squid in this study were age-matched among the groups, it is possible that early-life experience of injury shapes defensive behaviour into a more ‘adult-like’ state earlier in life. Alternatively, because the injury model we used was fin crush, it is possible that injured squid were adapting to lost manoeuvrability or impaired swimming ability by relying for longer on primary defence [4,17], although our previous work has not identified such motor impairments [4] and the same effect would presumably occur in mid-life injured squid, which our results do not support. Alternatively, it is possible that the simulated predator we used was not particularly salient to the squid, although other studies have used model predators to elicit defensive behaviour in other squid species effectively [8]. Although we have not tested visual acuity in E. scolopes directly, their eyes and eye position are largely similar to those of cuttlefish, which have excellent frontal vision and can see predators from a considerable distance [18]; thus it is unlikely that the frontal approach of the predator was the cause of the limited responsiveness to the predator mimic we observed in some squid.
Impairment in short-term memory was apparent in both the early-life injured and late-life injured groups. Distraction effects of acute injury and pain are well documented in mammalian models [19,20], and this is likely the cause of the learning deficits observed in the LLI group in our study, where the injury occurred 6 h prior to testing, when we have previously shown that acute effects on both behavioural and neural sensitization are at a peak [7,15,21]. Cognitive deficits in mammalian models of ELI have been reported, although the learning contexts differ somewhat from the aversive conditioning task we used here [22–24]. We had hypothesized that the mildly noxious nature of the ‘shrimp-in-the-tube’ procedure [13], coupled with the generalized sensitization to tactile stimuli previously found in cephalopods after injury [6,15], would lead to enhancement of learning in this operant task, because the unrewarded tentacle strikes at the glass should have been more aversive if the whole body surface was sensitized by ELI, and thus the negative reinforcement of each strike would be greater. However, although we found generalized sensitization of primary afferents after ELI in peripheral nerve recordings, this did not translate to enhanced aversive learning. In mammalian studies of tissue injury in neonates, there is evidence that there are multiple separate cellular mechanisms that are modified, some of which produce hyperalgesia when strong noxious stimulation is applied at the site of original injury, and others that produce hypoalgesia to mild noxious stimulation elsewhere on the body [2]; we suggest that a similar hypoalgesic mechanism might function in squid to increase nociceptive threshold in the tentacle clubs, a region distant from the fin injury site, and thus would impair the normal reinforcement learning that occurs in this mildly noxious reinforcement paradigm.
Within the same aversive conditioning test, we also looked at whether injuries affected risk-prone foraging decisions. Uninjured controls reduced foraging effort in the presence of a predator cue, which we expect would translate to a fitness benefit [25]. By contrast, both early-life and late-life injuries erased this reduction, which seems likely to be maladaptive; however, it is possible that an alternative, high-activity foraging strategy is employed after injury that offsets predation risk during exposure. Further tests are needed to identify the functional significance of the behaviours observed in injured groups in this test.
Data from nerve recordings showed that the Euprymna nervous system is capable of encoding nociceptive plasticity throughout life; both hatchlings and acutely injured adults showed the same patterns of long-term (24 h) sensitization after injury. In both age classes, in vivo injury also blocked the further enhancement of firing rates typically seen in uninjured animals after ex vivo tissue crush [7], suggesting that a maximum level of plasticity occurs after in vivo injury that cannot be further enhanced. This same pattern was apparent in squid injured in early life—baseline firing rates were significantly higher in adults with ELI compared with controls, but no further plasticity was apparent after ex vivo crush in the same location. This pattern differs somewhat from effects described in a number of studies of ELI in rodents, where neonatal injury drives pronounced hyperalgesia after secondary reinjury of tissue in later life [1–3,26,27].
The apparent mis-match between peripheral nerve effects and behaviour is somewhat unexpected. It is possible that this is due to ELI-induced re-wiring in the central brain or in descending, inhibitory circuits [2,28], neither of which was functional in the nerve recording preparation we used here. These central mechanisms may function to modulate evolutionarily ancient processes of primary nociceptor hyperexcitability after injury [29], resulting in an animal that gains the fitness benefit of persistent hypersensitivity while ameliorating the costs of inadvertent signalling to predators via hyper-responsive injury-induced behaviours. The fact that such complex effects appear to occur only in response to injury in early life, and are opposite to those observed in adults with acute injuries, suggests that there are unique selection pressures shaping early-life plasticity in response to injury. We suggest that injury that occurs in the early post-hatching period is likely an indication of a high-danger environment, and in such circumstances, injury-induced, lifelong changes to nervous system function may be an effective way of establishing an escalated baseline response to perceived threats, such that costs associated with hyperexcitability are outweighed by benefits of lifelong, protective behavioural effects.
Acknowledgements
We thank the Marine Biological Laboratory Cephalopod Initiative for supplying Euprymna eggs and for advice on culture. Members of the Crook laboratory assisted with animal husbandry and supplementary data analysis. Rockfish odour was supplied by Dr Karen Crow of San Francisco State University.
Ethics
In the USA, where this research was conducted, no IACUC protocol is required for invertebrates. Standards of humane care and handling were upheld according to Directive EU/2010/63, although no formal approval process occurred.
Data accessibility
Raw data associated with this study are available from Open Science Framework, under the project name ‘Early life injury in squid’. Link: https://osf.io/3gpfk/.
Authors' contributions
R.J.C.: designed study, conducted experiments, analysed data, wrote paper. R.B.H.: designed study, conducted experiments, analysed data. C.R.L., L.N.L. and P.P.P.: conducted experiments, analysed data.
Competing interests
We declare we have no competing interests.
Funding
This study was supported by NIH-R25-GM048972 to R.B.H., NIH-R25-GM059298 to P.P.P. and CSUPERB to R.J.C.
References
- 1.Fitzgerald M, McKelvey R. 2016. Nerve injury and neuropathic pain—a question of age. Exp. Neurol. 275, 296–302. ( 10.1016/j.expneurol.2015.07.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwaller F, Fitzgerald M. 2014. The consequences of pain in early life: injury-induced plasticity in developing pain pathways. Eur. J. Neurosci. 39, 344–352. ( 10.1111/ejn.12414) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hermann C, Hohmeister J, Demirakça S, Zohsel K, Flor H. 2006. Long-term alteration of pain sensitivity in school-aged children with early pain experiences. Pain 125, 278–285. ( 10.1016/j.pain.2006.08.026) [DOI] [PubMed] [Google Scholar]
- 4.Crook RJ, Dickson K, Hanlon RT, Walters ET. 2014. Nociceptive sensitization reduces predation risk. Curr. Biol. 24, 1121–1125. ( 10.1016/j.cub.2014.03.043) [DOI] [PubMed] [Google Scholar]
- 5.Perrot-Minnot M-J, Banchetry L, Cézilly F. 2017. Anxiety-like behaviour increases safety from fish predation in an amphipod crustacea. R. Soc. open sci. 4, 171558 ( 10.1098/rsos.171558) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alupay JS, Hadjisolomou SP, Crook RJ. 2014. Arm injury produces long-term behavioral and neural hypersensitivity in octopus. Neurosci. Lett. 558, 137–142. ( 10.1016/j.neulet.2013.11.002) [DOI] [PubMed] [Google Scholar]
- 7.Crook RJ, Hanlon RT, Walters ET. 2013. Squid have nociceptors that display widespread long-term sensitization and spontaneous activity after bodily injury. J. Neurosci. 33, 10 021–10 026. ( 10.1523/JNEUROSCI.0646-13.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oshima M, di Pauli von Treuheim T, Carroll J, Hanlon RT, Walters ET, Crook RJ. 2016. Peripheral injury alters schooling behavior in squid, Doryteuthis pealeii. Behav. Processes 128, 89–95. ( 10.1016/j.beproc.2016.04.008) [DOI] [PubMed] [Google Scholar]
- 9.Hanlon RT, Claes MF, Ashcraft SE, Dunlap PV. 1997. Laboratory culture of the sepiolid squid Euprymna scolopes: a model system for bacteria-animal symbiosis on JSTOR. Biol. Bull. 192, 364–374. ( 10.2307/1542746) [DOI] [PubMed] [Google Scholar]
- 10.Hanlon RT, Messenger JB. 1996. Cephalopod behaviour. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 11.Seehafer K, Brophy S, Tom SR, Crook RJ. 2018. Ontogenetic and experience-dependent changes in defensive behavior in captive-bred Hawaiian bobtail squid, Euprymna scolopes. Front. Physiol. 9, 299 ( 10.3389/fphys.2018.00299) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zepeda EA, Veline RJ, Crook RJ. 2017. Rapid associative learning and stable long-term memory in the squid Euprymna scolopes. Biol. Bull. 232, 212–218. ( 10.1086/693461) [DOI] [PubMed] [Google Scholar]
- 13.Cartron L, Darmaillacq AS, Dickel L. 2012. The ‘prawn-in-the-tube’ procedure: what do cuttlefish learn and memorize? Behav. Brain Res. 240, 29–32. ( 10.1016/j.bbr.2012.11.010) [DOI] [PubMed] [Google Scholar]
- 14.Butler-Struben HM, Brophy SM, Johnson NA, Crook RJ. 2018. In vivo recording of neural and behavioral correlates of anesthesia induction, reversal, and euthanasia in cephalopod molluscs. Front. Physiol. 9, 109 ( 10.3389/fphys.2018.00109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crook RJ, Lewis T, Hanlon RT, Walters ET. 2011. Peripheral injury induces long-term sensitization of defensive responses to visual and tactile stimuli in the squid Loligo pealeii, Lesueur 1821. J. Exp. Biol. 214, 3173–3185. ( 10.1242/jeb.058131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anand KJ, Coskun V, Thrivikraman KV, Nemeroff CB, Plotsky PM. 1999. Long-term behavioral effects of repetitive pain in neonatal rat pups. Physiol. Behav. 66, 627–637. ( 10.1016/S0031-9384(98)00338-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Staudinger MD, Buresch KC, Mathger LM, Fry C, Mcanulty S, Ulmer KM, Hanlon RT. 2013. Defensive responses of cuttlefish to different teleost predators. Biol. Bull. 225, 161–174. ( 10.1086/BBLv225n3p161) [DOI] [PubMed] [Google Scholar]
- 18.Watanuki N, Kawamura G, Kaneuchi S, Iwashita T. 2000. Role of vision in behavior, visual field, and visual acuity of cuttlefish Sepia esculenta. Fish. Sci. 66, 417–423. ( 10.1046/j.1444-2906.2000.00068.x) [DOI] [Google Scholar]
- 19.Ford GK, Moriarty O, McGuire BE, Finn DP. 2008. Investigating the effects of distracting stimuli on nociceptive behaviour and associated alterations in brain monoamines in rats. Eur. J. Pain 12, 970–979. ( 10.1016/j.ejpain.2008.01.002) [DOI] [PubMed] [Google Scholar]
- 20.Villemure C, Bushnell MC. 2002. Cognitive modulation of pain: how do attention and emotion influence pain processing? Pain 95, 195–199. ( 10.1016/S0304-3959(02)00007-6) [DOI] [PubMed] [Google Scholar]
- 21.Perez PVPV, Butler-Struben HMHM, Crook RJRJ. 2017. The selective serotonin reuptake inhibitor fluoxetine increases spontaneous afferent firing, but not mechanonociceptive sensitization, in octopus. Invertebr. Neurosci. 17, 10 ( 10.1007/s10158-017-0203-1) [DOI] [PubMed] [Google Scholar]
- 22.Henderson YO, Victoria NC, Inoue K, Murphy AZ, Parent MB. 2015. Early life inflammatory pain induces long-lasting deficits in hippocampal-dependent spatial memory in male and female rats. Neurobiol. Learn. Mem. 118, 30–41. ( 10.1016/j.nlm.2014.10.010) [DOI] [PubMed] [Google Scholar]
- 23.Duerden EG, et al. 2018. Early procedural pain is associated with regionally-specific alterations in thalamic development in preterm neonates. J. Neurosci. 38, 878–886. ( 10.1523/JNEUROSCI.0867-17.2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grunau RE, et al. 2009. Neonatal pain, parenting stress and interaction, in relation to cognitive and motor development at 8 and 18 months in preterm infants. Pain 143, 138–146. ( 10.1016/j.pain.2009.02.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown JS, Kotler BP. 2004. Hazardous duty pay and the foraging cost of predation. Ecol. Lett. 7, 999–1014. ( 10.1111/j.1461-0248.2004.00661.x) [DOI] [Google Scholar]
- 26.Benatti C, Alboni S, Capone G, Corsini D, Caggia F, Brunello N, Tascedda F, Blom JMC. 2009. Early neonatal inflammation affects adult pain reactivity and anxiety related traits in mice: genetic background counts. Int. J. Dev. Neurosci. 27, 661–668. ( 10.1016/j.ijdevneu.2009.07.009) [DOI] [PubMed] [Google Scholar]
- 27.Moriarty O, Tu Y, Sengar AS, Salter MW, Beggs S, Walker SM. 2019. Priming of adult incision response by early life injury: neonatal microglial inhibition has persistent but sexually dimorphic effects in adult rats. J. Neurosci. 39, 3081–3093. ( 10.1523/JNEUROSCI.1786-18.2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walker SM, Fitzgerald M, Hathway GJ. 2015. Surgical injury in the neonatal rat alters the adult pattern of descending modulation from the rostroventral medulla. Anesthesiology 122, 1391–1400. ( 10.1097/ALN.0000000000000658) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walters ET. 2009. Chronic pain, memory, and injury: evolutionary clues from snail and rat nociceptors. Int. J. Comp. Psychol. 22, 127–140. ( 10.1159/000258667) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data associated with this study are available from Open Science Framework, under the project name ‘Early life injury in squid’. Link: https://osf.io/3gpfk/.