Abstract
Objective
We implemented a stepwise antimicrobial stewardship program (ASP). This study evaluated the effect of each intervention and the overall economic impact on carbapenem (CAR) use.
Method
Carbapenem days of therapy (CAR-DOT) were calculated to assess the effect of each intervention, and antipseudomonal DOT were calculated to assess changes in use of broad-spectrum antibiotics. We carried out segmented regression analysis of studies with interrupted time series for 3 periods: Phase 1 (infectious disease [ID] consultation service only), Phase 2 (adding monitoring and e-mail feedback), and Phase 3 (adding postprescription review and feedback [PPRF] led by ID specialist doctors and pharmacists). We also estimated cost savings over the study period due to decreased CAR use.
Results
The median monthly CAR-DOT, per month per 100 patient-days, during Phase 1, Phase 2, and Phase 3 was 5.46, 3.69, and 2.78, respectively. The CAR-DOT decreased significantly immediately after the start of Phase 2, but a major decrease was not observed during this period. Although the immediate change was not apparent after Phase 3 started, CAR-DOT decreased significantly over this period. Furthermore, the monthly DOT of 3 alternative antipseudomonal agents also decreased significantly over the study period, but the incidence of antimicrobial resistance did not decrease. Cost savings over the study period, due to decreased CAR use, was estimated to be US $150 000.
Conclusions
Adding PPRF on the conventional ASP may accelerate antimicrobial stewardship. Our CAR stewardship program has had positive results, and implementation is ongoing.
Keywords: antimicrobial resistance, antimicrobial stewardship, carbapenem; stewardship program, cost savings, postprescription review and feedback
INTRODUCTION
Carbapenem-resistant Enterobacteriaceae and other carbapenem-resistant bacteria exhibiting antimicrobial resistance (AMR) have become a global concern in recent years, and the implementation of AMR control measures has become an urgent task for medical institutions. Studies have found that the development of antimicrobial stewardship programs (ASPs) [1] and proactive clinician support by antimicrobial stewardship teams (ASTs) have decreased the unnecessary use of broad-spectrum antibiotics and reduced medical costs, with no negative effect on the outcomes of infectious diseases (ID) (and sometimes even improving these outcomes) [2, 3]. At our hospital, which is a tertiary acute-care hospital, we implemented a stepwise ASP for patients administrating carbapenem (CAR) antibiotics, supervised by a team led by ID physicians and pharmacists. In this ASP, we implemented postprescription review and feedback (PPRF) as the effect of the conventional ASP plateaued. Prospective review and feedback [4] and PPRF [5–8] have been reported widely as important ASP strategies. However, most of the research was reported from the United States or from European countries; there have been few studies of the effectiveness of ASPs at the facility level in other geographic areas. To promote ASP globally, it is important to report the effect of ASP from multiple areas, especially Asia [9]. Thus, we carried out a retrospective study to determine the impact of the ASP in our hospital in Japan.
METHODS
Setting
The study was done at the National Center for Global Health and Medicine (NCGM) Hospital in Tokyo, Japan. The hospital is an acute hospital responsible for tertiary care, with 781 beds in 43 clinical departments. These departments include a department of infectious diseases, staffed by 10 ID physicians, and 8 nonspecialist doctors. Five pharmacists are involved directly with the ASP. The work of the AST is rotated among the doctors in the department and the ASP pharmacists. Since April 2018, 2 ID physicians, 2 pharmacists, 2 clinical laboratory technicians, and 1 infection control nurse have served as the core members of the AST.
Study Design
This study was a single-institution retrospective before-and-after study that was carried out over a 93-month period from September 2010 to May 2018.
Antimicrobial Stewardship Program Interventions
The program was implemented in 3 phases:
Phase 1: Establishing an ID consultation service (September 2010 to May 2013)
Phase 2: Monitoring and feedback by the infection control team (June 2013 to May 2016)
Phase 3: PPRF by the antimicrobial steward team (June 2016 to May 2018)
These are described below.
Phase 1: Establishing an ID consultation service (September 2010 to May 2013)
Before the ID intervention was introduced, the Infection Control Team (ICT) monitored antimicrobial use density on a weekly or monthly basis without intervening. At the start of the program, 4 CAR antibiotics (imipenem/cilastatin, meropenem, panipenem/betamipron, and doripenem), were available in our hospital. In April 2010, the hospital established a general ID course to train ID physicians and introduced an ID consultation service. The ID consultations were provided by 5 ID physicians. In June 2012, 2 of the 4 CAR antibiotics, panipenem/betamipron and imipenem/cilastatin were removed from the hospital formulary and imipenem/cilastatin use was permitted only when all alternatives were unavailable. Less frequently used 2 of the 4 carbapenems, panipenem/betamipron and imipenem/cilastatin were removed from hospital formulary because of similar potency as meropenem or doripenem.
Phase 2: Monitoring and feedback by the ICT (June 2013 to May 2016)
Infection control interventions were introduced in June 2013. They comprised introduction of monitoring, e-mail feedback, and a notification system. As the AST had not yet been established, the intervention team consisted of ID physicians belonging to the ICT and fellow doctors, pharmacists, and clinical laboratory technicians. (The role of ICT and AST was shown in Supplementary Table 1.) In practice, the ICT pharmacists identified patients receiving CAR (meropenem, doripenem, and imipenem/cilastatin) every weekday. The ICT doctors monitored 4 parameters: (1) whether 2 sets of blood cultures were taken before starting CAR; (2) the reason for using CAR; (3) whether there was phased withdrawal or dosing-down once patients improved (so-called “de-escalation”); and (4) whether CAR was used for more than 2 weeks. The ICT doctors tabulated these 4 parameters and used them to provide weekly feedback to clinical departments, using the e-mail system linked to the electronic medical records. The content of the e-mail was informed to the prescriber by the head of the department. From June 2014, the ICT doctors also started providing feedback to clinical departments by e-mail on the number of cases of carbapenemase-producing Enterobacteriaceae (CPE) and carbapenem-resistant Pseudomonas aeruginosa (CRPA) infection detected each week to raise the awareness of the appropriate use of CAR. These reports were monitored by clinical laboratory technicians. In September 2014, a notification system was introduced whereby a clinician starting a patient on CAR was required to notify the ICT. During this period, the ICT did not engage clinicians directly in its interventions. Another study using the Verigene system, which evaluated the impact of rapid identification of positive blood cultures on antibiotic prescriptions, was performed during this period [10].
Phase 3: PPRF by the antimicrobial steward team (June 2016 to May 2018)
The AST intervention, which was launched in June 2016, comprised PPRF. From June 2016 to April 2017, AST pharmacists identified patients who had been taking CAR continuously for at least 21 days, and the pharmacists telephoned the clinician concerned to discuss the CAR treatment strategy. From April 2017, an AST doctor carried out a review of each patient for whom CAR was prescribed, and if doctors considered that the information from medical records was not enough to make a decision regarding the necessity of CAR use, a member of the AST directly examined the patient. If such an examination was performed, an AST doctor telephoned the clinician concerned and made a note in the patient's medical record suggesting either the continuation of CAR use, phasing it out, or discontinuation. The appropriateness of the treatment was decided based on the results of culture, course of the treatment, and duration of treatment. If the clinician's opinion on CAR use differed from the recommendation of the AST, this was discussed, and the final decision was left to the discretion of the clinician. The decision of the necessity of direct examinations may vary because AST doctors rotate every 3 months during Phase 3. From May 2017, the intervention was expanded to include all patients who had been taking CAR continuously for at least 7 days. From April 2018, the intervention was expanded again to include all patients who had been taking CAR continuously for at least 3 days. The intervention team comprised an AST ID physician, a nonspecialist doctor, and a pharmacist. During this period, the Japanese national action plan on antimicrobial resistance developed in April 2016 as a governmental policy on antimicrobial resistance.
Outcomes
Primary outcome
The primary outcome measure of this study was the change in CAR-DOT, expressed as DOT per 100 patient-days per month.
Secondary outcomes
Days of therapy for antipseudomonal agents other than CAR.
The secondary outcome measure of this study was the total DOT per month per 100 patient-days of 3 antipseudomonal agents: CAR, piperacillin/tazobactam (PIPC/TAZ), and cefepime (CFPM), because these antibiotics are regarded as broad-spectrum, similar to CAR. This parameter was calculated to check whether CAR was simply being replaced with other broad-spectrum antipseudomonal agents. The total DOT per month per 100 patient-days of other antipseudomonal agents, such as piperacillin, ceftazidime, aztreonam, aminoglycosides, and fluoroquinolones, also were calculated.
Incidence of resistant bacteria detections, Clostridioides difficille infections, and candidaemia.
We measured the incidence of antibiotic-resistant bacteria detections and Clostridioides difficile infection (CDI) per 1000 patient-days between April 2012 and May 2018, the period for which data were available, as an indicator of the outcome of the ASP. Carbapenemase-producing Enterobacteriaceae was defined as a gram-negative Enterobacteriaceae-producing carbapenemase regardless of genotype. Carbapenem-resistant Pseudomonas aeruginosa was defined as Pseudomonas aeruginosa that was resistant to CAR and either aminoglycoside or fluoroquinolone in Clinical and Laboratory Standards Institute's standard antimicrobial susceptibility tests, regardless of whether carbapenemase was produced. Clostridioides difficile infection was defined as the number of patients with positivity of CD toxin. We also included the incidence of candidaemia, which was previously reported to be associated with CAR use in low-birthweight neonates [11]. Samples collected within 48 hours of admission were excluded. In order to exclude duplicates, only the first sample was considered if the same strain was detected from the same patient more than once. There were no major changes in infection control policy in the hospital during this period.
Cost of CAR.
We evaluated the cost of purchasing CAR each year from 2012 to 2017 in order to assess the economic impact of the ASP. As the costs were calculated annually according to the Japanese fiscal year (from April to March), we compared the costs in units of a fiscal year (ie, 2012 for Phase 1, 2015 for Phase 2, and 2017 for Phase 3). We calculated the actual cost of purchasing CAR (taking into consideration the cost of switching from branded to generics and fluctuations in drug prices) and the adjusted cost of purchasing CAR on the basis of the price of branded CAR in April 2012.
Statistical Analysis
To demonstrate the effect of each intervention on CAR-DOT and DOT with 3 antipseudomonal agents, we carried out segmented regression analysis of interrupted time series studies [12] for 3 periods from September 2010, for which DOT data were available: Phase 1 (September 2010 to May 2013), Phase 2 (June 2013 to May 2016), and Phase 3 (June 2016 to May 2018). Trends in the incidence of antibiotic-resistant bacterial infection, CDI, and candidaemia were evaluated using the Jonckheere-Terpstra test. Bivariate analysis was carried out using Fisher exact test and a χ 2 test (categorical variables) or the Mann-Whitney test (continuous variables), with P < .05 regarded as statistically significant. Stata version 15.1 (StataCorp, College Station, TX) was used for segmented regression analysis of interrupted time series, and EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan) was used for all the other analyses.
Ethical Considerations
This study was approved by the Institutional Review Board of the NCGM (approval number: NCGM-G-003220-00).
RESULTS
Trends in Carbapenem Use
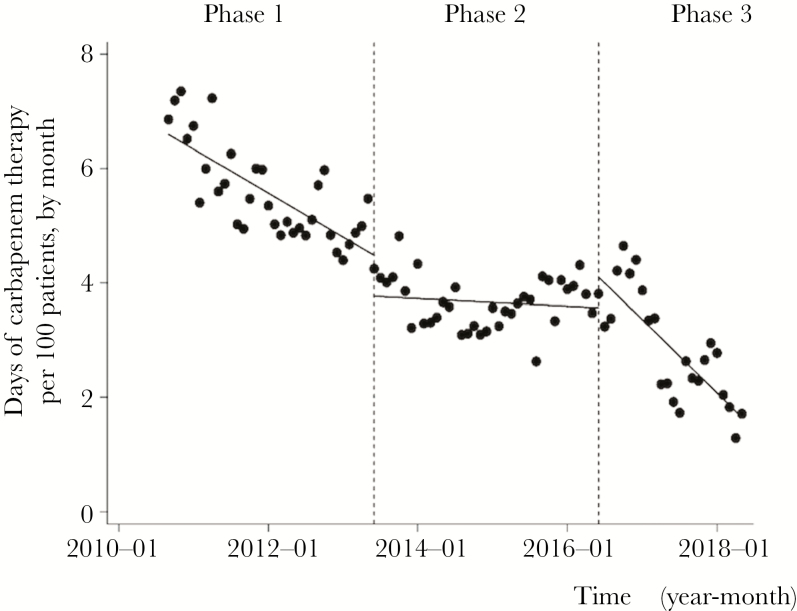
Figure 1 shows changes in the CAR-DOT during each phase. The median monthly CAR-DOT was 5.46 days per 100 patient-days (interquartile range [IQR], 4.99–6.03) during Phase 1 and 3.69 days per 100 patient-days (IQR, 3.37–4.06) during Phase 2. The CAR-DOT decreased significantly immediately after the start of Phase 2, but a major decrease was not observed during this period. Although the immediate change was not apparent after Phase 3 started, CAR-DOT decreased significantly over this period. During the Phase 3, the median CAR-DOT was 2.78 days per 100 patient-days (IQR, 2.22–3.52). Although the immediate change was not apparent after the Phase 3 started, CAR-DOT decreased significantly over this period (slope changed from 0.06 to –0.10, P < .001).
Figure 1.
Trends of Days of Carbapenem Therapy per 100 Patients, by Month, During Each Phase of the Intervention Period Each dot refers to the days of carbapenem therapy per 100 patients each month and a slope based on linear regression in each phase. The explanation of each phase is as follows: Phase 1 (establishing an infectious disease consultation service from April 2010 to May 2012); Phase 2 (monitoring and feedback by the infection control team from June 2012 to May 2016); and Phase 3 (postprescription and feedback review by the antimicrobial steward team from June 2016 to May 2018). Days of carbapenem therapy started to decrease immediately after the start of Phase 2 (P = .005), but the major decrease was not observed during this period (change of slope from –0.06 to 0.06, P < .01). Although no change was evident immediately after the Phase 3 started (P = .06), days of carbapenem therapy decreased significantly over this period (change of slope from 0.06 to –0.10, P < .001).
Use of Antipseudomonal Agents
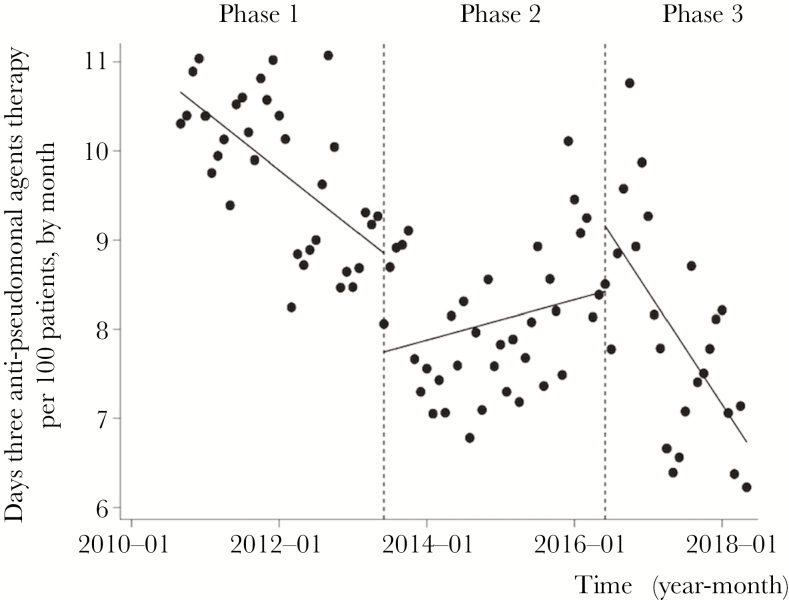
Figure 2 shows changes in the monthly DOT of 3 antipseudomonal agents (CAR, PIPC/TAZ, and CFPM), according to Figure 1. The median DOT was 9.99 days per 100 patient-days (IQR, 9.01–10.50) during Phase 1 and 8.09 days per 100 patient-days (IQR, 7.60–8.68) during Phase 2. Although there was a statistically significant decrease in the monthly DOT immediately after the start of Phase 2 (P = .003), the monthly DOT increased overall (change of slope from –0.05 to 0.07, P = .10). The median DOT during Phase 3 was 7.86 days per 100 patient-days (IQR, 7.15–8.82). Although no change was evident immediately after the beginning of Phase 3 (P = .14), the DOT decreased significantly over this period (change of slope from 0.07 to –0.12, P < .001). Although DOT of other antipseudomonal agents, such as piperacillin, ceftazidime, aztreonam, aminoglycosides, and fluoroquinolones, also were calculated, the DOT of these antibiotics did not increase followed by the decrease in CAR use (Supplementary Figure 1).
Figure 2.
Trends of Days of 3 Antipseudomonal Agents per 100 Patient Days, by Month, During Each Phase of the Intervention Period Each dot refers to days of 3 antipseudomonal agents therapy per 100 patients each month, and the slope is based on linear regression in each phase. The explanation of each phase is as follows: Phase 1 (establishing an infectious disease consultation service from April 2010 to May 2012), Phase 2 (monitoring and feedback by the infection control team from June 2012 to May 2016), and Phase 3 (postprescription review and feedback by the antimicrobial steward team from June 2016 to May 2018). Although there was a statistically significant decrease in days of 3 antipseudomonal agents therapy immediately after the start of Phase 2 (P = .003), days of 3 antipseudomonal agents therapy increased over this period (change of slope from –0.05 to 0.07, P = .10). No change was evident immediately after the beginning of Phase 3 (P = .14), and days of 3 antipseudomonal agents therapy decreased significantly over this period (change of slope from 0.07 to –0.12, P < .001).
Incidence of Antimicrobial-Resistant Bacterial Infection, CDI, and Candidaemia
Table 1 shows the incidence of CPE, CRPA infection, candidaemia, and CDI. The tests for trend found no significant decrease in the incidence of CPE (P = .26)), CRPA (P = .53), or candidaemia (P = .48) from Phase 1 to Phase 3, but it did show that the incidence of CDI decreased significantly during this period (P = .01).
Table 1.
Trends of Carbapenem Associated Outcome Indicators from 2012 to 2018
| Year | CPEa | CRPAa | Candidaemiaa | CDIa | Inpatient total number |
|---|---|---|---|---|---|
| 2012a | 0.074 | 0.074 | 0.114 | 0.22 | 175860 |
| 2013 | 0.073 | 0.105 | 0.105 | 0.17 | 246916 |
| 2014 | 0.118 | 0.134 | 0.049 | 0.20 | 245703 |
| 2015 | 0.074 | 0.095 | 0.066 | 0.22 | 243234 |
| 2016 | 0.106 | 0.119 | 0.059 | 0.10 | 235762 |
| 2017 | 0.051 | 0.060 | 0.120 | 0.09 | 233192 |
| 2018b | 0.021 | 0.053 | 0.137 | 0.06 | 94549 |
Abbreviations: CDI, Clostridioides difficile infection; CPE, carbapenemase-producing Enterobacteriaceae; CRPA, Carbapenem-resistant Pseudomonas aeruginosa.
a Data are presented per 1000 patient-days.
b The data of 2012 is for April–December, and of 2018 is for January–May.
Cost of CARs
Table 2 shows the actual and adjusted CAR purchase costs for each year. The actual CAR purchase cost in 2017 (Phase 3) was US $141 205, a savings of approximately US $300 000 compared to the actual CAR purchase cost of US $440 581 in 2012 (Phase 1). It also was approximately US $90 000 lower than the actual CAR purchase cost of US $231 861 in 2015 (Phase 2). The adjusted CAR purchase cost in 2017 (Phase 3) was US $300 957, a savings of approximately US $150 000 compared to the adjusted CAR purchase cost of US $493 952 in 2012 (Phase 1). It also was approximately US $130 000 lower than the adjusted CAR purchase cost of US $429 100 in 2015 (Phase 2).
Table 2.
Purchase Costs of Carbapenem from 2012 to 2017
| Year | Actual cost USD | Adjusted cost USD |
|---|---|---|
| 2012 | $440 581 | $493 952 |
| 2013 | $319 396 | $477 604 |
| 2014 | $226 791 | $408 716 |
| 2015 | $231 861 | $429 100 |
| 2016 | $239 388 | $495 581 |
| 2017 | $141 205 | $300 957 |
The actual cost includes the cost of switching to generic drugs and takes changing drug prices into account.
The adjusted cost is calculated based on the drug price in April 2012.
DISCUSSION
In this study, we evaluated the effects of a phased intervention on long ASP and demonstrated the effectiveness of PPRF as an ASP for CAR. A well-organized education system of infection control and prevention for doctors is not established in Japan, and the knowledge of its necessity is not prevalent among clinicians. Therefore, solid ASP (eg, PPRF) has not been established nationwide. Results in our study are consistent with previous reports from Japan and elsewhere of the efficacy of PPRF [2, 7]. However, our study demonstrated that the effectiveness of stewardship by infection control consultation (Phase 1) or monitoring (Phase 2) alone eventually wears off and that the addition of PPRF (Phase 3), based on ID consultation provided by a dedicated AST, further increased the effectiveness of the ASP.
During Phase 1, ID physicians optimized the antibiotic selection for individual patients at the request of the patients' clinicians. However, this intervention had no effect on the prescribing behavior of those clinicians who did not request an ID consultation; thus, its effectiveness eventually plateaued. There was no major change in CAR-DOT during Phase 2, but the DOT with the 3 antipseudomonal agents increased. This phenomenon was caused by clinicians simply replacing CAR with other broad-spectrum antibiotics without reducing their prescription of broad-spectrum antibiotics. However, PPRF, implemented in Phase 3, is a direct intervention targeting clinicians' CAR use, which engages all clinicians who use CAR irrespective of whether they request a consultation. Thus, PPRF is likely to have changed the prescribing behavior of a larger number of clinicians.
In this study, the incidence of CPE, CRPA, and candidaemia did not decrease, despite the large reduction in CAR-DOT. The incidence of these infections may have been affected by factors other than antibiotic use, such as the use of indwelling devices and previous invasive procedures [13–15]. This explains why the incidence of these infections did not decrease as a result of the ASP, which only targeted CAR. However, the incidence of CDI decreased. During the study period, antibiotics associated with the occurrence of CDI other than CAR were not decreased, except for fluoroquinolones, and the DOT of fluoroquinolones was relatively small compared to that of CARs (Supplementary Figure 2). Moreover, there was no major change in the infection control policy during the study period. Thus, the decrease in CAR-DOT may have led to the decreased incidence of CDI. The occurrence of CDI is likely to be more directly affected by CAR selection pressure than that of resistant bacteria and candidaemia. However, it is not possible to assess whether the decrease in the incidence of CDI decreased as a result of the ASP interventions, and this needs further investigation.
The decrease in CAR-DOT during Phase 1 and Phase 3 led to a large reduction in purchase costs. Even after correcting for factors, including switching to generics and changes in drug prices, this cost still decreased by a large amount, indicating that the decrease in CAR use, which is attributable to the ASP, had a major effect on hospital costs. Presenting the effects of ASP as a specific outcome is important for justifying the presence of an AST and for enhancing their credibility, enabling them to carry out further activities.
This study had several limitations. First, this ASP was implemented as a part of hospital quality improvement, not for clinical research. Thus, we could not remove various cofactors, such as the quality of infection control and prevention, other clinical research, staff turn-over, and other undocumented factors. In addition, the study design was a single-arm interrupted time series analysis; therefore, we could not assure the plausibility of the effect of interventions or the causal relationship between ASP and various outcomes. Second, we did not assess changes in the characteristics of patients taking CAR or the association between the content of PPRF interventions and patient outcomes. Third, it may not be possible for PPRF to achieve the same effect if hospitals do not have the resources for ID physicians and pharmacists to conduct PPRF together on a daily basis. Thus, hospital size and human resources limit the generalizability of this intervention. Finally, although this study suggests that PPRF may lead to further antibiotic cost savings, further studies are required to evaluate the costs of staff, time, and facilities required for ASPs, as well as those associated with patient outcomes. Nevertheless, prioritizing PPRFs focused on particular antibiotics, like the PPRF for CAR practiced in our hospital, may be a good strategy within an existing ASP [1, 4].
Our results in this study showed that an ASP consisting solely of monitoring the use of CAR and providing e-mail feedback had a transient effect and that their effectiveness eventually plateaued. ASPs that do not directly engage clinicians may cause simple replacement with other broad-spectrum antimicrobial agents. AMR to other antimicrobial agents increases if clinicians substitute CAR with other antibiotics without changing their prescribing practices. However, our study showed that the addition of PPRF based on ID consultations by an AST and led by ID physicians and pharmacists may decrease CAR use without triggering increases in the use of other antimicrobial agents.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We are grateful to the doctors of the Disease Control and Prevention Center, the pharmacists of the Department of Pharmacy, and all of the clinical staff of the National Center for Global Health and Medicine for their commitment to patient care.
Author contributions. T.A., Y.K., and K.H. performed conceptualization work for this paper. Data was curated by T.A.; T.A. and H.F. performed formal analysis. Funding was acquired by K.H. Investigations were made by Y.M., H.O., S.T., K.Y., R.H., A.S., M.O., M.I., and S.T. The methodology for this paper was created by Y.K. and K.H.; N.O. supervised. Validation was performed by S.K. and H.T. The original draft of this paper was written by T.A. It was reviewed and edited by T.A., Y.K., K.H., and N.O.
Finanacial support. This study was funded by a National Center for Global Health and Medicine grant for International Health Research and Development (no. 28S-1106). However, the funding provider played no role in the study design, data collection or analysis, decision on publication, or manuscript preparation.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Barlam TF, Cosgrove SE, Abbo LM, et al. . Implementing an antimicrobial stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 2016; 62:e51–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cai Y, Shek PY, Teo I, et al. . A multidisciplinary antimicrobial stewardship programme safely decreases the duration of broad-spectrum antibiotic prescription in Singaporean adult renal patients. Int J Antimicrob Agents 2016; 47:91–6. [DOI] [PubMed] [Google Scholar]
- 3. Campbell TJ, Decloe M, Gill S, Ho G, McCready J, Powis J. Every antibiotic, every day: maximizing the impact of prospective audit and feedback on total antibiotic use. PLOS ONE 2017; 12:e0178434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Doernberg SB, Abbo LM, Burdette SD, et al. . Essential resources and strategies for antimicrobial stewardship programs in the acute care setting. Clin Infect Dis 2018; 67:1168–74. [DOI] [PubMed] [Google Scholar]
- 5. Tamma PD, Avdic E, Keenan JF, et al. . What is the more effective antimicrobial stewardship intervention: preprescription authorization or postprescription review with feedback? Clin Infect Dis 2017; 64:537–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kimura T, Uda A, Sakaue T, et al. . Long-term efficacy of comprehensive multidisciplinary antibiotic stewardship programs centered on weekly prospective audit and feedback. Infection 2018; 46:215–24. [DOI] [PubMed] [Google Scholar]
- 7. Tagashira Y, Horiuchi M, Tokuda Y, Heist BS, Higuchi M, Honda H. Antimicrobial stewardship for carbapenem use at a Japanese tertiary care center: an interrupted time series analysis on the impact of infectious disease consultation, prospective audit, and feedback. Am J Infect Control 2016; 44:708–10. [DOI] [PubMed] [Google Scholar]
- 8. Honda H, Murakami S, Tagashira Y, et al. . Efficacy of a postprescription review of broad-spectrum antimicrobial agents with feedback a 4-year experience of antimicrobial stewardship at a tertiary care center. Open Forum Infect Dis 2018; 22;5:ofy314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Honda H, Ohmagari N, Tokuda Y, Mattar C, Warren DK. Antimicrobial stewardship in inpatient settings in the asia pacific region: a systematic review and meta-analysis. Clin Infect Dis 2017; 64:119–26. [DOI] [PubMed] [Google Scholar]
- 10. Hayakawa K, Mezaki K, Kobayakawa M, et al. . Impact of rapid identification of positive blood cultures using the Verigene system on antibiotic prescriptions: a prospective study of community-onset bacteremia in a tertiary hospital in Japan. PLOS ONE 2017; 12:e0181548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fu J, Wang X, Wei B, Jiang Y, Chen J. Risk factors and clinical analysis of candidemia in very-low-birth-weight neonates. Am J Infect Control 2016; 44:1321–5. [DOI] [PubMed] [Google Scholar]
- 12. Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther 2002; 27:299–309. [DOI] [PubMed] [Google Scholar]
- 13. Woerther PL, Lepeule R, Burdet C, Decousser JW, Ruppé É, Barbier F. Carbapenems and alternative β-lactams for the treatment of infections due to extended-spectrum β-lactamase-producing Enterobacteriaceae: what impact on intestinal colonisation resistance? Int J Antimicrob Agents 2018; 52:762–70. [DOI] [PubMed] [Google Scholar]
- 14. Raman G, Avendano EE, Chan J, Merchant S, Puzniak L. Risk factors for hospitalized patients with resistant or multidrug-resistant Pseudomonas aeruginosa infections: a systematic review and meta-analysis. Antimicrob Resist Infect Control 2018; 7:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kofteridis DP, Valachis A, Dimopoulou D, et al. . Factors influencing non-albicans candidemia: a case-case-control study. Mycopathologia. 2017; 182:665–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.