Abstract
Flexible ureteroscopy is an important therapeutic and diagnostic procedure and has seen rapid rise in its utilisation in recent years. There have been numerous developments in flexible ureteroscope (fURS) technology but scope fragility, and the associated high maintenance costs, remains a concern. A comprehensive Medline search for related publications from the last 20 years was undertaken to identify common causes of fURS damage and ascertain practices to minimise this. Flexible ureteroscopy can be due to intraoperative causes (loss of the deflection mechanism, damage to the working channel due and fibreoptic bundle injury) and non-operative damage which occur during cleaning, sterilisation and handling of the fURS. The review summarises the available literature to help highlight common mechanisms of scope damage, and outlines evidence-based measures to reduce the risk of damage and maximise durability. Scope fragility remains a problem with significant associated cost implications. In a culture of rising fURS use and reducing re-imbursement for endourologists, prolonging the longevity of the fURS is imperative for maintaining profitability. There are simple and inexpensive practices which can be immediately adopted to maximise fURS use and reduce the need for repairs.
Keywords: Flexible ureteroscopy, ureteroscope, scope durability, ureteroscope damage, ureteroscope repair
Introduction
Since the first report half a century ago, use of the flexible ureteroscope (fURS) in Urology for diagnostic and therapeutic indications has grown exponentially (1). This rapid upsurge in fURS usage is in no small part due to the rising prevalence of kidney stone disease as a ubiquitous, worldwide condition which poses a clinical and economic public health burden upon the population (2-4).
Where data is available, kidney stone disease is now the second most expensive urological disease (5). Data from the USA demonstrates that in a 30-year period from 1984 to 2014, the annual estimated direct healthcare costs of urolithiasis rose from $898 million to $5.3 billion (5). Similarly, prevalence data from Europe, Australia and Far-Eastern Asia show a significant rise in patients diagnosed with renal tract stones (6).
Exciting developments in endoscopic technology, including scope miniaturisation, advances in active and passive deflection, and high definition image digitalisation combined with a plethora of high-quality accessories now mean that no part of the upper urinary tract is out of reach with a fURS (7). For these, and other reasons, the popularity of fURS has risen rapidly in the UK (8,9). A recent systematic review shows similar worldwide trends over the past two decades charting the rapid rise of fURS utilisation in most parts of the world, often at the expense of extracorporeal shock wave lithotripsy (ESWL) (4).
fURSs have undergone significant engineering over the last 30 years. This progress has been influenced by two key factors; first, the drive to reduce scope diameter and tip size and second, in response to an increased awareness of scope damage with a need to maximise durability and reduce repair costs (10). Nevertheless, technological advancements have come at the expense of endoscope fragility. Expensive repair or replacement of a fURS in a high-volume centre can have a significant impact on the clinical and cost effectiveness of the institution. In this paper we review the causes of fURS damage and the steps that the urologist may take to minimise damage and prolong the life of their ureteroscopes.
Methods
We undertook a comprehensive Medline search using the following search keywords: “flexible ureteroscope/y”, “durability”, “handling”, “protecting”, “usage” and “damage”. The filter “humans” was applied, and the search limited to the last 20 years, given the rapid evolution in scope technology and innovation in the last two decades. In total, 255 articles were identified, from which 60 were deemed appropriate for abstract review and subsequent consideration in this review. We aim to summarise the literature regarding trends of ureteroscope damage and thus identify techniques to minimise malfunction and maximise usage.
Does ureteroscope durability really matter?
Ureteroscopy has consistently been reported as being a more cost-effective treatment with lower overall costs compared to other stone treatment modalities (6,11). Even beyond the capital investment to acquire a fURS, subsequent care, maintenance, breakages and repair become significant cost considerations. The total cost of providing a fURS service differs depending on multiple factors including instrument costs, ancillary care expenses, hospital overheads and local reimbursement tariffs thus making it difficult to establish precise costs. Nevertheless, early experience reports of fURS damage concluded that scopes required frequent repair after only 12–15 procedures, but even more recent published data with newer ureteroscopes suggest that failure will occur at an average of 9–50 cases, though some authors have described a higher usage in selected cases (12-14).
Data from large volume centres place an annual estimated repair cost for each institution ranging from $44,722 to $115,000, with an average cost per repair for a major repair ranging between $4,500 and $7,521 (15-17). Therefore, the estimated average repair cost per case varies from $355 to $605. This suggests that a concerted and intentional focus on improving ureteroscope durability and reducing repair/replacement costs is essential to maintain profitability against a background of rising fURS usage and decreasing reimbursement in many health economies (17).
fURS damage
Karl Storz (Tuttlingen, Germany) was a pioneer of miniaturisation, developing a 7.5-Fr scope with a 3.7-Fr working channel in 1993 (Model 11274AA) (7). Subsequent reduction in the scope diameter allowed for better manoeuvrability within the pelvicalyceal system, but was found to result in increased fragility regardless of make and model (18). Engineers have also had to contend with the challenge of retaining light and image quality while reducing size and this has been permitted by the development of digital scopes and light emitting diodes (LED).
Traditional analogue fURS rely on fibre optics for the passage of light from an external source down the scope and transmission of images back to the camera unit. The image quality depends on the number of fibres in the bundle which in turn limits the minimum scope diameter (7). The fragile fibres are subject to breakages and subsequent poor image quality over time. Digital videoscopes utilise an LED at the scope tip instead of an external light source and images are captured via a digital microchip (chip-on-the-tip) and transmitted via a single wire, negating the need for an optic lens or camera head. This leads to high resolution image quality and ergonomics which significantly reduce operating time (19). Combined data from four major fURS manufacturers showed that the most common types of damage requiring repair were due to working channel damage (52%), scope shaft malfunction (27%), impaired deflection components (15%), and eyepiece components damage (8%) (18).
fURS damage can broadly be divided into those related to:
Intra-operative scope handling and use of accessory equipment during surgical usage, and
Non-operative handling during ureteroscope cleaning, sterilization and transfer.
Additionally, the risk of damage also increases with scope age and the extent and number of previous repairs (20).
Intraoperative causes of ureteroscope damage
The three most common intraoperative causes of fURS damage are:
❖ Loss of deflection at the tip due to repetitive extreme deflection;
❖ Damage to the working channel secondary to frequent instrumentation or accidental laser firing (10); and
❖ Fibre optic bundle damage (21).
Other causes of damage include fluid intrusion, lens damage or ureteral access sheath (UAS) damage.
Deflection
The active deflection at the tip of a fURS is its most fragile moveable part. Most scopes now boast 270° bi-directional active deflection but with prolonged or excessive stress applied to the deflection mechanism, this range decreases over time (Figure 1) (7). The bending sheath, angulation cables and deflection mechanisms are all possible sites of damage (22). In an independent analysis of the Olympus fURSs by Canales et al., it was found that damage to the outer sheath (outer bending rubber of the distal deflection tip) rather than in the inner working channels at the distal end was the most common site (10). It is speculated that intra-operative fURS impact trauma, aggressive bending, improper access sheath usage and careless processing are likely causes of deflection mechanism damage (Figure 2) but there is a clear need for a robust prospective database in each unit to identify the role and frequency of each factor (10).
Figure 1.

Loss of active deflection range over time.
Figure 2.
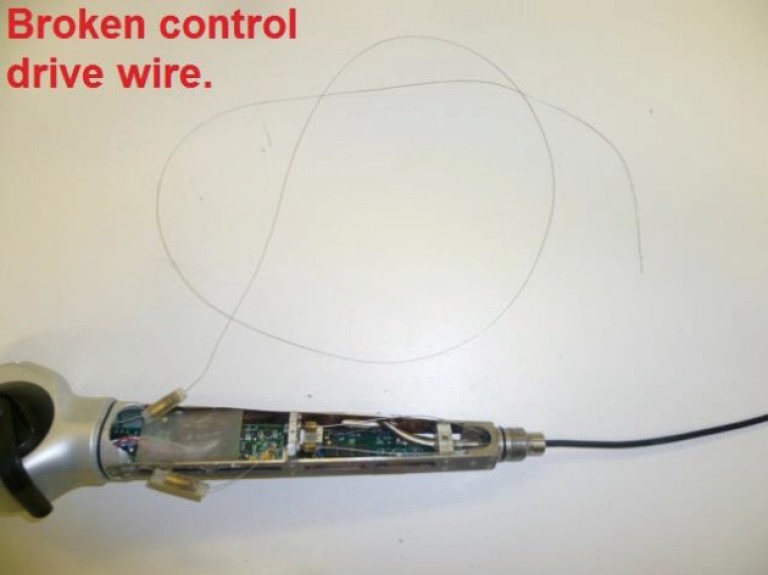
Example of the complete breakage of the deflection mechanism control wire due to excessive forced deflection.
Lower pole stones
Ozimek et al. found that a steep infundibulopelvic angle of 60° or less is an important risk factor for ureteroscope damage due to the prolonged and exaggerated deflection required to treat a stone in this location damage (23). A steep angle reduces efficiency of laser energy transfer down the fibre also making it more prone to fracture, increasing fragmentation time and stress on the fURS. Re-location of lower pole stones into a straighter middle or upper pole calyx for treatment has been demonstrated to reduce deflection mechanism damage (24).
UAS
The UAS splits opinions with some authors recommending routine use while others limit use to selected and challenging cases (19). Frequently extolled advantages of the UAS include ease of multiple fURS passage, reduced intra-renal pressures, better stone free rate, and shorter operative times (19). Nevertheless, in a recent systematic review debunking the “facts and myths” surrounding the UAS, authors have concluded that there is no convincing evidence to date that routine UAS use results in improved stone free rates or shorter operative times (25). In addition, the review also concludes that the effect of UAS use on fURS durability has not been appropriately evaluated. Most studies have simply stated theories for UAS-mediated fURS damage, with proposed mechanisms including removal of the fURS while deflected against the tip of the UAS, and damage to the outer coat if a small stone fragment is lodged between the shaft of the fURS and the UAS lumen while the fURS is removed forcibly (13,24,26). The risk of damage to the distal deflection tip against the end of the UAS is thought to be reduced if both the UAS and the ureteroscope are extracted together, with the distal end of the ureteroscope left outside the UAS in view (26,27).
Working channel damage
Nitinol devices
Numerous accessories traverse the fURS working channel including guidewires, Nitinol devices including baskets and forceps, and laser fibres. Seto and colleagues analysed damage to the working channel of the fURS caused by repeated insertion and removal (up to 100 times) of a variety of accessories (28). The fURS scopes were meticulously tested for pin-hole damage using an air-leak test as well as by direct visualisation using a fine (2.4 F) fibrescope. Remarkably, the Nitinol stone-retrieval basket and biopsy forceps (up to 3 F) caused no significant damage to the working channel despite a scope deflection of 120° (28). However, even the 200 µm holmium laser fibres caused visible damage to the inner channel surface of the channel with a fURS deflection of >60° and clear evidence of channel penetration at 120° deflection. Therefore, while care must be taken when using Nitinol baskets/forceps, it is imperative that laser fibre insertion is performed with the fURS tip in the neutral position.
Guidewires
Aside from the safety benefits of a guidewire during upper tract endoscopy, it is proposed that guidewire placement also leads to a straightening of the ureter, thus reducing its tortuosity while decreasing the risk of scope flexion on introduction into the ureter (29). Safety guidewire use was found to have a beneficial effect with an estimated reduction in the risk of fURS damage by around 50%, though this effect needs to be further investigated (30).
Conversely, careless use of guidewires has the propensity to facilitate endoscope damage. For example, “backloading” of the fURS over a working wire could also result in working channel puncture and flap creation (7). We propose that backloading, when necessary, is performed over a well lubricated, secured, hydrophilic wire without any kink, held in as straight a position as possible.
Laser fibre related damage
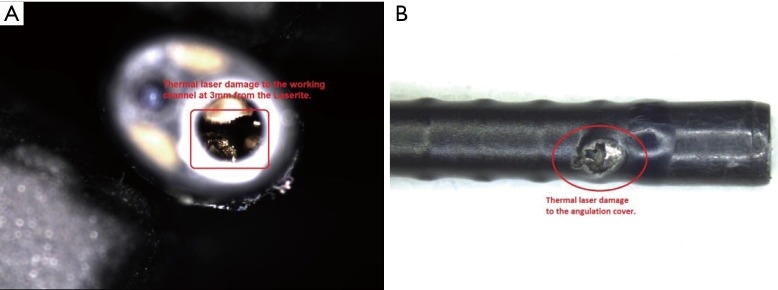
Ninety percent of laser damage is to be found in the distal 3 to 4 mm of the fURS (Figure 3) (18). Undoubtedly, thermal laser damage to the fURS working channel is a common cause of scope failure and every laser fibre must be checked for defects prior to use and must never be fired while inside the channel (13,31). Additionally, laser fibres can cause significant working channel damage when inserted in a fURS with a deflection of >45–60° (28,32). It is essential to keep the scope tip straight during insertion of a laser fibre.
Figure 3.
Examples of laser mediated damage. (A) Working channel laser damage; (B) laser damage to the distal angulation cover.
A modified fibre tip may help reduce working channel injury. Carlos et al. found that using a ball-tip laser fibre, such as the Flexiva TracTip (Boston Scientific, MA, USA) created less damage compared to the standard flat-tip laser fibres (32). Flat-tip fibres seemed to require >5-fold insertion forces at all angles and caused demonstrable fURS damage when deflected >45°, while a ball-tip fibre could be safely passed through an 180° deflected ureteroscope liner without causing trauma. However, because of the burn-back effect of any laser fibre, the ball tip may only last for a few seconds of laser lithotripsy, thus limiting its role in cases of multiple insertions and removals.
There is conflicting evidence regarding the safety and efficiency of reusable versus single use laser fibres. Previous reports have proposed re-usable fibres to be more cost effective, but they have not included data on laser related damage (33). Conversely, a UK-based study found that single use laser fibres showed consistent performance in energy transmission and fracture resistance and though associated with a higher initial cost, they caused less damage than reusable ones which were more likely to cleave or display micro-fractures inside the working channel and cause thermal damage (34). Based on their calculations, the overall cost of fURS repairs and sterilisation of reusable fibres more than justified the cost-effectiveness of routinely employing single use fibres (34). An American report, acknowledged that there may be reduced repair costs with a single-use laser fibre, but calculated that the fURS would need to be used for around 100 procedures before repair to justify the cost of routinely employing single-use laser fibres (16). It is clear that cost effectiveness of single versus reusable laser fibres is relation to fURS durability is dependent on many factors, including locally agreed reimbursement tariffs and therefore a wholesale recommendation is not possible.
Either way, all laser fibres—regardless of their diameter—are prone to degradation and may result in fURS damage. One technique to reduce this risk is to cleave the fibre after every 10 minutes of use (35). Furthermore, fibre cleaving can be undertaken using standard metallic scissors without retrieving the fibre through the fURS, to reduce the risk of damage to the fURS from repeated insertions (36).
Another concept to reduce the risk of laser damage to the fURS tip is that of keeping a “safety distance”. Due to the subtle differences in scope design, the appearance of the laser fibre on the viewing screen will vary between ureteroscopes with at least 1 mm of fibre protruding from the end of the 0° camera fURS before it is visualised (37). This distance however will vary, but in a study comparing seven scopes, investigators observed that in all cases, when advanced to one-quarter of the screen, the fibre was out 3 mm or more from the fURS tip (37). This >3 mm distance offers clear protection for the fURS tip from the laser-generated plasma bubble as well as stone fragments and is regarded as the “safety distance” to avoid fURS injury even when using high energy with a short pulse (37).
Laser protection technologies
Various technologies have been developed to reduce laser-related damage to the working channel. Karl Storz newer generation endoscopes utilise Laserite™ ceramic technology which reduces risk of thermal laser damage to the distal tip (7). Flexguard™ (LISA Laser Products, Katlenburg-Lindau, Germany) is a single use laser fibre sheath with an outer diameter of 0.9 mm designed for 3.6 F working channel scopes which can be used to reduce fibre-insertion related injury, though it is not designed to protect the scope from laser damage if inadvertently activated inside the working channel (38-40). A disadvantage of the Flexguard™ sheath, apart from the obvious additional cost implication, is the reduction in irrigant flow and fURS deflection tip when in situ, which can be minimised by removal of the sheath once the laser fibre has been inserted into position (38,39). The Endoscope Protection System (EPS) (Gyrus-ACMI, Southborough, MA, USA) relies on sensory feedback from the end of the digital fURS and identifies the blue colour of the laser fibre outer cover and automatically shuts down the laser firing when the fibre is withdrawn into the ureteroscope (41). In a clinical evaluation of 80 fURS cases, no laser energy-related fURS damage was noted despite fast (5 cm/s) or slow (2 cm/s) withdrawal of the fibre with the fURS in a flexed as well as straight position (42). The authors suggest that EPS should complement, rather than replace, the standard safe laser techniques. Nevertheless, all of the above technologies have not gained universal acceptance as there is insufficient published data to clarify their credential with regards to overall cost-effectiveness.
While the precise laser settings for stone fragmentation vary depending on stone location and size, surgeon preference and the desired outcome, we suggest using low energy (0.5 J) and long pulse duration to reduce the risk of fURS damage since the maximum radius of the resulting plasma bubble is a function of the energy and pulse duration of the laser pulse (43).
Fibre optic damage
Fibre optic damage can take the form of broken individual fibres resulting in black spots within the image and inadequate light transmission or moisture leakage into the fibres causing a blurry image (Figure 4). This may occur as a result of natural deterioration with increasing use, excessive deflection, advancing the fURS forcibly against resistance and poor handling and sterilisation techniques (7). All fURS should be routinely checked for fibre optic damage, and a greater than 20% fibre loss or any degree of moisture leak requires repair.
Figure 4.
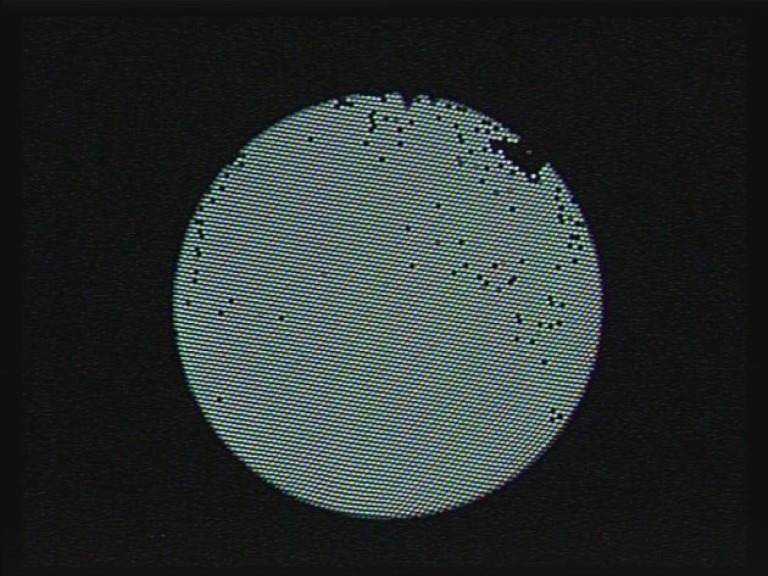
Fibre optics damage seen as black spots on the fURS view. fURS, flexible ureteroscope.
Fibre optic versus digital fURS
With regards to fibre optic scopes, refurbished fURSs are more fragile and prone to subsequent damage compared to new ones (22). There also appears to be very little difference between different makes of fibre optics ureteroscopes (31). All major endoscopy manufacturers supply a digital fURS which undoubtedly has advantages with regards to scope movement and image quality. The initial enthusiasm surrounding improved durability of the digital fURS compared to standard fibre optic ones has not been borne out (26). In fact, a recent report demonstrates significant variability in the durability of the digital and fibre optic scopes ranging from 10 to 79 uses prior to repair (44). Temiz and colleagues also concluded that the digital fURS was associated with a higher initial cost but offered no additional benefit with regards to scope durability or surgical outcomes compared to standard fibre optic scopes (45). Therefore, the evidence of increased longevity of the digital fURS is still lacking.
Non-operative causes of ureteroscope damage
Away from the patient, a competent endourology service requires dedicated staff to ensure correct fURS handling and robust reprocessing protocols to maintain scopes in good working order and ensure longevity. Every aspect of fURS care, including handling and transfer for sterilisation is critical to minimising damage, and requires cooperation between members of the clinical/operating room team and the sterilisation unit. Increasing the number of links in this chain, increases the probability for fURS damage, but the endourologist needs to demonstrate leadership to encourage appropriate fURS care.
Non-operative causes of fURS damage centre around the methods of cleaning, sterilisation and storage of scopes.
Pressure leak testing
Sung and colleagues reviewed instructions from four different manufacturers of ureteroscopes on how to reduce fURS damage (18). They recommended that operating room staff should perform a pressure leak test after every procedure before releasing the fURS for cleaning and sterilisation (18). Not only does this ensure the integrity of the working channel but can also help identify the site (intraoperative or during processing) where the fURS may have sustained damage, and can reduce overall repair costs by detecting damage early, so progression to more serious destruction, if the leaking scope is exposed to high irrigation pressures during sterilisation, can be avoided (46).
Cleaning and sterilisation
Scope cleaning and sterilisation must strictly adhere to manufacturer instructions using dedicated scope trays and staff committed to carefully processing these delicate instruments. A fURS cannot be handled in a similar way to a gastro- or colonoscope and sterilisation unit staff must be appropriately trained. Most of the major fURS manufacturers provide a free training and advice service for operating room and sterilisation room staff on request and this should be readily utilised.
We disagree with the conclusions of McDougall and colleagues who compared the effects of two different techniques of sterilisation [the Steris 20 (peroxyacetic acid 35%) technique and Cidex (glutaraldehyde 2.4%)] on fURS durability and found that neither the sterilisation technique nor the number of personnel involved had any significant effect on scope durability (47). On the contrary, our experience resonates with numerous other reports which demonstrate that when the correct sterilisation method is used, ideally by trained nurses within the endoscopy suite there is a demonstrable improvement in scope longevity and a reduction in cost per procedure (18,21,48).
Sterilisation techniques are not uniform and are largely dictated by local protocol or national guidance, but using the following method, Semins and co-workers prospectively used 11 ureteroscopes which were processed 478 times over a 12-month period, with no damage attributable to the cleaning and sterilisation process which was undertaken by dedicated urology staff. Their method is hand washing of the fURS after use and soaking it in a basin of Enzol Enzymatic Detergent (Advanced Sterilisation Products, Irvine, CA, USA), then 90 mL of Enzol is flushed through the working channel, followed by a manual brush scrubbing of the channel and double flush with 90 mL of Enzol (48). The fURS is rinsed with water thoroughly and sterilised in a Steris System 1 Endoscope Processing System (Steris Corporation, Mentor, Ohio, USA) for 30 minutes and following which it is stored in straight neutral position (48).
Finally, it is advisable to avoid using an ETO (Ethylene Oxide) venting cap during liquid sterilisation and to allow the fURS to be wiped and completely dry prior to storage to reduce the risk of chemical damage or corrosion (Figure 5) (18). The fURS should be stored in sterilised containers in a straight or lightly curled position, as careless storage can cause “trapping” injury to the shaft (Figure 6) (18).
Figure 5.

Inadequate drying following sterilisation can result in chemical damage to the hand-piece.
Figure 6.
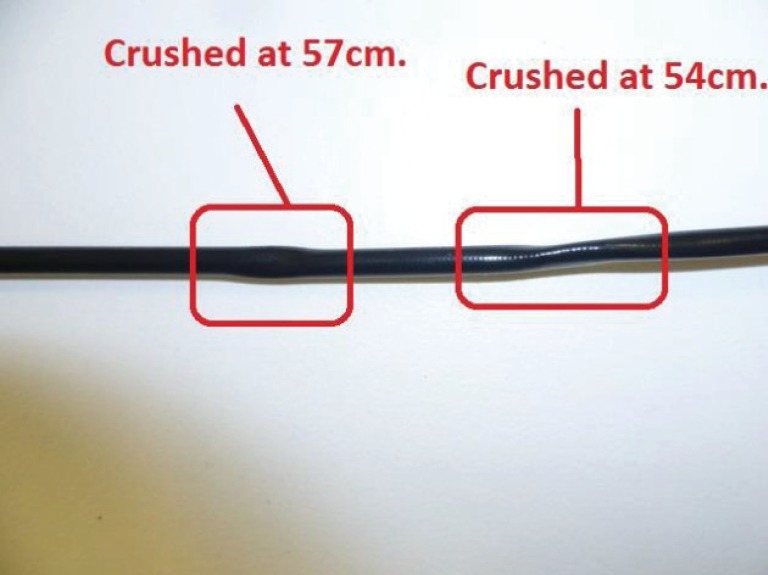
“Trapping injury” due to the fURS being stored in a poorly fitting case with undue care. fURS, flexible ureteroscope.
Operator experience
It is clear that surgeon experience and institution case volume have a direct correlation with scope durability, with high volume centres associated with fewer fURS repairs (24,49-51). Nevertheless, this has to be balanced with other confounding factors including the fact that tertiary centres undertake a larger proportion of more challenging cases perhaps requiring longer fURS use per case, thus adding wear and tear on the fURS (50). In addition, there is a new generation of endourology trainees who need to be trained in developing their experience and competency. There is emerging evidence that training does not need to jeopardise fURS durability. Karaolides and co-workers noted that following the adoption of simple and practical guidelines on fURS use, and in conjunction with simulated training and mentoring, there was a noticeable reduction in the rate of fURS damage even though most procedures were commenced by a trainee under supervision (51).
Limitations
We acknowledge the limitations in this review in terms of the quality of studies included. While there are some prospective randomised studies evaluating aspects of fURS, there are many facets of fURS care which have not been scrutinised adequately. So much of endourology practice is governed by the surgeon’s experience and preference and therefore determination of causal relations between affecting factors and fURS durability is challenging. Also, this is a rapidly evolving field and there is very little durability data on the newer 3rd generation of ureteroscopes.
In addition, our experience and intuition indicate that there are several aspects of flexible ureteroscopy practice which seem to be good practice and may help to prolong the life of the fURS, but which currently lack evidence from good quality studies. These techniques include:
❖ Routine semi-rigid ureteroscopy prior to flexible ureteroscopy, facilitating pre-dilatation of the ureteral orifice and lower ureter and reducing insertion pressure on the fURS;
❖ Holding the fURS by the hand piece with the tip relaxed in a dependent position;
❖ Liberal use of lubricant during fURS manipulation;
❖ Maintaining the scope loosely coiled in transit and taking extra care when placing the fURS in its storage compartment to avoid trapping of the shaft.
In conclusion, fURS longevity is a key concern and deserves careful attention and leadership from practising endourologists. It has significant implications for the cost effectiveness of the fURS programme and adoption of simple techniques can help maximise scope usage and reduce the need for expensive repairs, without compromising on high quality patient care. Finally, Table 1 summarises our top tips to improve durability and minimise damage to the fURS which can make an immediate impact with little or no additional financial investment necessary.
Table 1. Top tips to minimise fURS damage and improve durability.
| Prior to use |
| Develop and publicise guidelines for safe use of the fURS |
| Check fURS for external or fibre optic bindle damage |
| Ensure no damage to the laser fibre |
| Ureteroscope insertion |
| Semi-rigid ureteroscope immediately prior to fURS may help calibrate and dilate the lower ureter |
| Keep fURS as straight as possible during insertion |
| Ensure adequate lubrication on the fURS |
| Use a well lubricated hydrophilic guidewire if back-loading the fURS |
| Intra-operatively |
| Reducing deflection related injury |
| Use gentle manipulation of the hand-piece deflection lever |
| Avoid working with maximum deflection for prolonged periods |
| Move lower pole stones to a more accessible calyx prior to lithotripsy |
| Ensure that UAS is not restricting fURS deflection (ideally the UAS should be in the proximal ureter below the uretero-pelvic junction) |
| Remove the fURS and UAS together when finished |
| Laser fibres |
| Use a smaller diameter laser fibre if possible |
| Keep fURS in a straight, neural position during insertion of laser fibre. Avoid firing the laser unless the fibre is clearly visible (¼ of the screen). Avoid firing the laser at extreme fURS deflection |
| General |
| Hold the fURS by the hand piece |
| Always maintain the fURS in a neutral/loosely coiled position on standby |
| Following the procedure |
| Pressure leak test after every case (immediate repair if leak identified). Ensure cleaning and sterilisation adhere to manufacturers guidelines |
| If sterilisation is done by non-urology staff, ensure appropriate awareness and training (communication from the endourologist is paramount) |
| Ensure that the scope is stored carefully in dedicated cases to avoid trapping |
fURS, flexible ureteroscope; UAS, ureteral access sheath.
Acknowledgments
None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Marshall VF. Fiber optics in urology. J Urol 1964;91:110-4. 10.1016/S0022-5347(17)64066-7 [DOI] [PubMed] [Google Scholar]
- 2.Edvardsson VO, Indridason OS, Haraldsson G, et al. Temporal trends in the incidence of kidney stone disease. Kidney Int 2013;83:146-52. 10.1038/ki.2012.320 [DOI] [PubMed] [Google Scholar]
- 3.Sorokin I, Mamoulakis C, Miyazawa K, et al. Epidemiology of stone disease across the world. World J Urol 2017;35:1301-20. 10.1007/s00345-017-2008-6 [DOI] [PubMed] [Google Scholar]
- 4.Geraghty RM, Jones P, Somani BK. Worldwide trends of urinary stone disease treatment over the last two decades: A systematic review. J Endourol 2017;31:547-56. 10.1089/end.2016.0895 [DOI] [PubMed] [Google Scholar]
- 5.Ghani KR, Roghmann F, Sammon JD, et al. Emergency department visits in the United States for upper urinary tract stones: trends in hospitalization and charges. J Urol 2014;191:90-6. 10.1016/j.juro.2013.07.098 [DOI] [PubMed] [Google Scholar]
- 6.Raheem OA, Khandwala YS, Sur RL, et al. Burden of urolithiasis: Trends in prevalence, treatments, and costs. Eur Urol Focus 2017;3:18-26. 10.1016/j.euf.2017.04.001 [DOI] [PubMed] [Google Scholar]
- 7.Abbott JE, Sur RL. Ureterorenoscopy: current technology and future outlook. Minerva Urol Nefrol 2016;68:479-95. [PubMed] [Google Scholar]
- 8.Rukin NJ, Siddiqui ZA, Chedgy ECP, et al. Trends in upper tract stone disease in England: Evidence from the Hospital Episodes Statistics database. Urol Int 2017;98:391-6. 10.1159/000449510 [DOI] [PubMed] [Google Scholar]
- 9.Heers H, Turney BW. Trends in urological stone disease: a 5-year update of hospital episode statistics. BJU Int 2016;118:785-9. 10.1111/bju.13520 [DOI] [PubMed] [Google Scholar]
- 10.Canales BK, Gleason JM, Hicks N, et al. Independent analysis of Olympus flexible ureteroscope repairs. Urology 2007;70:11-5. 10.1016/j.urology.2007.02.065 [DOI] [PubMed] [Google Scholar]
- 11.Geraghty RM, Jones P, Herrmann TRW, et al. Ureteroscopy is more cost effective than shock wave lithotripsy for stone treatment: systematic review and meta-analysis. World J Urol 2018;36:1783-93. 10.1007/s00345-018-2320-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Landman J, Lee DI, Lee C, et al. Evaluation of overall costs of currently available small flexible uretereoscopes. Urology 2003;62:218-22. 10.1016/S0090-4295(03)00376-5 [DOI] [PubMed] [Google Scholar]
- 13.Traxer O, Dubosq F, Jamali K, et al. New-generation flexible ureterorenoscopes are more durable than previous ones. Urology 2006;68:276-9. 10.1016/j.urology.2006.02.043 [DOI] [PubMed] [Google Scholar]
- 14.Ziemba JB, Matlaga BR. Understanding the costs of flexible ureteroscopy. Minerva Urol Nefrol 2016;68:586-91. [PubMed] [Google Scholar]
- 15.Somani BK, Robertson A, Kata SG. Decreasing the cost of flexible ureterorenoscopic procedures. Urology 2011;78:528-30. 10.1016/j.urology.2010.12.073 [DOI] [PubMed] [Google Scholar]
- 16.Kramolowsky E, McDowell Z, Moore B, et al. Cost analysis of flexible ureteroscope repairs: Evaluation of 655 procedures in a community-based practice. J Endourol 2016;30:254-6. 10.1089/end.2015.0642 [DOI] [PubMed] [Google Scholar]
- 17.Tosoian JJ, Ludwig W, Sopko N, et al. The effect of repair costs on the profitability of a ureteroscopy program. J Endourol 2015;29:406-9. 10.1089/end.2014.0435 [DOI] [PubMed] [Google Scholar]
- 18.Sung JC, Springhart WP, Marguet CG, et al. Location and etiology of flexible and semirigid ureteroscope damage. Urology 2005;66:958-63. 10.1016/j.urology.2005.05.033 [DOI] [PubMed] [Google Scholar]
- 19.Bach C, Nesar S, Kumar P, et al. The new digital flexible ureteroscopes: 'size does matter'--increased ureteric access sheath use! Urol Int 2012;89:408-11. 10.1159/000341429 [DOI] [PubMed] [Google Scholar]
- 20.Carey RI, Gomez CS, Maurici G, et al. Frequency of ureteroscope damage seen at a tertiary care center. J Urol 2006;176:607-10. 10.1016/j.juro.2006.03.059 [DOI] [PubMed] [Google Scholar]
- 21.Defidio L, De Dominicis M, Di Gianfrancesco L, et al. Improving flexible ureterorenoscope durability up to 100 procedures. J Endourol 2012;26:1329-34. 10.1089/end.2012.0178 [DOI] [PubMed] [Google Scholar]
- 22.Carey RI, Martin CJ, Knego JR. Prospective evaluation of refurbished flexible ureteroscope durability seen in a large public tertiary care center with multiple surgeons. Urology 2014;84:42-5. 10.1016/j.urology.2014.01.022 [DOI] [PubMed] [Google Scholar]
- 23.Ozimek T, Cordes J, Wiessmeyer J, et al. Steep infundibulopelvic angle as a new risk factor for flexible ureteroscope damage and complicated postoperative course. J Endourol 2018;32:597-602. 10.1089/end.2018.0147 [DOI] [PubMed] [Google Scholar]
- 24.Pietrow PK, Auge BK, Delvecchio FC, et al. Techniques to maximize flexible ureteroscope longevity. Urology 2002;60:784-8. 10.1016/S0090-4295(02)01948-9 [DOI] [PubMed] [Google Scholar]
- 25.De Coninck V, Keller EX, Rodríguez-Monsalve M, et al. Systematic review of ureteral access sheaths: facts and myths. BJU Int 2018;122:959-69. 10.1111/bju.14389 [DOI] [PubMed] [Google Scholar]
- 26.Multescu R, Geavlete B, Georgescu D, et al. Improved durability of flex-Xc digital flexible ureteroscope: how long can you expect it to last? Urology 2014;84:32-5. 10.1016/j.urology.2014.01.021 [DOI] [PubMed] [Google Scholar]
- 27.Kaplan AG, Lipkin ME, Scales CD, Jr, et al. Use of ureteral access sheaths in ureteroscopy. Nat Rev Urol 2016;13:135-40. 10.1038/nrurol.2015.271 [DOI] [PubMed] [Google Scholar]
- 28.Seto C, Ishiura Y, Egawa M, et al. Durability of working channel in flexible ureteroscopes when inserting ureteroscopic devices. J Endourol 2006;20:223-6. 10.1089/end.2006.20.223 [DOI] [PubMed] [Google Scholar]
- 29.Taguchi K, Usawachintachit M, Tzou DT, et al. Micro-costing analysis demonstrates comparable costs for LithoVue compared to reusable flexible fiberoptic ureteroscopes. J Endourol 2018;32:267-73. 10.1089/end.2017.0523 [DOI] [PubMed] [Google Scholar]
- 30.Dutta R, Vyas A, Landman J, et al. Death of the Safety Guidewire. J Endourol 2016;30:941-4. 10.1089/end.2016.0314 [DOI] [PubMed] [Google Scholar]
- 31.Knudsen B, Miyaoka R, Shah K, et al. Durability of the next-generation flexible fiberoptic ureteroscopes: A randomized prospective multi-institutional clinical trial. Urology 2010;75:534-8. 10.1016/j.urology.2009.06.093 [DOI] [PubMed] [Google Scholar]
- 32.Carlos EC, Li J, Young BJ, et al. Let's Get to the Point: Comparing Insertion Characteristics and Scope Damage of Flat-Tip and Ball-Tip Holmium Laser Fibers. J Endourol 2019;33:22-6. 10.1089/end.2018.0229 [DOI] [PubMed] [Google Scholar]
- 33.Knudsen BE, Pedro R, Hinck B, et al. Durability of reusable holmium:YAG laser fibers: a multicenter study. J Urol 2011;185:160-3. 10.1016/j.juro.2010.08.020 [DOI] [PubMed] [Google Scholar]
- 34.Chapman RA, Somani BK, Robertson A, et al. Decreasing cost of flexible ureterorenoscopy: single-use laser fiber cost analysis. Urology 2014;83:1003-5. 10.1016/j.urology.2013.12.019 [DOI] [PubMed] [Google Scholar]
- 35.Kronenberg P, Traxer O. Are we all doing it wrong? Influence of stripping and cleaving methods of laser fibers on laser lithotripsy performance. J Urol 2015;193:1030-5. 10.1016/j.juro.2014.07.110 [DOI] [PubMed] [Google Scholar]
- 36.Giusti G, Proietti S, Villa L, et al. Current standard technique for modern flexible ureteroscopy: Tips and tricks. Eur Urol 2016;70:188-94. 10.1016/j.eururo.2016.03.035 [DOI] [PubMed] [Google Scholar]
- 37.Talso M, Emiliani E, Haddad M, et al. Laser fiber and flexible ureterorenoscopy: The safety distance concept. J Endourol 2016;30:1269-74. 10.1089/end.2016.0209 [DOI] [PubMed] [Google Scholar]
- 38.Durak E, Hruby G, Mitchell R, et al. Evaluation of a protective laser sheath for application in flexible ureteroscopy. J Endourol 2008;22:57-60. 10.1089/end.2006.0394 [DOI] [PubMed] [Google Scholar]
- 39.Herrmann TR, Bach T, Imkamp F, et al. Flexguard™: a new laser insertion sheath: functional aspects in ureterorenoscopy. World J Urol 2007;25:269-73. 10.1007/s00345-007-0176-5 [DOI] [PubMed] [Google Scholar]
- 40.Herrmann TR, Bach T, Imkamp F, et al. Insertion sheaths prevent breakage of flexible ureteroscopes due to laser fiber passage: a video-endoluminal study of the working channel. J Endourol 2010;24:1747-51. 10.1089/end.2009.0298 [DOI] [PubMed] [Google Scholar]
- 41.Sung C, Singh H, Schwartz M, et al. Evaluation of efficacy of novel optically activated digital endoscope protection system against laser energy damage. Urology 2008;72:57-60. 10.1016/j.urology.2008.01.011 [DOI] [PubMed] [Google Scholar]
- 42.Xavier K, Hruby GW, Kelly CR, et al. Clinical evaluation of efficacy of novel optically activated digital endoscope protection system against laser energy damage. Urology 2009;73:37-40. 10.1016/j.urology.2008.07.041 [DOI] [PubMed] [Google Scholar]
- 43.Jansen ED, Asshauer T, Frenz M, et al. Effect of pulse duration on bubble formation and laser-induced pressure waves during holmium laser ablation. Lasers Surg Med 1996;18:278-93. [DOI] [PubMed] [Google Scholar]
- 44.Legemate JD, Kamphuis GM, Freund JE, et al. Durability of Flexible Ureteroscopes: A Prospective Evaluation of Longevity, the Factors that Affect it, and Damage Mechanisms. Eur Urol Focus 2018. [Epub ahead of print]. 10.1016/j.euf.2018.03.001 [DOI] [PubMed] [Google Scholar]
- 45.Temiz MZ, Colakerol A, Ertas K, et al. Fiberoptic versus Digital: A Comparison of Durability and Cost Effectiveness of the Two Flexible Ureteroscopes. Urol Int 2019;102:181-6. 10.1159/000494385 [DOI] [PubMed] [Google Scholar]
- 46.Khan F, Mukhtar S, Marsh H, et al. Evaluation of the pressure leak test in increasing the lifespan of flexible ureteroscopes. Int J Clin Pract 2013;67:1040-3. 10.1111/ijcp.12149 [DOI] [PubMed] [Google Scholar]
- 47.McDougall EM, Alberts G, Deal KJ, et al. Does the cleaning technique influence the durability of the <9F flexible ureteroscope? J Endourol 2001;15:615-8. 10.1089/089277901750426409 [DOI] [PubMed] [Google Scholar]
- 48.Semins MJ, George S, Allaf ME, et al. Ureteroscope cleaning and sterilization by the urology operating room team: the effect on repair costs. J Endourol 2009;23:903-5. 10.1089/end.2008.0489 [DOI] [PubMed] [Google Scholar]
- 49.Martin CJ, McAdams SB, Abdul-Muhsin H, et al. The economic implications of a reusable flexible digital ureteroscope: A cost-benefit analysis. J Urol 2017;197:730-5. 10.1016/j.juro.2016.09.085 [DOI] [PubMed] [Google Scholar]
- 50.Kandasami SV, Mamoulakis C, El-Nahas AR, et al. CROES URS Global Study Group Impact of case volume on outcomes of ureteroscopy for ureteral stones: the clinical research office of the endourological society ureteroscopy global study. Eur Urol 2014;66:1046-51. 10.1016/j.eururo.2014.06.054 [DOI] [PubMed] [Google Scholar]
- 51.Karaolides T, Bach C, Kachrilas S, et al. Improving the durability of digital flexible ureteroscopes. Urology 2013;81:717-22. 10.1016/j.urology.2013.01.016 [DOI] [PubMed] [Google Scholar]