The acute respiratory distress syndrome (ARDS) is a diffuse inflammatory condition of the lungs presenting with hypoxemia and bilateral chest infiltrates, which is characterized by nonhydrostatic pulmonary edema, increased volume of collapsed alveolar units, decreased functional residual capacity and increased dead space (1). Generally, ARDS represents 10.4% of intensive care unit (ICU) admissions and 23.4% of patients requiring mechanical ventilation (2). Patients with ARDS have a high in-hospital mortality, ranging from 34.9% in milder cases to more than 45% in the most severe cases (2). Also, ARDS survivors report reduced health-related quality of life even after 2 years of hospital discharge (3). Its treatment remains mainly supportive and the avoidance of ventilator-induced lung injury (VILI) while keeping acceptable gas exchange through adequate adjustments of the mechanical ventilator plays a critical role (1).
The current guideline on the mechanical ventilation of ARDS patients endorses strong recommendations on limiting tidal volumes (4 to 8 mL/kg of predicted body weight) and plateau pressure (<30 cmH2O), and also recommends prone positioning for more than 12 h a day in severe cases (4). The guideline also states a recommendation on the use of higher levels of positive end-expiratory pressure (PEEP) in moderate-to-severe cases, but, the use of neuromuscular blocking agents (NMBA), is not addressed in the last update of the guideline (4). Despite the lack of formal recommendation, NMBA are used in 21.7% of ARDS patients (2).
The major concerns on the use of NMBA in this group of patients include increased mismatch between ventilation and perfusion due to paralysis, increased risk of infection and incidence of neuromuscular weakness, and even increased risk of death due to disconnection from the ventilator (5). On the other side, among the possible benefits of neuromuscular blockade in ARDS are: (I) reduction of oxygen consumption by decreasing work of breathing; (II) full control of the tidal volume size and minute ventilation, allowing the maintenance of a protective strategy of ventilation and decreasing the risk of VILI; (III) reduction of patient-ventilator asynchrony, potentially limiting both alveolar collapse and regional overdistention (4-6).
In a pilot randomized, clinical trial, Gainnier et al. evaluated the effects of 48 h of cisatracurium administration on gas exchange in 56 patients with ARDS and a PaO2/FiO2 (PF) ratio less than 150 (5). It was shown that patients in the cisatracurium group had higher PF ratio after 48 h of therapy, and this difference persisted for at least 120 h after randomization (5). In addition, patients in the cisatracurium group were ventilated with lower levels of PEEP and plateau pressure, probably due to a better oxygenation, allowing a more protective strategy of ventilation (5). This sustained improvement on gas exchange and the maintenance of a protective ventilation might have been related to the reduced release of inflammatory mediators found in the study (5). The relationship among NMBA administration and the kinetics of inflammatory biomarkers was demonstrated in another small pilot randomized clinical trial by the same group (7). In this trial, 48 h after randomization, patients in the cisatracurium group had lower levels of IL-1B, IL-6 and IL-8 measured in the bronchoalveolar lavage, and lower levels of serum IL-1B and IL-6 (7). As an additional finding, there was an association between lower levels of cytokines and improved gas exchange (7). Taking together, these studies suggested that NMBA could reduce inflammation, and improve oxygenation and lung protection in patients with ARDS even when compared to a control arm including patients under heavy sedation and receiving a protocolized protective strategy of ventilation. Finally, patients receiving NMBA in both trials had a trend toward lower mortality (5,7), and a larger and well-powered clinical trial was necessary to confirm the findings.
The ARDS et Curarisation Systematique (ACURASYS) trial was a multicenter, randomized, placebo-controlled trial conducted to evaluate whether administration of cisatracurium for 48 h in severe ARDS was associated with improved clinical outcomes (8). The study included 340 patients with moderate-to-severe ARDS, defined as PF ratio less than 150, and no more than 48 h of mechanical ventilation until randomization. The primary outcome was the 90–day mortality. As there was an imbalance on the baseline PF ratio between the groups, the authors included the PF ratio in the adjusted model of the primary outcome. All patients received low tidal volume ventilation based on the ARMA study protocol and deep sedation (9). At the end of follow–up, there was a reduction in the primary outcome of adjusted 90-day mortality in the cisatracurium group [31.6% vs. 40.7%; hazard ratio (HR) 0.68, 95% confidence interval (CI), 0.48–0.98; P=0.04]. However, it is important to emphasize that the crude 90-day mortality was similar in both groups and the study was underpowered as the mortality in the placebo group was lower than expected in the sample size calculation. In addition, there was also a benefit of cisatracurium in several secondary endpoints, like more ventilator–free days, more days spent out of ICU, and less barotrauma. Finally, a post-hoc analysis showed that the mortality benefit was restricted to patients with a PF ratio less than 120.
The practice of sedation in the ICU has changed in the last decade, with more patients receiving light sedation, defined as a target Richmond Agitation Sedation Scale (RASS) around 0 to ‒1. Thus, the possible benefits with the use of NMBA in this new scenario was unknown, since all patients in previous trials received heavy sedation and were ventilated without spontaneous breathing in the control arm (5,7,8). Indeed, the use of spontaneous breathing ventilation in ARDS was recently studied in a prospective multicenter study (10). In an adjusted analysis, spontaneous breathing during the first two days of ventilation was not associated with any effect on ICU or hospital mortality [33% vs. 37%; odds ratio (OR) 1.18, 95% CI, 0.92–1.51; P=0.19 and 37% vs. 41%; OR, 1.18, 95% CI, 0.93–1.50; P=0.196, respectively) but was associated with an increased number of ventilator-free days [13 (0 to 22) vs. 8 (0 to 20); P=0.014] and shorter duration of ICU stay [11 (6 to 20) vs. 12 (7 to 22); P=0.04] (10). Indeed, the early use of assisted modes of ventilation with spontaneous breathing may lead to recruitment of non-aerated areas increasing functional residual capacity and decreasing the risk of VILI, and also it can prevent the ventilator-induced diaphragmatic dysfunction (VIDD) by keeping respiratory muscles active (11). An observational study of 191 patients requiring mechanical ventilation for more than 24 h has shown that diaphragm thickness declined faster in patients with higher respiratory support and that the development of diaphragm atrophy was associated with less ventilator-free days, prolonged ICU stay, prolonged mechanical ventilation and higher incidence of complications (11).
However, on the other side, spontaneous breathing may worsen or even cause VILI if ARDS is severe and spontaneous effort is vigorous (11,12). Maintenance of spontaneous respiratory effort in positive pressure ventilation reduces the pleural pressure during inspiration which increases transpulmonary pressure, leading to increased stress (12). In ARDS the inspiratory reduction of pleural pressure in dependent lung regions is not uniformly distributed through the lung, leading to local increases in transpulmonary pressure (12). This causes exchange of air from non–dependent to dependent regions, known as the Pendelluft effect, and this rapid inflation and deflation of lung tissue is possibly related to VILI (12). Expiratory muscular efforts can also lead to VILI by displacing the diaphragm upwards and reducing the end-expiratory lung volume, resulting in more overdistention for the same tidal volume (12).
In 2019, the Prevention and Early Treatment of Acute Lung Injury (PETAL) Clinical Trials Network of the National Heart, Lung, and Blood Institute (NHLBI) published the “Reevaluation of Systemic Early Neuromuscular Blockade” (ROSE) trial (13). This multicenter, open label, randomized clinical trial of patients with moderate-to-severe ARDS, was performed in 48 ICUs in the United States and compared the use of NMBA combined with deep sedation versus usual care without NMBA and with lighter sedation targets. The primary outcome was 90-day mortality and secondary outcomes included, 28-day mortality, organ dysfunction-free days, days not in the ICU, days free of mechanical ventilation, and days not in the hospital at day 28 (13). The trial was stopped at second interim analysis for futility with 1,006 patients enrolled from a pre-planned sample size of 1,408 patients. No difference was found in the primary outcome (42.5% vs. 42.8%; risk difference, ‒0.3, 95% CI, ‒6.4 to 5.9; P=0.930) but more serious cardiovascular adverse effects were found with the use of cisatracurium (13).
One of the main limitations of the ROSE trial was the number of patients excluded due to previous use of NMBA. Of the 4,848 patients screened, 655 were already receiving continuous NMBA and were excluded, and this may have led to the exclusion of the patients whose clinicians identified as more likely to respond to this therapy, increasing the risk a selection bias. However, the sensitivity analysis in sites that rarely excluded patients did not show any benefit of the intervention. Another limitation was the awareness of the group assignments by the professionals, which could have affected some short–term assessments and care.
There are several reasons that might have driven the differences in the results of the ROSE and the ACURASYS trials. Although both trials ventilated the patients based on the PEEP table, the ROSE trial used higher levels of PEEP from the table modified from the ALVEOLI trial (14), while the ACURASYS used lower levels of PEEP based on the table from the ARMA trial (9). The use of higher levels of PEEP may reduce the atelectrauma and heterogeneity in alveolar expansion that possibly attenuates the effects of neuromuscular blocking. This effect of higher levels of PEEP can be observed in the lack of difference in the PF ratio among the groups during the first 7 days in the ROSE trial, which substantially differs from the findings from the previous studies (5,7,8). Another important difference is the proportion of patients under light sedation in the control arm of the ROSE trial. During the first three days of the trial there was a greater proportion of patients under light sedation in the control group than in the ACURASYS trial. This may have protected the control group from adverse events related to heavy sedation such as hypotension and bradycardia, reflected in a lower incidence of serious cardiovascular adverse events in patients not receiving NMBA.
Another possible explanation for the different results between the studies could be the fact that the ACURASYS had a higher percentage of patients who underwent prone positioning than those in the ROSE trial. It is known that prone positioning reduces mortality in severe ARDS, however, the effect of neuromuscular blockade in clinical outcomes when used combined with prone position is unknown. Furthermore, the incidence of patient-ventilator asynchronies was not reported in both studies, but deep sedation in the control group of ACURASYS trial may have led to reverse–triggered breaths, that can occur in heavily sedated patients, causing pulmonary overdistention and possibly worsening clinical outcomes (15), while the intervention group was protected from this effect since NMBA block diaphragmatic contraction. The ROSE trial had lighter sedation in control group, protecting patients from this mechanism of lung injury (16).
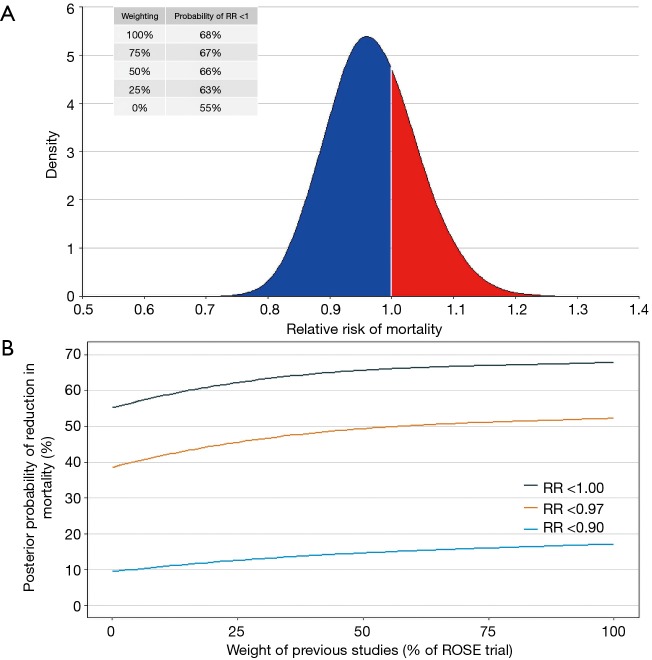
One of the best approaches to estimate the probability of benefit of an intervention is through a Bayesian hierarchical meta-analysis. Considering previous findings from three clinical trials summarized in two systematic reviews and meta-analysis (6,17), and using a minimally informative prior, the posterior probability of benefit of NMBA in patients with ARDS is 0.97 (95% credible interval, 0.84–1.11). The probability that NMBA can add any benefit in this group of patients (defined as a risk ratio <1.00) is 68% and, when a down-weighting of 50% is done in previous studies (due to the small sample sizes and the methodological problems described above), this probability decreases to 66% (Figure 1).
Figure 1.
Results of the Bayesian hierarchical meta-analysis. (A) Posterior probability distribution of the pooled results of the meta-analysis (full posterior density from the Markov chain Monte Carlo approach, the red area is where risk ratio >1 and NMBA increase mortality); (B) resulting estimated posterior probability that the risk ratio for mortality exceeds that threshold value according to different down-weightings of previous studies (if these weights are equal to 1, a meta-analysis of all studies are carried out, on the other hand, with the decrease in these weights, the importance of previous studies decrease and when these weights are equal to 0, then we learn nothing about the risk ratio for NMBA from previous studies, and with a minimally informative prior the results represent the analysis of the ROSE trial alone). ROSE trial, Reevaluation of Systemic Early Neuromuscular Blockade trial; NMBA, neuromuscular blocking agents.
In summary, the probability of benefit of NMBA in patients with ARDS after the publication of the ROSE trial is low, and it should not be used routinely in patients with ARDS, even in severe cases. The administration of these agents should be restricted to patients in which asynchronies fail to respond to ventilatory adjustments and deep sedation, and when NMBA is necessary to reduce the plateau pressure to recommended levels, increasing lung protection in patients already receiving low tidal volume ventilation.
Acknowledgments
None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Provenance: This is an invited article commissioned by the Section Editor Xue-Zhong Xing [National Cancer Center (NCC)/Cancer Hospital, Chinese Academy of Medical Sciences (CAMS) and Peking Union Medical College (PUMC), Beijing, China].
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Fan E, Brodie D, Slutsky AS. Acute Respiratory Distress Syndrome: Advances in Diagnosis and Treatment. JAMA 2018;319:698-710. 10.1001/jama.2017.21907 [DOI] [PubMed] [Google Scholar]
- 2.Bellani G, Laffey JG, Pham T, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016;315:788-800. 10.1001/jama.2016.0291 [DOI] [PubMed] [Google Scholar]
- 3.Bienvenu OJ, Colantuoni E, Mendez-Tellez PA, et al. Depressive symptoms and impaired physical function after acute lung injury: a 2-year longitudinal study. Am J Respir Crit Care Med 2012;185:517-24. 10.1164/rccm.201103-0503OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fan E, Del Sorbo L, Goligher EC, et al. An Official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: Mechanical Ventilation in Adult Patients with Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med 2017;195:1253-63. 10.1164/rccm.201703-0548ST [DOI] [PubMed] [Google Scholar]
- 5.Gainnier M, Roch A, Forel JM, et al. Effect of neuromuscular blocking agents on gas exchange in patients presenting with acute respiratory distress syndrome. Crit Care Med 2004;32:113-9. 10.1097/01.CCM.0000104114.72614.BC [DOI] [PubMed] [Google Scholar]
- 6.Neto AS, Pereira VG, Espósito DC, et al. Neuromuscular blocking agents in patients with acute respiratory distress syndrome: a summary of the current evidence from three randomized controlled trials. Ann Intensive Care 2012;2:33. 10.1186/2110-5820-2-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forel JM, Roch A, Marin V, et al. Neuromuscular blocking agents decrease inflammatory response in patients presenting with acute respiratory distress syndrome. Crit Care Med 2006;34:2749-57. 10.1097/01.CCM.0000239435.87433.0D [DOI] [PubMed] [Google Scholar]
- 8.Papazian L, Forel JM, Gacouin A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 2010;363:1107-16. 10.1056/NEJMoa1005372 [DOI] [PubMed] [Google Scholar]
- 9.Acute Respiratory Distress Syndrome Network , Brower RG, Matthay MA, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000;342:1301-8. 10.1056/NEJM200005043421801 [DOI] [PubMed] [Google Scholar]
- 10.van Haren F, Pham T, Brochard L, et al. Spontaneous Breathing in Early Acute Respiratory Distress Syndrome: Insights From the Large Observational Study to UNderstand the Global Impact of Severe Acute Respiratory FailurE Study. Crit Care Med 2019;47:229-38. 10.1097/CCM.0000000000003519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goligher EC, Dres M, Fan E, et al. Mechanical Ventilation-induced Diaphragm Atrophy Strongly Impacts Clinical Outcomes. Am J Respir Crit Care Med 2018;197:204-13. 10.1164/rccm.201703-0536OC [DOI] [PubMed] [Google Scholar]
- 12.Yoshida T, Fujino Y, Amato MB, et al. Fifty Years of Research in ARDS. Spontaneous Breathing during Mechanical Ventilation. Risks, Mechanisms, and Management. Am J Respir Crit Care Med 2017;195:985-92. 10.1164/rccm.201604-0748CP [DOI] [PubMed] [Google Scholar]
- 13.National Heart, Lung, and Blood Institute PETAL Clinical Trials Network, Moss M, Huang DT, et al. Early Neuromuscular Blockade in the Acute Respiratory Distress Syndrome. N Engl J Med 2019;380:1997-2008. [DOI] [PMC free article] [PubMed]
- 14.Brower RG, Lanken PN, MacIntyre N, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 2004;351:327-36. 10.1056/NEJMoa032193 [DOI] [PubMed] [Google Scholar]
- 15.Akoumianaki E, Lyazidi A, Rey N, et al. Mechanical ventilation-induced reverse-triggered breaths: a frequently unrecognized form of neuromechanical coupling. Chest 2013;143:927-38. 10.1378/chest.12-1817 [DOI] [PubMed] [Google Scholar]
- 16.Slutsky AS, Villar J. Early Paralytic Agents for ARDS? Yes, No, and Sometimes. N Engl J Med 2019;380:2061-3. 10.1056/NEJMe1905627 [DOI] [PubMed] [Google Scholar]
- 17.Alhazzani W, Alshahrani M, Jaeschke R, et al. Neuromuscular blocking agents in acute respiratory distress syndrome: a systematic review and meta-analysis of randomized controlled trials. Crit Care 2013;17:R43. 10.1186/cc12557 [DOI] [PMC free article] [PubMed] [Google Scholar]