Abstract
Background
Crizotinib has been the standard treatment for patients with anaplastic lymphoma kinase (ALK)-rearranged advanced non-small cell lung cancer (NSCLC). It demonstrated superior progression-free survival (PFS) and higher objective response rates (ORRs) vs. chemotherapy in previously treated and untreated patients with ALK-positive advanced NSCLC. This retrospective analysis reports real-world experience in treatment outcome and toxicity of crizotinib in this group of patients, with a focus on the cardiac toxicity and its management.
Methods
Twenty-two patients diagnosed with ALK-positive NSCLC, either by immunohistochemistry (IHC) or fluorescence in situ hybridization (FISH), treated at Johns Hopkins Singapore International Medical Centre (JHSIMC) and Tan Tock Seng Hospital (TTSH) in Singapore, were identified and followed for a median of 18 months. Data were collected and analyzed for baseline demographics, PFS, ORR, duration of response, toxicity and overall survival (OS).
Results
Clinical profile of patients included in the study was similar with clinical trials on crizotinib. Most patients were young of mean age 42, non-smokers and with good performance status. Fifty-nine percent had prior chemotherapy. Fifty percent of patients had brain metastases (BM), either de novo or on progression. ORR of crizotinib was 64% with median total duration of treatment of 8.5 months (range, 2–73+ months). Median PFS for patients treated with first-line crizotinib was 15 months. Most patients with BM had brain radiation. Median time for intracranial progression from the start of crizotinib was 11 months. Those with stable or responding extracranial disease continued crizotinib after radiotherapy to the brain. Median duration of response in this group of patients was 14 months (range, 2–31 months). Median OS among patients treated with upfront crizotinib was not reached, with 7 out of 11 patients still alive at the time of data analysis (n=11, range, 1–73+). Toxicity was manageable with moderate rate of grade 3 or worse toxicity (n=7, 31.8%). Three patients had grade 3–4 neutropenia. Eighteen percent (n=4) of patients developed cardiotoxicities such as bradycardia, prolonged QTc interval and complete heart block. One patient who developed complete heart block required pacemaker insertion. Two patients are long term responders who have been on crizotinib for 68+ and 73+ months.
Conclusions
This retrospective analysis of a real-world experience confirms the therapeutic benefit of crizotinib in advanced ALK-positive NSCLC. Our data showed crizotinib is tolerable and effective, comparable with literature report. Occasional serious cardiac toxicity requires attention.
Keywords: Anaplastic lymphoma kinase (ALK) positive advanced non-small cell lung cancer (NSCLC), crizotinib, treatment outcomes, brain metastases (BM), cardiotoxicity
Introduction
Targeted therapy has become the standard treatment for advanced non-small cell lung cancer (NSCLC) with oncogenic driver genotype. Discovery of the transforming echinoderm microtubule-associated protein like 4 and anaplastic lymphoma kinase (EML4-ALK) fusion genes in 2007 led to further identification of this subset of NSCLC which does not harbour the epidermal growth factor receptor (EGFR) mutation. ALK rearrangement has been found in about 5–10% of NSCLC. Patients in this subgroup are more frequently younger, never or ex- light smoker, and with adenocarcinoma histology (1,2). ALK-rearranged NSCLC tends to be more aggressive and usually presents at a late stage (3). Brain metastases (BM) and disease progression in the brain are very common and pose clinical challenges (4).
Crizotinib is an oral multitarget tyrosine kinase inhibitor (TKI) with clinical activity against ALK, c-ros oncogene 1 (ROS-1) and mesenchymal-epithelial transition factor (c-MET) positive NSCLC. It was originally developed as a c-MET inhibitor but was subsequently evaluated on ALK-positive NSCLC (5-8). Crizotinib gained accelerated approval by the US FDA in 2011 based on PROFILE 1005, a phase II trial on ALK-rearranged recurrent or advanced NSCLC who progressed after one or more lines of chemotherapy. It yielded 53% objective response rate (ORR, 95% CI, 47–60%), with median progression-free survival (PFS) of 8.5 months (95% CI, 6.2–9.9 months), and median duration of response of 43 weeks (95% CI, 36–50 weeks) (9). Similarly, patients with ROS-1 rearranged NSCLC also showed marked response to crizotinib. The dual inhibition of ALK and ROS-1 by crizotinib may be due to structural similarities between these two closely related tyrosine kinases. ORR of crizotinib in ROS-1 positive NSCLC was 72% (95% CI, 58–84%), with median PFS of 19.2 months (95% CI, 14.4 to NR). Overall survival (OS) rate at 12 months was 85% (95% CI, 72–93%); the median survival had not been reached (10).
Subsequently, two phase III trials were conducted in ALK-positive NSCLC treated with crizotinib. PROFILE 1007 was done in the second line setting comparing crizotinib with single-agent chemotherapy. Crizotinib significantly improved PFS from 3.0 to 7.7 months (HR 0.49, P<0.001). ORR was also significantly improved by 45%; 20% with chemotherapy vs. 65% with crizotinib (P<0.001). No significant improvement in overall survival, however, was noted (11). PROFILE 1014 included patients with no previous systemic treatment for advanced disease. It also showed significantly longer PFS of 10.9 months with crizotinib compared to 7.0 months with chemotherapy (HR 0.45, P<0.001). ORR was significantly higher with crizotinib than with chemotherapy (74% vs. 45%, respectively, P<0.001) (12). Final overall survival analysis after median follow up of 46 months, showed no significant OS benefit (HR 0.760, 2-sided P=0.978); the longest OS was observed in crizotinib-treated patients who received a subsequent ALK inhibitor. Median OS was not reached among patients in the crizotinib group who had at least one line of subsequent ALK inhibitor, compared to 47.5 months among those who received ALK inhibitor after chemotherapy, and 20.8 months among patients in the crizotinib group who did not receive subsequent ALK inhibitor treatment (13). Crizotinib was well tolerated in both trials. It provided greater reduction in symptoms and improvement in the quality of life compared with chemotherapy.
PROFILE 1029 is another phase 3 trial which investigated the efficacy and safety of crizotinib compared with chemotherapy as first-line treatment for advanced ALK-positive NSCLC in previously untreated East Asian patients. Crizotinib showed a significantly improved PFS compared to chemotherapy (HR 0.40, median 11.1 vs. 6.8 months, P<0.0001). ORR was also significantly higher with crizotinib compared to chemotherapy (88% vs. 46%, P<0.0001), more durable (44 vs. 18 weeks), and faster (median time to response 6 vs. 12 weeks). OS was also longer with crizotinib, though not statistically significant (28.5 months crizotinib arm vs. 27.7 months chemotherapy arm, P=0.33) (14).
Methods
Objective of the study
This study evaluates our own experience in comparison with reported literature data on treatment outcome of crizotinib in ALK-positive advanced NSCLC. We also report our observation of less common side effects such as cardiotoxicity and its management, as well as long term survivors.
Methodology
Twenty-two patients with ALK-rearranged advanced NSCLC treated with crizotinib at Johns Hopkins Singapore International Medical Centre (JHSIMC) and Tan Tock Seng Hospital (TTSH) in Singapore from 2011 up to December 2017 were identified using electronic health record and medical charts. Domain Specific Review Board (DSRB), a local institutional review board (IRB) approved our study protocol for retrospective analysis (DSRB Reference 2017/00025). ALK rearrangement was detected using either immunohistochemistry (IHC) or break-apart fluorescence in situ hybridization (FISH). Patients received crizotinib 250 mg twice daily until progression or development of intolerable side effects. Patient data included baseline demographics, presence or absence of BM, treatment response, toxicity profile, progression and survival outcomes. Radiological assessment for treatment response was usually performed every 3 months or earlier as clinically indicated.
ORR, duration of response, PFS and OS were assessed. PFS was defined as the time from initiation of crizotinib until disease progression, death from any cause or discontinuation for toxicity. OS was defined as the time from initiation of crizotinib until occurrence of death from any cause. Duration of response was defined as the time from documentation of first tumour response to crizotinib until disease progression or discontinuation.
Patients with BM were separately assessed for time to intracranial disease progression (TIDP) and 2nd intracranial PFS. TIDP is defined as time from start of crizotinib until either onset of first brain metastasis or intracranial progression for those with de novo BM. Second intracranial PFS refers to median time to second intracranial progression from the start of crizotinib. Adverse effects were documented and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 (NCCTC).
Results
Clinical presentation
Baseline characteristics of patients included in this analysis are shown in Table 1. Median age at diagnosis was 42 years. Mostly were non-smokers (55%) with good performance status (ECOG 0–1) at the time of diagnosis. Most patients were Asian, except for two Arabs. Eighty-six percent had stage IV disease at diagnosis. Majority had intracranial (50%) and bone (50%) metastases. Other sites of disease included lung (45%), liver (27%), pleura (27%), adrenal gland (14%), and peritoneum (4%). Fifty percent of patients had prior cytotoxic chemotherapy, mostly with one line, platinum doublet chemotherapy, prior to crizotinib. Median time interval from chemotherapy to crizotinib was 9 months (3–36 months). Another 50% of the patients started crizotinib as first-line systemic treatment.
Table 1. Baseline characteristics.
| Characteristics | No. [%] (n=22) |
|---|---|
| Age | |
| Median (year) | 42 |
| Range (year) | 29–76 |
| Sex | |
| Male | 12 [55] |
| Female | 10 [45] |
| Race | |
| Asian | 20 [91] |
| Arab | 2 [9] |
| ECOG | |
| 0 | 10 [45] |
| 1 | 9 [41] |
| 2 | 2 [9] |
| 3 | 1 [5] |
| Smoking history | |
| Yes | 10 [45] |
| No | 12 [55] |
| Stage at diagnosis | |
| I–III | 3 [14] |
| IV | 19 [86] |
| Lines of treatment | |
| Upfront crizotinib | 11 [50] |
| Prior chemotherapy | 11 [50] |
| 1 | 6 [55] |
| 2 | 4 [36] |
| ≥3 | 1 [9] |
| Brain metastases | 11 [50] |
| De novo | 5 [45] |
| Asymptomatic, no brain radiotherapy | 2 |
| Local treatment (WBRT, SRS) | 3 |
| Intracranial metastases on crizotinib (1st onset) | 6 [55] |
WBRT, whole brain radiotherapy; SRS, stereotactic radiosurgery.
Median follow up of patients was 18 months (range, 1–73+ months). 36% of patients (n=8) were still on ALK inhibitors, either crizotinib or second-generation ALK inhibitors at the time of data analysis. One patient started crizotinib for residual mediastinal disease after completing concurrent chemoradiation. Another patient underwent lower lobectomy for stage IIIA NSCLC but noted to have pleural nodule intra-operatively, hence given crizotinib. Both patients are still on crizotinib (68+, 73+ months).
Treatment outcomes of crizotinib
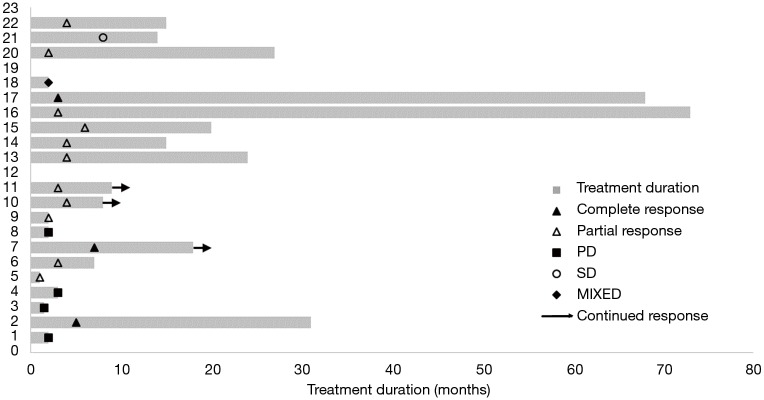
Clinical efficacy of crizotinib in our patient population is shown in Table 2. ORR, as determined by complete response and partial response documented by treating oncologists, was 64% (n=14) with median time to response of 3 months. Three patients (14%) had complete response. Four patients (18%) progressed on crizotinib with median duration of treatment of 2 months. Two of these patients received upfront crizotinib. One patient’s tumour was initially ALK-positive by IHC but turned out to be ALK-negative by FISH. Another patient was intolerant to crizotinib due to grade 3 vomiting and had to stop after 2 months. The other two patients who had disease progression on crizotinib had extensive lung and liver disease. Two patients cannot be evaluated; one died unexpectedly two weeks after starting crizotinib from a non-cancer related event, and another refused to continue after one day of treatment. One patient developed crizotinib- related pneumonitis. No radiologic disease progression was noted. (Figure 1).
Table 2. Treatment efficacy of crizotinib based on response rate, progression free survival and duration of treatment.
| Treatment efficacy | No. [%] (n=22) |
|---|---|
| Objective response | 14 [64] |
| Complete response | 3 [14] |
| Partial response | 11 [50] |
| Stable disease | 1 [5] |
| Disease progression | 4 [18] |
| Mixed response | 1 [5] |
| Not evaluable | 2 [9] |
| Duration of response, median (mo) | 8.5 |
| Sites of disease progression | |
| Intracranial | 7 [32] |
| Extracranial (lung, liver, adrenal, bone, nodes, peritoneum) | 6 [27] |
| Both intracranial & extracranial) | 2 [9] |
| Overall median PFS (mo) | 8 |
| Time to intracranial progression | 11 |
| Time to extracranial progression | 2.5 |
| Overall survival from start of crizotinib (mo) | 20 |
Figure 1.
Swimmer’s plot of clinical response to crizotinib. PD, progression of disease; SD, stable disease.
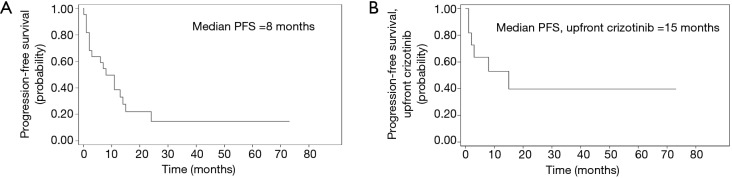
Median total duration of response was 8.5 months (n=22, range, 2–73+ months). Two patients are long-responders: one with residual mediastinal disease post-concurrent chemotherapy, and another with pleural disease post-lobectomy. Both continue to show durable response to crizotinib at the time of data analysis (68+ and 73+ months, respectively). Overall median PFS was 8 months (n=22). Median PFS for patients treated with 1st line crizotinib was 15 months (n=11) (Figure 2).
Figure 2.
Kaplan-Meier curves for PFS for all patients treated with crizotinib, n=22 (A), and for all patients treated with 1st line crizotinib, n=11 (B), with median PFS of 8 and 15 months, respectively. PFS, progression-free survival.
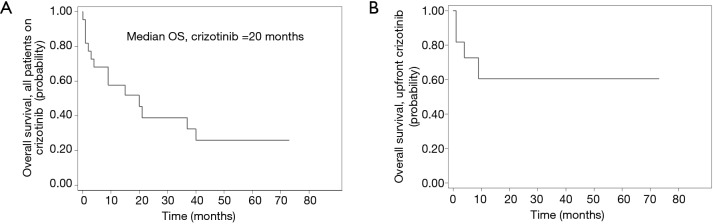
Fourteen of the 22 patients (64%) had died at the time of data analysis. Median OS from starting crizotinib was 20 months (n=22, range, 1–73+ months), while median OS for those treated with upfront crizotinib was not reached, with seven out of eleven patients still alive at the time of data analysis (Figure 3).
Figure 3.
Kaplan-Meier curves for OS. For all patients from time crizotinib was started, n=22, median OS of 20 months (A); median OS was not reached for patients treated with upfront crizotinib including 2 long responders (68+ and 73+ months) (B). OS, overall survival.
BM
Nine out of eleven patients with BM developed intracranial progression while on crizotinib: three with de novo BM and six who progressed with BM. Three patients with de novo BM received crizotinib upfront: one patient continued to respond at the time of data analysis; two patients stopped crizotinib, either due to toxicity or intracranial progression, and switched to a second-generation ALK inhibitor. Median TIDP and median duration of response for patients with BM were 11 months (range, 2–24 months) and 14 months (range, 2–31 months), respectively (n=11).
Apart from two asymptomatic patients with de novo BM, all the other patients completed local treatment such as whole brain radiotherapy (WBRT) or stereotactic radiosurgery (SRS). Patients with extracranial disease who either showed continued response or stable disease (SD), continued crizotinib after local treatment to the brain. Median duration of response in this subgroup was 25 months (n=4). Median time to second intracranial progression, from the first onset of brain metastasis while on crizotinib (2nd intracranial PFS) was 4 months (n=4; range, 3–6 months).
Median OS for patients with intracranial BM from initiation of crizotinib was 30 months (n=11; range, 7–64 months). One patient remains on crizotinib with good intracranial and extracranial disease control for 7 months at the time of data analysis.
Upfront crizotinib
Fifty percent (50%) of patients were treated with crizotinib upfront with median PFS of 15 months. Three patients with de novo BM had 9 months median duration of treatment response. At the time of data analysis, four patients (18%) remain on crizotinib. Another 18% was switched to second-line ALK inhibitor either due to disease progression or toxicity.
Toxicity
Adverse effects were generally manageable (Table 3). The most common side effect was gastrointestinal toxicity such as diarrhea, nausea and vomiting. Seven patients had grade 3 or more toxicities [cardiovascular, gastrointestinal (vomiting, elevated transaminases), pneumonitis and neutropenia]. Crizotinib was withheld in one patient due to an episode of grade 4 neutropenia. Three patients stopped crizotinib due to toxicity (persistent vomiting and abdominal pain despite dose reductions in two patients, and pneumonitis in one patient). Another patient who had history of hepatitis C had grade 3 transaminitis requiring dose reduction.
Table 3. Adverse events.
| Adverse events (n=22) | Graded toxicities | |||
|---|---|---|---|---|
| Grade 1–2, (n) | Grade 3, (n) | Grade 4, (n) | All grades, n [%] | |
| Cardiovascular | ||||
| Bradycardia | 2 | 0 | 1 | 3 [14] |
| Prolonged QTc | 1 | 1 | 0 | 2 [9] |
| Complete heart block | 0 | 0 | 1 | 1 [5] |
| Gastrointestinal | ||||
| Nausea, vomiting | 3 | 1 | 0 | 4 [18] |
| Diarrhea | 3 | 0 | 0 | 3 [14] |
| Constipation | 1 | 0 | 0 | 1 [5] |
| Abdominal pain | 1 | 0 | 0 | 1 [5] |
| Transaminitis* | 0 | 1 | 0 | 1 [5] |
| Pneumonitis | 0 | 1 | 0 | 1 [5] |
| Neutropenia | 0 | 2 | 1 | 3 [14] |
| Neurological | ||||
| Visual (photosensitivity, diplopia) | 3 | 0 | 0 | 3 [14] |
| Numbness | 1 | 0 | 0 | 1 [5] |
| Dizziness | 1 | 0 | 0 | 1 [5] |
| Peripheral edema | 2 | 0 | 0 | 2 [9] |
| Anorexia | 1 | 0 | 0 | 1 [5] |
| Easy fatigability | 2 | 0 | 0 | 2 [9] |
| Change in taste | 3 | 0 | 0 | 3 [14] |
*, history of hepatitis C infection.
Cardiotoxicity from crizotinib occured in four patients. This included bradycardia (grade 1/2-2; grade 4-1), QTc prolongation (grade 2/3-2), and complete heart block (grade 4-1). Both QTc prolongation and bradycardia were reversible after withholding crizotinib. Patient with the complete heart block required pacemaker insertion, and subsequently continued and tolerated the full dose of crizotinib.
Second line ALK inhibitor
Seven patients who progressed or developed crizotinib-induced toxicity continued treatment with second line ALK inhibitor. Six patients received ceritinib with median PFS of 6.5 months (range, 3-20 months). Side effects of ceritinib included gastrointestinal toxicity such as vomiting, diarrhea and abdominal discomfort (grade 1–3) and thrombocytopenia (grade 4) requiring dose reduction. Two patients had alectinib, either on disease progression on crizotinib or after 2nd line ceritinib.
Four out of seven patients continue to respond well with 2nd generation ALK inhibitors at the time of data analysis. One patient had good disease control for 20 months with ceritinib, then was switched to alectinib due to intracranial disease progression. However, he developed hemolytic anemia and is now on brigatinib. Another patient is on entrectinib. Overall, median combined PFS with sequential use of ALK inhibitors is 30 months (n=7, range, 8–43+ months).
Discussion
This case series represents a cohort of ALK-positive NSCLC patients treated in TTSH and JHSIMC. TTSH is a tertiary public hospital under the National Healthcare Group (NHG), that provides medical service to the central region of Singapore. JHSIMC was a joint venture of NHG and Johns Hopkins University (JHU), that provided medical oncology service for private patients. The combined practice, therefore, reflected real world experience in Singapore.
Clinical efficacy of crizotinib
Crizotinib has been the standard practice for ALK-positive advanced NSCLC at our institution at the start of this case series. In the advent of second-generation ALK inhibitors, it remains as one of the standard treatments in this molecular subtype of NSCLC. Our study is limited by the small number of patients with heterogeneous presentation. Some patients were diagnosed prior to routine ALK testing and when crizotinib was not yet readily available in Singapore. Majority of these patients received first-line chemotherapy which may have affected particularly the overall survival. Table 4 summarizes comparison among the different pivotal clinical trials on the use of crizotinib for ALK-positive advanced NSCLC and this case series.
Table 4. Comparison of PROFILE trials and real world experience.
| Clinical studies | PROFILE 1005 | PROFILE 1007 | PROFILE 1014 | PROFILE 1029 | Current case series |
|---|---|---|---|---|---|
| Study design | Phase 2, 2nd line | Phase 3, 2nd line | Phase 3, 1st line | Phase 3, 1st line, East Asian | Case series, 1st line, 2nd line & beyond |
| Treatment | Crizotinib | Crizotinib vs. pemetrexed or docetaxel | Crizotinib vs. pemetrexed with cisplatin or carboplatin | Crizotinib vs. pemetrexed-cisplatin/carboplatin | Crizotinib |
| ORR | 53% | 65% vs. 20% (P<0.001) | 74% vs. 45% (P<0.001) | 88% vs. 46% (P<0.0001) | 64% |
| Median DOR | 43 weeks | Not given | 11.3 vs. 5.3 months | 44 vs. 18 weeks (P<0.0001) | 34 weeks (8.5 months) |
| Median PFS | 8.5 months | 7.7 vs. 3.0 months (HR 0.49, P<0.001) | 10.9 vs. 7.0 months (HR 0.45, P<0.001) | 11.1 vs. 6.8 months (HR 0.40, P<0.0001) | 8 months, overall; 15 months, upfront crizotinib |
| Median OS | Not assessed | 20.3 vs. 22.8 months (HR 1.02, P=0.54, NS); median follow up 12 months | 45.8 to NR vs. 47.5 to NR months (HR 0.76, P=0.978); median follow up 46 months | 28.5 vs. 27.7 months (HR 0.09, P=0.33); median follow up 33 months | Not reached (for upfront crizotinib; median follow up 18 months |
ORR, higher objective response rates; DOR, duration of response; PFS, progression-free survival; OS, overall survival; NS, not significant; NR, not reached.
Median PFS, for the total population and those who received crizotinib as first-line treatment, were 8 months and 15 months, respectively. This is comparable to PROFILE 1029, first line crizotinib trial in the East Asian population (14). Shorter duration of response in this case series maybe due to non-cancer related death in 30% of patients in the first 3 months. ORR to crizotinib was also comparable to published landmark trials.
Median TIDP with locally treated BM who progressed on crizotinib was 11 months (n=9). This finding is similar to a retrospective multi-institutional analysis of patients with BM from ALK-rearranged NSCLC treated with crizotinib. 84 out of 90 patients in that study received RT to the brain (either SRS or WBRT). Median intracranial PFS was equivalent for both patients treated before metastatic disease in the brain and those who received ALK-targeted TKIs (either crizotinib or crizotinib followed by second-generation ALK inhibitor) after the diagnosis of BM (11.7 vs. 11.9 months, respectively). This showed no difference in the durability of intracranial disease control of ALK inhibitors regardless of onset of BM (15). Similarly, ceritinib, a second- generation ALK inhibitor, was shown in ASCEND-4 to have better intracranial disease control compared to platinum-based chemotherapy (median intracranial PFS 10.6 vs. 6.7 months) (16).
Although BM in ALK-positive NSCLC occur frequently and may indicate a poor prognosis, our study showed that patients with BM who received appropriate local therapy may continue to benefit from crizotinib after intracranial disease progression. One patient had good systemic disease control outside the CNS for 31 months despite locally treated BM, before developing liver metastases. Another patient had asymptomatic de novo BM and received cytotoxic chemotherapy without local brain treatment. He then had recurrent intracranial progression for which he received WBRT and SRS. Duration of response of the lung metastases to crizotinib was also 31 months.
Patients with ALK-rearranged advanced NSCLC enrolled onto PROFILE 1005 or 1007, with previously treated BM followed by crizotinib, had longer TIDP compared to those with previously untreated BM (13.2 vs. 7 months, respectively) (17). Ou and co-authors in a retrospective analysis showed the clinical benefit of continuation of crizotinib beyond RECIST-defined progression of disease (PD). Overall survival was significantly longer among patients who continued crizotinib compared to those who did not (median 16.4 vs. 3.9 months, respectively, P<0.0001) (18). Crizotinib, therefore, should still be considered as one of the first lines of treatment.
Sequential ALK-inhibitor therapy
Final overall survival of PROFILE 1014 was reported by Solomon et al. They showed that sequential ALK inhibition therapy had the best survival benefit as compared to other treatments (13).
Retrospective data on sequential use of ALK inhibitors among Japanese patients with ALK-rearranged NSCLC showed durable control and improved overall survival for those who received alectinib after crizotinib failure (19,20). Asao and co-authors reported median PFS of 35.2 months and a 5-year survival rate from diagnosis of 77.8% in 13 patients who received sequential ALK inhibitors (20). Similarly, in another retrospective analysis of sequential treatment with crizotinib and ceritinib in ALK-positive NSCLC, the median combined PFS was 17.4 months (21). This trend for a long combined PFS from sequential treatment is also reflected in our case series. For patients who had sequential therapy of crizotinib followed with either ceritinib or alectinib, combined PFS in our case series was 30 months. Although it is noteworthy to consider the use of crizotinib, followed by 2nd line generation ALK inhibitor to maximize the optimal benefit of sequential ALK inhibitor therapy, we still lack prospective, randomized trials to prove the benefit of sequential therapy vs. upfront 2nd generation ALK inhibitors.
More recent trials showed that 2nd generation ALK-inhibitors improved PFS. J-ALEX trial, a phase 3 trial on Japanese patients with advanced ALK-positive NSCLC showed alectinib was superior compared to crizotinib as first-line treatment in terms of PFS benefit and better control of BM. This trial showed that at 12-month median follow up, median PFS was significantly improved with alectinib [not estimable (NE), 95% CI, 20.3–NE] compared with crizotinib (10.2 months, 8.2–12 months; HR 0.35, P<0.0001) (22). Ceritinib, in an adjusted comparison study across separate clinical trials, was also associated with prolonged OS and PFS compared with crizotinib when used as initial ALK inhibitor in previously treated ALK-positive NSCLC (23). Results from these trials pose a challenge to the clinician on how to maximize use of available ALK inhibitors, whether to use 2nd generation ALK inhibitor (alectinib or ceritinib) upfront or use sequential therapy with crizotinib as first-line then either alectinib or ceritinib on progression, to overcome crizotinib resistance. The most updated NCCN guideline listed alectinib as the preferred 1st line choice for ALK-positive advanced NSCLC. However, brigatinib, ceritinib or crizotinib remain to be other choices for first- line therapy.
Crizotinib cardiotoxicity
Eighteen percent (18%, n=4) of patients in our case series developed cardiotoxicity including bradycardia, prolonged QTc, and onset of complete heart block. Similarly, grade 1 and 2 sinus bradycardia (SB) occurred in 5% of patients, according to the crizotinib package insert (24). Grade 3 or 4 QTc prolongation was also observed in 2–4% of patients, and grade 5 arrhythmia in 1% of patients in PROFILE 1007 and PROFILE 1014 (11,12). Patients are usually asymptomatic and do not require dose reductions. However, crizotinib should be withheld in cases of QTC prolongation ≥500 ms or an increase from baseline ≥60 ms, until recovery to baseline, or to a QTc ≤481 ms. Once recovered, crizotinib is reduced to 200 mg BD and if necessary, to 250 mg once daily. Crizotinib should be permanently discontinued if torsades de pointes (TdP), polymorphic ventricular tachycardia or signs/symptoms of serious arrhythmia are present (25,26).
In a retrospective analysis on the potential correlation of heart rate and clinical response to crizotinib, sinus bradycardia was associated with significantly higher response rates and maximum tumour shrinkage. Mechanism of sinus bradycardia maybe related to the chronotropic effect of crizotinib rather than inotropic. In terms of dose modification, crizotinib is withheld in symptomatic bradycardia (grade 2–3) until recovery to at least grade 1, or heart rate of 60 bpm or above (27). In our case series, one patient developed severe bradycardia (heart rate, 39) and 30% increase of QTc from baseline. Pacemaker was inserted and patient subsequently resumed full dose crizotinib.
Conclusions
Crizotinib is an effective and safe treatment for ALK-positive NSCLC, either in first line or second line therapy. Analysis of our small series of cases, the real world experience, showed very comparable results with randomized phase 3 trials in terms of ORR and PFS. This confirms the therapeutic benefit of crizotinib in our daily practice.
Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448:561-6. 10.1038/nature05945
Blackhall FH, Peters S, Bubendorf L, et al. Prevalence and clinical outcomes for patients with ALK-positive resected stage I to III adenocarcinoma: results from the European Thoracic Oncology Platform Lungscape Project. J Clin Oncol 2014;32:2780-7. 10.1200/JCO.2013.54.5921
Kim H, Jang SJ, Chung DH, et al. A comprehensive comparative analysis of the histomorphological features of ALK-rearranged lung adenocarcinoma based on driver oncogene mutations: frequent expression of epithelial-mesenchymal transition markers than other genotype. PLoS One 2013;8:e76999. 10.1371/journal.pone.0076999
Chun SG, Choe KS, Iyengar P, et al. Isolated central nervous system progression on Crizotinib: an Achilles heel of non-small cell lung cancer with EML4-ALK translocation?. Cancer Biol Ther 2012;13:1376-83. 10.4161/cbt.22255
Sahu A, Prabhash K, Noronha V, et al. Crizotinib: A comprehensive review. South Asian J Cancer 2013;2:91-7. 10.4103/2278-330X.110506
Cui JJ, Tran-Dubé M, Shen H, et al. Structure based drug design of crizotinib (PF-02341066), a potent and selective dual inhibitor of mesenchymal-epithelial transition factor (c-MET) kinase and anaplastic lymphoma kinase (ALK). J Med Chem 2011;54:6342-63. 10.1021/jm2007613
Zhang Y, Wang W, Wang Y, et al. Response to Crizotinib Observed in Lung Adenocarcinoma with MET Copy Number Gain but without a High-Level MET/CEP7 Ratio, MET Overexpression, or Exon 14 Splicing Mutations. J Thorac Oncol 2016;11:e59-62. 10.1016/j.jtho.2015.12.102
Ou SH, Kwak EL, Siwak-Tapp C, et al. Activity of crizotinib (PF02341066), a dual mesenchymal-epithelial transition (MET) and anaplastic lymphoma kinase (ALK) inhibitor, in a non-small cell lung cancer patient with de novo MET amplification. J Thorac Oncol 2011;6:942-6. 10.1097/JTO.0b013e31821528d3
Kim DW, Ahn MJ, Shi Y, et al. Results of a global phase II study with crizotinib in advanced ALK-positive non-small cell lung cancer. J Clin Oncol 2012;30:7533 10.1200/JCO.2011.39.9766
Shaw AT, Ou SHI, Bang YJ, et al. Crizotinib in ROS1-Rearranged Non-Small Cell Lung cancer. N Engl J Med 2014;371:1963-71. 10.1056/NEJMoa1406766
Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385-94. 10.1056/NEJMoa1214886
Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. 10.1056/NEJMoa1408440
Solomon BJ, Kim DW, Wu YL, et al. Final Overall Survival Analysis From a Study Comparing First-Line Crizotinib Versus Chemotherapy in ALK-Mutation-Positive Non-Small-Cell Lung Cancer. J Clin Oncol 2018;36:2251-8. 10.1200/JCO.2017.77.4794
Lu S, Mok T, Lu Y, et al. Phase 3 study of first-line crizotinib vs pemetrexed-cisplatin/carboplatin (PCC) in East Asian patients with ALK+ advanced non-squamous non-small cell lung cancer (NSCLC). J Clin Oncol 2016;34:abstr 9058.
Johung KL, Yeh N, Desai NB, et al. Extended Survival and Prognostic Factors for Patients With ALK-Rearranged Non-Small-Cell Lung Cancer and Brain Metastasis. J Clin Oncol 2016;34:123-9. 10.1200/JCO.2015.62.0138
Soria JC, Tan DSW, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet 2017;389:917-29. 10.1016/S0140-6736(17)30123-X
Costa DB, Shaw AT, Ou SH, et al. Clinical Experience With Crizotinib in Patients With Advanced ALK-Rearranged Non-Small-Cell Lung Cancer and Brain Metastases. J Clin Oncol 2015;33:1881-8. 10.1200/JCO.2014.59.0539
Ou SH, Jänne PA, Bartlett CH, et al. Clinical benefit of continuing ALK inhibition with crizotinib beyond initial disease progression in patients with advanced ALK-positive NSCLC. Ann Oncol 2014;25:415-22. 10.1093/annonc/mdt572
Watanabe S, Hayashi H, Okamoto K, et al. Progression-Free and Overall Survival of Patients With ALK Rearrangement-Positive Non-Small Cell Lung Cancer Treated Sequentially With Crizotinib and Alectinib. Clin Lung Cancer 2016;17:528-34. 10.1016/j.cllc.2016.05.001
Asao T, Fujiwara Y, Itahashi K, et al. Sequential Use of Anaplastic Lymphoma Kinase Inhibitors in Japanese Patients With ALK-Rearranged Non-Small-Cell Lung Cancer: A Retrospective Analysis. Clin Lung Cancer 2017;18:e251-8. 10.1016/j.cllc.2016.11.015
Gainor JF, Tan DS, De Pas T, et al. Progression-Free and Overall Survival in ALK-Positive NSCLC Patients Treated with Sequential Crizotinib and Ceritinib. Clin Cancer Res 2015;21:2745-52. 10.1158/1078-0432.CCR-14-3009
Hida T, Nokihara H, Kondo M, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet 2017;390:29-39. 10.1016/S0140-6736(17)30565-2
Tan DS, Araújo A, Zhang J, et al. Comparative Efficacy of Ceritinib and Crizotinib as Initial ALK-Targeted Therapies in Previously Treated Advanced NSCLC: An Adjusted Comparison with External Controls. J Thorac Oncol 2016;11:1550-7. 10.1016/j.jtho.2016.05.029
Pfizer Inc. Xalkori prescribing information [package insert]. Groton, CT: Pfizer Inc.; 2011. Available online: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/202570s0001b1.pdf
Tartarone A, Gallucci G, Lazzari C, et al. Crizotinib-induced cardiotoxicity: the importance of a proactive monitoring and management. Future Oncol 2015;11:2043-8. 10.2217/fon.15.47
Cappuzzo F, Moro-Sibilot D, Gautschi O, et al. Management of crizotinib therapy for ALK-rearranged non-small cell lung carcinoma: an expert consensus. Lung Cancer 2015;87:89-95. 10.1016/j.lungcan.2014.12.010
Ou SH, Tong WP, Azada M, et al. Heart rate decrease during crizotinib treatment and potential correlation to clinical response. Cancer 2013;119:1969-75. 10.1002/cncr.28040
Acknowledgments
We thank some of our colleagues from the Department of Medical Oncology, Tan Tock Seng Hospital, including Dr. Akhil Chopra, Dr. Jens Samol and Dr. Matthew Flook, as well as the pharmacists and research team, for assisting us in identifying patients for this study. Pfizer partially supported this study.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Domain Specific Review Board approved our study protocol for retrospective analysis (DSRB Reference 2017/00025).
Footnotes
Conflicts of Interest: Dr. Chang has served as a member of the local advisory board of Bristol Myers Squibb (BMS), Mercke Sharpe and Dohme (MSD), Pfizer, Novartis and Celgene. He has received clinical research grant support from BMS, MSD, Pfizer, Astra Zeneca, Exelixis and Merck KGaA. Dr. Del Valle has no conflicts of interest to declare.