Abstract
Aims
Most people with Type 1 diabetes have low levels of persistent endogenous insulin production. The Diabetes Control and Complications Trial showed that close to diagnosis preserved endogenous insulin was associated with lower HbA1c, hypoglycaemia and complication rates, when intensively treated. We aimed to assess the clinical impact of persistent C‐peptide on rate of hypoglycaemia and HbA1c in those with long duration (> 5 years) Type 1 diabetes.
Methods
We conducted a cross‐sectional case–control study of 221 people (median age 24 years) with Type 1 diabetes. We confirmed ongoing endogenous insulin secretion by measuring C‐peptide after a mixed‐meal tolerance test. We compared self‐reported hypoglycaemia (n = 160), HbA1c, insulin dose and microvascular complications (n = 140) in those with preserved and low C‐peptide.
Results
Stimulated median (IQR) C‐peptide was 114 (43, 273) pmol/l and < 3 (< 3, < 3) pmol/l in those with preserved and low C‐peptide respectively. Participants with preserved C‐peptide had lower reported monthly rates of hypoglycaemia, with 21% fewer symptomatic episodes, 5.9 vs. 7.5 [incidence rate ratio (IRR) 0.79, P = 0.001], and 65% fewer asymptomatic episodes, 1.0 vs. 2.9 (IRR 0.35, P < 0.001). Those with preserved C‐peptide had a lower insulin dose (0.68 vs. 0.81 units/kg, P = 0.01) but similar HbA1c (preserved 69 vs. low 67 mmol/mol, P = 0.06).
Conclusions
Adults with Type 1 diabetes and preserved endogenous insulin production receiving usual care in the UK have lower daily insulin doses and fewer self‐reported hypoglycaemic episodes, but no difference in HbA1c. This is consistent with non‐intensive treatment in previous studies, and suggests a need to consider therapy intensification to gain full benefit of preserved endogenous insulin.
What's new?
Little is known about the clinical impact of persistent C‐peptide in long‐duration Type 1 diabetes.
We found that in adults with long‐duration Type 1 diabetes, persistent C‐peptide was associated with reduced self‐reported hypoglycaemia and insulin dose but was not associated with reduced HbA1c.
This suggests that individuals with persistent C‐peptide receiving routine care are potentially undertreated.
Routinely testing C‐peptide and subsequently setting personalized targets for treatment intensification in adults with persistent C‐peptide may improve glycaemic control and complication rates in this group.
What's new?
Little is known about the clinical impact of persistent C‐peptide in long‐duration Type 1 diabetes.
We found that in adults with long‐duration Type 1 diabetes, persistent C‐peptide was associated with reduced self‐reported hypoglycaemia and insulin dose but was not associated with reduced HbA1c.
This suggests that individuals with persistent C‐peptide receiving routine care are potentially undertreated.
Routinely testing C‐peptide and subsequently setting personalized targets for treatment intensification in adults with persistent C‐peptide may improve glycaemic control and complication rates in this group.
Introduction
Recent work has shown that many individuals with long duration Type 1 diabetes continue to produce low levels of endogenous insulin; however, the clinical significance of this is uncertain. β‐Cell function declines with increasing disease duration in Type 1 diabetes and this was assumed to progress to total β‐cell loss 1. Recent studies have demonstrated persistent endogenous insulin in many people with long duration Type 1 diabetes 2, 3, 4, 5, 6. Although there is rapid initial decline post diagnosis, insulin secretion reaches a plateau after ~ 7 years post diagnosis 7. Using highly sensitive C‐peptide assays up to 80% of those with long duration Type 1 diabetes (median duration 18 years) have been shown to have low‐level, detectable endogenous insulin secretion 5. Furthermore, some people with long duration Type 1 diabetes have surprisingly high levels of endogenous insulin, with 8–15% of those diagnosed in adulthood having either a serum C‐peptide > 0.2 nmol/l or a urine C‐peptide creatinine ratio of > 0.2 nmol/mmol 5, 6. The strongest clinical associations of C‐peptide appear to be disease duration and age at diagnosis, with those diagnosed younger being much less likely to have persistent C‐peptide 2, 5, 6, 8.
The Diabetes Control and Complications Trial (DCCT) and islet cell transplant studies have provided evidence for the clinical significance of persistent C‐peptide. Grouped and continuous prospective analyses of DCCT showed higher C‐peptide levels were associated with lower insulin dose, improved glycaemic control, fewer microvascular complications and markedly lower rates of hypoglycaemia 9, 10, 11. These findings were seen only in the intensively treated arm of DCCT, where among intensively treated participants persistent postprandial blood C‐peptide > 200 pmol/l was associated with a reduction in HbA1c and a 65% risk reduction in severe hypoglycaemia when compared with those with C‐peptide < 200 pmol/l 9. Findings from DCCT highlight that the benefit of persistent C‐peptide may arise from allowing tighter glucose control with intensive treatment through protection from hypoglycaemia. Additional data from islet transplant recipients reveal that restoration of even partial β‐cell function improves glycaemic control, variability and hypoglycaemic awareness along with reducing rates of hypoglycaemia 12, 13, 14, 15, 16. The effects of improved β‐cell function in islet transplantation appear continuous and not linked to an absolute threshold, with hypoglycaemic episodes in particular often improving with minimal graft function 16. It is possible that C‐peptide may have direct protective effect on complications 17 although a recent therapeutic trial of C‐peptide did not achieve its primary endpoint 18. These results are important evidence for international efforts to prevent or reverse β‐cell loss 19. Although DCCT provides clear evidence of benefit from preserved endogenous insulin secretion in an intensively treated trial setting, and studies of islet cell transplants show the clear benefit of restoring relatively large amounts of endogenous insulin secretion, the impact of preserved endogenous insulin in people with long‐standing diabetes receiving usual clinical care is unclear.
We aimed to assess the clinical impact of preserved endogenous insulin secretion, measured using C‐peptide, in people with long duration Type 1 diabetes.
Methods
The TIGI (Type 1 diabetes, Immunology, Genetics and endogenous Insulin production) study is a cross‐sectional, observational case–control study of people with long duration Type 1 diabetes in the UK 7. We recruited participants from the cross‐sectional UNITED (Using pharmacogeNetics to Improve Treatment in Early onset Diabetes) study, a population‐based study of those diagnosed with diabetes before age 30 years (and aged under 50 at recruitment) 5. Potential TIGI participants were selected on the basis of diabetes duration > 5 years and being in either the top or bottom quintile of urinary C‐peptide to creatinine ratio for their diabetes duration in UNITED 5. All participants included in TIGI had clinically defined Type 1 diabetes diagnosed under the age of 30 years, were treated with insulin from diagnosis and lived in the south west of the UK. Those with renal impairment were excluded from the analysis because C‐peptide is not a reliable measure of endogenous insulin production due to its renal excretion 20. Potential participants with a urinary C‐peptide to creatinine ratio > 0.2 nmol/l had glutamic acid decarboxylase (GAD) and islet antigen 2 (IA2) autoantibody testing performed. If autoantibody testing was negative individuals were tested for monogenic diabetes as described previously, and were excluded if found to have monogenic diabetes 21. To avoid the inclusion of young‐onset Type 2 diabetes, those with a urinary C‐peptide to creatinine ratio > 0.2 nmol/l who were islet autoantibody negative were excluded if they had a BMI > 30 kg/m2. Some 96% of participants were white British. All participants provided informed consent and the National Research Ethics Service Committee South West approved the study (13/SW/0312).
Confirmation of C‐peptide status
C‐peptide status was confirmed using a standard mixed‐meal tolerance test. This test was either performed at the Exeter National Institute for Health Research Clinical Research Facility, or at home where participants were visited by the study nurse. All participants fasted from midnight and did not take their morning insulin. Individuals were given a standard mixed‐meal tolerance test (Fortisip Compact, Nutricia, Trowbridge, UK) consisting of 160 ml containing per 100 ml: 240 kcal, 9.6 g protein, 9.3 g fat and 29.7 g carbohydrate. Participants attending the clinical research facility had a full multiple time point mixed‐meal tolerance test, with samples taken at 0, 30, 60, 90 and 120 min post meal. Participants visited at home had an abbreviated single time point mixed‐meal tolerance test, with a blood sample taken at 90 min post meal. Serum C‐peptide was analysed using a direct electrochemiluminescence immunoassay (Roche Diagnostics, Mannheim, Germany). The limit of detection of the assay is 3.3 pmol/l. We confirmed C‐peptide group, preserved (> 20 pmol/l) or low (< 10 pmol/l) using serum C‐peptide post mixed‐meal tolerance test to ensure participants remained in the pre‐defined groups. Eight of the recruited participants were excluded from further analysis as a result of discordant C‐peptide on mixed‐meal tolerance testing. These participants all had high C‐peptide in the UNITED study, but serum C‐peptide < 20 pmol/l after mixed‐meal tolerance test.
Assessment of hypoglycaemia
Participants completed a modified Clarke's hypoglycaemia questionnaire to assess rate and awareness of hypoglycaemia at the time of mixed‐meal tolerance test. The questionnaire is comprised of eight multiple choice questions, with answers being scored as 0 (aware) or 1 (reduced awareness). The maximum score is 7 and a score > 4 indicates reduced hypoglycaemic awareness 22. Rates of hypoglycaemia were determined by response to questions 5 and 6, they record frequency of hypoglycaemic episodes in the last month (defined as blood glucose < 3.5 mmol/l) with and without symptoms during the episode respectively, as described previously 23. Frequency of episodes was taken as a monthly average; those answering ‘1–3 episodes in the last month’ were averaged to 2, ‘once a week’ to 4 (1 × 4), ‘2–3 episodes per week’ to 10 (2.5 × 4), ‘4–5 episodes per week’ to 18 (4.5 × 4), and ‘almost daily’ to 25.
Assessment of HbA1c and microvascular complications
HbA1c was measured at the study visit and a historic HbA1c mean calculated from a local laboratory records. With informed consent we collected historic glycaemic control data from a biochemistry laboratory download of all recorded samples over the preceding 12 years in participants from our local area. HbA1c was measured at this time using ion exchange chromatography HPLC on the TOSOH G8 analyser (TOSOH Diagnostics, Tokyo, Japan) and standardized to IFCC. Historic HbA1c mean was calculated for each participant from all available results prior to recruitment [median (IQR) of 18 (12, 26) observations over 8 (5, 10) years].
For participants from our local area, clinical data on microvascular complications were obtained from hospital laboratory and retinal screening records. These records were not available for participants whose general practice used the laboratory and retinal screening service of other regional hospitals. Retinopathy status was obtained from the participant's most recent retinal screening record. The worst grade of retinopathy identified at the retinal screening visit prior to recruitment was recorded. Nephropathy status was defined according to whether an individual had ever had clinically defined microalbuminuria, as based on their biochemistry records. Microalbuminuria was defined as having two of three consecutive albumin to creatinine ratios high (> 2.5 mg/mmol for men and > 3.5 mg/mmol for women).
Statistical analysis
Analysis was carried out using Stata Statistical Software: Release 14 (StataCorp, College Station, TX, USA). Continuous variables were assessed for normality on visual examination of a histogram. Subsequently, differences in all clinical parameters were assessed using the Mann–Whitney U‐test. Differences in the severity of retinopathy (characterized as none, background, pre‐proliferative or proliferative) were compared using a non‐parametric trend test. Hypoglycaemia rates were considered to follow a Poisson distribution. Therefore, results are displayed as rates and incidence rate ratios, with confidence intervals also in the Poisson distribution. Statistical significance was defined as P < 0.05 for all statistical tests. The study recruited people who were in the top or bottom 20th centile of stimulated C‐peptide adjusted for duration. This led to a large difference in C‐peptide between the two pre‐defined groups and we therefore performed comparative analyses of these groups.
Results
Some 70 participants with preserved C‐peptide and 151 with low C‐peptide were included in this analysis (characteristics presented in Table 1). The majority were adults, the median (IQR) age 24 (17, 39) years. Median (IQR) C‐peptide was 114 (43, 273) pmol/l in the preserved C‐peptide group, and < 3 (< 3, < 3) pmol/l in the low C‐peptide group. Although duration of diabetes was similar between the two groups (median 13 years in both groups, P = 0.2), the preserved C‐peptide group were diagnosed at an older age, 15 vs. 6 years (P < 0.0001).
Table 1.
Cohort characteristics
| Characteristics | Low C‐peptide | Preserved C‐peptide | P‐value |
|---|---|---|---|
| No. of participants | 151 | 70 | – |
| No. male (%) | 86 (57) | 29 (41) | 0.03* |
| Age at diagnosis (years) | 6.1 (3.0, 12.5) | 15.1 (12.2, 22.0) | < 0.0001 |
| Age at recruitment (years) | 19.9 (14.3, 36.5) | 30.9 (20.8, 42.1) | 0.0001 |
| BMI (kg/m²) | 23.3 (20.2, 26.5) | 25.2 (23.3, 27.3) | 0.0006 |
| BMI standard deviation score | 0.8 (0.1, 1.5) | 1.0 (0.5, 1.6) | 0.2 |
| Duration of diabetes (years) | 13.3 (8.5, 24.5) | 12.6 (7.5, 22.0) | 0.2 |
| C‐peptide (pmol/l) | <3 (<3, <3) | 114 (43, 273) | < 0.0001 |
| HbA1c (mmol/mol) | 67 (58, 76) | 69 (62, 81) | 0.6 |
| HbA1c (%) | 8.3 (7.5, 9.1) | 8.5 (7.8, 9.6) | 0.6 |
| Insulin dose (U/kg in 24 h) | 0.81 (0.67, 0.95) | 0.68 (0.54, 0.94) | 0.01 |
| No. in hypoglycaemia rate analysis (%) | 121 (80) | 39 (56) | – |
| No. in historic HbA1c analysis (%) | 86 (57) | 67 (96) | – |
Values are reported as n (%) or median (IQR). P‐values are Mann–Whitney U unless stated otherwise stated; *χ2.
Adults with preserved C‐peptide had lower rates of hypoglycaemia
Hypoglycaemia questionnaires were filled out by 160 participants (Table S1), median (IQR) age 21 (15, 38) years. Some 151 participants completed all parts of questionnaire and we were able to calculate both hypoglycaemia rates and awareness; 9 of 160 partially completed the questionnaire, enabling calculation of hypoglycaemia rates but not hypoglycaemic awareness. The preserved C‐peptide group had 21% fewer symptomatic episodes per month [incidence rate ratio (IRR) 0.79, 95% confidence interval (CI) 0.68–0.91, P = 0.001], 5.9 vs. 7.5 episodes/month; and 65% fewer asymptomatic episodes per month (IRR 0.35, 95% CI 0.25–0.48, P < 0.001), 1.0 vs. 2.9 episodes/month (Figs 1 and S1). There was no difference in Clarke score between the two groups (P = 0.3), or proportion with a reduced hypoglycaemic awareness (score > 4 of 7, 8% vs. 12%, P = 0.5; Fig. S2).
Figure 1.
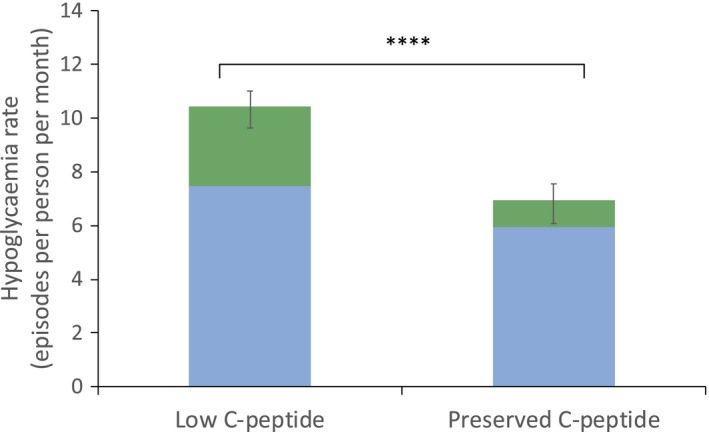
Total monthly rate of hypoglycaemia by C‐peptide group. Rates of aware (blue) and unaware (green) episodes with blood glucose < 3.5 mmol/l per month; derived from Clarke's hypoglycaemia questionnaire questions 5 and 6 respectively. ****P < 0.0001, for low (n = 118) vs. preserved (n = 39) C‐peptide. Error bars represent 95% confidence intervals.
Both the study visit HbA1c and historic HbA1c means were similar in both groups
Participants in the preserved group had a marginal trend towards higher study visit HbA1c, 69 vs. 67mmol/mol (8.5% vs. 8.3%), P = 0.06 (Figs 2 and S3). Historic HbA1c mean was calculated in 153 participants (Table 1). The historic HbA1c mean was also similar in both groups, 71 vs. 68mmol/mol (8.6% vs. 8.4%) in those with high and low C‐peptide respectively, P = 0.4 (Fig. S4).
Figure 2.
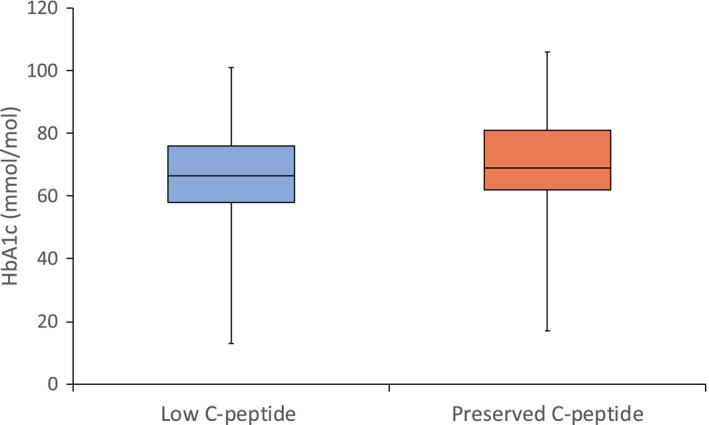
Boxplot of study visit HbA1c by C‐peptide group. Study visit HbA1c was similar in the low (n = 148) and preserved (n = 70) C‐peptide groups P = 0.06. Outliers are shown in Fig. S3.
Insulin dose was substantially lower in those with preserved C‐peptide production
Participants with preserved C‐peptide received a total daily dose of 0.68 (0.54, 0.94) units/kg, whereas those with low C‐peptide received 0.81 (0.67, 0.95) units/kg, for comparison P = 0.01 (Figs 3 and S5).
Figure 3.
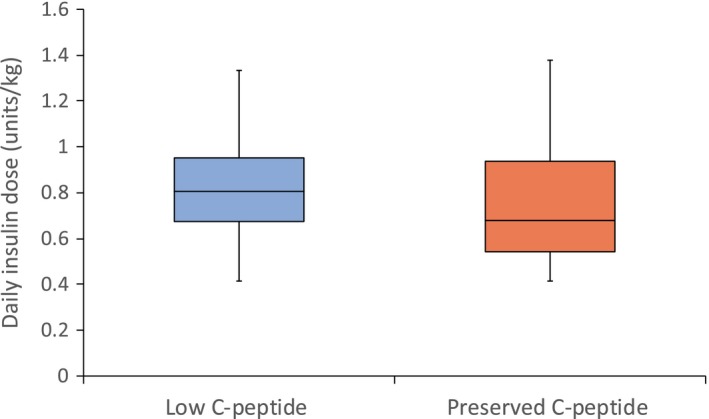
Boxplot of daily insulin dose by C‐peptide group. Insulin dose was lower in the preserved C‐peptide group (n = 70) vs. the low (n = 151) group, P = 0.01. Outliers are shown in Fig. S5.
Preservation of endogenous insulin secretion was not associated with differences in retinopathy or microalbuminuria
Retinopathy results were available on 130 participants (Table S2). Presence of retinopathy in the preserved C‐peptide group was similar to the low group, 66% vs. 74%, P = 0.3 (Fig. S6). There was no difference in grades of retinopathy between the two groups (P = 0.5).
The prevalence of microalbuminuria was also similar in participants with high and low C‐peptide. Records were available on 120 participants (Table S2). In these participants 20% and 23% of those with high and low C‐peptide met study criteria for microalbuminuria (P = 0.6) (Fig. S6).
Sensitivity analysis excluding people with C‐peptide > 200 pmol/l
To test whether the effects seen were due purely to those with C‐peptide > 200 pmol/l we performed analysis with these people excluded. The detectable (but < 200 pmol/l) C‐peptide group had 20% fewer symptomatic episodes per month (IRR 0.80, 95% CI 0.68–0.84, P = 0.005) and 61% fewer asymptomatic episodes per month (IRR 0.39, 95% CI 0.28–0.55, P < 0.001). HbA1c was similar between the two groups (P = 0.1), as was daily insulin dose (P = 0.1). Rates of retinopathy and microalbuminuria were similar in both groups (P = 0.9 and 0.4 respectively). Grade of retinopathy was also similar in both groups (P = 0.6).
Discussion
Our study showed that in adults, low levels of preserved C‐peptide production in long duration Type 1 diabetes in UK clinical practice were associated with reduced self‐reported hypoglycaemia without improvement in HbA1c. Rates of both symptomatic and asymptomatic hypoglycaemia were reduced in the preserved C‐peptide group, with similar hypoglycaemic awareness and without differences in either single measure or historic HbA1c mean. However, those with preserved C‐peptide were treated with a lower exogenous insulin dose. Consistent with a lack of difference in HbA1c, levels of retinopathy and microalbuminuria were not different when examined in a subset of our study population.
These findings mirror the conventionally treated arm of the DCCT. This suggests a lack of intensive treatment in UK practice and highlights the challenges in achieving tight control outside the closely monitored clinical trial setting. DCCT showed that where intensive diabetes treatment is given, higher levels of C‐peptide are associated with markedly lower hypoglycaemia, HbA1c and microvascular complications even with low levels of secretion (< 200 pmol/l). However, benefits were much less marked where conventional therapy was given 9, 10, 11. Although this may be explained in part by more rapid loss of endogenous insulin secretion in non‐intensively treated participants 11, it may also relate to reductions in hypoglycaemia risk associated with preserved C‐peptide allowing intensification of treatment to a tighter level of glycaemic control. The limitation of achieving optimal glycaemic control with intensive treatment in Type 1 diabetes is usually hypoglycaemia, which prevents up‐titration of insulin doses. The reduced glucose variability and better hypoglycaemia counter‐regulation associated with preservation of endogenous insulin secretion 16, 23, 24, 25 means that with intensive treatment an adult with retained endogenous insulin secretion can obtain a lower HbA1c at an acceptable level of hypoglycaemia than would be possible where endogenous insulin is absent.
Our findings are consistent with previous research on the clinical impact of C‐peptide. Hope et al. 23 observed an approximate doubling in self‐reported hypoglycaemia in those with Type 1 diabetes with C‐peptide < 200 pmol/l compared with > 200 pmol/l. This study focused on people who were older when diagnosed, with group durations of 25 vs. 10 years respectively 23. Kuhtreiber et al. 8 also used the Clarke score to assess hypoglycaemia. They categorized individuals as having mild, moderate or severe hypoglycaemia, showing that more severe hypoglycaemia was associated with lower levels of C‐peptide. In addition, they found that higher C‐peptide was associated with better glycaemic control and fewer complications. Their study had the benefit of a larger sample size and was thus better powered to assess a difference in complication rates 8. Our data are also aligned with studies of islet cell transplant recipients. In this setting, even minimal graft function, measured by C‐peptide or β score, correlates with reduced hypoglycaemia risk and improved glycaemic variability 12, 13, 14, 15, 16. Vantyghem et al. 13 showed that in islet cell transplant recipients partial β‐cell function reduced rates of hypoglycaemia however improvements in glycaemic control and variability required significantly better graft function. Combined these findings point toward endogenous insulin playing a key role in preventing hypoglycaemia; perhaps directly by stopping secretion when blood glucose levels fall or indirectly through counter‐regulatory hormones such as glucagon.
Strengths of our study include that we were able to utilize a highly sensitive C‐peptide assay, allowing for identification and classification of C‐peptide status at historically undetectable levels. We also robustly excluded individuals with both Type 2 and monogenic diabetes, ensuring that those with a high C‐peptide truly had Type 1 diabetes. Additionally, our recruitment process allowed the disease duration of both groups to be the same, removing a potential key confounder from this analysis. Our cohort were not part of a clinical intervention trial and received routine clinical care, making them reflective of current Type 1 diabetes management in the UK, both strengthening our findings and making them relevant to routine care in the UK.
Our study was limited by self‐reported hypoglycaemia and region‐restricted complication data reducing the power to assess a difference in complications. We used a validated hypoglycaemia questionnaire; however, this relied on participants both correctly identifying and recording hypoglycaemic episodes. It is likely that our data underestimate the frequency of hypoglycaemia, due to reduced hypoglycaemic awareness and nocturnal hypoglycaemia. Accurate identification of hypoglycaemia relies on frequent blood glucose self‐monitoring or continuous glucose monitoring, neither of which were part of the study protocol. It would be valuable to carry out continuous glucose monitoring on a cohort of similar participants, looking to remove participant bias and potentially validate our findings. A further limitation of our hypoglycaemia analysis is that participants with low C‐peptide were younger (18.4 vs. 29.3 years), differences in care, and frequency of hypoglycaemia in the paediatric population may potentially influence our findings. Despite studying 69 cases aged under 18 years overall (median age 14 years), with 62 cases in the hypoglycaemia rate analysis, the number of people under 18 years with preserved C‐peptide was very low (6 of 69 and 4 of 62). Because of the absence of young children in the preserved C‐peptide group, our suggestion of potentially intensifying treatment in those with preserved C‐peptide is not applicable to children. This additionally raises the question of whether the high hypoglycaemia rate in young children is related directly to this lack of preserved C‐peptide in those diagnosed young, or to a mixture of metabolic and behavioural factors. The differences in age of study participants with and without preserved C‐peptide could potentially bias our findings of different insulin doses in those with low and high C‐peptide: many (51 of 151) of our low C‐peptide participants were in the pubertal age range (10–16 years), which can affect insulin requirements. However, when we excluded those between the ages of 10 and 16 years, the differences were similar: median daily insulin dose was 0.67 units/kg in the preserved group compared with 0.75 units/kg in the low group (n = 166, P = 0.1). A further limitation is that we were only able to obtain data on complications on participants based in the Exeter area, due to the availability of medical records. This reduced our power to identify differing rates of retinopathy and microalbuminuria, so we could not rule out smaller differences that may still be clinically relevant. The sample size for our complication analysis provided 80% power (α = 0.05), to detect a difference in proportions of 30% for both retinopathy and microalbuminuria, therefore meaningful differences in complications may not be detected with our limited sample size. A further limiting factor to this analysis was the selection criteria for our study, which excluded people with renal impairment, as C‐peptide is renally excreted and therefore less reflective of endogenous beta cell function in those with impaired renal function 20. Our study assessed endogenous insulin by measuring serum C‐peptide, and is therefore unable to differentiate between an association with preserved endogenous insulin, or a direct association with C‐peptide rather than endogenous insulin.
Notwithstanding these limitations, our study suggests it may be possible to intensify treatment in adults with persistent C‐peptide. DCCT demonstrated that the benefits of persistent C‐peptide production could only be fully utilized when individuals received intensive therapy. Our study focused on those receiving routine clinical care and did not show the improvement of HbA1c associated with persistent C‐peptide in DCCT, with the benefit of maintained endogenous insulin secretion limited to lower insulin dose and less hypoglycaemia. A lack of impact on microvascular complications is therefore unsurprising considering >10 years of HbA1c records showed similar glycaemic control in both groups. We consider the most likely explanation for this finding to be a lack of intensive treatment, showing that factors other than hypoglycaemia limit achievement of HbA1c targets. Clinicians do not routinely test C‐peptide and there are currently no guidelines to treat those with persistent C‐peptide, who are protected from hypoglycaemia, more intensively. Therefore, these people may be considered to have acceptable glycaemic control in practice, when they would be able to achieve tighter glycaemia control and reduce risk of long‐term complications with more intensive treatment, without unacceptable hypoglycaemia. Cautious targeted intensification of treatment in adults with preserved C‐peptide could therefore be a potential clinical strategy to improve control and an important area for future study.
Our study highlights the association of persistent high C‐peptide with reduced hypoglycaemia in adults. Additionally, it demonstrates that higher C‐peptide does not always robustly associate with improved glycaemic control and reduced complications rates. This may be a consequence of all adults being treated to the same glycaemic targets, irrespective of C‐peptide production. The apparent under‐treatment of those with preserved C‐peptide production makes our assessment of any complication benefit difficult.
Funding sources
This study was funded by the JDRF (strategic research agreement 201301394). SH, BK, AH and RB are core members of the National Institute for Health Research Exeter Clinical Research Facility. ATH and BMS are supported by the NIHR Exeter Clinical Research Facility. BMS is supported as part of the MRC MASTERMIND consortium. TJM is funded by an NIHR clinical senior lecturer fellowship. ATH is supported by a Wellcome Trust Senior Investigator Award (WT098395/Z/12/Z) and an NIHR Senior Investigator award. AGJ is supported by an NIHR Clinician Scientist award. RAO is supported by a Diabetes UK Harry Keen Fellowship. This article presents independent research supported by JDRF, Diabetes UK, the Wellcome Trust and the NIHR Exeter Clinical Research Facility.
The views expressed are those of the authors and not necessarily of JDRF, Diabetes UK, the Wellcome Trust, the National Health Service, the National Institute for Health Research, or the Department of Health.
Competing interests
None declared.
Author contributions
RAO, AGJ, TJM and ATH conceived of and designed the study. TT and BR contributed to design of the study. AH and RB recruited participants for the study. SMM, SH, BK, AH and RB were involved in acquisition of data and clinical information. SMM, BMS, AGJ, ATH and RAO contributed to the data analysis. SMM, AGJ and RAO wrote the manuscript which was reviewed by all authors. All authors approved the final version of the manuscript.
Supporting information
Figure S1. Hypoglycaemia rates by C‐peptide group.
Figure S2. Reduced hypoglycaemic awareness by C‐peptide group.
Figure S3. Boxplot of study visit HbA1c by C‐peptide group.
Figure S4. Historic HbA1c mean analysis.
Figure S5. Boxplot of daily insulin dose by C‐peptide group.
Figure S6. Rates of retinopathy and microalbuminuria by C‐peptide group.
Table S1. Hypoglycaemia analysis cohort characteristics.
Table S2. Complication analysis cohort characteristics.
Acknowledgements
Diabet. Med. 36: 1092–1099(2019)
(Clinical Trials Registry No: NCT03490773)
References
- 1. Eisenbarth GS. Type I diabetes mellitus. A chronic autoimmune disease. N Engl J Med 1986; 314: 1360–1368. [DOI] [PubMed] [Google Scholar]
- 2. Palmer JP, Fleming GA, Greenbaum CJ, Herold KC, Jansa LD, Kolb H et al C‐peptide is the appropriate outcome measure for Type 1 diabetes clinical trials to preserve beta‐cell function: report of an ADA workshop, 21‐22 October 2001. Diabetes 2004; 53: 250–264. [DOI] [PubMed] [Google Scholar]
- 3. Wang L, Lovejoy NF, Faustman DL. Persistence of prolonged C‐peptide production in Type 1 diabetes as measured with an ultrasensitive C‐peptide assay. Diabetes Care 2012; 35: 465–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Oram RA, Jones AG, Besser REJ, Knight BA, Shields BM, Brown RJ et al The majority of patients with long‐duration Type 1 diabetes are insulin microsecretors and have functioning beta cells. Diabetologia 2014; 57: 187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oram RA, McDonald TJ, Shields BM, Hudson MM, Shepherd MH, Hammersley S et al Most people with long‐duration Type 1 diabetes in a large population‐based study are insulin microsecretors. Diabetes Care 2015; 38: 323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Davis AK, DuBose SN, Haller MJ, Miller KM, DiMeglio LA, Bethin KE et al Prevalence of detectable C‐peptide according to age at diagnosis and duration of Type 1 diabetes. Diabetes Care 2015; 38: 476–81. [DOI] [PubMed] [Google Scholar]
- 7. Shields BM, McDonald TJ, Oram R, Hill A, Hudson M, Leete P et al C‐peptide decline in Type 1 diabetes has two phases: an initial exponential fall and a subsequent stable phase. Diabetes Care 2018; 41: 1486–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kuhtreiber WM, Washer SLL, Hsu E, Zhao M, Reinhold P, Burger D et al Low levels of C‐peptide have clinical significance for established Type 1 diabetes. Diabet Med 2015; 32: 1346–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Effect of intensive therapy on residual beta‐cell function in patients with Type 1 diabetes in the diabetes control and complications trial. A randomized, controlled trial. The Diabetes Control and Complications Trial Research Group. Ann Intern Med 1998; 128: 517–523. [DOI] [PubMed] [Google Scholar]
- 10. Steffes MW, Sibley S, Jackson M, Thomas W. Beta‐cell function and the development of diabetes‐related complications in the diabetes control and complications trial. Diabetes Care 2003; 26: 832–836. [DOI] [PubMed] [Google Scholar]
- 11. Lachin JM, McGee P, Palmer JP; DCCT/EDIC Research Group . Impact of C‐peptide preservation on metabolic and clinical outcomes in the Diabetes Control and Complications Trial. Diabetes 2014; 63: 739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL et al Islet transplantation in seven patients with Type 1 diabetes mellitus using a glucocorticoid‐free immunosuppressive regimen. N Engl J Med 2000; 343: 230–238. [DOI] [PubMed] [Google Scholar]
- 13. Vantyghem M‐C, Raverdy V, Balavoine A‐S, Defrance F, Caiazzo R, Arnalsteen L et al Continuous glucose monitoring after islet transplantation in Type 1 diabetes: an excellent graft function (β‐score greater than 7) is required to abrogate hyperglycemia, whereas a minimal function is necessary to suppress severe hypoglycemia (β‐score greater than 3). J Clin Endocrinol Metab 2012; 97: E2078–E2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brooks AM, Walker N, Aldibbiat A, Hughes S, Jones G, de Havilland J et al Attainment of metabolic goals in the integrated UK islet transplant program with locally isolated and transported preparations. Am J Transplant 2013; 13: 3236–3243. [DOI] [PubMed] [Google Scholar]
- 15. Ryan EA, Paty BW, Senior PA, Lakey JRT, Bigam D, Shapiro AMJ. Beta‐score: an assessment of beta‐cell function after islet transplantation. Diabetes Care 2005; 28: 343–347. [DOI] [PubMed] [Google Scholar]
- 16. Brooks AM, Oram R, Home P, Steen N, Shaw JAM. Demonstration of an intrinsic relationship between endogenous C‐peptide concentration and determinants of glycemic control in Type 1 diabetes following islet transplantation. Diabetes Care 2015; 38: 105–112. [DOI] [PubMed] [Google Scholar]
- 17. Wahren J, Larsson C. C‐peptide: new findings and therapeutic possibilities. Diabetes Res Clin Pract 2015; 107: 309–319. [DOI] [PubMed] [Google Scholar]
- 18. Wahren J, Foyt H, Daniels M, Arezzo JC. Long‐acting C‐peptide and neuropathy in Type 1 diabetes: a 12‐month clinical trial. Diabetes Care 2016; 39: 596–602. [DOI] [PubMed] [Google Scholar]
- 19. Skyler JS. Hope vs. hype: where are we in Type 1 diabetes? Diabetologia 2017; 61: 509–516. [DOI] [PubMed] [Google Scholar]
- 20. Jones AG, Hattersley AT. The clinical utility of C‐peptide measurement in the care of patients with diabetes. Diabet Med 2013; 30: 803–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shepherd M, Shields B, Hammersley S, Hudson M, McDonald TJ, Colclough K et al Systematic population screening, using biomarkers and genetic testing, identifies 2.5% of the U.K. pediatric diabetes population with monogenic diabetes. Diabetes Care 2016; 39: 1879–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Clarke WL, Cox DJ, Gonder‐Frederick LA, Julian D, Schlundt D, Polonsky W. Reduced awareness of hypoglycemia in adults with IDDM. A prospective study of hypoglycemic frequency and associated symptoms. Diabetes Care 1995; 18: 517–522. [DOI] [PubMed] [Google Scholar]
- 23. Hope SV, Knight BA, Shields BM, Hill AV, Choudhary P, Strain WD et al Random non‐fasting C‐peptide testing can identify patients with insulin‐treated Type 2 diabetes at high risk of hypoglycaemia. Diabetologia 2018; 61: 66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fukuda M, Tanaka A, Tahara Y, Ikegami H, Yamamoto Y, Kumahara Y et al Correlation between minimal secretory capacity of pancreatic β‐cells and stability of diabetic control. Diabetes 1988; 37: 81–88. [DOI] [PubMed] [Google Scholar]
- 25. Pinckney A, Rigby MR, Keyes‐Elstein L, Soppe CL, Nepom GT, Ehlers MR. Correlation among hypoglycemia, glycemic variability, and C‐peptide preservation after alefacept therapy in patients with Type 1 diabetes mellitus: analysis of data from the Immune Tolerance Network T1DAL Trial. Clin Ther 2016; 38: 1327–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Hypoglycaemia rates by C‐peptide group.
Figure S2. Reduced hypoglycaemic awareness by C‐peptide group.
Figure S3. Boxplot of study visit HbA1c by C‐peptide group.
Figure S4. Historic HbA1c mean analysis.
Figure S5. Boxplot of daily insulin dose by C‐peptide group.
Figure S6. Rates of retinopathy and microalbuminuria by C‐peptide group.
Table S1. Hypoglycaemia analysis cohort characteristics.
Table S2. Complication analysis cohort characteristics.


