Abstract
Viral hepatitis is a leading cause of morbidity and mortality worldwide, but has long been neglected by national and international policymakers. Recent modelling studies suggest that investing in the global elimination of viral hepatitis is feasible and cost‐effective. In 2016, all 194 member states of the World Health Organization endorsed the goal to eliminate viral hepatitis as a public health threat by 2030, but complex systemic and social realities hamper implementation efforts. This paper presents eight case studies from a diverse range of countries that have invested in responses to viral hepatitis and adopted innovative approaches to tackle their respective epidemics. Based on an investment framework developed to build a global investment case for the elimination of viral hepatitis by 2030, national activities and key enablers are highlighted that showcase the feasibility and impact of concerted hepatitis responses across a range of settings, with different levels of available resources and infrastructural development. These case studies demonstrate the utility of taking a multipronged, public health approach to: (a) evidence‐gathering and planning; (b) implementation; and (c) integration of viral hepatitis services into the Agenda for Sustainable Development. They provide models for planning, investment and implementation strategies for other countries facing similar challenges and resource constraints.
Keywords: developing countries, disease elimination, hepatitis B, hepatitis C, investment case, organizational case studies
Abbreviations
- DAAs
direct‐acting antivirals
- DBS
dried blood spot
- GHSSH
Global Health Sector Strategy on Viral Hepatitis
- HBIg
hepatitis B immunoglobulin
- HBsAg
hepatitis B surface antigen
- HIV
human immunodeficiency virus
- MTCT
mother‐to‐child transmission
- NSP
needle and syringe program
- OAT
opioid antagonist treatment
- PWID
people who inject drugs
- RNA
ribonucleic acid
- SDG
Sustainable Development Goals
- TRIPS
Trade Related Aspects of Intellectual Property Rights
- WHO
World Health Organization
Key points.
Viral hepatitis is the 6th leading cause of death globally, surpassing all other chronic infectious diseases including HIV, tuberculosis and malaria
Elimination of viral hepatitis as a public health threat is achievable; all WHO member countries endorsed this goal formally in 2016
Planning, implementation and integration of national responses to viral hepatitis is ongoing, and many countries have adopted innovative approaches to address the diverse challenges of this endeavour in their local contexts
Existing approaches demonstrate that investing in viral hepatitis is affordable and cost‐effective, provides multisectoral cost‐benefits, and alleviates the human burden of the epidemic
1. INTRODUCTION
Viral hepatitis contributes substantially to the global burden of disease, with 248 million people infected with hepatitis B and 71 million infected with hepatitis C worldwide.1 If left untreated, chronic viral hepatitis can cause life‐threatening complications, such as cirrhosis and hepatocellular carcinoma.2 Despite this, the public health consequences of viral hepatitis have long been neglected.1 In contrast to the progress in combating many other communicable diseases in recent years, viral hepatitis‐related morbidity and mortality continue to rise.1, 3 In 2010 viral hepatitis was the 10th leading cause of death, but by 2015, with 1.2 million deaths, it had overtaken HIV, malaria and tuberculosis to rise to sixth.4 Most viral hepatitis deaths are avertable through increased access to prevention, diagnosis and treatment.
In areas of high hepatitis B endemicity (eg Southeast Asia and sub‐Saharan Africa), perinatal mother‐to‐child transmission (MTCT) and horizontal transmission during childhood are the most common routes of infection, while sexual contacts, unsafe injecting practices, and unhygienic medical or cosmetic procedures drive transmission elsewhere.5, 6, 7 Risk of developing chronic hepatitis B infection is inversely related to age at infection: around 90% of infants infected perinatally develop chronic infection, unless vaccinated at birth. This risk decreases to around 30% among children infected before the age of six years and to less than 5% of persons infected as adults.8, 9, 10
The hepatitis C epidemic is similarly geographically diverse and mode of transmission differs substantially between regions.11, 12, 13, 14 Globally, an estimated 52% of people who inject drugs (PWID) are hepatitis C antibody positive.15 Lack of access to needle and syringe programmes (NSPs) and opioid antagonist treatment (OAT) result in unsafe injecting practices, which are the major route of transmission in high‐income countries.15, 16 In low‐ and middle‐income countries, additional transmission occurs in healthcare settings through substandard infection control practices.17
In 2016, the 69th World Health Assembly adopted the Global Health Sector Strategy on Viral Hepatitis (GHSSH) 2016‐2021. The strategy outlines five synergistic prevention and treatment service coverage targets to achieve the elimination of viral hepatitis as a public health threat by 2030 (defined as 90% reduction in incidence and 65% in mortality, see Table 1).18 Implementation of the strategy is expected to strengthen health systems while enabling progress toward the United Nations' Sustainable Development Goal (SDG) 3 target of universal health coverage.19, 20 Modelling studies suggest that rapid investment in diagnostic, prevention, and treatment services could achieve the World Health Organization (WHO) targets by 2030.21, 22
Table 1.
Viral hepatitis service coverage and impact targets
| Target area | Baseline 2015 | 2020 Target | 2030 Target |
|---|---|---|---|
| Service coverage targets | |||
| Hepatitis B virus vaccination: childhood vaccine coverage (third dose coverage) | 82% of infants | 90% | 90% |
| Prevention of hepatitis B virus mother‐to‐child transmission: hepatitis B virus birth‐dose coverage or other approach to prevent mother‐to‐child transmission | 38% | 50% | 90% |
| Blood safety: donations screened with quality assurance | 89% | 95% | 100% |
| Injection safety: use of engineered devices | 5% | 50% | 90% |
| Sterile needle/syringe set distributed per person per year for people who inject drugs | 20 | 200 | 300 |
| Viral hepatitis B and C diagnosis (coverage %) | <5% of chronic hepatitis infections diagnosed | 30% | 90% |
| Viral hepatitis B and C treatment (coverage %) | <1% receiving treatment | 3 million | 80% eligible treated |
| Impact targets | |||
| Incidence: new cases of viral hepatitis B and C infections | Between 6 and 10 million infections are reduced to 0.9 million infections by 2030 (95% declined in hepatitis B virus infections, 80% decline in hepatitis C virus infections) | 30% reduction (equivalent to 1% prevalence of HBsAg among children) | 90% reduction (equivalent to 0.1% prevalence of HBsAg among children) |
| Mortality: viral hepatitis B and C deaths | 1.4 million deaths reduced to less than 500 000 by 2030 (65% for both viral hepatitis B and C) | 10% reduction | 65% reduction |
Source: Global Health Sector Strategy on Viral Hepatitis, 2016‐2021.18
1.1. How can viral hepatitis be eliminated by 2030?
Eliminating viral hepatitis requires substantial investments in health systems strengthening and the full continuum of hepatitis services.18 Investing in the prevention and treatment of viral hepatitis provides many direct, indirect and cross‐sectoral economic benefits through saving lives and alleviating the cost burden of disease to the individual, their families and the state.23, 24, 25, 26 To achieve elimination at a national level, the country‐specific context and its unique challenges must be considered. A multipronged approach comprising three main pillars is most effective in addressing the local context; comprising (a) evidence‐gathering and planning the response; (b) implementation of disease‐specific activities, including investments in the delivery of care; and (c) integration of the viral hepatitis response into SDG 3 by adopting a public health approach and embedding services into universal health coverage.27
The necessary tools for viral hepatitis elimination are already available, but worldwide implementation of a concerted viral hepatitis response is slow and faces many challenges. These include low levels of investments in health overall; inadequate data and weak surveillance systems; poor infrastructure; low awareness among policymakers, at‐risk populations and primary care practitioners; high prices of some diagnostics and treatments; and a lack of prioritisation of viral hepatitis.28, 29 While most countries are on track to meet the WHO's 2030 target of < 0.1% Hepatitis B surface antigen (HBsAg) prevalence among 5‐year‐olds, without substantial further investments this target is currently unachievable for 20 countries, mainly in Africa and the Western Pacific. Moreover, only 12 countries are currently on track to achieve the hepatitis C elimination goal that all WHO member states adopted in 2016.30
We have developed a Viral Hepatitis Investment Framework outlining the resourcing required to achieve elimination, the cost of the elimination of viral hepatitis globally, and methods for countries to address existing challenges.31 The Viral Hepatitis Investment Framework highlights key enablers to support a comprehensive viral hepatitis response and outlines priority national and international activities to maximise return on investment (Figure 1). Using the structure of the Investment Framework, this paper presents case studies from diverse countries (Table 2) that are successfully implementing innovative strategies to eliminate viral hepatitis (see Table 3). Additional case studies listed in Table 3 are summarised in the Appendix S1 (Figures 2, 3, 4).
Figure 1.
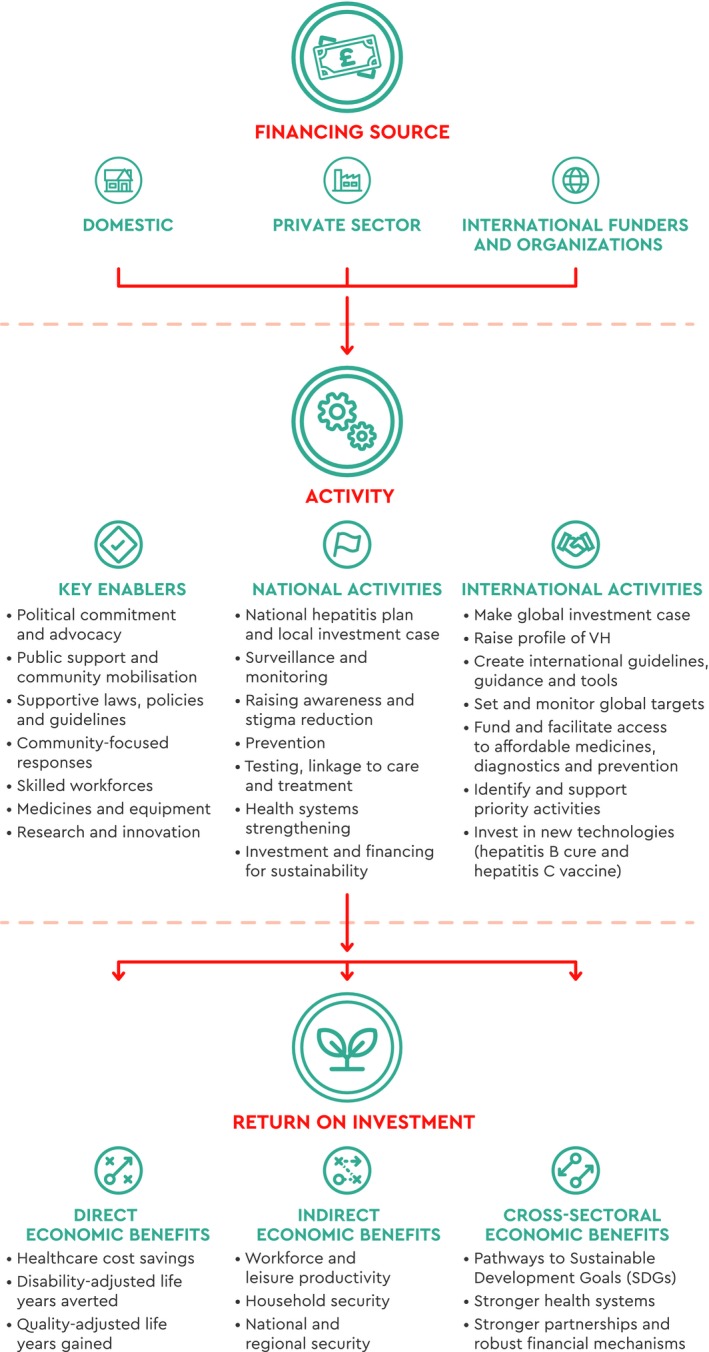
Investment framework for viral hepatitis elimination
Table 2.
Country characteristics
| Georgia | South Africa | Scotland | Brazil | China | Egypt | Rwanda | Australia | |
|---|---|---|---|---|---|---|---|---|
| Population total (million, 2017) | 3.72 | 56.72 | 5.3 (2011) | 208.49 | 1.386 billion | 97.55 | 12.21 | 24.6 |
| Life expectancy at birth (years) | 73 | 63 | 79 | 76 | 76 | 71 | 67 | 83 |
| GNI per capita (US$) | 3780 | 5430 | 42 370 (UK total) | 8840 | 8690 | 3010 | 720 | 51 360 |
| HBsAg positive population (%) | 115 948 (2.64%)2 | 3.5 million (6.7%)2 | 8700 (0.2%)2, 100 | 1.28 million (0.65%)2 | 74.6 million (5.49%)2 | 1.34 million (1.7%)2 | 722 449 (6.7%)2 | 83 121 (0.37%)2 |
| HCV‐RNA positive population (%) | 150 000 (5.4%)101 | 356 000 (0.7%)102 | 37 000 (0.8%)103 | 700 000 (0.71%)104 | 9.8 million (0.7%)105 | 3.81 million (7%)76 | 175 000 (3.1%)11 | 230 000 (1%) |
Table 3.
National activities and country examples aimed at elimination of viral hepatitis
| National activities | Country examples presented in this paper | |
|---|---|---|
| Evidence‐gathering and planning | National hepatitis plan (addressing hepatitis B, hepatitis C or both) | Georgia, Australia, Brazil, China, Egypt, Iceland, Malaysia, Portugal, Scotland, South Africa |
| Accurate data to inform the response (Surveillance and Monitoring) | Scotland, Portugal, Brazil, Egypt, Georgia, Iceland, Pakistan, South Africa | |
| Local investment case | South Africa, Rwanda | |
| Implementation | Raising awareness and stigma reduction | Brazil, Australia,, China, Egypt, Iceland, Malaysia, Portugal, Pakistan |
| Investment in prevention | China, Fiji, Pakistan, Australia, Brazil, Iceland, Georgia, Malaysia, Portugal, Scotland | |
| Testing, linkage to care and treatment | Egypt, Australia, China, Georgia, Iceland, Malaysia, Portugal, Scotland, South Africa | |
| Integration | Investment and financing for sustainability | Australia, China, Iceland, Malaysia, Rwanda |
| Health Systems Strengthening | Rwanda, Brazil, Fiji, Georgia, Malaysia, South Africa |
Source: Global Policy Report on the prevention and control of viral hepatitis.106
Figure 2.
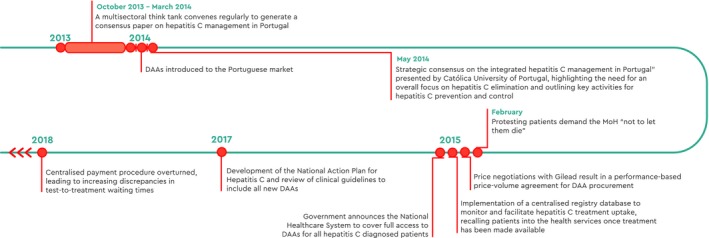
Timeline of national activities, Portugal
Figure 3.
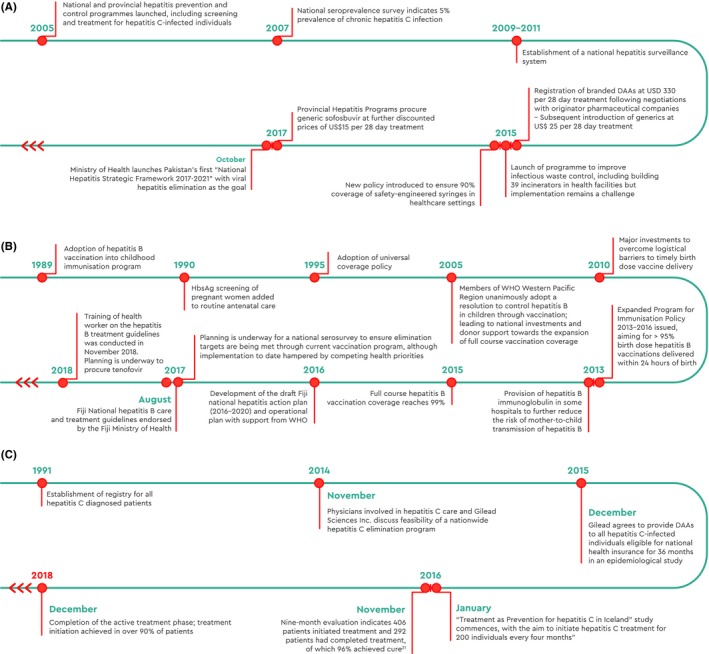
Timeline of national activities, Pakistan (A), Fiji (B), and Iceland (C)
Figure 4.
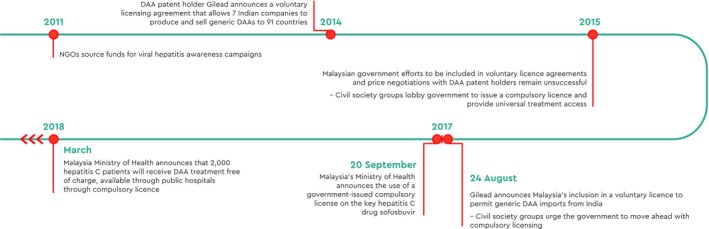
Timeline of national activities, Malaysia
1.2. Evidence‐gathering and planning
Low‐quality surveillance systems and a lack of reliable cause‐specific mortality data limit countries' capacity to guide, implement and monitor effective viral hepatitis responses.32, 33 To advocate for an adequate allocation of domestic resources and to mobilise external funding support, countries should develop a national plan that sets ambitious but achievable targets, informed by a robust local investment case for viral hepatitis. Gathering accurate data to inform a targeted approach can improve the cost‐effectiveness of specific interventions.34, 35, 36 Since the launch of the GHSSH 2016‐2021, more countries have developed national hepatitis plans1 and both local and global investment cases for the elimination of viral hepatitis have been built.31, 35, 37 Many countries have begun collecting epidemiological data through national seroprevalence surveys or by adding key hepatitis indicators into existing surveillance systems. Below, we give examples of countries that have gathered evidence and are developing a national plan (Georgia), produced an investment case for elimination (South Africa) and obtained accurate data to inform the response (Scotland).
1.3. Georgia: the development of a national plan
Georgia was the first country in the WHO European region to set a hepatitis C elimination goal and develop a national plan for viral hepatitis tailored to the local context. Georgia's significant experience with HIV prevention and control programmes and the existing human and technical capacities to implement large‐scale health programmes facilitated the implementation of their national hepatitis C elimination programme.38 An international Technical Advisory Group assisted with describing the local hepatitis C epidemiology and proposing strategies, objectives and actions to address gaps in advocacy and awareness, surveillance, harm reduction, blood safety, infection control, and evidence‐based screening and linkage to care. Gilead Science provided direct‐acting antiviral (DAA) therapy to Georgia at no cost after the elimination programme commenced; reportedly, a key reason for their decision was the Georgian Government's commitment to an elimination response.
The programme initially focused on increasing access to affordable diagnostics; providing free DAA treatment to persons with severe liver disease at highest‐risk of hepatitis C‐related mortality; and building capacity to achieve programme goals of preventing transmission and eliminating the disease.39 Initial obstacles included suboptimal alignment of programme development and implementation, leading to bottlenecks in patient flow and wait lists.40 Training for healthcare workers was only provided after the programme launched; however, doctors have subsequently received continuous technical support.
The programme has now expanded its scope to treat every person chronically infected with hepatitis C, as outlined in the “Strategic plan for the Elimination of Hepatitis C Virus in Georgia, 2016‐2020”. Hepatitis C treatment services are provided at treatment centres located throughout the country and treatment decentralisation in harm reduction centres and primary care is ongoing. Patient out‐of‐pocket fees for diagnostics and clinical monitoring are based on ability to pay. Georgia is working to integrate its hepatitis C elimination programme into the overall health system, because this will benefit the management of other health problems such as HIV and tuberculosis.41 This is primarily being achieved via treatment decentralisation into primary care and harm reduction services.
The implementation of the national action plan increased access to hepatitis C testing and linkage to care while driving improvements in monitoring and surveillance, infection control and prevention.38, 41 The evaluation of harm reduction‐based peer‐supported hepatitis C treatment demonstrated excellent treatment uptake and retention in care among PWID based in Tbilisi.42 By January 2019, 53 000 people had initiated treatment with the new DAAs, of whom almost 34 800 had already achieved hepatitis C cure (Figure 5A). Remaining challenges relate to the marginalised status of PWID, with stigma and discrimination preventing PWID from accessing hepatitis C services. Punitive drug laws (such as criminal responsibility for personal drug use) challenge the effectiveness of harm reduction programmes and lead to high rates of incarceration and hepatitis C transmission in prisons, where access to OST is limited. As well, as in other countries aiming for hepatitis C elimination, treatment numbers declined after the first two years of the programme, with many people being unaware of their hepatitis C status or not commencing treatment.
Figure 5.
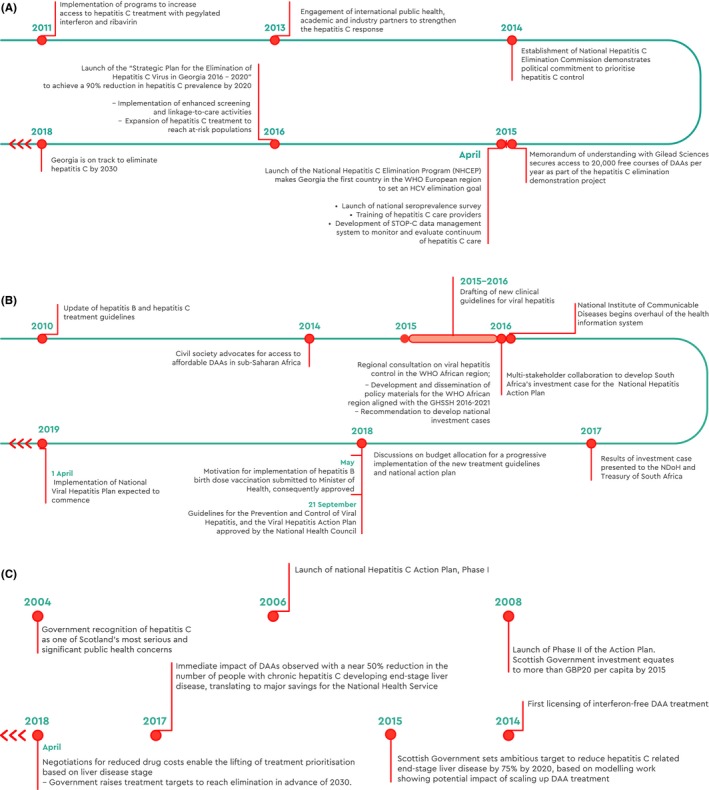
Timeline of national activities, Georgia (A), South Africa (B), and Scotland (C)
1.4. South Africa: The development of an investment case
South Africa's National Action Plan 2017‐2021 is one of the first examples of an investment case that combines tools for costing, impact modelling, cost‐effectiveness analysis, and fiscal space analysis for scaled‐up hepatitis B and hepatitis C disease control scenarios.35 The action plan was developed in collaboration with leading South African experts, Ministry of Health officials, and external specialists in global health policy and economics, who assessed cost and affordability, health impact and cost‐effectiveness for four priority interventions: hepatitis B birth dose vaccination, prevention of MTCT and treatment for hepatitis B and C.
The model suggests expanded hepatitis B prevention and treatment for hepatitis B and C (using DAAs for the latter) is cost‐effective and affordable in the South African context,35 noting that hepatitis B birth dose vaccination should be prioritised if funds are insufficient for the full implementation. The five‐year Action Plan was estimated to cost US$270 million, with the “testing, care, and treatment” component being the most costly. Whilst this is a significant amount of money, seen against 5‐year HIV expenditure, the cost of the Hepatitis Action Plan is estimated to be less than 4% of the projected HIV spend in South Africa.43 Integrating the action plan into the existing health system, particularly maternal and child health and HIV/AIDS services, was estimated to improve implementation feasibility.
The modelling data suggest the initial five‐year investment could avert an estimated 13 000 hepatitis B‐related deaths and 7000 hepatitis C‐related deaths. Moreover, a continued expansion of the treatment programme beyond 2021 has the potential to avert 672 000 hepatitis B‐infections and 60 000 deaths averted from hepatitis C‐related liver disease, which would put South Africa firmly on the path to achieve elimination by 2030 (Figure 5B).35
The multi‐stakeholder approach used to develop an investment case for the cost‐effectiveness and affordability of hepatitis control and elimination for South Africa provides a template for other countries.44 Implementation of the investment case‐informed Viral Hepatitis Action Plan is expected to commence on 1st April 2019, with five priority interventions during the first year: (a) hepatitis B birth dose vaccination; (b) healthcare worker hepatitis screening, vaccination and training in viral hepatitis (c) increasing awareness, diagnosis and management of Hepatitis B virus (Tenofovir is on the Essential Medicine list); (d) registration of DAAs and price negotiations; (e) a comprehensive package of viral hepatitis services for key populations – men who have sex with men and people who use/inject drugs.
Key obstacles to the response are a lack of funding being allocated to the Programme due to fiscal constraints; a shortage of trained health workers; lack of knowledge about viral hepatitis in the general public; viral hepatitis‐related stigma; limited access to harm reduction services; and punitive drug laws. There is a need to improve viral hepatitis services in other key populations, including prisoners, sex workers and men who have sex with men. Moreover, DAAs are yet to be registered in South Africa due to administrative delays at the South African Health Products Regulatory Authority, preventing broader hepatitis C treatment scale‐up.
In order to address these obstacles, the South African Viral Hepatitis Working Group has established three subcommittees to oversee implementation of the hepatitis B birth dose vaccine, training of healthcare workers in conjunction with training on new HIV treatment regimens, and hepatitis C micro‐elimination programmes.
1.5. Scotland: accurate data to inform the response
In Scotland, advocates used political pressure and scientific evidence to raise awareness of the human impact of hepatitis C and its links to inequalities, which generated political consensus to support significant funding and evidence‐based policy initiatives.45 Social and political recognition of the scale of the problem galvanised policymakers into action. Innovative strategies such as the introduction of dried blood spot (DBS) sampling in community drug services made the model of viral hepatitis care more acceptable to affected communities and helped overcome barriers to testing.46 Adopting a project management approach ensured achievable goal‐setting and controlled ongoing cost. Substantial investment in a robust monitoring and surveillance system – combined with ambitious treatment targets – facilitated progress and demonstrated immediate impact, which helped to sustain momentum.47 Scotland's response – the National Hepatitis C Action Plan – has been a phased one. Launched in 2006, Phase I focused on gathering evidence to inform and generate proposals for the development of hepatitis C services and identify the additional investment required. Subsequently, in Phase II the Scottish Government committed funds to substantially improve prevention (including increasing coverage of harm reduction services), diagnosis and treatment services and deliver evidence‐based actions throughout the country for improved hepatitis C prevention and control (Figure 5C). Since 2011, the Hepatitis C Action Plan has been integrated with other national policies within the Scottish Government's Framework on Sexual Health and Blood Borne Viruses, which adopts a multi‐agency outcomes‐based approach with a strong focus on challenging inequalities.48, 49
The national strategy to improve prevention, diagnosis and treatment services led to a significant decline in hepatitis C incidence, more new diagnoses, more people undergoing hepatitis C treatment and achieving cure, reductions in liver‐related morbidity and mortality, and a decreased population prevalence of chronic hepatitis C.47, 50, 51, 52 Scotland's example showcases the utility of evidence‐based national hepatitis C strategies in reducing the financial and societal burden of the epidemic52, 53 and provides a working model for other countries to follow.
Despite the progress made in improving harm reduction services in Scotland during the era of interferon‐based treatment, the prevalence of hepatitis C infection had remained stubbornly high. The recent scale‐up of DAA therapy to PWID is hoped to bring a treatment‐as‐prevention benefit.54 While the roll‐out of DBS testing was effective at diagnosing infection, a substantial minority of the infected population remains undiagnosed. It has proven difficult to fully engage general practitioners in case‐finding initiatives, with awareness‐raising campaigns having limited success.55, 56 However, it is hoped that the availability of DAAs within primary care and other community settings will increase treatment uptake as the utility of the new therapies is recognised.
1.6. Implementation
Globally, nine of the 10 people living with viral hepatitis are unaware of their infection,33 and lack of public knowledge is often compounded by viral hepatitis‐related stigma and discrimination. Implementation of a viral hepatitis strategy should therefore include awareness‐raising activities to generate demand for viral hepatitis care (eg through social media campaigns, such as in Brazil57) in conjunction with supportive laws, policy and guidelines that aim to reduce stigma and enable the establishment of community‐focused responses.46
Prevention activities should be implemented and scaled up to effectively eliminate viral hepatitis transmission. A highly effective hepatitis B vaccine has been available since the 1980s, and early immunisation plus the distribution of hepatitis B immunoglobulin (HBIg) to at‐risk infants prevents perinatal transmission, as China has demonstrated.58 Harm reduction interventions, including NSPs and provision of OAT, cost‐effectively reduce primary and reinfection incidence among PWID.59, 60, 61 Iatrogenic transmission can be eliminated through routine screening of blood supply62 and implementation of safe infection practices (including reducing unnecessary injections, staff training and effective waste management),63 while simultaneously contributing to health systems strengthening.4, 64
Finally, implementation of a viral hepatitis response must aim to optimise the viral hepatitis care cascade by substantially improving testing rates, linkage to care and treatment numbers. The case of Egypt (and Iceland, see Appendix S1) demonstrates that concerted efforts enable substantial advances towards the WHO targets of 90% of people diagnosed and 80% of eligible people treated.30, 65, 66
Below are examples of implementation: raising awareness and stigma reduction (Brazil), investment in prevention (China), and investment in testing, linkage to care and treatment (Egypt).
1.7. Brazil: raising awareness and stigma reduction
Brazil, a middle‐income country, has been providing universal access to antiretroviral therapy for HIV since 1996, driven by strong political will, multisectoral mobilisation and use of Trade Related Aspects of Intellectual Property Rights (TRIPS) flexibilities, and civil society engagement.67 It has championed the cause of viral hepatitis and advocated for an intensified global response for many years. Learning from its successes in reversing the trend of the HIV epidemic, Brazil established a national hepatitis programme informed by up‐to‐date estimates of disease prevalence, international guidelines and cost‐effectiveness in the Brazilian Unified Health System.57 Brazil invested in universal hepatitis B vaccination, increased capacity for hepatitis C testing in HIV services, expanded its laboratory network and set up a referral system for hepatitis patients. To reach the target population, the Ministry of Health conducted new public awareness and diagnosis campaigns using a variety of media with endorsement from civil society and the scientific community.57
Brazil was able to obtain an unprecedented discount for an upper‐middle‐income country through price negotiations with originator pharmaceutical companies. Between 2015 and 2018 it provided treatment to nearly 90 000 people, and is expected to treat another 50 000 patients in 2019, largely thanks to the strong advocacy of civil society.
The remarkable process applied in Brazil was based on epidemiological data and scientific evidence, and motivated by its engagement with the SDGs, which may inspire other countries to identify ways to achieve these goals by 2030.57 Brazil has pledged to provide free hepatitis C treatment to everyone infected and is one of 12 countries on track to achieve hepatitis C elimination by 2030 (Figure 6A).30
Figure 6.
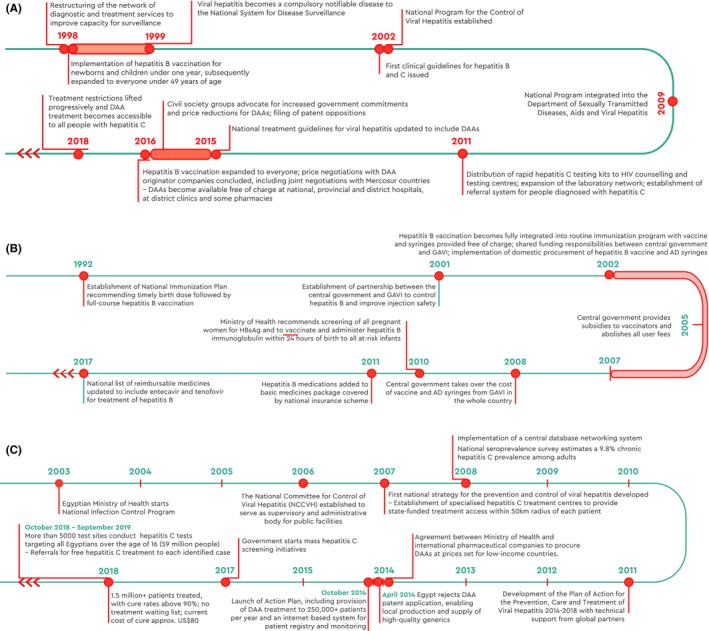
Timeline of national activities, Brazil (A), China (B), and Egypt (C)
Despite this progress, geographical, social and economic disparities in Brazil challenge the provision of equitable service access across varied geographical regions. Brazil is working to improve diagnosis rates and mitigate losses to follow‐up, resulting from the long delays between diagnosis and treatment initiation arising from small numbers of specialists who can provide DAA treatment.68
1.8. China: investment in prevention
China is home to nearly one third of all people living with hepatitis B infection globally. HBsAg prevalence is estimated at 5.5%2 and hepatitis B causes over 300 000 deaths annually due to liver diseases.69 The implementation of a universal hepatitis B vaccination programme for infants has reduced chronic hepatitis B incidence dramatically during the past two decades. The full implementation of a national programme for the prevention of MTCT guarantees adequate supply of HBIg for at‐risk newborns. Domestic procurement of the hepatitis B vaccine and auto‐disable syringes ensures sustainable supply chains and stimulates regional industry and technology markets.70
Driven by strong political commitment and with support from the Global Alliance on Vaccine and Immunization, including an investment of ~ USD76 million to subsidise the hepatitis B catch‐up vaccination programme for 15 million children through public‐private partnerships such as with Rotary and the ZeShan Foundation,71 multiple strategies were developed and implemented collaboratively (Figure 6B). As a result, >95% of infants receive the hepatitis B vaccine within 24 hours of birth.72, 73, 74, 75 This programme led to a nationwide catch‐up vaccination drive for children up to the age of 15, reaching 68 million people over a 3‐year period (2008‐2011) (private communication). Between 1992 and 2013, China's efforts have prevented 90 million cases of chronic hepatitis B infection and 24 million fewer people are carriers of the virus –a massive reduction in the global burden of viral hepatitis.70
Although China has made considerable progress with hepatitis B, systemic obstacles to the elimination of MTCT remain. The physician‐centred approach of the medical service infrastructure discourages affected pregnant women from seeking timely treatment, because physicians trained to provide treatment (ie obstetricians, gynaecologists, gastroenterologists and infectious disease specialists working in central hospitals) are often reluctant to do so. Moreover, China is yet to implement a comprehensive national strategy addressing its hepatitis C epidemic. Few DAAs have been approved and their high cost restricts inclusion in basic health insurance programmes; consequently, DAA treatment is not universally available. Policy changes and education campaigns are needed to overcome stigma and discrimination and improve diagnosis rates, and linkage to care needs improvement.
1.9. Egypt: testing, linkage to care and treatment
Egypt has a very high burden of hepatitis C infection and disease, with approximately 7% of Egyptians aged 18‐59 living with chronic hepatitis C infection in 2015.76 This large reservoir of active infection and continued unsafe medical practices contribute to ongoing transmission; in 2016, an estimated 150 000 Egyptians were newly infected.77
Egypt is committed to ending its generalised hepatitis C epidemic. It has developed one of the largest national programmes for hepatitis C treatment.78 Egypt provides free and universal access to locally produced DAA treatment, as part of a national action plan for the prevention and control of viral hepatitis. To maximise efficiencies, the country has rolled out mass screening since October 2018, providing direct linkage to hepatitis C care. Over six months, more than 49 million people were reached, of whom over 2 million were diagnosed as hepatitis C‐antibody positive (in addition, >2.5 million possible cases of diabetes and > 10 million possible cases of hypertension were identified and referred for further assessment and management). Of hepatitis C patients linked to care and confirmed as ribonucleic acid (RNA) positive, 750 000 started treatment.
By 2019, over 2.4 million Egyptians had been treated, and the country is on track to achieve WHO elimination targets in spite of its high hepatitis C prevalence (Figure 6C).78, 79 Egypt's response was facilitated by strong political will and government advocacy, effective price negotiations, removal of patent barriers on DAAs and ability to produce DAAs locally.66, 80
Despite great progress Egypt's response is challenged by difficulties in capturing non‐responders to treatment and lack of appropriate medications to initiate retreatment. Moreover, children under 12 years old cannot be treated because the medications have not yet been approved for this age group. Finally, plans and strategies for surveillance to reliably capture whether hepatitis C elimination targets have been met are not fully developed.
1.10. Integration
The cost burden of viral hepatitis diagnostic tests and treatment – in particular the new DAA treatment for hepatitis C – challenges the feasibility and sustainability of effective viral hepatitis elimination activities. Unlike for other major communicable diseases such as HIV, tuberculosis and malaria, there is little funding available for viral hepatitis at an international level and most countries lack dedicated hepatitis budgets or programmes.18 Although the private sector (such as pharmaceutical companies) and international funders and organisations are important actors in global elimination efforts, most funding will have to be mobilised from public, domestic sources to ensure the sustainability of viral hepatitis services as part of a broader effort to increase overall investments in health.29, 81, 82 Increasing investment in infrastructure and health service delivery (ie health systems strengthening) is not only a key enabler for viral hepatitis elimination but a requirement to reach the overarching SDG 3 for health and its target of universal health coverage.19 Ensuring that hepatitis services are integrated within these systems can reduce costs, compared to an isolated, non‐strategic approach,31 exemplified here in the case of Rwanda.
Integrating the viral response into the health system by utilising existing structures and trained workforces can save costs and generate efficiencies, as well as maximising access to services for key risk populations.83 For example conducting viral hepatitis testing at HIV services is likely to yield high diagnosis rates because people living with HIV have a higher risk of hepatitis B or hepatitis C co‐infection, and may improve their engagement in care.84 However, it is important to look beyond integrating the response into HIV programmes, because further opportunities exist to broaden the viral hepatitis response by integrating it within tuberculosis, maternal and child health, and diabetes programmes. Also such an approach may not be useful in countries with generalised epidemics (such as China and Egypt) that require population‐based approaches to testing and treatment.
Even when the response is integrated within the broader health system, there will be extra costs due to the need to expand services and to increase staffing levels to accommodate the increased activity. For example, additional time is needed to administer a hepatitis B vaccine or to provide post‐test counselling for positive test results.82 Due to concerns about extra costs and workload, efforts to integrate viral hepatitis responses into existing systems and platforms may receive substantial pushback, particularly initially. However, there is no evidence to support the notion that introducing viral hepatitis care into these systems causes existing structures to collapse.85
Moreover, multiple countries have been able to make treatment accessible to the broader population by successfully negotiating with patent holders (eg Australia), making use of patent licenses either available directly from the patent owner or those held by the Medicines Patent Pool (eg Rwanda),86 or using TRIPS flexibilities to circumvent patent barriers to accessing lower priced generic DAAs (eg Malaysia, see Table 4 and Appendix S1).87
Table 4.
Hepatitis B vaccination coverage and procurement status of hepatitis C medicines
| Country | Hepatitis B vaccination coverage (2019)75 | Hepatitis C treatment procurement (2017)107 | ||||
|---|---|---|---|---|---|---|
| Three‐dose vaccination <1 y | Timely birth dose | DAAs registered in country | Voluntary license (VL) or Compulsory/government‐use license (CL) | Generic local production | Support from originator company | |
| Australia | 94% | 91% | Yes | — | No | No |
| Brazil | 86% | 76% | Yes | No | No | No |
| China | 99% | 96% | Yes | No | No | No |
| Egypt | 95% | 13% | Yes | VL | Yes | Yes |
| Fiji | 93% | 95% | No | VL | No | No |
| Georgia | 92% | 94% | Yes | VL | No | Yes |
| Iceland | a | a | Yes | — | No | Yes |
| Malaysia | 98% | 88% | Yes | CL and VL | No | No |
| Pakistan | 86% | <1% | Yes | VL | Yes | Yes |
| Portugal | 98% | 97% | Yes | — | No | No |
| Rwanda | 98% | 0% | Yes | VL | No | Yes |
| Scotland | <1% (UK) | <1% (UK) | Yes | — | No | No |
| South Africa | 74%2 | n/a | Yes | VL | No | No |
Estimates of hepatitis B vaccination coverage were produced only for countries with universal birth dose policy.75
Below are examples of integration: health systems strengthening (Rwanda) and investment and financing for sustainability (Australia). Importantly, the health systems in both countries have coped with this considerable scale‐up of treatment and care.
1.11. Rwanda: expanding on universal health coverage
Rwanda is a low‐income country that is using a public health framework for hepatitis control and care to progress on its aim to achieve universal health coverage.
The country has made tremendous gains in maternal and child health, malaria, tuberculosis and HIV outcomes. The Rwandan Government now invests major resources in viral hepatitis, using programmatic steps that form a blueprint for other low‐income countries in the region.88 Key elements of Rwanda's programme for viral hepatitis prevention and treatment include:
Simplified treatment algorithms not requiring hepatitis C genotype or hepatitis B viral load and largely able to be delivered by nurses at health centre level
Selective partnerships and preferred suppliers to drive down price, consolidate the supply chain and streamline diagnostic platforms to avoid siloed approaches to healthcare89
Study of necessary resources for efficient implementation
Development of a training programme for health staff
Development, funding and implementation of birth dose vaccination for hepatitis B.
To ascertain feasibility and ensure financing for sustainability, a national operational plan was developed to demonstrate priority‐setting of key activities and provide costing estimates for different levels of coverage of screening, diagnosis, and treatment of both hepatitis B and C.88 Several initiatives were used to secure funding, including support from international donors, in particular the Clinton Health Access Initiative. Rwanda has a voluntary licensing agreement for DAAs and is therefore able to produce medication at reduced cost (approx. US$ 560 in 2017).66, 88 Rwanda's Essential Medicines List includes generic hepatitis B medicines treatment; this is subsidised by government for people with HIV coinfection. All major private health insurance companies (as well as military medical insurance) reimburse for the cost of DAAs, and the Rwanda social security board covers 85% of the cost. Ultimately, the aim is to provide reimbursement for hepatitis C diagnostics and treatment by the community‐based health insurance scheme.88 As of June 2017, 2500 patients had been treated with DAAs and treatment for 9000 additional patients had been procured (Figure 7A). Rwanda aims to establish treatment capacity at all 48 district hospitals countrywide by 2020.
Figure 7.
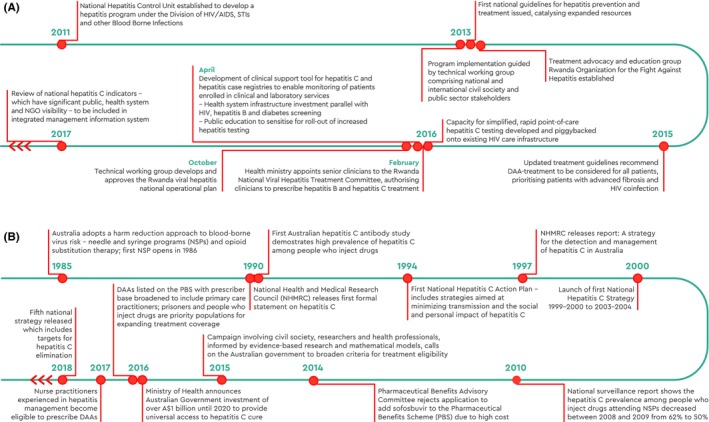
Timeline of national activities, Rwanda (A) and Australia (B)
Major ongoing barriers to addressing viral hepatitis in Rwanda include the lower awareness of, and priority given to, viral hepatitis compared to other infectious diseases (eg malaria and HIV) and the competing priorities for limited public‐sector health funding. A prior strategy (from 2011) that failed and has since been abandoned was to develop local viral hepatitis treatment guidelines based upon international consensus guidelines, without sufficient attention to the resources required for implementation (including particularly laboratory testing and availability of medications) or the skills and experience required of clinicians. These guidelines thus lacked local contextualisation and recommended unavailable or unaffordable management; consequently, they were impractical and did not influence daily clinical practice greatly.
1.12. Australia: a multipronged approach to elimination
In 1999, Australia was one of the first countries to implement and subsequently refine their national hepatitis C strategies and has since then become a best practice model for hepatitis C elimination. Key to Australia's response, including achieving universal treatment access (described below), has been strong community advocacy, health research, health sector and political leadership that foster continued commitment to the WHO 2030 elimination targets, including a timely response to new challenges.90 Australia has had a long and sustained harm reduction approach to injecting drug use, with engagement of civil society, the health sector and government. This is beneficial in reducing bloodborne virus transmission and cultivates a point of engagement with PWID in providing health and social services.59, 60, 61, 91 Strong engagement with PWID is crucial to Australia's response.
By negotiating a volume‐based, risk‐sharing agreement with originator pharmaceutical companies and committing over AUD1 billion to the purchase of DAAs between March 2016 and February 2021, Australia obtained major discounts on drug list prices and as a consequence limited its expenditure. With no cap on treatment numbers,92 there is an incentive to diagnose and treat as many people as possible to maximise Australia's investment and its public health benefits. This provides an enabling environment to prioritise high‐prevalence groups with ongoing risks for treatment, such as PWID and prisoners, necessary to achieve hepatitis C elimination. In addition, all registered medical practitioners are able to prescribe DAA therapy, enabling more convenient, patient‐centred care. In Australia, close collaboration between people living with hepatitis C, community organisations, clinicians and policymakers facilitated improved access to diagnosis and treatment scale‐up (Figure 7B).
Australia aims to treat around 15 000 to 20 000 hepatitis C patients per year, to reach the WHO target to eliminate viral hepatitis as a major public health threat by 2030. This early commitment to achieving elimination and provide unrestricted treatment access enabled rapid treatment uptake during the first two years of DAA availability.90 Between March 2016 and late 2018, over 70 000 patients (around 30% of all infected Australians) were treated. The proportion of individuals prescribed DAA treatment by general practitioners increased from 8% in March 2016 to 39% in June 2017.93 With the successful implementation of its hepatitis C strategy – a global benchmark for best practice94 – Australia is on track to achieve elimination by 2030.95
Of concern in Australia is the continuing drop off in the number of patients undergoing screening and confirmatory testing and treatment since March 2016.93 While treatment numbers have been sufficient to maintain the elimination targets, further decline could put the elimination effort at risk. The decline in treatment numbers demonstrates that universal availability of DAA treatment alone is not enough to improve access to diagnosis and retention in care. Continued political commitment and policy and health system interventions are needed to facilitate treatment access for key populations to sustain momentum and overcome ongoing programme challenges to treatment scale‐up.
2. DISCUSSION
The broader benefits of investing in the elimination of viral hepatitis – including progressing on the SDGs ‐ are increasingly being recognised. Countries with different income levels, public health infrastructures and policy environments are effectively responding to their respective epidemics.
Attaining the viral hepatitis elimination targets set by the global community in 2016 is achievable but also highly ambitious and comes with considerable challenges (see Appendix S1). These should not be ignored, but instead considered and addressed both at a global level and within the local context to invigorate and maintain national elimination efforts. Gathering sufficient funds to finance viral hepatitis programmes continues to be difficult among competing health priorities and budget constraints. Not all countries currently benefit from generic competition, with heavily burdened middle‐income countries (eg China, Malaysia, Thailand) struggling to afford higher drug prices. A further obstacle is the increasing cost of diagnostics; for example, in Egypt expenditure on diagnostics now exceeds that on hepatitis C treatment.68 There are few WHO prequalified point‐of‐care viral hepatitis tests and little production of low‐cost high‐quality generic tests. In many low‐income countries, strengthening primary health care systems for maternal and child health, developing laboratory capacity, and improving weak registration and procurement systems for essential medicines is an ongoing challenge. For hepatitis B, cold chain barriers to vaccination including birth dose delivery exist, and while the controlled temperature chain presents a cost‐effective alternative that could vastly improve coverage96 it is yet to be broadly adopted.
Even in countries such as Australia, where there is close collaboration between community, government and health practitioners to guide implementation, elimination cannot be guaranteed because many patients remain undiagnosed and/or do not access treatment.93, 97, 98 Identification of sufficient numbers of infected patients needing treatment remains a challenge globally; meanwhile, in countries where scale‐up of a viral hepatitis response is pending, demand for viral hepatitis testing and treatment can outstrip available testing and treatment facilities,85 creating bottlenecks within the care cascade leading to losses to follow‐up. High levels of stigma, discrimination, social marginalisation and legal impediments imposed on key populations at risk or infected with viral hepatitis (eg PWID, prisoners, men who have sex with men, sex workers) is a major issue preventing engagement in care and service access84 and in many countries legal protections remain insufficient.68 The impact of regressive policies and laws on the elimination response cannot be underestimated.
The country case studies presented here demonstrate that major gains are possible in spite of these challenges – across various epidemic profiles, within a diverse range of resource constraints and within relatively short‐time frames. The case studies illustrate that political will and commitment, civil society advocacy, donor support and community acceptance are crucial and can make a difference. From concerted screening efforts in Egypt and using innovative approaches to increase hepatitis C testing in Scotland, to building local investment cases in South Africa, to integrating viral hepatitis services into existing health infrastructure in Brazil and Rwanda, these pioneers provide important models for other countries to follow. In all countries multi‐stakeholder engagement of national and international experts, civil society organisations and affected communities form critical components across the three pillars of evidence‐gathering and planning, implementation and integration.
On a global level, civil society bodies such as the World Hepatitis Alliance are instrumental in generating pressure on governments and international organisations, providing an evidence‐based approach to the response.82 Locally, robust evidence and civil society advocacy helped to achieve political commitment and facilitated the development of national plans. Collaboration and cooperation between civil society, the pharmaceutical industry and government smoothed the introduction of prevention and control programmes. Such unified, evidence‐informed strategies at the political and technical levels are crucial to attract and sustain commitment and financing. Learnings from these country examples and other local projects demonstrating the feasibility of elimination (eg micro‐elimination projects36, 99) can help persuade policymakers in other countries to support viral hepatitis prevention and control plans. In‐country and global advocacy must be maintained to keep viral hepatitis high on the political agenda.82
3. CONCLUSION
At the 2016 World Health Assembly, the global community uniformly endorsed the unique opportunity to eliminate viral hepatitis as a public health threat. Although an ambitious goal, technological advancements have made it scientifically feasible and increasing recognition of the public health threat posed by viral hepatitis provides the grounds for substantial political and societal support. The broader benefits of investing in the elimination of viral hepatitis – including progress on the Agenda for Sustainable Development – are now well recognised. Sustaining political momentum will be critical if elimination efforts are to be successful and more countries will need to take action if global elimination of viral hepatitis is to be achieved. Looking to existing approaches that address viral hepatitis can facilitate political support, because they demonstrate that investing in viral hepatitis is cost‐effective and can be made affordable, provide multiple economic benefits, and above all alleviate the human burden of the epidemic. The case studies presented in this paper provide clear and feasible examples of successful approaches taken by low‐, middle‐ and high‐income countries with diverse epidemics of hepatitis B and C to achieve the WHO 2030 viral hepatitis elimination targets.
CONFLICT OF INTEREST
AHS reports grants and travel funding to her institution from ViiV Healthcare. ETH is the former director of the Medicines Patent Pool. NS has received investigator‐initiated research funding from Gilead Sciences. JVL reports grants and personal fees from AbbVie, Gilead Sciences and MSD, personal fees from CEPHEID and Janssen outside the submitted work. MES is principal investigator in an investigator‐initiated trial sponsored by Gilead Sciences (received no PI fees, trial closed April 17th 2019) and reports an educational grant to travel to EASL 2019 (Gilead Sciences). SJH received honoraria from Gilead, unrelated to submitted work. MH's institute receives investigator‐initiated research funding from Gilead Sciences, Abbvie and BMS. JH received the Gilead Sciences Australia fellowship (2017). DW, CK, RA, RBL, MB, LA, AG, SH, RH, WL, RBM, SO, RP, MS, CWS, TS, MT, TW and ESS have nothing to declare.
Supporting information
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the contribution to this work of the Qatar Foundation and of the Victorian Operational Infrastructure Support Program received by Burnet Institute. We are grateful to the Brazilian Ministry of Health for their assistance in developing the Brazilian case study. We thank Dr Gottfried Hirnschall and Dr Yvan J Hutin (WHO), Giten Khwairakpam (TREAT Asia/amfAR), Dr Manik Sharma (Hamad Medical Corporation) and Dr John Thwaites (Monash University) for their contributions to the WISH 2018 Viral Hepatitis Forum Report, on which this work is built. We would like to acknowledge the contributions of Mary Ribeiro Pombo (Imperial College London and World Innovation Summit for Health (WISH, Qatar), Walid Qoronfleh (WISH, Qatar), Deidre Thompson (Imperial College London and WISH, Qatar) to the WISH 2018 Viral Hepatitis Forum. We thank Stephanie Luketic (Burnet Institute).
Schröeder SE, Pedrana A, Scott N, et al. Innovative strategies for the elimination of viral hepatitis at a national level: A country case series. Liver Int. 2019;39:1818–1836. 10.1111/liv.14222
Handling Editor: Francesco Negro
Funding information
This work received funding from the Qatar Foundation as part of their support for the World Innovations Summit for Health, 2018. Burnet Institute receives funding from the Victorian Operational Institute Support Program. The funders had no role in decision to publish or preparation of the manuscript.
Contributor Information
Sophia E. Schröeder, Email: sophia.schroeder@burnet.edu.au.
Jessica Howell, Email: jess.howell@burnet.edu.au.
REFERENCES
- 1. World Health Organization . Global Hepatitis Report 2017. Geneva, Switzerland: World Health Organization; 2017. [Google Scholar]
- 2. Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546‐1555. [DOI] [PubMed] [Google Scholar]
- 3. GBD 2015 Mortality and Causes of Death Collaborators . Global, regional, and national life expectancy, all‐cause mortality, and cause‐specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016; 388:1459‐1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization . Global Hepatitis Report 2017. Geneva: WHO; 2017. [updated 2017; cited 2018 24 March]; Available from: http://apps.who.int/iris/bitstream/handle/10665/255016/9789241565455-eng.pdf;jsessionxml:id=9DECA1FF83BC4A8C41B74E3BE2649662?sequence=1. [Google Scholar]
- 5. Edmunds WJ, Medley GF, Nokes DJ, et al. Epidemiological patterns of hepatitis B virus (HBV) in highly endemic areas. Epidemiol Infect. 1996;117:313‐325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kiire CF. The epidemiology and prophylaxis of hepatitis B in sub‐Saharan Africa: a view from tropical and subtropical Africa. Gut. 1996;38(Suppl 2):S5‐S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cornberg M, Razavi HA, Alberti A, et al. A systematic review of hepatitis C virus epidemiology in Europe, Canada and Israel. Liver Int. 2011;31(Suppl 2):30‐60. [DOI] [PubMed] [Google Scholar]
- 8. WHO . Hepatitis B. WHO; 2018. [updated 18 July 2018; cited 2018 13 Dec 2018]; Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b. [Google Scholar]
- 9. Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age‐specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30:2212‐2219. [DOI] [PubMed] [Google Scholar]
- 10. Hyams KC. Risks of chronicity following acute hepatitis B virus infection: a review. Clin Infect Dis. 1995;20:992‐1000. [DOI] [PubMed] [Google Scholar]
- 11. Bruggmann P, Berg T, Ovrehus AL, et al. Historical epidemiology of hepatitis C virus (HCV) in selected countries. J Viral Hepat. 2014;21(Suppl 1):5‐33. [DOI] [PubMed] [Google Scholar]
- 12. Liakina V, Hamid S, Tanaka J, et al. Historical epidemiology of hepatitis C virus (HCV) in select countries ‐ volume 3. J Viral Hepat. 2015;22(Suppl 4):4‐20. [DOI] [PubMed] [Google Scholar]
- 13. Maaroufi A, Vince A, Himatt SM, et al. Historical epidemiology of hepatitis C virus in select countries‐volume 4. J Viral Hepat. 2017;24(Suppl 2):8‐24. [DOI] [PubMed] [Google Scholar]
- 14. Saraswat V, Norris S, de Knegt RJ, et al. Historical epidemiology of hepatitis C virus (HCV) in select countries ‐ volume 2. J Viral Hepat. 2015;22(Suppl 1):6‐25. [DOI] [PubMed] [Google Scholar]
- 15. Degenhardt L, Peacock A, Colledge S, et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Glob Health. 2017;5:e1192‐e1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Degenhardt L, Charlson F, Stanaway J, et al. Estimating the burden of disease attributable to injecting drug use as a risk factor for HIV, hepatitis C, and hepatitis B: findings from the Global Burden of Disease Study 2013. Lancet Infect Dis. 2016;16:1385‐1398. [DOI] [PubMed] [Google Scholar]
- 17. Umar M, Bilal M, Hepatitis C. A mega menace: a Pakistani perspective. J Pak Med Stud. 2012;2:68‐72. [Google Scholar]
- 18. World Health Organization . Global Health Sector Strategy on Viral Hepatitis 2016–2021. Geneva, Switzerland: WHO; 2016. [Google Scholar]
- 19. United Nations . Transforming our world: the 2030 Agenda for Sustainable Development; 2015. http://www.un.org/ga/search/view_doc.asp?symb. Accessed October 26, 2017.
- 20. Hutin YJ‐F, Bulterys M, Hirnschall GO. How far are we from viral hepatitis elimination service coverage targets? J Int AIDS Soc. 2018;21:e25050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Heffernan A, Cooke GS, Nayagam S, Thursz M, Hallett TB. Scaling up prevention and treatment towards the elimination of hepatitis C: a global mathematical model. Lancet. 2019;393(10178):1319‐1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nayagam S, Thursz M, Sicuri E, et al. Requirements for global elimination of hepatitis B: a modelling study. Lancet Infect Dis. 2016;16:1399‐1408. [DOI] [PubMed] [Google Scholar]
- 23. Scott N, McBryde ES, Thompson A, Doyle JS, Hellard ME. Treatment scale‐up to achieve global HCV incidence and mortality elimination targets: a cost‐effectiveness model. Gut. 2017;66:1507‐1515. [DOI] [PubMed] [Google Scholar]
- 24. Younossi ZM, Stepanova M, Janssen H, et al. Effects of treatment of chronic HBV infection on patient‐reported outcomes. Clin Gastroenterol Hepatol. 2018;16:1641‐1649.e6 [DOI] [PubMed] [Google Scholar]
- 25. Younossi Z, Brown A, Buti M, et al. Impact of eradicating hepatitis C virus on the work productivity of chronic hepatitis C (CH‐C) patients: an economic model from five European countries. J Viral Hepat. 2016;23:217‐226. [DOI] [PubMed] [Google Scholar]
- 26. DiBonaventura MD, Wagner J‐S, Yuan Y, L'Italien G, Langley P, Ray Kim W. The impact of hepatitis C on labor force participation, absenteeism, presenteeism and non‐work activities. J Med Econ. 2011;14:253‐261. [DOI] [PubMed] [Google Scholar]
- 27. Tracking universal health coverage: 2017 global monitoring report. World Health Organization and International Bank for Reconstruction and Development / The World Bank; 2017. Licence: CC BY-NC-SA 3.0 IGO.
- 28. Fitzsimons D. A viral hepatitis free future: how to make it feasible and affordable 2015. 2015 June 5–6.
- 29. Viral Hepatitis Prevention Board . Innovative financing into hepatitis B and C prevention and treatment in low and middle income countries. Geneva, Switzerland: IFPMA; 2016. [Google Scholar]
- 30. CDA Foundation . Just 12 countries worldwide on track to eliminate hepatitis C infection by 2030, with United Kingdom, Italy and Spain among those joining the list. 2018 [updated 16 June 2018; cited 2018 22 June 2018]; Available from: http://cdafound.org/just-12-countries-worldwide-on-track-to-eliminate-hepatitis-c-infection-by-2030-with-united-kingdom-italy-and-spain-among-those-joining-the-list/.
- 31. Pedrana A, Howell J, Schroeder S, et al. Eliminating Viral Hepatitis: The investment case. Doha, Qatar: World Innovation Summit for Health; 2018. [Google Scholar]
- 32. World Health Organization . Global health sector strategy on viral hepatitis 2016–2021 In: HIV/AIDS Do , ed. Towards ending viral hepatitis. Geneva, Switzerland: WHO Document Production Services; 2016. [Google Scholar]
- 33. World Hepatitis Alliance . Find the Missing Millions; 2018. http://www.worldhepatitisalliance.org/missing-millions/about/. Accessed January 16, 2019.
- 34. Scott N, Stoové M, Wilson DP, et al. Eliminating hepatitis C virus as a public health threat among HIV‐positive men who have sex with men: a multi‐modelling approach to understand differences in sexual risk behaviour. J Int AIDS Soc. 2018;21:e25059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hecht R, Hiebert L, Spearman WC, et al. The investment case for hepatitis B and C in South Africa: adaptation and innovation in policy analysis for disease program scale‐up. Health Policy Plan. 2018;33:528‐538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lazarus J, Safreed‐Harmon K, Thursz M, et al. The micro‐elimination approach to eliminating hepatitis C: strategic and operational considerations. Semin Liver Dis. 2018;38:181‐192. [DOI] [PubMed] [Google Scholar]
- 37. Nayagam S, Chan P, Zhao K, et al. Presentations: Posters Friday, 15 April 2016: EU and public health. FRI‐ 431: Investment Case for a Comprehensive Package of Interventions against Hepatitis B in China. J Hepatol. 2016;64:S469. [Google Scholar]
- 38. Gvinjilia L, Nasrullah M, Sergeenko D, et al. National progress toward hepatitis c elimination ‐ Georgia, 2015–2016. MMWR Morb Mortal Wkly Rep. 2016;65:1132‐1135. [DOI] [PubMed] [Google Scholar]
- 39. Mitruka K, Tsertsvadze T, Butsashvili M, et al. Launch of a Nationwide Hepatitis C Elimination Program‐Georgia, April 2015. MMWR Morb Mortal Wkly Rep. 2015;64:753‐757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chikovani I, Ompad DC, Uchaneishvili M, et al. On the way to Hepatitis C elimination in the Republic of Georgia—Barriers and facilitators for people who inject drugs for engaging in the treatment program: A formative qualitative study. PLoS ONE. 2019;14:e0216123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. World Health Organization Europe . Georgia's hepatitis C elimination programme setting an example in Europe. Health Topics 2017 [cited 2018 5 April]; Available from: http://www.euro.who.int/en/health-topics/communicable-diseases/pages/news/news/2017/08/georgias-hepatitis-c-elimination-programme-setting-an-example-in-europe.
- 42. Kikvidze T, Luhmann N, Avril E, et al. Harm reduction‐based and peer‐supported hepatitis C treatment for people who inject drugs in Georgia. Int J Drug Policy. 2018;52:16‐19. [DOI] [PubMed] [Google Scholar]
- 43. South Africa National AIDS Council . National Strategic Plan on HIV, TB, and STIs 2017–2022; 2017.
- 44. World Health Organization . Prevention, Care and Treatment of Viral Hepatitis in the African Region: Framework for Action, 2016–2020. Geneva, Switzerland: WHO Press; 2017. [Google Scholar]
- 45. Goldberg D, Brown G, Hutchinson S, et al. Hepatitis C action plan for Scotland: phase II (May 2008‐March 2011). Euro Surveill. 2008;13:18876 [DOI] [PubMed] [Google Scholar]
- 46. McLeod A, Weir A, Aitken C, et al. Rise in testing and diagnosis associated with Scotland's Action Plan on Hepatitis C and introduction of dried blood spot testing. J Epidemiol Community Health. 2014;68:1182‐1188. [DOI] [PubMed] [Google Scholar]
- 47. Hutchinson SJ, Dillon JF, Fox R, et al. Expansion of HCV treatment access to people who have injected drugs through effective translation of research into public health policy: Scotland's experience. Int J Drug Policy. 2015;26:1041‐1049. [DOI] [PubMed] [Google Scholar]
- 48. Scottish Government . The Sexual Health and Blood Borne Virus Framework 2011–2015; 2011. Available from: https://www.webarchive.org.uk/wayback/archive/20170702043339/http://www.gov.scot/Publications/2011/08/24085708/16.
- 49. Scottish Government . Sexual Health and Blood Borne Virus Framework 2015–2020 Update; 2015. Available from: https://www.gov.scot/publications/sexual-health-blood-borne-virus-framework-2015-2020-update/pages/0/.
- 50. Wylie L, Hutchinson S, Liddell D, Rowan N. The successful implementation of Scotland's Hepatitis C Action Plan: what can other European stakeholders learn from the experience? A Scottish voluntary sector perspective. BMC Infect Dis. 2014;14(Suppl 6):S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Palmateer NE, Taylor A, Goldberg DJ, et al. Rapid decline in HCV incidence among people who inject drugs associated with national scale‐up in coverage of a combination of harm reduction interventions. PLoS ONE. 2014;9:e104515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hutchinson S, Valerio H, Dillon J, et al. Reduction in the incidence of hepatitis C‐related decompensated cirrhosis associated with national scale‐up of direct‐acting antiviral therapies targeting patients with advanced liver fibrosis. J Hepatol. 2018;68:S67. [Google Scholar]
- 53. Global Commission on Drug Policy (GCDP) . The negative impact of the war on drugs on public health: The hidden hepatiits C epidemic. Geneva, Switzerland: GCDP; 2013. [Google Scholar]
- 54. Innes H, Goldberg D, Dillon J, Hutchinson SJ. Strategies for the treatment of Hepatitis C in an era of interferon‐free therapies: what public health outcomes do we value most? Gut. 2015;64:1800‐1809. [DOI] [PubMed] [Google Scholar]
- 55. McLeod A, Cullen BL, Hutchinson SJ, et al. Limited impact of awareness‐raising campaigns on hepatitis C testing practices among general practitioners. J Viral Hepat. 2017;24:944‐954. [DOI] [PubMed] [Google Scholar]
- 56. McLeod A, Weir A, Hutchinson SJ, Goldberg DJ. Hepatitis C test uptake among historic blood transfusion recipients following media coverage of the Penrose Inquiry and an awareness‐raising campaign. J Viral Hepat. 2019;26:93‐100. [DOI] [PubMed] [Google Scholar]
- 57. Mesquita F, Santos ME, Benzaken A, et al. The Brazilian comprehensive response to hepatitis C: from strategic thinking to access to interferon‐free therapy. BMC Public Health. 2016;16:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Centre for Disease Control . Epidemiology and Prevention of Vaccine‐Preventable Diseases 13th Edition Hamborsky J, Kroger A, Wolfe S, eds. Washington D.C.: Public Health Foundation; 2015. [Google Scholar]
- 59. Turner K, Hutchinson S, Vickerman P, et al. The impact of needle and syringe provision and opiate substitution therapy on the incidence of hepatitis C virus in injecting drug users: pooling of UK evidence. Addiction. 2011;106:1978‐1988. [DOI] [PubMed] [Google Scholar]
- 60. Aitken CK, Agius PA, Higgs PG, Stoové MA, Bowden DS, Dietze PM. The effects of needle‐sharing and opioid substitution therapy on incidence of hepatitis C virus infection and reinfection in people who inject drugs. Epidemiol Infect. 2017;145:796‐801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Platt L, Minozzi S, Reed J, et al. Needle and syringe programmes and opioid substitution therapy for preventing HCV transmission among people who inject drugs: findings from a Cochrane Review and meta‐analysis. Addiction. 2018;113:545‐563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. World Health Organization . Global database on blood safety. 2015. [cited 2019 13 march]; Available from: https://www.who.int/bloodsafety/global_database/tools2015/en/.
- 63. Lanini S, Easterbrook PJ, Zumla A, Ippolito G. Hepatitis C: global epidemiology and strategies for control. Clin Microbiol Infect. 2016;22:833‐838. [DOI] [PubMed] [Google Scholar]
- 64. World Health Organization .Global Policy Report on the Prevention and Control of Viral Hepatitis. 2012. http://wwwwhoint/csr/disease/hepatitis/global_report/en/.
- 65. El‐Akel W, El‐Sayed MH, El Kassas M, et al. National treatment programme of hepatitis C in Egypt: Hepatitis C virus model of care. J Viral Hepatitis. 2017;24:262‐267. [DOI] [PubMed] [Google Scholar]
- 66. Ayoub HH, Abu‐Raddad LJ. Impact of treatment on hepatitis C virus transmission and incidence in Egypt: A case for treatment as prevention. J Viral Hepat. 2017;24:486‐495. [DOI] [PubMed] [Google Scholar]
- 67. World Health Organization . The World health report 2004 ‐ changig history. Geneva, Switzerland: WHO; 2004. [Google Scholar]
- 68. Cooke GS, Andrieux‐Meyer I, Applegate TL, et al. Accelerating the elimination of viral hepatitis: a Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol Hepatol. 2019;4:135‐184. [DOI] [PubMed] [Google Scholar]
- 69. Lieu J, Liang W, Jing W, Liu M. Countdown to 2030: eliminating hepatitis B disease, China. Bull World Health Organ. 2019;2019:230‐238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Liang X, Cui F, Hadler S, et al. Origins, design and implementation of the China GAVI project. Vaccine. 2013;31(Suppl 9):J8‐J14. [DOI] [PubMed] [Google Scholar]
- 71. Centers for Disease Control and Prevention . Progress in hepatitis B prevention through universal infant vaccination–China, 1997–2006. MMWR Morb Mortal Wkly Rep. 2007;56:441‐445. [PubMed] [Google Scholar]
- 72. Zhou YH, Wu C, Zhuang H. Vaccination against hepatitis B: the Chinese experience. Chin Med J (Engl). 2009;122:98‐102. [PubMed] [Google Scholar]
- 73. Kane MA, Hadler SC, Lee L, et al. The inception, achievements, and implications of the China GAVI Alliance Project on Hepatitis B Immunization. Vaccine. 2013;31(Suppl 9):J15‐20. [DOI] [PubMed] [Google Scholar]
- 74. Hutin Y, Hennessey K, Cairns L, et al. Improving hepatitis B vaccine timely birth dose coverage: lessons from five demonstration projects in China, 2005–2009. Vaccine. 2013;31(Suppl 9):J49‐J55. [DOI] [PubMed] [Google Scholar]
- 75. WHO and UNICEF . WHO and UNICEF estimates of national immunisation coverage: China 2016 revision. 2017. [updated 3 July 2017; cited 2018 5 April]; 4 July 2017; Available from: https://data.unicef.org/wp-content/uploads/country_profiles/China/immunization_country_profiles/immunization_chn.pdf.
- 76. Kandeel A, Genedy M, El‐Refai S, Funk AL, Fontanet A, Talaat M. The prevalence of hepatitis C virus infection in Egypt 2015: implications for future policy on prevention and treatment. Liver Int. 2017;37:45‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. The World Bank . Eliminating Hepatitis C from Egypt : 2017 update on current trends and policy recommendations - policy brief (English). Egypt's viral hepatitis program. Washington, DC: World Bank Group; 2018. http://documents.worldbank.org/curated/en/164381517333701631/Eliminating-Hepatitis-Cfrom-Egypt-2017-update-on-current-trends-and-policyrecommendations-policy-brief [Google Scholar]
- 78. Elsharkawy A, El‐Raziky M, El‐Akel W, et al. Planning and prioritizing direct‐acting antivirals treatment for HCV patients in countries with limited resources: Lessons from the Egyptian experience. J Hepatol. 2018;68:691‐698. [DOI] [PubMed] [Google Scholar]
- 79. World Hepatitis Alliance . Nine countries now on track to eliminate hepatitis C Sao Paolo: World Hepatitis Alliance; 2017. [updated 1 November 2017; cited 2018 04 April]; Available from: http://www.worldhepatitisalliance.org/news/nov-2017/nine-countries-now-track-eliminate-hepatitis-c.
- 80. Assefa Y, Hill PS, Ulikpan A, Williams OD. Access to medicines and hepatitis C in Africa: can tiered pricing and voluntary licencing assure universal access, health equity and fairness? Global Health. 2017;13:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Rosenthal ES, Graham CS. Price and affordability of direct‐acting antiviral regimens for hepatitis C virus in the United States. Infect Agent Cancer. 2016;11:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. FitzSimons D, Hendrickx G, Hallauer J, et al. Innovative sources for funding of viral hepatitis prevention and treatment in low‐ and middle‐income countries: a roundtable meeting report. Hepatol Med Policy. 2016;1:16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Suthar AB, Harries AD. A public health approach to hepatitis c control in low‐ and middle‐income countries. PLoS Medicine. 2015;12:e1001795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Easterbrook P, Johnson C, Figueroa C, Baggaley R. HIV and hepatitis testing: global progress, challenges, and future directions. AIDS Rev. 2016;18:3‐14. [PubMed] [Google Scholar]
- 85. Popping S, Bade D, Boucher C, et al. The global campaign to eliminate HBV and HCV infection: International Viral Hepatitis Elimination Meeting and core indicators for development towards the 2030 elimination goals. J Virus Erad. 2019;5:60‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Medicines Patent Pool . Our work: hepatitis C. 2019. [cited 2019 05 March 2019]; Available from: https://medicinespatentpool.org/what-we-do/our-work/hepatitis-c/.
- 87. Douglass CH, Pedrana A, Lazarus JV, et al. Pathways to ensure universal and affordable access to hepatitis C treatment. BMC Med. 2018;16:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Mbituyumuremyi A, Van Nuil JI, Umuhire J, et al. Controlling hepatitis C in Rwanda: a framework for a national response. Bull World Health Organ. 2018;96:51‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Republic of Rwanda Ministry of Health . National Policy on Viral Hepatitis Prevention and Management in Rwanda. Kigali, Rwanda: Republic of Rwanda Ministry of Health, HIV-AIDS, STIS & OBBI DIVISION; 2015. [Google Scholar]
- 90. Doyle JS, Scott N, Sacks‐Davis R, Pedrana AE, Thompson AJ, Hellard ME. Treatment access is only the first step to hepatitis C elimination: experience of universal anti‐viral treatment access in Australia. Aliment Pharmacol Ther. 2019;49:1223. [DOI] [PubMed] [Google Scholar]
- 91. Mathers BM, Degenhardt L, Ali H, et al. HIV prevention, treatment, and care services for people who inject drugs: a systematic review of global, regional, and national coverage. Lancet. 2010;375:1014‐1028. [DOI] [PubMed] [Google Scholar]
- 92. Dore GJ, Grebely J. Negotiating better discounts for DAA therapy is critical to achieve HCV elimination by 2030. J Hepatol. 2017;67:419‐420. [DOI] [PubMed] [Google Scholar]
- 93. The Kirby Institute . Monitoring hepatitis C treatment uptake in Australia (Issue 8). Sydney, Australia: The Kirby Institute, UNSW Sydney; 2017. [Google Scholar]
- 94. The Boston Consulting Group . Road to elimination: Barriers and best practices in hepatitis C management. The Boston Consulting Group; 2017. [Google Scholar]
- 95. The Kirby Institute . Monitoring hepatitis C treatment uptake in Australia. Sydney, Australia: UNSW; 2017. [Google Scholar]
- 96. Scott N, Palmer A, Morgan C, et al. Cost‐effectiveness of the controlled temperature chain for the hepatitis B virus birth dose vaccine in various global settings: a modelling study. Lancet Glob Health. 2018;6:e659‐e667. [DOI] [PubMed] [Google Scholar]
- 97. Grebely J, Dore GJ, Morin S, Rockstroh JK, Klein MB. Elimination of HCV as a public health concern among people who inject drugs by 2030 ‐ What will it take to get there? J Int AIDS Soc. 2017;20:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Richmond JA, Wallace J. Implementation of hepatitis C cure in Australia: one year on. J Virus Erad. 2018;4:115‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Lazarus JV, Wiktor S, Colombo M, Thursz M. Micro‐elimination & #x2013; A path to global elimination of hepatitis C. J Hepatol. 2017;67:665‐666. [DOI] [PubMed] [Google Scholar]
- 100. Schnier C, Wallace L, Tempelton K, et al. Use of laboratory‐based surveillance data to estimate the number of people chronically infected with hepatitis B living in Scotland. Epidemiol Infect. 2014;142:2121‐2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Georgia Ministry of Health . Strategic Plan for the Elimination of Hepatitis C Virus in Georgia, 2016–2020 (Health Mo , ed.). Tbilisi, Georgia; 2016. [Google Scholar]
- 102. Polaris Observatory HCV Collaborators . Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2:161‐176. [DOI] [PubMed] [Google Scholar]
- 103. Public Health England . Hepatitis C in the UK London. London, UK: Public Health England; 2013. [Google Scholar]
- 104. Benzaken A, Catapan E, Girade R, et al. Hepatitis C elimination by 2030 is feasible in Brazil: a mathematical modelling approach. J Hepatol. 2018;68:S193. [Google Scholar]
- 105. Blach S, Zeuzem S, Manns M, et al. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2:161‐176. [DOI] [PubMed] [Google Scholar]
- 106. World Health Organization . Global policy report on the prevention and control of viral hepatitis in WHO member states. Geneva, Switzerland: WHO Press, World Health Organization; 2013. [Google Scholar]
- 107. World Health Organization . Progress report on access to hepatitis C treatment: focus on overcoming barriers in low- and middle-income countries. Geneva, Switzerland: World Health Organization; 2018. Report No.: WHO/CDS/HIV/18.4. Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


