Abstract
Background and Aims
Sporadic hepatitis E is an emerging indigenous disease in Europe induced by genotype 3 of the virus. While the disease takes an acute self‐limited course in immunocompetent individuals, under immunocompromised conditions chronic hepatitis E might develop. The histology of chronic hepatitis E has not been described in detail systematically.
Methods
Liver biopsies from 19 immunosuppressed patients with chronic hepatitis E were collected: 17 were organ transplant recipients, one had a CD4‐deficiency and one had received steroid therapy because of ulcerative colitis. Biopsies were processed with standard stains. Evaluation of histologic activity and fibrosis was performed according to Ishak. Additionally, immunohistochemistry with antibodies directed against open reading frame 2 and 3 of the virus was performed and liver biopsies were tested for hepatitis E virus RNA.
Results
Biochemical data showed an increase in alanine transaminase, aspartate transaminase, gamma‐glutamyl transferase and total bilirubin. Histopathology displayed typical features of chronic hepatitis with mild to moderate activity. The number of polymorphonuclear leucocytes was considerably increased and all patients had a florid cholangitis that presented as a destructive form in five of them. Hepatocytes and bile duct epithelia stained positive for hepatitis E virus by immunohistochemistry.
Conclusions
Chronic hepatitis E in immunocompromised individuals runs a similar course as hepatitis B and C and shows similar histopathology. However, the presence of destructive cholangitis in some cases accompanied by an increased number of polymorphonuclear leucocytes is markedly different. Immunohistochemically the virus is present in bile duct epithelia, seemingly the cause for cholangitis.
Keywords: HEV genotype 3, immunohistochemistry, immunosuppression, solid organ transplantation
Abbreviations
- ALT
Alanine Transaminase
- AP
Alkaline Phosphatase
- AST
Aspartate Transaminase
- CAB
Chromotrop‐Anilinblue
- CD
Cluster of Differentiation
- CHE
chronic hepatitis E
- CMV
Cytomegalovirus
- EBV
Epstein‐Barr virus
- GGT
Gamma‐Glutamyl Transferase
- H&E
Haematoxylin and Eosin
- HAV
Hepatitis A virus
- HBV
Hepatitis B virus
- HCV
Hepatitis C virus
- HDV
Hepatitis D virus
- HEV
Hepatitis E virus
- HIV
Human Immunodeficiency Virus
- IHC
Immunohistochemistry
- ORF
Open Reading Frame
- PAS‐D
Periodic acid–Schiff diastase
- PCR
Polymerase Chain Reaction
- PMN leucocytes
Polymorphonuclear leucocytes
- RNA
Ribonucleic acid
Key points.
This is the first comprehensive report on histopathology of chronic hepatitis E (CHE) in a larger cohort of 19 patients substantiated by PCR in liver tissue and localization of hepatitis E virus (HEV) by immunohistochemistry (IHC).
First description of patterns of HEV staining in CHE in hepatocytes with cytoplasmic and nuclear reaction and in bile ducts with cholangitis in a larger series.
Cholangitis seems to be an intrinsic lesion in CHE because of the presence of HEV in bile ducts.
1. INTRODUCTION
Hepatitis E is a worldwide disease with a global incidence of about 20 million cases of acute hepatitis each year and estimated 70 000 deaths.1 Among adults in Asia, the Middle East and Africa it proved to be the most or second most important cause of acute clinical hepatitis.2 On the contrary, hepatitis E virus (HEV) was rarely identified in industrialized countries; affected individuals have usually travelled to an endemic area a short time ago.3 In the past few years, there has been an increasing number of reports on hepatitis E cases in persons who had not travelled abroad, but were infected at home in Europe or the USA.4, 5
There exist at least four genotypes of HEV that are pathogenic for humans.3 In Europe and the USA, there is an increasing number of cases caused by genotype 3.6 Genotypes 1, 2 and 3 may induce acute, even fatal acute, liver failure. However, genotype 3 can run a chronic course especially in immunocompromised individuals.7 Most of the reported cases with a chronic disease occurred in the transplant setting when patients received solid organ transplants.8, 9, 10, 11 A strong association between HEV infection after transplantation and graft rejection was shown.12
The histopathology of acute hepatitis E has been described in several publications,13, 14, 15, 16, 17 whereas the histopathology of chronic hepatitis E has been described in case reports, but has not been evaluated in detail so far.8, 11, 18, 19, 20, 21, 22 Therefore, we analysed the liver biopsies from a cohort of 19 patients with chronic hepatitis E.
2. MATERIALS AND METHODS
Liver biopsies from 19 patients (17 male, 2 female; aged 23‐66 years) from four hospitals (Vienna, Hanover, Hamburg, Cologne), of whom 17 had undergone solid organ transplantation (6 liver transplantations, 3 kidney transplantations, 4 double‐lung transplantations and 4 heart transplantations) were obtained (see Tables 1 and 3 and Table S1.). In the six patients that received liver transplants, two had cirrhosis because of alcoholic liver disease, three had end‐stage liver disease and cirrhosis because of chronic hepatitis C and one woman had cirrhosis with autoimmune hepatitis. One human immunodeficiency virus (HIV)‐negative patient had CD4 impairment and the second individual without transplantation had received longstanding steroid therapy because of ulcerative colitis. Other liver diseases as autoimmune hepatitis, viral hepatitis A, B and C, steatohepatitis, alcoholic disease, calcineurin inhibitor toxicity, rejection and biliary duct diseases as primary sclerosing cholangitis and primary biliary cholangitis or obstruction were excluded by chemical and laboratory findings, nucleic acid testing and clinical data. All patients were positive for HEV in the serum for more than 6 months and positive for HEV in the liver when tested by PCR after RNA extraction. So in all patients, clinical data and histopathology showed chronic hepatitis when characterized by the classic definition of a hepatitis lasting at least for 6 months. All patients had given their written consent for the biopsy. Liver biopsies from five patients undergoing bariatric surgery and negative for HEV infection served as negative controls. The study was approved by the Ethics Commission of the Medical University of Vienna (1382/2016).
Table 1.
Mean (minimum‐maximum) age at time of biopsy and biochemical data by transplanted organ
| Transplanted organ | Number of patients and gender | Mean age at time of biopsy (years) | AST [U/L] | ALT [U/L] | AP [U/L] | GGT [U/L] | Total bilirubin [mg/dL] |
|---|---|---|---|---|---|---|---|
| Liver | 6 male | 41.2 (23‐57) | 147.7 (35‐324) | 255 (25‐646) | 149.7 (66‐281) | 130.3 (30‐362) | 1.0 (0.9‐1.5) |
| Kidney | 3 male | 58.3 (54‐65) | 475 (102‐1211) | 764 (86‐1690) | 179.3 (96‐337) | 233.7 (50‐371) | 13.3 (2.6‐30.98) |
| Double‐lung | 3 male, 1 female | 45.3 (23‐65) | 241 (80‐467) | 356.3 (148‐630) | 109.8 (90‐141) | 123 (26‐195) | 6.1 (0.55‐21.42) |
| Heart | 3 male, 1 female | 57.5 (49‐66) | 168 (91‐379) | 199.3 (143‐315) | 109.5 (50‐179) | 122.5 (63‐189) | 1.55 (0.9‐2.3) |
| None | 2 male | 37.5 (34, 41) | 303 (160, 446) | 1542 (510, 2574) | 113.5 (97, 130) | 278 (276, 280) | 9.75 (4.9, 14.6) |
Table 3.
Transplanted organ, immunosuppressive medication, laboratory parameters and RNA status of individual patients arranged by degree of cholangitis
| Cholangitis | Gender | Transplanted organ | Immunosuppressant | AST [U/L] | ALT [U/L] | AP [U/L] | GGT [U/L] | Total bilirubin [mg/dL] | HEV‐RNA |
|---|---|---|---|---|---|---|---|---|---|
| 3 | male | Double‐lung | Tacrolimus | 293 | 630 | 103 | 146 | 21.42 | Positive |
| 3 | male | Kidney | Ciclosporin, Mycophenolic acid, Prednisolone | 102 | 516 | 105 | 371 | 2.6 | Positive |
| 3 | male | Liver | Mycophenolic acid, Prednisolone, Tacrolimus | 40 | 64 | 71 | 30 | 1.5 | Positive |
| 3 | male | Liver | Mycophenolic acid, Prednisolone, Tacrolimus | 35 | 25 | 66 | 42 | 1 | Positive |
| 3 | male | Liver | Mycophenolic acid, Prednisolone, Tacrolimus | 246 | 469 | 235 | 362 | 0.9 | Positive |
| 2 | male | Double‐lung | Everolimus, Tacrolimus | 124 | 148 | 141 | 195 | 1.6 | Positive |
| 2 | female | Double‐lung | Everolimus, Tacrolimus | 467 | 497 | 105 | 125 | 0.55 | Positive |
| 2 | male | Heart | Azathioprine, Ciclosporin, Prednisolone | 100 | 174 | 50 | 111 | 2.3 | Positive |
| 2 | male | Heart | Everolimus, Mycophenolic acid, Prednisolone | 379 | 315 | 179 | 189 | 1.4 | Positive |
| 2 | male | Kidney | Mycophenolic acid, Prednisolone, Sirolimus | 1211 | 1690 | 337 | 280 | 6.4 | positive |
| 2 | male | Kidney | Data not available | 112 | 86 | 96 | 50 | 30.98 | Positive |
| 2 | male | Liver | Ciclosporin, Everolimus | 191 | 256 | 86 | 62 | 0.9 | Positive |
| 2 | male | Liver | Ciclosporin, Mycophenolic acid | 324 | 646 | 159 | 174 | 0.9 | Positive |
| 2 | male | Liver | Azathioprine, Ciclosporin, Prednisolone | 50 | 70 | 281 | 112 | 0.9 | Positive |
| 2 | male | none (Immunosuppression) | Longstanding steroid therapy | 446 | 2574 | 97 | 276 | 14.6 | Positive |
| 1 | male | Double‐lung | Ciclosporin, Mycophenolic acid, Prednisolone | 80 | 150 | 90 | 26 | 0.79 | Positive |
| 1 | male | Heart | Ciclosporin, Everolimus, Prednisolone | 91 | 165 | 79 | 127 | 0.9 | Positive |
| 1 | female | Heart | Ciclosporin, Everolimus, Prednisolone | 102 | 143 | 130 | 63 | 1.6 | Positive |
| 1 | male | none (CD4‐deficiency) | none | 160 | 510 | 130 | 280 | 4.9 | Positive |
2.1. Serology and Biochemistry
Laboratory tests were done for aspartate and alanine transaminase (AST and ALT), gamma‐glutamyl transferase (GGT), alkaline phosphatase (AP) and total bilirubin (see Tables 1 and 3). Infections such as hepatitis A, B, C and D by HAV, HBV, HCV and HDV were excluded as well as Epstein‐Barr virus (EBV) and Cytomegalovirus (CMV) by serology using commercial tests. Chronic HEV infection was diagnosed by PCR in the serum on two test occasions with an interval of at least 6 months in between (for IgG & IgM using an ELISA‐recomwell, Mikrogen, Neuried, Germany) and HEV‐RNA extraction from the tissue as reported earlier.13
2.2. Liver tissue preparation
Liver biopsies were processed at standard protocol with fixation in 4% neutral formaldehyde and embedding in paraffin. Four micrometre sections were stained with haematoxylin and eosin (H&E), Chromotrop‐Anilinblue (CAB) trichrome staining, Prussian blue and Periodic acid‐Schiff diastase (PAS‐D).
2.3. Histological evaluation
The histological evaluation of the inflammatory activity and the stage of fibrosis were performed by applying the Ishak grading and score.23 When observing that many of the biopsies displayed some degree of cholangitis, we set up a semi‐quantitative score for the bile duct lesions:
Grade 1: Some lymphocytes and/or polymorphonuclear (PMN) leucocytes between bile duct epithelia (analogue reactive cholangitis).
Grade 2: Lymphoepithelial lesions in bile ducts with degenerative changes of the epithelia.
Grade 3: Infiltration of bile ducts with lymphocytes and PMN leucocytes as well as necrosis of bile duct epithelia (equivalent to destructive cholangitis).
Emperipolesis and PMN leucocytes were also graded by a semi‐quantitative score: One emperipolesis or PMN leucocyte per high power field means one point, two to four per high power field results in two points, and five or more are graded with 3.
2.4. Immunohistochemistry
Biopsies were immunohistochemically stained with two different HEV antibodies using a Universal DAB Detection Kit (Ventana, Roche, 760‐500, Basel, Switzerland) on a Benchmark Ultra (Ventana, Roche, Basel, Switzerland), one antibody directed against open reading frame (ORF) 2.1 encoded region (LSBioSciences clone 4B2, LS‐C67674, Seattle, USA; dilution 1:50) and the other one directed against ORF3 (Bioss Antibodies, bs0212R, Woburn USA; dilution 1:100). Incubation of the primary antibody was performed for 32 minutes on paraffin sections after protease digestion for 8 minutes. Negative controls were taken from five patients with bariatric surgery negative for HEV testing in the blood. Additionally, we performed immunohistochemical staining with an antibody against cytokeratin 7 (Dako, M7018, Santa Clara, USA; dilution 1:200) to look for cholangiocellular metaplasia in hepatocytes as an indicator for chronic cholestasis.
3. RESULTS
The patients showed an increase in ALT (mean 480.42 U/L), AST (mean 239.63 U/L), GGT (mean 159 U/L) and total bilirubin (mean 5.06 mg/dL). The biochemical data of the patients are given in Tables 1 and 3. All of them were tested positive for anti‐HEV IgM. None of them was positive for HAV, HBV, HCV or HDV. Tests for infections with EBV or CMV were negative. There was no significant difference in GGT, bilirubin and AP levels when comparing patients with cholangitis grade 3 and grade 1 or 2 using the Jonckheere‐Terpstra test (GGT: P = 0.307, bilirubin: P = 0.141, AP: P = 0.454). HEV‐PCR was positive in the sera and tissues from all patients and could be determined by sequence analysis as genotype 3 in all specimens. All biopsies had at least 10 portal tracts and so were representative.
3.1. Histopathology
The overall histological activity showed a mean Ishak score of 6 with a range from 3 to 10. The periportal necroinflammatory lesions (Ishak A) were mild (1) to moderate (3) with interface hepatitis showing piecemeal necrosis with cell death and apoptosis of hepatocytes accompanied by densely infiltrating lymphocytes and activated Kupffer cells. Portal tracts were diffusely enlarged showing infiltration of lymphocytes, some plasma cells and also PMN leucocytes (see Figure 1, panel A). Bile ducts were involved and displayed cholangitis in all cases (see Tables 2 and 3). Bile duct epithelia were infiltrated by lymphocytes and a significant number of PMN leucocytes, which were also present to a lesser degree in zone I of the lobule. Destruction of bile duct epithelia (grade 3 cholangitis) was noted in 5 of the 19 biopsies (see Figure 1, panel B), however there was no ductopenia. The basement membranes of the ducts were preserved, though no bile duct loss with ductopenia was found. The necroinflammatory activity in the lobules was variable: In four of the biopsies confluent necrosis (Ishak B) was present. Lobular spotty necrosis (Ishak C) was obvious in all cases without zonal distribution, but mostly in midlobular and sometimes in perivenular localization. There was some cellular cholestasis in zone III. Steatosis was not observed, and no granulomas were found. Endothelitis was absent.
Figure 1.
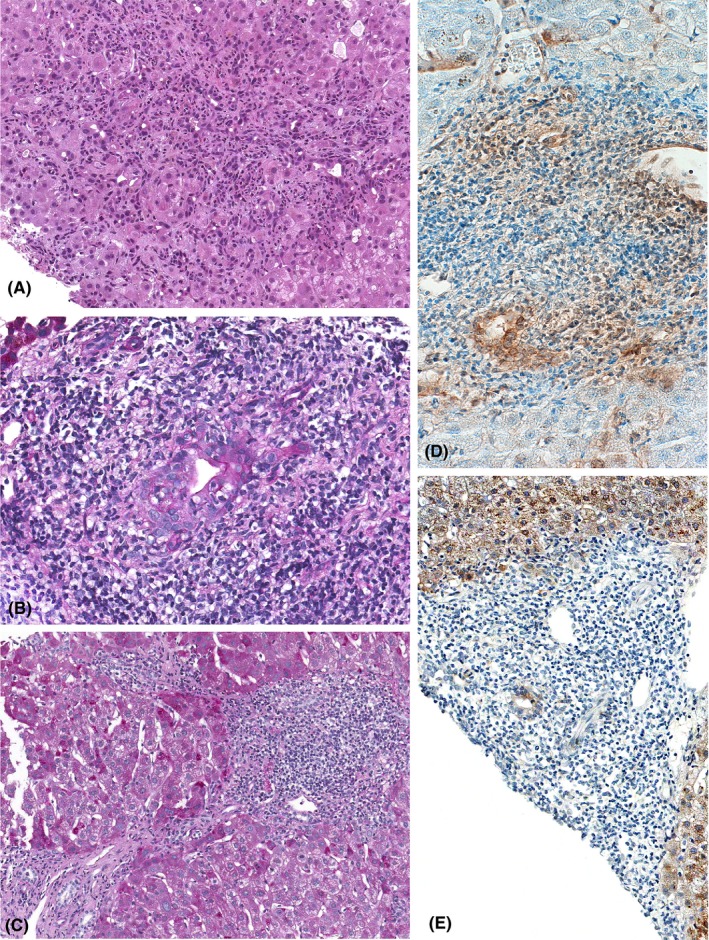
Histology and immunohistochemistry of patients with chronic hepatitis E. A, Portal and periportal area with a dense active inflammatory infiltrate, cholangitis and interface hepatitis (200x, H&E). B, Portal tract in chronic hepatitis E with interlobular bile duct sustaining destructive cholangitis (400x, PAS‐D). C, Chronic hepatitis E with three enlarged portal tracts densely infiltrated by inflammatory cells and connected by bridging septae (200x, PAS‐D). D, Portal tract with dense mononuclear infiltrates and a small bile duct with cholangitis staining positive for HEV ORF3 (200x, antibody dilution 1:50). E, Portal tract with dense mononuclear infiltrates and a small bile duct with cholangitis staining positive for HEV ORF2 (400x, antibody dilution 1:50)
Table 2.
Histological evaluation of the 19 biopsies arranged by grade of cholangitis
| Ishak A | Ishak B | Ishak C | Ishak D | Fibrosis | Emperipolesis | PMN Leucocytes | Cholestasis | Cholangitis |
|---|---|---|---|---|---|---|---|---|
| 2 | 0 | 2 | 2 | 2 | 2 | 2 | 1 | 3 |
| 1 | 1 | 3 | 2 | 1 | 3 | 2 | 1 | 3 |
| 2 | 0 | 2 | 2 | 1 | 1 | 2 | 0 | 3 |
| 3 | 1 | 2 | 3 | 2 | 2 | 2 | 0 | 3 |
| 3 | 0 | 3 | 3 | 3 | 3 | 3 | 1 | 3 |
| 2 | 0 | 2 | 2 | 2 | 2 | 2 | 1 | 2 |
| 2 | 0 | 2 | 3 | 1 | 2 | 2 | 0 | 2 |
| 2 | 2 | 3 | 3 | 1 | 3 | 3 | 1 | 2 |
| 3 | 1 | 3 | 3 | 2 | 3 | 3 | 1 | 2 |
| 1 | 0 | 2 | 1 | 2 | 2 | 2 | 0 | 2 |
| 1 | 0 | 2 | 2 | 1 | 2 | 1 | 0 | 2 |
| 1 | 0 | 1 | 1 | 2 | 2 | 2 | 0 | 2 |
| 1 | 0 | 1 | 1 | 1 | 1 | 2 | 1 | 2 |
| 1 | 0 | 2 | 2 | 2 | 1 | 2 | 1 | 2 |
| 2 | 0 | 1 | 2 | 5 | 2 | 2 | 0 | 2 |
| 2 | 0 | 2 | 1 | 1 | 2 | 2 | 0 | 1 |
| 2 | 0 | 2 | 2 | 1 | 2 | 1 | 0 | 1 |
| 1 | 0 | 2 | 1 | 1 | 1 | 1 | 1 | 1 |
| 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 |
Fibrosis was evident in all cases with a mean stage of 2. The portal tracts were enlarged with or without short septae (score 2) in seven patients. Fibrosis with bridging septae (score 3) was noted in one patient (see Figure 1, panel C) and one patient had incomplete cirrhosis (score 5). By trichrome stain the collagen tissue was restricted to portal tracts and septae in most cases; however, in three biopsies, there was a conspicuous fibrosis pattern with central vein sclerosis and pericellular fibrosis in the central area as also seen in alcoholic or non‐alcoholic steatohepatitis. There was no substantial difference of histopathology in patients who had received liver transplants and those who had received other solid organs.
3.2. Immunohistochemistry
All biopsies were positive for staining with the anti‐HEV antibodies. The pattern at the single cell level displayed a granular staining in the cytoplasm of the hepatocytes and some nuclei were also positive, as reported earlier.24, 25, 26 At the lobular level, a focal staining pattern was observed, sometimes extending into areas of the whole lobule. The amount of positive hepatocytes varied between 10% and 60% for antibodies against ORF2 and 40 and 50% with antibodies against ORF3. Bile ducts were positive as well. Remarkably, larger bile ducts did not show a significant cholangitis, whereas smaller bile ducts were affected by considerable, even destructive cholangitis (see Figure 1, panel D and E). In the bile ducts, the whole epithelia stained positive. Hepatocytes were negative for antibodies against cytokeratin 7, whereas all bile duct epithelia were positive. Liver biopsies from our control patients negative for HEV in the serum showed a mild background as been observed also by Lenggenhager,25 but no specific staining in immunohistochemistry with ORF2 and ORF3 antibodies (see Figure S1). An antibody reaction was interpreted as specific when the staining was bound to cell structures and subcellular structures, whereas an unspecific staining was sometimes noted at the rim of the section or background not bound to cell organelles.
4. DISCUSSION
The infection with HEV genotype 3 as an indigenous disease in Europe and the USA has been diagnosed with an increasing frequency in the recent years.3, 6, 27, 28 Transmission by blood transfusions and blood products has recently been reported in several cases.29, 30 Whereas most of the cases run an acute self‐limiting hepatitis, the disease may evolve into a chronic course in immunocompromised patients, especially in the setting of solid organ transplantation.8, 9, 10, 11, 27 Our patients received immunosuppression therapy (see Table 3). Also patients with HIV infection bear the risk to develop a chronic disease.20, 31, 32 The occurrence of chronic hepatitis E was also reported in an immunocompetent patient.33 Recently, Ingiliz P et al described a patient with persistence of an HEV infection, although the immunosuppressant therapy was terminated.34 We defined chronic hepatitis E in agreement with the classic recommendation of a course longer than 6 months although in the new guideline of EASL a chronic hepatitis E can be diagnosed already after 3 months of starting the therapy.35
The incidence of chronic hepatitis E in transplant recipients was estimated at about 66%36; however, this frequency is based on a single study, and more data are needed.
The histopathology of acute hepatitis E caused by genotype 3 has been described in several reports13, 14, 15, 16, 17; however, a detailed evaluation of the chronic lesions is lacking so far; there are only five short statements,8, 11, 18, 19, 20 and description of two further patients.22 Therefore, we analysed the histopathology of biopsies from 19 patients with chronic hepatitis E, 17 of whom had undergone solid organ transplantation, one HIV‐negative patient had CD4 lymphocyte deficiency and there was one patient who received longstanding steroid therapy because of ulcerative colitis.
The histopathology of chronic hepatitis fits into the pattern of chronic viral hepatitis with enlarged infiltrated portal tracts, lobular hepatitis to a variable extend and fibrosis of portal tracts extending as fibrotic septae into the parenchyma. There are no pathognomonic features as are ground glass hepatocytes for chronic hepatitis B. However, there were some lesions that were more pronounced than in the other types of chronic viral hepatitis. One is the obvious active cholangitis that surpassed a reactive cholangitis in type B and C hepatitis as described in the literature.15, 16, 37 Four biopsies of 19 showed grade 1 and ten displayed grade 2 cholangitis; five biopsies showed a florid active inflammation of the bile ducts with necrosis of bile duct epithelia (grade 3, cholangiodestructive cholangitis) as described by Wendum,14 although there was no destruction of the bile duct membrane and no ductopenia. Cholangitis in hepatitis E has also been reported by several authors.8, 13, 15, 37 The cause for this conspicuous bile duct involvement in hepatitis E may be that the virus is secreted via the bile ducts into the gut and the stool. The presence of HEV in about 10% to 60% of the hepatocytes (ORF2) is similar to the results of Lenggenhager et al25 In our cohort, we could confirm the presence of the virus in bile duct epithelia by immunohistochemistry. One group that performed immunohistochemistry with an in‐house‐made antibody on post‐mortem livers did not report a positive staining for HEV in bile duct epithelia,24 whereas HEV was recently found in bile canaliculi by another group.25 Our immunohistochemical staining was similar as observed in immunofluorescence staining in chimeric mice,38, 39, 40 and the authors Allweiss et al used the same antibody against ORF3.40 The presence of HEV in bile duct epithelia has even been demonstrated by electron microscopy and in situ hybridization.41, 42 The virus is present in the bile duct epithelia and elicits an inflammatory immune reaction. However, patients with grade 3 cholangitis do not present with higher cholestasis parameter than patients with grade 1 or 2 cholangitis. Other causes of cholangitis as recurrence of primary sclerosing cholangitis or primary biliary cholangitis as well as rejection, ischaemic cholangiopathy or autoimmune hepatitis were excluded by serology and cholangiography. Rejection with endothelitis was not observed and excluded clinically. Another feature of chronic hepatitis E is the increased number of PMN leucocytes compared to chronic hepatitis B and C that has been observed also by Agrawal et al37 The evaluation of the subtypes of infiltrating inflammatory cells suggests that the virus itself is not cytopathic but triggers a strong antiviral interferon response.16, 43, 44, 45, 46 The majority of the PMN leucocytes were seen in portal tracts in the vicinity of bile ducts as in florid cholangitis and some were also noted in the periportal area. In our cases, we did not observe a complete cirrhosis (stage 6) as it has been reported in the literature.20, 36 However, one biopsy showed advanced fibrosis in bridging septae (stage 5). Beyond that we noticed in three biopsies a conspicuous central vein sclerosis with adjacent pericellular fibrosis. This did not fit into the fibrosis pattern of chronic viral hepatitis and can be explained by a pre‐existing toxic injury. The impact of immunosuppression on histopathology could not be evaluated since there are no real controls. All patients received Ribavirin therapy after the diagnosis of chronic HEV was established after the second PCR test, according to Wedemeyer et al27 17 of the 19 patients cleared the virus after four to eight weeks of treatment, so follow‐up biopsies were not taken.
Histopathology of the two patients without solid organ transplantation was not different in comparison with the ones who have received transplantation, and also identical in patients with liver transplantation.
In summary, chronic hepatitis E shows a histopathological pattern consistent with a chronic hepatitis of viral origin. There are no pathognomonic features for HEV genotype 3; however, biopsies display a cholangitis that is active and sometimes destructive seemingly because of the presence of the virus in bile duct epithelia. Peculiar lesions with an increased number of PMN leucocytes are also present.
CONFLICT OF INTEREST
There is no conflict to disclose.
AUTHOR'S CONTRIBUTION
Andrea Beer involved in study concept and design, acquisition and interpretation of data, drafting of the manuscript. Heidemarie Holzmann involved in acquisition and interpretation of data, statistical analysis, critical revision of the manuscript. Sven Pischke and Michael Manns involved in aquisition and interpretation of data, critical revision of the manuscript, material support. Patrick Behrendt, Jerome Schlue, Uta Drebber, Hans Kreipe, Ansgar Lohse, Martina Sterneck, Heiner Wedemeyer involved in acquisition and interpretation of data, material support. Fritz Wrba involved in acquisition and interpretation of data, critical revision of the manuscript. Barbara Neudert and Emina Halilbasic involved in material support. Hans Peter Dienes involved in study concept and design, acquisition and interpretation of data, drafting of the manuscript, critical revision of the manuscript.
Supporting information
ACKNOWLEDGEMENTS
The study was supported by the Department of Pathology, Medical University of Vienna, General hospital, Vienna, Austria, by providing slides from routine diagnostics and processing immunohistochemical stains.
Beer A, Holzmann H, Pischke S, et al. Chronic Hepatitis E is associated with cholangitis. Liver Int. 2019;39:1876–1883. 10.1111/liv.14137
Handling Editor: Francesco Negro
REFERENCES
- 1. Rein DB, Stevens GA, Theaker J, Wittenborn JS, Wiersma ST. The global burden of hepatitis E virus genotypes 1 and 2 in 2005. Hepatology. 2012;55(4):988‐997. [DOI] [PubMed] [Google Scholar]
- 2. Purcell RH, Emerson SU. Hepatitis E: an emerging awareness of an old disease. J Hepatol. 2008;48(3):494‐503. [DOI] [PubMed] [Google Scholar]
- 3. Purcell RH, Emerson SU. Hidden danger: the raw facts about hepatitis E virus. J Infect Dis. 2010;202(6):819‐821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. De Keukeleire S, Reynders M. Hepatitis E: an underdiagnosed, emerging infection in nonendemic regions. J Clin Transl Hepatol. 2015;3(4):288‐291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sayed IM, Vercouter AS, Abdelwahab SF, Vercauteren K, Meuleman P. Is hepatitis E virus an emerging problem in industrialized countries? Hepatology. 2015;62(6):1883‐1892. [DOI] [PubMed] [Google Scholar]
- 6. Schiller D, Schoefl R, Holzmann H. Fatal acute liver failure in a kidney‐transplant recipient. Gastroenterology. 2014;146(1): 36, 325. [DOI] [PubMed] [Google Scholar]
- 7. Behrendt P, Steinmann E, Manns MP, Wedemeyer H. The impact of hepatitis E in the liver transplant setting. J Hepatol. 2014;61(6):1418‐1429. [DOI] [PubMed] [Google Scholar]
- 8. Kamar N, Selves J, Mansuy J‐M, et al. Hepatitis E virus and chronic hepatitis in organ‐transplant recipients. N Engl J Med. 2008;358(8):811‐817. [DOI] [PubMed] [Google Scholar]
- 9. Gérolami R, Moal V, Colson P. Chronic hepatitis E with cirrhosis in a kidney‐transplant recipient. N Engl J Med. 2008;358(8):859‐860. [DOI] [PubMed] [Google Scholar]
- 10. Haagsma EB, Niesters H, van den Berg AP, et al. Prevalence of hepatitis E virus infection in liver transplant recipients. Liver Transpl. 2009;15(10):1225‐1228. [DOI] [PubMed] [Google Scholar]
- 11. Pischke S, Suneetha PV, Baechlein C, et al. Hepatitis E virus infection as a cause of graft hepatitis in liver transplant recipients. Liver Transpl. 2010;16(1):74‐82. [DOI] [PubMed] [Google Scholar]
- 12. Sue PK, Pisanic N, Heaney CD, et al. Hepatitis E Virus infection among solid organ transplant recipients at a North American transplant center. Open Forum Infect Dis. 2016;3(1):ofw006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Drebber U, Odenthal M, Aberle SW, et al. Hepatitis E in liver biopsies from patients with acute hepatitis of clinically unexplained origin. Front Physiol. 2013;13(4):351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wendum D, Nachury M, Yver M, et al. Acute hepatitis E: a cause of lymphocytic destructive cholangitis. Hum Pathol. 2005;36(4):436‐438. [DOI] [PubMed] [Google Scholar]
- 15. Peron J‐M, Danjoux M, Kamar N, et al. Liver histology in patients with sporadic acute hepatitis E: a study of 11 patients from South‐West France. Virchows Arch. 2007;450(4):405‐410. [DOI] [PubMed] [Google Scholar]
- 16. Malcolm P, Dalton H, Hussaini HS, Mathew J. The histology of acute autochthonous hepatitis E virus infection. Histopathology. 2007;51(2):190‐194. [DOI] [PubMed] [Google Scholar]
- 17. Chijioke O, Bawohl M, Springer E, Weber A. Hepatitis e virus detection in liver tissue from patients with suspected drug‐induced liver injury. Front Med. 2015;30(2):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pischke S, Stiefel P, Franz B, et al. Chronic hepatitis e in heart transplant recipients. Am J Transplant. 2012;12(11):3128‐3133. [DOI] [PubMed] [Google Scholar]
- 19. Liu LI, Liu Y, Du Y, et al. Analysis of long‐term follow‐up and examination of pathological liver tissue in chronic hepatitis E. Dig Liver Dis. 2016;48(6):684‐686. [DOI] [PubMed] [Google Scholar]
- 20. Jagjit Singh GK, Ijaz S, Rockwood N, et al. Chronic Hepatitis E as a cause for cryptogenic cirrhosis in HIV. J Infect. 2013;66(1):103‐106. [DOI] [PubMed] [Google Scholar]
- 21. Miyoshi M, Kakinuma S, Tanabe Y, et al. Chronic Hepatitis E infection in a persistently immunosuppressed patient unable to be eliminated after Ribavirin therapy. Intern Med. 2016;55(19):2811‐2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Protzer U, Böhm F, Longerich T, et al. Molecular detection of hepatitis E virus (HEV) in liver biopsies after liver transplantation. Mod Pathol. 2015;28(4):523‐532. [DOI] [PubMed] [Google Scholar]
- 23. Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22(6):696‐699. [DOI] [PubMed] [Google Scholar]
- 24. Gupta P, Jagya N, Pabhu SB, Durgapal H, Acharya SK, Panda SK. Immunohistochemistry for the diagnosis of hepatitis E virus infection. J Viral Hepat. 2012;19(2):e177‐e183. [DOI] [PubMed] [Google Scholar]
- 25. Lenggenhager D, Gouttenoire J, Malehmir M, et al. Visualization of hepatitis E virus RNA and proteins in the human liver. J Hepatol. 2017;67(3):471‐479. [DOI] [PubMed] [Google Scholar]
- 26. Prost S, Crossan CL, Dalton HR, et al. Detection of viral hepatitis E in clinical liver biopsies. Histopathology. 2017;71(4):580‐590. [DOI] [PubMed] [Google Scholar]
- 27. Wedemeyer H, Pischke S, Manns MP. Pathogenesis and treatment of hepatitis e virus infection. Gastroenterology. 2012;142(6):1388‐1397.e1. [DOI] [PubMed] [Google Scholar]
- 28. Dalton HR, Pas SD, Madden RG, van der Eijk AA. Hepatitis e virus: current concepts and future perspectives. Curr Infect Dis Rep. 2014;16(4):399. [DOI] [PubMed] [Google Scholar]
- 29. Hewitt PE, Ijaz S, Brailsford SR, et al. Hepatitis E virus in blood components: a prevalence and transmission study in southeast England. Lancet. 2014;384(9956):1766‐1773. [DOI] [PubMed] [Google Scholar]
- 30. Kurihara T, Yoshizumi T, Itoh S, et al. Chronic hepatitis E virus infection after living donor liver transplantation via blood transfusion: a case report. Surg Case Rep. 2016;2(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kenfak‐Foguena A, Schöni‐Affolter F, Bürgisser P, et al. Hepatitis E Virus seroprevalence and chronic infections in patients with HIV, Switzerland. Emerg Infect Dis. 2011;17(6):1074‐1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dalton HR, Bendall RP, Keane FE, Tedder RS, Ijaz S. Persistent carriage of hepatitis E virus in patients with HIV infection. N Engl J Med. 2009;361(10):1025‐1027. [DOI] [PubMed] [Google Scholar]
- 33. Grewal P, Kamili S, Motamed D. Chronic hepatitis E in an immunocompetent patient: a case report. Hepatology. 2014;59(1):347‐348. [DOI] [PubMed] [Google Scholar]
- 34. Ingiliz P, Mayr C, Obermeier M, Herbst H, Polywka S, Pischke S. Persisting hepatitis E virus infection leading to liver cirrhosis despite recovery of the immune system in an HIV‐infected patient. Clin Res Hepatol Gastroenterol. 2016;40(3):e23‐e25. [DOI] [PubMed] [Google Scholar]
- 35. European Association for the Study of the Liver . EASL clinical practice guidelines on hepatitis E virus infection. J Hepatol. 2018;68(6):1256‐1271. [DOI] [PubMed] [Google Scholar]
- 36. Kamar N, Garrouste C, Haagsma EB, et al. Factors associated with chronic hepatitis in patients with hepatitis E virus infection who have received solid organ transplants. Gastroenterology. 2011;140(5):1481‐1489. [DOI] [PubMed] [Google Scholar]
- 37. Agrawal V, Goel A, Rawat A, Naik S, Aggarwal R. Histological and immunohistochemical features in fatal acute fulminant hepatitis E. Indian J Pathol Microbiol. 2012;55(1):22–27. [DOI] [PubMed] [Google Scholar]
- 38. van de Garde M, Pas SD, van der Net G, et al. Hepatitis E Virus (HEV) Genotype 3 infection of human liver chimeric mice as a model for chronic HEV infection. J Virol. 2016;90(9):4394–4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sayed IM, Verhoye L, Cocquerel L, et al. Study of hepatitis E virus infection of genotype 1 and 3 in mice with humanised liver. Gut. 2017;66(5):920–929. [DOI] [PubMed] [Google Scholar]
- 40. Allweiss L, Gass S, Giersch K, et al. Human liver chimeric mice as a new model of chronic hepatitis E virus infection and preclinical drug evaluation. J Hepatol. 2016;64(5):1033–1040. [DOI] [PubMed] [Google Scholar]
- 41. Asher LV, Innis BL, Shrestha MP, Ticehurst J, Baze WB. Virus‐like particles in the liver of a patient with fulminant hepatitis and antibody to hepatitis E virus. J Med Virol. 1990;31(3):229–233. [DOI] [PubMed] [Google Scholar]
- 42. Kawai HF, Koji T, Iida F, Kaneko S, Kobayashi K, Nakane PK. Shift of hepatitis E virus RNA from hepatocytes to biliary epithelial cells during acute infection of rhesus monkey. J Viral Hepat. 1999;6(4):287–297. [DOI] [PubMed] [Google Scholar]
- 43. Suneetha PV, Pischke S, Schlaphoff V, et al. Hepatitis E virus (HEV)‐specific T‐cell responses are associated with control of HEV infection. Hepatology. 2012;55(3):695–708. [DOI] [PubMed] [Google Scholar]
- 44. Husain MM, Aggarwal R, Kumar D, Jameel S, Naik S. Effector T cells immune reactivity among patients with acute hepatitis E. J Viral Hepat. 2011;18(10):e603–e608. [DOI] [PubMed] [Google Scholar]
- 45. Srivastava R, Aggarwal R, Bhagat MR, Chowdhury A, Naik S. Alterations in natural killer cells and natural killer T cells during acute viral hepatitis E. J Viral Hepat. 2008;15(12):910–916. [DOI] [PubMed] [Google Scholar]
- 46. Wang W, Wang Y, Qu C, et al. The RNA genome of hepatitis E virus robustly triggers an antiviral interferon response. Hepatology. 2018;67(6):2096–2112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


