Abstract
Purpose
Patients with monoclonal gammopathy of undetermined significance (MGUS) have an increased risk of developing infections. Streptococcus pneumoniae vaccinations are recommended for immunocompromised patients, including patients with lymphoproliferative disorders such as MGUS. The objective of the study was to assess the immune response to the 13-valent pneumococcal conjugate vaccine (PCV13) in treatment-naive MGUS patients versus healthy subjects. All study groups were evaluated for the levels of specific pneumococcal antibodies, the levels of IgG and IgG subclasses, and selected peripheral blood lymphocyte subpopulations, including the proportion of plasmablasts before and after immunization.
Patients and methods
A total of 22 previously untreated patients with MGUS and 15 healthy age- and sex-matched volunteers were included in the study. All participants were immunized with PCV13 Prevenar13 (Pfizer). The following parameters were assessed: 1) serum-specific pneumococcal antibody titers before and 30 days after vaccination, 2) percentage of plasmablasts, defined as CD19+/IgD−/CD27++, before and 7 days after vaccination, 3) serum total IgG and IgG1, IgG2, IgG3, IgG4 levels before and 30 days after vaccination.
Results and conclusion
PCV13 vaccination in MGUS patients is safe and effectively protects against S. pneumoniae infection. In unvaccinated individuals, vaccination should be carried out as soon as possible after diagnosis. It can protect patients against serious infectious complications, which can contribute to extending the time to progression and transformation into more aggressive diseases. PCV13 vaccination is more effective in MGUS patients with a lower concentration of M protein. Serum M protein concentration in patients diagnosed with MGUS may be a useful predictor of the effectiveness of vaccination.
Keywords: 13-valent pneumococcal conjugate vaccine, immune response, monoclonal gammopathy of undetermined significance, M protein, plasmablasts, pneumococcal antibodies
Introduction
Monoclonal gammopathy of undetermined significance (MGUS) results from clonal proliferation of differentiated plasma cells producing homogeneous immunoglobulin or its light (or, rarely, heavy) chain.1 The M component, which appears as a sharp band on serum protein electrophoretic analysis, may be typed by immunofixation.1 Even low-level production of the M component in a formerly healthy individual is associated with an increased risk of multiple myeloma (MM), Waldenström macroglobulinemia, and other malignant lymphoproliferative disorders.1,2 MGUS incidence increases with advancing age.2 It is usually diagnosed by chance and is characterized by the presence of a serum monoclonal immunoglobulin (M protein) (<30 g/L).2 MGUS is present in 3% of people over 50 years and in up to 5% of people over 70 years.3,4
Patients with MGUS have a twofold increased risk of developing any infection at 5- and 10-year follow-up.5 More specifically, these patients have an increased risk of bacterial (pneumonia, osteomyelitis, septicemia, pyelonephritis, cellulitis, endocarditis, and meningitis) and viral (influenza and herpes zoster) infections.5 MGUS patients with M protein concentrations over 2.5 g/dL at diagnosis have the highest risks of infections.5 However, the risk also increases among those with concentrations below 0.5 g/dL.5
In both the European and US guidelines, Streptococcus pneumoniae (S. pneumoniae) vaccinations are recommended for all elderly people with comorbidities. In the US, the vaccinations are recommended for immunocompromised patients, including patients with lymphoproliferative disorders such as MGUS.6 Nowadays, both the 23-valent pneumococcal polysaccharide vaccine (PPV23) and the 13-valent pneumococcal conjugate vaccine (PCV13) have been recommended for pneumococcal infection prevention in patients with lymphoproliferative-associated immunodeficiency.7 Twelve of the PCV13 serotypes are also included in PPV23. PCV13 likely provides better protection against pneumococcal disease due to different immunogenic properties.8 The effectiveness of PCV13 in MGUS patients has not been assessed.
The objective of the study was to assess the immune response to PCV13 in treatment-naive MGUS patients with no comorbidities versus healthy subjects. All study groups were evaluated for the levels of specific pneumococcal antibodies, the levels of IgG and IgG subclasses, and selected peripheral blood lymphocyte subpopulations, including the proportion of plasmablasts before and after immunization.
Materials And Methods
Study And Control Group
A total of 22 previously untreated patients with MGUS, aged between 53 and 85 years, who were diagnosed in the Hematology Department at Holy Cross Cancer Centre in Kielce, Poland, were included in the study. The control group consisted of 15 healthy individuals representing demographic (sex and age) characteristics similar to those observed within the study group. The same control group was used as a reference in our earlier study.9 Table 1 presents the characteristics of the study and control groups. According to the criteria revised by the International Myeloma Working Group in 2010, MGUS was diagnosed by an M protein concentration of <30 g/L, fewer than 10% of plasma cells in the bone marrow, and no related organ or tissue impairment (no end organ damage, such as hypercalcemia, renal insufficiency, anemia or lytic bone lesions related to the plasma cell disorder – the CRAB criteria),10,11 and based on SLiM criteria, which included the following: ≥60% clonal bone marrow plasma cells, serum-free light chain ratio involved:uninvolved ≥100, more than one focal lesion (≥5 mm each) detected by MRI.12
Table 1.
The Characteristics Of The Study And Control Groups
| Parameter | MGUS Patients | Control Group | |
|---|---|---|---|
| Gender | Female (n) | 9 | 6 |
| Male (n) | 13 | 9 | |
| Age | Mean±SD | 69.23±9.68 | 68.6±8.21 |
| Median (min.–max.) | 67.5 (53–85) | 68.5 (54–83) | |
| Lymphocyte count (x103/mL) | Mean±SD | 2163.1±364.34 | 2044±600.88 |
| Median (min.–max.) | 2165 (1140–3120) | 1995 (1130–3310) | |
| LDH serum concentration (U/L) | Mean±SD | 329.77±66.67 | 343.81±50.07 |
| Median (min.–max.) | 324 (164–435) | 347.5 (251–435) | |
| Beta-2-microglobulin serum concentration (µg/L) | Mean±SD | 1924.5±364.34 | 1870.69±339.56 |
| Median (min.–max.) | 1875 (1436–2798) | 1957 (1178–2285) | |
| M-protein serum concentration (g/dL) | Mean±SD | 0.83±0.59 | N/A |
| Median (min.–max.) | 0.72 (0.1–2.78) | N/A | |
| Type of M protein | IgG | 77% (n=17) | N/A |
| IgM | 14% (n=3) | N/A | |
| IgA | 9% (n=2) | N/A | |
| FLC ratio | Mean±SD | 1.61±1.9 | N/A |
| Median (min.–max.) | 0.97 (0.03–7.03) | N/A |
Abbreviations: MGUS, monoclonal gammopathy of undetermined significance; N/A, not applicable; LDH, lactate dehydrogenase; FLC, free light chain; SD, standard deviation.
None of the patients and controls had been receiving drugs affecting the immune system, and none had received blood transfusions. None showed any signs of infection at least 3 months prior to the study or of allergic or autoimmune disease. All participants gave their prior written informed consent for participation. The study protocol was approved by the Bioethics Committee of the Regional Chamber of Physicians in Kielce (No. KB7/2012).9 The study was conducted in accordance with the Declaration of Helsinki.
During the follow-up, all patients and controls underwent control visits at the Hematology Department, Holy Cross Cancer Centre at least every three months, or more often, every time they had an infection. To determine causative agent of each infection, we collected swabs and run microbial cultures, according to the current guidelines. Additionally, to further confirm etiology of infection, ELISA and PCR tests were conducted.
Study Material
Peripheral blood samples were obtained from the basilic vein and were used to measure the following parameters: 1) serum-specific pneumococcal antibody titers before vaccination (3 mL of peripheral blood collected to tubes with a clotting activator) and 30 days after vaccination (3 mL of peripheral blood collected to tubes with a clotting factor), 2) percentage of plasmablasts, defined as CD19+/IgD−/CD27++, before and 7 days after vaccination (5 mL of peripheral blood collected to tubes with the anticoagulant EDTA at each draw), 3) total serum IgG and IgG1, IgG2, IgG3, and IgG4 levels before (5 mL of peripheral blood collected to tubes with a clotting activator) and 30 days after vaccination (5 mL of peripheral blood collected to tubes with a clotting factor).9 According to the studies published before, days 7 and 30 post-vaccination were chosen to monitor plasmablast levels and antibody titers. Detection of plasmablasts in peripheral blood, described previously as a marker of an early response to vaccination,13–16 is usually conducted at day 7 following vaccination.14,17–19 Immune response to the vaccine, e.g., determination of serum antibody titers, is carried out one month (or 30 days) post-vaccination.20–24 Serum samples were stored at –70°C until use. The percentages of plasmablasts were assessed using fresh peripheral blood samples from MGUS patients and healthy volunteers. Serum total IgG and IgG1, IgG2, IgG3, and IgG4 levels were measured using fresh serum samples. Complete blood count tests and measurements of serum IgA, IgM, IgG, IgG1, IgG2, IgG3, and IgG4 levels were conducted before and one month after vaccination. Routine immunophenotyping of peripheral blood lymphocytes, β2microglobulin, IL-6, complement components 3 and 4 (C3 and C4), lactate dehydrogenase (LDH), albumin, total protein, M protein, free light chains (FLC), IgG, IgM, IgA serum levels, plasmablast evaluation, serum pneumococcal antibody assessment, and IgG subclasses assessment were performed as described previously.9,25 Assessment of serum pneumococcal antibody was done before vaccination (on the vaccination day) and 30 days after vaccination, using a commercial ELISA test (ELIZEN Pneumococcus IgG Assay, Zentech, Belgium), which determines the concentration of anti-capsular-polysaccharide antibody specific for 23 pneumococcal serotypes (1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F, 33F). For details, see the work by Pasiarski et al.9
Vaccine
MGUS patients and controls were immunized with the PCV13 Prevenar13 (Pfizer) vaccine, which contains polysaccharide antigens of pneumococcal serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F, conjugated to the carrier protein CRM197. The vaccine was injected via the intramuscular route. A single dose of PCV13 was administered. None of the patients and controls had received PPV23 or any other S. pneumoniae vaccine previously.9
Statistical Analysis
The normal distribution of continuous variables was verified with the Shapiro–Wilk Test. The statistical characteristics of continuous variables are presented as median and extreme values (minimum and maximum), as well as arithmetic means and standard deviations (SD). Intergroup comparisons were conducted with the Mann–Whitney U-test, as well as a univariate ANOVA followed by the post-hoc Tukey’s test, or the Kruskal–Wallis test followed by the post-hoc Dunn test. Pearson’s chi-squared test or Fisher’s exact test were used for intergroup comparisons of discontinuous variables. All calculations were conducted with Statistica 10 (StatSoft, US), with the significance level set at p<0.05.9
Results
Study Group Characteristics
Patients from both the study and the control group received the vaccination between January and February 2014. The follow-up time was 5 years. During the follow-up, no deaths and no cases of S. pneumoniae infections were stated. In MGUS patients, none of the patients progressed to MM, Waldenström’s macroglobulinemia, or other major oncological/hematological condition. All infections during the follow-up time were recorded. In the MGUS group, two patients had pharyngitis of adenovirus etiology twice a year from 2015, two patients – pharyngitis of respiratory syncytial virus (RSV) etiology, two patients had parainfluenza virus infection, one patient had bronchitis of Mycoplasma pneumoniae in 2017, and one bronchitis of Chlamydophila pneumonia in 2018. In the control group, two patients had urinary bladder infection of Escherichia coli etiology twice a year from 2016, two patients had pharyngitis of RSV etiology in 2016 and in 2018, one patient had pharyngitis of adenovirus etiology in 2017, and one patient had bronchitis of Mycoplasma pneumoniae etiology. All bacterial infections were treated with targeted antibiotic therapy.
The response to vaccination with PCV13 was assessed by determining the concentration of anti-pneumococcal antibodies. A positive response was defined as a minimum twofold increase in the baseline concentration of anti-pneumococcal antibodies, as described previously.9,26,27 This response was obtained by 95% of MGUS patients and 100% of healthy controls. The difference in the response to vaccination was not statistically significant (p=0.7). No side effects related to the vaccination were reported in either the control group or the study group.
Specific Anti-Pneumococcal Antibodies
The concentration of specific anti-pneumococcal antibodies before vaccination did not differ significantly in MGUS patients compared with controls either before (p=0.57) or after (p=0.48) vaccination. The concentration of specific anti-pneumococcal antibodies in both groups increased statistically significantly after vaccination (p<0.0001 for both groups) (Table 2).
Table 2.
Specific Anti-Pneumococcal Antibody Concentrations In Patients With MGUS And Control Group Before And After PCV13 Vaccination
| Specific Anti-Pneumococcal Antibodies Concentration (mU/mL) |
MGUS Patients Before Vaccination | MGUS Patients After Vaccination | Control Group Before Vaccination | Control Group After Vaccination |
|---|---|---|---|---|
| Mean ± SD | 401.6±286.1 | 2824.7±1499.9 | 491.4±521.1 | 2825.4±1316.4 |
| Median | 303.55 | 3127.3 | 297.3 | 3197 |
| Min.–Max. | 79–1018.5 | 230.5–4400 | 82.2–1919.9 | 748.2–4400 |
| p-value | p<0.0001 | p<0.0001 | ||
Abbreviations: MGUS, monoclonal gammopathy of undetermined significance; PCV13, 13-valent pneumococcal conjugate vaccine; SD, standard deviation.
Plasmablast Percentages
Next, the population of plasmablasts was evaluated in the MGUS patients and in the control group. The percentage of plasmablasts before vaccination in the MGUS group was statistically significantly higher compared with the control group (p<0.0001). The percentage of plasmablasts after vaccination in the MGUS group and the control group increased statistically significantly (p=0.0002 and p=0.0001, respectively) (Table 3). After vaccination, the percentage of plasmablasts in the MGUS patients and control group did not differ statistically significantly (p=0.7211).
Table 3.
Percentage Of Plasmablasts In Patients With MGUS And Control Group Before And After PCV13 Vaccination
| Plasmablasts (%) | MGUS Patients Before Vaccination | MGUS Patients After Vaccination | Control Group Before Vaccination | Control Group After Vaccination |
|---|---|---|---|---|
| Mean ± SD | 1.605±1.268 | 15.667±19.993 | 0.349±0.200 | 13.014±15.004 |
| Median | 1.435 | 5.855 | 0.26 | 6.95 |
| Min.–Max. | 0.47–6.81 | 2.06–69.6 | 0.14–0.72 | 1.73–47.28 |
| p-value | p=0.0002 | p=0.0001 | ||
Abbreviations: MGUS, monoclonal gammopathy of undetermined significance; PCV13, 13-valent pneumococcal conjugate vaccine; SD, standard deviation.
Median Response Expressed As The Concentration Of Anti-Pneumococcal Antibodies After PCV13
In the MGUS group, the median response expressed as the concentration of anti-pneumococcal antibodies after PCV13 vaccination was 3,127.35 mU/mL. Because only one patient with MGUS did not fulfil the criterion of a positive response to vaccination (i.e., two-fold post-vaccination anti-pneumococcal antibodies increase), to analyze possible differences in clinical characteristics related to vaccine response, selected parameters were compared between MGUS patients above and below the median response. The results are shown below.
In the group of MGUS patients above the median response, the serum M protein concentration was 0.57 g/dL±0.36 g/dL (median=0.6 g/dL; min. 0.1 g/dL; max. 1.37 g/dL). It was statistically significantly lower (p=0.0418) than in the MGUS patients below the median response, who had a serum M protein concentration of 1.09 g/dL±0.67 g/dL (median=0.78 g/dL; min. 0.4 g/dL; max: 2.78 g/dL).
Then, the increase in the percentage of plasmablasts after vaccination in the MGUS patients above and below the median response was compared. The increase in the percentage was defined as the difference in the percentage of plasmablasts after and before vaccination. A statistically significantly greater change in the percentage of plasmablasts after PCV13 vaccination was found in the MGUS group above the median response (p=0.0488).
The increase in the concentration of antibodies after vaccination compared with the pre-vaccination concentration was also statistically significantly greater in the MGUS group above the median response relative to the MGUS group below the median response (p<0.0001).
When assessing the concentration of IgG2, a statistically significant increase was demonstrated after vaccination in MGUS patients above (p=0.0186) and below (p=0.0244) the median response.
Correlations
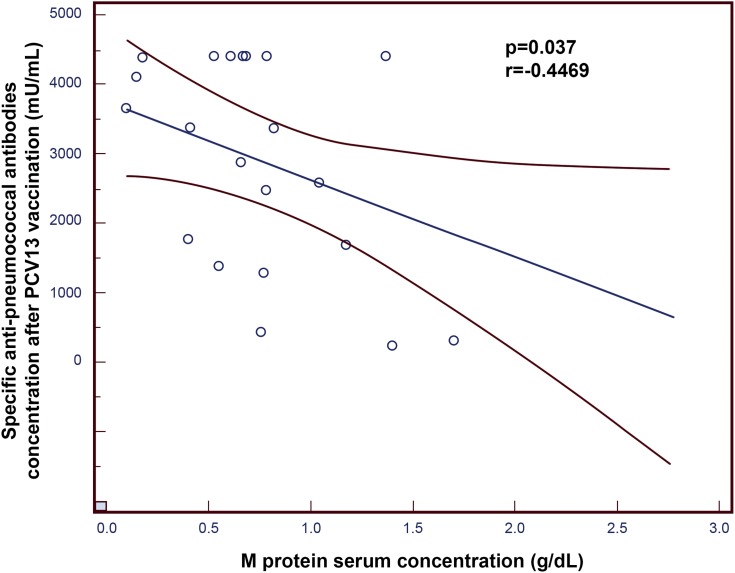
There was a statistically significant negative correlation between the concentration of specific anti-pneumococcal antibodies after vaccination and the concentration of M protein in the serum of patients with MGUS (p=0.037; r=−0.4469; Figure 1).
Figure 1.
Negative correlation between the concentration of specific anti-pneumococcal antibodies after 13-valent pneumococcal conjugate vaccine (PCV13) vaccination and the concentration of M protein in the serum of patients with monoclonal gammopathy of undetermined significance (MGUS).
Similarly, there was a statistically significant negative correlation between the increase in specific anti-pneumococcal antibodies after vaccination (when compared with pre-vaccination antibody titer) and serum M protein levels in patients with MGUS (p=0.0356; r=−0.4501; Figure 2).
Figure 2.
Negative correlation between the difference in the concentration of anti-pneumococcal antibodies after vs before 13-valent pneumococcal conjugate vaccine (PCV13) vaccination, and the concentration of M protein in the serum of patients with monoclonal gammopathy of undetermined significance (MGUS).
In addition, the relationships between the effectiveness of vaccination and parameters such as β2 microglobulin, LDH, albumin concentration, total protein concentration, calcium and phosphorus concentration, FLC, FLC ratio, and plasma cell infiltration were investigated. However, no relationships were found.
Discussion
Numerous studies have confirmed the association of MGUS with infections, particularly pneumonia, infections caused by viruses (cytomegalovirus, Epstein-Barr virus, chronic viral hepatitis), and tuberculosis.28 Additionally, the mean survival for patients with MGUS is shorter compared with the healthy population. The major reason for early mortality in this group is infection. Despite a frequently asymptomatic clinical course, MGUS leads to the development of secondary immunodeficiency. The incidence of infections in patients with MGUS is twice as high as that of the healthy population. Patients with MGUS have a 2.4-fold higher risk of pneumonia compared with healthy subjects,5 and an association between infections and rapid progression from MGUS to MM was proposed.29 Due to the shorter expected survival, greater incidence of infections, and the potential impact of infection on the progression from MGUS to MM, it seems reasonable to search for methods to prevent dangerous complications of infectious diseases, such as pneumococcal pneumonia.
In the present study, patients with MGUS were vaccinated with PCV13. A positive response to vaccination was observed in 95% of patients with MGUS, and this result did not differ significantly from the control group. Concentrations of specific anti-S. pneumoniae antibodies did not differ significantly among both groups, either before or after vaccination. In both groups, a statistically significant increase in the concentration of specific anti-S. pneumoniae antibodies was observed after vaccination. Prior to vaccination, the frequency of plasmablasts was significantly higher in patients with MGUS compared with the control group, which may be the result of the ongoing neoplastic process in the course of MGUS. The percentage of plasma cells in the bone marrow in patients with MGUS may be slightly increased (however not exceeding 10%)30 and is perhaps related to the presence of a higher proportion of plasmablasts in the peripheral blood of these patients. We did not find any available literature concerning this issue. Comparison of the frequencies of plasmablasts after vaccination in patients and controls did not reveal any statistically significant differences. We observed an adequate raise of plasmablasts on the 7th day after vaccination in both groups, which indicates that early stimulation of the immune system was appropriate.14 Next, we divided MGUS patients into 2 groups; the cut-off point for their separation was the median level of specific antipneumococcal antibodies after vaccination. The group of patients with higher levels of antibodies had a lower serum concentration of M protein. This group also had a greater difference between the pre- and postvaccination antibody titers, which indicates a better immune response. Both groups experienced a statistically significant increase in the serum IgG2 level after vaccination. Also, both the concentration of specific anti-pneumococcal antibodies and the increase in the concentration of their titers pre- vs postvaccination in the whole population of MGUS patients correlated negatively with the concentration of M protein. At the same time, no relationship between the percentage of plasmacytes in the bone marrow and the FLC ratio, and the parameters evaluating the response to vaccination were found. In this study, however, we were not able to determine the concentration of antibodies following the vaccination that is enough to protect against S. pneumoniae in patients with MGUS, as no cases of S. pneumoniae infection were observed over the 5-year follow-up. Additionally, antigens with a protein carrier, present in a PCV13 vaccine, elicit a T-cell-dependent antibody response. Therefore, apart from induction of antibody production, stimulation of the cell immune response is also present post-vaccination.31,32
Based on the analyses carried out, it can be concluded that the response to immunization worsens with the increase of M protein in the serum. This dependence agrees with the observations of Kristinsson et al who found that the risk of infection is higher in patients with MGUS in whom the M protein concentration exceeds 2.5 g/dL.5 The increase in the risk of bacteremia has also been demonstrated by Gregersen et al.33 Karlsson et al also demonstrated that the concentration of specific anti-pneumococcal antibodies in MGUS patients was significantly lower compared with healthy controls. However, it was higher compared with MM patients. The same study showed that the specific anti-Moraxella catarrhalis, anti-Candida sp., anti-Aspergillus sp., and anti-measles virus antibodies were similarly decreased in MGUS and MM patients.34 This may indicate a gradual impairment of the immune system with the progression of the disease from MGUS to MM. The lack of available literature on the effectiveness of vaccinations among patients with MGUS suggests the need to deepen the study on this subject and plan further research on a larger group of patients. This is particularly important because, as Augustson et al showed in their research, 45% of early deaths (deaths that occurred up to 60 days from the diagnosis of MM) are caused by infections, mainly by pneumonia and sepsis.35 It is likely that the introduction of preventive vaccinations against S. pneumoniae, inter alia, for patients with MGUS would contribute to mortality reduction in those patients in whom MGUS progresses to MM. Although anti-pneumococcal vaccine is recommended in MGUS and MM patients, vaccination coverage is low. Preliminary data from the Insight-MM study, including 650 patients with MM, revealed that only 17% of newly diagnosed and 24% of relapsed/refractory MM patients received the recommended anti-pneumococcal vaccination in the past 5 years.36 Our observations on the relationship between the response to vaccination and the concentration of M protein in the blood serum indicate that the immunodeficiency worsens as MGUS progresses.
Patients diagnosed with MGUS are not a homogenous group. They include patients with stable or low levels of M protein, as well as patients in whom quite rapid transformation into MM or Waldenström’s macroglobulinemia is observed. Therefore, immune system suppression in this population varies in individual patients.34 One of the factors contributing to the unfavorable prognosis in the Mayo Clinic risk assessment model is serous M protein concentration exceeding 1.5 g/dL.37 In light of the presented results, it may also be a predictor of the response to vaccination, although further research is required to confirm this.
A limitation of our study is related to the bacterial serotypes. Immunization was carried out with PCV13, which contains 13 capsular antigens, although 23 vaccine serotypes exist. We suspect the difference in antibody titers we observed before and after vaccination correspond to the antibodies against the PCV13 serotypes. However, we did not assess the serum titers of serotype-specific antibodies, but only measured the overall subclasses of the total antibodies in the serum. Therefore, we plan to assess serotype-specific antibodies (i.e., IgG1, IgG2, IgG3, and IgG4) in future work, both in MGUS patients and controls. A small sample size can be recognized as another limitation of this study. Strict inclusion criteria applied, mainly older age and lack of severe comorbidities, rendered it impossible to enroll a larger group of patients.
Conclusion
PCV13 vaccination in MGUS patients is safe and effective in protecting against S. pneumoniae infection. Vaccination should be carried out as soon as possible after the diagnosis. It can protect patients against serious infectious complications, which may contribute to extending the time to progression and transformation into more aggressive diseases like MM. In patients with MGUS who have a lower concentration of M protein, PCV13 vaccination is more effective. Serum M protein concentration in patients diagnosed with MGUS may be a useful predictor of the effectiveness of vaccination.
Funding Statement
This work was supported by the Polish National Science Center (grant no. UMO-2016/21/B/NZ6/02279) and the Medical University of Lublin (grant no. DS460). The sponsors had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Ethics Approval And Informed Consent
The study protocol was approved by the Bioethics Committee of the Regional Chamber of Physicians in Kielce (No. KB7/2012). All participants gave their prior written informed consent for participation. The study was conducted in accordance with the Declaration of Helsinki.
Author Contributions
All authors contributed to data analysis, drafting, and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Babel N, Schwarzmann F, Pruss A, Volk HD, Reinke P. Monoclonal gammopathy of undetermined significance (MGUS) is associated with an increased frequency of Epstein-Barr Virus (EBV) latently infected B lymphocytes in long-term renal transplant patients. Transplant Proc. 2004;36(9):2679–2682. doi: 10.1016/j.transproceed.2004.09.046 [DOI] [PubMed] [Google Scholar]
- 2.Tete SM, Bijl M, Sahota SS, Bos NA. Immune defects in the risk of infection and response to vaccination in monoclonal gammopathy of undetermined significance and multiple myeloma. Front Immunol. 2014;5:257. doi: 10.3389/fimmu.2014.00257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kyle RA, Therneau TM, Rajkumar SV, et al. Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med. 2006;354(13):1362–1369. doi: 10.1056/NEJMoa054494 [DOI] [PubMed] [Google Scholar]
- 4.Blade J, Rosinol L, Cibeira MT, de Larrea CF. Pathogenesis and progression of monoclonal gammopathy of undetermined significance. Leukemia. 2008;22(9):1651–1657. doi: 10.1038/sj.leu.2404889 [DOI] [PubMed] [Google Scholar]
- 5.Kristinsson SY, Tang M, Pfeiffer RM, et al. Monoclonal gammopathy of undetermined significance and risk of infections: a population-based study. Haematologica. 2012;97(6):854–858. doi: 10.3324/haematol.2011.054015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Isturiz RE, Schmoele-Thoma B, Scott DA, et al. Pneumococcal conjugate vaccine use in adults. Expert Rev Vaccines. 2016;15(3):279–293. doi: 10.1586/14760584.2016.1132171 [DOI] [PubMed] [Google Scholar]
- 7.European Medicines Agency. Human medicine European public assessment report (EPAR): Prevenar 13. Amsterdam, The Netherlands: European Medicines Agency; 2009. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/001104/WC500152448.pdf. Accessed December20, 2018.
- 8.Bonten M, Bolkenbaas M, Huijts S, et al. Community acquired pneumonia immunisation trial in adults (CAPiTA). Pneumonia. 2014;3(ISPPD special issue):95. [Google Scholar]
- 9.Pasiarski M, Rolinski J, Grywalska E, et al. Antibody and plasmablast response to 13-valent pneumococcal conjugate vaccine in chronic lymphocytic leukemia patients – preliminary report. PLoS One. 2014;9(12):e114966. doi: 10.1371/journal.pone.0114966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kyle RA, Durie BG, Rajkumar SV, et al.; for International Myeloma Working Group. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia. 2010;24(6):1121–1127. doi: 10.1038/leu.2010.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cabrera Q, Macro M, Hebert B, Cornet E, Collignon A, Troussard X. Epidemiology of monoclonal gammopathy of undetermined significance (MGUS): the experience from the specialized registry of hematologic malignancies of Basse-Normandie (France). Cancer Epidemiol. 2014;38(4):354–356. doi: 10.1016/j.canep.2014.04.006 [DOI] [PubMed] [Google Scholar]
- 12.Rajkumar SV. Updated diagnostic criteria and staging system for multiple myeloma. Am Soc Clin Oncol Educ Book. 2016;35:e418–23. doi: 10.1200/EDBK_159009 [DOI] [PubMed] [Google Scholar]
- 13.Chovancova Z, Vlkova M, Litzman J, Lokaj J, Thon V. Antibody forming cells and plasmablasts in peripheral blood in CVID patients after vaccination. Vaccine. 2011;29(24):4142–4150. doi: 10.1016/j.vaccine.2011.03.087 [DOI] [PubMed] [Google Scholar]
- 14.He XS, Sasaki S, Narvaez CF, et al. Plasmablast-derived polyclonal antibody response after influenza vaccination. J Immunol Methods. 2011;365(1–2):67–75. doi: 10.1016/j.jim.2010.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Camous X, Visan L, Ying CTT, et al. Healthy elderly Singaporeans show no age-related humoral hyporesponsiveness nor diminished plasmablast generation in response to influenza vaccine. Immun Ageing. 2018;15:28. doi: 10.1186/s12979-018-0137-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ju CH, Blum LK, Kongpachith S, et al. Plasmablast antibody repertoires in elderly influenza vaccine responders exhibit restricted diversity but increased breadth of binding across influenza strains. Clin Immunol. 2018;193:70–79. doi: 10.1016/j.clim.2018.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Obermoser G, Presnell S, Domico K, et al. Systems scale interactive exploration reveals quantitative and qualitative differences in response to influenza and pneumococcal vaccines. Immunity. 2013;38(4):831–844. doi: 10.1016/j.immuni.2012.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rahman A, Rashu R, Bhuiyan TR, et al. Antibody-secreting cell responses after Vibrio cholerae O1 infection and oral cholera vaccination in adults in Bangladesh. Clin Vaccine Immunol. 2013;20(10):1592–1598. doi: 10.1128/CVI.00347-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lavinder JJ, Wine Y, Giesecke C, et al. Identification and characterization of the constituent human serum antibodies elicited by vaccination. Proc Natl Acad Sci USA. 2014;111(6):2259–2264. doi: 10.1073/pnas.1317793111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Mens SP, Meijvis SC, Endeman H, et al. Longitudinal analysis of pneumococcal antibodies during community-acquired pneumonia reveals a much higher involvement of Streptococcus pneumoniae than estimated by conventional methods alone. Clin Vaccine Immunol. 2011;18(5):796–801. doi: 10.1128/CVI.00007-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pauksens K, Nilsson AC, Caubet M, et al. Randomized controlled study of the safety and immunogenicity of pneumococcal vaccine formulations containing PhtD and detoxified pneumolysin with alum or adjuvant system AS02V in elderly adults. Clin Vaccine Immunol. 2014;21(5):651–660. doi: 10.1128/CVI.00807-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kitchin N, Remich SA, Peterson J, et al. A phase 2 study evaluating the safety, tolerability, and immunogenicity of two 3-dose regimens of a clostridium difficile vaccine in healthy US adults aged 65 to 85 years. Clin Infect Dis. 2019; ciz153 Epub 2019 May 24. doi 10.1093/cid/ciz153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang L, Chen Z, Hu Y, et al. Safety, immunogenicity, and lot-to-lot consistency of live attenuated varicella vaccine in 1-3 years old children: a double-blind, randomized phase III trial. Hum Vaccin Immunother. 2019;15(4):822–827. doi: 10.1080/21645515.2018.1551701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vanderkooi OG, Scheifele DW, Girgenti D, et al.; for Canadian PCV13 Study Group. Safety and immunogenicity of a 13-valent pneumococcal conjugate vaccine in healthy infants and toddlers given with routine pediatric vaccinations in Canada. Pediatr Infect Dis J. 2012;31(1):72–77. doi: 10.1097/INF.0b013e318233049d. [DOI] [PubMed] [Google Scholar]
- 25.Nelson LS, Steussy B, Morris CS, Krasowski MD. Effect of specimen type on free immunoglobulin light chains analysis on the Roche Diagnostics cobas 8000 analyzer. Springerplus. 2015;4:760. doi: 10.1186/s40064-015-1546-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hartkamp A, Mulder AH, Rijkers GT, van Velzen-Blad H, Biesma DH. Antibody responses to pneumococcal and haemophilus vaccinations in patients with B-cell chronic lymphocytic leukaemia. Vaccine. 2001;19(13–14):1671–1677. doi: 10.1016/s0264-410x(00)00409-6 [DOI] [PubMed] [Google Scholar]
- 27.Safdar A, Rodriguez GH, Rueda AM, et al. Multiple-dose granulocyte-macrophage-colony-stimulating factor plus 23-valent polysaccharide pneumococcal vaccine in patients with chronic lymphocytic leukemia: a prospective, randomized trial of safety and immunogenicity. Cancer. 2008;113(2):383–387. doi: 10.1002/cncr.23561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bida JP, Kyle RA, Therneau TM, et al. Disease associations with monoclonal gammopathy of undetermined significance: a population-based study of 17,398 patients. Mayo Clin Proc. 2009;84(8):685–693. doi: 10.4065/84.8.685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Landgren O, Rapkin JS, Mellemkjaer L, Gridley G, Goldin LR, Engels EA. Respiratory tract infections in the pathway to multiple myeloma: a population-based study in Scandinavia. Haematologica. 2006;91(12):1697–1700. [PubMed] [Google Scholar]
- 30.Rajkumar SV, Dimopulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):538–548. doi: 10.1016/S1470-2045(13)70510-2 [DOI] [PubMed] [Google Scholar]
- 31.Shah D, Mustafa S, Bress J, Jamshed S. Comparison of response to vaccination with PCV 13 in patients with multiple myeloma versus healthy controls. Blood. 2017;130(Suppl 1):5418. [Google Scholar]
- 32.Hayward S, Thompson LA, McEachern A. Is 13-valent Pneumococcal Conjugate Vaccine (PCV13) combined with 23-valent Pneumococcal Polysaccharide Vaccine (PPSV23) superior to PPSV23 alone for reducing incidence or severity of pneumonia in older adults? A clin-IQ. J Patient Cent Res Rev. 2016;3(2):111–115. doi: 10.17294/2330-0698.1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gregersen H, Madsen KM, Sørensen HT, Schønheyder HC, Ibsen JS, Dahlerup JF. The risk of bacteremia in patients with monoclonal gammopathy of undetermined significance. Eur J Haematol. 1998;61(2):140–144. doi: 10.1111/j.1600-0609.1998.tb01075.x [DOI] [PubMed] [Google Scholar]
- 34.Karlsson J, Andréasson B, Kondori N, et al. Comparative study of immune status to infectious agents in elderly patients with multiple myeloma, Waldenstrom’s macroglobulinemia, and monoclonal gammopathy of undetermined significance. Clin Vaccine Immunol. 2011;18(6):969–977. doi: 10.1128/CVI.00021-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Augustson BM, Begum G, Dunn JA, et al. Early mortality after diagnosis of multiple myeloma: analysis of patients entered onto the United Kingdom Medical Research Council trials between 1980 and 2002–Medical Research Council Adult Leukaemia Working Party. J Clin Oncol. 2005;23(36):9219–9226. doi: 10.1200/JCO.2005.03.2086 [DOI] [PubMed] [Google Scholar]
- 36.Terpos E, Chari A, Rifkin RM, et al. Uncovering the blind spot of clinical trials: first report of baseline characteristics of Newly Diagnosed (ND) and Relapsed/Refractory (RR) Multiple Myeloma (MM) Patients (Pts) in Insight-MM, a Global, Prospective, Observational Study. Blood. 2017;130(Suppl 1):5419. [Google Scholar]
- 37.Merlini G, Palladini G. Differential diagnosis of monoclonal gammopathy of undetermined significance. Hematology Am Soc Hematol Educ Program. 2012;2012:595–603. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- European Medicines Agency. Human medicine European public assessment report (EPAR): Prevenar 13. Amsterdam, The Netherlands: European Medicines Agency; 2009. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/001104/WC500152448.pdf. Accessed December20, 2018.