Abstract
The aim of this study was to quantify bisphenol A (BPA) concentrations in saliva and urine before and after treatment with dental polymer‐based restorative materials to assess if placement of this material is associated with increased BPA levels in saliva and urine. Twenty individuals in need of at least one dental restoration with polymer‐based restorative material were included in this study. The participants were instructed to abstain from eating, drinking, and brushing their teeth for at least 10 h prior to sampling. Saliva and urine were collected before and 10 min (saliva only), 1 h, 24 h, and 1 wk after treatment. Samples were stored at −80°C before analyses. BPA in saliva and urine was determined with liquid chromatography/mass spectrometry. Linear mixed effects regression models were used for statistical analyses. There was a statistically significant increase of salivary BPA concentration directly after placement of the dental polymer‐based restorations. Following placement, the concentration of BPA decreased exponentially with time. One week after treatment the BPA level in saliva was only marginally higher than before treatment. In urine, no statistically significant change of the BPA concentration was detected after treatment.
Keywords: composite filling, exposure to BPA, resin‐based material, saliva, urine
Bisphenol A (BPA; CAS no. 80‐05‐7) is a synthetic chemical substance, produced in large quantities and widely used in the production of polycarbonate plastics, epoxy resins, dental monomers, thermal paper, and numerous other products 1. Bisphenol A is known as an endocrine disruptor with the ability to interfere with, and mimic, estrogenic hormones 2, 3, 4. Concern has been raised about low‐level exposure to BPA and the possible association with adverse health effects. In‐vitro and animal studies have linked BPA exposure to a variety of negative outcomes 5. In several epidemiological studies, BPA levels in human populations have been associated with reproductive abnormalities, adverse developmental effects, metabolic disease, and breast cancer, among other health conditions 6, 7, 8. Although these findings are controversial 9, the current opinion of risk‐assessment agencies, such as the European Food Safety Authority (EFSA), is that negative health effects from BPA exposure cannot be excluded 10. Of greatest concern is potential exposure during vulnerable periods, such as fetal and early postnatal development 4. In humans, the free (unconjugated) estrogenic form of BPA generally is conjugated to a non‐estrogenic form via ‘the first pass metabolism’ in the liver and is eliminated in urine 11. However, the ability to metabolize and excrete BPA from the body may not be fully developed in the fetus and the neonate 12. In January 2015, the EFSA revised the recommended limit of ‘tolerable daily intake’ of BPA from 50 to 4 μg kg−1 of bodyweight per day 10 and in December 2017 the European Chemicals Agency reclassified BPA as a chemical of very high concern 13. In November 2018, a working group from the EFSA started re‐evaluating the potential hazards of BPA in food based on studies and data published after 2012. This new assessment is expected to be completed in 2020 (https://www.efsa.europa.eu/en/press/news/180904, downloaded 22.02.2019).
There is wide interest in the sources of BPA exposure. The primary source of human exposure is assumed to be through the diet because BPA can leach into food and beverages from containers made of polycarbonate plastic or lined with epoxy resin coatings 1, 14, 15. However, results from studies have indicated human exposure also from numerous non‐dietary sources, including dust and indoor air, thermal paper, cosmetics, and dental materials 1, 10, 15, 16.
In dentistry, BPA is used as a raw material in the synthesis of several resin monomers and may be found as an impurity in dental materials 17. The most frequently used monomers synthesized from BPA include bisphenol A glycidyl methacrylate (Bis‐GMA; CAS no. 1565‐94‐2), bisphenol A ethoxylate dimethacrylate (Bis‐EMA, CAS no. 41637‐38‐1), and bisphenol A dimethacrylate (Bis‐DMA; CAS no. 3253‐39‐2) 18, 19. It has been shown that Bis‐DMA‐based materials, such as Delton LC fissure sealant (Ash/Dentsply, York, PA, USA), may release BPA as a result of hydrolysis at the ester bond 20, whereas Bis‐GMA‐based materials, which typically comprise restorative materials, do not undergo this form of biodegradation because its ether bond is resistant to hydrolysis 20, 21, 22, 23, 24. Several in‐vitro and some in‐vivo studies have focused on BPA leakage from dental polymer‐based filling materials and have attempted to quantify the amounts detected in different solutions or biological media 17, 19, 25, 26, 27, 28, 29, 30. However, the amounts reported vary, probably because of differences in the materials examined and the methodological approaches 19, 31. Moreover, exposure from other sources, e.g., participants' diet and its contribution to the BPA concentration in biological samples (e.g., urine), has not been thoroughly considered in previous clinical studies.
The aim of this study was to quantify BPA concentrations in saliva and urine, before and after treatment with dental polymer‐based restorative materials, to assess if placement of these materials is associated with increased BPA levels in saliva and urine.
Material and methods
Study population
Patients in need of at least one dental restoration, involving two or more tooth surfaces, with a polymer‐based restorative material were informed about the study by their dental hygienist or dentist at their regular dental examination at two public dental clinics in Bergen, Norway. Patients who chose to participate were given written information about the study for perusal at home. Individuals with removable dentures, dental splints, and those who currently were undergoing orthodontic treatment were excluded. Smokers, snuff users, and drug abusers were also excluded. We did not include individuals who had received polymer‐based dental fillings during the previous 3 months, dental students, and dental health workers. Twenty volunteers, aged between 16 and 40 yr, without any known diseases or medications at the time of the study, were included in the study from January 2016 to November 2017. All participants provided written informed consent.
Ethical approval
All procedures involving human participants were performed in accordance with the ethical standards of the 1964 Helsinki declaration and its later amendments, or comparable ethical standards. The study protocol was reviewed and approved by The Regional Committee for Medical Research Ethics, South‐East Norway (reference number 2014/1529). The study is registered at ClinicalTrials.gov (no.: NCT02575118).
Dental treatment procedures
One dentist (TLLB) recorded the number of tooth surfaces previously filled with tooth‐colored restorative materials. The same dentist (TLLB) provided the dental treatment at a public dental clinic in Bergen, Norway. The treatment was performed according to standardized procedures and materials used at the clinic. Local anesthesia (Xylocain Dental adrenalin; Dentsply, Weybridge, UK) was used in 19 of the participants; one participant preferred treatment without anesthesia. Cavity preparations were performed with diamond burs (Horico, Berlin, Germany) and round steel burs (Meisinger, Neuss, Germany). Cotton rolls and low‐volume evacuator equipment (Hygoformic Saliva Ejector; Orsing, Helsingborg, Sweden) were used for moisture control. High‐volume evacuator equipment (Hygovac aspirator tube; Orsing) was used during cavity preparations and etching, bonding, and finishing procedures. Rubber dam isolation was not used. Contoured anatomical steel matrices (Polydentia, Mezzovico, Switzerland) were used to support and shape the restorations in premolars/molars, and transparent curved strips (Hawe Neos; Kerr, Orange, CA, USA) were used in front teeth. To create a tighter seal, preventing gingival overhang, dental approximal wooden wedges (Hawe Sycamore; Kerr) were used in all cases. Etching with 37% phosphoric acid (ANA Etching Gel 37%; Directa, Upplands Väsby, Sweden) was performed according to the principles of the total‐etch technique 32. A two‐part primer adhesive system (OptiBond FL; Kerr) was used as the bonding agent. The cavities were restored with a widely used filling material (Tetric EvoCeram, 0.2 g compules, Color A2, LOT 14504; Ivoclar Vivadent, Schaan, Liechtenstein), tested to be assured it was Bis‐GMA‐based. The bonding procedure and the application of filling material were carried out according to the manufacturers’ instructions. For each participant, a new compule with filling material was used. The material was applied in incremental layers of <2.0 mm, and each layer was cured for 20–30 s. Care was taken to avoid application of excessive amounts of material. Any surplus was removed and put back into the compule. Each compule was weighed before and after treatment, using an analytical balance (AG204 DeltaRange; Mettler Toledo, Greifensee, Switzerland). The amount (weight in g) of polymer‐based material used in each participant was estimated by the difference between the two measurements. The curing lamp (Satelec Mini LED; Aceton, Meriganac, France) emitted 600–700 mW cm−2 light intensity at a range of 440–460 nm. The lamp was controlled prior to each treatment using a hand‐held curing radiometer (Model 100, Dementron; Kerr, Danbury, CT, USA). After curing, the fillings were polished according to standard procedures using polishing diamond burs (Foss Viking, Fetsund, Norway), polishing disks (Sof‐Lex XT Pop; 3M Espe, St Paul, MN, USA), and silicone polishers (Identoflex Composite Polisher; Kerr, Bioggio, Switzerland).
The restorations differed in size depending on the tooth size and the extent of the prepared lesion. To adjust for differences, each filling surface was given scores from 1 to 3, depending on its area 33. Small restorations were given the lowest score of 1. Restorations of intermediate size, typically the approximal or occlusal surfaces of Class II restorations in premolars, were given a score of 2. The highest score, 3, was used for molars to denote restorations extending over the total occlusal fissure pattern or over the approximal surface of Class II restorations. The scores for all polymer‐based filling surfaces treated in each patient were summed and yielded the variable ‘filling points’. The tooth surfaces treated and the estimated ‘filling points’ were recorded.
Sample collection
All treatment sessions were scheduled in the morning before 9 am. Prior to the treatment, the participants were asked about their dental hygiene habits and if they had work that involved handling of receipts (thermal paper). Each participant provided a total of five saliva samples and four urine samples. The first saliva and urine samples were collected immediately before treatment, after a 10‐h fast. Sampling of a second saliva sample was started 10 min after placement of the polymer‐based fillings, and subsequent saliva and urine samples were collected 1 h, 24 h, and 1 wk after placement of the fillings (Fig. 1). On each day of sampling, the participants also answered questions regarding consumption of canned and microwaved food during the previous week and within the previous 24 h. To reduce exposure from other potential sources of BPA, the participants were instructed to abstain from eating, drinking, and brushing their teeth for at least 10 h prior to sampling. Only tap‐water was allowed for drinking. The participants were asked not to use lip balm or lipstick during the same period. To identify possible contamination during sampling, transport, and storage, field blanks were collected using ultra‐pure water (Synergy Water Purification Systems; Millipore, Billerica, MA, USA) instead of saliva and urine. The field blanks were treated like the biological samples in all aspects.
Figure 1.
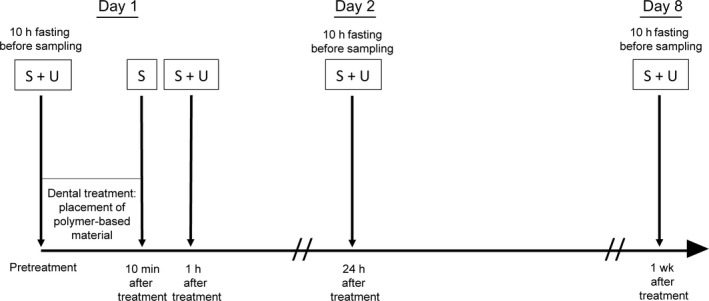
Time schedule of saliva (S) and urine (U) sampling.
For saliva sampling, the participants were sitting in a relaxed position in the dental unit chair. They were instructed to spit into the dental unit sink, but not to rinse their mouth. Immediately afterwards, they were instructed to perform active tongue and cheek movements for 60 s and then to spit the accumulated saliva into a polypropylene tube (15 ml, order number 62.554.001; Sarstedt, Nümbrecht, Germany) until about 2 ml of saliva had been sampled. To avoid contamination from the ambient air, participants were asked to put the cap back on the tube between each spit. The sampling time was recorded.
As the first morning urine void should not be collected, the participants were instructed to empty their bladder in the morning at home before entering the clinic. Urine specimens were collected in 100‐ml polypropylene cups (order number 75.562.300; Sarstedt), and aliquots were transferred into 15‐ml polypropylene tubes. Immediately after collection, the samples were refrigerated (at 4°C) and within the same day were stored frozen at −80°C until they were sent for analysis.
Determination of BPA in saliva and urine
Urine and saliva samples were analyzed at the laboratory of the Division of Occupational and Environmental Medicine at Lund University, Sweden, using liquid chromatography‐triple quadrupole mass spectrometry (LC/MS/MS; QTRAP 5500; AB Sciex, Foster City, CA, USA). Saliva samples were analyzed using a method described by berge et al. 34, and urine samples were analyzed using a modified method described by gyllenhammar et al. 35. Briefly, for the determination of total BPA in saliva, 100‐μl aliquots of saliva were digested with 10 µl glucuronidase added with 1 ng of isotopically labeled internal standard for BPA (D16‐BPA), and proteins were precipitated using acetonitrile. Free (unconjugated) BPA in saliva was determined without using glucuronidase. For the analysis of total BPA in urine samples, aliquots of 200 μl were digested with 10 µl glucuronidase and added with 1 ng D16‐BPA. The concentration of BPA in urine was adjusted for urinary density 36. Two different in‐house prepared quality control (QC) samples and chemical blanks were analyzed in the analytical batches. The limit of detection (LOD) was determined to be 0.1 ng ml−1. The method had acceptable between‐day and within‐run precision. The laboratory used is a European reference laboratory for BPA in urine (http://www.eu-bm.info/democophes) and a reference laboratory for BPA in urine in the Erlangen Round Robin inter‐laboratory control program. A detailed description of the analytical method is given in Appendix S1.
Statistical method
Continuous variables were summarized using mean values, minimum and maximum values, and standard deviations (SDs), whereas categorical variables were described using frequencies. For analyses of repeated measures for saliva and urine samples, linear mixed‐effects regression models were applied. In the mixed‐effects model, the repeated nature of the measurements of the data was accounted for using the patient ID, entered as a random factor, with an additional factor for time to account for the difference in variation (SD) over time.
Time point when a measurement was made was entered as a categorical variable in the models, comparing the succeeding measurements with the baseline (before treatment) measure. All available data, and hence data for participants with missing observations at some time points, were included in the model.
The decrease over time of the mean posttreatment BPA concentration in saliva was described using the equation: Y = aX b, where Y is the BPA concentration and X is time. The equation was estimated by applying mixed‐effects regression on a log transform of the equation (which transforms the equation to a linear model).
For the descriptive statistics, SPSS for Windows (IBM Corp, Version 25, Armonk, NY, USA) was applied, while stata for Windows (StataCorp, LLC., Version 15, College Station, TX, USA) was used for the mixed‐effect analyses. Bisphenol A values below the LOD were set to one half of the LOD in the statistical analysis 37. Values of P <0.05 were considered to indicate statistical significance.
Results
Background and dental treatment characteristics
Background characteristics of the participants are shown in Table 1. Data regarding dental hygiene habits and consumption of canned and microwaved food are listed in Table 2. Details regarding dental treatment are shown in Table 3. Three of the participants attended the study without having any pre‐existing polymer‐based dental fillings, and nine participants had polymer‐based fillings removed during treatment.
Table 1.
Background characteristics of the participants (n = 20)
| Variables | |
|---|---|
| Sex | |
| Female/Male | 13/7 |
| Age (yr) | |
| Mean (SD) | 23.4 (5.7) |
| Min‐Max | 17–36 |
| Education (yr) | |
| Mean (SD) | 13.8 (1.8) |
| Min‐Max | 10–17 |
| Daily use of chewing gum | |
| Yes/No | 4/16 |
| Daily use of toothpaste | |
| Yes/No | 20/0 |
| Use of rinsing agents | |
| Yes/No | 11/9 |
| Job handling receipts | |
| Yes/No | 5/15 |
Max, Maximum; Min, Minimum.
Table 2.
Data regarding participants’ dental hygiene habits and intake of canned and microwaved food, stratified according to sampling day (n = 20)
| Variables | Day 1 (day of treatment) | Day 2 (24 h after treatment) | Day 8 (1 wk after treatment) |
|---|---|---|---|
| Refrained from food and drink at least 10 h before sampling | |||
| Yes/No | 20/0 | 20/0 | 19/1 |
| Brushed teeth during the morning before sampling | |||
| Yes/No | 5/15 | 2/18 | 2/18 |
| Use of mouthrinse before sampling | |||
| Yes/No | 1/19 | 1/19 | 0/20 |
| Intake of canned food last 24 h | |||
| Yes/No | 4/16 | 2/18 | 4/16 |
| Intake of microwaved food last 24 h | |||
| Yes/No | 2/18 | 2/18 | 1/19 |
| Intake of canned food last week | |||
| Yes/No | 13/7 | NA | 12/8 |
| Intake of microwaved food last week | |||
| Yes/No | 7/13 | NA | 2/18 |
NA, not applicable.
Table 3.
Data regarding previous and current dental restorative treatment of the participants (n = 20)
| Variables | Mean (SD) | Min | Max |
|---|---|---|---|
| Number of pre‐existing tooth‐colored filling surfaces | 11.8 (9.6) | 0 | 26 |
| Number of pre‐existing tooth‐colored filling points | 25.7 (21.7) | 0 | 68 |
| Number of polymer‐based filling surfaces removed during treatment | 0.70 (0.92) | 0 | 3 |
| Number of polymer‐based filling points removed during treatment | 1.8 (2.7) | 0 | 9 |
| Number of treated filling surfaces | 3.7 (1.9) | 2 | 10 |
| Number of treated filling points | 7.7 (0.7) | 4 | 15 |
| Weight of polymer‐based material used (g) | 0.158 (0.067) | 0.065 | 0.309 |
Max, Maximum; Min, Minimum.
Concentration of BPA in saliva
The saliva samples collected 10 min after treatment showed a statistically significant increase in BPA levels compared with the pretreatment samples. The concentrations remained significantly elevated 1 h, 24 h, and 1 wk after placement (Table 4; Fig. 2). After the immediate post‐treatment increase, the concentration of BPA in saliva (Y; ng ml−1) decreased exponentially. We estimated the relationship between the decrease and time (X; h) to be Y = 54.2X −1.12 (Fig. 3). In saliva, no conjugated BPA was detected. Pretreatment levels of BPA in saliva were low, and the mean value was estimated to be 0.11 ng ml−1 (Table 4). Before treatment, 11 (55%) of 20 participants had salivary BPA levels below the limit of detection (0.1 ng ml−1). In one saliva sample collected before treatment, the BPA concentration was more than 100 times higher (11.6 ng ml−1) than the mean value and more than 100 SD from the mean of the remaining 19 samples. This saliva sample was excluded from the statistical analysis because it was probably contaminated. One participant had breakfast before the sample time point 1 wk after treatment, and thus the samples collected from this participant at this time point were not included in the statistical analysis.
Table 4.
Estimated bisphenol A (BPA) concentration (ng ml−1) in saliva and urine samples before and after treatment with polymer‐based dental restorative material
| Biological matrix | Time point | N | Mean | 95% CI | P value |
|---|---|---|---|---|---|
| Saliva | Before treatment | 19 | 0.11 | <LOD–0.16 | Reference |
| 10 min after treatment | 20 | 385 | 205–565 | <0.001 | |
| 1 h after treatment | 20 | 88.2 | 42.4–134 | <0.001 | |
| 24 h after treatment | 20 | 1.85 | 0.72–2.98 | 0.003 | |
| 1 wk after treatment | 19 | 0.25 | 0.17–0.33 | 0.002 | |
| Urine* | Before treatment | 20 | 1.41 | 0.42–2.41 | Reference |
| 1 h after treatment | 20 | 1.17 | 0.18–2.16 | 0.674 | |
| 24 h after treatment | 20 | 1.53 | 0.54–2.53 | 0.834 | |
| 1 wk after treatment | 19 | 1.99 | 0.97–3.00 | 0.321 |
Limit of detection (LOD) = 0.1 ng ml−1, values <0.1 ng ml−1 were set to half of the LOD.
*Density‐adjusted urine concentrations (1.016 g l−1).
Figure 2.
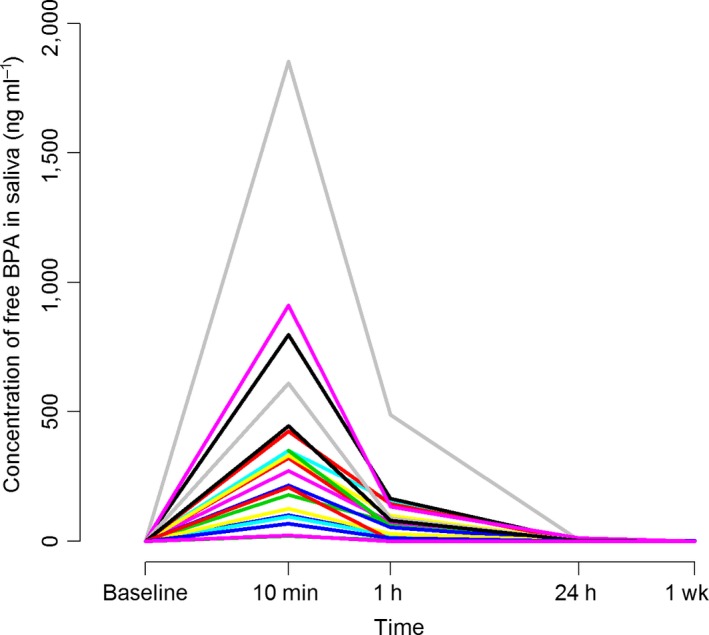
Salivary concentrations (ng ml−1) of bisphenol A (BPA) among participants (individual patterns are shown) before treatment (baseline), and 10 min, 1 h, 24 h, and 1 wk after treatment with polymer‐based filling material (n = 20).
Figure 3.
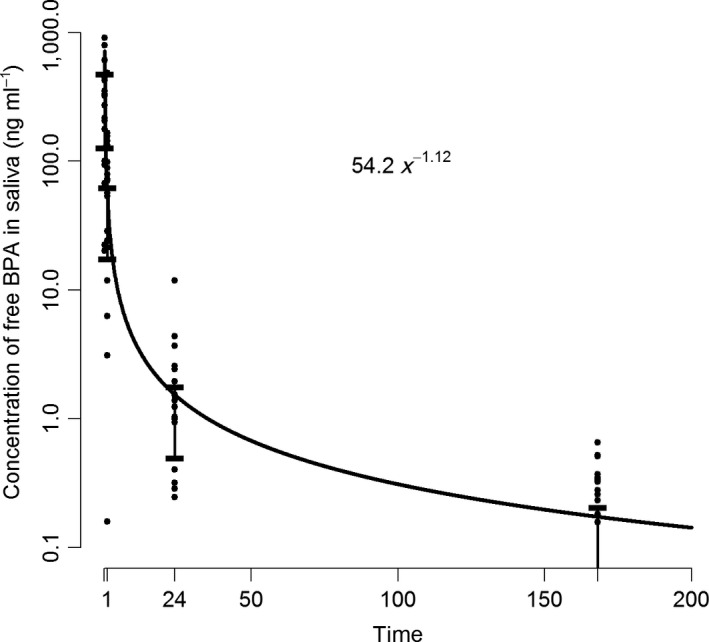
Concentration (ng ml−1) of bisphenol A (BPA) in saliva after treatment with dental polymer‐based restorative materials as a function of time (h).
The levels of BPA concentrations in saliva were confirmed by analyzing nine samples using LC‐MS/MS at an independent laboratory (Nordic Institute of Dental Materials, Oslo, Norway). These samples were selected to represent the full range of the values from the laboratory in Lund. The interclass correlation was high (0.91; 95% CI: 0.72–0.98), which indicates strong agreement between the measurements.
Secondary explorative analysis showed that the number of filling points was associated with the BPA levels in saliva, 24 h (P = 0.011) and 1 wk (P = 0.029) after treatment. However, neither the number of filling surfaces nor the amount (weight) of dental polymer‐based material placed was associated with the salivary BPA concentration at any time point (all P > 0.05). Moreover, there were no statistically significant associations between the other covariates tested and the salivary BPA levels at the different time points (see Tables 1, 2, 3 for tested variables).
Concentration of BPA in urine
Table 4 presents density‐adjusted concentrations of BPA in urine before and after treatment. Before treatment, 19 (95%) of 20 participants had detectable levels of BPA in their urine. There were no statistically significant differences between urinary BPA levels before and after placement of the dental polymer‐based restorations (Table 4; Fig. 4). The BPA levels in the urine samples collected 1 h after treatment did not show a statistically significant association with the BPA level in the saliva samples collected 10 min after treatment (data not shown).
Figure 4.
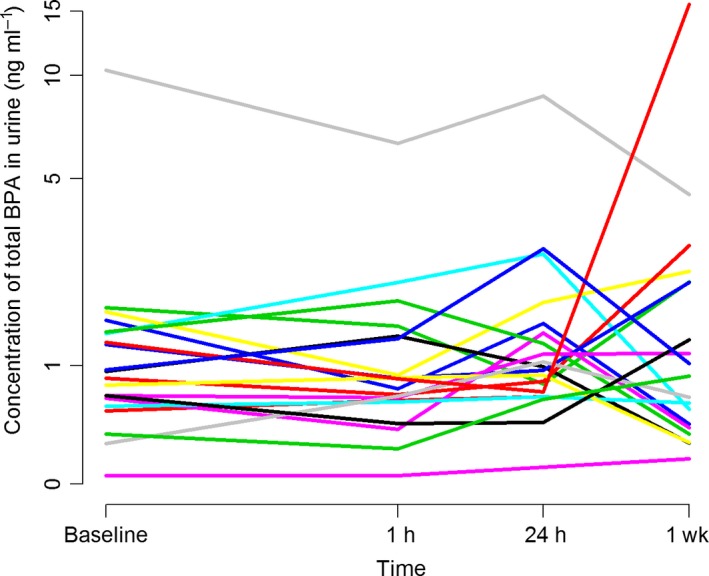
Urinary concentrations (ng ml−1) of bisphenol A (BPA) among participants (individual patterns are shown) before treatment (baseline), and 1 h, 24 h, and 1 wk after treatment with polymer‐based filling material (n = 20).
Figure 4, presenting the urinary BPA levels over time, illustrates that two of the participants showed considerably higher BPA levels than the others. One of these participants had higher BPA levels at all time points, while the other only had an elevated BPA concentration at the 1‐wk time point. These participants were identified as being two of five participants who handled cash register receipts at work. The participant with highest levels in urine was also the one with the highest levels of BPA in saliva at all time points. Using receipts as a group variable (yes, n = 5/no, n = 15) we found an overall elevated average level of urinary BPA in the group of participants who handled receipts (P = 0.031, mean difference 0.83 ng ml−1; 95% CI: 0.08–1.57).
Regression models testing additional potential factors that could contribute to the BPA concentration in urine (see Tables 1, 2, 3 for tested variables) did not show a statistically significant influence at any time point (all P > 0.05).
All field blanks had BPA concentrations below the detection limit.
Discussion
There was a considerable increase in the BPA concentration in saliva directly after placement of a dental polymer‐based dental restorative material. The concentration of BPA then decreased exponentially with time. One week after treatment the concentration of BPA in saliva was only marginally higher than before treatment. This is in agreement with the results from other studies of BPA leakage from existing polymer‐based fillings 29, 34. The time‐course of the salivary BPA concentration after treatment is in accordance with other studies and supports a plausible pattern, which suggests that the main exposure to BPA from polymer‐based dental filling materials is limited to a short period after placement 21, 26, 38, 39. In urine, no change of the BPA concentration was detected after treatment.
In the last two decades, the amounts of BPA present in saliva and urine after placement of dental polymer‐based materials have been examined in several studies 26, 28, 30, 39. However, there are wide differences in the materials tested regarding composition, brands, and application modes. Moreover, the size and number of tooth surfaces filled, sampling procedures, measurement time points and intervals, analytical methods, sensitivity of methods, and detection limits differ between studies. These differences may account for diverging results, which make comparison between studies and calculations of the actual amounts released difficult 19, 31. KLOUKOS et al. 31, when searching the literature to assess the short‐ and long‐term release of BPA in human tissues after treatment with dental sealants, concluded that only qualitative evaluation may be performed.
In the present study, the mean BPA concentration detected in saliva immediately after treatment was higher than expected. Our results are comparable with previous studies assessing salivary BPA concentrations directly after 21 and 1–3 h after 38 placement of a Bis‐DMA‐based dental fissure sealant (Delton LC). The range in BPA concentrations from these studies was 0.3–2.8 ppm and 5.8–105.6 ppb, respectively. OLEA et al. 25 reported that the cumulative salivary BPA concentration 1 h after sealant placement was approximately 100 times higher than the BPA concentration detected directly after treatment in our study. However, the reliability of the analytical method used by OLEA et al. has been questioned 22 and the levels have not been confirmed by later studies. Other authors have evaluated the release of BPA in saliva after placement of Bis‐GMA‐based dental materials used as restoratives 26, 29, 40, 41 and in orthodontic treatment, bonding lingual retainers 28, or brackets 30, 42. The salivary BPA levels reported in these studies are considerably lower than the levels reported in the present study.
The potential of dental polymer‐based restorative materials to release BPA may differ depending on the BPA derivatives included in the material. Because of the hydrolysis of Bis‐DMA, the BPA concentration in saliva collected directly after treatment with Bis‐DMA‐based materials is expected to be higher than the amount of BPA found in salivary samples collected directly after placement of Bis‐GMA‐based material 22, 24. However, the wide divergence in the salivary BPA concentration reported may have other explanations. The source of BPA in Bis‐GMA‐based materials includes residual BPA present in the raw materials used in the manufacturing process 43. The amount of BPA remaining after the synthesis of Bis‐GMA may vary between different producers. A notable batch‐to‐batch variation could also be expected if different raw materials are used 43.
The release of components from dental polymer‐based materials after curing could be a consequence of incomplete polymerization 44. During the polymerization process, most of the monomers should be converted into polymers to form a polymer network. However, the degree of conversion is reported never to be complete 45, 46. The extent to which monomers are converted may be influenced by several factors, including the curing time, the light intensity, the composition of the polymer‐based material, and the thickness of the incremental layer 47. In the present study, the dental polymer‐based material was placed in layers and cured according to the manufacturer's instructions. No association was found between the amount (weight) of material placed and salivary BPA concentration. This finding is in agreement with other studies and may be explained by the observation period used in the present study, which was too short to allow degradation and subsequent release of unbound compounds trapped in the polymer network 17. However, the exposure of materials to air inhibits polymerization and leads to a thin, liquid layer on the filling surface 48, 49. From this layer, uncured components have the potential to leak immediately into the oral environment for a short period of time 17. Some studies have indicated that the release of BPA from dental polymer‐based fillings depends on the extent of the exposed filling surface 50, 51. Our study found no association between the surface area of the polymer‐based material placed and the salivary BPA levels immediately after and 1 h after treatment. However, 24 h and 1 wk after treatment, the BPA concentration was associated with the number of filling points.
Moisture control during restorative dental treatment may include cotton rolls and a rubber dam combined with a saliva ejector. It has been reported that the increase in salivary BPA concentrations directly after placement of dental polymer‐based material may be lower when a rubber dam is used 26; however, studies indicate that most dentists do not use rubber dam isolation during operative dentistry procedures 52, 53. We expected the leakage of BPA from newly placed polymer‐based fillings to be low, so in an effort to perform the most commonly used clinical procedures and detect the maximum amount of BPA released after treatment, a rubber dam was not used during treatment in our study. Immediately after treatment, the participants were instructed to spit once into the unit sink. They then performed cheek and tongue movements and consecutively spat the accumulated saliva into tubes. Neither rinsing nor gargling was allowed before saliva sampling. Thus, the saliva samples collected immediately after treatment in our study may include BPA exposure from the uncured layer as well as surplus material from the grinding and polishing procedure. Probably the association between surface area (filling points) and BPA concentration immediately after treatment would have been stronger if gargling had been permitted. Rinsing the mouth with water for 30 s after polymer‐based filling placement has been shown to decrease the BPA concentration in saliva to nearly baseline levels 41. In some studies, it was not reported whether the participants were instructed to rinse their mouth before sampling. Moreover, the time interval reflecting ‘immediately after treatment’ may vary from 5 to 60 min across studies 26, 28, 29. Thus, the varied sampling procedures could influence the differences in the BPA concentrations detected and complicate estimation of the amount of BPA released immediately after treatment.
Clinical studies examining the saliva concentration of leachable chemicals from dental polymer‐based materials show generally wide variations between participants and the relative SD is usually high 25, 41, 54. Thus, the homogeneity and representativeness of the saliva samples could be questioned 55.
In the present study, the participants were instructed to refrain from food and beverage intake for 10 h before sample collection. Urine samples were collected prior to treatment and 1 h, 24 h, and 1 wk after treatment. The salivary BPA concentrations detected immediately after treatment were high and could be expected to be reflected in the subsequent urinary BPA concentrations. However, neither the first post‐treatment urine samples (collected 1 h after treatment), nor any other post‐treatment samples showed significant increases in BPA concentrations compared with pretreatment levels. This is in contrast to the findings by JOSKOW et al. 39, which indicated that urinary BPA levels were highest 1 h after sealant placement. Over time, there is a large variation of BPA in urine 56, and intake of food has a considerable influence on the concentration of BPA in urine 57. Thus, it could be speculated that the elevated urinary concentrations 1 h after treatment, as reported in other studies, may have been influenced by food intake before treatment. However, 10 h of fasting may decrease the BPA exposure, and subsequently the urinary BPA level, and thus partly mask a potential increase in the urinary BPA concentration caused by the dental treatment. The BPA concentration in urine reflects the absorbed dose of BPA 58. Because saliva samples were collected immediately after treatment, we may have removed a significant amount of BPA, which otherwise would have been absorbed, metabolized in the liver, and excreted via the urine. Thus, saliva sampling directly after treatment could have influenced the BPA concentrations in urine considerably 39.
In the present study, we used LC/MS/MS with an isotopically labeled internal standard (D16‐BPA) for the analysis of BPA. This is the method of choice for measuring the content of BPA in biological samples 59. Quality control samples and field blanks were analyzed together with the samples of saliva and urine. In addition, a limited number of saliva samples were analyzed at a second laboratory and showed similar results. Thus, we evaluated the analysis to be reliable and the accuracy and precision acceptable for the present purpose.
To standardize the procedures as much as possible, the dental treatment was provided by one dentist at one dental clinic using the same procedures for all patients. Moreover, the same batch of filling material was used for all fillings.
The participants in the present study acted as their own control, as reflected by pretreatment sampling. Including an unexposed control group could have facilitated the interpretation of the contribution from the polymer‐based material to urinary BPA levels. Although data from the study by JOSKOW et al. 39 indicate an increase of BPA in urine 1 h after treatment, it is possible that the peak concentration of BPA in urine after exposure is between 1 and 4 h 58. Thus, sample collection at 1 h may have been too early to detect the maximum concentration of BPA in urine.
Two participants showed significantly higher urinary BPA levels compared with the other participants (Fig. 4). One had elevated BPA levels in urine at all time points. Both participants handled receipts at work and consequently thermal paper could be one source of exposure to BPA. Regarding the group of participants who handled receipts, there was substantial between‐ and within‐subject variability, but average levels were elevated, as was also found by THAYER et al. 60.
There may be considerable differences regarding potential BPA exposure, depending on the materials used. Because only one batch of one material was tested in this study, the amounts of BPA detected must be interpreted with care. Materials with a lower level of contamination with BPA may result in lower exposure. Hence, the daily dose of BPA from dental polymer‐based restorative materials is probably relatively low compared with the total exposure from food and other sources 10. However, the biologic effects of BPA have been reported to occur even within the range of the detection threshold of most analytical procedures, and its influence on tissues may show a non‐monotonic dose–response curve pattern 61. This is characterized by intense reactivity at low levels and no response at high levels 62.
Data on the chemical composition of dental polymer‐based material from the material Safety Data Sheets are incomplete 19. Therefore, manufacturers should be required to provide more exact information about the composition of their products 19. Ideally, dental polymer‐based materials should be produced without components that have estrogenic effects. However, materials introduced as BPA free have also shown estrogenic activity 63. Thus, methods to reduce the release of BPA after placement of dental polymer‐based materials should be provided. Using a rubber dam to control the operative field would limit the potential exposure 26. Moreover, rinsing with water directly after treatment should be highly recommended 41.
In conclusion, the findings in this study confirm that placement of dental polymer‐based restorative materials may cause a substantial increase in the concentration of salivary BPA after treatment. The results indicate that exposure to BPA is relatively short and transient. After 1 wk, the concentration of BPA in saliva was only slightly elevated compared with the levels before treatment. This study did not show changes in the BPA concentration in urine after treatment with a dental polymer‐based restorative material.
Conflicts of interest
The authors declare no conflicts of interest.
Supporting information
Appendix S1. Analysis of bisphenol A.
Fig. S1. Total ion chromatogram for the BPA peak from the standard sample of 50 ng/ml.
Fig. S2. Quadropole time of flight mass spectra for the BPA peak from the standard sample of 50 ng/ml.
Fig. S3. Total ion chromatogram for the BPA peak from a saliva sample with a concentration of 800 ng BPA/ml.
Fig. S4. Quadropole time of flight mass spectra for the BPA peak from the saliva sample shown in Fig. S3.
Acknowledgements
The authors want to give special thanks to the participants of this study. We also appreciate the support from The Public Dental Services in Hordaland County and skillful help from personnel at the dental clinics (Årstad and Sammen/Student Welfare Organization in Bergen). We acknowledge Carina S Nilsson and Margareta Maxe, Lund University, Sweden, for technical assistance with the analyses of BPA. Hilde M Kopperud and Lene A Grutle, Nordic Institute of Dental Materials, Norway, are acknowledged for skillful help with the additional analyses of BPA. The Norwegian Dental Biomaterials Adverse Reaction Unit and the Oral Health Centre of Expertise in Western Norway are funded by the Norwegian Ministry of Health and Care Services. The study was supported by the Norwegian Directorate of Health.
Berge TLL, Lygre GB, Lie SA, Lindh CH, Björkman L. Bisphenol A in human saliva and urine before and after treatment with dental polymer‐based restorative materials. Eur J Oral Sci 2019; 127: 435–444. © 2019 The Authors. Eur J Oral Sci published by John Wiley & Sons Ltd
References
- 1. Geens T, Goeyens L, Covaci A. Are potential sources for human exposure to bisphenol‐A overlooked? Int J Hyg Environ Health 2011; 214: 339–347. [DOI] [PubMed] [Google Scholar]
- 2. Crain DA, Eriksen M, Iguchi T, Jobling S, Laufer H, LeBlanc GA, Guillette LJ Jr. An ecological assessment of bisphenol‐A: evidence from comparative biology. Reprod Toxicol 2007; 24: 225–239. [DOI] [PubMed] [Google Scholar]
- 3. Rubin BS. Bisphenol A: an endocrine disruptor with widespread exposure and multiple effects. J Steroid Biochem Mol Biol 2011; 127: 27–34. [DOI] [PubMed] [Google Scholar]
- 4. Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM. Bisphenol‐A and the great divide: a review of controversies in the field of endocrine disruption. Endocr Rev 2009; 30: 75–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, Vandenbergh JG, Walser‐Kuntz DR, vom Saal FS. In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol 2007; 24: 199–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mikolajewska K, Stragierowicz J, Gromadzinska J. Bisphenol A ‐ Application, sources of exposure and potential risks in infants, children and pregnant women. Int J Occup Med Environ Health 2015; 28: 209–241. [DOI] [PubMed] [Google Scholar]
- 7. Mustieles V, Perez‐Lobato R, Olea N, Fernandez MF. Bisphenol A: Human exposure and neurobehavior. Neurotoxicology 2015; 49: 174–184. [DOI] [PubMed] [Google Scholar]
- 8. Rochester JR. Bisphenol A and human health: a review of the literature. Reprod Toxicol 2013; 42: 132–155. [DOI] [PubMed] [Google Scholar]
- 9. Beronius A, Ruden C, Hakansson H, Hanberg A. Risk to all or none? A comparative analysis of controversies in the health risk assessment of Bisphenol A. Reprod Toxicol 2010; 29: 132–146. [DOI] [PubMed] [Google Scholar]
- 10. Scientific EFSA. Opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA J 2015; 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Volkel W, Colnot T, Csanady GA, Filser JG, Dekant W. Metabolism and kinetics of bisphenol A in humans at low doses following oral administration. Chem Res Toxicol 2002; 15: 1281–1287. [DOI] [PubMed] [Google Scholar]
- 12. Divakaran K, Hines RN, McCarver DG. Human hepatic UGT2B15 developmental expression. Toxicol Sci 2014; 141: 292–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. European Chemicals Agency . Agreement of the member state committee on the identification of 4,4’‐isopropylidenediphenol (Bisphenol A) A substance of very high concern. 2017.
- 14. Lorber M, Schecter A, Paepke O, Shropshire W, Christensen K, Birnbaum L. Exposure assessment of adult intake of bisphenol A (BPA) with emphasis on canned food dietary exposures. Environ Int 2015; 77: 55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wilson NK, Chuang JC, Morgan MK, Lordo RA, Sheldon LS. An observational study of the potential exposures of preschool children to pentachlorophenol, bisphenol‐A, and nonylphenol at home and daycare. Environ Res 2007; 103: 9–20. [DOI] [PubMed] [Google Scholar]
- 16. Stahlhut RW, Welshons WV, Swan SH. Bisphenol A data in NHANES suggest longer than expected half‐life, substantial nonfood exposure, or both. Environ Health Perspect 2009; 117: 784–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Van Landuyt KL, Nawrot T, Geebelen B, De Munck J, Snauwaert J, Yoshihara K, Scheers H, Godderis L, Hoet P, Van Meerbeek B. How much do resin‐based dental materials release? A meta‐analytical approach Dent Mater 2011; 27: 723–747. [DOI] [PubMed] [Google Scholar]
- 18. Bowen RL. Properties of a silica‐reinforced polymer for dental restorations. J Am Dent Assoc 1963; 66: 57–64. [DOI] [PubMed] [Google Scholar]
- 19. Fleisch AF, Sheffield PE, Chinn C, Edelstein BL, Landrigan PJ. Bisphenol A and related compounds in dental materials. Pediatrics 2010; 126: 760–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schmalz G, Preiss A, Arenholt‐Bindslev D. Bisphenol‐A content of resin monomers and related degradation products. Clin Oral Investig 1999; 3: 114–119. [DOI] [PubMed] [Google Scholar]
- 21. Arenholt‐Bindslev D, Breinholt V, Preiss A, Schmalz G. Time‐related bisphenol‐A content and estrogenic activity in saliva samples collected in relation to placement of fissure sealants. Clin Oral Investig 1999; 3: 120–125. [DOI] [PubMed] [Google Scholar]
- 22. Atkinson JC, Diamond F, Eichmiller F, Selwitz R, Jones G. Stability of bisphenol A, triethylene‐glycol dimethacrylate, and bisphenol A dimethacrylate in whole saliva. Dent Mater 2002; 18: 128–135. [DOI] [PubMed] [Google Scholar]
- 23. Chen L, Suh BI. Bisphenol A in dental materials: a review. JSM Dent 2013; 1. [Google Scholar]
- 24. Kadoma Y, Tanaka M. Acid and base‐catalyzed hydrolysis of bisphenol A‐related compounds. Dent Mater J 2000; 19: 139–152. [DOI] [PubMed] [Google Scholar]
- 25. Olea N, Pulgar R, Perez P, Olea‐Serrano F, Rivas A, Novillo‐Fertrell A, Pedraza V, Soto AM, Sonnenschein C. Estrogenicity of resin‐based composites and sealants used in dentistry. Environ Health Perspect 1996; 104: 298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kingman A, Hyman J, Masten SA, Jayaram B, Smith C, Eichmiller F, Arnold MC, Wong PA, Schaeffer JM, Solanki S, Dunn WJ. Bisphenol A and other compounds in human saliva and urine associated with the placement of composite restorations. J Am Dent Assoc 2012; 143: 1292–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maserejian NN, Hauser R, Tavares M, Trachtenberg FL, Shrader P, McKinlay S. Dental composites and amalgam and physical development in children. J Dent Res 2012; 91: 1019–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kang YG, Kim JY, Kim J, Won PJ, Nam JH. Release of bisphenol A from resin composite used to bond orthodontic lingual retainers. Am J Orthod Dentofacial Orthop 2011; 140: 779–789. [DOI] [PubMed] [Google Scholar]
- 29. Lee JH, Yi SK, Kim SY, Kim JS, Son SA, Jeong SH, Kim JB. Salivary bisphenol A levels and their association with composite resin restoration. Chemosphere 2017; 172: 46–51. [DOI] [PubMed] [Google Scholar]
- 30. Moreira MR, Matos LG, de Souza ID, Brigante TA, Queiroz ME, Romano FL, Nelson‐Filho P, Matsumoto MA. Bisphenol A release from orthodontic adhesives measured in vitro and in vivo with gas chromatography. Am J Orthod Dentofacial Orthop 2017; 151: 477–483. [DOI] [PubMed] [Google Scholar]
- 31. Kloukos D, Pandis N, Eliades T. In vivo bisphenol‐a release from dental pit and fissure sealants: a systematic review. J Dent 2013; 41: 659–667. [DOI] [PubMed] [Google Scholar]
- 32. Sofan E, Sofan A, Palaia G, Tenore G, Romeo U, Migliau G. Classification review of dental adhesive systems: from the IV generation to the universal type. Ann Stomatol (Roma) 2017; 8: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Olstad ML, Holland RI, Wandel N, Pettersen AH. Correlation between amalgam restorations and mercury concentrations in urine. J Dent Res 1987; 66: 1179–1182. [DOI] [PubMed] [Google Scholar]
- 34. Berge TLL, Lygre GB, Jonsson BAG, Lindh CH, Bjorkman L. Bisphenol A concentration in human saliva related to dental polymer‐based fillings. Clin Oral Investig 2017; 21: 2561–2568. [DOI] [PubMed] [Google Scholar]
- 35. Gyllenhammar I, Glynn A, Jonsson BA, Lindh CH, Darnerud PO, Svensson K, Lignell S. Diverging temporal trends of human exposure to bisphenols and plastizisers, such as phthalates, caused by substitution of legacy EDCs? Environ Res 2017; 153: 48–54. [DOI] [PubMed] [Google Scholar]
- 36. Cone EJ, Caplan YH, Moser F, Robert T, Shelby MK, Black DL. Normalization of urinary drug concentrations with specific gravity and creatinine. J Anal Toxicol 2009; 33: 1–7. [DOI] [PubMed] [Google Scholar]
- 37. Hornung R, Reed L. Estimation of average concentration in the presence of nondetectable values. Appl Occup Hyg 1990; 5: 46–51. [Google Scholar]
- 38. Fung EYK, Ewoldsen NO, St. Germain HA, Marx DB, Miaw C‐L, Siew C, Chou H‐N, Gruninger SE, Meyer DM. Pharmacokinetics of Bisphenol a Released from a Dental Sealant. J Am Dent Assoc 2000; 131: 51–58. [DOI] [PubMed] [Google Scholar]
- 39. Joskow R, Barr DB, Barr JR, Calafat AM, Needham LL, Rubin C. Exposure to bisphenol A from bis‐glycidyl dimethacrylate–based dental sealants. J Am Dent Assoc 2006; 137: 353–362. [DOI] [PubMed] [Google Scholar]
- 40. Polydorou O, Huberty C, Wolkewitz M, Bolek R, Hellwig E, Kummerer K. The effect of storage medium on the elution of monomers from composite materials. J Biomed Mater Res B Appl Biomater 2012; 100: 68–74. [DOI] [PubMed] [Google Scholar]
- 41. Sasaki N, Okuda K, Kato T, Kakishima H, Okuma H, Abe K, Tachino H, Tuchida K, Kubono K. Salivary bisphenol‐A levels detected by ELISA after restoration with composite resin. J Mater Sci Mater Med 2005; 16: 297–300. [DOI] [PubMed] [Google Scholar]
- 42. Kloukos D, Sifakakis I, Voutsa D, Doulis I, Eliades G, Katsaros C, Eliades T. BPA qualtitative and quantitative assessment associated with orthodontic bonding in vivo. Dent Mater 2015; 31: 887–894. [DOI] [PubMed] [Google Scholar]
- 43. American Dental Association Council on Scientific Affairs . Determination of bisphenol a released from resin‐based composite dental restoratives. J Am Dent Assoc 2014; 145: 763–765. [DOI] [PubMed] [Google Scholar]
- 44. Gupta SK, Saxena P, Pant VA, Pant AB. Release and toxicity of dental resin composite. Toxicol Int 2012; 19: 225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sideridou I, Tserki V, Papanastasiou G. Effect of chemical structure on degree of conversion in light‐cured dimethacrylate‐based dental resins. Biomaterials 2002; 23: 1819–1829. [DOI] [PubMed] [Google Scholar]
- 46. Peutzfeldt A. Resin composites in dentistry: the monomer systems. Eur J Oral Sci 1997; 105: 97–116. [DOI] [PubMed] [Google Scholar]
- 47. AlShaafi MM. Factors affecting polymerization of resin‐based composites: A literature review. Saudi Dent J 2017; 29: 48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ruyter IE. Unpolymerized surface layers on sealants. Acta Odontol Scand 1981; 39: 27–32. [DOI] [PubMed] [Google Scholar]
- 49. Anusavice KJ, Shen C, Rawls HR. Phillips’ science of dental materials, 12th ed. St. Louis, MO: Elsevier/Saunders, 2013. [Google Scholar]
- 50. Hamid A, Hume WR. A study of component release from resin pit and fissure sealants in vitro. Dent Mater 1997; 13: 98–102. [DOI] [PubMed] [Google Scholar]
- 51. Komurcuoglu E, Olmez S, Vural N. Evaluation of residual monomer elimination methods in three different fissure sealants in vitro. J Oral Rehabil 2005; 32: 116–121. [DOI] [PubMed] [Google Scholar]
- 52. Gilbert GH, Litaker MS, Pihlstrom DJ, Amundson CW, Gordan VV, DPBRN Collaborative Group . Rubber dam use during routine operative dentistry procedures: findings from the Dental PBRN. Oper Dent 2010; 35: 491–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lynch CD, McConnell RJ. Attitudes and use of rubber dam by Irish general dental practitioners. Int Endod J 2007; 40: 427–432. [DOI] [PubMed] [Google Scholar]
- 54. Michelsen VB, Kopperud HB, Lygre GB, Bjorkman L, Jensen E, Kleven IS, Svahn J, Lygre H. Detection and quantification of monomers in unstimulated whole saliva after treatment with resin‐based composite fillings in vivo. Eur J Oral Sci 2012; 120: 89–95. [DOI] [PubMed] [Google Scholar]
- 55. Mullangi R, Agrawal S, Srinivas NR. Measurement of xenobiotics in saliva: is saliva an attractive alternative matrix? Case studies and analytical perspectives. Biomed Chromatogr 2009; 23: 3–25. [DOI] [PubMed] [Google Scholar]
- 56. Ye X, Wong LY, Bishop AM, Calafat AM. Variability of urinary concentrations of bisphenol A in spot samples, first morning voids, and 24‐hour collections. Environ Health Perspect 2011; 119: 983–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA). Reprod Toxicol 2007; 24: 139–177. [DOI] [PubMed] [Google Scholar]
- 58. Thayer KA, Doerge DR, Hunt D, Schurman SH, Twaddle NC, Churchwell MI, Garantziotis S, Kissling GE, Easterling MR, Bucher JR, Birnbaum LS. Pharmacokinetics of bisphenol A in humans following a single oral administration. Environ Int 2015; 83: 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Putzeys E, Cokic SM, Chong H, Smet M, Vanoirbeek J, Godderis L, Van Meerbeek B, Van Landuyt KL, Duca RC. Simultaneous analysis of bisphenol A based compounds and other monomers leaching from resin‐based dental materials by UHPLC‐MS/MS. J Sep Sci 2017; 40: 1063–1075. [DOI] [PubMed] [Google Scholar]
- 60. Thayer KA, Taylor KW, Garantziotis S, Schurman SH, Kissling GE, Hunt D, Herbert B, Church R, Jankowich R, Churchwell MI, Scheri RC, Birnbaum LS, Bucher JR. Bisphenol A, Bisphenol S, and 4‐Hydroxyphenyl 4‐Isoprooxyphenylsulfone (BPSIP) in Urine and Blood of Cashiers. Environ Health Perspect 2016; 124: 437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR Jr, Lee DH, Shioda T, Soto AM, vom Saal FS, Welshons WV, Zoeller RT, Myers JP. Hormones and endocrine‐disrupting chemicals: low‐dose effects and nonmonotonic dose responses. Endocr Rev 2012; 33: 378–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Welshons WV, Nagel SC, vom Saal FS. Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology 2006; 147: S56–S69. [DOI] [PubMed] [Google Scholar]
- 63. Bittner GD, Yang CZ, Stoner MA. Estrogenic chemicals often leach from BPA‐free plastic products that are replacements for BPA‐containing polycarbonate products. Environ Health 2014; 13: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Analysis of bisphenol A.
Fig. S1. Total ion chromatogram for the BPA peak from the standard sample of 50 ng/ml.
Fig. S2. Quadropole time of flight mass spectra for the BPA peak from the standard sample of 50 ng/ml.
Fig. S3. Total ion chromatogram for the BPA peak from a saliva sample with a concentration of 800 ng BPA/ml.
Fig. S4. Quadropole time of flight mass spectra for the BPA peak from the saliva sample shown in Fig. S3.


