Abstract
Aim
Abnormal lipid metabolism plays a dual role in tumorigenesis, specifically in the occurrence and development of cancers. Monoacylglycerol lipase (MAGL), a hydrolase that is important for lipid metabolism, plays a vital role in different aspects of tumorigenesis. Many studies have shown that MAGL is highly elevated in a variety of cancers and plays an active role. However, its potential role in supporting endometrial cancer (EC) growth and progression has not yet been explored in depth.
Methods
Immunohistochemistry and quantitative real‐time reverse transcription polymerase chain reaction were performed to estimate the protein and messenger RNA (mRNA) levels of MAGL in tumor tissues. Then, JZL184 and small interfering RNA (siRNA) were used to decrease the expression of MAGL in EC cells. The gene and protein expression levels of MAGL were measured using quantitative real‐time PCR and western blotting, respectively. Additionally, the effect of MAGL on tumor growth in EC was detected by 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide , cell cycle and western blotting assay in vitro.
Results
We found that MAGL was overexpressed in EC and was significantly correlated with surgical‐pathological stage, myometrial invasion, number of pregnancies and body mass index. The growth and cell cycle progression of tumor cells were significantly impaired in vitro by the pharmacological and siRNA‐mediated MAGL inhibition. In addition, MAGL inhibition seemed to repress two target genes, Cyclin D1 and Bcl‐2.
Conclusion
In summary, we have demonstrated that MAGL is involved in EC growth and progression. Our results suggest that targeting MAGL may be a novel and valid treatment for EC.
Keywords: Bcl‐2, cyclin D1, endometrial cancer, lipid metabolism, MAGL
Introduction
Endometrial cancer (EC) is the most common gynecologic malignant neoplasm, and its incidence is gradually increasing worldwide. Abnormal lipid metabolism is a known to contribute to various features of chronic diseases and tumorigenesis. The malignant behavior of tumor cells is accompanied by reprogramming of metabolic pathways, including the development of a lipogenic phenotype.1 Recently, monoacylglycerol lipase (MAGL), a key metabolic enzyme in the development of cancer, has attracted increasing attention. Many studies have shown that MAGL is highly elevated in human cancer cells and primary tumors and that its inhibition impairs the cell migration, invasion, survival and tumor growth of invasive breast, ovarian and melanoma cancer cells.2, 3 The role of obesity in the etiology of EC has been documented.4, 5, 6 However, whether MAGL plays crucial roles in the progression and malignancy of EC remains unclear.
MAGL is a ubiquitous 33 kDa enzyme, and its gene locus is on human chromosome 3q21.3.7 MAGL, a member of the serine hydrolase superfamily, is a key enzyme in the production of free fatty acids (FFA) in lipid metabolism via its hydrolysis of triglycerides. Previous research has shown that MAGL plays an essential role in many pathological and physiological processes, such as inflammation, pain, neuroprotection, neurodegenerative disorders, metabolic disorders and cancer.8, 9 In addition, recently, an increasing number of scholars have paid attention to the role of MAGL in lipid metabolism and tumor development. In cancer cells, MAGL hydrolyzes triglycerides into FFA and induces an increase in a series of lipid signaling molecules, such as lysophosphatidic acid (LPA), prostaglandin E2 (PGE2), lysophospholipid , lysophosphatidyl ethanolamine and phosphatidic acid ; some of these molecules (LPA and PGE2) induce carcinogenesis and tumor progression.10 MAGL is functionally expressed in multiple aggressive tumors, such as colorectal cancer,11 gastrointestinal stromal tumors,12 prostate cancer,13 neuroblastoma,14 nasopharyngeal carcinoma,15 hepatocellular carcinoma,16 malignant melanoma,17 ovarian cancer18 and breast cancer.19 Naturally, the effect of MAGL on EC attracted our attention.
Hence, in the present study, we investigated MAGL expression in EC and its effect on tumor cells. Our findings establish a novel function of MAGL in tumor promotion and uncover a potential therapeutic target in human EC.
Methods
Patients and specimens
One study group consisted of 134 paraffin‐embedded specimens, 28 specimens of normal endometrium in the proliferative phase, 31 specimens of atypically hyperplastic endometrium and 75 specimens of EC placed in the pathology archives of Qilu Hospital of Shandong University from 2010 to 2012. The age of patients with EC ranged from 30 to 77 (mean age, 55). According to the cut‐off points of body mass index (BMI) of Chinese criteria,20 obesity was defined as a BMI of 28 or higher in our present analysis. Another 16 specimens of fresh uterine endometrial adenocarcinoma tissues and 16 specimens of the corresponding adjacent normal endometrial tissues were obtained, and each tissue specimen was confirmed by an expert gynecologic pathologist who reviewed hematoxylin and eosin (H&E)‐stained sections of the specimens. All specimens were preserved at −80°C. All tumors were primary, were collected before treatment and surgery and had complete clinicopathological information. The procedure was approved by the Ethics Committee of Qilu Hospital of Shandong University.
Immunohistochemistry
Formalin‐fixed, paraffin‐embedded sections (5 μm) were deparaffinized by heating the slides to 75°C for 40 min and then subjecting them to two 15‐min washes in 100% xylene; the sections were then rehydrated through a graded alcohol series. The sections were immersed in a heat‐resistant plastic box filled with sodium citrate buffer (pH 6.0) and heated in a microwave oven for 15 min at low heat for antigen retrieval. The slides were removed from the oven and cooled to room temperature. The slides were then washed ?keyword=three ?keyword= ?keyword=times in phosphate‐buffered saline (PBS). Endogenous peroxidase activity was eliminated by incubation in 3% hydrogen peroxide for 15 min, and the sample was subsequently incubated in 5% bovine serum albumin to block nonspecific binding. The slides were then incubated with a polyclonal antibody to MAGL (1:100 dilution, Abcam) overnight at 4°C. After washing with PBS, the sections were incubated in goat antirabbit antibody (1:1000 dilution, Abcam) for 20 min at 37°C, followed by a wash. The sections were incubated with the avidin–biotin peroxidase complex at 37°C for 15 min, and visualization was performed with the 3,3′‐diaminobenzidine (DAB) substrate chromogen for 1 min after washing with PBS. Finally, the slides were counterstained with hematoxylin.
All stained tissues were assessed by two independent pathologists blinded to the origin of the samples. Quantifications were recorded according to the following criteria: 0–25% positive cells, 0; 26–50% positive cells, 1; 51–75% positive cells, 2; and 76–100% positive cells, 3; Staining intensity was scored as follows: negative, 0; weak, 1; moderate, 2; and strong, 3. The final score was the multiplication of the quantification and the staining intensity. A final score of 0–3 was considered negative MAGL expression, and ≥ 3 was considered positive.
RNA isolation and quantitative real‐time reverse transcription polymerase chain reaction
Total RNA was obtained using TRIzol (Invitrogen) according to the manufacturer's instructions. cDNA was synthesized from the total RNA using the SuperScript II Reverse Transcriptase Kit (Invitrogen). Then, polymerase chain reaction (PCR) was performed using SYBR‐Green Real‐Time PCR Master Mix (TaKaRa, Japan), and data were analyzed with StepOne Software V2.2 (Applied Biosystems). β‐Actin was used as a standardization control, and the primer sequences for the genes are listed in Table 1.
Table 1.
Primer sequence of the genes
| Gene | Primer sequence | Product size (bp) |
|---|---|---|
| MAGL |
5′‐CAA CTG CTG AAT GCC GTC TC‐3′ 5′‐GGT AGG CAC CTT CAT AAA TCT TG‐3′ |
172 |
| Cyclin D1 |
5′‐ATG CCA ACC TCC TCA ACG AC‐3′ 5′‐TCT GTT CCT CGC AGA CCT CC‐3′ |
159 |
| Bcl‐2 |
5′‐CCA GAG CCT ACT GGA CTG C‐3′ 5′‐AGC CAG CAT TGC CAT AAA AGA‐3′ |
197 |
| β‐Actin |
5′‐CTG AGA GGG AAA TCG TGC GT‐3′ 5′‐CCA CAG GAT TCC ATA CCC AAG A‐3′ |
208 |
Cell culture
The human EC cell lines Ishikawa, RL95‐2, and AN3CA were friendly provided by Professor Beihua Kong (Qilu Hospital of Shandong University, Jinan, China), and were cultured in modified RPMI‐1640 medium, Dulbecco's Modified Eagle Medium / Nutrient Mixture F‐12 (DMEM/F‐12) 1:1 (1X), and DMEM/high glucose, respectively. The basal medium was supplemented with 10% fetal bovine serum. All cells were maintained at 37°C in a humid atmosphere with 5% CO2.
Small interfering RNA and transfection
Contraposing the different encoding regions of monoacylglycerol lipase transcript, two pairs of small interfering RNA (siRNA) were designed and chemically synthesized (GenePharma). These two small interfering nucleotide sequences were as follows: siMAGL1 (sense 5′‐GGA UGG UAG UGU CUG ACU UTT‐3′, antisense 5′‐AAG UCA GAC ACU ACU ACC AUC CTT‐3′); siMAGL2 (sense 5′‐GCC AAU CCU GAA UCU GCA ATT‐3′, antisense 5′‐UUG CAG AUU CAG GAU UGG CTT‐3′). A scrambled siRNA sequence (sense 5′‐UUC UCC GAA CGU GUC ACG UTT‐3′, antisense 5′‐ACG UGA CAC GUU CGG AGA ATT‐3′) was used as a negative control. The cells were placed in six‐well plates and then transfected with siRNA using Lipofectamine 2000 reagent (Invitrogen) following the manufacturer's instructions. Each experiment was repeated at least twice.
3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide assay and cell growth analysis
JZL184, a selective inhibitor of MAGL in experimental use was selected in our study. Our previous preliminary experiments demonstrated that 1 μmoL/L JZL184 had the most obvious effect on EC cell proliferation. To investigate the influence of MAGL on cell growth, the cells that had been treated with JZL184 and transfected with the indicated siRNA were seeded onto 96‐well plates at a density of 4000 cells/well in 200 μL of culture medium. After a 24 h incubation, 20 μL of 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide (MTT) (5 mg/mL, Sigma) was added to each well and incubated for 4 h; the reaction was then terminated by the addition of 150 μL of dimethyl sulfoxide. The optical density values at 490 nm were measured on a microplate reader. After different incubation times (48 and 72 h), the same MTT method was used to analyze cell proliferation after the MAGL level was modulated.
Cell cycle analysis
For cell cycle analysis, tumor cells were seeded in six‐well plates, treated with JZL184 or transfected with siRNA (siMAGL1, siMAGL2, siControl), then digested by trypsin, suspended and washed twice by pre‐chilled PBS buffer, fixed in ice‐cold 70% ethanol at 4°C overnight and stained with propidium iodide for DNA cell cycle analysis. The results were analyzed and expressed using ModFit LT software (BD). Each experiment was repeated three times.
Western blot analysis
The cells were treated with JZL184 for 24 and 48 h post‐transfection and then harvested and lysed by cell lysis buffer. Protein concentrations were measured by the BCA protein quantitative analysis kit (Tiangen Biotech Co., Ltd.). Then, 30 μg/lane cellular protein was electrophoresed through 10% sodium dodecyl sulfate‐Polyacrylamide gel electrophoresis and then blotted onto a polyvinylidene difluoride membrane. The membranes were blocked in 5% nonfat milk for 2 h at room temperature and then incubated with primary antibody against MAGL at 4°C overnight. The membrane was then incubated with antirabbit IgG (1:10 000 dilution), and the membranes were scored using an ECL Plus kit (GE Healthcare Life Sciences). β‐Actin was used as the internal control protein. The primary antibodies used in this experiment were for MAGL (1:800 dilution, Abcam), Cyclin D1 (1:10 000 dilution, Abcam) and Bcl‐2 (1:1000 dilution, Santa Cruz Biotechnology, Inc.).
Statistical analysis
All data are expressed as the mean ± standard deviation. The 2−ΔΔCt method was used to assess the relative quantification of RNA expression. Statistical significance (P < 0.05) was analyzed through Student's t‐test, Variance analysis or the χ² test using GraphPad Prism version 6.01 (GraphPad Software Inc.).
Results
High expression of MAGL in EC and its relationship with clinicopathological factors
We used immunohistochemistry staining to estimate the expression of MAGL and correlated this expression with the patient characteristics. The cytoplasm and cytomembrane, which had brownish‐yellow staining, were positive for MAGL expression. Compared to the normal controls and the atypical hyperplasia endometrium, the tumor tissues had significantly higher expression of MAGL (Fig. 1a‐c). The percentages of normal endometrium, atypical hyperplasia endometrium and EC specimens that were positive for MAGL expression were 25.0, 51.61 and 76.0%, respectively, as shown in Table 2, with significant differences among these specimens (P < 0.05).
Figure 1.
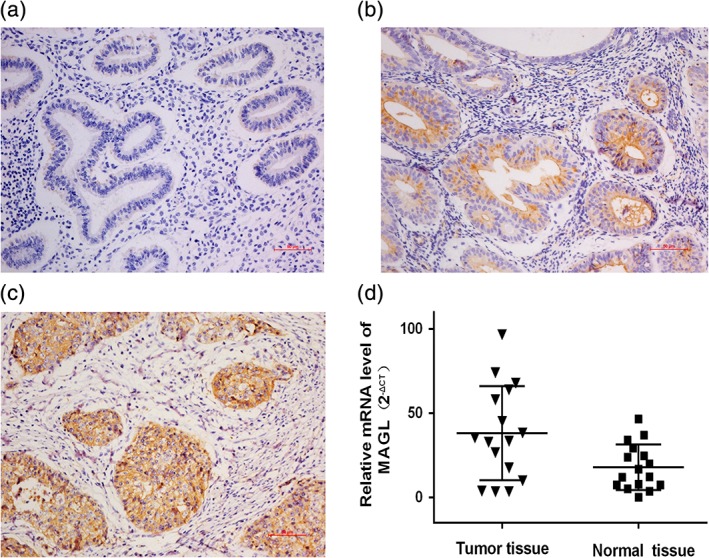
Expression of MAGL in normal endometrium, atypical hyperplasia endometrium and EC tissue. Histological evaluation of MAGL protein in normal (a), atypical hyperplasia endometrium (b) and in EC tissue (c) (scale bars, 50 μm). Our research indicates that MAGL was overexpressed in EC tissues, while low expression was found in the normal endometrium and the atypically hyperplastic endometrium. Examination of the mRNA levels by qRT‐PCR (d) also demonstrated that MAGL expression is significantly elevated in tumor tissue compared with that in the adjacent normal endometrium (P < 0.05).
Table 2.
Expression of monoacylglycerol lipase (MAGL) in normal endometrium (N), atypical endometrial hyperplasia (AH) and endometrial cancer (EC) specimens
| Subject | No. | Expression of MAGL | Positive Percentage (%) | P‐value | |
|---|---|---|---|---|---|
| Positive | Negative | ||||
| N | 28 | 7 | 21 | 25.0 | <0.05* |
| AH | 31 | 16 | 15 | 51.6 | |
| EC | 75 | 57 | 18 | 76.0 | |
Stands for pairwise comparisons among the subjects.
Moreover, the surgical‐pathological stage, myometrial invasion, number of pregnancies, and BMI in the patients were significantly correlated with the MAGL expression (P < 0.05). However, the pathological grade, lymph node metastasis, lymphovascular space invasion, age and presence or absence of the combination of hypertension and diabetes had no correlation with MAGL expression (Table 3).
Table 3.
Association between MAGL expression and clinicopathological characteristics in EC
| Clinicopathological factor | No. | Expression of MAGL | χ2 | P‐value | |
|---|---|---|---|---|---|
| Positive | Negative | ||||
| FIGO stage | |||||
| I | 50 | 34 | 16 | 4.030 | 0.045 |
| II–IV | 25 | 23 | 2 | ||
| Differentiation | |||||
| G1 | 28 | 20 | 8 | 0.512 | 0.474 |
| G2/3 | 47 | 37 | 10 | ||
| Depth of myometrial invasion | |||||
| <1/2 | 45 | 30 | 15 | 4.170 | 0.041 |
| ≥1/2 | 30 | 27 | 3 | ||
| Lymphovascular space invasion | |||||
| No | 63 | 49 | 14 | 0.209 | 0.647 |
| Yes | 12 | 8 | 4 | ||
| Lymph node metastasis | |||||
| No | 68 | 51 | 17 | 0.028 | 0.867 |
| Yes | 7 | 6 | 1 | ||
| Number of pregnancies | |||||
| <3 | 45 | 38 | 7 | 4.398 | 0.036 |
| ≥3 | 30 | 19 | 11 | ||
| BMI | |||||
| <28 kg/m2 | 25 | 15 | 10 | 5.263 | 0.022 |
| ≥28 kg/m2 | 50 | 42 | 8 | ||
| Age | |||||
| <60 | 58 | 43 | 15 | 0.140 | 0.708 |
| ≥60 | 17 | 14 | 3 | ||
| Combination of hypertension/diabetes | |||||
| No | 46 | 35 | 11 | 0.000 | 0.982 |
| Yes | 29 | 22 | 7 | ||
Then, we evaluated the quantity of MAGL in endometrioid adenocarcinoma tissues and adjacent normal endometrial tissues by qRT‐PCR, and this observation also demonstrated that MAGL was upregulated in EC at the mRNA level (P < 0.05) (Fig. 1d). These experimental results indicated that MAGL expression positively correlates with the tumorigenesis and progression of EC.
Silencing the expression of MAGL by siRNA and pharmacological inhibition
Ishikawa, RL95‐2 and AN3CA cells were transfected with siRNA (siMAGL1 and siMAGL2) or with a scrambled siRNA as a negative control (siControl), and untransfected cells were considered the blank control cells (normal). From these three cell lines, a significant decrease in the transcriptional level of MAGL was found via qRT‐PCR (P < 0.05, Fig. 2a‐c), and a decrease in the protein level was also found by western blotting. Then, JZL184 was used to inhibit MAGL pharmacologically, and the MAGL expression was still downregulated significantly (P < 0.05, Fig. 2d‐i). In addition, there was no significant difference in the inhibition of MAGL mediated by siRNA and by JZL184. In general, both siRNA and pharmacological regulation can inhibit MAGL expression, and the MAGL gene level directly regulated the MAGL protein level.
Figure 2.
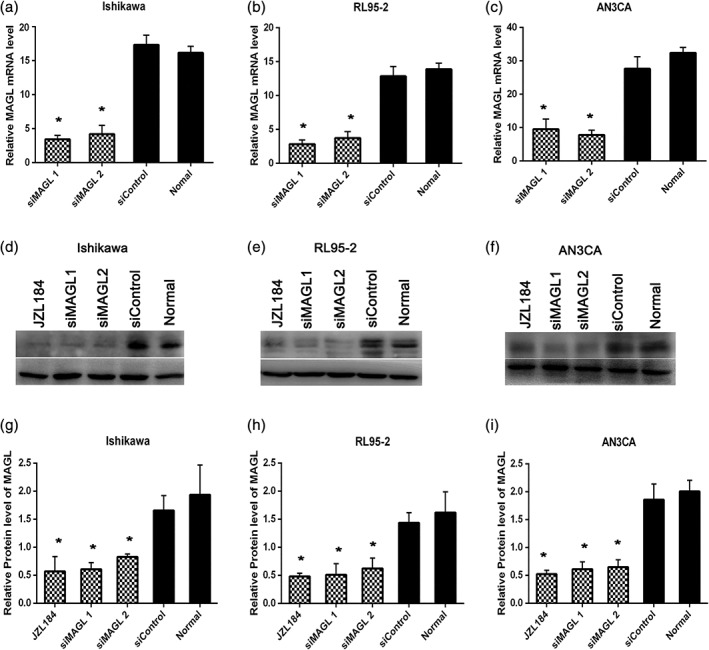
SiRNA‐mediated and pharmacological inhibition of MAGL in EC cell lines. qRT‐PCR was performed to evaluate the expression of MAGL at the transcriptional level. Ishikawa (a), RL95‐2 (b) and AN3CA (c) cells 48 h following transfection with siRNA targeted against MAGL (siMAGL1, siMAGL2) or with a scrambled siRNA as a negative control (siControl). Untransfected cells were used as a blank control (normal). A significant decrease in the transcriptional level of MAGL was found via by the qRT‐PCR assay, with decreases of nearly 89.3% in Ishikawa cells, 86.1% in RL95‐2 cells and 74.7% in AN3CA cells (mean of siMAGLs) (P < 0.05). A decrease in the protein level was also found by western blotting (d‐i), with decreases of nearly 56.7% in Ishikawa cells, 60.6% in RL95‐2 cells and 66.1% in AN3CA cells (mean of siMAGLs) (P < 0.05). Then, a specific pharmacological inhibitor of MAGL, JZL184 (1 μmoL/L, 24 h) was used to inhibit MAGL, and the MAGL expression decreased approximately 70.6% in Ishikawa cells, 70.3% in RL95‐2 cells and 74.0% in AN3CA cells (D‐I) (P < 0.05). *Indicates a significant difference (P < 0.05) between JZL184 or siMAGL treatment and siControl or normal.
Tumor growth capacity impaired by inhibition of MAGL
In this study, we inhibited MAGL in EC cells by JZL184 and siRNA. Then, the MTT assay was performed to investigate the effect of MAGL inhibition on cell growth. We found that both the pharmacological (JZL184) inhibition and the siRNA (siMAGL1, siMAGL2)‐mediated downregulation of MAGL decreased the EC cell growth (Fig. 3a‐c). The inhibitory effect in JZL184‐treated cells was more unstable than the effect in siRNA‐treated cells and decreased gradually over time. However, there was no significant difference in the reduction of cell growth between these two types of inhibition. In short, these findings implied that MAGL is indispensable for EC cell growth and progression.
Figure 3.
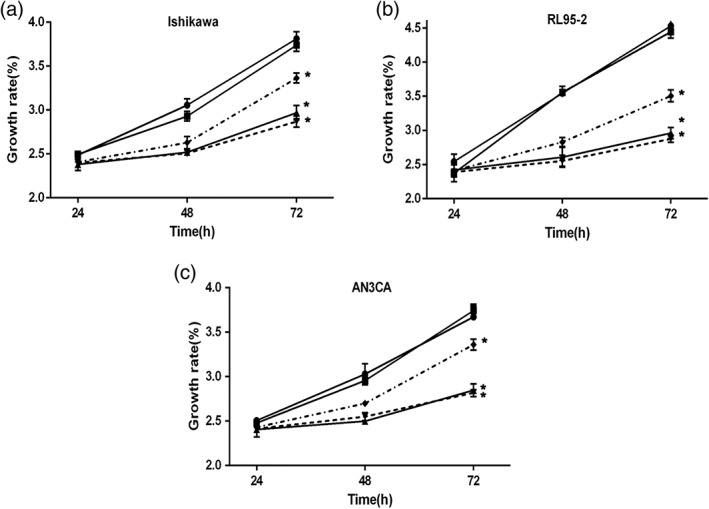
Tumor cell growth is impaired by MAGL inhibition. a‐c EC cells (Ishikawa, RL95‐2 and AN3CA) were treated with JZL184 (1 μmoL/L, 24 h) or transfected with the indicated siRNA and then seeded in 96‐well plates; untransfected cells served as a black control (normal). The MTT assay was performed to estimate tumor cell growth, and significantly less growth was found in all three cell lines following inhibition of MAGL with either JZL184 or siRNA. However, the growth‐inhibiting effect induced by JZL184 was less stable than that induced by siRNA, but there was no significant difference in the tumor growth reduction between these two types of MAGL inhibition. * P < 0.05 compared with siControl and normal. (a)–(c) ( ) Normal, (
) Normal, ( ) siControl, (
) siControl, ( ) siMAGL 1, (
) siMAGL 1, ( ) siMAGL 2 and (
) siMAGL 2 and ( ) JZL184
) JZL184
Inhibition of MAGL induces cell cycle arrest
To observe whether MAGL could affect the cell cycle, we performed flow cytometric assays. As revealed in Fig. 4, compared with the control groups, the tumor cells subjected to either pharmacological or siRNA inhibition of MAGL were arrested in the G0/G1 phase (Fig. 4a‐c). Compared with that in the untreated control group, the G0/G1 phase of the siMAGL‐transfected cells increased by 20.6% in Ishikawa cells, 41.5% in RL95‐2 cells and 30.3% in AN3CA cells 48 h post‐transfection. In addition, the percentage of G0/G1 phase cells in the JZL184‐treated cells increased by 16.2% in Ishikawa cells, 28.6% in RL95‐2 cells and 11.8% in AN3CA cells 24 h later (Fig. 4d‐f). Additionally, the percentage of S phase cells and G2/M phase cells decreased as the percentage of G0/G1 phase cells increased (data not shown). In summary, the results show that inhibition of MAGL expression in EC cells induced G0/G1 arrest and that the effect mediated by siMAGLs was more obvious than the JZL184‐mediated effect. Moreover, this result further confirmed the inhibitory effect of MAGL downregulation on tumor cell growth.
Figure 4.
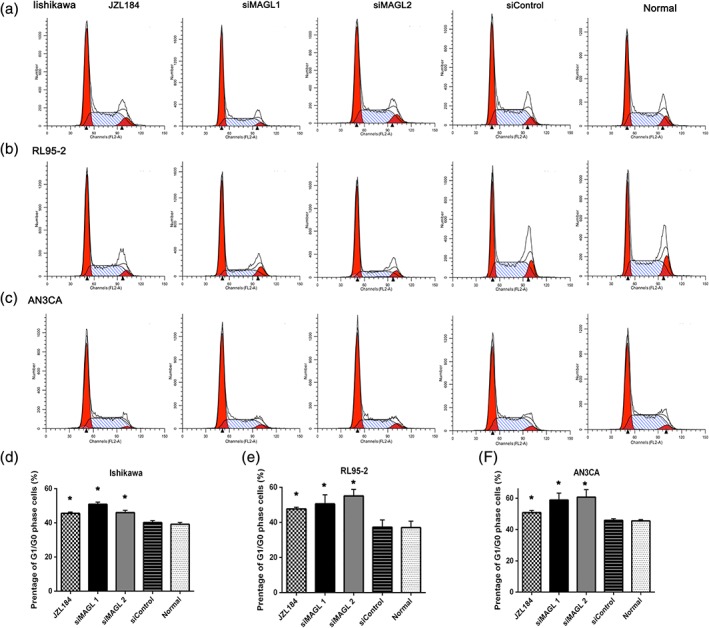
MAGL inhibition leads to G0/G1 arrest in EC. Tumor cells (a, Ishikawa; b, RL95‐2; c, AN3CA) were seeded in six‐well plates and treated with JZL184 (1 μmoL/L) or transfected with siRNA, with untransfected cells serving as a control (normal). Flow cytometric assays were performed to analyze the cell cycle. The proportion of G0/G1 phase cells was increased significantly in all cell lines. The effect of siRNA was greater than the effect of pharmacological inhibition. The proportion of G2/M phase cells decreased concurrently with the increase in G0/G1 phase cells. *Indicates a significant (P < 0.05) increase relative to siControl and normal. (d)–(f) ( ) JZL184, (
) JZL184, ( ) siMAGL 1, (
) siMAGL 1, ( ) siMAGL 2, (
) siMAGL 2, ( ) siControl and (
) siControl and ( ) normal
) normal
Inhibition of MAGL decreases the expression of cyclin D1 and Bcl‐2
Cyclin D1 and Bcl‐2 have been considered to be associated with the tumor pathogenicity of cancer cells. Further investigation was performed to evaluate the effect of MAGL inhibition on the mRNA and protein levels of Cyclin D1 and Bcl‐2. As shown in Fig. 5a‐c, a significant decrease in cyclin D1 (52.2% in Ishikawa cells, 65.9% in RL95‐2 cells, and 49.8% in AN3CA cells [mean of siMAGLs]) and Bcl‐2 (59.6% in Ishikawa cells, 67.4% in RL95‐2 cells, and 56.6% in AN3CA cells [mean of siMAGLs]) was found in all three cell lines. Then, the protein expression levels of Cyclin D1 and Bcl‐2 were estimated after treatment with JZL184, a direct pharmacological inhibitor of MAGL expression. Cyclin D1 expression decreased nearly 33.2% in Ishikawa cells, 34.4% in RL95‐2 cells and 49.8% in AN3CA cells, and the Bcl‐2 expression decreased significantly by nearly 35.1% in Ishikawa cells, 36.2% in RL95‐2 cells and 29.3% in AN3CA cells (Fig. 5c,d). Our experiment demonstrated that both cyclin D1 and Bcl‐2 could be downregulated by siRNA and pharmacological inhibition of MAGL, another line of evidence proving that targeting MAGL could decrease the tumor growth of EC.
Figure 5.
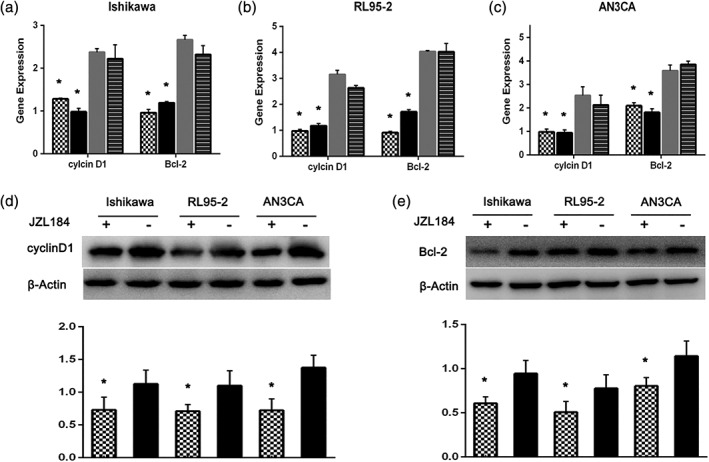
Inhibition of MAGL decreases the expression of Cyclin D1 and Bcl‐2. Ishikawa (a), RL95‐2 (b) and AN3CA (c) cells were transfected with siRNA, with untransfected cells serving as a blank control. The expression of Cyclin D1 and Bcl‐2 mRNA was evaluated by the qRT‐PCR assay. In all three cell lines, both Cyclin D1 and Bcl‐2 levels were significantly decreased. EC cells (Ishikawa, RL95‐2 and AN3CA) were treated with JZL184 (1 μmoL/L, 24 h). The protein levels were evaluated by western blotting. The expression levels of Cyclin D1 (d) and Bcl‐2 (e) were significantly suppressed in JZL184‐treated cells. *P < 0.05 compared with the normal siControl or/and siControl groups ( ) siMAGL 1, (
) siMAGL 1, ( ) siMAGL 2, (
) siMAGL 2, ( ) siControl, (
) siControl, ( ) Nomal
) Nomal
Discussion
Abnormal lipid metabolism can cause many human diseases, including diabetes, obesity, neurodegenerative diseases and cancers. The occurrence and development of tumors is a complex biological process with multiple mechanisms and multiple factors. Energy metabolism deregulation has become a concern in the research of various cancers recently. With the development of lipidomics technology, accumulating evidence shows that abnormal lipid metabolism plays a dual role in tumorigenesis, specifically in the occurrence and development of cancers. Our research group focused on this field of EC in recent years, and the lipid metabolism‐related molecular markers include sterol regulatory element‐binding protein 1, sirtuins 1 and stearoyl‐CoA desaturase 1).6, 21, 22, 23, 24 Previous research has confirmed that the energy supply of lipids comes from de novo synthesis rather than circulating lipids during tumor development.25 MAGL, as an important hydrolase involved in lipid metabolism, plays a vital role in different aspects of tumorigenesis.26
Our experiments confirmed that the expression of MAGL, a cancer‐promoting factor, was higher in EC tissue than in normal endometrium, which was consistent with the findings for some other cancers, such as breast cancer, colorectal cancer, prostate cancer, nasopharyngeal carcinoma, hepatocellular carcinoma, melanoma and ovarian cancer. We also showed that the expression of MAGL in normal endometrium, atypical endometrium and EC was gradually increased, and the difference was significant. In addition, the immunohistochemistry staining of MAGL in EC was related to the surgical–pathological stage and the depth of myometrial invasion in patients, suggesting that MAGL is closely related to the carcinogenesis and development of EC. Infertility, delayed menopause, obesity, high blood pressure and diabetes are known risk factors of EC,5, 27 and pregnancy is a protective factor for EC. This experiment indicated that the expression of MAGL in the high‐risk EC group (pregnancy <3 group and the overweight and obese group) was significantly higher than that in the low‐risk group, suggesting that high expression of MAGL may increase the risk of EC and promote the development of EC. Adult obesity and BMI increases are risk factors for EC. Our data also suggest that the expression of MAGL was notably correlated with BMI. However, the mechanisms underlying the effect of overweight and obesity on cancer progression are still poorly understood. We speculate that a possible mechanism is that high levels of MAGL break down lipids, producing large amounts of FFA and thus decreasing the binding of estrogen to sex hormone binding globulin via the increased free estrogen levels; this change would ultimately exert long‐term effects on the endometrium. The effects of estrogens and the lack of a progesterone antagonistic action lead to long‐term hyperplasia of the endometrium and greatly increase the risk of EC.28 Here, we need to note that our experiment only supports the link between obesity and EC; the specific mechanisms still need further exploration.
We also inhibited MAGL to explore its possible role in EC. Pharmacological and siRNA treatments were performed to inhibit MAGL, and the tumor cell growth was significantly suppressed. These results support our postulate that MAGL plays an important role in EC development and progression. Similar results have also been reported for melanoma, colorectal and ovarian cancer.1, 11 However, it is still not clear how MAGL contributes to the promotion of tumorigenesis and growth in EC. Previous studies have shown that MAGL supports tumor growth through a mechanism of tumor‐promoting fatty acid pathways, while silencing MAGL induces weak expression of its products, such as FFA, in aggressive human cancer cell lines.26 So, the enzyme MAGL may regulate a lipid network including monoacylglycerides, FFA and secondary lipid metabolites, such as LPA and PGE2 to support cancer malignancy. In addition, these possible mechanisms all require confirmation via further experiments in EC.
Studies in colorectal cancer have confirmed that downregulation of MAGL leads to decreased expression of Cyclin D1 and Bcl‐2, thereby inhibiting the proliferation of colorectal cancer and promoting tumor apoptosis or/and cell cycle arrest.11 Cyclin D1, the protein encoded by CCND1, plays an important and positive role during the key rate‐limiting point, the G1 → S phase transition, in the cell cycle,29, 30 and Bcl‐231 is an antagonistic protein in the process of apoptosis or programmed death and is thought to prolong the survival of cells in tumors. In our study, we used the same experimental scheme to confirm whether MAGL inhibition MAGL resulted in a reduction in Cyclin D1 and Bcl‐2 in EC, which would lead to impairment of tumor pathogenicity. Our observation illuminated that MAGL inhibition suppressed tumor growth and increased tumor apoptosis or/and inhibited cell cycle progression by targeting Cyclin D1 and Bcl‐2 in EC. These findings are similar to those for colorectal cancer. Regulation of Cyclin D1 and Bcl‐2 is required for tumor development and progression, and this information may provide a novel therapeutic target for EC. In addition, the changes in the two target genes may help us to identify the signaling pathway underlying MAGL‐induced tumor regulation.
In summary, our research confirmed that MAGL expression is significantly upregulated and strongly associated with an increased risk of EC and provides new evidence for the cross‐talk between obesity and EC. In addition, treatment with MAGL siRNA and with a MAGL inhibitor significantly suppressed the tumor cell growth and induced tumor cell cycle arrest in vitro. Although these conclusions need to be confirmed in vivo experiments and several potential mechanisms require further investigation, our results suggest that MAGL plays a substantial role in tumor growth and progression and that targeting MAGL may be a novel and valid treatment for EC.
Disclosure
None declared.
Acknowledgments
This study was partly funded by the National Nature Science Foundation of China (81772778 to J.J.), Shandong Province Key Research & Development Program (2017GSF18175 to J.J.) and Fundamental Research Funds of Qilu Hospital of Shangdong University (2082015QLMS44 to J.J.).
References
- 1. Nomura DK, Long JZ, Niessen S, Hoover HS, Ng SW, Cravatt BF. Monoacylglycerol lipase regulates a fatty acid network that promotes cancer pathogenesis. Cell 2010; 140: 49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Granchi C, Caligiuri I, Minutolo F, Rizzolio F, Tuccinardi T. A patent review of Monoacylglycerol lipase (MAGL) inhibitors (2013‐2017). Expert Opin Ther Pat 2017; 27: 1341–1351. [DOI] [PubMed] [Google Scholar]
- 3. Labar G, Wouters J, Lambert DM. A review on the Monoacylglycerol lipase: At the Interface between fat and Endocannabinoid signalling. Curr Med Chem 2010; 17: 2588–2607. [DOI] [PubMed] [Google Scholar]
- 4. Weiderpass E, Persson I, Adami HO, Magnusson C, Lindgren A, Baron JA. Body size in different periods of life, diabetes mellitus, hypertension, and risk of postmenopausal endometrial cancer (Sweden). Cancer Causes and Control 2000; 11: 185–192. [DOI] [PubMed] [Google Scholar]
- 5. Park SL, Goodman MT, Zhang ZF, Kolonel LN, Henderson BE, Setiawan VW. Body size, adult BMI gain and endometrial cancer risk: The multiethnic cohort. Int J Cancer 2010; 126: 490–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li WH, Tai YH, Bai HM et al Targeting stearoyl‐CoA desaturase 1 to repress endometrial cancer progression. Oncotarget 2018; 9: 12064–12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Labar G, Bauvois C, Borel F, Ferrer JL, Wouters J, Lambert DM. Crystal structure of the human monoacylglycerol lipase, a key actor in endocannabinoid signaling. Chembiochem 2010; 11: 218–227. [DOI] [PubMed] [Google Scholar]
- 8. Scalvini L, Piomelli D, Mor M. Monoglyceride lipase: Structure and inhibitors. Chem Phys Lipids 2016; 197: 13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gil‐Ordonez A, Martin‐Fontecha M, Ortega‐Gutierrez S et al Monoacylglycerol lipase (MAGL) as a promising therapeutic target. Biochem Pharmacol 2018; 157: 18–32. [DOI] [PubMed] [Google Scholar]
- 10. Dorsam RT, Gutkind JS. G‐protein‐coupled receptors and cancer. Nat Rev Cancer 2007; 7: 79–94. [DOI] [PubMed] [Google Scholar]
- 11. Ye L, Zhang B, Seviour EG et al Monoacylglycerol lipase (MAGL) knockdown inhibits tumor cells growth in colorectal cancer. Cancer Lett 2011; 307: 6–17. [DOI] [PubMed] [Google Scholar]
- 12. Li CF, Chuang IC, Liu TT et al Transcriptomic reappraisal identifies MGLL overexpression as an unfavorable prognosticator in primary gastrointestinal stromal tumors. Oncotarget 2016; 7: 49986–49997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cipriano M, Gouveia‐Figueira S, Persson E, Nording M, Fowler CJ. The influence of monoacylglycerol lipase inhibition upon the expression of epidermal growth factor receptor in human PC‐3 prostate cancer cells. BMC Res Notes 2014; 7: 441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Matuszak N, Hamtiaux L, Baldeyroux B et al Dual inhibition of MAGL and type II topoisomerase by N‐phenylmaleimides as a potential strategy to reduce neuroblastoma cell growth. Eur J Pharm Sci 2012; 45: 263–271. [DOI] [PubMed] [Google Scholar]
- 15. Hu WR, Chen LZ, Lian YF et al Monoacylglycerol lipase promotes metastases in nasopharyngeal carcinoma. Int J Clin Exp Pathol 2014; 7: 3704–3713. [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang J, Liu Z, Lian Z et al Monoacylglycerol lipase: A novel potential therapeutic target and prognostic indicator for hepatocellular carcinoma. Sci Rep 2016; 6: 35784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Baba Y, Funakoshi T, Mori M et al Expression of monoacylglycerol lipase as a marker of tumour invasion and progression in malignant melanoma. J Eur Acad Dermatol Venereol 2017; 31: 2038–2045. [DOI] [PubMed] [Google Scholar]
- 18. Roy D, Mondal S, Wang C et al Loss of HSulf‐1 promotes altered lipid metabolism in ovarian cancer. Cancer Metab 2014; 2: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Oliveras‐Ferraros C, Vazquez‐Martin A, Cuyas E et al Acquired resistance to metformin in breast cancer cells triggers transcriptome reprogramming toward a degradome‐related metastatic stem‐like profile. Cell Cycle 2014; 13: 1132–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhou BF. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults‐study on optimal cut‐off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci 2002; 15: 83–96. [PubMed] [Google Scholar]
- 21. Li WH, Tai YH, Zhou J et al Repression of endometrial tumor growth by targeting SREBP1 and lipogenesis. Cell Cycle 2012; 11: 2348–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Qiu C, Dongol S, Lv QT, Gao X, Jiang J. Sterol regulatory element‐binding protein‐1/fatty acid synthase involvement in proliferation inhibition and apoptosis promotion induced by progesterone in endometrial cancer. Int J Gynecol Cancer 2013; 23: 1629–1634. [DOI] [PubMed] [Google Scholar]
- 23. Qiu CP, Lv QT, Dongol S, Wang C, Jiang J. Single nucleotide polymorphism of SREBF‐1 gene associated with an increased risk of endometrial cancer in Chinese women. PLoS One 2014; 9: e90491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lin L, Zheng X, Qiu C et al SIRT1 promotes endometrial tumor growth by targeting SREBP1 and lipogenesis. Oncol Rep 2014; 32: 2831–2835. [DOI] [PubMed] [Google Scholar]
- 25. Medes G, Thomas A, Weinhouse S. Metabolism of neoplastic tissue. IV. A study of lipid synthesis in neoplastic tissue slices in vitro. Cancer Res 1953; 13: 27–29. [PubMed] [Google Scholar]
- 26. Qin H, Ruan ZH. The role of monoacylglycerol lipase (MAGL) in the cancer progress. Cell Biochem Biophys 2014; 70: 33–36. [DOI] [PubMed] [Google Scholar]
- 27. Modesitt SC, Hsu JY, Chowbina SR, Lawrence RT, Hoehn KL. Not all fat is equal: Differential gene expression and potential therapeutic targets in subcutaneous adipose, visceral adipose, and endometrium of obese women with and without endometrial cancer. Int J Gynecol Cancer 2012; 22: 732–741. [DOI] [PubMed] [Google Scholar]
- 28. Enriori CL, Reforzo‐Membrives J. Peripheral aromatization as a risk factor for breast and endometrial cancer in postmenopausal women: A review. Gynecol Oncol 1984; 17: I–21. [DOI] [PubMed] [Google Scholar]
- 29. Liang S, Mu K, Wang Y et al CyclinD1, a prominent prognostic marker for endometrial diseases. Diagn Patho 2013; 8: 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Khabaz MN, Abdelrahman AS, Butt NS, al‐Maghrabi B, al‐Maghrabi J. Cyclin D1 is significantly associated with stage of tumor and predicts poor survival in endometrial carcinoma patients. Ann Diagn Pathol 2017; 30: 47–51. [DOI] [PubMed] [Google Scholar]
- 31. Rouette A, Parent S, Girouard J, Leblanc V, Asselin E. Cisplatin increases B‐cell‐lymphoma‐2 expression via activation of protein kinase C and Akt2 in endometrial cancer cells. Int J Cancer 2012; 130: 1755–1767. [DOI] [PubMed] [Google Scholar]


