Abstract
Background
Desmoid tumors are rare locally invasive, benign neoplasms that develop along aponeurotic structures. Current treatment is complicated by associated morbidity and high recurrence rates.
Methods
A retrospective, single‐institution review identified 23 patients (age: 16‐77) with extra‐abdominal desmoid tumors who received CT‐guided percutaneous cryoablation as either a first‐line (61%) or salvage (39%) treatment in 30 sessions between 2014 and 2018. Median maximal lesion diameter was 69 mm (range: 11‐209). Intent was curative in 52% and palliative in 48%. Contrast‐enhanced cross‐sectional imaging was obtained before and after treatment in addition to routine clinical follow‐up.
Results
Technical success was achieved in all patients. The median follow‐up was 15.4 months (3.5‐43.4). Symptomatic improvement was demonstrated in 89% of patients. At 12 months, the average change in viable volume was −80% (range −100% to + 10%) and response by modified response evaluation criteria in solid tumors (mRECIST) was CR 36%, PR 36%, and SD 28% No rapid postablation growth or track seeding was observed. Four patients underwent repeat cryoablation for either residual or recurrent disease. Two patients sustained a major procedural complication consisting of significant neuropraxia.
Conclusion
Cryoablation for desmoid tumors demonstrates a high degree of symptom improvement and local tumor control on early follow‐up imaging with relatively low morbidity.
Keywords: ablation, aggressive fibromatosis, cryoablation, desmoid, sarcoma
Abbreviations
- APC
adenomatous polyposis coli
- CT
computed tomography
- CR
complete response
- DLP
dose‐length product
- FAP
familial adenomatous polyposis
- MAC
monitored anesthesia care
- MCW
Medical College of Wisconsin
- mRECIST
modified response evaluation criteria in solid tumors
- NCCN
National Comprehensive Cancer Network
- NSAID
non‐steroidal anti‐inflammatory
- PD
progressive disease
- PR
partial response
- SD
stable disease
- SERM
selective estrogen receptor modulator
- TKI
tyrosine kinase inhibitor
- TLV
total lesion volume
- VTV
viable tumor volume
1. INTRODUCTION
Desmoid tumors, also known as aggressive fibromatosis, represent a rare form of benign neoplasms that develop along aponeurotic structures. By definition, these tumors have no potential for metastatic spread, although they can be locally invasive. Desmoid tumors exhibit a widely variable natural history, ranging from asymptomatic and quiescent to rapidly growing and locally destructive, with the potential to threaten limb and occasionally life. As a result, these tumors have long challenged specialists to devise a treatment paradigm that will cause the least harm.
Desmoid tumors typically arise from mutations in the adenomatous polyposis coli (APC) or CTNNB1 genes, both of which are fundamental components of the wingless/Integrated signaling pathway that regulates turnover and degradation of β‐catenin. Mutations along this pathway result in inhibition of β‐catenin degradation, leading to its accumulation in the cytoplasm and the nucleus of affected cells. The majority of cases, reportedly 85% to 93%, arise sporadically from β‐catenin‐activation mutations in the CTNNB1 gene.1 Sporadic cases have a predilection for female patients (2:1). The age at diagnosis can be variable, although typically peaks at 25 to 35.2 Desmoid tumors can develop throughout the body. Intra‐abdominal desmoids are more often related to familial APC gene mutations associated with familial adenomatous polyposis (FAP), and management frequently differs from that of extra‐abdominal desmoid tumors. Extra‐abdominal disease is most commonly located in the limb or limb girdle (50%), trunk (43%), or head and neck (7%).3 Desmoid tumor occurrence has also been associated with pregnancy, trauma, and surgery.
NCCN guidelines currently recommend the use of surgical resection, radiation, systemic therapy, and observation for the treatment of both primary and recurrent desmoid tumors. Although wide local excision has served as the gold standard treatment for desmoid tumors in the past, no individual study or meta‐analysis has proven that surgical resection yields superior outcomes over other modalities.4 While resection can be effective in achieving local control, notable disadvantages include a high long‐term recurrence rate of up to 40%,4 destruction of native tissue planes, neurovascular injury, potential activation of residual tumor along dissection planes, and duration of postoperative recovery.
Active surveillance can be a reasonable treatment option in lieu of targeted local therapy for many lesions, as several large, multi‐institutional studies3, 5, 6 have demonstrated spontaneous regression rates of 28% to 50% in recent years. However, identifying these lesions prospectively can be difficult even when accounting for features with high risk for progression, which include large tumor size, young age, and extremity site tumors.3, 7 Surveillance as a first‐line therapy may be contraindicated for patients who are symptomatic or for lesions where further growth would alter future treatment options or lead to functional limitations.
The dual possibility of spontaneous regression and high postoperative recurrence rates has led to a paradigm shift where nonsurgical therapies are often pursued before attempting surgical resection. In this context, percutaneous cryoablation has been explored as a less invasive option for local control and potential cure of desmoid tumors with promising early results.8 Cryoablation causes direct injury to cellular membranes and vascular endothelium through the alternating formation and thawing of intracellular ice.9 Collectively, these processes result in immediate apoptosis at the epicenter of the ablation zone and vascular thrombosis leading to additional ischemic injury. Uniquely, connective tissue structure is often preserved with this ablative therapy. Interestingly, despite many centers migrating toward cryoablation as an alternative treatment option for these patients, outcome studies are limited, and there is currently no mention of ablative therapies in the NCCN treatment algorithm for desmoid tumors.10
Our study presents the largest, most heterogeneous cohort of patients treated with cryoablation to date. While several smaller series have previously described institutional experience in the treatment of extra‐abdominal desmoid tumors,11, 12 most patients in these studies were treated with cryoablation only after previous treatment failure or for inoperable lesions. The purpose of this study is to evaluate the efficacy, safety, and unique advantages of cryoablation for both first‐line and salvage treatment of extra‐abdominal desmoid tumors.
2. MATERIALS AND METHODS
2.1. Patient selection
After gaining institutional review board approval, a retrospective search of our single‐institution database was performed to identify patients with biopsy‐proven desmoid tumors who were treated with percutaneous cryoablation from July 2014 to May 2018, with follow‐up through January 2019. Patients were discussed at our multidisciplinary tumor board and all treatment options were considered before proceeding with cryoablation.
2.2. Data collection and analysis
Data collection was performed through a retrospective chart review and managed using the Research Electronic Data Capture database.13 Variables recorded for analysis included demographic information, previous treatment modalities, adjunctive therapy, imaging parameters, symptom characteristics, and complications.
The total lesion volume (TLV) was estimated by a calculation of ½ × (length × width × height), measured consistently in the same orientation for each patient on all imaging studies. This measurement included both viable disease, defined as nodular enhancing tissue on postcontrast imaging, and ablated tumor, defined as any non‐enhancing component following ablation. The viable tumor volume (VTV), representing only viable (enhancing) disease, was calculated in the same manner. These two values were identical on all preoperative imaging.
Ablation success was determined based on residual tumor enhancement on early postprocedural imaging. Paralleling the surgical literature, an A0 ablation was defined as complete ablation of the tumor without the residual enhancing disease. No procedures were defined as A1 due to the inherent inability to identify the presence of microscopically positive margins without histologic analysis. An A2 ablation was defined as residual enhancing disease on follow‐up imaging and was further stratified arbitrarily into A2a, indicating residual viable disease of less than or equal to 15% by volume compared to the index scan, and A2b, indicating residual viable disease of greater than 15%. In cases without early follow‐up contrast‐enhanced imaging, success was established based on close comparison between the intra‐procedural noncontrast CT obtained at maximal ablation zone size and preprocedural imaging.
All clinic visits with members of our multidisciplinary tumor board, including medical oncology, interventional radiology, radiation oncology, orthopedic oncology, and surgical oncology, were reviewed for change in symptoms and evidence of complications. Given the rarity of this disease and novelty of the treatment approach, a standard follow‐up interval was not established with the current cohort. Follow‐up time was largely based on provider preference, location of the lesion, and patient factors and symptoms. Two patients had not received any follow‐up imaging at the time of analysis. One patient received follow‐up imaging with contrast‐enhanced CT rather than magnetic resonance imaging.
Complications were recorded and classified according to the Society of Interventional Radiology Adverse Event Severity Scale, which was designed to translate to the surgical Clavien‐Dindo scale.14 The severity scale is based on the degree of care escalation, ranging from mild events requiring no therapy or unsubstantial therapy to patient death.
2.3. Cryoablation procedure
After obtaining informed consent, all patients were brought into the CT scanner suite where either general anesthesia or monitored anesthesia care (MAC) was provided for the duration of the procedure. Cryoablation probes were advanced into the target mass using a combination of ultrasound and CT guidance (Figure 1). The operator determined the number of probes used at the time of the procedure based on the size of the intended treatment area and the manufacturer's predicted ablation volumes per instructions for use. If critical structures such as neurovascular bundles, skin, bowel or bladder were within close proximity of the projected treatment zone, special precautionary measures were performed to prevent injury, including hydrodissection, passive skin warming, and/or intraoperative nerve monitoring.
Figure 1.
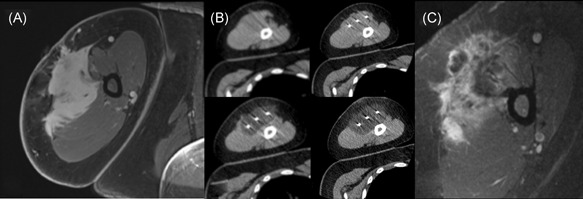
(A) Pre‐ablation axial T1 postcontrast MRI and (B) procedural images obtained for a patient undergoing cryoablation of an upper extremity desmoid tumor. Sequential axial noncontrast CT images obtained intermittently throughout the procedure reveal a progressive increase in the ablation zone that ultimately encompasses the mass. (C) First follow‐up axial T1 (fat sat.) postcontrast MRI revealing a small area of residual enhancing tumor at the posterior ablation margin, which was treated with a second cryoablation procedure, with extensive heterogenous enhancement anteriorly consistent with expected posttreatment change. MRI, magnetic resonance imaging
Our standardized ablation protocol consisted of two consecutive cycles using a 10‐minute freeze followed by a 5‐minute passive thaw, which is frequently used in other solid organ ablations.15 Modifications such as decreased freeze time or decreased energy were used at the discretion of the treating physician if there was imaging or neurologic evidence that the ablation zone was encroaching upon a critical structure. Periodic CT and ultrasound imaging were performed during ablation to ensure that nontarget structures were safely protected from the ablation zone. A noncontrast CT was obtained at the end of the final freeze cycle to define the maximum ablation zone, to identify any untreated area of the mass, and to verify there was no visible injury to surrounding structures. After a routine postprocedural monitoring period, patients were either discharged home on the same day or admitted overnight for observation, depending on the complexity of the procedure and the patient's medical comorbidities.
3. RESULTS
During the study period, 23 individual patients (61% female, 39% male) presenting with a single desmoid lesion were treated with cryoablation (Table 1). The median patient age at the time of the procedure was 40.5 years (range: 16‐77). One patient had FAP. Lesions were located in the abdominal wall (rectus abdominis), superficial soft tissue and deep musculature of the chest wall, shoulder girdle, upper extremity, hip girdle, lower extremity, and head and neck. The median pretreatment tumor size by greatest dimension was 69 mm (range: 11‐209), and median TLV was 69.2 cm3 (range: 0.35‐456.5). Nine patients (39%) had received previous therapy including surgery, radiation therapy, and/or systemic therapy while 14 patients (61%) had received no prior therapy.
Table 1.
Patient demographics
| First‐line | Salvage | Total | |
|---|---|---|---|
| Sex | |||
| Male | 5 | 4 | 9 |
| Female | 9 | 5 | 14 |
| Median age at treatment | 38.5 (17‐77) | 44 (16‐62) | 40.5 (16‐77) |
| Number of patients per treatment intent | |||
| Cure | 9 | 3 | 12 |
| Palliation | 5 | 6 | 11 |
| Location | |||
| Abdominal wall | 4 | 0 | 4 |
| Chest wall | 2 | 1 | 3 |
| Shoulder girdle | 4 | 3 | 7 |
| Upper extremity | 1 | 1 | 2 |
| Gluteal/hip | 2 | 2 | 4 |
| Lower extremity | 0 | 2 | 2 |
| Head/neck | 1 | 0 | 1 |
| Median tumor size (range) | |||
| LD, mm | 69.5 (29‐154) | 69 (11‐209) | 69 (11‐209) |
| Volume, cm3 | 83.3 (4.35‐261) | 82.8 (0.35‐456.5) | 69.2 (0.35‐456.5) |
| Prior treatment | |||
| Surgery | … | 6 | 6 |
| Radiotherapy | … | 5 | 5 |
| TKIs | … | 4 | 4 |
| SERMs | … | 1 | 1 |
| NSAIDs | … | 2 | 2 |
| Tumor development in the scar tissue of a previous, unrelated surgical procedure | 2 | 1 | 3 |
| Antecedent trauma in the region of the first‐line tumor | 3 | 1 | 4 |
Abbreviations: LD, longest cross‐sectional dimension; NSAIDs, non‐steroidal anti‐inflammatories; SERM, selective estrogen receptor modulator; TKI: tyrosine kinase inhibitor.
Thirty cryoablation procedures were performed in these 23 patients (Table 2). Cryoablation was performed with a goal of complete A0 ablation in 12 patients (52%), with an A0 ablation confirmed on follow‐up imaging in 5 patients and presumed based on intra‐procedural imaging in 1 patient (Figure 2 ). For the remaining 11 patients (48%), the decision was made preoperatively not to attempt complete ablation due to either lesion complexity or proximity to critical structures, in which case treatment was performed for palliative symptom control. Four patients underwent a second ablation procedure for the treatment of unintended residual and/or recurrent disease, either in an attempt to achieve A0 ablation or improve local disease control. Average procedure time from initiation of anesthetic to emergence was 173 minutes (SD ± 46), with 57% of cases using MAC and 43% using general endotracheal anesthesia. Average radiation dose defined by DLP was 1108 mGy*cm (SD ± 993). Mean hospital stay was 0.23±0.50 days (range: 0‐2). The mean length of imaging follow‐up was 16.8 ± 10.4 months (range: 3.5‐43.4).
Table 2.
Tumor characteristics before and after initial cryoablation
| Procedural site | Treatment line | Prior therapy | Goal of therapy | Index lesion characteristics pretreatment | Ablation success | ||
|---|---|---|---|---|---|---|---|
| LD, mm | Volume, cm3 | ||||||
| 1 | Abdominal wall | First‐line | … | Cure | 59 | 15.9 | A0 |
| 2 | Shoulder girdle | First‐line | … | Palliative | 94 | 172.3 | A2b (>15%) |
| 3 | Upper extremity | Salvage | XRT, SR | Cure | 11 | 0.4 | A0 |
| 4 | Shoulder girdle | Salvage | SR | Palliative | 69 | 82.8 | A2b (>15%) |
| 5 | Upper extremity | First‐line | … | Cure | 100 | 178.5 | A2b (>15%) |
| 6 | Chest wall | First‐line | … | Cure | 79 | 159.0 | A0 1 |
| 7 | Lower extremity | Salvage | XRT, SR, TKI, S | Palliative | 59 | 21.5 | A2b (>15%) |
| 8 | Shoulder girdle | First‐line | … | Palliative | 54 | 43.0 | A2a (<15%) |
| 9 | Abdominal wall | First‐line | … | Cure | 62 | 47.9 | A2a (<15%) |
| 10 | Abdominal wall | First‐line | … | Cure | 57 | 31.4 | A0 |
| 11 | Chest wall | Salvage | XRT, SR, TKI, S, SERM | Cure | 57 | 34.6 | A2a (<15%) |
| 12 | Gluteal/hip | First‐line | … | Cure | 83 | 24.3 | A2a 2 (<15%) |
| 13 | Shoulder girdle | Salvage | XRT, TKI | Palliative | 69 | 85.9 | A2b 1 (>15%) |
| 14 | Shoulder girdle | First‐line | … | Cure | 29 | 4.4 | A0 |
| 15 | Gluteal/hip | First‐line | … | Palliative | 90 | 26.1 | A2b 3 (>15%) |
| 16 | Abdominal wall | First‐line | … | Cure | 63 | 20.8 | A0 |
| 17 | Head/neck | First‐line | … | Palliative | 74 | 111.0 | A2b (>15%) |
| 18 | Lower extremity | Salvage | SR | Palliative | 69 | 85.7 | A2b 1 (>15%) |
| 19 | Shoulder girdle | Salvage | SR | Cure | 88 | 41.6 | A2a (<15%) |
| 20 | Shoulder girdle | First‐line | … | Palliative | 81 | 158.2 | A2b (>15%) |
| 21 | Chest wall | First‐line | … | Cure | 58 | 7.9 | A2b (>15%) |
| 22 | Gluteal/hip | Salvage | TKI | Palliative | 140 | 455.7 | A2a (<15%) |
| 23 | Gluteal/hip | Salvage | XRT | Palliative | 91 | 456.5 | A2b (>15%) |
Note: Additional follow‐up after third ablation is pending.
Abbreviations: LD, longest cross‐sectional dimension; S, sulindac; SERM, selective estrogen receptor modulator; SR, surgical resection; TKI, tyrosine kinase inhibitor; XRT, radiation therapy.
Based on intra‐procedural imaging, post‐procedure contrast‐enhanced magnetic resonance imaging not available.
Performed as 2 staged procedures, A2a ablation at the completion of the second stage.
Performed as 3 staged procedures, A2b ablation at the completion of the second stage.
Figure 2 .
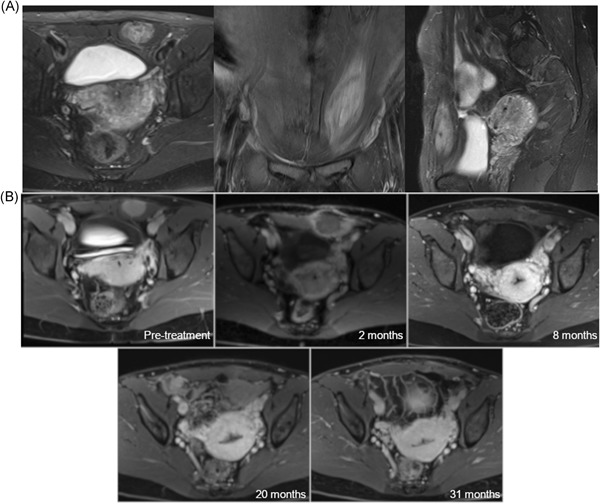
A, Representative pretreatment axial, coronal, and sagittal plane T2‐weighted fat‐suppressed MR images in a patient with a desmoid tumor involving the rectus abdominus. B, Axial T1‐weighted MR images five minutes postcontrast administration at preprocedural evaluation and at 2, 8, 20, and 31 months postablation demonstrating an A0 ablation. MR, magnetic resonance
Of the 20 patients presenting with pain or functional impairment before ablation, 18 (90%) demonstrated symptomatic improvement after cryoablation on early clinical follow‐up.
Follow‐up imaging was available for 21 patients overall, with 14 patients having diagnostic follow‐up imaging of 12 months or more (Figure 3). The average change in VTV at 12 months was −80% (range: −100% to +10%). The average change in TLV at 12 months was −56% (range: −100% to +51%). By modified response evaluation criteria in solid tumors (mRECIST) criteria, response at 12 months was as follows: CR 36% (5 of 14), PR 36% (5 of 14), and SD 28% (4 of 14).
Figure 3.
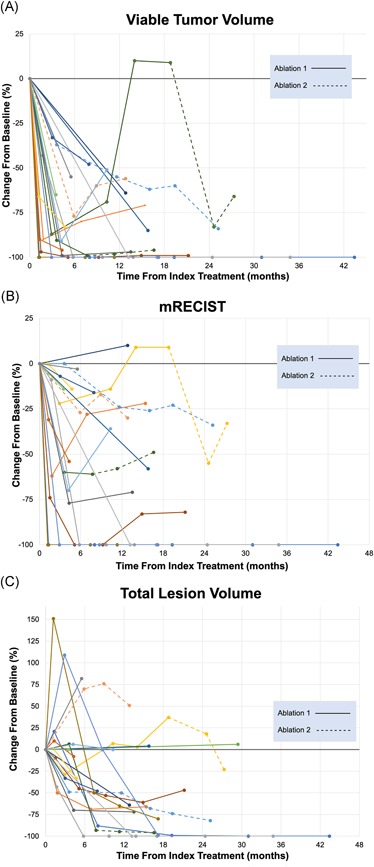
Tumor response following cryoablation by (A) viable tumor volume, (B) mRECIST criteria, and (C) total lesion volume. Given the irregular shape and tumor margins, evaluation on the basis of the single longest cross‐sectional dimension often underestimates response. mRECIST, modified response evaluation criteria in solid tumors [Color figure can be viewed at wileyonlinelibrary.com]
No patients had rapid progression of residual disease after cryoablation. All patients who received an A0 ablation with follow‐up imaging (n = 5) remained disease free at 1 year.
Of the 17 patients treated with incomplete ablation, 11 patients had two or more follow‐up studies available for the evaluation of disease progression using VTV. Of these 11 patients, 7 went on to have progression of untreated disease at the ablation margin.
One patient received adjuvant radiation therapy and another received adjuvant combination systemic and radiation therapy, both due to persistent symptoms and residual disease near neurovascular bundles following intentional A2 ablations. No patients received adjuvant surgical intervention during the study period.
Two procedures (6.7%, 2 of 30) resulted in major complications according to the SIR Adverse Event Classification Guidelines, both significant neuropraxia (Table 3). One patient experienced deltoid weakness after ablation of a neck lesion that resolved after 6 months. A second patient experienced a foot drop from sciatic nerve injury with a near‐total resolution at 9 months. Four additional procedures (13.3%, 4 of 30) resulted in minor complications.
Table 3.
Adverse events
| Treatment location | Complication | SIR classification | Timing | Risk factors | Intervention | Outcome |
|---|---|---|---|---|---|---|
| Shoulder girdle | Hematoma | Grade 1 | Periprocedural | Aspirin use | Observation | Self‐limited |
| Abdominal wall | Skin injury | Grade 1 | Immediate | Tumor location | Observation | Self‐limited |
| Gluteal/hip | Skin injury | Grade 1 | Periprocedural | None | Observation | Self‐limited |
| Abdominal wall | Skin injury | Grade 1 | Peri‐procedural | None | Observation | Self‐limited |
| Head/neck | Weakness, neuropathic pain | Grade 4 | Immediate | Tumor location | Physical therapy | Symptom resolution (after ~6 mo) |
| Gluteal/hip | Nerve injury, foot drop | Grade 4 | Immediate | Tumor location | Physical therapy | Improved but residual symptoms |
Note: Grade 1, mild; Grade 2: moderate; Grade 3, severe; Grade 4, life‐threatening or disabling; Grade 5, patient death.
4. DISCUSSION
The current dataset is one of the largest reported utilizing cryoablation in the treatment of desmoid tumors, and the first cohort with a majority of patients receiving cryoablation as first‐line therapy.
Primarily, our study demonstrates the ability of cryoablation to achieve a very high rate of symptom improvement. The 90% clinical response rate of this study is even more significant when considering over two‐thirds of patients received only partial ablations, suggesting that complete tumor ablation is not necessary to achieve significant improvement in clinical symptoms and that partial ablation can be offered with confidence.
Moreover, we achieved a very high rate of local disease control. Follow‐up imaging at 12 months demonstrated an average tumor volume reduction of 81%, with 71% of patients demonstrating CR or PR by mRECIST criteria. Additionally, a complete ablation was achieved in a large proportion of patients treated with curative intent.
When compared to the existing literature, our series reaffirms the use of cryoablation as a valid treatment for both symptom management and local control of desmoid tumors (Table 4). Most recently, Schmitz et al11 described a series of 18 patients with 26 discrete tumors who underwent a total of 31 treatments. In their study, imaging follow‐up was available for 23 tumors, although only nine tumors (39%) had a follow‐up of 12 months or more. Two notable differences can be observed between our cohorts. First, in their experience, only two patients received cryoablation as first‐line therapy, with the remaining patients having failed one or more prior treatment modalities. Second, their series was more homogeneous in comparison to our patient cohort, as it included a large percentage of paraspinal tumors, which were not well‐represented in our cohort. Given that tumor location can be a prognostic indicator of behavior, this difference may account for some degree of variability between the two studies, particularly with regard to disease recurrence and progression if incompletely treated.
Table 4.
Comparison studies
| MCW | Schmitz et al11 | Havez et al12 | |
|---|---|---|---|
| Number of patients | 23 | 18 | 13 |
| Number of discrete lesions | 23 | 26 | 17 |
| Number of treatments | 30 | 31 | 17 |
| History of FAP | 4% (1/23) | 44% (8/18) | 15% (2/13) |
| Mean follow‐up time (mos.) | 16.8 ± 10.4 | 16.2 ± 20.0 | 11.3 ± 8.1 |
| Number of tumors with follow‐up imaging | 21* | 23 a | 17 |
| Number of tumors with ≥12‐mo follow‐up | 15 | 9 | 8 |
| Mean pretreatment lesion LD, mm | 80.5 ± 41.3 b | 64 ± 3.1 (17‐140) | 53 (17‐142) |
| Mean pretreatment lesion volume, cm3 | 114.8 ± 129.7 b (0.4‐456.5) | 38.1 ± 38.1 (1.6‐118.3) | unknown |
| Mean Δ lesion LD (at last follow‐up) | −50.3% (−100% to +10%) | −53% (−100% to +69% | −37.6% (−100% to +17%) |
| Mean Δ lesion volume (at last follow‐up) | −80.8% (−100% to −48%) | −70% (−100% to +20%) | −87% (−100% to +40%) |
| First‐line lesions | 61% (14/23) | 8% (2/26) | 6% (1/17) |
| Salvage lesions | 39% (9/23) | 92% (24/26) | 94% (16/17) |
| A0 ablation | 26% (6/23) | unknown | 53% (9/17) |
| mRECIST response b (CR/PR/SD/PD) | 5/9/6/0 | 9/11/4/2 | 1/7/9/0 |
| Symptom control | 90% (18/20) | 80% (4/5) | 82% (14/17) |
| Complications—major | 6.7% (2/30) | 0% (0/31) | 5.8% (1/17) |
| Complications—all | 20% (6/30) | 9% (3/31) | 18% (3/17) |
Abbreviations: CR, complete response; CT, computed tomography; FAP, familial adenomatous polyposis; LD, longest cross‐sectional dimension; MCW, Medical College of Wisconsin; MR, magnetic resonance; PD, progressive disease; PR, partial response; SD, stable disease.
MR performed for 20 patients, CT performed for 1 patient (excluded from mRECIST analysis).
MR performed for 12 patients, CT performed for 6 patients.
Longest enhancing dimension.
Enhancing lesion volume.
An earlier series published by Havez et al12 in 2014 evaluated cryoablation of 17 desmoid tumors in 13 patients for a total of 17 sessions. Similar to our series, this group reported that 83% of patients were pain‐free at 1 year. This study also demonstrated encouraging local control rates despite a high rate of incomplete ablation (47%). Follow‐up imaging was available at 6 months for all patients, but only four patients had a follow‐up of greater than 12 months. Given the variable course of desmoid tumors and prolonged response following ablation, results at short‐term follow‐up are viewed with caution.
In both cohorts, the patients who ultimately had a progression of disease shared similar features, which included multiple prior treatment failures, potentially a reflection of individual tumor biology, and close proximity to critical structures, potentially limiting aggressive ablation margins.
The efficacy of cryoablation compares favorably to the treatment modalities included in the current NCCN guidelines: surgical resection, radiation (definitive or adjuvant), and systemic therapy. The absence of recurrent disease after complete A0 ablation on follow‐up imaging at 1 year is promising, especially when viewed in the context of a 23% recurrence rate at 2 years following an R0 surgical resection.4 Longer term follow‐up is needed to assess how A0 cryoablation compares to the R0 5‐year recurrence rate of almost 40%.4 Radiation therapy has been shown to provide benefit in cases of definitive therapy, adjuvant therapy following surgical resection, and retreatment in cases of recurrence.16, 17 A recent Phase II trial of 44 patients receiving 56 Gy in 28 fractions for primary and recurrent extra‐abdominal lesions achieved 3‐year imaging responses of CR 14%, PR 36%, SD 41%, and PD 7% by RECIST criteria, similar to the results of the current study despite differences in assessment.18 Several small studies investigating systemic therapy have shown moderate benefit with NSAIDs, antiestrogen therapy, and cytotoxic chemotherapy with widely variable rates of partial imaging response in the ranges of 15% to 50%.19, 20 More recently, tyrosine kinase inhibitors have been evaluated with moderate effect as well with imaging response rates of 5% to 20% at 1 year and a larger majority exhibiting progression‐free disease between 60% and 70%.21, 22
In six patients, a curative A0 ablation was attempted, but unfortunately not initially achieved. For some lesions, it can be quite challenging to ensure the ablation zone completely covers the tumor. A major reason for this is poor tumor visibility during ablation due to the minimal attenuation difference between tumor and surrounding muscle on the unenhanced CT imaging used during a cryoablation procedure, especially in lesions that are large with invasive or multi‐focal morphologies. Advanced imaging techniques such as the fusion of preoperative contrast‐enhanced imaging with intra‐procedural imaging (either ultrasound, CT, or magnetic resonance) and the placement of ablation probes using needle guidance and ablation planning software can minimize this. Significant improvements in these workflows will be necessary before adoption is widespread; however, we believe the rate of unintentional, incomplete ablations will continue to shrink as these advanced imaging technologies mature and become more efficient and user‐friendly in the future.
Several features of cryoablation provide a distinct advantage over alternative treatment options. Unlike surgical resection, cryoablation generally preserves connective tissue planes. As a result, delayed recurrence after ablation is usually in the same anatomic location or compartment, while recurrence after surgical resection can occur in adjacent tissue compartments that were previously uninvolved, often complicating subsequent treatment. Cosmetically, surgical resections may produce a large soft tissue defect necessitating complex reconstruction techniques whereas cryoablation causes only minimal scarring as a result of small skin nicks. Unlike radiation therapy, cryoablation does not subject patients to the risk of skin and soft tissue fibrosis, chronic lymphedema, or radiation‐induced malignancy. Given that younger patients have disproportionately worse local control with radiation therapy, the risk of severe radiation‐associated toxicities23 should be thoughtfully considered when choosing a treatment modality. Additionally, cryoablation can be used repeatedly within the same treatment area if needed without any effective “dose limit.”
In general, cryoablation is well tolerated by patients with minimal impact on daily life. Procedural recovery is relatively easy as demonstrated by our high proportion of same‐day outpatient procedures. Many times, general anesthesia can be avoided for straightforward cases away from critical structures. Surgical recovery and postoperative limitations will be proportional to the complexity of the excision and reconstruction, while nearly all cryoablation patients can gradually return to normal activity within one to 2 weeks. Arguably, one or two cryoablation procedures causes significantly less disruption to a patient's employment and personal obligations than radiation therapy, which often requires close to 30 therapeutic visits, or systemic therapy which may require daily medications and frequent office visits for drug administration or toxicity monitoring.
Our low rate of procedural complications compares favorably to surgical and radiation therapy complication rates.19 The heterogeneity of our dataset underscores the unique risk profile of cryoablation for desmoid tumors. In our experience, the most common structures at risk for injury during cryoablation of extra‐abdominal desmoid tumors include the skin, bowel, and adjacent nerves. In some cases, we have utilized intraoperative neurologic monitoring with motor and somatosensory evoked potentials to provide real‐time feedback regarding nerve function during ablation. While we have found these techniques helpful, the accuracy of neurologic monitoring feedback can be limited by many intra‐procedural parameters, most notably type of anesthesia. In our study, we experienced two major procedural complications (7%) that were both nerve injuries, one despite neurologic monitoring. The targeted tumors were in close proximity to or invading adjacent neurologic structures. In both cases, the injury occurred in part due to a small margin of error and difficult visualization of the nerve on unenhanced CT imaging with some degree of image degradation from the metallic ablation probes.
Our study has several limitations as a small, retrospective series with variable follow‐up times. It is important to highlight that the current patient cohort is heterogenous and comprised of several distinct patient groups which should be considered somewhat separately. A key distinction is between the subset in which a complete A0 ablation with an intent to cure can be performed safely and the subset in which it cannot, usually due to pre‐existing proximity to or invasion of critical structures, most often nerves, which would carry unacceptable morbidity if injured. In these cases, only partial ablation was performed with the intent to debulk the tumor mass for palliative symptom control, which inherently biases the collective imaging response of the cohort. Notably, our series did not include any patients with intra‐abdominal desmoid lesions, which are typically are more aggressive, more difficult to treat with ablation, and more prone to recurrence.
5. CONCLUSION
In summary, cryoablation should be considered safe and effective as both a first‐line and salvage treatment option for desmoid tumors requiring local disease control. While early recurrence rates are favorable, additional long‐term follow‐up will be essential in determining whether disease control rates compare favorably with current standards of care in this very persistent disease. However, cryoablation offers many advantages over therapies currently recommended by national guidelines as a minimally‐invasive, single‐session, and repeatable procedure with low morbidity and swift recovery times.
As in many sarcoma centers worldwide, our institutional treatment paradigm has changed over the last several years. If decided by our multidisciplinary tumor board that treatment is preferable to active surveillance, cryoablation is often used in both first‐line and salvage situations, while reserving systemic therapy and radiation therapy for the adjunct setting in cases with residual or recurrent disease that cannot be safely ablated. Surgery is generally reserved for intra‐abdominal lesions unresponsive to alternative therapies.
While clinical trials evaluating cryoablation of desmoid tumors are ongoing outside of the United States, they currently do not address its use as a first‐line treatment modality, but rather as a means of salvage therapy.24 We believe that this study provides sufficient evidence to support a prospective clinical trial with long‐term follow‐up evaluating cryoablation as first‐line therapy for patients that would benefit from local tumor control.
SYNOPSIS
Percutaneous cryoablation is a safe and effective treatment for desmoid tumors as both a first‐line and salvage treatment option. Short‐term follow‐up demonstrates effective symptom and disease control on par with traditional therapy with significantly reduced morbidity and quick recovery.
Redifer Tremblay K, Lea WB, Neilson JC, King DM, Tutton SM. Percutaneous cryoablation for the treatment of extra‐abdominal desmoid tumors. J Surg Oncol. 2019;120:366‐375. 10.1002/jso.25597
References
REFERENCES
- 1. Nieuwenhuis MH, Casparie M, Mathus‐Vliegen LMH, Dekkers OM, Hogendoorn PC, Vasen HF. A nation‐wide study comparing sporadic and familial adenomatous polyposis‐related desmoid‐type fibromatoses. Int J Cancer. 2011;129:256‐261. 10.1002/ijc.25664 [DOI] [PubMed] [Google Scholar]
- 2. Molloy AP, Hutchinson B, O'toole GC. Extra‐abdominal desmoid tumours: a review of the literature. Sarcoma. 2012;2012:1‐9. 10.1155/2012/578052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eastley N, McCulloch T, Esler C, et al.Extra‐abdominal desmoid fibromatosis: a review of management, current guidance and unanswered questions. Eur J Surg Oncol. 2016;42:1071‐1083. 10.1016/j.ejso.2016.02.012 [DOI] [PubMed] [Google Scholar]
- 4. Salas S, Dufresne A, Bui B, et al. Prognostic factors influencing progression‐free survival determined from a series of sporadic desmoid tumors: A wait‐and‐see policy according to tumor presentation. J Clin Oncol. 2011;29:3553‐3558. 10.1200/JCO.2010.33.5489 [DOI] [PubMed] [Google Scholar]
- 5. Bonvalot S, Ternès N, Fiore M, et al. Spontaneous regression of primary abdominal wall desmoid tumors: More common than previously thought. Ann Surg Oncol. 2013;20:4096‐4102. 10.1245/s10434-013-3197-x [DOI] [PubMed] [Google Scholar]
- 6. Colombo C, Miceli R, Le Péchoux C, et al. Sporadic extra abdominal wall desmoid‐type fibromatosis: surgical resection can be safely limited to a minority of patients. Eur J Cancer. 2015;51:186‐192. 10.1016/j.ejca.2014.11.019 [DOI] [PubMed] [Google Scholar]
- 7. Crago AM, Denton B, Salas S, et al. A prognostic nomogram for prediction of recurrence in desmoid fibromatosis. Ann Surg. 2013;258:347‐353. 10.1097/SLA.0b013e31828c8a30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kurup AN, Callstrom MR. Expanding role of percutaneous ablative and consolidative treatments for musculoskeletal tumours. Clin Radiol. 2017;72:645‐656. 10.1016/j.crad.2017.02.019 [DOI] [PubMed] [Google Scholar]
- 9. Cazzato RL, Garnon J, Ramamurthy N, et al. Percutaneous image‐guided cryoablation: current applications and results in the oncologic field. Med Oncol. 2016;33:140 10.1007/s12032-016-0848-3 [DOI] [PubMed] [Google Scholar]
- 10. von Mehren M, Randall RL, Benjamin RS, et al. Soft tissue sarcoma, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:536‐563. 10.6004/jnccn.2018.0025 [DOI] [PubMed] [Google Scholar]
- 11. Schmitz JJ, Schmit GD, Atwell TD, et al. Percutaneous cryoablation of extraabdominal desmoid tumors: a 10‐year experience. Am J Roentgenol. 2016;207:190‐195. 10.2214/AJR.15.14391 [DOI] [PubMed] [Google Scholar]
- 12. Havez M, Lippa N, Al‐Ammari S, et al. Percutaneous image‐guided cryoablation in inoperable extra‐abdominal desmoid tumors: a study of tolerability and efficacy. Cardiovasc Intervent Radiol. 2014;37:1500‐1506. 10.1007/s00270-013-0830-9 [DOI] [PubMed] [Google Scholar]
- 13. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)–A metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khalilzadeh O, Baerlocher MO, Shyn PB, et al. Proposal of a new adverse event classification by the society of interventional radiology standards of practice committee. J Vasc Interv Radiol. 2017;28:1432‐1437. 10.1016/j.jvir.2017.06.019 [DOI] [PubMed] [Google Scholar]
- 15. Littrup PJ, Ahmed A, Aoun HD, et al. CT‐guided percutaneous cryotherapy of renal masses. J Vasc Interv Radiol. 2007;18:383‐392. 10.1016/j.jvir.2006.12.007 [DOI] [PubMed] [Google Scholar]
- 16. Lev D, Kotilingam D, Wei C, et al. Optimizing treatment of desmoid tumors. J Clin Oncol. 2007;25:1785‐1791. 10.1200/JCO.2006.10.5015 [DOI] [PubMed] [Google Scholar]
- 17. Nuyttens JJ, Rust PF, Thomas CR, Turrisi AT. Surgery versus radiation therapy for patients with aggressive fibromatosis or desmoid tumors: a comparative review of 22 articles. Cancer. 2000;88:1517‐1523. [DOI] [PubMed] [Google Scholar]
- 18. Keus RB, Nout RA, Blay J‐Y, et al. Results of a phase II pilot study of moderate dose radiotherapy for inoperable desmoid‐type fibromatosis—an EORTC STBSG and ROG study (EORTC 62991‐22998). Ann Oncol. 2013;24:2672‐2676. 10.1093/annonc/mdt254 [DOI] [PubMed] [Google Scholar]
- 19. Hansmann A, Adolph C, Vogel T, et al. High‐dose tamoxifen and sulindac as first‐line treatment for desmoid tumors. Cancer. 2004;100:612‐620. 10.1002/cncr.11937 [DOI] [PubMed] [Google Scholar]
- 20. Fiore M, Colombo C, Radaelli S, et al. Hormonal manipulation with toremifene in sporadic desmoid‐type fibromatosis. Eur J Cancer. 2015;51:2800‐2807. 10.1016/j.ejca.2015.08.026 [DOI] [PubMed] [Google Scholar]
- 21. Penel N, Le Cesne A, Bonvalot S, et al. Surgical versus non‐surgical approach in primary desmoid‐type fibromatosis patients: a nationwide prospective cohort from the French Sarcoma Group. Eur J Cancer. 2017;83:125‐131. 10.1016/j.ejca.2017.06.017 [DOI] [PubMed] [Google Scholar]
- 22. Kasper B, Gruenwald V, Reichardt P, et al. Imatinib induces sustained progression arrest in RECIST progressive desmoid tumours: Final results of a phase II study of the German Interdisciplinary Sarcoma Group (GISG). Eur J Cancer. 2017;76:60‐67. 10.1016/j.ejca.2017.02.001 [DOI] [PubMed] [Google Scholar]
- 23. Bishop AJ, Zarzour MA, Ratan R, et al. Long‐term outcomes for patients with desmoid fibromatosis treated with radiation therapy: a 10‐year update and re‐evaluation of the role of radiation therapy for younger patients. Int J Radiat Oncol. 2019;103:1167‐1174. 10.1016/j.ijrobp.2018.12.012 [DOI] [PubMed] [Google Scholar]
- 24. Gangi A Evaluation of the cryodestruction of non abdominopelvic desmoid tumors in patients progressing despite medical treatment‐tabular view‐clinicaltrials.gov. ClinicalTrials.Gov. 2018. https://clinicaltrials.gov/ct2/show/record/NCT02476305 (accessed August 14, 2018).


