Abstract
Objective
There is no curative treatment for osteoarthritis (OA), which is the most common form of arthritis. This study was undertaken to identify causal risk factors of knee, hip, and hand OA.
Methods
Individual‐level data from 384,838 unrelated participants in the UK Biobank study were analyzed. Mendelian randomization (MR) analyses were performed to test for causality for body mass index (BMI), bone mineral density (BMD), serum high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol, and triglyceride levels, type 2 diabetes, systolic blood pressure (BP), and C‐reactive protein (CRP) levels. The primary outcome measure was OA determined using hospital diagnoses (all sites, n = 48,431; knee, n = 19,727; hip, n = 11,875; hand, n = 2,330). Odds ratios (ORs) with 95% confidence intervals (95% CIs) were calculated.
Results
MR analyses demonstrated a robust causal association of genetically determined BMI with all OA (OR per SD increase 1.57 [95% CI 1.44–1.71]), and with knee OA and hip OA, but not with hand OA. Increased genetically determined femoral neck BMD was causally associated with all OA (OR per SD increase 1.14 [95% CI 1.06–1.22]), knee OA, and hip OA. Low systolic BP was causally associated with all OA (OR per SD decrease 1.55 [95% CI 1.29–1.87]), knee OA, and hip OA. There was no evidence of causality for the other tested metabolic factors or CRP level.
Conclusion
Our findings indicate that BMI exerts a major causal effect on the risk of OA at weight‐bearing joints, but not at the hand. Evidence of causality of all OA, knee OA, and hip OA was also observed for high femoral neck BMD and low systolic BP. However, we found no evidence of causality for other metabolic factors or CRP level.
INTRODUCTION
Osteoarthritis (OA) is the most common form of arthritis in developed countries, representing an increasing health economic burden 1. Patients with knee or hip OA have excess all‐cause mortality compared with the general population, and there is no curative treatment for OA 2. Besides age and sex, modifiable factors have been shown to be associated with OA risk, the highest level of evidence being for obesity and joint injury 3, 4. Obesity is a major risk factor for OA incidence and progression at the knee, and to a lesser extent, at the hip 5. In addition, the role of obesity is strongly supported by findings of improvement of knee symptoms in patients undergoing weight loss 6, 7.
Besides body mass index (BMI), other factors have been described as being associated with either prevalence, incidence or progression of OA. High bone mineral density (BMD) was determined to be associated with increased risk of radiographic hip OA 8 or knee OA 9, and with total joint replacement 10. However, results from prospective studies of incident radiographic OA or OA progression are controversial 11. The concept of a “metabolic OA” phenotype was recently proposed 4. However, as metabolic factors are closely related to BMI, their BMI‐independent contribution to the risk of OA is unknown. Previous studies have shown that the presence of the metabolic syndrome and many of its components are associated with the risk of hand and knee OA 12, 13, 14. Most associations with each of the metabolic syndrome components, however, become nonsignificant after adjustment for BMI 13, 15. In addition, conflicting data regarding type 2 diabetes as a BMI‐independent predictor of OA have been reported 16, 17. The results from classic epidemiologic studies may be affected by residual confounding or reverse causation. Thus, there is an unmet need for a well‐powered study to identify causal risk factors of OA. We hypothesized that causal associations may differ by OA site.
Mendelian randomization (MR) can be used to test for a causal association between a risk factor and a particular outcome 18. Two recent MR studies have demonstrated that overweight or high BMI is causally associated with increased risk of OA 19, 20. Therefore, to reduce potential bias through genetic associations with confounders, MR analyses of other causal factors should include sensitivity analyses without genetic instrument variables also associated with BMI.
The present study aimed to identify causal risk factors for site‐specific OA in the complete UK Biobank data set. Using genetic instrument variables from previously published genome‐wide association study (GWAS) meta‐analysis, the possible causality of BMI, BMD, serum high‐density lipoprotein (HDL) cholesterol, low‐density lipoprotein (LDL) cholesterol, and triglyceride levels, type 2 diabetes, systolic blood pressure (BP), and C‐reactive protein (CRP) levels in the risk of developing knee, hip, and hand OA was evaluated. The primary outcome measure was hospital diagnosis of OA. In secondary analyses, more severe cases of knee and hip OA, as identified by joint replacement, were also evaluated.
SUBJECTS AND METHODS
UK Biobank study subjects and ethics approval
In this study, conducted using the UK Biobank resource (http://www.ukbiobank.ac.uk/), 502,647 individuals between the ages of 37 and 76 years were recruited from across the UK from 2006 to 2010 21. Participants provided a range of information regarding health status, demographics, and lifestyle via questionnaires and interviews. In addition, they were physically examined for anthropometric measurements, BP readings, and an estimation of BMD of the heel using a noninvasive method. The full data set was downloaded in April 2018. We included 384,838 unrelated participants of white European descent with valid data on the outcome measure and relevant covariates (age, sex, BMI). Pairs of individuals up to third‐degree relatives were identified using the robust estimated kinship coefficients from the King software 22. The UK Biobank has ethical approval from the North West Multi‐Centre Research Ethics Committee, and informed consent was obtained from all participants. The present research was approved by the UK Biobank Research and Access Committee (application no. 26952).
Outcome measure definitions
The outcome measure was obtained from the UK Biobank database downloaded in April 2018. The primary outcome measure in the present study was OA as defined by hospital diagnoses (http://biobank.ctsu.ox.ac.uk/crystal/label.cgi?id=2022) using the International Classification of Diseases, Ninth Revision (ICD‐9) codes or the Tenth Revision (ICD‐10) for all OA (n = 48,431), knee OA (n = 19,727), hip OA (n = 11,875), and hand OA (n = 2,330) (see Supplementary Table 1, available on Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.40928/abstract). Of the total of 48,731 all OA cases, 11 were identified with ICD‐9 codes and 48,420 with ICD‐10 codes. In secondary analyses, we evaluated self‐reported OA from the UK Biobank questionnaires and more severe cases of OA defined by a history of knee replacement or hip replacement. Patients with valid information from operative procedures summary information for knee replacement (W40‐42) or hip replacement (W37‐39), according to the Office of Population Censuses and Surveys Classification of Interventions and Procedures, version 4 (http://biobank.ctsu.ox.ac.uk/crystal/label.cgi?id=2025), were included. We excluded patients who had a concomitant diagnosis of chronic inflammatory arthritis or aseptic osteonecrosis at the time of intervention. For hip replacement, we further excluded patients who had concomitantly self‐reported hip fracture (Supplementary Table 1, http://onlinelibrary.wiley.com/doi/10.1002/art.40928/abstract). In the UK Biobank, the hospital admissions registers began in 1981 and were updated until February 29, 2016. The last OA case date registered in our data set was February 25, 2016.
As expected, there was a substantial overlap between the different OA outcomes (Supplementary Table 2, http://onlinelibrary.wiley.com/doi/10.1002/art.40928/abstract). Interestingly, among the 11,875 individuals with a hospital diagnosis of hip OA, only 4,445 (37%) were identified in the self‐reported OA questionnaire. Therefore, we selected outcome measure definitions using hospital diagnoses for our primary analyses.
Causal associations using MR
To assess causal associations between risk markers and OA, we performed MR analyses. We used genetic instrument variables obtained from selected GWAS as proxies for BMI 23, femoral neck BMD and lumbar spine BMD 24, serum HDL cholesterol, LDL cholesterol, and triglyceride levels 25, type 2 diabetes 26, systolic BP 27, and CRP levels 28 (Supplementary Table 3, http://onlinelibrary.wiley.com/doi/10.1002/art.40928/abstract). As genetic variants are randomly distributed at birth, they are unaffected by confounders. We then regressed the association of these single‐nucleotide polymorphisms (SNPs) on the outcome measure, weighing their effect by the magnitude of their effect upon the corresponding exposure.
Our primary MR method was the 2‐sample inverse‐variance weighting, using the effect estimates for the exposure from the corresponding GWAS. Sensitivity analyses were performed using the MR‐Egger method to preclude pleiotropy 29 (Supplementary Figure 1, http://onlinelibrary.wiley.com/doi/10.1002/art.40928/abstract), the weighted median MR method, and the penalized weighted median MR method. When the exposure parameter was available (for BMI, type 2 diabetes, and systolic BP), we also performed 1‐sample MR methods using the effect estimates for exposure in the UK Biobank. Power analyses were performed for each exposure and OA outcome (Supplementary Table 4, http://onlinelibrary.wiley.com/doi/10.1002/art.40928/abstract). Early on we identified a robust causal effect of BMI on the risk of OA and therefore for other candidate causal traits, we performed additional MR sensitivity analyses excluding genetic instrument variables that are also associated (P < 0.05) with BMI (Supplementary Tables 5–13, http://onlinelibrary.wiley.com/doi/10.1002/art.40928/abstract). Detailed methods for MR studies are described in Supplementary Methods, http://onlinelibrary.wiley.com/doi/10.1002/art.40928/abstract.
We evaluated 9 candidate causal risk factors, and for all these traits, we first evaluated the association with all OA, as this was the outcome measure with the highest power for the 2‐sample inverse‐variance weighting method (required Bonferroni‐corrected P value <0.0056 [P = 0.05/9]). If this threshold was met for all OA, a nominal P value of 0.05 was required for the site‐specific analyses of knee, hip, or hand OA. Therefore, 95% confidence intervals (95% CIs) for all OA are presented before and after adjustment for multiple comparisons (n = 9).
Comorbidities and covariates
For BMI, we used the calculated measures of weight/height² available in the anthropometrics data at recruitment. Two measures of systolic BP were obtained at the inclusion interview. We used the mean value of the 2 available measures for each individual. Information on the use of antihypertensive medication was collected during the same visit. For the population description at baseline, individuals with prevalent type 2 diabetes were identified using self‐reported diabetes at recruitment in combination with a hospital diagnosis of type 2 diabetes by ICD‐10 code (E11). For the MR analyses, we included prevalent and incident cases of type 2 diabetes, using any diagnosis of type 2 diabetes by ICD‐10 codes in the database. Information on current smoking status (yes or no) was collected at the time of the interview.
RESULTS
The mean age of the 384,838 unrelated individuals of European descent included in the present study was 56.8 years. Patient characteristics and main comorbidities at recruitment are presented in Table 1. Our primary analyses were inverse‐variance weighting by 2‐sample MR for all OA, and after adjustment for multiple comparisons (9 traits; P < 0.0056), causal evidence was observed for high BMI (P = 3.0 × 10−24), high femoral neck BMD (P = 3.0 × 10−4), and low systolic BP (P = 2.3 × 10−6), as shown in Figure 1 (also see Supplementary Table 14, http://onlinelibrary.wiley.com/doi/10.1002/art.40928/abstract). For all OA, there was no clear evidence of pleiotropy, and the MR‐Egger intercept demonstrated no significance for any of the tested exposures.
Table 1.
Participant characteristics and main comorbidities at recruitmenta
| All subjects at recruitment | |
| Age, years | 56.8 ± 8.0 |
| Female sex, no. (%) | 207,789 (54.0) |
| Body mass index, kg/m2 | 27.4 ± 4.8 |
| Cholesterol‐lowering medication users, no. (%) | 66,379 (17.3) |
| Prevalent type 2 diabetes, no. (%) | 10,170 (2.6) |
| Prevalent and incident type 2 diabetes, no. (%) | 16,946 (4.4) |
| Antihypertensive medication users, no. (%) | 79,081 (20.6) |
| Systolic BP, mm Hg | 138.0 ± 18.6 |
| Systolic BP, excluding antihypertensive medication users, mm Hg | 136.2 ± 18.3 |
| Current smokers, no. (%) | 39,840 (10.4) |
| OA | |
| All locations combined, no. (%) | |
| All OA | 48,431 (12.6) |
| Self‐reported OA | 34,229 (8.9) |
| Knee, no. (%) | |
| Knee OA | 19,727 (5.1) |
| Knee replacement | 9,716 (2.6) |
| Hip, no. (%) | |
| Hip OA | 11,875 (3.1) |
| Hip replacement | 9,932 (2.6) |
| Hand OA, no. (%) | 2,330 (0.6) |
The n value for each variable was 384,838, except as follows: for cholesterol‐lowering medication users and antihypertensive medication users, n = 384,468; for systolic blood pressure (BP), n = 384,515; for systolic BP, excluding antihypertensive medication users, n = 305,170; for current smokers, n = 383,470; for knee replacement, n = 379,840; and for hip replacement, n = 379,197. Except where indicated otherwise, values are the mean ± SD. OA = osteoarthritis.
Figure 1.
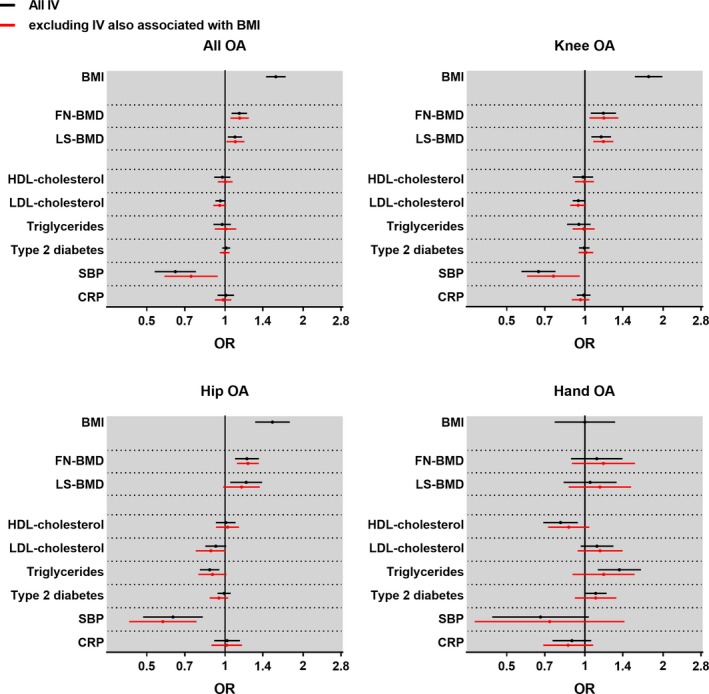
Causal associations between genetically determined risk factors and osteoarthritis (OA) by site. For each site, the odds ratio (OR) and 95% confidence interval for the risk of OA are represented for each factor, as determined using the 2‐sample Mendelian randomization inverse‐variance weighting method for body mass index (BMI; per SD increase), femoral neck bone mineral density (FN‐BMD; per SD increase), lumbar spine BMD (LS‐BMD; per SD increase), serum levels of high‐density lipoprotein (HDL) cholesterol, low‐density lipoprotein (LDL) cholesterol, and triglyceride (per SD increase), systolic blood pressure (SBP; per SD increase), C‐reactive protein (CRP) level (per ln[mg/liter] increase), or presence of type 2 diabetes. The total number of subjects is 384,838, with 48,431 all OA, 19,727 knee OA, 11,875 hip OA, and 2,330 hand OA cases. IV = instrument variable.
Evidence of a causal association of BMI with knee and hip OA, but not hand OA
The 2‐sample MR revealed that an increase in genetically determined BMI was causally associated with all OA (odds ratio [OR] per SD increase 1.57 [95% CI 1.44–1.71; adjusted for 9 comparisons 1.39–1.77]), as shown in Figure 1 and Supplementary Table 14. As individual‐level data were available and as the UK Biobank was well‐powered for the outcome of all OA, we performed stratified analyses to compare the causal association of BMI in different subgroups. The causal association of genetically determined BMI with all OA was robust and similar in young individuals (below the median age of cases) and older individuals (above the median age of cases), in males and females, as well as in subpopulations stratified by smoking status or type 2 diabetes (Figure 2). Causal associations for BMI were also found at weight‐bearing joints (OR per SD increase 1.76 [95% CI 1.56–1.99] in knee OA and 1.52 [95% CI 1.31–1.78] in hip OA), but not at the hand (OR 1.00 [95% CI 0.76–1.31]). Similar results were obtained with the other 2‐sample MR methods and 1‐sample MR methods (see Supplementary Tables 14–17, http://onlinelibrary.wiley.com/doi/10.1002/art.40928/abstract).
Figure 2.
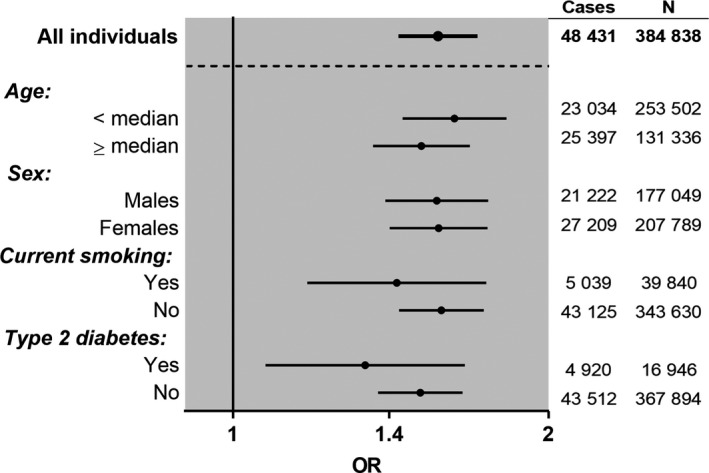
Stratified analyses of the causal associations of body mass index (BMI) with all osteoarthritis (OA). Odds ratios (ORs) and 95% confidence intervals are shown for the causal associations between BMI (per SD increase) and the risk of all OA, as determined using the 2‐sample Mendelian randomization inverse‐variance weighting method in analyses stratified by age (median of cases), sex, current smoking status, or type 2 diabetes.
Evidence of a causal association of BMD with knee and hip OA
Genetically determined femoral neck BMD was causally associated with all OA (OR per SD increase 1.14 [95% CI 1.06–1.22; adjusted for 9 comparisons 1.03–1.25]; Figure 1). The causal association was found to be of similar magnitude at the knee (OR 1.18 [95% CI 1.05–1.32]) and hip (OR 1.22 [95% CI 1.09–1.35]), but not at the hand (OR 1.11 [95% CI 0.88–1.39]; Figure 1 and Supplementary Table 14). Sensitivity analyses, excluding BMI‐associated SNPs (Figure 1), as well as analyses using other MR methods, revealed similar results (Supplementary Table 14). For lumbar spine BMD, the causal association with all OA was less pronounced and only nominally significant. Site‐specific analyses demonstrated a causal association of lumbar spine BMD at the knee (OR 1.15 [95% CI 1.06–1.26]), which remained after the exclusion of genetic instrument variables also associated with BMI (Supplementary Table 14).
Evidence of a causal association of systolic BP with hip OA
Systolic BP was causally inversely associated with all OA (OR per SD increase 0.64 [95% CI 0.54–0.77; adjusted for 9 comparisons 0.50–0.83]), knee OA (OR 0.66 [95% CI 0.57–0.77]), and hip OA (OR 0.63 [95% CI 0.48–0.82], Figure 1 and Supplementary Table 14). The associations remained unchanged when subjects receiving antihypertensive medication were excluded from the analyses (see Supplementary Figure 2, http://onlinelibrary.wiley.com/doi/10.1002/art.40928/abstract). Similar results were obtained in sensitivity analyses excluding BMI‐associated SNPs, and with other MR methods (Supplementary Tables 14–17, http://onlinelibrary.wiley.com/doi/10.1002/art.40928/abstract).
No evidence of a causal association of other tested metabolic markers or CRP level with OA
No other tested candidate traits (HDL cholesterol, LDL cholesterol, triglyceride level, type 2 diabetes, or CRP level) demonstrated any evidence of a causal association with all OA, knee OA, hip OA, or hand OA that remained after exclusion of genetic instrument variables also associated with BMI or correction for multiple comparisons (Figure 1). Although failing to pass our prespecified threshold for adjustment for multiple comparisons, weak causal inverse associations between LDL cholesterol level and risk of knee OA and hip OA were observed (for knee OA, OR per SD increase 0.94 [95% CI 0.90–0.99] for all SNPs and OR 0.94 [95% CI 0.88–1.01] without BMI SNPs; for hip OA, OR per SD increase 0.92 [95% CI 0.84–1.01] for all SNPs and OR 0.88 [95% CI 0.77–1.01] without BMI SNPs) (Supplementary Table 14).
Secondary analyses using knee and hip joint replacement as definitions of severe OA
We repeated the analyses using knee or hip replacement definitions of severe OA and found causal associations for genetically determined high BMI (knee replacement: OR per SD increase 2.30 [95% CI 1.93–2.75]; hip replacement: OR per SD increase 1.65 [95% CI 1.41–1.92]), and high femoral neck BMD (knee replacement: OR per SD increase 1.27 [95% CI 1.09–1.48]; hip replacement: OR per SD increase 1.17 [95% CI 1.05–1.31]). Systolic BP was causally inversely associated with joint (knee replacement: OR per SD increase 0.64 [95% CI 0.50–0.83]; hip replacement: OR per SD increase 0.64 [95% CI 0.48–0.85]), which was in accordance with the findings from our primary analyses using hospital diagnoses (Figure 3 and Supplementary Tables 14–17, http://onlinelibrary.wiley.com/doi/10.1002/art.40928/abstract).
Figure 3.
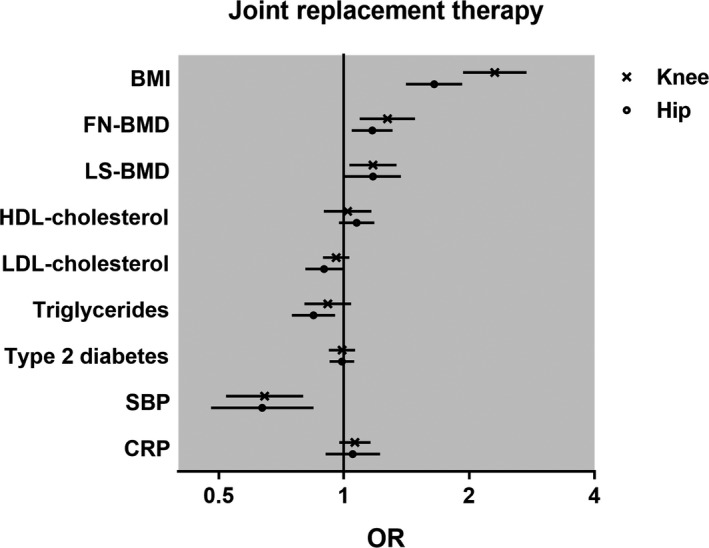
Causal associations between genetically determined risk factors and knee or hip replacement. For each joint replacement osteoarthritis (OA) definition, the odds ratio (OR) and 95% confidence interval for the risk of OA are represented for each factor, as determined using the 2‐sample Mendelian randomization inverse‐variance weighting method for body mass index (BMI; per SD increase), femoral neck bone mineral density (FN‐BMD; per SD increase), lumbar spine BMD (LS‐BMD; per SD increase), serum levels of high‐density lipoprotein (HDL) cholesterol, low‐density lipoprotein (LDL) cholesterol, and triglyceride (per SD increase), systolic blood pressure (SBP; per SD increase), C‐reactive protein (CRP) level (per ln[mg/liter] increase), or presence of type 2 diabetes. The total number of subjects is 384,838 (9,716 knee replacement and 9,932 hip replacement cases).
DISCUSSION
This study used the largest known data set to date with individual‐level clinical, genetic, and outcome data to assess causal factors for OA. In the UK Biobank setting, we found 3 factors (high BMI, high femoral neck BMD, and low systolic BP) that were causally associated with increased risk of OA. High BMI was shown to be causal for knee OA and hip OA, but not hand OA. We also report the first clear evidence of a causal role of high femoral neck BMD, predominantly reflecting cortical bone mass, on the risk of knee OA and hip OA. The most novel finding from this study is that low systolic BP was causally associated with all OA, knee OA, and hip OA. In contrast, the other metabolic factors and CRP levels were not causally associated with the risk of developing OA, after exclusion of the genetic instrument variables that are also associated with BMI.
Weight loss is reported to reduce knee OA symptoms and structural damage 7, 30. The causal role of BMI in both knee and hip OA has recently been demonstrated in a previous study using first‐release data from the UK Biobank 19. In this study, we replicated these causal BMI associations at weight‐bearing joints. In addition, because in the current well‐powered study we had access to individual‐level data, we also performed stratified analyses, demonstrating that the causal effect of BMI on OA was robust and similar in young and old individuals, in males and females, as well as in subpopulations stratified by smoking status or type 2 diabetes. Importantly, we observed no evidence of a causal effect of BMI on the risk of hand OA. Collectively, these findings demonstrate that it is the body weight–induced loading of the joints that causes the increased risk of OA observed at weight‐loaded, but not non–weight‐loaded OA sites.
The importance of subchondral bone in the pathophysiology of OA has been extensively debated 31, 32, 33, 34. So far, the supposed inverse association between osteoporosis and OA 35, 36, and the observation that high bone mass is associated with osteophyte volume 37, radiographic OA 8, 9, and increased prevalence of joint replacement 10, led to the general hypothesis that increased BMD could be deleterious to the joint. The findings of these observational studies are in accordance with the results from the present MR analyses, which demonstrated a robust causal role of femoral neck BMD on knee and hip OA. We confirmed these associations in sensitivity analyses excluding the genetic instrument variables that were also associated with BMI, to preclude pleiotropy. Similarly, as previously described 19, we observed a modest causal association between lumbar spine BMD and knee OA. The previous lumbar spine BMD MR analyses, performed in an early‐released subset of the UK Biobank, did not comply with the MR assumptions that genetic instrument variables should not be associated with the known confounder (BMI). In addition, the previous UK Biobank study was restricted by the phenotypes available in the MR‐base platform (http://www.mrbase.org/), and therefore the most powerful lumbar spine BMD GWAS was not used. Also, the femoral neck BMD trait with the most robust causal association in the present study was not available for selection of genetic instrument variables 24.
Our present finding that low systolic BP is causally associated with OA is novel. Previous limited observational studies have evaluated the association between hypertension, defined as a combination of antihypertensive medication use and high BP, and OA. Hypertension was associated with increased risk of radiographic knee OA both in a prospective analysis in the Framingham Osteoarthritis Study 15 and in a large cross‐sectional South Korean study 38. However, after adjustment for BMI, hypertension was not significantly associated with incident OA in the Framingham Osteoarthritis Study. It is possible that earlier prospective studies have been underpowered to identify systolic BP as a BMI‐independent predictor of OA. Further studies are needed to confirm the causal link between low systolic BP and OA, and to identify the underlying mechanisms.
The previous MR study, which used a less powered subsample from the UK Biobank, indicated that some metabolic factors may display modest, but significant, causal associations with OA 19. However, BMI‐associated SNPs were not excluded in those analyses, which therefore most likely were confounded by pleiotropic effects related to the known strong causal effect of BMI on OA. The present study, performing sensitivity analyses with BMI‐associated SNPs excluded, did not identify any causal association of metabolic risk factors except for systolic BP. The absence of a causal association for type 2 diabetes on OA in the present study indicates that the results of the previous observational association studies could be biased by remaining confounding factors or by reverse causation. Indeed, low physical activity caused by impaired mobility with knee or hip OA may increase insulin resistance. Finally, the lack of evidence of a causal role of lipids in knee or hip OA is supported by the results of 2 studies that showed no beneficial effect of statin use on OA risk or OA progression 39, 40. However, a recent MR analysis in the Malmö Diet and Cancer Study showed that genetically determined increased LDL cholesterol was causally associated with reduced risk of OA 41. This latter MR analysis also confirmed that high BMI is causally associated with increased risk of OA. Although low‐grade inflammation has been associated with increased risk of severe OA in observational studies 13, no causal effect of CRP levels was observed in the present study, which is in accordance with the negative results of intervention studies targeting inflammatory cytokines 42.
The major strength of the present study is its large sample size with individual‐level data available. With respect to the second and third MR assumptions, we considered possible pleiotropy in sensitivity analyses in addition to the MR‐Egger method, by excluding instrument variables that were also associated with BMI, since BMI was early shown to be a strong causative factor for knee and hip OA. We also performed detailed power calculations to ascertain the interpretation of negative results. Finally, we replicated our main analyses using other definitions for severe OA cases, using a joint replacement register that was independent of the ICD‐9 and ICD‐10 codes and found very similar results.
However, this study has some limitations. As we used hospital diagnoses, we could not assess any radiographic structural progression or pain as is recommended for clinical trials 43. While MR as an approach is appealing to address concerns about confounding, we cannot fully exclude the possibility that the null findings for some of the MR analyses could be due to misclassification of the diagnosis. This limitation may mostly affect hand OA, for which we acknowledge an underestimation of cases. Moreover, for knee OA, posttraumatic OA may be overrepresented when using hospital diagnosis. A hospital contact could also impact the chance of being classified as an OA case. However, we consider that our case definitions, being classified by hospital physicians, reflect symptomatic cases. ICD‐10 code validation was not conducted in the present study using the UK Biobank; however, previous validation studies in other cohorts showed good positive predictive value 44, 45. Another limitation is that the impact of physical activity could not be analyzed in this study, as no large published GWAS met our criteria to be used as a source of genetic instrument variables. Finally, our conclusions can only apply to a white European population.
In conclusion, BMI exerts a major causal effect on the risk of OA at weight‐bearing joints, but not at the hand. Evidence of causality of knee OA and hip OA was observed for high femoral neck BMD and low systolic BP. These results should be considered in the future research of OA and for the elaboration of prevention or therapeutic strategies for the different OA sites.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Funck‐Brentano had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Funck‐Brentano, Nethander, Ohlsson.
Acquisition of data
Nethander.
Analysis and interpretation of data
Funck‐Brentano, Nethander, Movérare‐Skrtic, Richette, Ohlsson.
Supporting information
ACKNOWLEDGMENT
We thank all the participants in the UK Biobank.
Supported by the European Commission's Horizon 2020 Framework Programme Marie Skłodowska‐Curie Actions (GOTBONE‐750579), the French Society of Rheumatology, Vinnova, the Swedish Research Council, the Swedish Foundation for Strategic Research, the ALF/LUA research grant in Gothenburg, the Lundberg Foundation, the Knut and Alice Wallenberg Foundation, the Torsten Söderberg Foundation, and the Novo Nordisk Foundation.
1Thomas Funck‐Brentano, MD, PhD, Maria Nethander, MSc, Sofia Movérare‐Skrtic, PhD, Claes Ohlsson MD, PhD: University of Gothenburg, Gothenburg, Sweden; 2Pascal Richette, MD, PhD: AP‐HP, Hospital Lariboisière, INSERM U1132, Université Paris Diderot, Université de Paris, Paris, France.
No potential conflicts of interest relevant to this article were reported.
Anonymized phenotype and genetic data are available from UK Biobank on application.
References
- 1. Arden N, Nevitt MC. Osteoarthritis: epidemiology. Best Pract Res Clin Rheumatol 2006;20:3–25. [DOI] [PubMed] [Google Scholar]
- 2. Nüesch E, Dieppe P, Reichenbach S, Williams S, Iff S, Jüni P. All cause and disease specific mortality in patients with knee or hip osteoarthritis: population based cohort study. BMJ 2011;342:d1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cooper C, Snow S, McAlindon TE, Kellingray S, Stuart B, Coggon D, et al. Risk factors for the incidence and progression of radiographic knee osteoarthritis. Arthritis Rheum 2000;43:995–1000. [DOI] [PubMed] [Google Scholar]
- 4. Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. Lancet 2011;377:2115–26. [DOI] [PubMed] [Google Scholar]
- 5. Lohmander LS, Gerhardsson de Verdier M, Rollof J, Nilsson PM, Engström G. Incidence of severe knee and hip osteoarthritis in relation to different measures of body mass: a population‐based prospective cohort study. Ann Rheum Dis 2009;68:490–6. [DOI] [PubMed] [Google Scholar]
- 6. Messier SP, Mihalko SL, Legault C, Miller GD, Nicklas BJ, DeVita P, et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA 2013;310:1263–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Richette P, Poitou C, Garnero P, Vicaut E, Bouillot JL, Lacorte JM, et al. Benefits of massive weight loss on symptoms, systemic inflammation and cartilage turnover in obese patients with knee osteoarthritis. Ann Rheum Dis 2011;70:139–44. [DOI] [PubMed] [Google Scholar]
- 8. Hardcastle SA, Dieppe P, Gregson CL, Hunter D, Thomas GE, Arden NK, et al. Prevalence of radiographic hip osteoarthritis is increased in high bone mass. Osteoarthritis Cartilage 2014;22:1120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hardcastle SA, Dieppe P, Gregson CL, Arden NK, Spector TD, Hart DJ, et al. Individuals with high bone mass have an increased prevalence of radiographic knee osteoarthritis. Bone 2015;71:171–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hardcastle SA, Gregson CL, Deere KC, Davey Smith GD, Dieppe P, Tobias JH. High bone mass is associated with an increased prevalence of joint replacement: a case–control study. Rheumatology (Oxford) 2013;52:1042–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hardcastle SA, Dieppe P, Gregson CL, Davey Smith G, Tobias JH. Osteoarthritis and bone mineral density: are strong bones bad for joints? [review]. Bonekey Rep 2015;4:624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sowers M, Karvonen‐Gutierrez CA, Palmieri‐Smith R, Jacobson JA, Jiang Y, Ashton‐Miller JA. Knee osteoarthritis in obese women with cardiometabolic clustering. Arthritis Rheum 2009;61:1328–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Engström G, Gerhardsson de Verdier M, Rollof J, Nilsson PM, Lohmander LS. C‐reactive protein, metabolic syndrome and incidence of severe hip and knee osteoarthritis: a population‐based cohort study. Osteoarthritis Cartilage 2009;17:168–73. [DOI] [PubMed] [Google Scholar]
- 14. Berenbaum F, Griffin TM, Liu‐Bryan R. Metabolic regulation of inflammation in osteoarthritis. Arthritis Rheumatol 2017;69:9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Niu J, Clancy M, Aliabadi P, Vasan R, Felson DT. Metabolic syndrome, its components, and knee osteoarthritis: the Framingham Osteoarthritis Study. Arthritis Rheumatol 2017;69:1194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schett G, Kleyer A, Perricone C, Sahinbegovic E, Iagnocco A, Zwerina J, et al. Diabetes is an independent predictor for severe osteoarthritis: results from a longitudinal cohort study. Diabetes Care 2013;36:403–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nielen JT, Emans PJ, Dagnelie PC, Boonen A, Lalmohamed A, de Boer A, et al. Severity of diabetes mellitus and total hip or knee replacement: a population‐based case–control study. Medicine (Baltimore) 2016;95:e3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol 2013;37:658–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zengini E, Hatzikotoulas K, Tachmazidou I, Steinberg J, Hartwig FP, Southam L, et al. Genome‐wide analyses using UK Biobank data provide insights into the genetic architecture of osteoarthritis. Nat Genet 2018;50:549–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Panoutsopoulou K, Metrustry S, Doherty SA, Laslett LL, Maciewicz RA, Hart DJ, et al. The effect of FTO variation on increased osteoarthritis risk is mediated through body mass index: a Mendelian randomisation study. Ann Rheum Dis 2014;73:2082–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Collins R. What makes UK Biobank special? Lancet 2012;379:1173–4. [DOI] [PubMed] [Google Scholar]
- 22. Manichaikul A, Mychaleckyj JC, Rich SS, Daly K, Sale M, Chen WM. Robust relationship inference in genome‐wide association studies. Bioinformatics 2010;26:2867–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015;518:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Estrada K, Styrkarsdottir U, Evangelou E, Hsu YH, Duncan EL, Ntzani EE, et al. Genome‐wide meta‐analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat Genet 2012;44:491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Willer CJ, Schmidt EM, Sengupta S, Peloso GM, Gustafsson S, Kanoni S, et al. Discovery and refinement of loci associated with lipid levels. Nat Genet 2013;45:1274–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Morris AP, Voight BF, Teslovich TM, Ferreira T, Segrè AV, Steinthorsdottir V, et al. Large‐scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat Genet 2012;44:981–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ehret GB, Munroe PB, Rice KM, Bochud M, Johnson AD, Chasman DI, et al, for the International Consortium for Blood Pressure Genome‐Wide Association Studies . Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 2011;478:103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dehghan A, Dupuis J, Barbalic M, Bis JC, Eiriksdottir G, Lu C, et al. Meta‐analysis of genome‐wide association studies in >80,000 subjects identifies multiple loci for C‐reactive protein levels. Circulation 2011;123:731–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol 2015;44:512–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gersing AS, Schwaiger BJ, Nevitt MC, Joseph GB, Chanchek N, Guimaraes JB, et al. Is weight loss associated with less progression of changes in knee articular cartilage among obese and overweight patients as assessed with MR imaging over 48 months? Data from the Osteoarthritis Initiative. Radiology 2017;284:508–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lories RJ, Corr M, Lane NE. To Wnt or not to Wnt: the bone and joint health dilemma. Nat Rev Rheumatol 2013;9:328–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Funck‐Brentano T, Cohen‐Solal M. Subchondral bone and osteoarthritis. Curr Opin Rheumatol 2015;27:420–6. [DOI] [PubMed] [Google Scholar]
- 33. Geusens PP, van den Bergh JP. Osteoporosis and osteoarthritis: shared mechanisms and epidemiology. Curr Opin Rheumatol 2016;28:97–103. [DOI] [PubMed] [Google Scholar]
- 34. Felson DT, Neogi T. Osteoarthritis: is it a disease of cartilage or of bone? [editorial]. Arthritis Rheum 2004;50:341–4. [DOI] [PubMed] [Google Scholar]
- 35. Hart DJ, Mootoosamy I, Doyle DV, Spector TD. The relationship between osteoarthritis and osteoporosis in the general population: the Chingford Study. Ann Rheum Dis 1994;53:158–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hart DJ, Cronin C, Daniels M, Worthy T, Doyle DV, Spector TD. The relationship of bone density and fracture to incident and progressive radiographic osteoarthritis of the knee: the Chingford Study. Arthritis Rheum 2002;46:92–9. [DOI] [PubMed] [Google Scholar]
- 37. Hardcastle SA, Dieppe P, Gregson CL, Arden NK, Spector TD, Hart DJ, et al. Osteophytes, enthesophytes, and high bone mass: a bone‐forming triad with potential relevance in osteoarthritis. Arthritis Rheumatol 2014;66:2429–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kim HS, Shin JS, Lee J, Lee YJ, Kim M, Bae YH, et al. Association between knee osteoarthritis, cardiovascular risk factors, and the Framingham risk score in South Koreans: a cross‐sectional study. PLoS ONE 2016;11:e0165325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Eymard F, Parsons C, Edwards MH, Petit‐Dop F, Reginster JY, Bruyère O, et al. Statin use and knee osteoarthritis progression: results from a post‐hoc analysis of the SEKOIA trial. Joint Bone Spine 2018;85:609–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Michaëlsson K, Lohmander LS, Turkiewicz A, Wolk A, Nilsson P, Englund M. Association between statin use and consultation or surgery for osteoarthritis of the hip or knee: a pooled analysis of four cohort studies. Osteoarthritis Cartilage 2017;25:1804–13. [DOI] [PubMed] [Google Scholar]
- 41. Hindy G, Åkesson KE, Melander O, Aragam KG, Haas ME, Nilsson PM, et al. Cardiometabolic polygenic risk scores and osteoarthritis outcomes: a Mendelian randomization study using data from the Malmö Diet and Cancer Study and the UK Biobank. Arthritis Rheumatol 71:925–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chevalier X, Eymard F, Richette P. Biologic agents in osteoarthritis: hopes and disappointments. Nat Rev Rheumatol 2013;9:400–10. [DOI] [PubMed] [Google Scholar]
- 43. Leyland KM, Gates LS, Nevitt M, Felson D, Bierma‐Zeinstra SM, Conaghan PG, et al. Harmonising measures of knee and hip osteoarthritis in population‐based cohort studies: an international study. Osteoarthritis Cartilage 2018;26:872–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rahman MM, Kopec JA, Goldsmith CH, Anis AH, Cibere J. Validation of administrative osteoarthritis diagnosis using a clinical and radiological population‐based cohort. Int J Rheumatol 2016;2016:6475318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Turkiewicz A, Petersson IF, Björk J, Hawker G, Dahlberg LE, Lohmander LS, et al. Current and future impact of osteoarthritis on health care: a population‐based study with projections to year 2032. Osteoarthritis Cartilage 2014;22:1826–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


