Abstract
Background
Patients with postural tachycardia syndrome (POTS) experience chronic symptoms of orthostatic intolerance. There are minimal data detailing the demographics, clinical features and clinical course of this condition. This online, community‐based survey highlights patients’ experience with POTS. It consists of the largest sample of POTS patients reported to date.
Objectives
To describe the demographics, past medical history, medications, treatments and diagnostic journey for patients living with POTS.
Methods
Postural tachycardia syndrome patients completed an online, community‐based, cross‐sectional survey. Participants were excluded if they had not received a diagnosis of POTS from a physician. The questions focused on the patient experience and journey, rather than physiological responses.
Results
The final analysis included 4835 participants. POTS predominantly affects white (93%) females (94%) of childbearing age, with approximately half developing symptoms in adolescence (mode 14 years). POTS is a chronic multisystem disorder involving a broad array of symptoms, with many patients diagnosed with comorbidities in addition to POTS. POTS patients often experience lengthy delays [median (interquartile range) 24 (6–72) months] and misdiagnosis, but the diagnostic delay is improving. POTS patients can present with a myriad of symptoms most commonly including lightheadedness (99%), tachycardia (97%), presyncope (94%), headache (94%) and difficulty concentrating (94%).
Conclusions
These data provide important insights into the background, clinical features and diagnostic journey of patients suffering from POTS. These data should serve as an essential step for moving forward with future studies aimed at early and accurate diagnoses of these patients leading to appropriate treatments for their symptoms.
Keywords: autonomic nervous system, orthostatic intolerance
Introduction
Postural tachycardia syndrome (POTS) is a clinical condition in which patients experience chronic symptoms of orthostatic intolerance. Consensus documents define POTS by a significant heart rate increase (≥30 beats per minute within 10 min of upright posture or ≥40 beats per minute if under 18 years), with no orthostatic hypotension (drop in blood pressure >20/10 mmHg within 3 min of upright posture) 1, 2, 3, 4, 5. POTS can be associated with significant morbidity, poor sleep quality and diminished quality of life 6, 7, 8. There is no single treatment that can ‘cure’ a patient of symptoms 9. A multidisciplinary approach to treatment is often needed 9.
Postural tachycardia syndrome is estimated to affect between 500 000 and 3 000 000 people in the United States alone 10, 11. It also has a strong female predominance (5:1) 12, 13. Despite this, there are few published data detailing the demographics, features and clinical course of this condition. Most studies are limited by small sample sizes, referral bias and comprise populations from single, highly specialized tertiary care centres.
Here, we present data from an international survey of patients with POTS. These data provide unique information from the perspective of patients suffering from POTS. We aimed to highlight patients’ long‐term experiences in managing POTS, including background demographics, comorbidities, symptom burden and their diagnostic journey with this illness. This online, community‐based survey consists of the largest sample of POTS patients reported to date.
Materials and methods
Participants
This online, community‐based, cross‐sectional survey of POTS patients was conducted in partnership between academic institutions and Dysautonomia International, a patient advocacy organization. The ‘Diagnosis and Impact of POTS’ study was approved by the Vanderbilt University Institutional Review Board and the Calgary Conjoint Health Research Ethics Board. All participants 18 years or older provided informed consent. Parents of POTS patients under age 18 years could also provide their informed consent and complete the survey on behalf of their child. Participants were excluded if they did not consent or had not received a diagnosis of POTS from a physician.
Survey design and administration
The survey contained multiple question types including multiple choice, Likert scale and short answer. The questions covered patient demographics, past medical history, nonpsychiatric comorbidities, medications, treatments, diagnostic journey, as well as social and economic impacts of living with POTS. The questions addressed the patient experience and journey, rather than physiological or pharmacological responses.
The survey was iteratively developed with beta testing performed by members of the Dysautonomia International Patient Advisory Board. The final survey took the beta testers about 40 min to complete. The online survey was advertised on the Dysautonomia International website and on their Facebook page (over 43 000 followers), and was designed to be suitable for sharing on social media with a short URL. Data collection was done in a self‐reported manner. Individual patients were not formally assessed by the research team for this study. Data were collected from July 2015 to July 2017.
Data analysis
Survey results were collected and stored on a REsearch Data CAPture (REDCap) 14 secure online database housed at Vanderbilt University (Nashville, Tennessee, USA). Data were exported to SPSS Version 22 (IBM Corporation, Armonk, New York, USA) for data cleaning and formal analysis. Clear outlier and implausible data (e.g. age at symptom onset of 120 years) were removed. There were a number of survey questions with numerical responses that allowed a participant to choose to not respond, and these non‐responses were recoded to ‘missing at random’ for data analyses. All other responses were included in the final statistical analysis.
We compared the comorbidities reported by a subgroup of patients that had been seen at an Autonomic Disorders Consortium (‘Consortium’) site to those not seen at the Consortium sites to determine if the broader patient community reported a higher or lower rate of comorbidities than those identified by physicians with significant POTS expertise. The Consortium sites include the leading autonomic disorders clinical and research centres in the United States. These sites included: Beth Israel Deaconess Hospital (Boston, MA), Mayo Clinic (Jacksonville, FL), Mayo Clinic (Rochester, MN), National Institutes of Health (Bethesda, MD), New York University (New York, NY), Vanderbilt University (Nashville, TN) and University of Texas Southwestern (Dallas, TX).
Statistical analysis
All participants who met inclusion and exclusion criteria were included in the final analysis. Data are presented as mean ± standard deviation for parametric data, median and interquartile range (IQR) for non‐parametric data, and number and percentages for categorical data. Differences between continuous variables were assessed using a Student's t‐test for parametric data or Mann–Whitney U‐test for non‐parametric data. The chi‐square test was used for categorical variables. The level of statistical significance was set at P < 0.05. Figures were produced using Adobe Illustrator CC (Adobe Systems Incorporated, San Jose, California, USA) and GraphPad Prism 7 (GraphPad Software Incorporated, La Jolla, California, USA).
Results
Patient characteristics
Consent was provided by 6475 participants (Fig. 1). Of these, 4835 participants reported being diagnosed with POTS by a physician. The total ‘n’ used for each analysis section varied, as some patients chose not to answer certain questions, and is listed accordingly in tables or text. The demographics for survey participants are displayed in Table 1. A subset of the cohort (n = 430; 9%) reported being assessed by clinicians at expert ‘consortium’ sites in autonomic disorders. The overall cohort responses were similar to the responses from those seen at the consortium sites (Table S1). Where there were statistically significant differences, the consortium patients reported a slightly higher symptom burden than the overall cohort.
Figure 1.
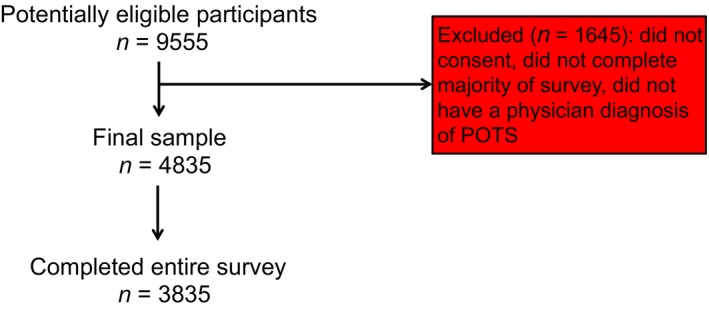
Participant flow diagram. There were 4835 participants that met inclusion/exclusion criteria and were included in the final analysed sample.
Table 1.
POTS patient demographics
| Patient characteristic | Number (%) or mean (SD) |
|---|---|
| Gender | Total = 4835 |
| Female | 4539 (94%) |
| Male | 296 (6%) |
| Age | Total = 4243 |
| Present age – over 18 years | 3726 (88%) |
| Age at symptom onset | 20.7 (12.0) years |
| Country of birth | Total = 4830 |
| United States | 3976 (82%) |
| United Kingdom | 331 (7%) |
| Canada | 147 (3%) |
| Australia | 144 (3%) |
| Other | 232 (5%) |
| Race (self‐described) | Total = 4835 |
| White | 4487 (93%) |
| Black | 35 (1%) |
| Asian | 25 (0.5%) |
| Other | 44 (1%) |
| Mixed | 170 (4%) |
| Ethnicity (self‐described) | Total = 4835 |
| Hispanic | 221 (5%) |
| Non‐Hispanic | 4435 (92%) |
| Years of formal education | 14.8 (3.2) years |
Co‐morbid conditions with POTS
There were 3276 (83%) participants who reported being diagnosed by a physician with another medical condition in addition to POTS (Table 2). A number of co‐morbid diagnoses were highly prevalent (≥20%). Only 290 (7%) of participants reported having all three of the most common comorbidities (migraine headaches, irritable bowel syndrome and Ehlers–Danlos syndrome). Only a minority of patients (n = 430; 9%) had been seen at an Autonomic Disorders Consortium site. Respondents seen at these sites reported a higher prevalence of multiple comorbidities (Table S1). For comorbidities such as migraine headaches (P = 0.017), mast cell activation disorder (P < 0.001), inappropriate sinus tachycardia (P = 0.021) and vasovagal syncope (P < 0.001), these differences were statistically significant.
Table 2.
Common comorbidities in POTS patients
| Comorbidity | Number (%) (of 3933 respondents) |
|---|---|
| Migraine headaches | 1557 (40%) |
| Irritable bowel syndrome | 1192 (30%) |
| Ehlers–Danlos syndrome | 994 (25%) |
| Chronic fatigue syndrome | 809 (21%) |
| Asthma | 798 (20%) |
| Fibromyalgia | 786 (20%) |
| Raynaud's phenomena | 610 (16%) |
| Iron deficiency anaemia | 628 (16%) |
| Gastroparesis | 548 (14%) |
| Vasovagal syncope | 499 (13%) |
| Inappropriate sinus tachycardia | 448 (11%) |
| Mast cell activation disorder | 353 (9%) |
| Autoimmune disease | 616 (16%) |
| Hashimoto's thyroiditis | 228 (6%) |
| Coeliac disease | 133 (3%) |
| Sjögren's syndrome | 112 (3%) |
| Rheumatoid arthritis | 93 (2%) |
| Lupus | 81 (2%) |
| Other | 160 (4%) |
Other autoimmune conditions included the following: idiopathic thrombocytopenic purpura, Addison's disease, Grave's disease, Behcet's disease, autoimmune pancreatitis, autoimmune hepatitis, vasculitis, multiple sclerosis, myasthenia gravis and type 1 diabetes mellitus.
POTS symptom triggers
The mean age of POTS symptom onset in the participants was 21 ± 12 years, with a median of 17 (IQR 13–28) years. The most common (modal) age of onset was 14 years (Fig. 2). There were 2220 (47%) participants who reported the onset of POTS symptoms after the age of 18 years. There were 1933 (41%) participants who reported that their symptoms started within 3 months of a specific event. Of these, the most common triggers were an infection (n = 788; 41%), surgery (n = 237; 12%) or pregnancy (n = 163; 9%). Others included a vaccination (n = 119; 6%), an accident (n = 105; 6%), puberty (n = 86; 5%), a concussion (n = 82; 4%), or emotional stress or trauma (n = 49; 3%).
Figure 2.
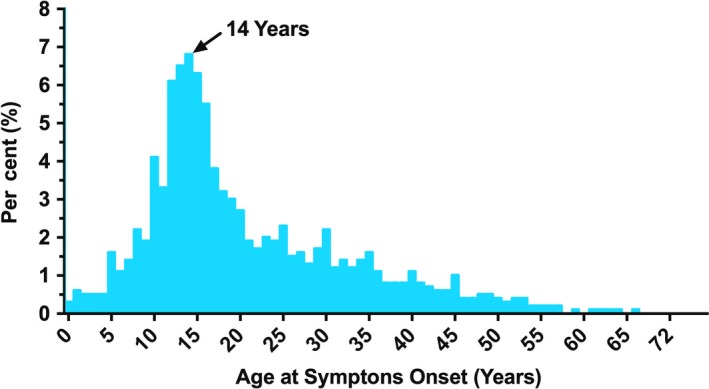
Age of symptom onset. Distribution of reported symptom onset age amongst survey participants.
Diagnostic journey travelled by POTS patients
Key information pertaining to the diagnostic journey of survey participants is displayed in Table 3. Participants frequently reported being misdiagnosed with other diagnoses, seeing many physicians prior to being diagnosed with POTS, and having to suggest POTS as a potential diagnosis to their physician. On average, patients saw 7 ± 11 [median 5 (IQR 3–8)] physicians prior to a diagnosis of POTS. Furthermore, 21% (n = 627) of participants reported having seen more than 10 doctors before a diagnosis was made.
Table 3.
Diagnostic journey in POTS patients
| Number (%) or mean (SD) | |
|---|---|
| Misdiagnosed prior to POTS diagnosis | 3421 (75%) |
| POTS diagnosis suggested by patient | 1557 (34%) |
| Number of physicians seen prior to diagnosis | 7 (11) |
| Number of ED visits prior to diagnosis | 9 (16) |
| Specialty of physician who made diagnosis | |
| Cardiologist | 1973 (41%) |
| Cardiac electrophysiologist | 696 (15%) |
| Neurologist | 889 (19%) |
| Family physician | 392 (8%) |
| Emergency room physician | 79 (2%) |
| Rheumatologist | 74 (2%) |
| Other | 711 (15%) |
Emergency department, ED. The total number of respondents to this question was 4760. Additional physicians who made the diagnosis under the category ‘other’ included nephrologists, gynaecologists, otolaryngologists and unsure/not specified.
Survey respondents frequently reported lengthy delays until diagnosis (Table S2). Respondents waited a median time of 24 (IQR 6–72) months (4.9 ± 7.1 years) after initial presentation to a physician before a POTS diagnosis was made (Fig. 3a). Approximately 15% of patients were not diagnosed for more than 10 years after initially visiting a physician for their symptoms. Female participants reported longer diagnostic delays (5.0 ± 7.2 years) compared with males (3.0 ± 4.4 years) with POTS (P < 0.001). There were no differences in diagnostic delay between races (P = 0.59).
Figure 3.
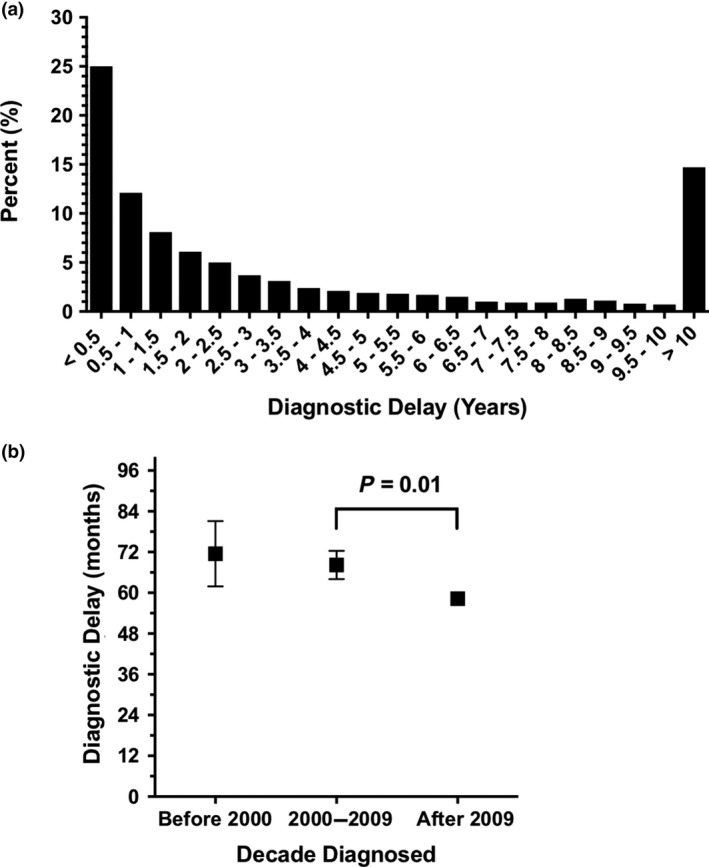
Delay in postural tachycardia syndrome (POTS) diagnosis. (a) Number of years of diagnostic delay prior to receiving final diagnosis of POTS and (b) Mean ± standard error of the mean diagnostic delay per decade since 1990. Diagnostic delay refers to the time between initial presentation to a physician and final diagnosis.
There has been a recent improvement in diagnostic delay. The delay period was 11.6 (95% CI: 2.2, 21.0) months shorter for patients diagnosed after 2009 compared to those diagnosed from 2000 to 2009 (P = 0.01; Fig. 3b). Despite this improvement, the average diagnostic delay since 2009 is still over 4.7 ± 6.9 years [median 23 (IQR 6–69) months].
Postural tachycardia syndrome was most commonly diagnosed by a general cardiologist (n = 1973; 41%), neurologist (n = 889; 19%), cardiac electrophysiologist (n = 696; 15%) and family physician (n = 392; 8%). Other specialties included emergency room physicians (n = 79; 2%), rheumatologists (n = 74; 2%), nephrologists (n = 19; <1%), gynaecologists (n = 8; <1%) and otolaryngologists (n = 8; <1%).
Misdiagnosis in POTS
Three‐quarters (n = 3421; 75%) of patients report that their POTS symptoms were misdiagnosed by a physician prior to being diagnosed with POTS. Prior to POTS diagnosis, many participants (n = 3257; 67%) encountered physicians who acknowledged a physical illness but were unsure how to proceed. There were 3471 (77%) respondents who encountered a physician who suggested their symptoms were due to a psychiatric or psychological problem before they were diagnosed with POTS. In contrast, only 1247 (28%) respondents report they were actually suffering from a psychiatric or psychological problem before they were diagnosed with POTS. After the diagnosis of POTS, only 1656 (37%) participants reported being told that they were suffering from a psychiatric or psychological problem, and 1392 (31%) reported that they were being actively treated for a psychiatric or psychological problem.
Symptom burden in POTS patients
Symptoms of POTS most frequently reported by survey respondents are detailed in Table 4. The most common symptoms include lightheadedness (n = 3992; 99%), tachycardia (n = 3901; 97%), presyncope (n = 3789; 94%), headache (n = 3797; 94%) and difficulty concentrating (n = 3794; 94%). Of these symptoms, the triad of lightheadedness, tachycardia and presyncope was concomitantly present in 3677 (91%) of respondents. A pentad of all five symptoms was reported by 3331 (83%) of respondents.
Table 4.
Symptom profile
| Symptoms | Number | Total numbera | % Total |
|---|---|---|---|
| Cardiovascular symptoms | |||
| Lightheadedness | 3992 | 4034 | 99 |
| Tachycardia | 3901 | 4032 | 97 |
| Presyncope | 3789 | 4032 | 94 |
| Shortness of breath | 3562 | 4032 | 88 |
| Palpitations | 3033 | 4031 | 87 |
| Chest pain | 3164 | 4032 | 79 |
| Low blood pressure | 2864 | 4033 | 71 |
| Syncope | 1452 | 4033 | 36 |
| Gastrointestinal symptoms | |||
| Nausea | 3618 | 4032 | 90 |
| Stomach pains | 3357 | 4032 | 83 |
| Bloating | 3184 | 4031 | 79 |
| Constipation | 2845 | 4032 | 71 |
| Diarrhoea | 2783 | 4032 | 69 |
| Neurological symptoms – head and brain | |||
| Headache | 3797 | 4032 | 94 |
| Difficulty concentrating | 3794 | 4032 | 94 |
| Memory problems | 3538 | 4032 | 87 |
| Tremulousness | 3124 | 4039 | 78 |
| Neurological symptoms – eyes and ears | |||
| Blurred vision | 3015 | 4032 | 75 |
| Dry mouth | 2662 | 4031 | 66 |
| Dry eyes | 2383 | 4030 | 60 |
| Neurological symptoms – extremities | |||
| Muscle pains | 3374 | 4029 | 84 |
| Foot coldness | 3377 | 4030 | 84 |
| Muscle weakness | 3344 | 4030 | 83 |
| Hand coldness | 3311 | 4029 | 82 |
| Hand tingling | 3060 | 4029 | 76 |
| Foot tingling | 2701 | 4028 | 67 |
| Hand numbness | 2627 | 4029 | 65 |
| Foot numbness | 2350 | 4029 | 58 |
| Skin symptoms | |||
| Skin flushing | 2774 | 4029 | 69 |
| Bladder symptoms | |||
| Frequent urination | 2733 | 4031 | 68 |
The denominator (or total n number) varies between symptom categories because there were several respondents for this section who did not select a yes or no response for all listed symptoms.
Symptom changes over time in POTS patients
Compared to when their symptoms started, 1702 (42%) patients reported that their symptoms had generally improved a little (n = 1167; 29%) or a lot (n = 535; 13%). Ten per cent (n = 400) of participants reported no change in symptoms. Additionally, 1756 (44%) patients reported that their symptoms had worsened a lot (n = 1197; 30%) or a little (n = 559; 14%). Almost two‐thirds of respondents (n = 2552; 63%) believed they have always had a tendency to have POTS‐like symptoms for most of their lives.
Nearly, one‐third (n = 490; 29%) of participants whose symptoms improved a little or a lot reported that medications were the predominant reason for this improvement. A majority of participants reporting some improvement (n = 876; 52%) reported that nonpharmacological factors had also been helpful (in addition to medications). A larger proportion (P < 0.001) of participants seen at an Autonomic Disorders Consortium site reported symptom improvement (n = 187/347; 54%) in comparison with those who were seen elsewhere (n = 1974/3476; 43%).
Participants reported having to go to the emergency department significantly fewer times after their diagnosis of POTS (paired difference in visits: 1.7 (95% CI: 0.9, 2.7; P < 0.001), although they still averaged 8 ± 20 visits postdiagnosis.
Discussion
The key findings from the study are as follows: (i) POTS predominantly affects white females of childbearing age, with approximately half developing symptoms in adolescence and half developing symptoms in adulthood; (ii) POTS is a chronic multisystem disorder involving a broader array of symptoms than the orthostatic tachycardia that defines it, with many patients diagnosed with comorbidities in addition to POTS; (iii) although the diagnostic delay for POTS is improving, patients often experience lengthy delays and misdiagnosis prior to POTS diagnosis, seeing multiple physicians before the POTS diagnosis is confirmed; and (iv) whilst POTS patients can present with a myriad of symptoms, most commonly these include the following: lightheadedness, tachycardia, presyncope, headache and difficulty concentrating.
Demographics of POTS
Postural tachycardia syndrome is a chronic condition that can be functionally debilitating. Our study found that white females of childbearing age are those predominantly affected by POTS, similar to other published studies 15, 16, 17, 18. This is the first study to demonstrate these findings in such a large cohort of patients from different places and who are cared for in different institutions 18, 19, 20, 21, 22. These findings further illustrate that POTS affects a young population in their formative years.
Co‐morbid conditions in POTS patients
This survey revealed that approximately 83% of respondents with POTS suffered from at least one additional medical condition. Migraine headaches, irritable bowel syndrome and Ehlers–Danlos syndrome (EDS) were the most commonly reported co‐morbid conditions. Additionally, we found a large proportion of participants reported a number of other conditions, including asthma, autoimmune diseases, iron deficiency anaemia and gastroparesis (Table 2). For some of these comorbidities, the estimated prevalence rates for POTS patients are higher than those reported in the general population 1, 23, 24, 25, 26.
The rate of physician‐diagnosed EDS was 25% in our POTS population. A small patient‐reported survey has documented EDS in 26% of POTS patients, whilst clinic‐based studies have documented EDS between 4% and 25% 19, 22, 27. Interestingly, these rates are significantly less than a survey conducted in the UK (POTS UK) that found EDS prevalence in 779 POTS patients to be nearly 50% 18. These differences may reflect different diagnostic criteria and terminology used, varied EDS diagnostic skills of the physicians making the diagnosis, and small study cohorts.
Fewer patients in our survey reported having a diagnosis of chronic fatigue syndrome (CFS) (21%) or vasovagal syncope (13%) in comparison with the POTS UK study (reported as CFS: 29%; vasovagal syncope: ~30%) 18. The rate of fibromyalgia was much higher (20%) in our group relative to the POTS UK study (~5%). The differences in the two surveys may reflect regional differences in physician diagnostic practices and expertise. POTS is clearly a multisystem disorder that can be associated with a variety of comorbidities 5, 22. It is unclear if the high prevalence of certain comorbidities is a result of a drawn‐out diagnostic journey in this population (patients see many physicians along their diagnostic journey) or if these co‐morbid diagnoses are related by a state of underlying autonomic dysfunction. Interestingly, patients seen at an Autonomic Disorders Consortium site (whose records are verifiable) had a significantly higher prevalence of multiple diagnosed comorbidities, compared with patients not seen at those sites (whose survey records are not verifiable). These data suggest that the survey responses globally were not inconsistent with the findings from only those patients seen at ‘expert sites’.
A difficult diagnostic journey
Postural tachycardia syndrome patients often experience long diagnostic delays and a lack of physician awareness of POTS. In fact, many respondents (34%) first suggested the diagnosis to their physicians, and many had to present to several healthcare practitioners prior to receiving a POTS diagnosis. This raises concerns that many individuals suffering from POTS may not receive a diagnosis at all, since many people cannot afford to see multiple doctors, and some healthcare systems or insurance providers do not allow multiple ‘second opinions’. Only 9% of participants presented to an Autonomic Disorders Consortium site, which are tertiary referral centres. This may suggest that the majority of POTS patients are either managed in the community by either general practitioners or specialists, or they are not actively followed. In our study, cardiologists most commonly made this diagnosis, followed by neurologists, cardiac electrophysiologists and family physicians. This is similar to the POTS UK study 18. Although the symptom profile of patients suggests that a majority report some neurological features of POTS, only one‐fifth of participants were diagnosed by a neurologist. This continues to highlight that a lack of awareness appears to impede a diagnosis of POTS, regardless of the specialty 28.
Overall, the majority of these diagnoses were clearly made by nontertiary care experts. This means that we cannot verify that the diagnoses of POTS are correct in each case. However, these data do reflect the current state of recognition and diagnosis of this condition in the ‘real world’, where there is a limited ability to access specialized centres well versed in assessing and managing patients with this condition. We believe these data emphasize the importance of strategies to broadly increase primary care physician education about the diagnosis and treatment of POTS.
Diagnostic delay in POTS
The results of this survey demonstrate that POTS patients frequently experience a lengthy diagnostic delay. We found that patients waited a median 24 months from initial presentation to diagnosis, whilst the mean diagnostic delay was 4.9 ± 7.1 years, reflecting that some patients had extraordinarily long waits. These wait times are not unusual. Boris et al. 22 found that in 704 paediatric patients diagnosed with POTS, patients waited over 2 years on average prior to diagnosis. Despite the fact that POTS is a very female‐predominant medical condition, females waited nearly 2 years longer (5.0 ± 7.2 years) for a POTS diagnosis than males (3.0 ± 4.4 years; P < 0.001), suggesting that gender bias may be influencing the diagnostic process. Future studies should explore factors that may be contributing to the significant difference in the diagnostic delay experienced by male and female POTS patients. The recent improvement in overall diagnostic delay might suggest improved medical awareness of POTS as a clinical condition.
Psychiatric diagnoses and POTS
A high percentage (77%) of participants reported being told they were suffering from a psychiatric or psychological problem before their POTS diagnosis. After the POTS diagnosis was made, only 37% of participants continued to have a diagnosis of a psychiatric or psychological disorder. When formally assessed, POTS patients have no increased lifetime prevalence of psychiatric disorders as compared to the general population, 29 and POTS patients have mental health scores similar to national norms whether they have improved or not 5 years after diagnosis 20. However, other studies have also suggested that patients with POTS may have increased prevalence of depression and anxiety 30. It is unknown how much of the psychological health problems that exist in POTS patients may be due to living with a chronic medical illness 31 or exacerbated by the stressful prolonged diagnostic process and misdiagnosis the majority of POTS patients experience. Improving the recognition of POTS symptoms may be helpful in avoiding misdiagnosis and reducing diagnostic delays, which could potentially improve mental health outcomes.
Symptoms in POTS patients
The three most common symptoms reported by survey participants were lightheadedness, tachycardia and presyncope (Table 4). Other studies have reported similar findings 17, 18. This symptom triad was present in 91% of respondents. The symptoms of headache and difficulties concentrating were also quite prevalent (94% of respondents). These are recognized symptoms of POTS 5, 32, 33, 34. The high prevalence of headaches identified is in keeping with a retrospective evaluation of symptoms in paediatric patients presenting to a POTS clinic 22. The autonomic symptoms associated with POTS can negatively impact many aspects of daily life including cognitive function, sleep, energy levels and the ability to exercise, work and attend school 18, 19, 21, 30, 35. A history of syncope was reported in only 36% of survey participants, which is much lower than that reported in the smaller POTS UK study (58%) 18. Overall, this survey describes that a wide variety of symptoms (>10) were reported to be present by a large proportion (>80%) of survey respondents. These confirm prior findings, but now establish them in a cohort that is many times larger than prior studies that have detailed the clinical characteristics of POTS patients 17, 18, 22, 36.
Limitations
There are several potential limitations to the present study. First, the majority of patients in this study were born in the USA. Although our study did have respondents from over 15 countries, they made up less than 20% of the total sample. This may limit the generalizability of these results for patient populations in other countries. This may also explain, in part, the differences in results between our survey and those presented in the POTS UK study 18 which sampled a United Kingdom cohort.
Second, our survey may have a selection bias for certain patients, particularly English‐speaking patients, those with Internet access and those already connected to Dysautonomia International, a patient advocacy group that played an important role in patient recruitment. Dysautonomia International has over 43 000 people linked to their Facebook page; so a high number of POTS patients were exposed to the online survey.
Third, since the survey was based on cross‐sectional patient self‐reports, it is likely that some co‐morbid diagnoses were under‐reported if the patient had not been diagnosed with a comorbidity that was present, or if they had been diagnosed with the comorbidity, but were unaware of the details of their medical records.
Finally, given the anonymous nature of this survey, it relied on honest self‐reporting, which carries with it both some limitations and the potential for introducing bias. Importantly, the responses were not independently verified. There were no barriers for a participant to complete the survey multiple times or to fraudulently complete the survey. We feel this is unlikely, however, given the time required to fill out this lengthy survey. These are known issues with survey‐based data 37. Overall, we feel that the obvious benefits of being able to access a large, global patient respondent audience outweigh the limitations of using an online survey for data collection and provide a novel perspective on the diagnostic journey faced by these patients.
Conclusions
Here, we highlight the results of the largest known survey of patients with a diagnosis of POTS. We believe these results provide important insights into the background, clinical features and diagnostic journey of patients suffering from this condition. We believe these data will serve as an essential step for moving forward with future studies aimed at better identifying these patients and establishing better treatments for their symptoms.
Conflict of interest statement
This work was supported in part by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number P01 HL056693, by the National Center for Advancing Translational Sciences Award UL1 TR000445. SRR receives research support from the Canadian Institutes of Health Research (CIHR; Ottawa, ON, Canada) grant MOP142426 and the Cardiac Arrhythmia Network of Canada (CANet; London, ON, Canada) grants SRG‐15‐P01‐001 and SRG‐17‐P27‐001.
Disclosures
BS, No disclosures to report. LS, No disclosures to report. KB, No disclosures to report. EAG, No disclosures to report. CAS, Consultant for Lundbeck NA Ltd. LEO, No disclosures to report. EMG, No disclosures to report. AG, No disclosures to report. AD, No disclosures to report. VR, No disclosures to report. RSS, No disclosures to report. IB, Consultant for Lundbeck NA Ltd., and Theravance. DR, Consultant for Lundbeck NA Ltd. SRR, Consultant for Lundbeck NA Ltd. GE Healthcare, Abbott and Allergan.
Supporting information
Table S1. Co‐morbidities in patients assessed at Autonomic Disorders Consortium sites.
Table S2. Diagnostic delay.
Acknowledgements
We would like to thank that POTS patients and the parents of POTS patients who took the time to complete this lengthy online survey.
Shaw BH, Stiles LE, Bourne K, Green EA, Shibao CA, Okamoto LE, Garland EM, Gamboa A, Diedrich A, Raj V, Sheldon RS, Biaggioni I, Robertson D, Raj SR (University of Calgary, Calgary, AB, Canada; Stony Brook University School of Medicine, Stony Brook, NY; Dysautonomia International, East Moriches, NY; Vanderbilt University Medical Center, Nashville, TN, USA; University of Calgary, Calgary, AB, Canada). The face of postural tachycardia syndrome – insights from a large cross‐sectional online community‐based survey. J Intern Med 2019;286:438–448.
References
- 1. Sheldon RS, Grubb BP 2nd, Olshansky B et al 2015 heart rhythm society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm 2015; 12: e41–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shen WK, Sheldon RS, Benditt DG et al 2017 ACC/AHA/HRS Guideline for the Evaluation and Management of Patients With Syncope: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2017; 70: 620–63. [DOI] [PubMed] [Google Scholar]
- 3. Brignole M, Moya A, de Lange FJ et al 2018 ESC Guidelines for the diagnosis and management of syncope. Eur Heart J 2018; 39: 1883–948. [DOI] [PubMed] [Google Scholar]
- 4. Freeman R, Wieling W, Axelrod FB et al Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res 2011; 21: 69–72. [DOI] [PubMed] [Google Scholar]
- 5. Fedorowski A. Postural Orthostatic Tachycardia Syndrome: clinical presentation, aetiology, and management. J Intern Med 2019; 285: 352–66. [DOI] [PubMed] [Google Scholar]
- 6. Bagai K, Song Y, Ling JF et al Sleep disturbances and diminished quality of life in postural tachycardia syndrome. J Clin Sleep Med 2011; 7: 204–10. [PMC free article] [PubMed] [Google Scholar]
- 7. Benrud‐Larson LM, Dewar MS, Sandroni P, Rummans TA, Haythornthwaite JA, Low PA. Quality of life in patients with postural tachycardia syndrome. Mayo Clin Proc 2002; 77: 531–7. [DOI] [PubMed] [Google Scholar]
- 8. Jones PK, Shaw BH, Raj SR. Clinical challenges in the diagnosis and management of postural tachycardia syndrome. Pract Neurol 2016; 16: 431–8. [DOI] [PubMed] [Google Scholar]
- 9. Raj S, Sheldon R. Management of postural tachycardia syndrome, inappropriate sinus tachycardia and vasovagal syncope. Arrhythm Electrophysiol Rev 2016; 5: 122–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Robertson D. The epidemic of orthostatic tachycardia and orthostatic intolerance. Am J Med Sci 1999; 317: 75–7. [DOI] [PubMed] [Google Scholar]
- 11. Garland EM, Celedonio JE, Raj SR. Postural tachycardia syndrome: beyond orthostatic intolerance. Curr Neurol Neurosci Rep 2015; 15: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arnold AC, Ng J, Raj SR. Postural tachycardia syndrome – Diagnosis, physiology, and prognosis. Auton Neurosci 2018; 215: 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hamrefors V, Spahic JM, Nilsson D et al Syndromes of orthostatic intolerance and syncope in young adults. Open Heart 2017; 4: e000585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garland EM, Raj SR, Black BK, Harris PA, Robertson D. The hemodynamic and neurohumoral phenotype of postural tachycardia syndrome. Neurology 2007; 69: 790–8. [DOI] [PubMed] [Google Scholar]
- 16. Low PA, Sandroni P, Joyner M, Shen WK. Postural tachycardia syndrome (POTS). J Cardiovasc Electrophysiol 2009; 20: 352–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thieben MJ, Sandroni P, Sletten DM et al Postural orthostatic tachycardia syndrome: the Mayo clinic experience. Mayo Clin Proc 2007; 82: 308–13. [DOI] [PubMed] [Google Scholar]
- 18. Kavi L, Nuttall M, Low DA et al A profile of patients with postural tachycardia syndrome and their experience of healthcare in the UK. Br J Cardiol 2016; 23: 1–6. [Google Scholar]
- 19. McDonald C, Koshi S, Busner L, Kavi L, Newton JL. Postural tachycardia syndrome is associated with significant symptoms and functional impairment predominantly affecting young women: a UK perspective. BMJ Open 2014; 4: e004127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bhatia R, Kizilbash SJ, Ahrens SP et al Outcomes of adolescent‐onset postural orthostatic tachycardia syndrome. J Pediatr 2016; 173: 149–53. [DOI] [PubMed] [Google Scholar]
- 21. Pederson CL, Brook JB. Health‐related quality of life and suicide risk in postural tachycardia syndrome. Clin Auton Res 2017; 27: 75–81. [DOI] [PubMed] [Google Scholar]
- 22. Boris JR. Postural orthostatic tachycardia syndrome in children and adolescents. Auton Neurosci 2018; 215: 97–101. [DOI] [PubMed] [Google Scholar]
- 23. Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta‐analysis. Clin Gastroenterol Hepatol 2012; 10: e4. [DOI] [PubMed] [Google Scholar]
- 24. Lipton RB, Bigal ME, Diamond M et al Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 2007; 68: 343–9. [DOI] [PubMed] [Google Scholar]
- 25. Mazurek JM, Syamlal G. Prevalence of asthma, asthma attacks, and emergency department visits for asthma among working adults – national health interview survey, 2011–2016. MMWR Morb Mortal Wkly Rep 2018; 67: 377–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Suter LG, Murabito JM, Felson DT, Fraenkel L. The incidence and natural history of Raynaud's phenomenon in the community. Arthritis Rheum 2005; 52: 1259–63. [DOI] [PubMed] [Google Scholar]
- 27. Wallman D, Weinberg J, Hohler AD. Ehlers‐Danlos syndrome and postural tachycardia syndrome: a relationship study. J Neurol Sci 2014; 340: 99–102. [DOI] [PubMed] [Google Scholar]
- 28. Kavi L. Consider postural orthostatic tachycardia syndrome in patients with syncope. Am Fam Physician 2012; 86: 4. [PubMed] [Google Scholar]
- 29. Raj V, Haman KL, Raj SR et al Psychiatric profile and attention deficits in postural tachycardia syndrome. J Neurol Neurosurg Psychiatry 2009; 80: 339–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Anderson JW, Lambert EA, Sari CI et al Cognitive function, health‐related quality of life, and symptoms of depression and anxiety sensitivity are impaired in patients with the postural orthostatic tachycardia syndrome (POTS). Front Physiol 2014; 5: 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stanners MN, Barton CA, Shakib S, Winefield HR. Depression diagnosis and treatment amongst multimorbid patients: a thematic analysis. BMC Family Pract 2014; 15: 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kavi L, Gammage MD, Grubb BP, Karabin BL. Postural tachycardia syndrome: multiple symptoms, but easily missed. Br J Gen Pract 2012; 62: 286–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Benarroch EE. Postural tachycardia syndrome: a heterogeneous and multifactorial disorder. Mayo Clin Proc 2012; 87: 1214–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stewart JM, Boris JR, Chelimsky G et al Pediatric disorders of orthostatic intolerance. Pediatrics 2018; 141: e20171673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Raj SR. Row, row, row your way to treating postural tachycardia syndrome. Heart Rhythm 2016; 13: 951–2. [DOI] [PubMed] [Google Scholar]
- 36. Sandroni P, Opfer‐Gehrking TL, McPhee BR, Low PA. Postural tachycardia syndrome: clinical features and follow‐up study. Mayo Clin Proc 1999; 74: 1106–10. [DOI] [PubMed] [Google Scholar]
- 37. Safdar N, Abbo LM, Knobloch MJ, Seo SK. Research methods in healthcare epidemiology: survey and qualitative research. Infect Control Hosp Epidemiol 2016; 37: 1272–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Co‐morbidities in patients assessed at Autonomic Disorders Consortium sites.
Table S2. Diagnostic delay.


