Abstract
Mucosal‐associated invariant T (MAIT) cells are unconventional T cells that recognize antigens derived from riboflavin biosynthesis. In addition to anti‐microbial functions, human MAIT cells are associated with cancers, autoimmunity, allergies and inflammatory disorders, although their role is poorly understood. Activated MAIT cells are well known for their rapid release of Th1 and Th17 cytokines, but we have discovered that chronic stimulation can also lead to potent interleukin (IL)‐13 expression. We used RNA‐seq and qRT‐PCR to demonstrate high expression of the IL‐13 gene in chronically stimulated MAIT cells, and directly identify IL‐13 using intracellular flow cytometry and multiplex bead analysis of MAIT cell cultures. This unexpected finding has important implications for IL‐13‐dependent diseases, such as colorectal cancer (CRC), that occur in mucosal areas where MAIT cells are abundant. We identify MAIT cells near CRC tumors and show that these areas and precancerous polyps express high levels of the IL‐13 receptor, which promotes tumor progression and metastasis. Our data suggest that MAIT cells have a more complicated role in CRC than currently realized and that they represent a promising new target for immunotherapies where IL‐13 can be a critical factor.
Keywords: Colorectal cancer, human immunity, IL‐13, MAIT cells, tumor immunity
Mucosal‐associated invariant T (MAIT) cells are regarded as proinflammatory lymphocytes with a Th1/Th17 cytokine response. This study shows human MAIT cells can also be prominent IL‐13‐producing cells. The findings shed new light on the potential role of MAIT cells in tumor immunity and their potential as new targets for immunotherapies in colorectal cancer and other IL‐13‐dependent diseases.

Introduction
Mucosal‐associated invariant T (MAIT) cells are MR1‐restricted unconventional T cells that express an invariant TCR α‐chain (Vα7.2‐Jα33 (TRAV1‐2–TRAJ33)) paired with a narrow set of TCR β‐chains (TRBV6 and TRBV20). They recognize riboflavin metabolites (e.g. 5‐[2‐oxopropylideneamino]‐6‐D‐ribitylaminouracil (5‐OP‐RU)) produced by bacteria and yeast,1, 2 although MAIT cells can also be stimulated independently of the TCR by interleukin (IL)‐12 and IL‐18, which are associated with microbial infection.1, 3, 4, 5 Human MAIT cells are abundant in mucosal tissues such as the lung and colon and also comprise approximately 5% of T cells in peripheral blood.6, 7, 8
The functioning of MAIT cells includes the potential for cytotoxicity,5 but they are especially known for their potent cytokine response.7, 9 Stimulated MAIT cells rapidly release TNF, IFN‐γ and IL‐17, but most studies have reported that they do not produce Th2 cytokines.7, 10, 11 One study detected IL‐13 from a small proportion of human CD4+CD161+TRAV1‐2+ MAIT‐like cells5; however, this feature was not observed in a later study of CD4+ MAIT cells that were more stringently identified using MR1‐5‐OP‐RU tetramer.12
Aside from anti‐microbial responses, MAIT cells have been linked to anti‐tumor responses, inflammatory and autoimmune disorders, asthma and type 1 and type 2 diabetes.8, 13, 14, 15, 16, 17, 18, 19, 20 Many of these diseases manifest themselves near mucosal tissues where MAIT cells are abundant, including respiratory and gut regions, but the role of MAIT cells is often poorly defined. In some cases, including colorectal cancer, activated MAIT cells have been identified in affected tissues, but it is not known whether they have a protective role or exacerbate disease and a caveat to these studies has been their reliance on surrogate markers to identify MAIT cells, rather than the more definitive use of MR1‐5‐OP‐RU tetramers.21, 22, 23
The cytokine profile of MAIT cells from healthy individuals has mostly been characterized after short‐term stimulation assays that take advantage of the MAIT cells’ rapid cytokine response.7, 10, 11, 24 Consistently strong cytokine responses have been reliably detected, but we reasoned that it was important to study MAIT cells over a longer period of stimulation because they could be chronically stimulated in some settings. One important example is in colorectal cancer, where tumors and precancerous polyps often cause breaches in epithelial barriers that exposes tissues to commensal bacteria and their degradative products for extended periods and often causes significant local inflammation.25, 26 Several studies report activated MAIT cells at high frequency near colorectal tumors,21, 22, 23 so it is important to determine their potential impact, particularly as MAIT cell accumulation in tumors has been identified as an indicator of poor prognosis for colorectal cancer (CRC).21
In this study, we demonstrate that one of the most prominent changes in gene expression by MAIT cells after 7–10 days of stimulation is the upregulation of IL‐13. Prodigious release of IL‐13 protein begins after several days, which explains why earlier studies of shorter duration did not report this feature. Our findings indicate that the cytokine profile of MAIT cells is more complex than currently recognized and we provide important new information about the potential involvement of MAIT cells in colorectal cancer and other diseases where IL‐13 is an important factor.27, 28
Results
Most studies have assessed cytokine production by MAIT cells after short‐term stimulation with the 5‐OP‐RU antigen, bacterial infection or preparations, or with pan‐T cell activators such as phorbol 12‐myristate 13‐acetate (PMA) and ionomycin7, 10, 11, 24. This induces a robust cytokine response dominated by TNF, IFN‐γ and IL‐17, but it may not reflect conditions where MAIT cells are stimulated for longer periods.
We sorted MAIT cells from blood of six healthy adult donors and cultured them with anti‐CD3/CD28 mAbs in the presence of Phytohaemagglutinin, IL‐2 and IL‐7 for ~2 weeks. MAIT cells were defined either as Vα7.2TCR+CD161+ T cells, or as T cells that bound MR1 tetramer loaded with 5‐OP‐RU (hereafter MR1 tetramer), with both approaches identifying near‐identical cell populations (Figure 1a). The cells were processed for RNA‐seq analysis immediately after harvesting cultures (“activated MAIT cells”), or after FACS‐sorting of ex vivo (“not cultured”) cell suspensions. A comparison of activated and nonactivated MAIT cells revealed high transcription of IL‐5 and IL‐13 in the activated groups, alongside expected increases in IFN‐γ, IL‐2Rα (CD25) and Granzyme B (Figure 1b). These results were surprising because MAIT cells have not been thought to produce Th2 cytokines.10
Figure 1.

Chronic stimulation induces IL‐13 expression by human Mucosal‐associated invariant T (MAIT) cells. MAIT cells were FACS‐sorted from blood of healthy donors for culturing and RNA analysis. We obtained similar results from MAIT cells defined as CD3+Vα7.2+CD161hi or as CD3+Vα7.2+MR1‐5‐OP‐RU tetramer+ lymphocytes and confirmed (a) that both methods of identification identified similar populations of cells. We conducted RNA sequencing of unstimulated MAIT cells (RNA extracted post FACS sort) and cells stimulated for 2–3 weeks (“stimulated”). (b) Glimma plot compares gene expression for stimulated versus unstimulated CD3+Vα7.2+CD161hi MAIT cells, where black dots signify genes with significant upregulation and light gray significant downregulation. Dark gray dots indicate no significant difference in gene expression. Data for each group represent RNA analysis of MAIT cells from six healthy donors. (c) Quantitative polymerase chain reaction (RT‐qPCR) confirmed upregulation of IL‐13 gene expression in CD3+Vα7.2+MR1‐tetramer+ MAIT cells stimulated for 8 days. MAIT cell samples were from healthy donors (n = 4), with each sample tested in triplicate for each gene.
We focused on IL‐13 because of its well‐established role in colorectal cancer, and because MAIT cells have been implicated in several CRC studies, although their role has not been defined.21, 22, 23 Importantly, we found that MAIT cells showed high IL‐13 responses irrespective of whether they were defined as Vα7.2TCR+CD161+ T cells, or as Vα7.2TCR+ T cells that bound MR1 tetramer (Figure 1b and data not shown). We confirmed the expression of IL‐13 by MAIT cells from four independent healthy donors using qRT‐PCR performed on cells stimulated under the same conditions as the RNA‐seq assay (Figure 1c).
We next sought to establish whether IL‐13 protein was actively secreted by MAIT cells. Supernatants from four of the stimulated cultures of sorted MAIT cells used for RNA‐seq analysis were tested for cytokines using a multiplexed bead‐based assay and confirmed high levels of IL‐13 protein alongside the expected TNF and IFN‐γ cytokines (Figure 2a). The results were closely aligned with the RNA‐seq data and a kinetic analysis showed that IL‐13 and IL‐5 secretion were substantially delayed compared with TNF and IFN‐γ, which were evident from the first day (Figure 2b).
Figure 2.

Prodigious IL‐13 production from chronically stimulated MAIT cells. (a) Cytokines from supernatants of four of the stimulated MAIT cell cultures used for RNAseq analysis were measured using the LEGENDplex T helper multi‐analyte flow assay kit. Each supernatant showed high levels of IL‐13. (b) A time course experiment of cytokines produced by expanding CD3+Vα7.2+MR1‐tetramer+ MAIT cells revealed an initial Th1 response dominated by IFN‐γ and TNF. After approximately 5 days, this profile changed to a Th1/Th2 profile with high concentrations of IL‐13 and IL‐5 (n = 7 separate healthy donors). Error bars signify mean and s.e.m.
The finding of high amounts of IFN‐γ and TNF early in the MAIT cell response was consistent with earlier reports; however, IL‐13 and IL‐5 expression was not detected for several days after stimulation, after which their concentrations rose rapidly. This was also true of IL‐4, although always at lower concentrations than for IL‐13.
We next assessed IL‐13 expression using intracellular flow cytometry. Human anti‐IL‐13 staining is notoriously weak for flow cytometry analysis, but we reliably demonstrated IL‐13 expression by MAIT cells from healthy human donors that were stimulated with Phytohaemagglutinin, IL‐2, IL‐7 and anti‐CD3/28 for 6–9 days, and with PMA and ionomycin for 5 h prior to harvest. The cells were surface stained for CD3, CD4, CD8, CD161 and CD69, and stained intracellularly for TNF and IL‐13. We consistently identified IL‐13+ MAIT cells among the archetypal CD8+ MAIT cell subset (Figure 3a).
Figure 3.
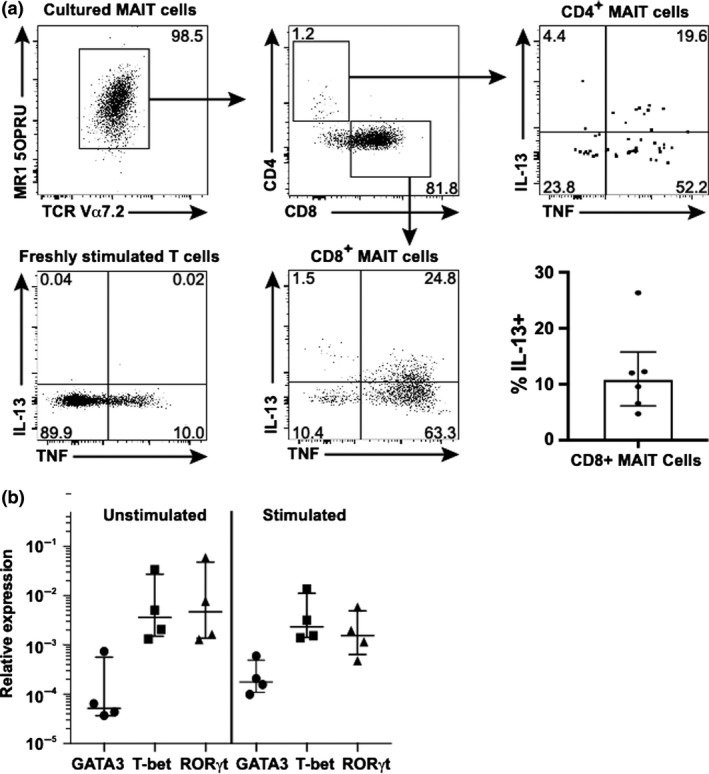
IL‐13 is produced by CD4+ and CD8+ MAIT cell subsets. FACS‐sorted MAIT cells were cultured in stimulation media for 8 days and further stimulated with phorbol 12‐myristate 13‐acetate (PMA) and ionomycin for the last 5 h of the culture. (a) Cultured MAIT cells (defined as CD3+Vα7.2+MR1‐5‐OP‐RU tetramer+ cells) were stained for flow cytometry analysis, gated into CD4+ and CD8+ subpopulations and analyzed for intracellular TNF and IL‐13 expression. A 6‐h control culture of conventional T cells was similarly analyzed (“freshly stimulated T cells”). Collective data showing the proportion of IL‐13+ CD8+ MAIT cells after stimulation with PMA and ionomycin after 8 day culture with PHA, anti‐CD3/CD28, IL‐7 and IL‐2 are shown for 5 independent donors (middle right graph). (b) qPCR analysis of sorted unstimulated MAIT cells (“not cultured”) and MAIT cells stimulated for 8 days revealed similar expression of GATA3, T‐bet (TBX21) and RORγt (RORC) between groups. qPCR reactions were conducted in triplicate for each gene. Each group shows analysis of MAIT cells isolated from the same four healthy donors. The median and interquartile range are shown. Wilcoxon matched‐pairs and Mann–Whitney analysis revealed no significant differences between groups.
In light of a recent report,5 we separately examined CD4+ MAIT cells and confirmed they were also producing IL‐13, although most IL‐13 production came from the numerically dominant CD8+ MAIT cell subset. An interesting observation was that despite the high expression of IL‐13 detected by qRT‐PCR and in culture media of sorted MAIT cells, the proportion of IL‐13+ cells detected at any one time with flow cytometry was usually relatively modest. For instance, the frequency of IL‐13+ stimulated MAIT cells from different donors was rarely above 20% in flow cytometry analysis (Figure 3a and data not shown), although this may be an underestimate as the anti‐human IL‐13 mAb has quite weak staining intensity compared with mAbs specific for cytokines such as TNF, or to IL‐13 in mice.
IL‐13 is associated with GATA‐3 expression by T cells and we tested whether GATA‐3 expression was increased in chronically stimulated MAIT cells. We FACS‐sorted MAIT cells from the blood of healthy donors and analyzed them using qRT‐PCR for expression of GATA‐3, T‐bet and RORγt before and after stimulation. Consistent with other reports, the expression of RORγt and T‐bet were higher than GATA‐3 among MAIT cells,12 and interestingly, there was no significant change in gene expression between unstimulated and stimulated MAIT cells (Figure 3b).
Previous studies have reported that MAIT cells are activated and higher in frequency in colorectal tumors21, 22, 23 and one study found that high frequencies of MAIT cells in CRC tumors were an indicator of poor prognosis.21 The role and importance of MAIT cells in this setting remains unclear, but it is noteworthy that IL‐13 signaling is a poor prognostic indicator in CRC.27, 28
We wanted to confirm that MAIT cells were present near CRC tumors because earlier studies had relied on surrogate markers such as anti‐Vα7.2 or anti‐CD161 mAbs that do not stringently identify MAIT cells.21, 22, 23 To address this, we used a combination of flow cytometry and immunofluorescent staining to analyze human CRC tumors and associated regions of otherwise healthy colon to determine whether MAIT cells were present. We stained lymphocytes from enzymatically‐digested tumor and colon samples and used flow cytometry to demonstrate that most Vα7.2+ T cells were MAIT cells that also bound the MR1 tetramer (Figure 4a and data not shown). While MAIT cells appeared higher in frequency in tumor infiltrated colon than in normal colon (expressed as a % of T cells), this difference was not significant.
Figure 4.

MAIT cell presence near colorectal tumors, and IL‐13 receptor α1 expression by colorectal tumors and neoplastic lesions. (a) CRC tumors were enzymatically digested and stained for flow cytometry analysis. MAIT cells were defined as viable lymphocytes that co‐expressed CD3 (left) and Vα7.2 and which bound the MR1 tetramer loaded with 5‐OP‐RU. A comparison of MAIT cell frequency as a proportion of total T cells (CD3+) in normal colon tissue (n = 6) and colorectal tumor samples (n = 10) is shown (far right) (Mann–Whitney U‐test; P > 0.31, n.s.). Patient matched samples were used where available. Data set error bars represent median and IQR (b) Representative immunofluorescence frozen section staining of human colorectal cancer tissue. Cells staining dual positive for CD3 and Vα7.2 were identified at the border between the normal mucosal tissue and the colorectal tumor mass. Representative immunohistochemistry staining is shown for IL‐13Rα1 in (c) normal colonic mucosa, (d) a dysplastic adenomatous polyp and (e) the border between normal mucosa and invasive cancer. (f, g) HT‐29 colorectal cancer cells were incubated for 60 min at 37°C, 5% CO2, with the supernatant from stimulated MAIT cells (MAIT S/N) or the same media that had not been cultured (Exp. Media). Phosphorylation of the nuclear transcription factor STAT6 (pSTAT6) was determined by flow cytometry. (f) Histograms of HT‐29 CRC cells show phosphorylation of STAT6 after exposure to MAIT cell supernatant. The addition of anti‐IL‐13 mAbs to the MAIT cell supernatant prior to culture with the HT‐29 cells inhibited STAT6 phosphorylation (MAIT S/N + α‐IL‐13). The experiment was repeated with MAIT cells from three separate healthy donors and included the controls RPMI + 10% FBS (Media), RPMI + 10% FBS containing 30 ng mL−1 recombinant IL‐13 (IL‐13) and RPMI + 10% FBS containing 30 ng mL−1 recombinant IL‐13 plus 10 μg mL−1 anti‐IL‐13 mAbs (IL‐13 + α‐IL‐13) (g), with IL‐13‐induced STAT6 phosphorylation occurring in each test. Data set error bars represent mean and s.e.m.
Having confirmed that MAIT cells were present, we used anti‐Vα7.2 mAbs for localization studies of MAIT cells on frozen sections of colon tissue from patients with CRC. We used anti‐Vα7.2 mAbs because they are more technically reliable than the MR1 tetramer for section staining, and we had already established that ~2/3 of Vα7.2+CD3+ T cells were MAIT cells that bound MR1 tetramer loaded with 5‐OP‐RU (Figure 4a). Costaining for Vα7.2, CD3 and DAPI demonstrated that Vα7.2+CD3+ T cells were overwhelmingly congregated near the margins of colorectal tumors and near the breaches in gut epithelium caused by the tumors (Figure 4b). Although some non‐MAIT cells would be present,12 flow cytometry showed that most Vα7.2+CD3+ T cells were MAIT cells that were colocalizing with CRC tumor cells. Importantly, we found high expression of the IL‐13 receptor (IL‐13Rα1) on CRC tumor cells and on polyps that can develop into tumors (Figure 4c–e), which highlighted the potential significance of IL‐13 release by MAIT cells in these regions.
Lastly, we tested the effect of supernatant from 7‐10 day cultures of sorted MAIT cells on STAT6 signaling in the HT‐29 human colorectal tumor cell line (Figure 4f, g). We detected stronger signaling through STAT6 in cells treated with MAIT cell supernatant than with untreated samples, indicating that physiologically significant amounts of IL‐13 had been released by the FACS‐sorted MAIT cells. STAT6 signaling can potentially be triggered by IL‐13 or IL‐4, but only low levels of IL‐4 were detected in MAIT cell cultures and STAT6 signaling appeared to be specifically inhibited by pretreating the assays with anti‐IL‐13 mAbs. These data identify a potential IL‐13/IL‐13R axis between chronically stimulated MAIT cells and nearby CRC tumor cells.
Discussion
The relative abundance of MAIT cells in blood and mucosal tissues suggest that they have an evolutionarily conserved role in human immunity. They clearly have anti‐microbial functions, but MAIT cells are now also associated with cancers, asthma, diabetes, inflammatory disorders and cardiometabolic disorders.18, 29, 30, 31, 32 Our data provide a more complete understanding of the functional potential of MAIT cells, which may shed light on their importance in these settings. Early reports that activated MAIT cells rapidly release proinflammatory cytokines such as IFN‐γ, TNF and IL‐17 have been confirmed by many groups, but our new data show that MAIT cells can also produce Th2 cytokines such as IL‐5 and IL‐13 after prolonged stimulation, with IL‐13 especially significant given its links to colorectal cancer and some respiratory conditions. This adds a new dimension to the functional potential of MAIT cells in healthy tissues and disease settings.
The delayed production of IL‐13 compared with IFN‐γ and TNF likely explains why this feature has not been observed in earlier studies that used short term stimulation. Our findings do not contradict the earlier studies and indeed our kinetic analysis of cytokine releases confirms that analysis up to 48 h poststimulation identifies significant expression of TNF and IFN‐γ, but longer stimulation sees IL‐13 (and IL‐5) become readily detectable. It is intriguing that the analysis of RNA and culture media from sorted MAIT cells indicated high expression of IL‐13 because flow cytometry identified fewer IL‐13+ than TNF+ MAIT cells, although a contributing factor could be that the anti‐IL‐13 mAb has comparatively weak staining. It is therefore possible (1) that flow cytometry does not detect all IL‐13+ cells; (2) that only a small subset of MAIT cells can express IL‐13; or (3) that the kinetics of IL‐13 release differs between MAIT cells. Cultured MAIT cells did show increased expression of some exhaustion markers, but only viable cells were analyzed and the proportion of IL‐13+ MAIT cells was relatively consistent across different timepoints (data not shown).
These findings suggest that it may be important to re‐appraise the role of MAIT cells in different settings. Obvious examples are in the gut and respiratory tract where MAIT cells are abundant and where there is clear potential for MAIT cells to be chronically activated through exposure to bacterial antigens, or IL‐12 and IL‐18. MAIT cells in these tissues are likely to have anti‐bacterial activities, but IL‐13 release could also see them impact smooth muscle contraction, mucin production and trafficking of innate immune cells.33
Of perhaps greater significance is that MAIT cells may have an unrecognized role in diseases where IL‐13 is important. For example, at least three studies have associated MAIT cells with colorectal cancer in humans21, 22, 23 and one also reported that the accumulation of MAIT cell in CRC tumors was correlated with a poor clinical outcome.21 These are intriguing findings, but the role of MAIT cells in CRC has not been defined, and the stringency of MAIT cell identification used in those studies has been challenged.
We have used MR1 tetramers loaded with 5‐OP‐RU to address the latter concern and unequivocally demonstrate that MAIT cells were present in tumor samples from patients with CRC. We also showed that most Vα7.2+ T cells were 5‐OP‐RU‐MR1‐tetramer+ MAIT cells, which meant we could use anti‐Vα7.2 mAbs to show MAIT cells colocalized with CRC tumor cells, which expressed high levels of IL‐13Rα1. Our data did not fully support the finding of earlier studies that reported significant increases in MAIT cell frequency in tumors, but these studies did not use the MR1 tetramer.21, 22, 23 However, we do not exclude this as a possibility (we saw a trend toward an increased frequency in tumors) and more importantly, we can now unequivocally confirm MAIT cells are present. Our findings suggest IL‐13 release by MAIT cells could potentiate CRC and thus explain why MAIT cells are associated with a poor clinical outcome because IL‐13 promotes epithelial to mesenchymal transition, M2 macrophage polarization and CRC tumor cell metastasis and proliferation.27, 28
Activated MAIT cells have been identified in CRC tumors, but the trigger(s) for their activation are not known. Our finding that MAIT cells are colocalized with CRC tumors may offer new clues because the gut epithelial layer is often breached by CRC tumor growth, which chronically exposes the lamina propria to commensal bacteria that can be recognized by resident MAIT cells.25, 26, 34, 35 We further demonstrated that supernatant from chronically stimulated MAIT cells induced IL‐13‐dependent STAT6 signaling in a CRC tumor cell line, illustrating the potency of the MAIT cell IL‐13 response. IL‐13 has immune‐suppressive activities and can support CRC tumor growth and metastasis through the STAT6 pathway, so the capacity of MAIT cells to produce very high levels of IL‐13 and their presence in and around tumors identifies them as a potentially significant factor in CRC pathogenesis and a potential new target for immunotherapies.
MAIT cells have also been implicated in other diseases where IL‐13 is an important cytokine mediator, including chronic obstructive pulmonary disease and asthma.13, 17, 36, 37, 38, 39 Given the chronic inflammation associated with these diseases and the involvement of mucosal tissues prone to bacterial infection, MAIT cells could again represent an important and previously unrecognized source of IL‐13, so targeting them could be clinically beneficial. We also found that IL‐5 and to a lesser extent, IL‐4, were unexpectedly expressed by MAIT cells days after TNF and IFN‐γ responses were detected. This is also intriguing because IL‐5 and IL‐4 have both been implicated in the pathogenesis of some forms of asthma and other respiratory conditions, so it will be important to establish if MAIT cells express these cytokines in vivo.40, 41
Our data shed new light on the functions of MAIT cells. We have shown that MAIT cells can express IL‐13 in addition to IFN‐γ, TNF and IL‐17, which means they are more versatile in function and influence than previously realized. In light of this, it is important to reconsider what roles MAIT cells play in various disease settings and whether it is feasible to modulate their cytokine profile to improve clinical outcomes.
Methods
Human blood and tissue
Peripheral blood and small samples of large intestine (tumor and/or tissue) were donated by patients undergoing surgery for colorectal cancer. Patient ages ranged from 36 to 84 years and healthy samples of colon were collected from the far end of the surgically removed specimen generally at least 30 cm from the tumor and well away from any areas of inflammation. Informed consent was provided by patients in compliance with Ethics approval from the Ballarat Health Services and Saint John of God Ballarat Hospital Human Research Ethics Committee (HREC/15/BHSSJOG/5 and HREC/10/BHSSJOG/57) and Federation University Australia HREC (A08‐100). Peripheral blood lymphocytes from healthy donors were isolated from buffy coat samples provided by the Australian Red Cross Blood Service.
PBMCs from patients were isolated by density gradients using Histopaque‐1077 (Sigma‐Aldrich, Sydney, Australia) and cryopreserved for later analysis. Donated colorectal tissue removed at surgery was sent to diagnostic pathology for routine pathological examination, with excess tissue either processed for lymphocyte isolation using a Miltenyi Tissue Dissociator (Miltenyi Biotec, Auburn, CA, USA), or frozen in Tissue Tek OCT (Sakura, Alphen aan den Rijn, Netherlands) chilled by liquid nitrogen and stored at −80°C for section/immunofluorescence staining. Additional colorectal formalin fixed, paraffin‐embedded tissue blocks were retrieved from pathology archive upon request.
Flow cytometry
Human PBMCs were stained in PBS + 1% Bovine Calf Serum for 15 min on ice with Fixable Viability Stain 700 (Becton Dickinson Horizon, San Jose, CA, USA), Human Fc Block (Becton Dickinson Pharmingen, San Jose, CA, USA) and anti‐human antibodies from the following list: Vα7.2‐FITC or APC (3C10; Becton Dickinson Biosciences, San Jose, CA, USA), CD3‐PE‐Cy7 (UCHT1; eBioScience, Waltham, MA, USA) or Pacific Blue (UCHT1; Becton Dickinson Pharmingen), CD4‐APC‐Cy7 (RPA‐T4; Becton Dickinson Biosciences) or BV510 (SK3; Becton Dickinson Horizon), CD8‐BV605 (SK1; Becton Dickinson Horizon), CD161‐PE‐Dazzle or APC (HP‐3G10; Biolegend, San Diego, CA, USA). Biotinylated 5‐OP‐RU‐loaded MR1 monomers were generated as previously described and tetramerized in‐house with streptavidin‐PE.2 Cytokine staining was performed with a BD Cytofix/Cytoperm kit (Becton Dickinson Biosciences) as per the manufacturer's instructions. Briefly, surface labeled cells were fixed and permeabilized, then stained with antibodies for TNFα‐FITC (MAb11; eBioscience) and IL‐13‐PE‐Cy7 (JES105A2; Biolegend).
Flow cytometry and fluorescence‐activated cell sorting
Flow cytometry analysis was performed using an LSR Fortessa (BD Biosciences, San Jose, CA, USA) and cell sorting was performed using a FACS Aria II cell sorter (BD Biosciences). Sorted populations were typically analyzed after sorting and populations greater than 97% purity were utilized for the stimulation and RNAseq experiments. FlowJo software (v. 10.5.0 (FlowJo, LLC, Ashland, OR, USA) was used to analyse flow cytometric data. Unless otherwise stated, analysis was of viable lymphocytes with nonviable cells, autofluorescent cells and doublets excluded. Gating of positive populations was made after comparisons with controls, including appropriate unstained and unstimulated samples.
MAIT cell stimulation assays
Ninety‐six‐well round bottom plates (Sigma‐Aldrich, Castle Hill, NSW, Australia) were coated with 50 μL of 5 μg mL−1 anti‐CD3 (HIT3a; BD Pharmingen, San Jose, CA, USA) in PBS at 37°C for 4 h prior to stimulation experiments. Purified MAIT cells were cultured in TexMacs (Miltenyi Biotec) supplemented with 2% (v/v) fetal bovine serum (FBS) (Sigma‐Aldrich) and 1× Penicillin‐Streptomycin (Sigma‐Aldrich) in the presence of IL‐2 (50 U mL−1), IL‐7 (10 U mL−1), Phytohaemagglutinin) (1:1000; Gibco, Grand Island, NY, USA) and CD28 (1 μg mL−1, clone CD28.2; BD Pharmingen). This media is herein referred to as “stimulation media.”
For time course experiments, approximately 100 000 purified MAIT cells were cultured in 100 μL of stimulation media, with 35 μL of supernatant removed at each time point and replaced with 35 μL of fresh stimulation media. For flow cytometry analysis, MAIT cells were usually stimulated as above, with the addition of 10 ng mL−1 phorbol 12‐myristate 13‐acetate (Sigma, Castle Hill, NSW, Australia) and 1 μg mL−1 ionomycin (Sigma, Castle Hill, NSW, Australia) in the presence of GolgiPlug (1:1000; BD Biosciences) for the final 5 h prior to harvesting.
Multiplex bead cytokine detection immunoassays
LEGENDplex (BioLegend) bead‐based cytokine detection immunoassays were used to identify secreted cytokines following in vitro cell culture and stimulation. Cell culture supernatants were collected and stored at −80°C before use. The Human Th Cytokine Panel and the Human Cytokine 2 Panel (BioLegend) were used to detect secreted cytokines as per the manufacturer's instructions. Analyses were performed using LEGENDplex Data Analysis Software (BioLegend) with cytokines quantified by comparing samples to a set of standard curves prepared in parallel with supernatant samples.
RNA extraction
To analyze gene expression by MAIT cells from healthy donors, total RNA was extracted from 100 000 freshly sorted MAIT cells, or from MAIT cells harvested from stimulation cultures, using the RNeasy mini kit (QIAGEN, Frederick, MD, USA) according to the manufacturer's instructions. Extracted RNA was either immediately converted to cDNA as described below or stored at −80°C prior to transportation on dry ice to the Australian Genome Research Facility (Parkville, VIC, Australia).
RNAseq
RNA sequencing was performed at the Australian Genome Research Facility using Lexogen's QuantSeq 3” mRNA‐seq kit. Briefly, DNase treated total RNA was assessed on the Agilent Bioanalyzer 2100 and QuantSeq library preparation was used according to the manufacturer's specifications. Briefly, libraries were initiated by oligodT priming. The primer already contains Illumina‐compatible linker sequences. After first strand synthesis, the RNA was removed and second strand synthesis initiated by random priming and a DNA polymerase. The random primer also contained Illumina‐compatible linker sequences. No purification was required between first‐ and second‐strand synthesis. Second‐strand synthesis was followed by a magnetic bead‐based purification step. The library was then amplified, introducing the sequences required for cluster generation. Prepared libraries were then quality controlled using qPCR and pooled to normalize prior to sequencing. Sequencing was carried out on the Illumina HiSeq 2500 using 50 bp cycles and V4 chemistry.
The resultant sequence reads went through quality control and trimming using trim‐galore. The STAR aligner (v2.5.3a) was used to map reads to the human reference genome (hg38). featureCounts (v1.5.3) was used to summarize the number of reads aligned to each region at the gene level. A single count was provided for each gene across each sample. Counts per million of 2 was used as the cut‐off to remove genes with low counts. edgeR (version 3.22.5) was used to perform differential expression analysis. The standard TMM normalization method from edgeR was applied to the count matrix to adjust for varying library sizes between samples. The differences in expression between groups for a variety of housekeeping genes were minor (<2 fold). The Quasi‐likelihood F‐test was used to assign P‐values (and False Discovery Rate/adjusted P‐values) for each gene. A gene was considered differentially expressed if its False Discovery Rate was less than 0.05. Data from this analysis will be made freely available after publication of the data set.
RT‐qPCR
RNA was converted to cDNA using the RT2 first strand kit (QIAGEN) as per the manufacturer's instructions. Quantitative real‐time PCR reactions were carried out on a Rotor‐Gene Q (QIAGEN) using cDNA from the above reaction, RT2 SYBR Green qPCR Mastermix (QIAGEN) and primers RPLP0 (housekeeping gene, Cat PPH21138F) IL‐4 (CAT PPH00565B), IL‐13 (CAT PPH00688F), TNF (CAT PPH00341F), IFNγ (CAT PPH00380C), RORC (CAT PPH05877A), TBX21 (Cat PPH00396A) and GATA3 (CAT PPH02143A, all purchased as commercial primers (QIAGEN). Reaction wells each contained 20 μL of reaction mix with cycling conditions as per the manufacturer's instructions. Each qPCR reaction was tested in triplicated for each gene. Fold change was expressed relative to the housekeeper gene (which was reliably detected in each experiment) and determined by the calculation 2−ΔCT.
Immunofluorescence
Immunofluorescence staining was performed based on a previously described method.42 Frozen sections were cut at a thickness of 6 μm on a Miles Tissue‐Tek II cryostat and subsequently fixed for 10 min in ice‐cold 2% formaldehyde in PBS. The sections were incubated overnight at 4°C with mouse anti‐Vα7.2 (3C10; Biolegend) and rabbit anti‐CD3 (polyclonal; Dako, Santa Clara, CA, USA), washed then stained with Alexa Fluor 488 goat anti‐mouse and Alexa Fluor 568 goat anti‐rabbit for 30 min at room temperature. The sections were then counterstained with DAPI (4’,6‐diamidino‐2‐phenylindole) and mounted in Prolong Diamond (both Invitrogen, Carlsbad, CA, USA). Fluorescence images were acquired using a Nikon C2+ confocal microscope and Nikon A1 camera equipped with a CFI plan apochromat 20X/0.75 objective (Nikon, Melville, NY, USA). Nikon NIS Elements software was used for image acquisition and images were processed with ImageJ/Fiji software (Version 2.0.0; (National Institutes of Health, Bethesda, MD, USA).
Immunohistochemistry
Immunohistochemistry for IL‐13Rα1 was performed on 3‐μm thick sections of formalin fixed, paraffin‐embedded tissue. The sections were mounted on positively coated slides and underwent high pH (Dako) microwave heat‐induced antigen retrieval (HIER) for 10 min. The IL‐13Rα1 primary antibody (polyclonal AB79277 1:500; Abcam, Cambridge, UK) was incubated for 30 min at room temperature and then visualized using the Dako Envision FLEX peroxidase detection system as per the manufacturer's instructions (Dako). The sections were counterstained with hematoxylin and mounted in DePeX.
STAT6 signaling
The human colorectal cancer cell line, HT‐29 (a gift from Professor Phil Darcy), was grown to near confluence, trypsinized, and re‐seeded at 100 000 cells per well in a 96‐well U‐bottom plate. The cells were rested overnight at 37°C, 5% CO2, in 200 μL of RPMI 1640 supplemented with 10% FBS. The plate was then centrifuged and media replaced with 50 μL of fresh MAIT cell stimulation media, or stimulation media harvested as supernatant from MAIT cells stimulated as above (“MAIT S/N”). The HT‐29 cells were incubated for 60 min at 37°C, then fixed and permeabilized before staining for pSTAT6 (pSTAT6‐PE, clone pY641; BD Bioscience). STAT6 phosphorylation was determined by analysis of mean cell fluorescence on an LSR Fortessa (BD Biosciences). Similar assays were established that included purified anti‐IL‐13 (LEAF, clone JES10‐5A2; BioLegend) at a concentration of 10 μg mL−1 (MAIT S/N + α‐IL‐13). Controls included HT‐29 cells cultured in RPMI + FBS (Media), 30 ng mL−1 recombinant IL‐13 (carrier free; BioLegend) in RPMI + 10% FBS (“IL‐13”) or 50 μL of 30 ng mL−1 recombinant IL‐13 in RPMI + FBS + 10 μg mL−1 anti‐Il‐13 (IL‐13 + α‐IL‐13) (Figure 4).
Statistical analysis
PRISM software (GraphPad Software, San Diego, CA, USA) was used for in‐house statistical analysis and graph generation. Nonparametric distribution was assumed unless otherwise stated.
Conflict of Interest
The authors declare no competing financial interests.
Acknowledgments
This research was supported by the National Health and Medical Research Council of Australia (NHMRC) (1113293, 1140126, 1145888 and 1121325), Australian Research Council (ARC) (CE140100011), CASS Foundation (8510) and FECRI. JK was supported by a PhD scholarship co‐funded by Lung Foundation Australia and an Australian Government Research Training Program (RTP) Fee‐Offset Scholarship through Federation University Australia. MP was supported by the Deutsche Forschungsgemeinschaft‐funded graduate school GRK2168 and the The Bonn and Melbourne Research and Graduate School (Bo&MeRanG) Program. DP is supported by a CSL Centenary Fellowship. AC is an inventor on patents describing MR1‐tetramers and is supported by an ARC Future Fellowship FT160100083. DHDG was supported by Australian NHMRC Senior Research Fellowship (1158024). DG is supported by an NHMRC Senior Principal Research Fellowship (1117766). SB is supported by a Dorevitch Cancer Research Fellowship. Mr Bruce Stewart performed the surgeries that provided colon tissue. The authors acknowledge The Department of Histology at Australian Clinical Labs, St John of God Hospital, Ballarat, for processing of paraffin embedded tissue and Professor James McCluskey for provision of MR1 reagents. The authors acknowledge the generous contributions of donors and clinicians who helped to provide human tissue samples.
[The copyright line for this article was changed on 23 August 2019 after original online publication.]
References
- 1. Kjer‐Nielsen L, Patel O, Corbett AJ, et al MR1 presents microbial vitamin B metabolites to MAIT cells. Nature 2012; 491: 717–723. [DOI] [PubMed] [Google Scholar]
- 2. Corbett AJ, Eckle SB, Birkinshaw RW, et al T‐cell activation by transitory neo‐antigens derived from distinct microbial pathways. Nature 2014; 509: 361–365. [DOI] [PubMed] [Google Scholar]
- 3. Awad W, Le Nours J, Kjer‐Nielsen L, McCluskey J, Rossjohn J. Mucosal‐associated invariant T cell receptor recognition of small molecules presented by MR1. Immunol Cell Biol 2018; 96: 588–597. [DOI] [PubMed] [Google Scholar]
- 4. Ussher JE, Bilton M, Attwod E, et al CD161++ CD8+ T cells, including the MAIT cell subset, are specifically activated by IL‐12+IL‐18 in a TCR‐independent manner. Eur J Immunol 2014; 44: 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kurioka A, Jahun AS, Hannaway RF, et al Shared and distinct phenotypes and functions of human CD161++ Vα7.2+T cell subsets. Front Immunol 2017; 8: 1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Treiner E, Duban L, Bahram S, et al Selection of evolutionarily conserved mucosal‐associated invariant T cells by MR1. Nature 2003; 422: 164–169. [DOI] [PubMed] [Google Scholar]
- 7. Salio M, Silk JD, Yvonne Jones E, Cerundolo V. Biology of CD1‐ and MR1‐restricted T cells. Annu Rev Immunol 2014; 32: 323–366. [DOI] [PubMed] [Google Scholar]
- 8. Godfrey DI, Uldrich AP, McCluskey J, Rossjohn J, Moody DB. The burgeoning family of unconventional T cells. Nat Immunol 2015; 16: 1114–1123. [DOI] [PubMed] [Google Scholar]
- 9. Wang H, D'Souza C, Lim XY, et al MAIT cells protect against pulmonary Legionella longbeachae infection. Nat Commun 2018; 9: 3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dusseaux M, Martin E, Serriari N, et al Human MAIT cells are xenobiotic‐resistant, tissue‐targeted, CD161hi IL‐17‐secreting T cells. Blood 2011; 117: 1250–1259. [DOI] [PubMed] [Google Scholar]
- 11. Tang XZ, Jo J, Tan AT, et al IL‐7 licenses activation of human liver intrasinusoidal mucosal‐associated invariant T cells. J Immunol 2013; 190: 3142–3152. [DOI] [PubMed] [Google Scholar]
- 12. Gherardin NA, Souter MN, Koay HF, et al Human blood MAIT cell subsets defined using MR1 tetramers. Immunol Cell Biol 2018; 96: 507–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wills‐Karp M, Luyimbazi J, Xu X, et al Interleukin‐13: central mediator of allergic asthma. Science 1998; 282: 2258–2261. [DOI] [PubMed] [Google Scholar]
- 14. Gold MC, Cerri S, Smyk‐Pearson S, et al Human mucosal associated invariant T cells detect bacterially infected cells. PLoS Biol 2010; 8: e1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Le Bourhis L, Martin E, Peguillet I, et al Antimicrobial activity of mucosal‐associated invariant T cells. Nat Immunol 2010; 11: 701–708. [DOI] [PubMed] [Google Scholar]
- 16. Booth JS, Salerno‐Goncalves R, Blanchard TG, et al Mucosal‐associated invariant T cells in the human gastric mucosa and blood: role in Helicobacter pylori infection. Front Immunol 2015; 6: 466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. D'Souza C, Pediongco T, Wang H, et al Mucosal‐associated Invariant T Cells augment immunopathology and gastritis in chronic Helicobacter pylori infection. J Immunol 2018; 200: 1901–1916. [DOI] [PubMed] [Google Scholar]
- 18. Rouxel O, Da Silva J, Beaudoin L, et al Cytotoxic and regulatory roles of mucosal‐associated invariant T cells in type 1 diabetes. Nat Immunol 2017; 18: 1321–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van Wilgenburg B, Scherwitzl I, Hutchinson EC, et al MAIT cells are activated during human viral infections. Nat Commun 2016; 7: 11653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hinks TS, Zhou X, Staples KJ, et al Innate and adaptive T cells in asthmatic patients: relationship to severity and disease mechanisms. J Allergy Clin Immunol 2015; 136: 323–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zabijak L, Attencourt C, Guignant C, et al Increased tumor infiltration by mucosal‐associated invariant T cells correlates with poor survival in colorectal cancer patients. Cancer Immunol Immunother 2015; 64: 1601–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ling L, Lin Y, Zheng W, et al Circulating and tumor‐infiltrating mucosal associated invariant T (MAIT) cells in colorectal cancer patients. Sci Rep 2016; 6: 20358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sundstrom P, Ahlmanner F, Akeus P, et al Human mucosa‐associated invariant T cells accumulate in colon adenocarcinomas but produce reduced amounts of IFN‐γ. J Immunol 2015; 195: 3472–3481. [DOI] [PubMed] [Google Scholar]
- 24. Reantragoon R, Corbett AJ, Sakala IG, et al Antigen‐loaded MR1 tetramers define T cell receptor heterogeneity in mucosal‐associated invariant T cells. J Exp Med 2013; 210: 2305–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Grivennikov SI, Wang K, Mucida D, et al Adenoma‐linked barrier defects and microbial products drive IL‐23/IL‐17‐mediated tumour growth. Nature 2012; 491: 254–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen J, Pitmon E, Wang K. Microbiome, inflammation and colorectal cancer. Semin Immunol 2017; 32: 43–53. [DOI] [PubMed] [Google Scholar]
- 27. Barderas R, Bartolome RA, Fernandez‐Acenero MJ, Torres S, Casal JI. High expression of IL‐13 receptor α2 in colorectal cancer is associated with invasion, liver metastasis, and poor prognosis. Cancer Res 2012; 72: 2780–2790. [DOI] [PubMed] [Google Scholar]
- 28. Cao H, Zhang J, Liu H, et al IL‐13/STAT6 signaling plays a critical role in the epithelial‐mesenchymal transition of colorectal cancer cells. Oncotarget 2016; 7: 61183–61198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Godfrey DI, Le Nours J, Andrews DM, Uldrich AP, Rossjohn J. Unconventional T cell targets for cancer immunotherapy. Immunity 2018; 48: 453–473. [DOI] [PubMed] [Google Scholar]
- 30. Chandra S, Wingender G, Greenbaum JA, et al Development of asthma in inner‐city children: possible roles of MAIT cells and variation in the home environment. J Immunol 2018; 200: 1995–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lezmi G, Leite‐de‐Moraes M. Invariant natural killer T and mucosal‐associated invariant T cells in asthmatic patients. Front Immunol 2018; 9: 1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Touch S, Assmann KE, Aron‐Wisnewsky J, et al Mucosal‐associated invariant T (MAIT) cells are depleted and prone to apoptosis in cardiometabolic disorders. FASEB J 2018; 32: 5078–5089. [DOI] [PubMed] [Google Scholar]
- 33. Wynn TA. Type 2 cytokines: mechanisms and therapeutic strategies. Nat Rev Immunol 2015; 15: 271–282. [DOI] [PubMed] [Google Scholar]
- 34. Martin E, Treiner E, Duban L, et al Stepwise development of MAIT cells in mouse and human. PLoS Biol 2009; 7: e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ghazarian L, Caillat‐Zucman S, Houdouin V. Mucosal‐associated invariant T cell interactions with commensal and pathogenic bacteria: potential role in antimicrobial immunity in the child. Front Immunol 2017; 8: 1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Grunig G, Warnock M, Wakil AE, et al Requirement for IL‐13 independently of IL‐4 in experimental asthma. Science 1998; 282: 2261–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kuperman DA, Huang X, Koth LL, et al Direct effects of interleukin‐13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat Med 2002; 8: 885–889. [DOI] [PubMed] [Google Scholar]
- 38. Ingram JL, Kraft M. IL‐13 in asthma and allergic disease: asthma phenotypes and targeted therapies. J Allergy Clin Immunol 2012; 130: 829–842; quiz 843–844. [DOI] [PubMed] [Google Scholar]
- 39. Ntontsi P, Papathanassiou E, Loukides S, Bakakos P, Hillas G. Targeted anti‐IL‐13 therapies in asthma: current data and future perspectives. Expert Opin Investig Drugs 2018; 27: 179–186. [DOI] [PubMed] [Google Scholar]
- 40. Chung KF. Targeting the interleukin pathway in the treatment of asthma. Lancet 2015; 386: 1086–1096. [DOI] [PubMed] [Google Scholar]
- 41. Chambliss JM, Sur S, Tripple JW. Asthma versus chronic obstructive pulmonary disease, the Dutch versus British hypothesis, and role of interleukin‐5. Curr Opin Allergy Clin Immunol 2018; 18: 26–31. [DOI] [PubMed] [Google Scholar]
- 42. Gibbs A, Leeansyah E, Introini A, et al MAIT cells reside in the female genital mucosa and are biased towards IL‐17 and IL‐22 production in response to bacterial stimulation. Mucosal Immunol 2017; 10: 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]


