Abstract
Hirayama disease, a juvenile muscular atrophy of the distal upper extremity, is a rare form of cervical flexion myelopathy characterized by insidiously progressive weakness of the hands and forearm muscles (i.e., painless amyotrophy). The pathognomonic finding is a markedly forward-shifted spinal cord during neck flexion, demonstrated by dynamic magnetic resonance imaging (MRI), as in a young man with muscle atrophy in the bilateral distal upper extremities. In this report, the authors describe a 31-year-old man who had the classic radiological and clinical presentations of Hirayama disease. Since prior medical treatment had been ineffective for years, he underwent multilevel instrumented anterior cervical discectomy and fusion (ACDF) to keep his subaxial cervical spine slightly-lordotic (nonflexion). His motor evoked potential amplitude improved immediately during the operation, and there were improvements of myelopathy and a modest reversal of muscle wasting at 1 year postoperatively. Postoperative dynamic cervical spine MRI also demonstrated minimal cord compression and elimination of the venous plexus engorgement dorsal to the thecal sac. Although Hirayama disease is benign in nature and frequently self-limiting, multilevel instrumented ACDF could be a reasonable management option.
Keywords: Hirayama disease, Monomelic amyotrophy, Spinal cord diseases
INTRODUCTION
Hirayama disease, also known as juvenile muscular atrophy of the distal upper extremity, was first described in 1959 [1]. The disease is a rare cervical flexion myelopathy characterized by an insidious progressive subacute unilateral or bilateral weakness of the hand and forearm muscles leading to a painless amyotrophy [2,3]. It is male predominant (mainly in their teens and early twenties) and usually sporadic. Most patients have unilateral muscular atrophy and some others have bilateral but asymmetric symptoms. They usually have only slight or no subjective or objective sensory disturbances. Lower-extremity signs might also be seen in rare severe cases, representing extensive cervical cord injury beyond the anterior horn [4,5]. The worsening course usually ceases spontaneously within several years after onset [3].
This cervical myelopathy is related to the forward displacement of the posterior thecal sac during flexion movements of the neck, causing compression of the cervical cord. Diagnosis is based on flexion cervical magnetic resonance imaging (MRI) by forward displacement of the posterior thecal sac, enhancing the posterior epidural mass from the engorged epidural venous plexus, and cord compression with or without associated cord edema or atrophy [6]. For those dynamic MRIs were not performed, loss of attachment of the posterior dura mater is another important sign in the neutral position [7].
Neural electrophysiological studies of patients with Hirayama disease have revealed an amplitude decrease in cervical somatosensory evoked potential (SSEP), F-wave, and motor evoked potentials (MEPs) [8-11]. However, the relationship between the signal change in neural electrophysiological studies and surgical positioning, or surgical outcome, is not clear. In this report, we present a case of Hirayama disease and describe the clinical observation of a neural electrophysiological study and the clinical outcome.
CASE REPORT
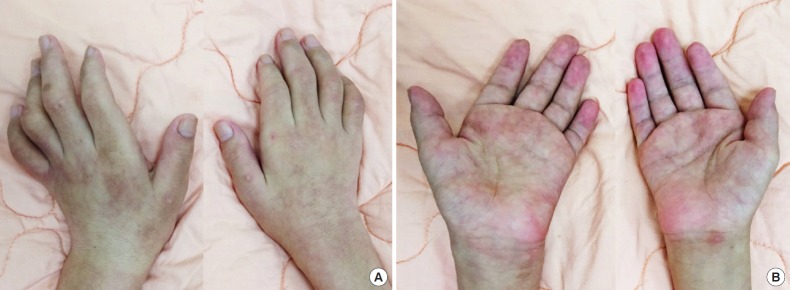
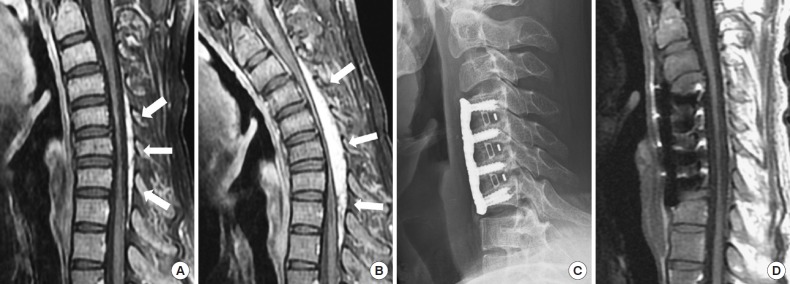
A 31-year-old man had had insidious weakness in both upper extremities since his teen years. He presented with various degrees of intrinsic hand muscle wasting (Fig. 1) bilaterally without sensory deficits, but an unsteady gait which resulted in him barely able to walk. Cervical MRIs taken in a neutral supine position demonstrated forward-shifting and asymmetric atrophy of the spinal cord at the lower cervical spine with engorged epidural venous plexus dorsal to the thecal sac (Figs. 2A, 3A). Moreover, during neck flexion, dilatation of the venous plexus became more prominent and remarkably, and the detached dura caused more compression to the lower cervical and upper thoracic spinal cord (Fig. 2B). The electrophysiological tests indicated lower amplitudes in MEP and decreased cortical SSEP; cervical myelopathy was concluded. These radiological and clinical findings were compatible with Hirayama disease.
Fig. 1.
(A, B) Intrinsic hand muscle wasting.
Fig. 2.
In preoperative cervical magnetic resonance imagings (MRIs), forward-shifting and asymmetric atrophy of the lower cervical spinal cord were seen in a neutral supine position (A, T1-weighted with contrast enhancement). Dilated venous plexus (arrows) became more prominent and more compressive to the lower cervical and upper thoracic spinal cord during neck flexion (B, T1-weighted with contrast enhancement, flexion position). The patient received ACDF over C4 to C7, and segmental kyphosis of the lower cervical spine decreased (C, cervical X-ray 1 year after surgery). Postoperative MRI (D, T1-weighted, neutral position) showed no cervical cord compression and a smaller size of the venous plexus.
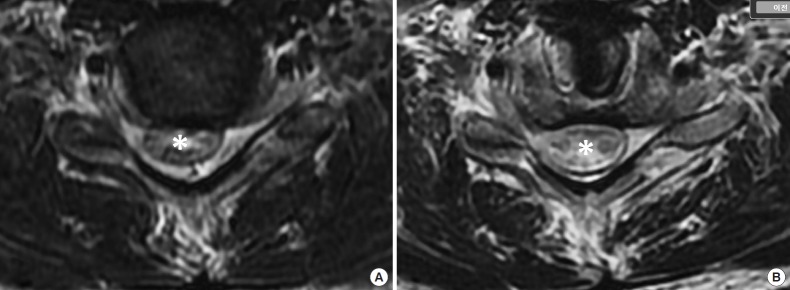
Fig. 3.
An axial view of a preoperative cervical magnetic resonance imaging in nonflexion position (A, T2-weighted) disclosed forward-shifting of the cervical cord (asterisk) and loss of attachment of the posterior dura mater, which improved after surgery (B, T2-weighted).
He received anterior cervical discectomy and fusion (ACDF) from C4 to C7 levels for decompression and to reduce neck flexion. He was placed supine with his neck in a nonflexion position before the operation. After positioning, the amplitude of intraoperative MEPs increased compared with the baseline signals and persisted till the end of the surgery (Fig. 4). The postoperative course was smooth and he could walk with some assistance 1 year after the surgery; however, there was no improvement in the wasting of his upper extremities. Postoperative X-rays revealed decreased segmental kyphosis (Fig. 2C), and MRIs showed no cervical cord compression and better attachment of the posterior dura mater in the nonflexion position (Figs. 2D, 3B).
Fig. 4.
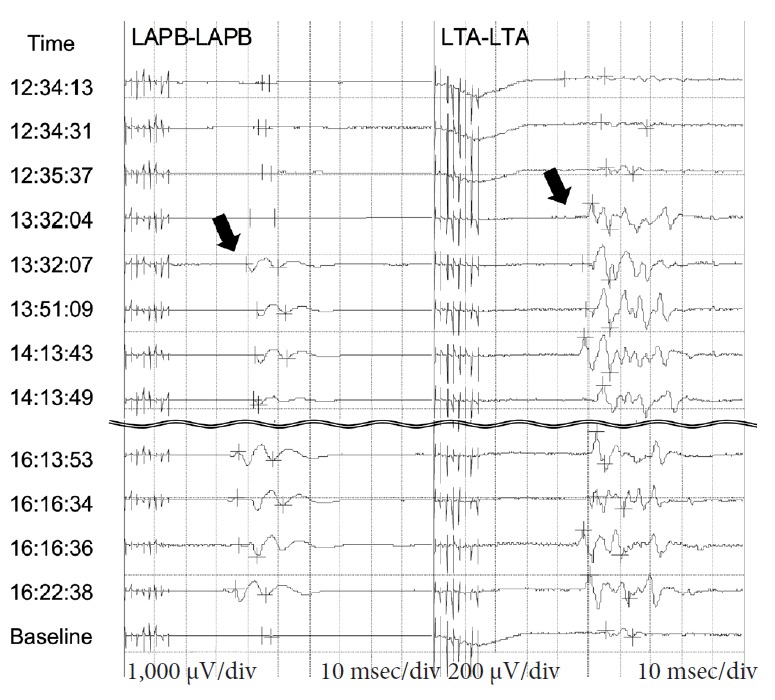
Intraoperative motor evoked potentials (MEPs) of abductor pollicis brevis (APB) and tibialis anterior (TA). The amplitude of MEPs started to magnify at around 13:32 (arrows) and persisted till the end of the surgery. LAPB, left APB; LTA, left TA.
DISCUSSION
Hirayama disease is caused by dynamic compression of the lower cervical cord due to repeated or sustained neck flexion [2,3]. An amplitude decrease in cervical SSEP, F-wave, and MEP are typically found in Hirayama patients. Some studies have shown differences during neck flexion [8,9], while others have reported no changes when compared with a neutral position [10,11]. In this case, we found an increased MEP amplitude after just lordotic cervical posture, and subsequent decompression as well. The MEP amplitude might be useful in predicting the effectiveness of adequate positioning and decompression, on which more studies should focus in the future.
The cervical flexion myelopathy might worsen within several years after onset. The contact pressure between the spinal cord and anterior structures (vertebral bodies and intervertebral discs) of the kyphotic segments in neck flexion could contribute to the ischemic necrosis of the anterior part of the cervical cord [12]. To date, there has not been a standard treatment protocol, and many proposals have demonstrated some effectiveness, although inconsistent. However, the commonly accepted treatment goal is to avoid, or at least mitigate, neck flexion injury to the cervical cord. To maintain the neck in a nonflexion position, some collar immobilization alone has been suggested [13,14]. In fact, neck collar is the most common conservative therapy which might significantly shorten the duration of disease progression as compared with the natural spontaneous arrest of Hirayama disease. There have been some improvements occasionally reported, in which the success was frequently attributed to the relatively shorter duration of illness and mild cord atrophy in a neutral neck position [3,13]. For those cases whose disease progressed with deterioration of neurological functions after medical treatment, surgery might have a role. Nevertheless, there are so many strategies in surgical approaches, including anterior or posterior arthrodesis, duroplasty, and resection of the congested venous plexuses. Spinal fusion procedures, either via an anterior or posterior approach, that fuse segments would certainly limit mobility (i.e., range of motion) and neck flexion, and which could be helpful to interrupt the progression of Hirayama disease [15]. In the literature, various surgical interventions have reportedly been performed, including multilevel anterior fixation, posterior fixation, laminoplasty, or laminectomy with dura tenting [4,12,16-22]. Some of the results were promising and could not only stop the progression of Hirayama disease but also alleviate patients’ previous symptoms. However, all the emerging techniques should warrant future investigation and corroboration.
Our patient received anterior fixation at 3 disc levels to reduce segmental kyphosis, to decompress the spinal cord at the responsible segment, and to keep the neck in a lordotic posture. Most surgery-treated patients have reported improvements or a stationary condition of muscle strength. In this case, the long-tract signs improved after surgery but his upper extremity wasting did not recover. This might be due to delayed treatment after the initial onset of symptoms (at least 10 years in our case) resulting in prolonged cord compressive injury. Therefore, early diagnosis is important for Hirayama disease.
A review of the literature in English revealed several reports of Hirayama cases treated by ACDF at various disc levels, as listed in Table 1 [4,16,17,19-22]. Paredes et al. [4] described a 19-year-old man whose electromyography revealed acute demyelination initially and chronic denervation changes during postoperative follow-up. There was no report of increased MEP amplitude after ACDF or real-time improvement during the surgery. We described a case of Hirayama disease whose MEP amplitude improved immediately intraoperatively, and there was improvement of myelopathy and modest reversal of the muscle wasting at 1-year postoperation. The postoperative dynamic cervical spine MRIs also demonstrated minimal cord compression and elimination of the venous plexus engorgement dorsal to the thecal sac. Although our case showed a satisfactory outcome, further study is still needed for a treatment plan of Hirayama disease.
Table 1.
Cases reported in English that diagnosed Hirayama disease, and treated with ACDF
| Author | Case No. (male) | Age (yr) | Time from onset to operation (yr) | ACDF disc levels | Preoperative abnormal NCS | Postoperative improved NCS | Clinical outcome | Postoperative improved MRI |
|---|---|---|---|---|---|---|---|---|
| Wang et al., [21] 2018 | 17 (16) | 18–28 | 0.5–4 | 2 | NA | NA | Improving | NA |
| McGregor et al., [20] 2017 | 2 (1) | 19–22 | 1–5 | 1–2 | Abnormal | NA | Improving | NA |
| Salome et al., [22] 2017 | 1 (1) | NA | 2 | 2 | Abnormal | NA | Improving | NA |
| Agundez et al., [17] 2015 | 1 (1) | 19 | 1.5 | 1 | Abnormal | NA | Stationary | NA |
| Guo et al., [19] 2014 | 4 (3) | 17–24 | 1.5–3 | 4 | NA | NA | Improving or minimal improving | NA |
| Paredes et al., [4] 2014 | 1 (1) | 19 | 2 | 1 | Abnormal | Not improved | Improving | NA |
| Lin et al., [16] 2010 | 4 (4) | 23–35 | 3–10 | 1–2 | NA | NA | Minimal improving or stationary | NA |
| Our study | 1 (1) | 31 | 10+ | 3 | Abnormal | Improved | Improving | Improved |
ACDF, anterior cervical discectomy and fusion; NCS, nerve conduction study; MRI, magnetic resonance imaging; NA, not available.
CONCLUSION
ACDF may be an effective treatment option to keep the patient’s neck in a nonflexion position, and intraoperative MEP amplitude can be helpful in defining an adequate position of fixation.
Footnotes
The authors have nothing to disclose.
REFERENCES
- 1.Hirayama K, Tookura Y, Tsubaki T. Juvenile muscular atrophy of unilateral upper extremity: a new clinical entity. Psychiatr Neurol Jpn. 1959;61:2190–8. doi: 10.1212/wnl.13.5.373. [DOI] [PubMed] [Google Scholar]
- 2.Hirayama K, Tokumaru Y. Cervical dural sac and spinal cord in juvenile muscular atrophy of distal upper extremity. Neurology. 2000;54:1922–6. doi: 10.1212/wnl.54.10.1922. [DOI] [PubMed] [Google Scholar]
- 3.Hirayama K. Juvenile muscular atrophy of distal upper extremity (Hirayama disease) Intern Med. 2000;39:283–90. doi: 10.2169/internalmedicine.39.283. [DOI] [PubMed] [Google Scholar]
- 4.Paredes I, Esteban J, Ramos A, et al. A severe case of Hirayama disease successfully treated by anterior cervical fusion. J Neurosurg Spine. 2014;20:191–5. doi: 10.3171/2013.10.SPINE13508. [DOI] [PubMed] [Google Scholar]
- 5.Sakai K, Ono K, Okamoto Y, et al. Cervical flexion myelopathy in a patient showing apparent long tract signs: a severe form of Hirayama disease. Joint Bone Spine. 2011;78:316–8. doi: 10.1016/j.jbspin.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Lai V, Wong YC, Poon WL, et al. Forward shifting of posterior dural sac during flexion cervical magnetic resonance imaging in Hirayama disease: an initial study on normal subjects compared to patients with Hirayama disease. Eur J Radiol. 2011;80:724–8. doi: 10.1016/j.ejrad.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 7.Chen CJ, Hsu HL, Tseng YC, et al. Hirayama flexion myelopathy: neutral-position MR imaging findings--importance of loss of attachment. Radiology. 2004;231:39–44. doi: 10.1148/radiol.2311030004. [DOI] [PubMed] [Google Scholar]
- 8.Restuccia D, Rubino M, Valeriani M, et al. Cervical cord dysfunction during neck flexion in Hirayama’s disease. Neurology. 2003;60:1980–3. doi: 10.1212/01.wnl.0000068017.75780.44. [DOI] [PubMed] [Google Scholar]
- 9.Zheng C, Zhu Y, Yang S, et al. A study of dynamic F-waves in juvenile spinal muscular atrophy of the distal upper extremity (Hirayama disease) J Neurol Sci. 2016;367:298–304. doi: 10.1016/j.jns.2016.06.032. [DOI] [PubMed] [Google Scholar]
- 10.Ammendola A, Gallo A, Iannaccone T, et al. Hirayama disease: three cases assessed by F wave, somatosensory and motor evoked potentials and magnetic resonance imaging not supporting flexion myelopathy. Neurol Sci. 2008;29:303–11. doi: 10.1007/s10072-008-0987-1. [DOI] [PubMed] [Google Scholar]
- 11.Misra UK, Kalita J, Mishra VN, et al. Effect of neck flexion on F wave, somatosensory evoked potentials, and magnetic resonance imaging in Hirayama disease. J Neurol Neurosurg Psychiatry. 2006;77:695–8. doi: 10.1136/jnnp.2005.082362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watanabe K, Hasegawa K, Hirano T, et al. Anterior spinal decompression and fusion for cervical flexion myelopathy in young patients. J Neurosurg Spine. 2005;3:86–91. doi: 10.3171/spi.2005.3.2.0086. [DOI] [PubMed] [Google Scholar]
- 13.Tokumaru Y, Hirayama K. Cervical collar therapy for juvenile muscular atrophy of distal upper extremity (Hirayama disease): results from 38 cases. Rinsho Shinkeigaku. 2001;41:173–8. [PubMed] [Google Scholar]
- 14.Lee KH, Choi DS, Lee YS, et al. Clinical experiences of uncommon motor neuron disease: Hirayama disease. Korean J Spine. 2016;13:170–2. doi: 10.14245/kjs.2016.13.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song J, Wang HL, Zheng CJ, et al. Risk factors for surgical results of Hirayama disease: a retrospective analysis of a large cohort. World Neurosurg. 2017;105:69–77. doi: 10.1016/j.wneu.2017.05.097. [DOI] [PubMed] [Google Scholar]
- 16.Lin MS, Kung WM, Chiu WT, et al. Hirayama disease. J Neurosurg Spine. 2010;12:629–34. doi: 10.3171/2009.12.SPINE09431. [DOI] [PubMed] [Google Scholar]
- 17.Agundez M, Rouco I, Barcena J, et al. Hirayama disease: is surgery an option? Neurologia. 2015;30:502–9. doi: 10.1016/j.nrl.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Goel A, Dhar A, Shah A. Multilevel spinal stabilization as a treatment for Hirayama disease: report of an experience with five cases. World Neurosurg. 2017;99:186–91. doi: 10.1016/j.wneu.2016.11.143. [DOI] [PubMed] [Google Scholar]
- 19.Guo X, Lu M, Xie N, et al. Multilevel anterior cervical discectomy and fusion with plate fixation for juvenile unilateral muscular atrophy of the distal upper extremity accompanied by cervical kyphosis. J Spinal Disord Tech. 2014;27:E241–6. doi: 10.1097/BSD.0000000000000098. [DOI] [PubMed] [Google Scholar]
- 20.McGregor S, Joswig H, Duggal N, et al. Hirayama disease: a diagnostic and therapeutic challenge. Can J Neurol Sci. 2017;44:754–6. doi: 10.1017/cjn.2017.219. [DOI] [PubMed] [Google Scholar]
- 21.Wang HL, Wu YW, Song J, et al. Cortical activation changes in Hirayama disease after anterior cervical decompression and fusion. World Neurosurg. 2018;116:e588–94. doi: 10.1016/j.wneu.2018.05.045. [DOI] [PubMed] [Google Scholar]
- 22.Salome M, Barkhof F, Visser L. Hirayama disease; an atypical clinical manifestation of a cervical myelopathy with typical MRI features. BMJ Case Rep. 2017 Jan 4;2017:pii: bcr2016217780. doi: 10.1136/bcr-2016-217780. https://doi.org/10.1136/bcr-2016-217780. [DOI] [PMC free article] [PubMed] [Google Scholar]