Abstract
Objective
Anterior cervical plating in anterior cervical discectomy and fusion (ACDF) procedures are associated with improved outcomes compared to stand-alone cages. However, concerns exist regarding increased rates of postoperative dysphagia following an ACDF. This study aims to quantify the effect of anterior plating on swallowing-quality of life (SWAL-QOL) scores and radiographic swelling assessments following a primary, single-level ACDF.
Methods
Patients retrospectively reviewed. Patients grouped into those receiving a cage or anterior plate. SWAL-QOL scores were recorded preoperatively and 6 weeks and 12 weeks postoperatively. Lateral radiographs were used to create a swelling index with a ratio of the prevertebral swelling distance to the anterior-posterior diameter of each involved vertebral body. An air index was created using the same methodology. Statistical analysis was performed using chi-square analysis and independent t-tests for categorical and continuous variables.
Results
Sixty-eight primary, single-level ACDF patients were included. Forty-one (60.3%) received a stand-alone cage and 27 (39.7%) received a cage with anterior plating. No differences in demographics, comorbidities, operative time, estimated blood loss, or length of hospital stay were identified between Cage and Plate cohorts. Finally, no differences were observed in postoperative SWAL-QOL scores or swelling and air indices between groups.
Conclusion
The results demonstrate that patients undergoing a primary, single-level ACDF with or without anterior plating experience similar operative times and lengths of stay. Patients that receive a cage with anterior plating did not experience significant increases in dysphagia as measured by the SWAL-QOL questionnaire compared to patients that received a stand-alone cage. Furthermore, radiographic assessments of swelling are comparable.
Keywords: Spine, Surgery, Dysphagia, Swallowing, Medical device
INTRODUCTION
Anterior cervical discectomy and fusion (ACDF) has become widely utilized for the treatment of degenerative cervical pathology [1]. ACDF has demonstrated high rates of arthrodesis, as well as proving to be a safe and cost-effective surgical therapy [2-7]. However, the risk for surgical complications still exists. Postoperative dysphagia secondary to soft tissue swelling is a common complication, with rates as high as 79% identified in the anterior cervical spine literature [8-14]. The potential for complications has prompted the development of surgical techniques to mitigate these risks and improve patient outcomes [15-17].
Established by Smith and Robinson, the utilization of anterior plating has been proven as an effective instrumentation method in ACDF [18]. This technique can provide adequate decompression and restoration of segmental anatomy using a biomechanically favorable construct [19,20]. Although anterior plating affords structural stability, studies have associated the technique with a higher risk of postoperative dysphagia [21-23]. This may potentially be due to increased retraction of the esophagus during anterior cervical plating, leading to greater tissue irritation and swelling. Recently, the zero-profile stand-alone interbody cage for ACDF has received increasing attention. This construct has been reported to avoid plate-related complications in addition to decreasing operative times and intraoperative blood loss [24-27].
Previous studies have reported on the relationship between ACDF instrumentation and postoperative dysphagia [28,29]. However, the clinical evaluation of dysphagia has varied greatly in the literature, as the gold standard modified barium swallow is not consistently performed [30]. There is a paucity of evidence regarding the association between anterior plating and swallowing function using a questionnaire such as the swallowing-quality of life (SWAL-QOL), or radiographic assessments of swelling. As such, the purpose of this study is to quantify the effect of anterior plating on SWAL-QOL scores and radiographic swelling assessments following a primary, single-level ACDF.
MATERIALS AND METHODS
1. Patient Population
Following Institutional Review Board approval (ORA#14051301), a prospectively-maintained surgical registry was retrospectively reviewed to identify patients undergoing primary, single-level ACDF for degenerative pathology from 2014–2017. All patients underwent ACDF by a single surgeon at a single academic institution. Patients were excluded if undergoing ACDF for non-degenerative etiologies, or if postoperative survey data was incomplete.
2. Surgical Technique
The Smith-Robinson approach was utilized for all procedures [18]. Patients either received a stand-alone cage (Cage) or a cage with anterior plating (Plate) at the discretion of the treating surgeon. The Cage cohort received a polyetheretherketone (PEEK) interbody cage with integrated screw fixation. The Plate cohort received a PEEK interbody cage in addition to anterior plating with screw fixation. In both cohorts, cages were packed with local bone graft obtained from anterior osteophyte resection and nanocrystalline hydroxyapatite was used as a bone graft extender.
3. Data Collection
Patients were grouped according to ACDF instrumentation utilized; either a stand-alone cage (Cage) or a cage with anterior plating (Plate). Demographic and comorbidity information was obtained from the surgical registry, including age, sex, body mass index, insurance status, and comorbidity burden as defined by Charlson Comorbidity Index. Recorded perioperative characteristics included operative time (minute), intraoperative blood loss (mL), length of hospital stay (hour), and time to discharge (postoperative day, POD). For time to discharge, POD 0 indicates discharge on the same day of surgery, POD 1 indicates discharge on the day following surgery, and so forth. Any complications during the intraoperative, immediate postoperative, and up to 12 weeks postoperative were also recorded.
SWAL-QOL scores were recorded at preoperative and 6-week and 12-week postoperative time points. Surveys were completed on paper or electronically via a computer or tablet at each clinical visit. The SWAL-QOL survey is composed of 13 multipart questions. For our analysis, we limited our focus on the third question, which contains 14 responses regarding physical problems associated with swallowing difficulty [31]: “coughing,” “choking when you eat food,” “choking when you take liquids,” “having thick saliva or phlegm,” “gagging,” “drooling,” “problems chewing,” “having excess saliva or phlegm,” “having to clear your throat,” “food sticking in your throat,” “food sticking in your mouth,” “food or liquid dribbling out of your mouth,” “food or liquid coming out of your nose,” or “coughing food or liquid out of your mouth when it gets stuck.” This approach to the SWAL-QOL questionnaire for postoperative dysphagia assessment has been previously described by Siska et al. [32] Each response indicated how frequently a patient-reported problem associated with swallowing, using a scale of 1 (“almost always”) to 5 (“never”). Scores were then scaled to a percentage out of 100 by dividing the total number of points scored by the maximum point score of 70. A scaled score of 100 indicated patients did not exhibit swallowing difficulty, whereas a minimum scaled score of 20 indicated patients suffered consistently from each related dysphagia component.
4. Radiographic Outcomes
Preoperative and 6-week and 12-week postoperative plain lateral radiographs were used to assess changes in swelling from baseline following ACDF. All radiographic measurements were taken at the midvertebral body, parallel to the intervertebral disc, for each index-level vertebrae, as well as one vertebral level above and below the level of fusion (Fig. 1). The prevertebral soft tissue swelling was defined as the distance from the anterior cortex of the vertebral body to the posterior tracheal air window. The anteriorposterior (AP) diameter of the tracheal air window was also measured. The ratio of the prevertebral soft tissue swelling to the vertebral body AP diameter at all measured levels were averaged and multiplied by 100 to obtain a swelling index [33]. An air index was calculated in the same manner, utilizing the tracheal air window in place of the prevertebral soft tissue swelling.
Fig. 1.
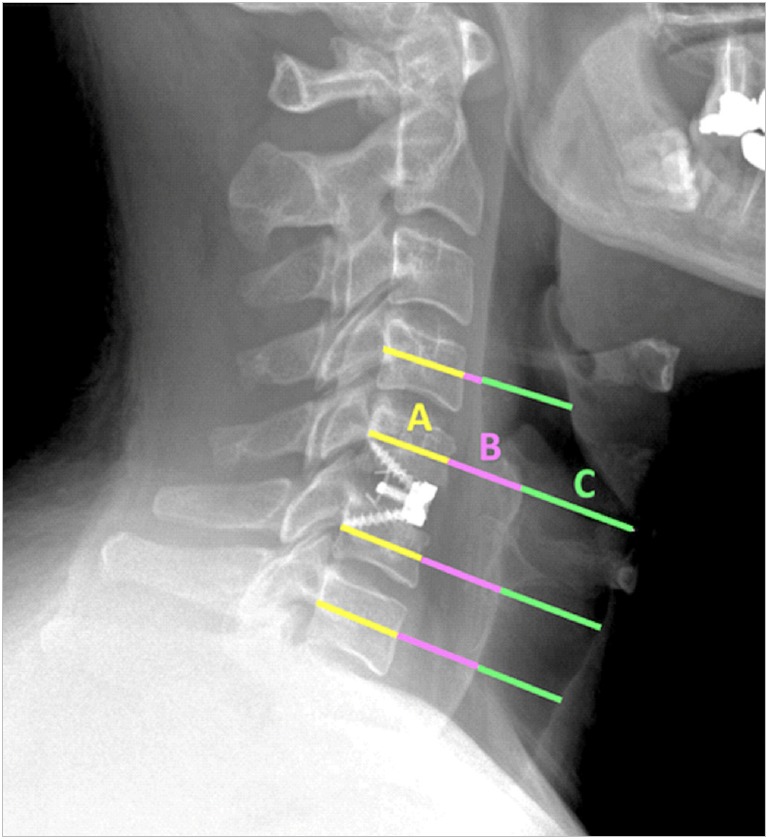
Postoperative lateral cervical radiograph of an anterior cervical discectomy and fusion patient who received a standalone cage demonstrates swelling and air index measurements at the operative level as well as one level above and below the fusion. All measurements are in the plane of the midvertebral body, parallel to the intervertebral discs. The measurements include anteriorposterior (AP) diameter of the vertebral body (A); prevertebral soft tissue swelling (B), from anterior cortex of the vertebral body to the posterior tracheal air window; and AP diameter of the tracheal air window (C).
5. Statistical Analysis
Statistical analysis was performed using Stata/SE 13.0 (Stata-Corp LP, College Station, TX, USA). Differences in patient demographics and perioperative characteristics were compared between cohorts using chi-square analysis and independent t-tests for categorical and continuous variables, respectively. Changes in postoperative SWAL-QOL scores and swelling and air indices from preoperative values were compared between groups using independent t-tests. Statistical significance was set at p<0.05.
6. Power Analysis
A post hoc power analysis revealed the present study has 81.3% power to detect a 7.5-point difference in scaled SWAL-QOL score at 6-week follow-up between Plate and Cage groups.
RESULTS
A total of 68 primary, single-level ACDF patients were included in this analysis. Of these, 41 (60.3%) received a standalone cage, while 27 (39.7%) received a cage plus anterior plating. The majority of patients in both groups were male (Cage=68.3%, Plate=59.3%; p=0.446). A greater percentage of Plate patients were smokers (18.5% vs. 4.9%, p=0.070) than Cage patients; however, this did not reach statistical significance. No differences in baseline characteristics were observed between cohorts (Table 1).
Table 1.
Baseline characteristics
| Characteristic | Cage (n = 41) | Plate (n = 27) | p-value† |
|---|---|---|---|
| Age (yr) | 46.3 ± 8.1 | 48.7 ± 9.5 | 0.275 |
| Sex | 0.446 | ||
| Female | 13 (31.7) | 11 (40.7) | |
| Male | 28 (68.3) | 16 (59.3) | |
| BMI (kg/m2) | 0.183 | ||
| Nonobese (BMI < 30) | 20 (48.8) | 17 (65.4) | |
| Obese (BMI ≥ 30) | 21 (51.2) | 9 (34.6) | |
| Smoking status | 0.070 | ||
| Nonsmoker | 39 (95.1) | 22 (81.5) | |
| Smoker | 2 (4.9) | 5 (18.5) | |
| Charlson Comorbidity Index | 0.9 ± 1.1 | 1.4 ± 1.2 | 0.103 |
Values are presented as mean±standard deviation or number (%).
BMI, body mass index.
p-value calculated using Student t-test and chi-square analysis.
The perioperative outcomes for Plate and Cage patients are detailed in Table 2. Similar operative time, estimated intraoperative blood loss, and lengths of postoperative stay were observed for Plate and Cage groups (p>0.05 for each). The majority of patients in both cohorts were discharged on POD 0 (Plate=80.5%, Cage=85.2%; p=0.686). No perioperative or postoperative complications were reported in either cohort.
Table 2.
Perioperative outcomes
| Variable | Cage (n = 41) | Plate (n = 27) | p-value† |
|---|---|---|---|
| Operative time (min) | 39.2 ± 8.9 | 43.6 ± 12.7 | 0.100 |
| Estimated blood loss (mL) | 27.3 ± 9.2 | 29.8 ± 14.2 | 0.386 |
| Length of hospital stay (hr) | 11.2 ± 9.6 | 9.8 ± 6.8 | 0.512 |
| Day of discharge | 0.686 | ||
| POD 0 | 33 (80.5) | 23 (85.2) | |
| POD 1 | 7 (17.1) | 4 (14.8) | |
| POD 2 | 1 (2.4) | 0 (0) | |
| Complications | - | ||
| Intraoperative | 0 (0) | 0 (0) | |
| Inpatient | 0 (0) | 0 (0) | |
| Postoperative | 0 (0) | 0 (0) |
Values are presented as mean±standard deviation or number (%).
POD, postoperative day.
p-value calculated using Student t-test or chi-square analysis.
Patient-reported swallowing function was assessed using the SWAL-QOL and compared between groups (Table 3). The average preoperative scaled SWAL-QOL score was 95.6 in the Plate cohort and 93.3 in the Cage cohort (p=0.245). No differences in SWAL-QOL scores were observed at 6-week or 12-week postoperative visits between Plate and Cage patients (p>0.05 each). Additionally, changes in postoperative SWAL-QOL scores from preoperative values were similar between groups (p>0.05 each).
Table 3.
SWAL-QOL outcomes
| SWAL-QOL | Cage (n = 41) | Plate (n = 27) | p-value† |
|---|---|---|---|
| SWAL-QOL | |||
| Preoperative | 93.3 ± 8.1 | 95.6 ± 7.5 | 0.245 |
| 6-Week postoperative | 89.2 ± 14.1 | 93.5 ± 7.1 | 0.149 |
| 12-Week postoperative | 89.5 ± 13.0 | 92.4 ± 10.4 | 0.387 |
| Changes in SWAL-QOL | |||
| Preoperative | 93.3 ± 8.1 | 95.6 ± 7.5 | - |
| ∆ 6-Week postoperative | -4.1 ± 12.4 | -2.1 ± 8.0 | 0.457 |
| ∆ 12-Week postoperative | -3.1 ± 11.7 | -3.0 ± 12.0 | 0.974 |
Values are presented as mean±standard deviation.
SWAL-QOL, Swallowing-quality of life.
p-value calculated using Student t-test or chi-square analysis.
Table 4 describes the radiographic outcomes between Plate and Cage groups. Preoperative swelling and air indices were similar between cohorts (p>0.05 each). At 6-week follow-up, Plate patients demonstrated a higher swelling index than Cage patients (87.6 vs. 75.4, p=0.010). However, no differences in swelling indices were identified at 12-week follow-up (p>0.05). Additionally, changes in swelling index from preoperative to postoperative time points were similar between Plate and Cage groups (p>0.05 each). Plate and Cage patients also experienced similar air indices and changes in air indices from preoperative values at both 6- and 12-week postoperative time points (p>0.05 for each).
Table 4.
Radiographic outcomes
| Variable | Cage (n = 41) | Plate (n = 27) | p-value† |
|---|---|---|---|
| Swelling index average‡ | |||
| Preoperative | 67.9 ± 18.4 | 75.0 ± 13.1 | 0.107 |
| 6-Week postoperative | 75.4 ± 16.4 | 87.6 ± 18.3 | 0.010* |
| 12-Week postoperative | 72.0 ± 15.9 | 80.5 ± 19.3 | 0.091 |
| Swelling index ∆ | |||
| ∆ 6-Week postoperative | 7.5 ± 11.6 | 12.6 ± 13.5 | 0.124 |
| ∆ 12-Week postoperative | 5.2 ± 9.3 | 6.0 ± 12.7 | 0.785 |
| Air index average‡ | |||
| Preoperative | 107.1 ± 20.6 | 115.5 ± 14.2 | 0.088 |
| 6-Week postoperative | 106.6 ± 22.4 | 109.9 ± 18.0 | 0.551 |
| 12-Week postoperative | 105.3 ± 22.4 | 108.6 ± 17.7 | 0.575 |
| Air index ∆ | |||
| ∆ 6-Week postoperative | -0.5 ± 14.8 | -5.6 ± 14.1 | 0.189 |
| ∆ 12-Week postoperative | -1.4 ± 14.8 | -7.3 ± 17.8 | 0.209 |
Values are presented as mean±standard deviation.
Air index Δ/swelling index Δ=postoperative air/swelling index average – preoperative air/swelling index average.
p<0.05, statistically significant difference.
p-value calculated using Student t-test.
Air index average/swelling index average=average of tracheal air/pretracheal swelling measurement for index, index±1-level.
DISCUSSION
ACDF with anterior plating is thought to improve structural stability of the fusion construct. However, concerns exist regarding postoperative complications such as increased rates of dysphagia. The aim of the present study was to assess postoperative swallowing function after ACDF with or without anterior plating. The results of this study indicate that patients undergoing ACDF with anterior plating do not experience greater postoperative swallowing dysfunction. Additionally, postoperative changes in radiographic swelling and air indices are similar regardless of instrumentation used. These findings suggest that anterior plating can be utilized in ACDF procedures without concern for a higher risk of postoperative dysphagia.
While the results of the current study indicate that anterior plating does not lead to greater postoperative dysphagia, the literature has exhibited mixed results. Nambiar et al. [28] performed a meta-analysis of reported outcomes following a single-level ACDF with or without anterior plating. Four of the 6 included studies reported rates of postoperative dysphagia. Anterior plating was associated with higher rates of dysphagia at follow-up (odds ratio [OR], 6.17; 95% confidence interval [CI], 1.04–36.64; p=0.05). A subsequent meta-analysis by Oliver et al. [29] also assessed ACDF outcomes with and without anterior plating among studies with at least 6 months of postoperative follow-up. Five of the fifteen included studies reported postoperative dysphagia rates. There was no significant difference in the incidence of dysphagia between Plate and No Plate cohorts, with pooled rates of 11.18% and 9.14%, respectively (OR, 1.21; 95% CI, 0.57–2.56; p=0.963). The opposing results of these meta-analyses may be due to variations in timing of dysphagia assessments or study selection criteria, as only one investigation reporting dysphagia rates met inclusion criteria for both studies. Nevertheless, this highlights the paucity of high-quality aggregate data comparing clinical outcomes of ACDF with and without anterior plating.
The inconsistent reports of increased dysphagia rates with anterior plating are likely a result of variable methodology used in the literature. The majority of studies in the ACDF literature determine dysphagia rates through review of clinical documentation, patient self-reporting, or unspecified methodology [34]. This approach makes reported results less reliable or amenable to critical analysis. Furthermore, it prevents accurate comparison and pooling of data within the literature. The Bazaz Dysphagia Score is one criterion used to define dysphagia in ACDF literature, which classifies dysphagia as severe, moderate, mild, or none [21]. While this assessment tool may be utilized because of its easy and rapid administration, it may be too simple to detect smaller variations in dysphagia severity [8,10]. The use of the SWAL-QOL in the present study may have allowed for an enhanced ability to assess subtle differences in swallowing function between Plate and Cage patients. While dysphagia severity in the present study was similar with and without the use of anterior plating, continued efforts to standardize dysphagia reporting with an assessment tool that is both comprehensive and efficient will remain important.
The present study determined that postoperative changes in radiographic swelling and air indices are not different following ACDF with or without anterior plating. Prevertebral soft tissue swelling (PSTS) has been previously reported to correlate with dysphagia after ACDF [35,36]. As postoperative swallowing function was similar for Plate and Cage patients, it is intuitive that swelling and air indices would also be similar between cohorts. However, these results are in contrast with previous reports in the literature. Prior studies have identified greater postoperative PSTS following ACDF with anterior plating compared to standalone cages [37-39]. However, these investigations employed different techniques for radiographic assessment, limiting the ability to compare results to the present study. As such, determination of the optimal PSTS calculation method may be an important aim of future investigations.
This study has several limitations. First, all patients were treated by a single surgeon at a single institution. This may limit the generalizability. Second, the retrospective nature of this study may impart a degree of selection bias. Third, postoperative swallowing assessments were only performed at 6-week and 12-week follow-up. It is possible patients experienced more significant impairments earlier in their postoperative course that subsided prior to survey administration. Furthermore, differences in swallowing function were not evaluated beyond 12-week follow-up. However, the chosen time points were considered to be of greatest relevance as dysphagia most often resolves within the first 3 months [10]. Fourth, although our analysis was adequately powered to demonstrate a 7.5-point difference in scaled SWAL-QOL score, this may not indicate a clinically relevant difference; however, a clinically relevant difference in SWAL-QOL score remains unclear at this time. Fifth, due to the low incidence of swallowing difficulty that occurs in patients following ACDF performed by an experienced surgeon, it may be difficult to distinguish the true differences between the 2 cohorts in the current study. An analysis of a larger population with more study subjects would be required in order to obtain a large enough sample size of patients that experienced swallowing difficulty after ACDF. As such, the conclusions drawn from our study are limited due to the low rate at which swallowing difficulty develops after ACDF regardless of whether an anterior plate was used or not. Finally, as the SWAL-QOL survey is optimized for use in oropharyngeal oncologic and otolaryngology populations, sections of the survey regarding quality of life relating to severe swallowing dysfunction have limited utility among spine surgery populations. As such, our evaluation utilized only the most relevant questions in the survey corresponding to physical effects of swallowing dysfunction.
CONCLUSIONS
The results of this study demonstrate that patients undergoing a primary, single-level ACDF with or without anterior plating experience similar operative times, estimated blood loss, and lengths of stay. Furthermore, patients that receive a cage with anterior plating do not experience significant increases in dysphagia as measured by the SWAL-QOL questionnaire compared to patients that received a stand-alone cage. Finally, the use of anterior plating does not lead to greater radiographic swelling and air indices. Patients should be counselled to expect similar postoperative swallowing function following a primary, single-level ACDF regardless of instrumentation used. A future investigation of a larger sample size is necessary to corroborate the similarity demonstrated by the study.
Footnotes
The authors have nothing to disclose.
REFERENCES
- 1.Lukasiewicz AM, Basques BA, Bohl DD, et al. Myelopathy is associated with increased all-cause morbidity and mortality following anterior cervical discectomy and fusion: a study of 5256 patients in American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) Spine (Phila Pa 1976) 2015;40:443–9. doi: 10.1097/BRS.0000000000000785. [DOI] [PubMed] [Google Scholar]
- 2.Barnes B, Haid RW, Rodts GE, et al. Early results using the Atlantis anterior cervical plate system. Neurosurg Focus. 2002;12:E13. doi: 10.3171/foc.2002.12.1.14. [DOI] [PubMed] [Google Scholar]
- 3.Papadopoulos EC, Huang RC, Girardi FP, et al. Three-level anterior cervical discectomy and fusion with plate fixation: radiographic and clinical results. Spine (Phila Pa 1976) 2006;31:897–902. doi: 10.1097/01.brs.0000209348.17377.be. [DOI] [PubMed] [Google Scholar]
- 4.Samartzis D, Shen FH, Matthews DK, et al. Comparison of allograft to autograft in multilevel anterior cervical discectomy and fusion with rigid plate fixation. Spine J. 2003;3:451–9. doi: 10.1016/s1529-9430(03)00173-6. [DOI] [PubMed] [Google Scholar]
- 5.Samartzis D, Shen FH, Lyon C, et al. Does rigid instrumentation increase the fusion rate in one-level anterior cervical discectomy and fusion? Spine J. 2004;4:636–43. doi: 10.1016/j.spinee.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 6.Samartzis D, Shen FH, Goldberg EJ, et al. Is autograft the gold standard in achieving radiographic fusion in one-level anterior cervical discectomy and fusion with rigid anterior plate fixation? Spine (Phila Pa 1976) 2005;30:1756–61. doi: 10.1097/01.brs.0000172148.86756.ce. [DOI] [PubMed] [Google Scholar]
- 7.Shen FH, Samartzis D, Khanna N, et al. Comparison of clinical and radiographic outcome in instrumented anterior cervical discectomy and fusion with or without direct uncovertebral joint decompression. Spine J. 2004;4:629–35. doi: 10.1016/j.spinee.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Skeppholm M, Ingebro C, Engström T, et al. The Dysphagia Short Questionnaire: an instrument for evaluation of dysphagia: a validation study with 12 months’ follow-up after anterior cervical spine surgery. Spine (Phila Pa 1976) 2012;37:996–1002. doi: 10.1097/BRS.0b013e31823a7a5b. [DOI] [PubMed] [Google Scholar]
- 9.Tervonen H, Niemelä M, Lauri ER, et al. Dysphonia and dysphagia after anterior cervical decompression. J Neurosurg Spine. 2007;7:124–30. doi: 10.3171/SPI-07/08/124. [DOI] [PubMed] [Google Scholar]
- 10.Anderson KK, Arnold PM. Oropharyngeal dysphagia after anterior cervical spine surgery: a review. Global Spine J. 2013;3:273–86. doi: 10.1055/s-0033-1354253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buttermann GR. Prospective nonrandomized comparison of an allograft with bone morphogenic protein versus an iliac-crest autograft in anterior cervical discectomy and fusion. Spine J. 2008;8:426–35. doi: 10.1016/j.spinee.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Kalb S, Reis MT, Cowperthwaite MC, et al. Dysphagia after anterior cervical spine surgery: incidence and risk factors. World Neurosurg. 2012;77:183–7. doi: 10.1016/j.wneu.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Lee MJ, Bazaz R, Furey CG, et al. Risk factors for dysphagia after anterior cervical spine surgery: a two-year prospective cohort study. Spine J. 2007;7:141–7. doi: 10.1016/j.spinee.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 14.Joaquim AF, Murar J, Savage JW, et al. Dysphagia after anterior cervical spine surgery: a systematic review of potential preventative measures. Spine J. 2014;14:2246–60. doi: 10.1016/j.spinee.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 15.Phan K, Kim JS, Lee NJ, et al. Relationship between ASA scores and 30-day readmissions in patients undergoing anterior cervical discectomy and fusion. Spine (Phila Pa 1976) 2017;42:85–91. doi: 10.1097/BRS.0000000000001680. [DOI] [PubMed] [Google Scholar]
- 16.Puvanesarajah V, Jain A, Cancienne JM, et al. Complication and reoperation rates following surgical management of cervical spondylotic myelopathy in medicare beneficiaries. Spine (Phila Pa 1976) 2017;42:1–7. doi: 10.1097/BRS.0000000000001639. [DOI] [PubMed] [Google Scholar]
- 17.Finn MA, MacDonald JD. C2-C3 anterior cervical fusion: technical report. Clin Spine Surg. 2016;29:E536–41. doi: 10.1097/BSD.0b013e318292b3ca. [DOI] [PubMed] [Google Scholar]
- 18.Smith GW, Robinson RA. The treatment of certain cervicalspine disorders by anterior removal of the intervertebral disc and interbody fusion. J Bone Joint Surg Am. 1958;40-A:607–24. [PubMed] [Google Scholar]
- 19.Brodke DS, Klimo P, Jr, Bachus KN, et al. Anterior cervical fixation: analysis of load-sharing and stability with use of static and dynamic plates. J Bone Joint Surg Am. 2006;88:1566–73. doi: 10.2106/JBJS.E.00305. [DOI] [PubMed] [Google Scholar]
- 20.Fraser JF, Härtl R. Anterior approaches to fusion of the cervical spine: a metaanalysis of fusion rates. J Neurosurg Spine. 2007;6:298–303. doi: 10.3171/spi.2007.6.4.2. [DOI] [PubMed] [Google Scholar]
- 21.Bazaz R, Lee MJ, Yoo JU. Incidence of dysphagia after anterior cervical spine surgery: a prospective study. Spine (Phila Pa 1976) 2002;27:2453–8. doi: 10.1097/00007632-200211150-00007. [DOI] [PubMed] [Google Scholar]
- 22.Fountas KN, Kapsalaki EZ, Nikolakakos LG, et al. Anterior cervical discectomy and fusion associated complications. Spine (Phila Pa 1976) 2007;32:2310–7. doi: 10.1097/BRS.0b013e318154c57e. [DOI] [PubMed] [Google Scholar]
- 23.Riley LH, 3rd, Skolasky RL, Albert TJ, et al. Dysphagia after anterior cervical decompression and fusion: prevalence and risk factors from a longitudinal cohort study. Spine (Phila Pa 1976) 2005;30:2564–9. doi: 10.1097/01.brs.0000186317.86379.02. [DOI] [PubMed] [Google Scholar]
- 24.Ji GY, Oh CH, Shin DA, et al. Stand-alone cervical cages versus anterior cervical plates in 2-level cervical anterior interbody fusion patients: analysis of adjacent segment degeneration. J Spinal Disord Tech. 2015;28:E433–8. doi: 10.1097/BSD.0b013e3182a355ad. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Hao D, He B, et al. The efficiency of zero-profile implant in anterior cervical discectomy fusion: a prospective controlled long-term follow-up study. J Spinal Disord Tech. 2015;28:398–403. doi: 10.1097/BSD.0000000000000032. [DOI] [PubMed] [Google Scholar]
- 26.Nemoto O, Kitada A, Naitou S, et al. Stand-alone anchored cage versus cage with plating for single-level anterior cervical discectomy and fusion: a prospective, randomized, controlled study with a 2-year follow-up. Eur J Orthop Surg Traumatol. 2015;25 Suppl 1:S127–34. doi: 10.1007/s00590-014-1547-4. [DOI] [PubMed] [Google Scholar]
- 27.Njoku I, Jr, Alimi M, Leng LZ, et al. Anterior cervical discectomy and fusion with a zero-profile integrated plate and spacer device: a clinical and radiological study: Clinical article. J Neurosurg Spine. 2014;21:529–37. doi: 10.3171/2014.6.SPINE12951. [DOI] [PubMed] [Google Scholar]
- 28.Nambiar M, Phan K, Cunningham JE, et al. Locking standalone cages versus anterior plate constructs in single-level fusion for degenerative cervical disease: a systematic review and meta-analysis. Eur Spine J. 2017;26:2258–66. doi: 10.1007/s00586-017-5015-9. [DOI] [PubMed] [Google Scholar]
- 29.Oliver JD, Goncalves S, Kerezoudis P, et al. Comparison of outcomes for anterior cervical discectomy and fusion with and without anterior plate fixation: a systematic review and meta-analysis. Spine (Phila Pa 1976) 2018;43:E413–22. doi: 10.1097/BRS.0000000000002441. [DOI] [PubMed] [Google Scholar]
- 30.Brady S, Donzelli J. The modified barium swallow and the functional endoscopic evaluation of swallowing. Otolaryngol Clin North Am. 2013;46:1009–22. doi: 10.1016/j.otc.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 31.McHorney CA, Bricker DE, Robbins J, et al. The SWAL-QOL outcomes tool for oropharyngeal dysphagia in adults: II. Item reduction and preliminary scaling. Dysphagia. 2000;15:122–33. doi: 10.1007/s004550010013. [DOI] [PubMed] [Google Scholar]
- 32.Siska PA, Ponnappan RK, Hohl JB, et al. Dysphagia after anterior cervical spine surgery: a prospective study using the swallowing-quality of life questionnaire and analysis of patient comorbidities. Spine (Phila Pa 1976) 2011;36:1387–91. doi: 10.1097/BRS.0b013e31822340f2. [DOI] [PubMed] [Google Scholar]
- 33.Haws BE, Khechen B, Narain AS, et al. Impact of local steroid application on dysphagia following an anterior cervical discectomy and fusion: results of a prospective, randomized single-blind trial. J Neurosurg Spine. 2018;29:10–7. doi: 10.3171/2017.11.SPINE17819. [DOI] [PubMed] [Google Scholar]
- 34.Shriver MF, Lewis DJ, Kshettry VR, et al. Dysphagia rates after anterior cervical diskectomy and fusion: a systematic review and meta-analysis. Global Spine J. 2017;7:95–103. doi: 10.1055/s-0036-1583944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamagata T, Naito K, Yoshimura M, et al. Influence of prevertebral soft tissue swelling on dysphagia after anterior cervical discectomy and fusion using a rectangular titanium stand-alone cage. J Craniovertebr Junction Spine. 2017;8:179–86. doi: 10.4103/jcvjs.JCVJS_57_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song KJ, Choi BW, Kim HY, et al. Efficacy of postoperative radiograph for evaluating the prevertebral soft tissue swelling after anterior cervical discectomy and fusion. Clin Orthop Surg. 2012;4:77–82. doi: 10.4055/cios.2012.4.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Son DK, Son DW, Kim HS, et al. Comparative study of clinical and radiological outcomes of a zero-profile device concerning reduced postoperative Dysphagia after single level anterior cervical discectomy and fusion. J Korean Neurosurg Soc. 2014;56:103–7. doi: 10.3340/jkns.2014.56.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alimi M, Njoku I, Hofstetter CP, et al. Anterior cervical discectomy and fusion (ACDF): comparison between zero profile implants and anterior cervical plate and spacer. Cureus. 2016;8:e573. doi: 10.7759/cureus.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hofstetter CP, Kesavabhotla K, Boockvar JA. Zero-profile anchored spacer reduces rate of dysphagia compared with ACDF with anterior plating. J Spinal Disord Tech. 2015;28:E284–90. doi: 10.1097/BSD.0b013e31828873ed. [DOI] [PubMed] [Google Scholar]


