Abstract
Objective
Current literature has not shown if using either allograft or autograft differentially affects postoperative cervical sagittal parameters. The goal of this study was to compare sagittal alignment and patient-reported outcomes following anterior cervical discectomy and fusion (ACDF) with allograft versus autograft.
Methods
A retrospective cohort analysis of patients who underwent single-level ACDF was conducted. Preoperative, immediate postoperative, and final follow-up radiographic assessments were conducted and included: change in C2–7 lordosis, T1 slope, levels fused, sagittal vertical axis (SVA), fusion mass lordosis, and proximal and distal adjacent segment degeneration (ASD). Patient-reported outcomes were obtained using the Neck Disability Index and visual analogue scale scores for neck and arm.
Results
A total of 404 patients were assessed; 353 using allograft and 51 using autograft. No significant differences existed in demographics. Cervical lordosis improved in both groups without significant changes in SVA. Autograft group had a significantly greater amount of lordosis at the proximal segment on immediate postoperative radiographs and less overall cervical lordosis at final follow-up. Sagittal parameters were similar at each time point without significant changes between the 3-time points. No significant differences existed in radiographic ASD or reoperation rates. Fusion rates exceeded 96% in both groups. No significant differences existed between preoperative, postoperative, or change in patient-reported outcomes between the 2 groups.
Conclusion
Sagittal alignment is maintained following ACDF when using either allograft or autograft. Radiographic evidence of ASD is present in both groups; however, this was not considered clinically significant, given low rates of pseudarthrosis or reoperation. No significant differences exist between groups in terms of patient-reported outcomes.
Keywords: Anterior cervical discectomy and fusion, Allografts, Autografts, Sagittal parameters, Clinical outcomes
INTRODUCTION
Iliac crest bone autograft (ICBG) is the gold standard for anterior cervical discectomy and fusion (ACDF) due to its osteoinductive, osteogenic, and osteoconductive properties [1,2]. However, because the harvesting of ICBG may increase patient discomfort, surgical complications, infection rates, and operative times, the use of allografts and synthetic grafts has increased in recent years [1-5]. Investigators have shown that biologic alternatives achieve similar rates of radiographic fusion to autograft while also achieving similar clinical outcomes and limiting subsidence and graft collapse [6-8]. Unfortunately, little is known about how the use of allograft or autograft differentially affects the cervical sagittal parameters following ACDF, nor is it well known how graft choice impacts patient-reported outcomes, including Neck Disability Index (NDI) or visual analogue scale (VAS) scores.
In addition to recording fusion rates and measuring clinical outcomes according to Odom’s criteria, some investigators have studied additional radiographic measurements, finding that alterations in sagittal radiographic parameters may correlate with changes in patient-reported outcomes. Maintenance or improvement of overall cervical or fusion segment lordosis has been shown to decrease NDI scores, while increased kyphosis may lead to greater VAS scores [9-12]. In particular, Lee et al. [11] found that alterations in C2–7 sagittal vertical axis (SVA) could predict changes in NDI and VAS measurements. To our knowledge, no investigation has analyzed the impact of graft choice on sagittal radiographic parameters while also measuring patient-reported outcomes throughout the follow-up period.
Our study aimed to determine the impact of graft choice (ICBG versus allograft) on various sagittal radiographic parameters in patients undergoing ACDF with plating. In addition to collecting sagittal parameters via radiographic measurements, we recorded NDI and VAS scores at preoperative, immediate postoperative, and final follow-up visits. We hypothesized that there would be no significant difference in sagittal parameters or patient-reported outcomes between ACDF with autograft versus allograft.
MATERIALS AND METHODS
1. Study Design
We retrospectively reviewed the records of consecutive patients who underwent ACDF between January 1998 and December 2015. This study was approved by the Institutional Review Board of Rush University Medical Center (approval number: 18072091). All surgeries were performed at a single quaternary referral medical center by one of 2 senior surgeons (HSA, EG). Surgery indications included: failure of conservative treatments for radiculopathy, myelopathy, or myeloradiculopathy. Exclusions criteria included: age under 18 years old, previous cervical fusion or concomitant posterior surgery, postoperative follow-up of less than one year, cervical spine fracture, or infection. Of 467 patients originally identified, 404 fulfilled the criteria.
2. Surgical Details
During the study period, senior authors transitioned from the use of autograft to cortico-cancellous allograft. Both surgeons used the following surgical technique. Patients were placed supine on a radiolucent table after induction of general endotracheal anesthesia. Once a bump was placed midline between the scapulae, Gardner-Wells tongs were placed with 15 pounds of traction. Standard Smith-Robinson approach to the anterior cervical spine was utilized. Subsequently, a discectomy was performed in standard fashion. Disc space was distracted with laminar spreaders placed in disc space to allow for adequate visualization and access to perform foraminotomies. The posterior longitudinal ligament was not routinely resected. If the patient was receiving autograft, an incision was made over the iliac crest, and a cortical bone window was created to gain access to the cancellous bone between the outer and inner table of the pelvis. Series of curettes were used to obtain the cancellous bone graft. For patients obtaining an allograft for fusion, after implants were trialed in standard fashion, a fresh-frozen VG2 cortico-cancellous allograft was placed in the disc space, and traction was removed. A rigid (Eagle, DepuySpine, Raynham, MA, USA) or semi-rigid plate (Vuelock, Biomet, Parsippany, NJ, USA) was applied to the anterior cervical spine and screws were then placed. A 3-0 Vicryl suture was used to close the platysma layer and subcutaneous tissue, followed by a 4-0 Vicryl suture and dermabond for the skin.
Postoperatively, all patients were placed into a soft cervical collar and admitted to the hospital for observation. Anteroposterior and lateral cervical spine radiographs were taken at postoperative visit within the first 4 weeks and then again at 3 months, 6 months, and 12 months.
3. Demographic and Radiographic Measurement Analysis
Demographic information collected for all patients included: age, sex, body mass index, diabetes, smoking status, and American Society of Anesthesiologists (ASA) physical status classification system. Preoperative, immediate postoperative, and final follow-up radiographic assessments were conducted and included: C2–7 lordosis, proximal and distal adjacent segment lordosis, height of the fusion mass, adjacent segment degeneration (ASD), T1 angle, SVA, fusion mass lordosis, and the presence of a fusion (Fig. 1). Lordosis of the fusion mass was the Cobb angle between inferior endplate of the superior vertebral body to the inferior endplate of inferior vertebral body encompassed into the fusion. T1 slope was the angle created from a line tangential to the superior endplate of T1 and a horizontal line. SVA was obtained through measuring the distance from posterior-superior corner of C7 to a vertical line that bisected C2 centroid. Distal adjacent segment lordosis was the angle between inferior endplate of the vertebral body caudal to the fusion mass (or proposed fusion mass) and superior endplate of the most caudal vertebral body of the fusion mass. Similarly, proximal adjacent segment lordosis was the angle between superior endplate of the vertebral body cephalad to the fusion mass and inferior endplate of the most cephalad vertebral body of the fusion mass. Height of the fusion mass/proposed fusion mass was determined by calculating the average of the distance between superior endplate of the cephalad vertebral body and inferior endplate of the cephalad vertebral at the anterior edge, posterior edge, and middle of the endplates. Subsidence was deemed present if there was a loss of height in more than 2 mm at any of the 2 measured disc heights [13]. Radiographic fusion was determined by bone bridging anteriorly and posteriorly across the fused level; no visible motion between the vertebral body and fusion adjunct on flexion/extension radiographs; the absence of a radiographic halo around the autograft/allograft on both anteroposterior and lateral views [14]. Radiographic diagnosis of ASD was determined by any one or more of the following: presence of new or enlarged anterior osteophyte; presence of spondylolisthesis >2 mm; endplate sclerosis; disc space narrowing >50%; increased calcification of the anterior longitudinal ligament (ALL) [15-17]. Evaluation of successful fusion and assessment of ASD were completed using radiographs as opposed to computed tomography (CT) or magnetic resonance imaging (MRI) due to institution protocol. Our protocol dictates that CT or MRI are used in postoperative assessment only if there is a concern for pseudarthrosis.
Fig. 1.
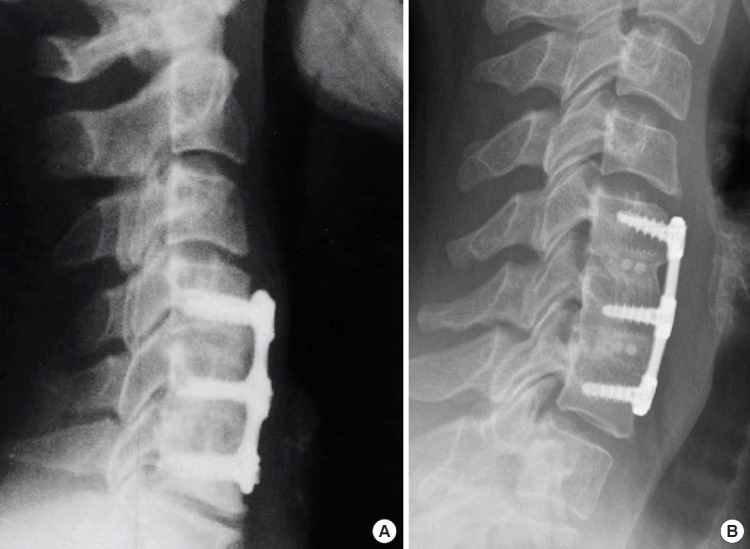
Lateral cervical plain radiographs are taken 12 months following a 2-level ACDF in a 56-year-old male who underwent ACDF with iliac crest bone autograft (A) and a 54-year-old male that underwent ACDF with fresh-frozen cortico-cancellous allograft (B). ACDF, anterior cervical discectomy and fusion.
Sagittal parameters were assessed at preoperative, postoperative, and at the final follow-up appointment with the operating spinal surgeon. Preoperative radiographs were obtained in the 1 month preceding operation, postoperative radiographs were taken in the 1 month following the operation, and final follow-up radiographs were obtained at the most recent visit with the surgeon of record.
4. Clinical Outcome
Postoperative data were collected regarding the presence of new radicular or myelopathic symptoms indicative of pathology at adjacent levels, indicating a clinical diagnosis of ASD. Patient-reported outcomes included NDI scores and VAS scores for neck and arm preoperatively and at the most recent follow-up. Patient charts were also reviewed for evidence of symptomatic pseudarthrosis and any reoperations in the cervical spine.
5. Statistical Analysis
Baseline patient characteristics were compared using chi-square analysis and independent sample t-tests for categorical and continuous data, respectively. Bivariate and multivariate regressions were applied to compare clinical outcomes between groups. Multivariate analyses controlled for differences in baseline patient characteristics.
RESULTS
A total of 404 patients fulfilled our inclusion criteria; of these, 353 (87.4%) underwent allograft placement, and 51 (12.6%) underwent ICBG (Table 1). Average clinical and radiographic follow-up was 24.3 months. There were no significant differences between allograft and autograft groups in demographic variables of age, female sex, body mass index, smoking, diabetes, or ASA physical status classification grade III. Number of levels fused via ACDF differed between groups, with a higher percentage of 1-level fusions performed using allograft (33.1% vs. 25.5%, p=0.026), and a greater proportion of 3-level fusions performed via autograft (23.5% vs. 19.0%, p=0.026); multivariate analysis was used to control for number of levels fused. The majority of patients underwent 2-level fusion procedures (47.5%).
Table 1.
Demographics
| Demographic | Allograft (n = 353) | Autograft (n = 51) | All patients (n = 404) | p-value |
|---|---|---|---|---|
| Age (yr) | 49.6 ± 10.8 | 52.4 ± 12.5 | 49.9 ± 11.1 | 0.094 |
| Female sex | 49.70 | 49.00 | 49.60 | 0.926 |
| BMI (kg/m2) | 28.7 ± 6.2 | 28.7 ± 6.9 | 28.7 ± 6.3 | 0.963 |
| Smoking | 20.40 | 11.80 | 19.30 | 0.144 |
| Diabetes | 11.30 | 14.00 | 11.70 | 0.582 |
| ASA grade ≥ III | 19.30 | 19.60 | 19.30 | 0.896 |
| No. of levels | 0.026* | |||
| 1 | 33.1 | 25.5 | 32.2 | |
| 2 | 47.6 | 47.1 | 47.5 | |
| 3 | 19.0 | 23.5 | 19.6 | |
| 4 | 0.3 | 3.9 | 0.7 |
Values are presented as mean±standard deviation or %.
BMI, body mass index; ASA, American Society of Anesthesiologists physical status classification system.
p<0.05, statistically significant differences.
Cervical lordosis was improved in both groups, without significant changes in SVA (Table 2). On bivariate analysis, the autograft group had a significantly greater amount of lordosis at the proximal segment on preoperative radiographs (4.1°±11.1° vs. 1.4°±7.4°, p=0.031), immediate postoperative radiographs (4.5°±12.2° vs. 0.8°±7.8°, p=0.004), and final follow-up radiographs (4.6°±11.2° vs. 1.8°±7.7°, p=0.025). On multivariate analysis, the autograft group had a significantly greater amount of lordosis at the proximal segment on immediate postoperative radiographs (4.5°±12.2° vs. 0.8°±7.8°, p=0.015) and less overall cervical lordosis at final follow-up (6.6°±9.6° vs. 9.5°±10.1°, p=0.010). Otherwise, fusion segment lordosis, T1 slope, and amount of lordosis at the distal segment were similar between the 2 groups at each time point. Similarly, there was no significant difference in the change of the sagittal alignment parameters at any time point among both groups (Table 3).
Table 2.
Preoperative and postoperative sagittal parameters
| Variable | Allograft | Autograft | All patients | Bivariate |
Multivariate |
||
|---|---|---|---|---|---|---|---|
| Beta | p-value | Beta | p-value | ||||
| Preoperative | |||||||
| Lordosis (°) | 4.7 ± 11.5 | 2.6 ± 10.9 | 4.4 ± 11.5 | -2.1 | 0.238 | -3.0 | 0.083 |
| SVA (mm) | 27.4 ± 11.1 | 27.6 ± 12.3 | 27.4 ± 11.2 | 0.2 | 0.919 | -0.4 | 0.816 |
| Fusion seg lordosis (°) | -0.1 ± 7.1 | 0.6 ± 8.4 | 0.0 ± 7.3 | 0.7 | 0.530 | 0.3 | 0.784 |
| T1 slope (°) | 26.0 ± 8.5 | 25.2 ± 7.9 | 25.9 ± 8.4 | -0.8 | 0.603 | -1.3 | 0.413 |
| Proximal lordosis (°) | 1.4 ± 7.4 | 4.1 ± 11.1 | 1.7 ± 8.0 | 2.7 | 0.031* | 1.7 | 0.145 |
| Distal lordosis (°) | 3.9 ± 4.4 | 2.4 ± 5.1 | 3.7 ± 4.5 | -1.5 | 0.099 | -1.2 | 0.190 |
| Immediate postoperative | |||||||
| Lordosis (°) | 7.7 ± 10.0 | 6.0 ± 10.2 | 7.5 ± 10.0 | -1.7 | 0.265 | -2.8 | 0.050 |
| SVA (mm) | 29.4 ± 10.7 | 31.6 ± 10.5 | 29.7 ± 10.7 | 2.1 | 0.189 | 2.0 | 0.189 |
| Fusion seg lordosis (°) | 6.26 ± 5.6 | 7.4 ± 6.8 | 6.4 ± 5.8 | 1.1 | 0.199 | 0.3 | 0.689 |
| T1 slope (°) | 27.9 ± 7.5 | 28.2 ± 1.5 | 27.9 ± 0.5 | 0.4 | 0.801 | -0.4 | 0.769 |
| Proximal lordosis (°) | 0.8 ± 7.8 | 4.5 ± 12.2 | 1.2 ± 8.5 | 3.7 | 0.004* | 3.0 | 0.015* |
| Distal lordosis (°) | 2.6 ± 4.6 | 1.6 ± 6.1 | 2.5 ± 4.8 | -1.0 | 0.244 | -0.7 | 0.470 |
| Final | |||||||
| Lordosis (°) | 9.5 ± 10.1 | 6.6 ± 9.6 | 9.1 ± 10.1 | -2.9 | 0.054 | -3.8 | 0.010* |
| SVA (mm) | 27.1 ± 10.2 | 28.6 ± 12.7 | 27.3 ± 10.5 | 1.5 | 0.347 | 0.9 | 0.548 |
| Fusion seg lordosis (°) | 5.8 ± 5.8 | 6.0 ± 6.2 | 5.9 ± 5.8 | 0.2 | 0.841 | -0.4 | 0.606 |
| T1 slope (°) | 28.7 ± 7.7 | 28.1 ± 8.0 | 28.6 ± 7.7 | -0.6 | 0.683 | -0.6 | 0.688 |
| Proximal lordosis (°) | 1.8 ± 7.7 | 4.6 ± 11.2 | 2.1 ± 8.3 | 2.8 | 0.025* | 2.0 | 0.093 |
| Distal lordosis (°) | 3.7 ± 5.0 | 2.2 ± 5.6 | 3.5 ± 5.1 | -1.5 | 0.105 | -1.1 | 0.262 |
Values are presented as mean±standard deviation.
SVA, sagittal vertical axis.
p<0.05, statistically significant differences.
Table 3.
Change in parameters at different time points
| Variable | Allograft | Autograft | All patients | Bivariate |
Multivariate |
||
|---|---|---|---|---|---|---|---|
| Beta | p-value | Beta | p-value | ||||
| Change preoperative to postoperative | |||||||
| Lordosis (°) | 3.1 ± 8.0 | 3.0 ± 8.4 | 3.1 ± 8.1 | -0.1 | 0.949 | -0.4 | 0.717 |
| SVA (mm) | 2.2 ± 7.5 | 4.1 ± 9.1 | 2.4 ± 7.8 | 2.0 | 0.118 | 1.9 | 0.121 |
| Fusion seg lordosis (°) | 6.4 ± 6.5 | 6.6 ± 6.6 | 6.4 ± 6.5 | 0.3 | 0.781 | 0.0 | 0.970 |
| T1 slope (°) | 2.0 ± 5.3 | 3.2 ± 6.5 | 2.2 ± 5.5 | 1.2 | 0.289 | 0.6 | 0.576 |
| Proximal lordosis (°) | -0.5 ± 3.9 | 0.3 ± 4.3 | -0.4 ± 4.0 | 0.8 | 0.169 | 0.8 | 0.214 |
| Distal lordosis (°) | -1.4 ± 4.1 | -0.4 ± 5.6 | -1.2 ± 4.3 | 1.0 | 0.254 | 1.0 | 0.261 |
| Change postoperative to final | |||||||
| Lordosis (°) | 1.7 ± 5.6 | 0.6 ± 5.8 | 1.5 ± 5.6 | -1.1 | 0.182 | -0.8 | 0.317 |
| SVA (mm) | -2.7 ± 7.2 | -3.7 ± 7.5 | -2.8 ± 7.3 | -1.1 | 0.343 | -1.4 | 0.209 |
| Fusion seg lordosis (°) | -0.4 ± 3.7 | -1.4 ± 3.8 | -0.6 ± 3.7 | -0.9 | 0.099 | -0.8 | 0.175 |
| T1 slope (°) | -0.2 ± 5.9 | -0.3 ± 4.4 | -0.1 ± 5.7 | -0.2 | 0.860 | 0.0 | 0.977 |
| Proximal lordosis (°) | 1.1 ± 3.7 | 0.1 ± 3.9 | 1.0 ± 3.8 | -1.0 | 0.081 | -1.0 | 0.080 |
| Distal lordosis (°) | 1.1 ± 3.8 | 0.5 ± 4.3 | 1.0 ± 3.9 | -0.6 | 0.413 | -0.4 | 0.575 |
| Change preoperative to final | |||||||
| Lordosis (°) | 4.8 ± 8.0 | 3.7 ± 6.6 | 4.7 ± 7.8 | -1.1 | 0.364 | -1.0 | 0.395 |
| SVA (mm) | -0.4 ± 8.1 | 0.5 ± 7.3 | -0.3 ± 8.0 | 0.9 | 0.460 | 0.7 | 0.588 |
| Fusion seg lordosis (°) | 5.9 ± 6.7 | 5.2 ± 6.4 | 5.8 ± 6.7 | -0.7 | 0.492 | -0.7 | 0.462 |
| T1 slope (°) | 2.1 ± 7.0 | 2.9 ± 5.2 | 2.2 ± 6.8 | 0.8 | 0.572 | 0.8 | 0.590 |
| Proximal lordosis (°) | 0.6 ± 4.6 | 0.6 ± 4.8 | 0.6 ± 4.6 | 0.0 | 0.992 | -0.1 | 0.930 |
| Distal lordosis (°) | -0.3 ± 4.3 | -0.5 ± 5.0 | -0.3 ± 4.4 | -0.2 | 0.780 | 0.0 | 0.970 |
Values are presented as mean±standard deviation.
SVA, sagittal vertical axis.
Clinical outcomes, as measured using VAS Neck, VAS Arm, and NDI scores, were similar between allograft and autograft groups, except preoperative NDI score (Table 4). On bivariate analysis, preoperative NDI score was higher in the allograft group (48.2±20.5 points vs. 36.8±16.4 points, p=0.038). This finding was not replicated in multivariate analysis. VAS Neck, VAS Arm, and NDI scores improved from preoperative levels in both groups. On multivariate analysis, there was no significant difference in the amount of patient-reported outcome improvement between groups in VAS Neck score (5.1±3.9 points vs. 5.5±3.6 points, p=0.434), VAS Arm score (4.2±3.8 points vs. 3.0±3.9 points, p=0.586), or NDI score (27.5±25.4 points vs. 22.9±17.5 points, p=0.866).
Table 4.
Comparing clinical outcomes
| Variable | Allograft | Autograft | All patients | Bivariate |
Multivariate |
||
|---|---|---|---|---|---|---|---|
| Beta | p-value | Beta | p-value | ||||
| Preoperative | |||||||
| VAS neck | 7.0 ± 3.3 | 7.1 ± 2.7 | 7.1 ± 3.2 | 0.0 | 0.969 | 0.2 | 0.819 |
| VAS arm | 5.7 ± 3.8 | 4.7 ± 3.8 | 5.6 ± 3.8 | -1.0 | 0.329 | -0.8 | 0.460 |
| NDI | 48.2 ± 20.5 | 36.8 ± 16.4 | 46.4 ± 20.3 | -11.4 | 0.038* | -8.0 | 0.141 |
| Final | |||||||
| VAS neck | 2.0 ± 2.4 | 2.1 ± 2.3 | 2.1 ± 2.4 | 0.1 | 0.922 | -0.2 | 0.735 |
| VAS arm | 1.6 ± 2.6 | 2.1 ± 3.1 | 1.7 ± 2.7 | 0.5 | 0.421 | 0.3 | 0.647 |
| NDI | 21.8 ± 20.7 | 17.0 ± 20.3 | 20.9 ± 20.6 | -4.8 | 0.343 | -5.2 | 0.311 |
| Change preoperative to final | |||||||
| VAS neck | 5.1 ± 3.9 | 5.5 ± 3.6 | 5.1 ± 3.8 | 0.5 | 0.669 | 0.9 | 0.434 |
| VAS arm | 4.2 ± 3.8 | 3.0 ± 3.9 | 4.1 ± 3.8 | -1.2 | 0.280 | -0.6 | 0.586 |
| NDI | 27.5 ± 25.4 | 22.9 ± 17.5 | 26.7 ± 24.2 | -4.6 | 0.492 | -1.1 | 0.866 |
Values are presented as mean±standard deviation (score).
VAS, visual analogue scale; NDI, Neck Disability Index.
p<0.05, statistically significant differences.
There were no significant differences between groups in radiographic ASD (allograft 19.6%, autograft 29.4%; odds ratio [OR], 1.75; p=0.101) or reoperation rates (allograft 4.3% vs. autograft 7.8%; OR, 1.98, p=0.250) at final follow-up (Table 5). Fusion rates, similarly, were high in both groups (allograft 97.4%, autograft 96.1%). Lastly, there was no significant difference in the rate of subsidence between the 2 groups (allograft 5.97%, autograft 9.80%; OR, 2.02; p=0.199).
Table 5.
Comparison of ASD, reoperations, fusion, and subsidence
| Variable | Allograft (%) | Autograft (%) | All patients (%) | Bivariate |
Multivariate |
||
|---|---|---|---|---|---|---|---|
| OR* | p-value | OR* | p-value | ||||
| Any ASD | 19.60 | 29.41 | 20.84 | 1.71 | 0.110 | 1.75 | 0.101 |
| Proximal | 14.49 | 21.57 | 15.38 | 1.62 | 0.194 | 1.69 | 0.171 |
| Distal | 9.38 | 13.73 | 9.93 | 1.54 | 0.335 | 1.48 | 0.390 |
| Proximal and distal | 4.26 | 5.88 | 4.47 | 1.40 | 0.602 | 1.32 | 0.678 |
| Reoperations | 4.26 | 7.84 | 4.71 | 1.91 | 0.267 | 1.98 | 0.250 |
| Fusion | 97.44 | 96.08 | 97.27 | 0.64 | 0.579 | 0.61 | 0.551 |
| Subsidence | 5.97 | 9.80 | 6.45 | 1.71 | 0.302 | 2.02 | 0.199 |
ASD, radiographic evidence of adjacent segment degeneration.
Subsidence as measured by a decrease in intervertebral disc height of ≥2 mm from immediate postoperative radiographs to final follow-up radiographs.
Odds ratio (OR) represents odds of ASD per one-unit increase in each sagittal parameter.
DISCUSSION
Although allograft is regularly utilized by surgeons performing ACDF with anterior plating, the superiority of allograft or autograft in maintaining or improving sagittal alignment has yet to be determined. It is also not well known if choosing allograft or autograft affects patient-reported outcomes. Our study determined that patients receiving autograft had significantly more lordosis at the proximal segment on immediate postoperative radiographs, but had significantly less overall cervical lordosis than the allograft group on final follow-up radiographs. We also found that there were no significant differences between groups in VAS Neck, VAS Arm, or NDI scores, nor significant differences in rates of fusion, subsidence, reoperations, or ASD when using allograft or autograft in ACDF.
Gum et al. [9] determined that cervical lordosis was an important factor in the prediction of postoperative health-related quality of life, finding that NDI scores decreased by 8 points for every 6 degrees of increased lordosis in the cervical spine. As a result of this finding, we expected to see significantly lower NDI scores in the allograft group as opposed to the autograft group, given the allograft group had more cervical lordosis than those receiving autograft. Our results did not corroborate this hypothesis. The lack of clinical improvement despite increased lordosis could be due to an increased level of proximal lordosis above the fusion segment relative to the increase in fusion segment lordosis. Villavicencio et al. found that improvement in fusion segment lordosis was more important in improving NDI and Short Form 36 Survey scores than overall cervical lordosis [12]. If our patients had improved proximal lordosis without much alteration in fusion segment lordosis, this could explain why there was not a significant difference in improvement of NDI scores between allograft and autograft groups. In terms of sagittal alignment, the allograft group in our study maintained greater cervical lordosis, although this did not necessarily correlate with overall outcomes, which may have a more significant impact on graft choice.
When we evaluated changes in sagittal alignment from preto postoperative period, and from postoperative to final follow-up, we found no significant differences between groups. In a 2012 investigation of 38 patients, Kim et al. [18] discovered that allograft use in ACDF with anterior plating leads to a higher level of graft collapse, but this was not borne out by our results. Although we did not directly measure graft collapse in this study, we expected to see a greater change in allograft sagittal parameters given previous results. This dissimilarity could be due to differences in patient selection or surgical technique. A separate study of 79 patients completed by Suchomel et al. [8] found no significant differences in graft collapse between autograft and allograft, which we believe is more in line with our results. Our results suggest that neither autograft nor allograft is superior in maintaining nor improving sagittal parameters in the postoperative period.
VAS Arm, VAS Neck, and NDI measurements all improved in both allograft and autograft groups, although neither graft option showed a significant benefit over the other. We could not find studies which directly measured patient-reported outcomes comparing allograft and autograft in ACDF, though most studies recorded fusion rate, clinical outcomes according to Odom’s criteria, rate of subsidence, or rate of graft collapse [6-8,18]. We conclude that both allograft and autograft are suitable choices from the patient perspective given similar patient-reported outcomes. Should patients or surgeons wish to avoid donor site complications or pain from ICBG harvesting, we recommend the use of allograft.
Patients who underwent ACDF with anterior plating using autograft or allograft had similar rates of fusion, reoperation, ASD, and subsidence. Both allograft group and autograft group had high rates of fusion (97.4% and 96.1%, respectively). This high level of fusion is similar to previously published research comparing allograft and autograft in ACDF with anterior plating [6-8]. While most investigations of allograft versus autograft for ACDF compare fusion rates, monitoring of reoperation and adjacent segments are not as common. We found no significant differences between groups in these parameters, suggesting that graft type is not essential when considering avoiding reoperations or ASD.
There are several limitations to this study. The study was retrospective, so patients were not randomly assigned to either group. During the study period, senior authors transitioned from use of autograft to allograft. However, the remaining fundamental techniques did not change. Although this could influence outcomes, the demographic variables were very similar in both groups, other than percentage of female patients, and all demographic variables were controlled for during multivariate statistical analysis. Another limitation is the short follow-up length in comparison to similar studies. Additionally, follow-up radiographs were not taken at specific intervals, but rather radiographs were selected based on their status as preoperative, immediate postoperative, or final follow-up radiographs. Instrumentation was somewhat varied between patients, based on surgeon preference which may impact outcomes. Half of the patients in the cohort received semi-rigid plates, while the other half received rigid plates.
CONCLUSION
Before this investigation, it was unknown if use of allograft or autograft in ACDF with anterior plating would lead to significant differences in sagittal parameters or patient-reported outcomes. By multivariate analysis, we found that while autograft use leads to significantly greater proximal segment lordosis in the immediate postoperative period, the use of autograft leads to significantly less cervical lordosis at final follow-up. Besides there was no difference between groups in terms of VAS Arm, VAS Neck, or NDI scores, nor did we find significant differences between groups in fusion rate, subsidence, reoperation rate, or rate of ASD. Overall, both graft choices are acceptable treatments for maintaining cervical sagittal parameters and improving patient-reported outcomes in patients undergoing ACDF for cervical radiculopathy or myelopathy.
Footnotes
The authors have nothing to disclose.
REFERENCES
- 1.Epstein NE. Iliac crest autograft versus alternative constructs for anterior cervical spine surgery: Pros, cons, and costs. Surg Neurol Int. 2012;3(Suppl 3):S143–56. doi: 10.4103/2152-7806.98575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Floyd T, Ohnmeiss D. A meta-analysis of autograft versus allograft in anterior cervical fusion. Eur Spine J. 2000;9:398–403. doi: 10.1007/s005860000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chau AM, Mobbs RJ. Bone graft substitutes in anterior cervical discectomy and fusion. Eur Spine J. 2009;18:449–64. doi: 10.1007/s00586-008-0878-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chau AM, Xu LL, Wong JH, et al. Current status of bone graft options for anterior interbody fusion of the cervical and lumbar spine. Neurosurg Rev. 2014;37:23–37. doi: 10.1007/s10143-013-0483-9. [DOI] [PubMed] [Google Scholar]
- 5.Fischer CR, Cassilly R, Cantor W, et al. A systematic review of comparative studies on bone graft alternatives for common spine fusion procedures. Eur Spine J. 2013;22:1423–35. doi: 10.1007/s00586-013-2718-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samartzis D, Shen FH, Goldberg EJ, et al. Is autograft the gold standard in achieving radiographic fusion in one-level anterior cervical discectomy and fusion with rigid anterior plate fixation? Spine (Phila Pa 1976) 2005;30:1756–61. doi: 10.1097/01.brs.0000172148.86756.ce. [DOI] [PubMed] [Google Scholar]
- 7.Samartzis D, Shen FH, Matthews DK, et al. Comparison of allograft to autograft in multilevel anterior cervical discectomy and fusion with rigid plate fixation. Spine J. 2003;3:451–9. doi: 10.1016/s1529-9430(03)00173-6. [DOI] [PubMed] [Google Scholar]
- 8.Suchomel P, Barsa P, Buchvald P, et al. Autologous versus allogenic bone grafts in instrumented anterior cervical discectomy and fusion: a prospective study with respect to bone union pattern. Eur Spine J. 2004;13:510–5. doi: 10.1007/s00586-003-0667-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gum JL, Glassman SD, Douglas LR, et al. Correlation between cervical spine sagittal alignment and clinical outcome after anterior cervical discectomy and fusion. Am J Orthop (Belle Mead NJ) 2012;41:E81–4. [PubMed] [Google Scholar]
- 10.Kwon B, Kim DH, Marvin A, et al. Outcomes following anterior cervical discectomy and fusion: the role of interbody disc height, angulation, and spinous process distance. J Spinal Disord Tech. 2005;18:304–8. doi: 10.1097/01.bsd.0000167359.10683.b1. [DOI] [PubMed] [Google Scholar]
- 11.Lee JS, Youn MS, Shin JK, et al. Relationship between cervical sagittal alignment and quality of life in ankylosing spondylitis. Eur Spine J. 2015;24:1199–203. doi: 10.1007/s00586-014-3491-8. [DOI] [PubMed] [Google Scholar]
- 12.Villavicencio AT, Babuska JM, Ashton A, et al. Prospective, randomized, double-blind clinical study evaluating the correlation of clinical outcomes and cervical sagittal alignment. Neurosurgery. 2011;68:1309–16. doi: 10.1227/NEU.0b013e31820b51f3. [DOI] [PubMed] [Google Scholar]
- 13.Gercek E, Arlet V, Delisle J, et al. Subsidence of stand-alone cervical cages in anterior interbody fusion: warning. Eur Spine J. 2003;12:513–6. doi: 10.1007/s00586-003-0539-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Selby MD, Clark SR, Hall DJ, et al. Radiologic assessment of spinal fusion. J Am Acad Orthop Surg. 2012;20:694–703. doi: 10.5435/JAAOS-20-11-694. [DOI] [PubMed] [Google Scholar]
- 15.Hilibrand AS, Robbins M. Adjacent segment degeneration and adjacent segment disease: the consequences of spinal fusion? Spine J. 2004;4(6 Suppl):190S–194S. doi: 10.1016/j.spinee.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Park MS, Kelly MP, Lee DH, et al. Sagittal alignment as a predictor of clinical adjacent segment pathology requiring surgery after anterior cervical arthrodesis. Spine J. 2014;14:1228–34. doi: 10.1016/j.spinee.2013.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Eck CF, Regan C, Donaldson WF, et al. The revision rate and occurrence of adjacent segment disease after anterior cervical discectomy and fusion: a study of 672 consecutive patients. Spine (Phila Pa 1976) 2014;39:2143–7. doi: 10.1097/BRS.0000000000000636. [DOI] [PubMed] [Google Scholar]
- 18.Kim SY, Park KS, Jung SS, et al. An early comparative analysis of the use of autograft versus allograft in anterior cervical discectomy and fusion. Korean J Spine. 2012;9:142–6. doi: 10.14245/kjs.2012.9.3.142. [DOI] [PMC free article] [PubMed] [Google Scholar]


