Abstract
Objective
Cervical disc replacement (CDR) is an effective long-term treatment for both cervical radiculopathy and myelopathy. However, there may be unique differences in the early postoperative clinical improvement for patients with and without myelopathy. In addition, previous studies using CDR to treat cervical myelopathy were underpowered to determine risk factors for relatively postoperative medical complications.
Methods
Two different cohorts were studied. A local cohort of patients undergoing CDR by a single surgeon was utilized to study the early postoperative course of clinical improvement. In addition, a national cohort of patients undergoing CDR in the 2015 and 2016 National Surgical Quality Improvement Program database was utilized to study differences in postoperative medical complications after CDR. Patients with a preoperative diagnosis of cervical myelopathy were identified in both cohorts, and perioperative outcomes and complications were compared to patients without myelopathy.
Results
A total of 43 patients undergoing CDR were included in the institutional cohort, of those 16 patients (37% of cohort) had a preoperative diagnosis of cervical myelopathy. A total of 3,023 patients undergoing CDR were included in the national cohort, of those 411 (13% of cohort) had a preoperative diagnosis of cervical myelopathy. In the institutional cohort, the nonmyelopathy group had a lower initial Neck Disability Index (NDI) and saw a faster improvement in NDI by 2 weeks postoperative. However, at 24 weeks there was no significant difference between groups in terms of NDI. Interestingly, only the nonmyelopathy cohort had a significant improvement in modified Japanese Orthopaedic Association score by 6 weeks (p<0.05). In the national cohort, myelopathy was associated with longer operative time and length of stay (p<0.05). However, there was no significant difference in perioperative complications (p>0.05) between myelopathy and nonmyelopathy patients.
Conclusion
Significant improvements in NDI, visual analogue scale (VAS)-arm pain, and VAS-neck pain are seen in both myelopathy and nonmyelopathy populations undergoing CDR by 6 weeks postoperatively. However, nonmyelopathy populations improve faster by 2 weeks postoperatively. In the national cohort analysis, medical complications were similarly low in both myelopathy and nonmyelopathy groups.
Keywords: Cervical disc arthroplasty, Myelopathy, Radiculopathy, Outcomes
INTRODUCTION
Cervical disc replacement (CDR) is an increasingly common treatment for cervical radiculopathy and myelopathy. Compared to the more traditional anterior cervical discectomy and fusion (ACDF) procedure, CDR preserves cervical motion while still decompressing the spinal canal and neuroforamen [1]. In recent series, CDR has been shown to result in better immediate postoperative range of motion and improved long-term outcomes compared to ACDF [2-9]. In addition, there is evidence suggesting lower rates of adjacent segment degeneration with CDR compared with ACDF [7,10,11].
For patients with myelopathy, both ACDF and CDR are known to be effective long-term treatment options. However, previous studies have compared postoperative complications and outcomes of patient with cervical radiculopathy versus cervical myelopathy [12-14]. In one large cohort of ACDF patients, myelopathy was associated with increased rates of serious medical adverse events and death [12]. In a smaller study of CDR patients, no significant differences were identified in 7-year clinical outcomes or adjacent level surgery for patients with or without myelopathy [13]. However, this small study was not powered to demonstrate differences in the rates of postoperative medical complications, nor did the study look at the variable course of improvement in outcomes during the early postoperative period. Finally, another study of 125 CDR patients similarly demonstrated improvement in patient-reported outcomes starting at 6-month follow-up, but again did not look at course of symptomatic improvement during the early postoperative period [14].
While CDR and has already been shown to be an effective long-term treatment for both cervical myelopathy and radiculopathy, we hypothesize that there may be unique differences in early postoperative course of symptomatic improvement seen between these different populations. This information would be useful to counsel patients regarding the time to clinical improvement following CDR for either cervical myelopathy or radiculopathy. The current study will track the early symptomatic improvement in patient-reported outcomes measures for CDR patients with and without myelopathy. In addition, previous studies of CDR for cervical myelopathy, in addition to our local cohort, are underpowered to determine risk factors for uncommon medical complications. Therefore, the current study will also utilize a large national cohort of patients undergoing CDR to compare uncommon medical complications between cohorts.
MATERIALS AND METHODS
1. Research Plans and Objective
A retrospective cohort study was conducted using both a local cohort and a large national cohort from the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database. The objective of the current study was to compare short-term complications and early postoperative outcomes in patients undergoing CDR for a diagnosis of cervical myelopathy or radiculopathy.
2. Methods – Institutional Cohort
In the institutional cohort, all consecutive adult patients undergoing CDR by a single surgeon during the study period of March 2017 and September 2018 were included. Patients were identified using a prospectively collected registry of spine surgery patients. All patients were consented for inclusion in the registry and local investigational review board approval was obtained prior to creation of the registry (IRB No. 2019-1126). This population was divided into 2 cohorts based the presence or absence of a preoperative diagnosis of cervical myelopathy. The diagnosis of myelopathy is made based on a combination of both radiological findings of spinal cord compression and clinical symptoms of hyperreflexia, ataxia, or loss of fine motor skills. Patients who had both radiographic and clinical findings documented in the preoperative medical chart were given a diagnosis of myelopathy. The severity of preoperative myelopathy was quantified based on the modified Japanese Orthopaedic Association score (mJOA).
Patient demographics including age, body mass index, American Society of Anesthesiologists (ASA) physical status classification, Charlson Comorbidity Index, and smoking status were collected. Operative data including number of disc levels treated (1 or 2), operative time, estimated blood loss, postoperative length of stay, postoperative transfusions, maximum postanesthesia care unit pain score within 3 hours postoperatively, and complications including neurological injury, surgical site infection, and incidental durotomy were recorded. Patient-reported outcomes measures (PROMs) were collected for patients preoperatively and at 2-, and 6-week postoperative visits. The PROMs collected include visual analogue scale (VAS)-arm pain, VAS-neck pain, Neck Disability Index (NDI), and mJOA.
3. Methods – National Cohort
In the national cohort, Current Procedural Terminology (CPT) procedure codes were used to identify adult patients undergoing cervical disc arthroplasty (CPT 22856, 22858). The diagnosis of myelopathy was determined using International Classification of Disease, Ninth Revision (ICD-9) and Tenth Revision (ICD-10) diagnosis codes. ICD-9 and ICD-10 principle diagnosis codes were used to identify all patients with preoperative diagnosis of myelopathy (ICD-9 721.1, 722.7, 721.91, ICD-10 M50.5, G99.2, M47.1). Demographic data (age, body mass index, ASA physical status classification, Charlson Comorbidity Index, smoker status), operative data (levels of CDR, operative time, hospital length of stay, discharge disposition, blood transfusions), and postoperative complication data (serious medical complications, surgical site infections, venous thromboembolisms) was also collected.
This cohort was then divided into 2 groups based on the presence of a preoperative diagnosis of myelopathy. Operative time, length of stay, discharge disposition, and complications were then compared between groups. The rate of serious medical complications was measured by aggregating the incidence of cardiac arrest, cerebrovascular accident, myocardial infarction, pulmonary embolism, renal failure requiring dialysis, and sepsis. In addition, the rate of surgical site infection (including superficial, deep, and organ space infections), venous thromboembolism (pulmonary embolism or deep vein thrombosis), and postoperative blood transfusion were compared.
All patients included in the NSQIP database are followed for 30 days postoperatively with regular chart review and phone interviews by NSQIP-trained clinical data reviewers. Data reviewers collect data on postoperative complications, reoperations, and readmission. Complications are all strictly defined by the NSQIP user guide [15], and data collection is regularly vetted by internal data audits confirming accuracy and completeness of registry data.
4. Statistical Analyses
In the institutional cohort Mann-Whitey Wilcoxon rank-sum testing was used to compare demographic data, operative data, and patient outcomes between myelopathy and nonmyelopathy patients, because a normal distribution cannot be assumed due to smaller sample size. Postoperative outcome measures were also compared with preoperative values using Mann-Whitey Wilcoxon rank-sum testing.
In the national cohort, Student t-test was utilized to compare age, body mass index, ASA physical status classification, Charlston Comorbidity Index, operative time, and length of stay. Chi-square testing was used to compared discharge disposition and rates of complications. In the study an alpha level of 0.05 was taken as statistically significant. Statistical test was conducted using Stata ver. 13.0 (StataCorp, LP, College Station, TX, USA).
RESULTS
1. Institutional Cohort
A total of 43 patients undergoing CDR were identified in the institutional cohort. Of those patients, 16 (37%) had a preoperative diagnosis of myelopathy. There were no statistically significant differences in age, body mass index, ASA physical status classification, or Charlson Comorbidity Index between myelopathy and nonmyelopathy groups (Table 1) (p>0.05). However, current smoking status was more common in myelopathy patients. In both the myelopathy and nonmyelopathy groups, there were no intraoperative complications in any patients, including incidental durotomy, hematoma, neurological deficit, or surgical site infection (Table 2). There was also no significant difference in operative time, blood loss, length of stay, or postoperative opioid or patient-controlled analgesia use between myelopathy and nonmyelopathy groups (Table 2) (p>0.05).
Table 1.
Institutional cohort demographic data (n=43)
| Variable | Nonmyelopathy (n = 27) | Myelopathy (n = 16) | p-value |
|---|---|---|---|
| Sex | 0.35 | ||
| Female | 8 (30) | 7 (44) | |
| Male | 19 (70) | 9 (56) | |
| Age (yr) | 43.8 ± 1.7 | 42.8 ± 2.6 | 0.77 |
| Charlson Comorbidity Index | 0.7 ± 0.3 | 0.3 ± 0.1 | 0.11 |
| Body mass index (kg/m2) | 26.5 ± 0.6 | 27.4 ± 1.6 | 0.90 |
| Current smoker | 1 (4) | 4 (25) | 0.04* |
| Preoperative diagnosis | |||
| Myelopathy | 0 (0) | 16 (100) | |
| Radiculopathy | 21 (78) | 13 (81) | |
| Herniated nucleus pulposus | 21 (78) | 11 (69) | |
| Degenerative disc disease | 5 (19) | 7 (44) | |
| Preoperative narcotic usage | 4 (15) | 3 (19) | 0.74 |
| Preoperative motor dysfunction | 8 (30) | 5 (31) | 0.91 |
Values are presented as number (%) or mean±standard error.
p<0.05, statistically significant difference between groups.
Table 2.
Institutional cohort surgical data (n=43)
| Variable | Nonmyelopathy (n = 27) | Myelopathy (n = 16) | p-value |
|---|---|---|---|
| Levels of disc arthroplasty | |||
| 1 | 23 (85) | 9 (56) | |
| 2 | 4 (15) | 7 (44) | |
| Disc spaces | |||
| C3–4 | 0 (0) | 2 (12) | |
| C4–5 | 2 (7) | 3 (19) | |
| C5–6 | 17 (63) | 10 (62) | |
| C6–7 | 12 (44) | 8 (50) | |
| Estimated blood loss | |||
| 1-Level | 26.1 ± 1.7 | 27.8 ± 2.8 | 0.49 |
| 2-Level | 25.0 ± 0.0 | 42.9 ± 10.5 | 0.14 |
| Operative time | |||
| 1-Level | 50.1 ± 2.1 | 54.0 ± 3.7 | 0.39 |
| 2-Level | 85.8 ± 4.3 | 107.9 ± 8.9 | 0.18 |
| Postop oral morphine equivalents (POD0) | 81.4 ± 9.8 | 115.9 ± 38.5 | 0.43 |
| Postop PCA (POD0) | 1 (4) | 2 (12) | 0.27 |
| Postoperative length of stay (hr) | 15.7 ± 2.3 | 15.7 ± 5.0 | 0.56 |
| Complications | |||
| Durotomy | 0 (0) | 0 (0) | |
| Hematoma | 0 (0) | 0 (0) | |
| Neurological deficit | 0 (0) | 0 (0) | |
| Surgical site infection | 0 (0) | 0 (0) |
Values are presented as number (%) or mean±standard deviation.
PCA, patient-controlled analgesia; POD0, postoperative day 0, day of surgery.
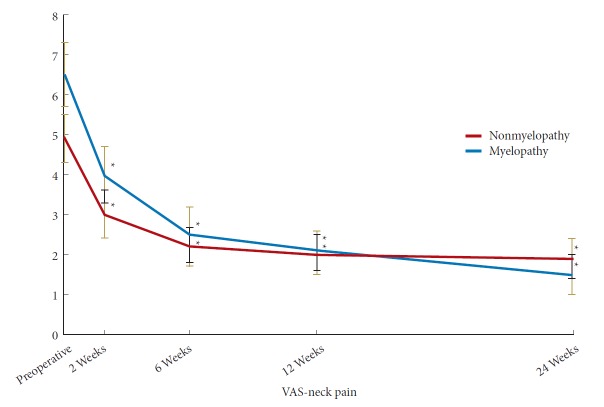
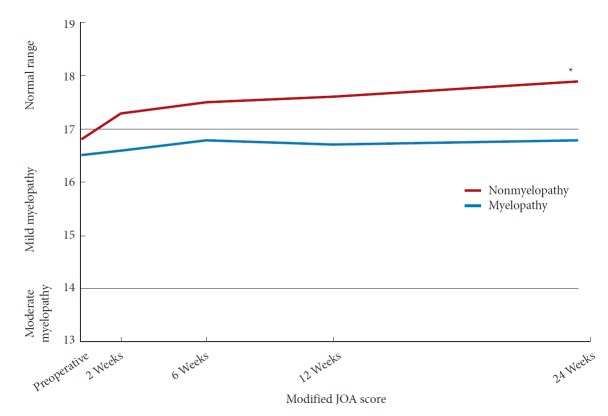
In the myelopathy group, there was a statistically significant improvement in NDI by 6 weeks postoperatively while in the nonmyelopathy group there was a significant improvement by 2 weeks postoperatively (Fig. 1). At 6 months postoperatively, there was no significant different in NDI between the 2 groups (Table 3) (p=0.58). In both the myelopathy and nonmyelopathy groups, there was a statistically significant improvement in VAS-neck pain score by 2 weeks postoperatively (Fig. 2). At 6 months postoperatively, there was no significant different in VAS-neck pain score between the groups (Table 3) (p=0.75). In the myelopathy group, there was a statistically significant improvement in VAS-arm pain score by 6 weeks postoperatively while in the nonmyelopathy group there was a significant improvement by 2 weeks postoperatively (Fig. 3). At 6 months postoperatively, there was no significant different in VAS-arm pain score between the groups (Table 3) (p=0.68). Interestingly, the myelopathy group did not see a statistically significant improvement in mJOA score by 6 months postoperatively, while the nonmyelopathy group demonstrated a significant improvement at 6 weeks and at 6 months postoperatively (Fig. 4).
Fig. 1.
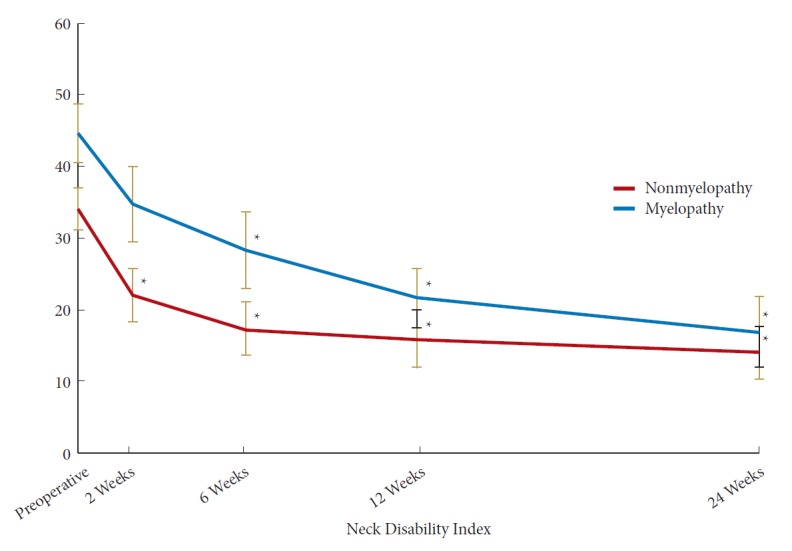
Postoperative improvement in Neck Disability Index. *Significant difference from preoperative value.
Table 3.
Institutional cohort postoperative outcomes (n=43)
| Variable | Nonmyelopathy (n=27) | Myelopathy (n=16) | p-value |
|---|---|---|---|
| Neck Disability Index | |||
| Preoperative (n = 43) | 33.9 ± 2.9 | 44.5 ± 4.1 | 0.06 |
| 2 Weeks postoperative (n = 40) | 22.0 ± 3.8† | 34.6 ± 5.2 | 0.06 |
| 6 Weeks postoperative (n = 33) | 17.3 ± 3.7† | 28.2 ± 5.3† | 0.11 |
| 12 Weeks postoperative (n = 33) | 15.9 ± 4.0† | 21.6 ± 4.1† | 0.26 |
| 6 Months postoperative (n = 29) | 14.0 ± 3.6† | 16.9 ± 4.9† | 0.58 |
| Visual analogue scale - neck pain | |||
| Preoperative (n = 43) | 4.9 ± 0.6 | 6.5 ± 0.8 | 0.09 |
| 2 Weeks postoperative (n = 40) | 3.0 ± 0.6† | 4.0 ± 0.7† | 0.16 |
| 6 Weeks postoperative (n = 33) | 2.2 ± 0.5† | 2.5 ± 0.7† | 0.58 |
| 12 Weeks postoperative (n = 33) | 2.0 ± 0.5† | 2.1 ± 0.5† | 0.74 |
| 6 Months postoperative (n = 29) | 1.9 ± 0.5† | 1.5 ± 0.5† | 0.75 |
| Visual analogue scale - arm pain | |||
| Preoperative (n = 43) | 4.9 ± 0.6 | 5.1 ± 1.0 | 0.79 |
| 2 Weeks postoperative (n = 40) | 1.3 ± 0.5† | 2.7 ± 0.8 | 0.21 |
| 6 Weeks postoperative (n = 33) | 0.8 ± 0.3† | 2.5 ± 0.8† | 0.18 |
| 12 Weeks postoperative (n = 33) | 1.0 ± 0.4† | 2.3 ± 0.7† | 0.26 |
| 6 Months postoperative (n = 29) | 0.9 ± 0.4† | 1.2 ± 0.5† | 0.68 |
| Modified Japanese Orthopaedic Association | |||
| Preoperative (n = 21) | 16.8 ± 0.5 | 16.5 ± 0.6 | 0.66 |
| 2 Weeks postoperative (n = 20) | 17.3 ± 0.3 | 16.6 ± 0.6 | 0.44 |
| 6 Weeks postoperative (n = 22) | 17.5 ± 0.2† | 16.8 ± 0.5 | 0.59 |
| 12 Weeks postoperative (n = 22) | 17.6 ± 0.2 | 16.7 ± 0.4 | 0.10 |
| 6 Months postoperative (n = 21) | 17.9 ± 0.1† | 16.8 ± 0.5 | 0.09 |
Values are presented as mean±standard error.
p<0.05, statistically significant difference between groups.
p<0.05. statistically significant difference from preoperative value.
Fig. 2.
Postoperative Improvement in visual analogue scale (VAS)-neck pain. *Significant difference from preoperative value.
Fig. 3.
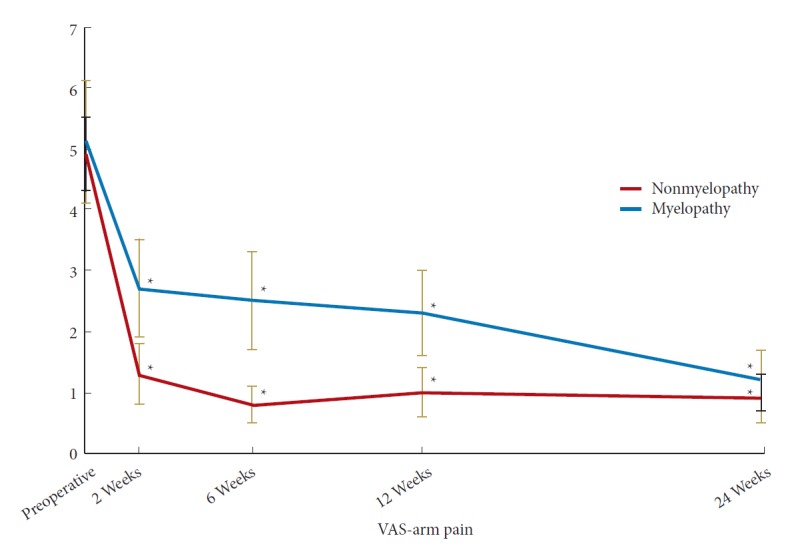
Postoperative Improvement in visual analogue scale (VAS)-arm pain. *Significant difference from preoperative value.
Fig. 4.
Postoperative Improvement in modified Japanese Orthopaedic Association (JOA) score. *Significant difference from preoperative value.
2. National Cohort
A total of 3,023 patients were identified in the national cohort, 411 (13%) with myelopathy. There were no statistically significant differences in age (mean: 45.7 vs. 46.2, p=0.42), body mass index (29.2 vs. 29.3, p=0.74), ASA physical status classification (2.2 vs. 2.2, p=0.21), Charlson Comorbidity Index (0.5 vs. 0.6, p=0.31), and smoking status (23.1% vs. 24.1%, p=0.66) between myelopathy and nonmyelopathy groups. In the national cohort (Table 4), myelopathy was associated with longer operative time and length of stay, even after controlling for the number of levels treated (p<0.05). However, there was no significant difference in discharge disposition to outside facility (1.2% vs. 1.7%, p=0.76), serious medical complications (0.2% vs. 0.2%, p=0.96), venous thromboembolism (0.1% vs. 0.2%, p=0.50), surgical site infection (0.4% vs. 0.2%, p=0.66), or blood transfusion (0.5% vs. 0.0%, p=0.15). Long-term data outside of 30 days postoperatively and patient-reported outcome measures are not available in the NSQIP database.
Table 4.
National cohort demographics and postoperative short-term outcomes
| Variable | Nonmyelopathy (n=2,612) | Myelopathy (n=411) | p-value |
|---|---|---|---|
| Age (yr) | 45.7 ± 0.2 | 46.2 ± 0.5 | 0.42 |
| Body mass index (kg/m2) | 29.2 ± 0.1 | 29.3 ± 0.3 | 0.74 |
| ASA physical status classification | 2.2 ± 0.0 | 2.2 ± 0.0 | 0.21 |
| Charlson Comorbidity Index | 0.5 ± 0.0 | 0.6 ± 0.0 | 0.31 |
| Current smoker | 603 (23.1) | 99 (24.1) | 0.66 |
| Levels of cervical disc replacement | < 0.01* | ||
| 1-Level | 2,320 (88.8) | 345 (83.9) | |
| 2-Level | 292 (11.2) | 66 (16.1) | |
| Operative time (min) | 113.9 ± 1.2 | 129.1 ± 2.7 | < 0.01* |
| Total hospital length of stay (day) | 1.2 ± 0.0 | 1.4 ± 0.1 | < 0.01* |
| Discharge to facility, not home | 31 (1.2) | 7 (1.7) | 0.76 |
| Serious medical complications | 6 (0.2) | 1 (0.2) | 0.96 |
| Surgical site infections | 10 (0.4) | 1 (0.2) | 0.66 |
| Venous thromboembolism | 3 (0.1) | 1 (0.2) | 0.50 |
| Blood transfusion | 13 (0.5) | 0 (0.0) | 0.15 |
Values are presented as mean±standard error or number (%).
ASA, American Society of Anesthesiologists.
p<0.05, statistically significant difference between groups.
DISCUSSION
CDR is an effective treatment for both cervical myelopathy and radiculopathy [2]. Biomechanically, compared to ACDF, CDR better approximates normal spine kinematics by preserving cervical motion segments [1,8]. Over the last 2 decades the use of CDR has increased, with 3 times more procedures performed in 2006 than in 2013 [16]. While CDR has been shown to have good long-term outcomes for treatment of both myelopathy and radiculopathy [2-7,10,11], further study of the early postoperative period is warranted as there may be unique differences in early symptomatic improvement between these populations. This information would be valuable to counsel patients preoperatively regarding the time to clinical improvement following CDR for either cervical myelopathy or radiculopathy. Using a local institutional cohort, the current study tracked early symptomatic improvement in PROMs for CDR patients with and without myelopathy. In addition, previous studies of cervical myelopathy treated using CDR were underpowered to identify any association of medical complications with myelopathy versus radiculopathy. To answer this question the current study utilized a large national cohort of patients undergoing CDR similar to previous studies of ACDF patients with myelopathy.
The current study found that in myelopathy patients, CDR resulted in a significant improvement in VAS-neck pain by 2 weeks postoperatively, and significant improvements in NDI and VAS-arm pain by 6 weeks postoperatively (Figs. 5, 6). Improvements were maintained at 6 months postoperatively Interestingly, radiculopathy patients, actually saw a more rapid improvement in outcomes, with significant improvements in NDI, VAS-arm pain, and VAS-neck pain by 2 weeks postoperatively, and maintained at 6 months postoperatively. No myelopathy or radiculopathy patients suffered from postoperative complications.
Fig. 5.
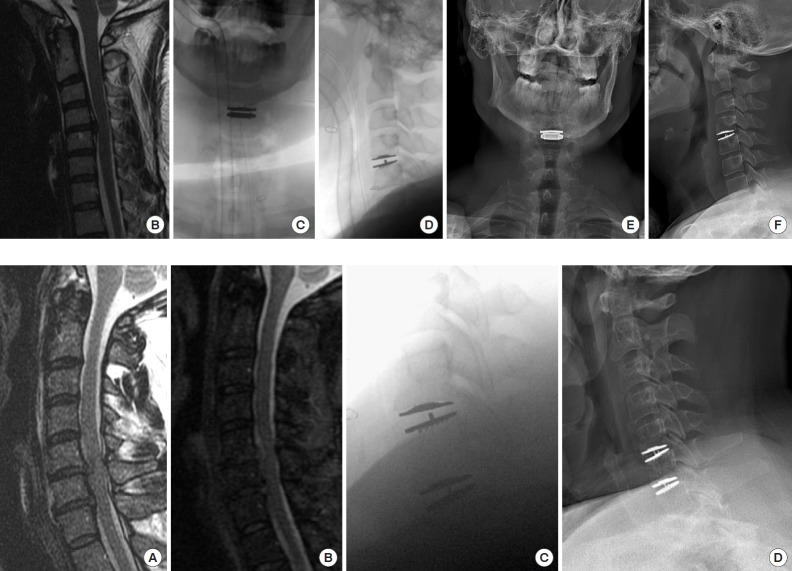
(A-F) Representative imaging from a 39-year-old male patient with a herniated nucleus pulposus and central stenosis at C4–5 disc space causing cervical myelopathy. Patient underwent a 1-level cervical disc replacement with postoperative improvement in Neck Disability Index. (A) Preoperative axial magnetic resonance imaging (MRI), fluid sensitive, T2-weighted pulse sequence showing disc herniation, central stenosis, and myelomalacia with increased cord signal. (B) Preoperative T2-weighted sagittal MRI. (C) Intraoperative coronal fluoroscopy. (D) Intraoperative sagittal fluoroscopy. (E) Six-month postoperative coronal radiograph. (F) Six-month postoperative sagittal radiograph.
Fig. 6.
(A-D) Representative imaging from a 51-year-old male patient with herniated nuclei pulposus and central stenosis at C5–6 and C6–7 disc spaces causing cervical myelopathy. Patient underwent a 2-level cervical disc replacement with postoperative improvement in Neck Disability Index. (A) Preoperative sagittal T2-weighted magnetic resonance imaging (MRI) sequence. (B) Preoperative sagittal fat saturated MRI sequence showing myelomalacia and cord signal change. (C) Intraoperative sagittal fluoroscopy. (D) Six-month postoperative sagittal radiograph.
Previous studies comparing outcomes after CDR in patients with and without myelopathy did not consider any possible differences in symptomatic improvement during this early postoperative period. These studies largely all show similar longterm improvements in outcomes for patients with and without myelopathy. In a study of approximately 400 CDR patients, no significant differences were identified in 7-year clinical outcomes or adjacent level surgery for patients with or without myelopathy [13]. However, outcomes earlier than 2 years were not studied. While long-term outcomes are ultimately most important for determining sustained effectiveness of the CDR procedure, understanding the early postoperative course of clinical improvement is additionally valuable for patient counseling. Another study of 125 CDR patients also demonstrated similar improvement in patient-reported outcomes between myelopathy and nonmyelopathy patients at 6-month follow-up, but again did not look at course of symptomatic improvement during the early postoperative period [14]. Finally, another study of 55 CDR patients found significant improvements in patient-reported outcomes in both myelopathy and radiculopathy patients at long-term follow-up (median, 48 months), but no significant differences between the groups [17].
In our national cohort analysis, even in a well-powered study of over 3,000 CDR patients, the myelopathy and nonmyelopathy groups did not differ significantly in the rate of postoperative medical complications or in discharge disposition. In addition, the overall rate of serious or moderate medical complications was very low. This is in contrast to a previous large national cohort study of ACDF patients with and without myelopathy, which found that myelopathy was associated with a nine-fold increase in postoperative mortality [12]. This may be due to the overall older age of ACDF patients compared with CDR patients, with a mean age of 53 years in the previous study (compared with 46 years in the current study) and with one-third of patients having a ASA physical status classification or CCI of at least 3. Interestingly, our national cohort analysis found that myelopathy was associated with increased operative time and length of stay. This significant association was not identified in our small institutional cohort, highlighting the relative strengths of the 2 different types of patient registry analyses.
As previously noted, much of the existing literature regarding use of CDR for myelopathy has focused on long-term outcomes [13,14,17]. CDR has also been shown to be effective for specific types of cervical myelopathy, such as ossification of the posterior longitudinal ligament (OPLL) [18]. One previous study of patients with severe 3-level cervical myelopathy from OPLL found that hybrid surgery with CDR combined with 1-level anterior cervical corpectomy resulted in improvement in NDI, mJOA, and preserved range of motion across the CDR level at 12 months postoperatively [19]. Another study of patients with 3-level myelopathy from congenital spinal stenosis found that hybrid 2-level CDR with 1-level ACDF had similar long-term outcomes as 3-level ACDF, albeit with improved postoperative neck range of motion [20].
A number of studies have previous compared CDR with ACDF, the current standard of care for treatment of myelopathy [21-23]. One randomized controlled trial examined short- and long-term outcomes by studying 2 total arthroplasty implant systems in patients with myelopathy compared with traditional ACDF in patients with myelopathy. The authors found that both ACDF and CDR improved NDI at 6 weeks postoperatively and that this improvement that was durable to 2 years [24]. However, the study did not specifically compare CDR patients with and without myelopathy. A recent retrospective study examined outcomes for CDR versus ACDF in patients with myelopathy and also found surgery time was longer for CDR but with lower rates of serious adverse events. Rates of reoperation and improvements in gait and neurologic function were also similar between CDR and ACDF at both 2- and 7-year follow-up [13].
Similarly, a number of other studies have specifically compared outcomes after ACDF and CDR in patients with cervical radiculopathy. One randomized controlled trial of 142 radiculopathy patients treated with CDR or ACDF found no significant different in NDI at long-term follow-up, or significant differences in secondary PROMs [25]. A study of 60 consecutive cervical radiculopathy patients found that CDR was associated with similar improvements in VAS-neck pain and Short-Form 12 questionnaire compared with ACDF, however cost-effectiveness was best with ACDF with cage alone [26]. Interestingly, one economic and decision analysis found that for radiculopathy patients, CDR resulted in lower long-term costs compared with ACDF [27]. Finally, a cohort study of CDR compared with posterior microendoscopic laminoforaminotomy for radiculopathy patients found no significant difference in improvements in NDI, VAS-neck pain, or VAS-arm pain at 3-, 6-, 12-, and 24-month follow-up [28].
There are a number of strengths to the current study. First, our institutional cohort analysis is the first study to specifically study both the early postoperative course of symptomatic improvement in CDR patients with or without myelopathy. In addition, the national cohort analysis is the first study powered to compare uncommon medical complications between CDR patients with or without myelopathy.
There are also several limitations of this study that must be considered when analyzing conclusions. The first of which is the small sample size of our institutional data. A sample of 43 patients, although similar to some previous studies of long-term outcomes, is not powered to detect differences in certain variables such as medical complications. Therefore, variables such as operative time and length of stay were found to be associated with myelopathy in our national cohort but not in our institutional cohort. In addition, our national cohort data did not include patient-reported outcomes, although this was studied in the institutional cohort. In this way, the current study highlights the relative strengths of a small versus large patient registries. While large national databases are well powered to analyze rare postoperative complications, they often do not include granular patient-reported outcome data. Finally, we recognize that the current study does not describe long-term outcomes greater than 6 months. We recognize that long-term outcomes are ultimately of significant importance when assessing the efficacy of surgery and for preoperative patient counseling. However, as numerous studies exist reporting long-term outcomes after CDR up to 7 years postoperatively, it is valuable to also now understand differences during the early postoperative course of symptomatic improvement.
CONCLUSION
CDR results in significant improvements in NDI, VAS-arm pain, and VAS-neck pain in both myelopathy and nonmyelopathy populations. However, significant improvements are seen faster in nonmyelopathy populations. In a large national cohort, medical complications were similarly low in both myelopathy and nonmyelopathy groups.
Myelopathy is not associated with increased perioperative morbidity and complications after CDR, contrary to what has been shown with ACDF patients. Significant improvement in patient-reported outcomes is seen at 6 weeks in myelopathy patients, although more rapid improvement is seen in patients without myelopathy.
Footnotes
Dr. Steven McAnany: Currently receiving Consulting Fees from Titan, Nuvasive, Stryker K2M. Dr. Todd Albert: Currently receiving Royalties from Biomet, DePuy Synthes; Consulting Fees from Nuvasive; Ownership Interests in ASIP, Augmedics, Biomerix, Bonovo Orthopedics Inc., Crosstrees Medical, Cyto-Dyn Inc., Gentis, Innovative Surgical Designs Inc., InVivo Therapeutics, Invuity, Morphogenesis, Paradigm Spine, Physician Recommended Neutriceuticals, PMIG, Pulse Equity, Spinicity, Strathspey Crown, Surg.IO LLC, Vital 5. Dr. Sravisht Iyer: Currently receiving Support from Healthgrades; Grant support from NASS. Dr. Sheeraz Qureshi: Currently receiving Consulting Fees from Stryker K2M, Globus Medical, Inc., Paradigm Spine; Royalties from RTI, Globus Medical Inc., Stryker K2M; Ownership Interest in Avaz Surgical, Vital 5; Medical/Scientific Advisory Board member at Spinal Simplicity, Lifelink.com; Board membership at Healthgrades, Minimally Invasive Spine Study Group; Honoraria from AMOpportunities. The other authors have nothing to disclose.
REFERENCES
- 1.Miller J, Sasso R, Anderson P, et al. Adjacent level degeneration: bryan total disc arthroplasty versus anterior cervical discectomy and fusion. Clin Spine Surg. 2018;31:E98–101. doi: 10.1097/BSD.0000000000000598. [DOI] [PubMed] [Google Scholar]
- 2.Bono CM, Ghiselli G, Gilbert TJ, et al. An evidence-based clinical guideline for the diagnosis and treatment of cervical radiculopathy from degenerative disorders. Spine J. 2011;11:64–72. doi: 10.1016/j.spinee.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 3.Gornet MF, Burkus JK, Shaffrey ME, et al. Cervical disc arthroplasty: 10-year outcomes of the Prestige LP cervical disc at a single level. J Neurosurg Spine. 2019 May 10;:1–9. doi: 10.3171/2019.2.SPINE1956. [Epub]. [DOI] [PubMed] [Google Scholar]
- 4.Gornet MF, Lanman TH, Burkus JK, et al. Two-level cervical disc arthroplasty versus anterior cervical discectomy and fusion: 10-year outcomes of a prospective, randomized investigational device exemption clinical trial. J Neurosurg Spine. 2019 Jun 21;:1–11. doi: 10.3171/2019.4.SPINE19157. [Epub]. [DOI] [PubMed] [Google Scholar]
- 5.Zou S, Gao J, Xu B, et al. Anterior cervical discectomy and fusion (ACDF) versus cervical disc arthroplasty (CDA) for two contiguous levels cervical disc degenerative disease: a meta-analysis of randomized controlled trials. Eur Spine J. 2017;26:985–97. doi: 10.1007/s00586-016-4655-5. [DOI] [PubMed] [Google Scholar]
- 6.Lavelle WF, Riew KD, Levi AD, et al. Ten-year outcomes of cervical disc replacement with the BRYAN cervical disc: results from a prospective, randomized, controlled clinical trial. Spine (Phila Pa 1976) 2019;44:601–8. doi: 10.1097/BRS.0000000000002907. [DOI] [PubMed] [Google Scholar]
- 7.Gao X, Yang Y, Liu H, et al. A comparison of cervical disc arthroplasty and anterior cervical discectomy and fusion in patients with two-level cervical degenerative disc disease: 5-year follow-up results. World Neurosurg. 2019;122:e1083–9. doi: 10.1016/j.wneu.2018.10.231. [DOI] [PubMed] [Google Scholar]
- 8.Chang CC, Huang WC, Wu JC, et al. The option of motion preservation in cervical spondylosis: cervical disc arthroplasty update. Neurospine. 2018;15:296–305. doi: 10.14245/ns.1836186.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tu TH, Lee CY, Kuo CH, et al. Cervical disc arthroplasty for less-mobile discs. J Neurosurg Spine. 2019 May 10;:1–7. doi: 10.3171/2019.2.SPINE181472. [Epub]. [DOI] [PubMed] [Google Scholar]
- 10.Latka D, Kozlowska K, Miekisiak G, et al. Safety and efficacy of cervical disc arthroplasty in preventing the adjacent segment disease: a meta-analysis of mid- to long-term outcomes in prospective, randomized, controlled multicenter studies. Ther Clin Risk Manag. 2019;15:531–9. doi: 10.2147/TCRM.S196349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu S, Liang Y, Zhu Z, et al. Adjacent segment degeneration or disease after cervical total disc replacement: a meta-analysis of randomized controlled trials. J Orthop Surg Res. 2018;13:244. doi: 10.1186/s13018-018-0940-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lukasiewicz AM, Basques BA, Bohl DD, et al. Myelopathy is associated with increased all-cause morbidity and mortality following anterior cervical discectomy and fusion: a study of 5256 patients in American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) Spine (Phila Pa 1976) 2015;40:443–9. doi: 10.1097/BRS.0000000000000785. [DOI] [PubMed] [Google Scholar]
- 13.Gornet MF, McConnell JR, Riew KD, et al. Treatment of cervical myelopathy: long-term outcomes of arthroplasty for myelopathy versus radiculopathy, and arthroplasty versus arthrodesis for myelopathy. Clin Spine Surg. 2018;31:420–7. doi: 10.1097/BSD.0000000000000744. [DOI] [PubMed] [Google Scholar]
- 14.Fay LY, Huang WC, Wu JC, et al. Arthroplasty for cervical spondylotic myelopathy: similar results to patients with only radiculopathy at 3 years' follow-up. J Neurosurg Spine. 2014;21:400–10. doi: 10.3171/2014.3.SPINE13387. [DOI] [PubMed] [Google Scholar]
- 15.MBSAQIP operations manual . Chicago (IL): American College of Surgeons; 2014. Chapter 2: The data collection process. [Google Scholar]
- 16.Saifi C, Fein AW, Cazzulino A, et al. Trends in resource utilization and rate of cervical disc arthroplasty and anterior cervical discectomy and fusion throughout the United States from 2006 to 2013. Spine J. 2018;18:1022–9. doi: 10.1016/j.spinee.2017.10.072. [DOI] [PubMed] [Google Scholar]
- 17.Khong P, Bogduk N, Ghahreman A, et al. Cervical disc arthroplasty for the treatment of spondylotic myelopathy and radiculopathy. J Clin Neurosci. 2013;20:1411–6. doi: 10.1016/j.jocn.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 18.Wu JC, Chen YC, Huang WC. Ossification of the posterior longitudinal ligament in cervical spine: prevalence, management, and prognosis. Neurospine. 2018;15:33–41. doi: 10.14245/ns.1836084.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang HC, Tu TH, Chang HK, et al. Hybrid corpectomy and disc arthroplasty for cervical spondylotic myelopathy caused by ossification of posterior longitudinal ligament and disc herniation. World Neurosurg. 2016;95:22–30. doi: 10.1016/j.wneu.2016.07.065. [DOI] [PubMed] [Google Scholar]
- 20.Chang PY, Chang HK, Wu JC, et al. Is cervical disc arthroplasty good for congenital cervical stenosis? J Neurosurg Spine. 2017;26:577–85. doi: 10.3171/2016.10.SPINE16317. [DOI] [PubMed] [Google Scholar]
- 21.Janssen ME, Zigler JE, Spivak JM, et al. ProDisc-C total disc replacement versus anterior cervical discectomy and fusion for single-level symptomatic cervical disc disease: seven-year follow-up of the prospective randomized U.S. Food and Drug Administration Investigational Device Exemption Study. J Bone Joint Surg Am. 2015;97:1738–47. doi: 10.2106/JBJS.N.01186. [DOI] [PubMed] [Google Scholar]
- 22.Wu TK, Liu H, Wang BY, et al. Minimum four-year subsequent surgery rates of cervical disc replacement versus fusion: a meta-analysis of prospective randomized clinical trials. Orthop Traumatol Surg Res. 2017;103:45–51. doi: 10.1016/j.otsr.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 23.Bae HW, Davis RJ, Hisey MS, et al. Comparing one-level versus two-level cervical TDR and one-level versus two-level ACDF at seven-year follow-up. Spine J. 2016;16(10 Suppl):S203–4. [Google Scholar]
- 24.Riew KD, Buchowski JM, Sasso R, et al. Cervical disc arthroplasty compared with arthrodesis for the treatment of myelopathy. J Bone Joint Surg Am. 2008;90:2354–64. doi: 10.2106/JBJS.G.01608. [DOI] [PubMed] [Google Scholar]
- 25.Donk RD, Verbeek ALM, Verhagen WIM, et al. What’s the best surgical treatment for patients with cervical radiculopathy due to single-level degenerative disease? A randomized controlled trial. PLoS One. 2017;12:e0183603. doi: 10.1371/journal.pone.0183603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhadra AK, Raman AS, Casey AT, et al. Single-level cervical radiculopathy: clinical outcome and cost-effectiveness of four techniques of anterior cervical discectomy and fusion and disc arthroplasty. Eur Spine J. 2009;18:232–7. doi: 10.1007/s00586-008-0866-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghori A, Konopka JF, Makanji H, et al. Long term societal costs of anterior discectomy and fusion (ACDF) versus cervical disc arthroplasty (CDA) for treatment of cervical radiculopathy. Int J Spine Surg. 2016;10:1. doi: 10.14444/3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu GM, Wang YJ, Wang DS, et al. Comparison of one-level microendoscopy laminoforaminotomy and cervical arthroplasty in cervical spondylotic radiculopathy: a minimum 2-year follow-up study. J Orthop Surg Res. 2013;8:48. doi: 10.1186/1749-799X-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]